Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.102690
Revised: January 23, 2025
Accepted: February 17, 2025
Published online: April 15, 2025
Processing time: 151 Days and 4.7 Hours
Although targeted therapy provides survival benefits for patients with metastatic colorectal cancer, some patients develop resistance to these treatments. Human epidermal growth factor receptor 2 (HER2) is overexpressed in a subset of pa
This case report describes a Chinese patient with HER2-amplified advanced rectal cancer who showed no response to chemotherapy and targeted therapies against epidermal growth factor receptor and vascular endothelial growth factor but achieved a remarkable response following treatment with immune checkpoint inhibitors (ICIs) in combination with pyrotinib. The combination of oxaliplatin and ICIs with pyrotinib demonstrates synergistic effects after late-stage disease progression.
ICIs and pyrotinib may be effective in treating HER2-amplified advanced rectal cancer. Chemotherapy following disease progression could enhance efficacy syn
Core Tip: Human epidermal growth factor receptor 2-amplified advanced rectal cancer was unresponsive to conventional chemotherapy and targeted therapy in a Chinese patient. This study demonstrated the synergistic effect of combining an immune checkpoint inhibitor with pyrotinib, providing a new treatment strategy for patients with advanced rectal cancer. Following treatment with an immune checkpoint inhibitor in combination with pyrotinib, the patient achieved significant therapeutic effects as evidenced by a marked reduction in tumour size.
- Citation: Xiao X, Wang QW, Zhou ZY, Wang LS, Huang P. Precision treatment for human epidermal growth factor receptor 2-amplified advanced rectal cancer: A case report. World J Gastrointest Oncol 2025; 17(4): 102690
- URL: https://www.wjgnet.com/1948-5204/full/v17/i4/102690.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i4.102690
The incidence and mortality rates of colorectal cancer rank third and second, respectively, among all malignant tumours, and its incidence is increasing annually[1]. Due to the non-specificity of early symptoms, 30%-40% of patients with colorectal cancer present with distant metastases at the time of diagnosis[2]. Although the advent of targeted combination therapies has significantly prolonged patient survival, the options for therapeutic drugs and their efficacy after drug-resistant progression remain limited[3]. In our department, targeted drugs are selected for patients with advanced metastatic rectal cancer who have progressed after conventional treatment based on the results of precision genetic testing in conjunction with immune checkpoint inhibitor therapy. This approach yielded good efficacy and was well-tolerated. When the disease progressed, we opted to combine single-agent chemotherapy, which remained effective.
The patient’s main complaints were altered bowel habits for more than two months and haematochezia for one week.
Two months earlier, the 59-year-old male experienced a change in bowel habits that was not given adequate attention. One week prior to presentation, this was accompanied by haematochezia; however, there were no symptoms of nausea, vomiting, or fatigue.
The patient denied any history of chronic conditions such as hypertension, diabetes, or other malignancies.
The patient reported occasional smoking and minimal alcohol consumption. The patient’s medical history was unremarkable.
Upon admission, the patient’s vital signs were as follows: Body temperature 36.9°C, blood pressure 120/70 mmHg, respiratory rate 18 breaths per minute, and heart rate 80 beats per minute. An abdominal examination revealed no tenderness or rebound pain. The systemic superficial lymph nodes were not enlarged. A digital rectal examination was not performed.
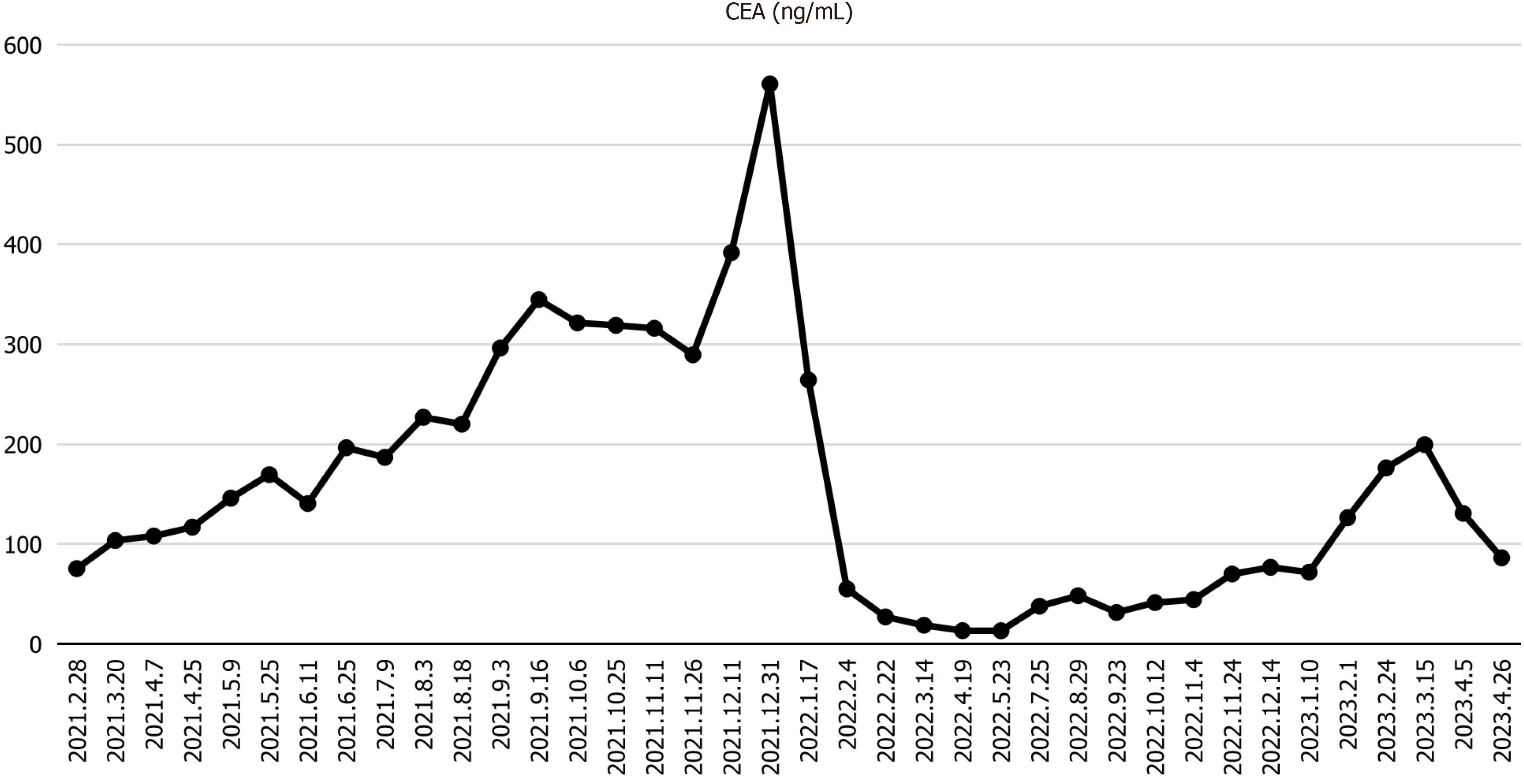
The carcinoembryonic antigen (CEA) level was 75.38 ng/mL, which was slightly elevated above the normal limits, and other routine blood tests were within normal limits.
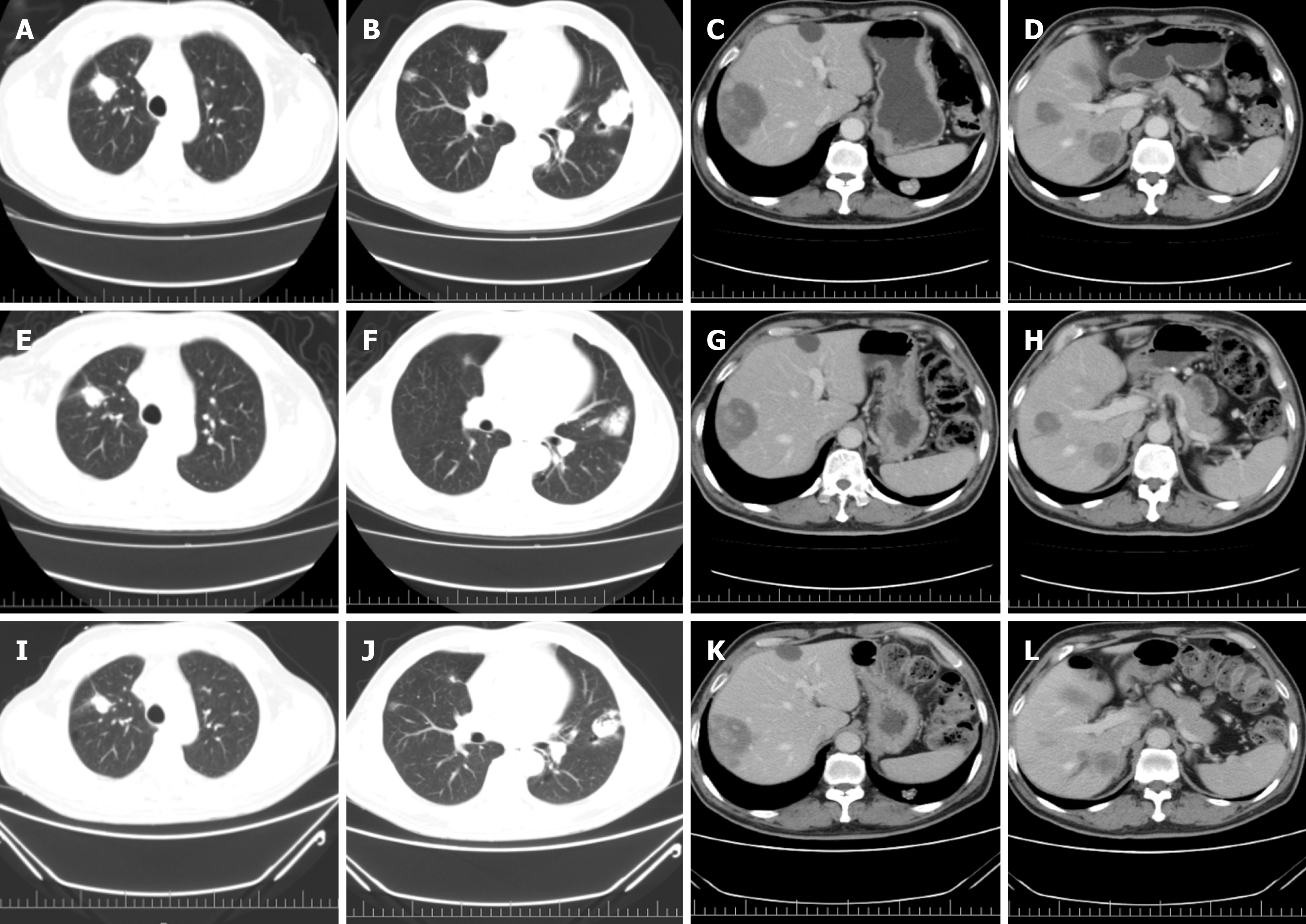
Colonoscopy performed in March 2021 revealed a large, irregular mass with an uneven surface, friable texture, and a tendency to bleed upon contact. The mass was located 15 cm from the anal verge and occupied the entire intestinal lumen. Therefore, rectal cancer was suspected. Colonoscopic biopsy revealed rectal adenocarcinoma. Magnetic resonance imaging demonstrated a rectal tumour with surrounding infiltration and enlarged lymph nodes suggestive of rectal cancer. Computed tomography (CT) of the chest, abdomen, and pelvis revealed rectal cancer with surrounding fatty interstitial infiltration, lymph node metastasis, and multiple metastases to both the lungs and liver.
The clinical diagnosis was stage IV rectal cancer with metastases to the liver, lungs, and lymph nodes.
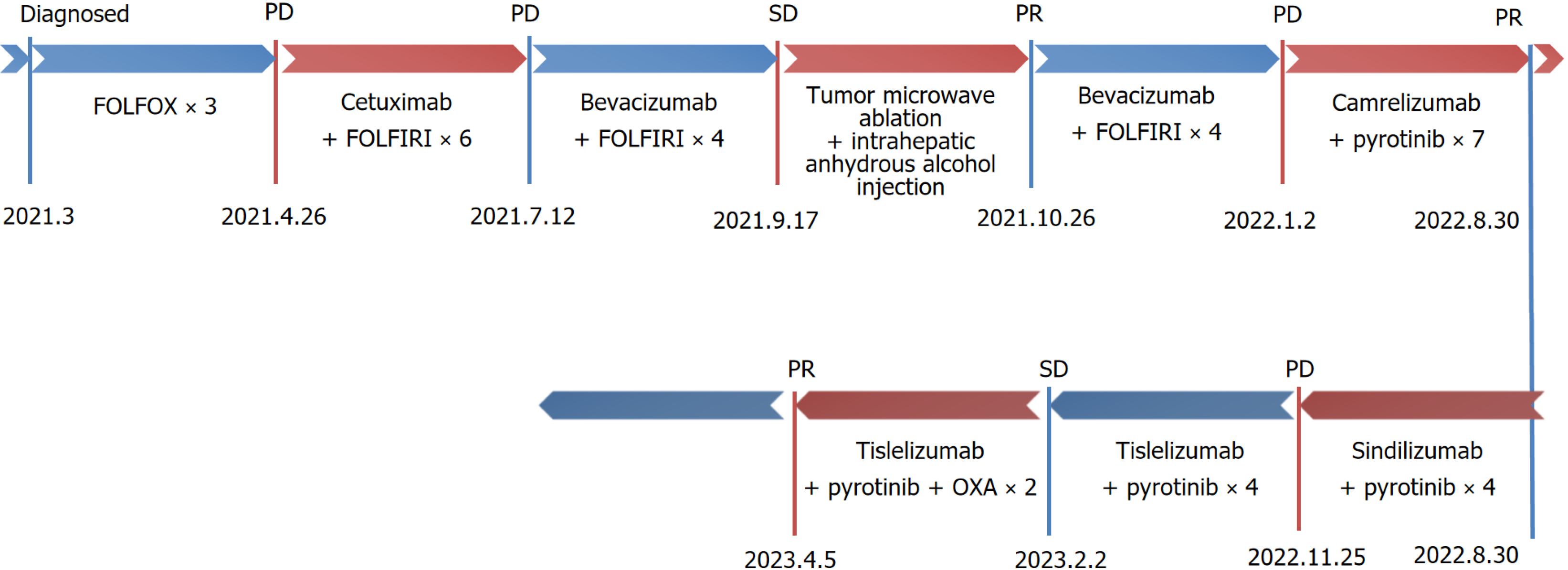
Figure 1 illustrates the treatment process. Palliative chemotherapy with the 5-fluorouracil (5-FU), leucovorin, oxaliplatin (OXA) regimen was initiated on March 4, 2021, for a total of three cycles. Specifically, the treatment consisted of OXA 150 mg administered intravenously on day 1, 5-FU 0.6 g given as an intravenous bolus on day 1, and 5-FU (4 g) delivered via continuous intravenous infusion over 46 hours, repeated every two weeks (q2w). Following chemotherapy, the patient experienced severe gastrointestinal reactions, including nausea and vomiting, and grade IV bone marrow suppression, which were intolerable. Repeat CT imaging suggested disease progression.
In April 2021, next-generation sequencing of the colorectal cancer tissue using a panel of 639 genes (method: Gene capture with high-throughput sequencing) revealed the following results: Low tumour mutation burden (2.2 mutations/mb), microsatellite low instability (36.36%), no mutations in Kirsten rat sarcoma viral oncogene homolog (KRAS), neu
| Gene | Outcome | Abundance of mutations/copy number |
| KRAS | Negative | - |
| NRAS | Negative | - |
| BRAF | Negative | - |
| ERBB2 | Amplification | 33 |
| APC | Exon16, c4348C>T, p.R1450 | 15.9% |
| CDK12 | Amplification | 7.5 |
| TP53 | Exon4, c.325T>A, p.F109I | 16.8% |
| TMB | TMB-L | 2.2 mutations/mb |
| MSI | MSI-L | 36.36% |
Palliative treatment with cetuximab in combination with the irinotecan plus 5-fluorouracil (FOLFIRI) regimen was initiated on April 26, 2021, for a total of six cycles. Specifically, the treatment consisted of cetuximab 800 mg administered intravenously on day 1, irinotecan (CPT-11) 300 mg administered intravenously on day 1, 5-FU 0.6 g administered as an intravenous bolus on day 1, and 5-FU 4 g delivered via continuous intravenous infusion over 46 hours repeated q2w. A repeat CT scan on July 12, 2021, indicated progression of the liver and lung lesions. Consequently, the treatment was changed to bevacizumab in combination with the FOLFIRI regimen on August 4, 2021, for four cycles. Specifically, the treatment consisted of bevacizumab 400 mg on day 1, CPT-11 300 mg on day 1, 5-FU 0.6 g administered as an intravenous bolus on day 1, and 5-FU 4 g delivered via continuous intravenous infusion over 46 hours, repeated q2w. On September 17, 2021, follow-up evaluation revealed stable disease. On September 23, 2021, hepatic microwave ablation was per
Following the failure of multiple conventional treatments, a comprehensive literature review indicated that the combination of immunotherapy and small-molecule targeted drugs exhibits a synergistic effect[4]. With the patient's informed consent, camrelizumab 200 mg was administered intravenously on day 1 every three weeks (q3w) in com
Due to intolerance to capillary hyperplasia, the dosing regimen was adjusted. Sindilizumab 200 mg on day 1 q3w and pyrotinib 400 mg qd were administered four times on August 30, 2022, September 24, 2022, October 13, 2022, and November 4, 2022. During the fourth administration of sindilizumab, the patient experienced sudden onset of generalised urticaria. Symptoms were promptly treated with corticosteroids and resolved following treatment. CEA levels initially decreased but subsequently increased following treatment. A repeat CT examination on October 17, 2022 revealed that some lesions were slightly enlarged compared to their previous size.
Owing to the development of systemic urticaria during the last administration of sindilizumab, the medication regimen was adjusted. Tislelizumab 200 mg on day 1 q3w and pyrotinib 400 mg qd were administered four times on November 25, 2022, December 15, 2022, January 11, 2023, and February 2, 2023. Following treatment, CEA levels began to increase compared to pre-treatment levels, and anal cramping worsened. A repeat CT examination on January 11, 2023, indicated that the lesion was similar to the previous findings. Adverse effects included skin rashes and pruritus. To improve efficacy, tislelizumab in combination with pyrotinib and OXA was administered twice, on March 15, 2023, and April 5, 2023. Following this treatment, CEA levels decreased with partial relief of anal swelling. Adverse effects included rashes with pruritus, gastrointestinal reactions, and malaise.
Colorectal cancer is a common malignancy in China, and its incidence is increasing annually. Chemotherapy remains the standard treatment for patients with advanced colorectal cancer. Although targeted therapies provide survival benefits to patients with advanced metastatic colorectal cancer, most patients inevitably develop drug resistance. Small-molecule tyrosine kinase inhibitors such as regorafenib offer limited therapeutic benefits when used as third-line treatments[5,6]. In this case, the patient achieved stable disease as the best outcome following targeted combination chemotherapy, and the therapeutic agents demonstrated limited efficacy in refractory colorectal cancer.
With advancements in precision testing, the application of targeted therapies in clinical practice has increased, enabling more patients with tumours to benefit from precision medicine. It has been found that human epidermal growth factor receptor 2 (HER2) amplification occurs in approximately 2%-6% of colorectal cancers and 5%-14% of rat sarcoma viral oncogene homolog (RAS)/BRAF wild-type intestinal cancers[7,8]. HER2 amplification can lead to the formation of homodimers or heterodimers, which further autophosphorylate intracellular tyrosine residues, thereby activating receptor tyrosine kinases and initiating downstream signalling cascades. It has been suggested that HER2 overexpression correlates with tumour pathological features, including the primary site of the tumour and KRAS/BRAF/neuroblastoma rat sarcoma viral oncogene homolog oncogene homologue gene status. Additionally, HER2 overexpression may play a role in the growth, proliferation, and invasion of colorectal cancer[9]. Although there are currently no studies supporting the correlation between HER2 overexpression and the prognosis of intestinal cancer, HER2 overexpression can lead to resistance to anti-epidermal growth factor receptor monoclonal antibodies. Tumor regression after resistance can be achieved with anti-HER2 therapy[10,11]. The HER2-positive metastatic colorectal cancer study evaluated the use of trastuzumab plus lapatinib in patients with HER2-positive and wild-type RAS/BRAF advanced colorectal cancer who progressed after standard therapy[12]. The My Pathway study confirmed the benefits of a pertuzumab plus trastuzumab regimen in patients with HER2-amplified KRAS wild-type metastatic colorectal cancer[13]. Based on these studies, the 2021 edition of the National Comprehensive Cancer Network guidelines recommends trastuzumab plus pertuzumab or trastuzumab plus lapatinib for the third-line treatment of HER2-amplified advanced colorectal cancer. Emerging evidence suggests that cyclin-dependent kinase 12 functions as a novel regulator of tumour stem cells, promoting tumour initiation and contributing to anti-HER2 therapeutic resistance in human breast cancer[14]. Currently, limited data are available on anti-HER2-targeted therapies for HER2-amplified colorectal cancer in China.
Pyrotinib is a novel irreversible small-molecule tyrosine kinase inhibitor that inhibits the formation of HER family homodimers by targeting adenosine triphosphate-binding sites in the intracellular kinase domains of HER1, HER2, and HER4. This inhibition blocks the activation of downstream signalling pathways, thereby suppressing tumour cell growth[15]. It has been approved for use in patients with HER2-positive recurrent or metastatic breast cancer; however, its application in HER2-positive colorectal cancer remains underexplored. In our patient, the best response was stable disease with limited benefits during the application of standard first- and second-line therapies. The disease subsequently progressed but showed significant remission with the administration of pyrotinib. Some studies have suggested that anti-HER2 therapy can be initiated at an early stage in patients with HER2-amplified advanced metastatic colorectal cancer patients[16], but more clinical data are needed to substantiate its specific advantages.
Immunotherapy has demonstrated efficacy in the treatment of multiple tumours[17]. The KEYNOTE-164 study found that patients with microsatellite instability-high/mismatch repair-deficient colorectal cancer benefited from pembrolizumab treatment, achieving a 1-year overall survival rate of 76% and 1-year progression-free survival rate of 41%[18]. Based on available data from international clinical studies and the 2021 edition of the National Comprehensive Cancer Network guidelines, it is recommended that patients with microsatellite instability-high/mismatch repair-deficient advanced colorectal cancer receive treatment with immune checkpoint inhibitors (ICIs). Studies have suggested that KRAS-mutant colorectal cancer derives limited benefits from immunotherapy, and the impact of HER2 expression status on immunotherapy efficacy remains unclear[4]. Emerging evidence indicates that patients harbouring tumour protein P53 mutations receive substantial clinical benefits from ICIs[19]. Immunotherapy and targeted therapy have synergistic effects in clinical oncology[20]. Small-molecule targeted drugs have been shown to activate specific cytotoxic T lym
In this case, the patient exhibited a minimal reduction in lesion size following multiple lines of targeted therapy and chemotherapy. The lesion progressively enlarges in the absence of an effective therapeutic intervention. Following treatment with an ICI in combination with pyrotinib, based on the results of precision medicine testing, the lesion exhibited significant shrinkage and a marked reduction in tumour marker compared to previous levels. This approach yields improved efficacy and symptom relief without serious, life-threatening adverse effects. During this period, various ICIs were altered due to intolerable cutaneous adverse reactions, including capillary naevus and generalised severe urticaria. The disease remained stable over a total treatment duration of 14 months. Subsequently, OXA monotherapy was initiated in response to progressive elevation of CEA levels and worsening symptoms. Although chemotherapy administered as monotherapy was less effective, a trend toward decreased CEA levels was observed following combination therapy. This effect may be attributed to synergistic interactions between the ICIs, pyrotinib, and OXA. The patient received this combination regimen twice and tolerated it well. Although this case represents an individual instance, it underscores the importance of precision diagnostics and individualized treatment strategies. It also highlights the potential of combining immunotherapy with targeted therapy and chemotherapy as well as the synergy among these modalities. However, further clinical research is needed to identify the optimal patient population for such combinations and determine the most effective sequence of drug administration.
We report a case of refractory colorectal cancer in which, despite the failure of conventional targeted therapy and chemotherapy, a precise individualized treatment plan was formulated based on genetic testing, resulting in significant clinical improvement.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64389] [Article Influence: 16097.3] [Reference Citation Analysis (176)] |
| 2. | Moreno CC, Mittal PK, Sullivan PS, Rutherford R, Staley CA, Cardona K, Hawk NN, Dixon WT, Kitajima HD, Kang J, Small WC, Oshinski J, Votaw JR. Colorectal Cancer Initial Diagnosis: Screening Colonoscopy, Diagnostic Colonoscopy, or Emergent Surgery, and Tumor Stage and Size at Initial Presentation. Clin Colorectal Cancer. 2016;15:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Qin S, Li J, Wang L, Xu J, Cheng Y, Bai Y, Li W, Xu N, Lin LZ, Wu Q, Li Y, Yang J, Pan H, Ouyang X, Qiu W, Wu K, Xiong J, Dai G, Liang H, Hu C, Zhang J, Tao M, Yao Q, Wang J, Chen J, Eggleton SP, Liu T. Efficacy and Tolerability of First-Line Cetuximab Plus Leucovorin, Fluorouracil, and Oxaliplatin (FOLFOX-4) Versus FOLFOX-4 in Patients With RAS Wild-Type Metastatic Colorectal Cancer: The Open-Label, Randomized, Phase III TAILOR Trial. J Clin Oncol. 2018;36:3031-3039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 170] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 4. | Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P, Li J, Wang G, Lan Z, Li J, Tang M, Jiang S, Ma X, Chen P, Katkhuda R, Korphaisarn K, Chakravarti D, Chang A, Spring DJ, Chang Q, Zhang J, Maru DM, Maeda DY, Zebala JA, Kopetz S, Wang YA, DePinho RA. KRAS-IRF2 Axis Drives Immune Suppression and Immune Therapy Resistance in Colorectal Cancer. Cancer Cell 2019; 35: 559-572.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 452] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 5. | 5 Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L, Yeh KH, Bi F, Cheng Y, Le AT, Lin JK, Liu T, Ma D, Kappeler C, Kalmus J, Kim TW; CONCUR Investigators. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 568] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 6. | Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, Yang L, Deng Y, Chen ZD, Zhong H, Pan H, Guo W, Shu Y, Yuan Y, Zhou J, Xu N, Liu T, Ma D, Wu C, Cheng Y, Chen D, Li W, Sun S, Yu Z, Cao P, Chen H, Wang J, Wang S, Wang H, Fan S, Hua Y, Su W. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319:2486-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 7. | DeStefanis RA, Kratz JD, Emmerich PB, Deming DA. Targeted Therapy in Metastatic Colorectal Cancer: Current Standards and Novel Agents in Review. Curr Colorectal Cancer Rep. 2019;15:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Ross JS, Fakih M, Ali SM, Elvin JA, Schrock AB, Suh J, Vergilio JA, Ramkissoon S, Severson E, Daniel S, Fabrizio D, Frampton G, Sun J, Miller VA, Stephens PJ, Gay LM. Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer. 2018;124:1358-1373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 9. | Martin V, Landi L, Molinari F, Fountzilas G, Geva R, Riva A, Saletti P, De Dosso S, Spitale A, Tejpar S, Kalogeras KT, Mazzucchelli L, Frattini M, Cappuzzo F. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer. 2013;108:668-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Sawada K, Nakamura Y, Yamanaka T, Kuboki Y, Yamaguchi D, Yuki S, Yoshino T, Komatsu Y, Sakamoto N, Okamoto W, Fujii S. Prognostic and Predictive Value of HER2 Amplification in Patients With Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2018;17:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda M, Fujisaka Y, Philips J, Shimizu T, Maenishi O, Cho Y, Sun J, Destro A, Taira K, Takeda K, Okabe T, Swanson J, Itoh H, Takada M, Lifshits E, Okuno K, Engelman JA, Shivdasani RA, Nishio K, Fukuoka M, Varella-Garcia M, Nakagawa K, Jänne PA. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3:99ra86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 519] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 12. | Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E, Troiani T, Ciardiello F, Racca P, Bertotti A, Siravegna G, Torri V, Amatu A, Ghezzi S, Marrapese G, Palmeri L, Valtorta E, Cassingena A, Lauricella C, Vanzulli A, Regge D, Veronese S, Comoglio PM, Bardelli A, Marsoni S, Siena S. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 13. | Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, Swanton C, Kurzrock R, Burris H, Sweeney C, Bose R, Spigel DR, Beattie MS, Blotner S, Stone A, Schulze K, Cuchelkar V, Hainsworth J. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 394] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 14. | Choi HJ, Jin S, Cho H, Won HY, An HW, Jeong GY, Park YU, Kim HY, Park MK, Son T, Min KW, Jang KS, Oh YH, Lee JY, Kong G. CDK12 drives breast tumor initiation and trastuzumab resistance via WNT and IRS1-ErbB-PI3K signaling. EMBO Rep. 2019;20:e48058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 15. | Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J, Luo Y, Xing P, Lan B, Li M, Yi Z, Cai R, Yuan P, Zhang P, Li Q, Xu B. Phase I Study and Biomarker Analysis of Pyrotinib, a Novel Irreversible Pan-ErbB Receptor Tyrosine Kinase Inhibitor, in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J Clin Oncol. 2017;35:3105-3112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 16. | Nakamura Y, Sawada K, Fujii S, Yoshino T. HER2-targeted therapy should be shifted towards an earlier line for patients with anti-EGFR-therapy naïve, HER2-amplified metastatic colorectal cancer. ESMO Open. 2019;4:e000530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Oliveira AF, Bretes L, Furtado I. Review of PD-1/PD-L1 Inhibitors in Metastatic dMMR/MSI-H Colorectal Cancer. Front Oncol. 2019;9:396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 18. | Le DT, Diaz LA Jr, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, Burge M, O'Neil BH, Kavan P, Yoshino T, Guimbaud R, Taniguchi H, Élez E, Al-Batran SE, Boland PM, Cui Y, Leconte P, Marinello P, André T. Pembrolizumab for previously treated, microsatellite instability-high/mismatch repair-deficient advanced colorectal cancer: final analysis of KEYNOTE-164. Eur J Cancer. 2023;186:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 19. | Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, Tu HY, Chen HJ, Sun YL, Zhou Q, Yang JJ, Yang XN, Lin JX, Yan HH, Zhai HR, Yan LX, Liao RQ, Wu SP, Wu YL. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res. 2017;23:3012-3024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 733] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 20. | Colli LM, Machiela MJ, Zhang H, Myers TA, Jessop L, Delattre O, Yu K, Chanock SJ. Landscape of Combination Immunotherapy and Targeted Therapy to Improve Cancer Management. Cancer Res. 2017;77:3666-3671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Moser JC, Chen D, Hu-Lieskovan S, Grossmann KF, Patel S, Colonna SV, Ying J, Hyngstrom JR. Real-world survival of patients with advanced BRAF V600 mutated melanoma treated with front-line BRAF/MEK inhibitors, anti-PD-1 antibodies, or nivolumab/ipilimumab. Cancer Med. 2019;8:7637-7643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17:569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 23. | Zhu H, Shan Y, Ge K, Lu J, Kong W, Jia C. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol (Dordr). 2020;43:1203-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 24. | Hong X, Dong T, Yi T, Hu J, Zhang Z, Lin S, Niu W. Impact of 5-Fu/oxaliplatin on mouse dendritic cells and synergetic effect with a colon cancer vaccine. Chin J Cancer Res. 2018;30:197-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17:725-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 867] [Article Influence: 173.4] [Reference Citation Analysis (0)] |
| 26. | Mathew M, Enzler T, Shu CA, Rizvi NA. Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther. 2018;186:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Noordam L, Kaijen MEH, Bezemer K, Cornelissen R, Maat LAPWM, Hoogsteden HC, Aerts JGJV, Hendriks RW, Hegmans JPJJ, Vroman H. Low-dose cyclophosphamide depletes circulating naïve and activated regulatory T cells in malignant pleural mesothelioma patients synergistically treated with dendritic cell-based immunotherapy. Oncoimmunology. 2018;7:e1474318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Wanderley CW, Colón DF, Luiz JPM, Oliveira FF, Viacava PR, Leite CA, Pereira JA, Silva CM, Silva CR, Silva RL, Speck-Hernandez CA, Mota JM, Alves-Filho JC, Lima-Junior RC, Cunha TM, Cunha FQ. Paclitaxel Reduces Tumor Growth by Reprogramming Tumor-Associated Macrophages to an M1 Profile in a TLR4-Dependent Manner. Cancer Res. 2018;78:5891-5900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 265] [Article Influence: 37.9] [Reference Citation Analysis (0)] |