INTRODUCTION
Gastric cancer (GC) is a significant health concern, ranking as the fifth most common cancer and the fifth leading cause of cancer-related deaths worldwide in 2022[1]. It is estimated that in 2040, there will be 1.8 million cases of GC and 1.3 million deaths, an increase of 66% and 71%, respectively, compared with 2020[2]. GC has received much attention in recent years because of its increasing global incidence. In most GC patients, the primary clinical manifestation is an intragastric mass lesion, typically in the absence of significant clinical signs. Radical surgery is primarily indicated for patients with resectable cancer lesions[3]. However, GC often remains asymptomatic in its early stages, leading to its predominant diagnosis at an advanced stage, where vascular invasion and distant metastasis have occurred. As a result, patients with advanced GC are typically ineligible for surgical intervention and face a grim prognosis.
Surgical resection remains the cornerstone of curative treatment for GC, with lymphadenectomy being pivotal to the success of radical surgery[4]. For resectable locally advanced GC, the 2024 National Comprehensive Cancer Network and Chinese Society of Clinical Oncology (CSCO) guidelines recommend neoadjuvant chemoradiotherapy or immunotherapy prior to surgery[5]. For unresectable locally advanced or metastatic GC, conversion therapy is recommended to increase the potential for surgical resection. Systemic chemotherapy remains the cornerstone of treatment for advanced GC and is equally vital for managing unresectable locally advanced cases[6,7]. Chemotherapy regimens combining fluoropyrimidines with platinum-based drugs are recommended as the preferred first-line treatment strategy, but patient survival is typically approximately one year[8-10]. Combinations of chemotherapy drugs such as S-1 plus oxaliplatin (SOX), capecitabine plus oxaliplatin, leucovorin calcium plus fluorouracil plus oxaliplatin, and docetaxel plus oxaliplatin plus capecitabine, among others, have not overcome the efficacy limitations of chemotherapy. In recent years, immune checkpoint inhibitor (ICI)-based immunotherapy has shown significant efficacy in treating gastrointestinal malignancies. ICIs combined with chemotherapy and/or targeted therapies have demonstrated substantial benefits in both locally advanced and advanced GC[11-13]. Furthermore, compared with cisplatin, programmed cell death protein 1 (PD-1) inhibitors with oxaliplatin significantly prolong progression-free survival (PFS) in advanced GC patients[14]. On the basis of phase III clinical trial results, the Food and Drug Administration (FDA) has approved certain PD-1 inhibitors in combination with oxaliplatin and fluoropyrimidines for first-line treatment of advanced or metastatic GC, adenocarcinoma of the esophagogastric junction (AEG), and esophageal adenocarcinoma[12]. In 2022, the National Medical Products Administration (NMPA) approved the combination of sintilimab with fluoropyrimidines and platinum-based drugs as a first-line treatment for unresectable locally advanced, recurrent, or metastatic GC/gastroesophageal junction cancer (GEJC). Recent studies indicate that the combination of sintilimab with nab-paclitaxel exhibits promising antitumor activity and an acceptable safety profile as a second-line treatment for advanced or metastatic AEG/GC[15].
While a PD-1 inhibitor combined with chemotherapy can prolong the survival of unresectable advanced or metastatic GC patients, radical resection is the key approach for achieving cancer cure and long-term survival. Conversion therapy refers to unresectable or borderline resectable tumors for surgical, technical and/or oncological reasons. After active and effective chemotherapy, immunotherapy, radiotherapy and other comprehensive treatments, primary gastric lesions can be reduced to a lower stage, while metastatic lesions can be effectively controlled to achieve R0 resection and improve the long-term survival rate[16,17]. In recent years, conversion therapy has been extensively implemented in advanced hepatocellular carcinoma and colorectal cancer patients and has demonstrated significant clinical efficacy[18,19]. In 2021, data from the phase II CO-STAR trial, published by the American Society of Clinical Oncology, revealed that the combination of sintilimab, apatinib, and chemotherapy (nab-paclitaxel plus S-1) demonstrated substantial efficacy as a conversion therapy in patients with advanced GC. The trial achieved an impressive objective response rate (ORR) of 61.1%, accompanied by an R0 resection rate of 94.4%[20]. These results emphasize the potential of this therapeutic regimen to convert initially unresectable tumors into resectable tumors, significantly improving the prognosis and offering a path toward a surgical cure for advanced GC patients. Notably, approximately 80% of GC patients exhibit a human epidermal growth factor receptor 2 (HER2)-negative status, rendering them ineligible for trastuzumab-targeted therapy. In light of robust evidence from multiple phase III clinical trials, the FDA has approved the use of certain PD-1 inhibitors in combination with chemotherapy as a first-line treatment strategy for HER2-negative GC[12,21-23]. Additionally, the CSCO guidelines advocate the use of nab-paclitaxel as a first-line therapeutic option for advanced metastatic malignancies, including esophageal cancer, GEJC, and pancreatic cancer. PD-1 inhibitors, especially sintilimab, in combination with chemotherapy or targeted therapy have great potential in the treatment of advanced-stage GC patients. However, the efficacy and safety of PD-1 inhibitors, nab-paclitaxel, oxaliplatin, and S-1 in conversion therapy for HER2-negative unresectable locally advanced or advanced GC remain unclear.
Here, we report a case of unresectable locally advanced gastric adenocarcinoma treated with sintilimab in combination with nab-paclitaxel, S-1, and oxaliplatin, demonstrating the efficacy and safety of the quadruple-drug conversion therapy regimen. Encouragingly, this patient achieved a pathological complete response (pCR) and R0 resection after successful conversion therapy, with tumor marker levels returning to normal. To our knowledge, this is the first reported case of unresectable locally advanced gastric adenocarcinoma treated with this quadruple-drug conversion therapy regimen.
CASE PRESENTATION
Chief complaints
A 55-year-old male was admitted to our hospital on August 21, 2023, due to intermittent epigastric pain for 3 months, which had worsened over the past 2 months and was accompanied by melena.
History of present illness
The patient developed upper abdominal pain without obvious cause three months prior, characterized by dull distension, which typically occurred at night when hungry. The pain worsened after spicy foods were consumed and was accompanied by acid reflux and belching. Self-administered omeprazole provided minimal relief, and the symptoms recurred. Over the next two months, the symptoms intensified, with the addition of melena. Since the onset of illness, the patient maintained a stable mental state and sleep, with normal bowel and urinary functions, poor appetite, and approximately 7 kg of weight loss.
History of past illness
The patient had no history of acute or chronic infectious diseases, heart disease, hypertension, diabetes, abdominal trauma, or surgeries.
Personal and family history
The patient had no pertinent family medical history.
Physical examination
Physical examination revealed mild epigastric tenderness with no other positive signs.
Laboratory examinations
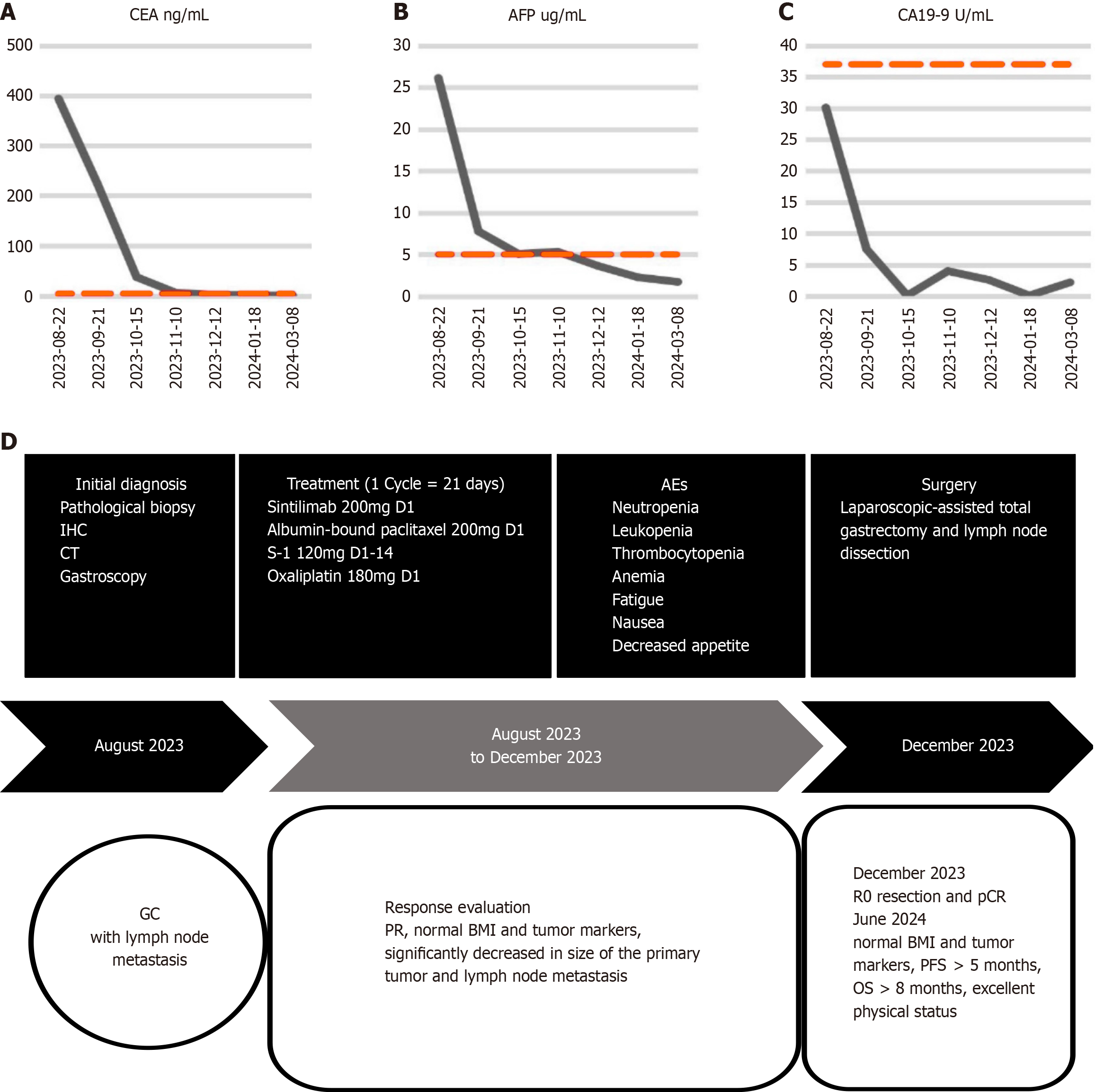
The carcinoembryonic antigen (CEA) level significantly increased to 394.12 ng/mL (normal reference range: 0-5.00 ng/mL), and the alpha-fetoprotein (AFP) level increased to 26.12 µg/mL (normal reference range: 0-15.00 µg/mL). The value of cancer antigen 19-9 (CA19-9) was 30.12 U/mL (normal reference range: 0-37.00 U/mL), which is near the upper limit of the normal range. Other tumor biomarkers, such as cancer antigen 125 (CA125) and cancer antigen 153, were within the normal range. The body mass index (BMI) decreased to 17.6 kg/m2 (normal reference range: 18.5-23.9 kg/m2).
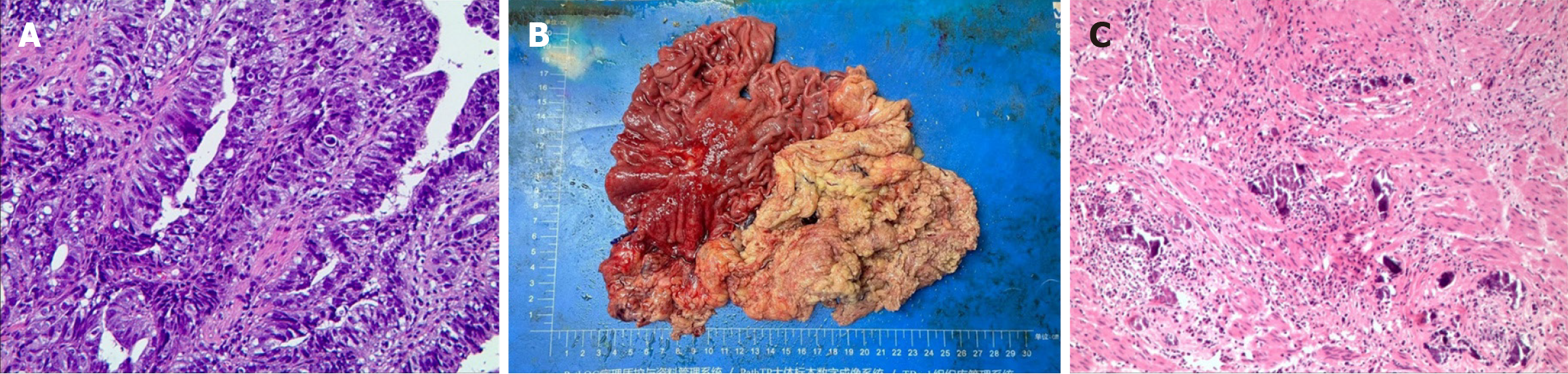
A pathological biopsy was performed on August 23, 2023, for pathological evaluation of the tumor mass. The pathological diagnosis was poorly differentiated adenocarcinoma of the gastric body (Figure 1A). On immunohistochemical evaluation, the tumor tissue was negative for Ki67, CKp, TP53, CK7, and HER2.
Figure 1 Hematoxylin-eosin staining and immunohistochemical evaluation of the obtained using a pathological biopsy.
A: The preoperative pathology image showed poorly differentiated adenocarcinoma of the gastric body; B: The specimen of the entire stomach, lymph node and tumor tissue removed during surgery; C: The postoperative pathology image showed no residual tumor cells.
Imaging examinations
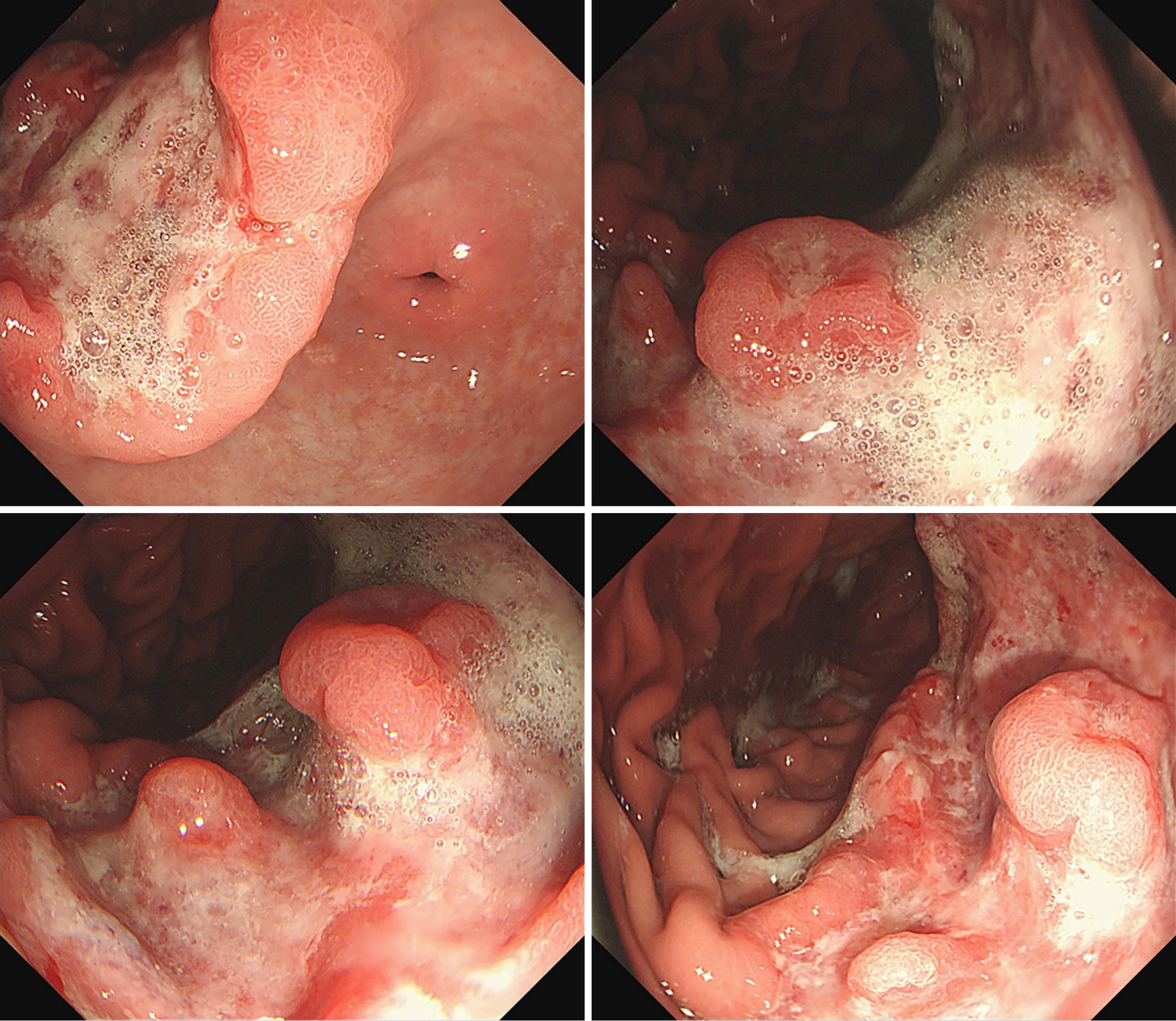
Enhanced abdominal computed tomography (CT) revealed irregular thickening of the gastric wall at the lesser curvature and gastric angle, with the thickest part measuring approximately 1.9 cm. The tumor infiltrated the full thickness of the gastric wall but did not involve the serosa. Multiple metastatic lymph nodes were observed in the abdominal cavity, with the largest measuring approximately 4.9 cm × 2.9 cm. Lymph node metastases were observed in the right paracardial lymph nodes, lesser curvature lymph nodes, proximal greater curvature lymph nodes, superior pyloric lymph nodes, and inferior pyloric lymph nodes surrounding the gastric cavity. Metastases were also present in the lymph nodes adjacent to the left gastric artery (perivascular) and in the lymph nodes surrounding organs, particularly those along the hepatoduodenal ligament. A total of seven groups of lymph nodes in the perigastric region exhibited metastases, with the metastatic tumors in the distal greater curvature lymph nodes being the largest in volume and most numerous. Electronic gastroscopy revealed a large ulcer on the lesser curvature of the stomach, with a foul, bleeding, and uneven base; the margins were raised, grayish in color, and rigid, accompanied by bleeding and erosion (Figure 2).
Figure 2 Endoscopic images.
MULTIDISCIPLINARY EXPERT CONSULTATION
A comprehensive treatment strategy for unresectable, locally advanced, or advanced GC integrates pathological, radiological, and immunohistochemical assessments with the patient’s clinical status, guided by current guidelines and the literature. Following a thorough evaluation by a multidisciplinary team (MDT) comprising experts in surgery, oncology, pathology, radiology, and pharmacology, factors such as pathology, clinical staging, genomics, treatment history, risk stratification, and patient preferences were analyzed. The MDT unanimously recommended first-line conversion therapy with a personalized quadruple regimen combining PD-1 inhibitors and chemotherapy to downstage the tumor and achieve R0 resection. The treatment plan, approved by the patient and family with informed consent, highlights the potential of this approach to halt tumor progression. This case highlights the importance of personalized therapy, multidisciplinary collaboration, and continuous follow-up in managing advanced GC and offers a framework applicable to other advanced cancers.
FINAL DIAGNOSIS
The patient was diagnosed with unresectable locally advanced gastric adenocarcinoma on the basis of his medical history and the imaging examination and pathology biopsy results. According to the American Joint Committee on Cancer/Union for International Cancer Control/International Gastric Cancer Association eighth edition guidelines, the patient was diagnosed with grade cT3N3M0 gastric adenocarcinoma.
TREATMENT
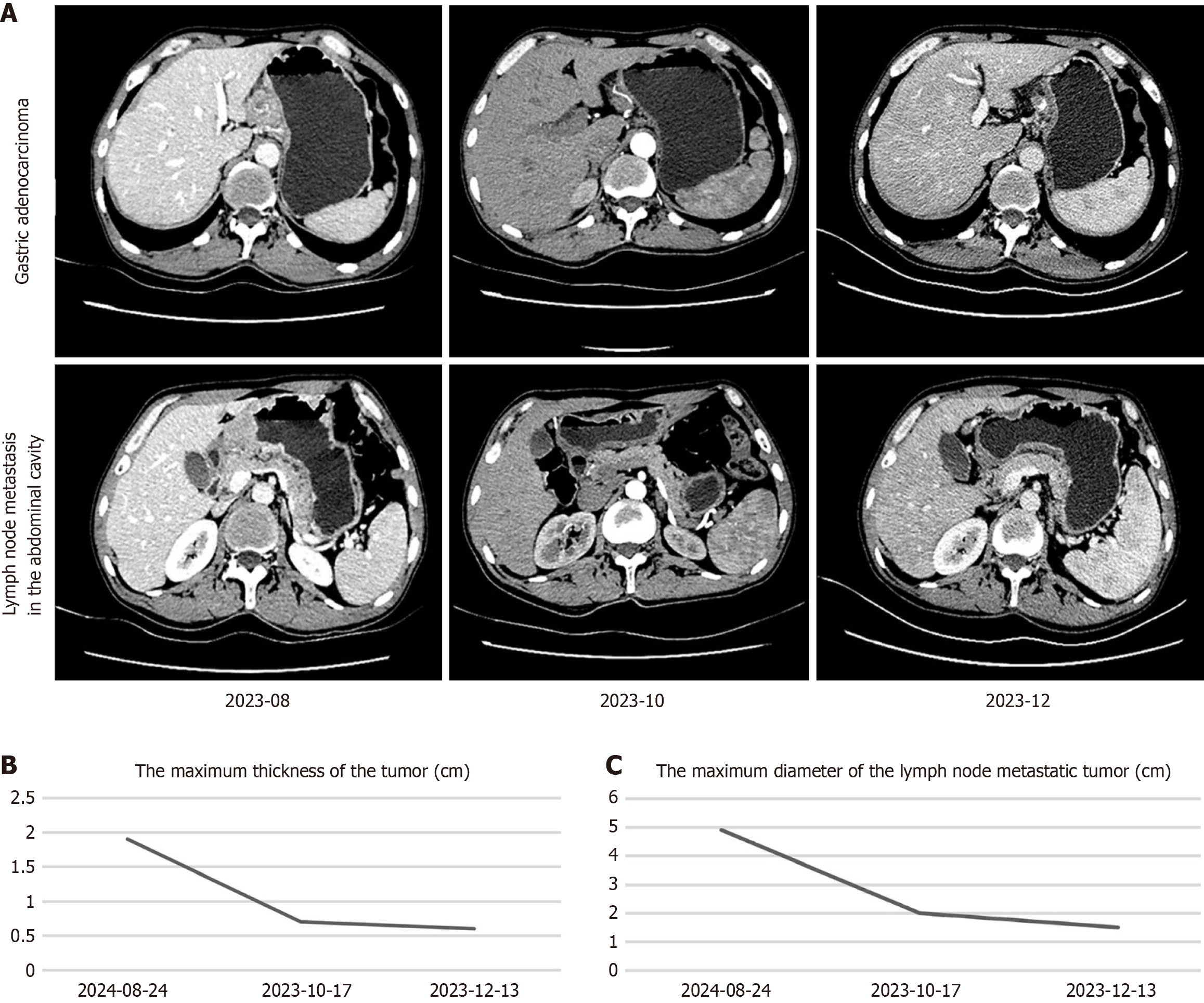
Following an MDT assessment, it was determined that the patient had the following conditions: (1) Severe upper abdominal pain accompanied by melena, indicating serious clinical symptoms; (2) The presence of multiple lymph node metastases in the abdominal cavity, with significantly enlarged nodes, making surgical treatment unsuitable; (3) Poorly differentiated adenocarcinoma with high malignancy; and (4) An acceptable general condition and eagerness for a cutting-edge and effective treatment approach. Therefore, it was decided to administer the quadruple-drug regimen for first-line conversion therapy to the patient. The treatment consisted of a PD-1 inhibitor, nab-paclitaxel, S-1 and oxaliplatin. Sintilimab (200 mg iv drip, on d1), nab-paclitaxel (200 mg iv drip, on d1), S-1 (60 mg orally, twice daily, on d1-14), and oxaliplatin (180 mg iv drip, on d1) were used for 1 cycle of 21 days, for a total of 4 cycles. Imaging evaluations were conducted on the first day of the first and third cycles, as well as on the day before surgery. After nearly 4 months of treatment, enhanced abdominal CT images revealed a significant decrease in the volume of the adenocarcinoma mass of the gastric body compared with the previous scan on August 21, 2023. The maximum thickness of the tumor decreased from 1.9 cm to 0.6 cm (Figure 3A and B). The sizes and numbers of multiple lymph node metastases in the abdominal cavity also significantly decreased, with the largest measuring 1.5 cm in diameter (Figure 3A and C). The serum levels of CEA decreased significantly from 394.12 ng/mL to 2.51 ng/mL (Figure 4A), those of AFP decreased from 26.12 µg/mL to 3.64 µg/mL (Figure 4B), and those of CA19-9 decreased from 30.10 U/mL to 2.60 U/mL (Figure 4C), with all tumor marker levels within the normal range. The BMI increased from 17.6 (weight: 51.0 kg, height: 1.70 m) to 19.0 (weight: 55.0 kg, height: 1.70 m) kg/m2. After 4 cycles of conversion therapy, the patient's abdominal pain and melena resolved, with a significant reduction in the size and volume of the primary tumor, as well as a marked decrease in the size and number of lymph node metastases, and the patient's overall condition remained good. Following discussions with the treatment team and with agreement from the patient and family members, the patient underwent laparoscopic total gastrectomy with abdominal lymph node dissection on December 19, 2023, achieving R0 resection. The postoperative pathological findings revealed fibrosis and calcification at the tumor bed of the gastric body, along with fibrosis and calcification in some of the dissected lymph nodes, with no residual cancerous tissue observed (Figure 1B and C). The tumor demonstrated complete regression [tumor regression grade (TRG) 0] with a pathological stage of pT0N0M0, indicating pCR and R0 resection. Postoperatively, the patient received sintilimab and SOX adjuvant therapy according to the treatment protocol. Sintilimab (200 mg iv drip, on d1), S-1 (60 mg orally, twice daily, on d1-14), and oxaliplatin (180 mg iv drip, on d1) were used for 1 cycle of 21 days, for a total of 2 cycles. The patient recovered well during the 6.5-month follow-up period. He had a PFS of more than 3.6 months and an overall survival of more than 6.5 months after receiving combination therapy. The timeline of treatment is shown in Figure 4D.
Figure 3 Enhanced abdominal computed tomography images for evaluating the treatment response.
A: Upper-row images show the changes in the size of the adenocarcinoma tumor tissue at the gastric body; Lower-row images show the changes in the size of the lymph node metastasis in the abdominal cavity; B: The maximum thickness of the tumor at the gastric body; C: The maximum diameter of the lymph node metastatic tumor.
Figure 4 Dynamics of carcinoembryonic antigen, alpha-fetoprotein, and cancer antigen 19-9 and timeline of treatment options.
A-C: Diagram depicting the dynamic of carcinoembryonic antigen (A), alpha-fetoprotein (B) and cancer antigen 19-9 (C); D: The timeline of the treatment protocol. CEA: Carcinoembryonic antigen; AFP: Alpha-fetoprotein; CA19-9: Cancer antigen 19-9; IHC: Immunohistochemistry; CT: Computed tomography; AEs: Adverse events; GC: Gastric cancer; PR: Partial response; BMI: Body mass index; pCR: Pathological complete response; PFS: Progression-free survival; OS: Overall survival.
Adverse events (AEs) were observed during first-line conversion therapy. The main AEs included neutropenia, leukopenia, thrombocytopenia, moderate anemia, fatigue, nausea, and decreased appetite. During the quadruple therapy, neutropenia occurred 4 times, with the lowest value of 0.54 × 109/L (grade 3 AE), which returned to normal levels after recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) injections; leukopenia occurred 3 times, with the lowest value of 1.22 × 109/L (grade 3 AE), which returned to normal levels after rhGM-CSF injections; thrombocytopenia occurred 1 time, with the lowest value of 36 × 109/L (grade 3 AE), which returned to normal levels after recombinant human thrombopoietin injections and interleukin-11 injections; hemoglobin decreased once, with the lowest value of 88 g/L (grade 2 AE), which returned to normal levels after compound ferrous sulfate folic acid tablets, vitamin B12, and vitamin B6; and mild fatigue, nausea, and decreased appetite were observed but were left untreated, resolving on their own. No immune-related AEs occurred. During conversion therapy, the patient experienced grade 2-3 hematologic AEs, likely due to chemotherapy-induced myelosuppression. These issues were resolved with symptomatic treatment, enabling uninterrupted completion of therapy.
OUTCOME AND FOLLOW-UP
This patient was miserable when he was diagnosed with unresectable locally advanced gastric adenocarcinoma. He consented to the treatment scheme. After receiving conversion therapy, the patient noticed rapid improvement in his condition. In the treatment process, first-line conversion therapy with a quadruple regimen eradicated the patient's clinical symptoms, downstaged the tumor, and led to complete tumor regression (TRG 0), pCR, and R0 resection. No grade 4 or higher AEs occurred. The patient was relieved upon being informed that no tumor cells remained and that his serum CA125 and AFP levels were within the normal range. The patient recovered well during the 6.5-month follow-up period. He had a PFS of more than 3.6 months and an OS of more than 6.5 months after receiving combination therapy. The patient and family members were satisfied with the efficacy and safety of this treatment approach. He gladly agreed to reveal his case.
DISCUSSION
The quadruple combination therapy of PD-1 inhibitors with nab-paclitaxel, S-1, and oxaliplatin represents a promising approach as a first-line conversion therapy for unresectable locally advanced gastric adenocarcinoma. This therapeutic strategy is designed to leverage the synergistic effects of immunotherapy and chemotherapy to maximize tumor reduction and potentially enable R0 resection and pCR.
A substantial proportion of GCs are diagnosed at advanced stages, precluding surgical intervention. Before 2021, systemic chemotherapy was the standard treatment for unresectable or metastatic GC, but modifications in regimens yielded limited improvements in OS. Recently, immunotherapy has emerged as a transformative approach. Pivotal phase III trials, including ORIENT-16, CheckMate-649, and KEYNOTE-590, have demonstrated that adding PD-1 inhibitors to chemotherapy as first-line treatment significantly improves survival in advanced or metastatic AEG and GC patients compared with chemotherapy alone[11,12,24]. On the basis of these findings, the FDA approved PD-1 inhibitors combined with chemotherapy in 2021 as first-line treatments for advanced AEG and GC, marking a major milestone in immunotherapy. In 2022, the NMPA approved sintilimab with fluoropyrimidine and platinum-based chemotherapy for unresectable locally advanced, recurrent, or metastatic AEG and GC, further highlighting its efficacy. These approaches establish PD-1 inhibitors, particularly sintilimab, combined with chemotherapy as the standard first-line treatment for advanced GC, offering significant survival benefits.
Advanced GC is associated with a poor prognosis, with a 5-year survival rate of approximately 10%[25]. Despite advancements in immunotherapy, targeted therapies, oncolytic viruses, and cancer vaccines, advanced GC remains largely incurable and presents significant therapeutic challenges. Recent studies suggest that conversion therapy offers a potential pathway to cure this disease, providing new hope in this context. However, no consensus exists on optimal regimens, and evidence is limited to retrospective analyses and phase II trials, with a lack of phase III studies specifically on conversion therapy for GC. Retrospective studies have reported R0 resection rates of 56.4%-84.8% after conversion surgery and a median OS of 22.5-50.0 months[26-31]. Dual and triplet chemotherapy regimens are commonly used, with triplet regimens offering slightly better efficacy but limited by toxicity and poor tolerance. The median OS remains at 1-2 years. Although promising, conversion immunotherapy is understudied, with limited clinical data. Two retrospective studies revealed that combining PD-1 inhibitors (e.g., sintilimab) with dual chemotherapy or targeted therapies improved the conversion and R0 resection rates, prolonging postoperative survival[32,33]. The CO-STAR phase II trial evaluated sintilimab and apatinib with dual chemotherapy for stage IV metastatic GC. The results revealed an ORR of 61.7%, an R0 conversion rate of 59.6%, and a high R0 resection rate of 94.4% among those who underwent surgery. Additionally, an ongoing phase III trial (NCT05002686) is assessing sintilimab with nab-paclitaxel, oxaliplatin, capecitabine, and radiotherapy as a first-line treatment for advanced GC with retroperitoneal lymph node metastasis, followed by D2 gastrectomy[34]. In summary, conversion therapy, particularly PD-1 inhibitors such as sintilimab combined with chemotherapy or targeted agents, shows promise in improving conversion rates and achieving R0 resections in advanced GC. However, further studies are needed to validate its safety and efficacy.
Conventional chemotherapy has shown limited efficacy in the treatment of advanced or metastatic GC. Meta-analyses suggest that ICIs as monotherapies may increase the risk of early mortality[35]. However, combining ICIs with chemotherapy significantly improves OS and PFS[36]. Sintilimab, a PD-1-targeted antibody, achieves remarkable efficacy in advanced GC by blocking the PD-1/PD-L1 pathway[24,37-39]. Nab-paclitaxel addresses the limitations of traditional paclitaxel, significantly enhances efficacy and reduces toxicity, and is widely used in advanced GC treatment[40,41]. Studies have demonstrated that the combination of nab-paclitaxel with S-1 produces superior outcomes[42], markedly increasing R0 resection rates in the neoadjuvant setting[43]. Retrospective studies reported a pCR rate of 21.52% with the combination of camrelizumab, S-1, and nab-paclitaxel, surpassing SOX and SAP (S-1 plus nab-paclitaxel) regimens[44]. The CO-STAR study further revealed that sintilimab combined with nab-paclitaxel, S-1, and apatinib achieved an R0 resection rate of 94.4% and an ORR of 61.7%[20]. These findings highlight the potential of the quadruple regimen, comprising sintilimab, nab-paclitaxel, oxaliplatin, and S-1, to synergistically enhance efficacy, reduce resistance, and optimize outcomes. Previously, our team employed a triple regimen of sintilimab, S-1, and oxaliplatin for conversion therapy in advanced unresectable GC, but the results were suboptimal. To improve efficacy, nab-paclitaxel was introduced into the regimen. Owing to its demonstrated efficacy and manageable toxicity in studies such as CO-STAR, this quadruple regimen holds great promise. Conversion therapy aims to downstage unresectable or borderline-resectable tumors to facilitate R0 resection, thereby improving long-term survival. Our patient presented with extensive lymph node metastases and large metastatic lesions, precluding surgical intervention. Following MDT evaluation, the patient underwent the quadruple regimen. Postoperative pathology revealed a pCR, with a final stage of T0N0M0. The patient recovered well. For adjuvant therapy, we considered sintilimab combined with SOX or SOX monotherapy at the same dose. Studies have shown that sintilimab combined with SOX achieves higher pCR rates and better major pathological responses than does SOX alone in the perioperative treatment of advanced GC[45]. Additionally, sintilimab combined with chemotherapy has demonstrated superior PFS and OS in HER2-negative advanced GC patients[46]. On the basis of the patient’s excellent response and supporting evidence, two cycles of sintilimab combined with SOX were administered as adjuvant therapy. In summary, the quadruple regimen integrates the complementary mechanisms of immunotherapy and chemotherapy, significantly enhancing efficacy, reducing resistance, and minimizing chemotherapy-related toxicity. This approach improves patient outcomes and quality of life. Although challenges such as drug interactions, potential side effects, individual variability, and treatment costs exist, these can be effectively managed through rational therapeutic strategies. The success of this regimen further validates its potential as a first-line treatment for advanced GC. Continued research and clinical optimization are expected to expand its applicability and therapeutic value across diverse clinical settings.
Owing to its personalized and experimental nature, this regimen has not yet been incorporated into clinical guidelines. Large-scale studies are still needed to definitively establish its efficacy, safety, and appropriate indications. The treatment team recommends that candidates for the quadruple regimen fulfill the following criteria: (1) Patients with unresectable, locally advanced, recurrent, or metastatic HER2-negative GC; (2) Those who experience clinical symptoms that significantly impair their quality of life; and (3) Patients who are relatively young, in good overall health, without major comorbidities or underlying conditions, and are expected to tolerate potential adverse reactions well. The severity of adverse reactions plays a crucial role in determining the overall effectiveness of treatment and patient adherence. In the sole reported study on conversion therapy for advanced GC using a four-drug regimen combining immunotherapy and chemotherapy, the most commonly observed adverse reactions with sintilimab, oxaliplatin, S-1, and apatinib were grade 2-3, indicating that the toxicity of the sintilimab-based quadruple regimen remained tolerable. During the treatment of this patient, the observed AEs included neutropenia, leukopenia, thrombocytopenia, moderate anemia, fatigue, nausea, and loss of appetite, all of which were classified as grade 3 or lower. These effects are relatively mild and are likely attributed to postchemotherapy bone marrow suppression. The patient successfully completed the treatment cycle on schedule, exhibiting excellent adherence and tolerance, further underscoring the favorable safety profile of the quadruple regimen comprising sintilimab, nab-paclitaxel, oxaliplatin, and S-1 as a first-line conversion therapy for unresectable, locally advanced gastric adenocarcinoma. Although the quadruple regimen demonstrated a favorable safety profile in this case, ensuring its broader safety across a larger patient population necessitates personalized treatment approaches, continuous monitoring, multidisciplinary collaboration, and proactive strategies for the prevention and management of AEs. To mitigate and manage adverse reactions, the treatment team recommends the following: (1) Pretreatment assessment: Comprehensive evaluation of health, medical history, organ function, and risk factors to guide personalized treatment and identify high-risk patients; (2) Dynamic monitoring: Regular checks of blood counts, liver/kidney function, electrolytes, heart, and thyroid function to detect early signs of adverse reactions, with a focus on hematologic and hepatotoxicity during immunotherapy and chemotherapy; and (3) Management of AEs: IrAEs: These include skin, gastrointestinal, hepatic toxicity, or immune-mediated thyroid disorders. Grade 1 irAEs are managed with supportive care; Grade 2 irAEs require pausing immunotherapy and administering corticosteroids or immunosuppressants; and Grade 3-4 irAEs necessitate the immediate discontinuation of immunotherapy, administration of high-dose immunosuppressants, and hospitalization for monitoring. Chemotherapy-related AEs: These include hematologic, neurotoxic, and renal toxicities. Grade 1 AEs are managed symptomatically; Grade 2 AEs involve dose adjustments and enhanced monitoring; Grade 3 AEs require pausing or adjusting chemotherapy and hospitalization for observation; and Grade 4 AEs require immediate cessation of chemotherapy and urgent hospitalization for life support. To balance efficacy and toxicity, the treatment team recommends the following: (1) Individual variations: Personalized treatment is crucial because of differences in immune response and drug metabolism. Healthy, young patients tolerate combination therapies better, whereas those with comorbidities may require dose adjustments; (2) Treatment adjustments: Early detection and monitoring help balance efficacy and toxicity, optimize outcomes and minimize harm; (3) Patient education and adherence: Enhancing patients’ understanding of side effects improves adherence and treatment success; and (4) Prospective research: Large-scale trials will further assess the efficacy-toxicity balance of combination therapies, providing solid evidence for treatment optimization and clinical decision-making. During the patient's treatment, although most adverse reactions were chemotherapy-related events of grades 2-3, we successfully reduced them to Grades 0-1 through timely monitoring and symptomatic treatment. This ensured the smooth execution of the treatment plan and achieved the desired therapeutic outcomes. This process not only reflects the clinical expertise of our team but is also supported by studies such as CO-STAR, providing valuable insights into the diagnosis and treatment of gastric and other cancers.
A comprehensive treatment strategy for unresectable, locally advanced, or advanced GC integrates pathological, radiological, and immunohistochemical assessments with the patient’s clinical status, guided by current guidelines and the literature. Following a thorough evaluation by an MDT comprising experts in surgery, oncology, pathology, radiology, and pharmacology, factors such as pathology, clinical staging, genomics, treatment history, risk stratification, and patient preferences were analyzed. The MDT unanimously recommended first-line conversion therapy with a personalized quadruple regimen combining PD-1 inhibitors and chemotherapy to downstage the tumor and achieve R0 resection. The treatment plan, approved by the patient and family with informed consent, highlights the potential of this approach to halt tumor progression. This case highlights the importance of personalized therapy, multidisciplinary collaboration, and continuous follow-up in managing advanced GC and offers a framework applicable to other advanced cancers.
CONCLUSION
To the best of our knowledge, this is the first reported case of first-line conversion therapy in an unresectable locally advanced gastric adenocarcinoma patient utilizing PD-1 inhibitors in combination with nab-paclitaxel, S-1, and oxaliplatin. The patient was downstaged to pT0N0M0, achieving pCR and R0 resection, with a PFS of more than 3.6 months, an OS of more than 6.5 months, and no grade 4 or higher AEs. After 6.5 months of treatment, the patient achieved excellent physical status. The accumulation of cases and evidence from prospective studies involving locally advanced GC, especially unresectable or advanced GC, are needed to validate the safety and efficacy of this quadruple therapy in conversion therapy. Based on the current preliminary results, the combination of PD-1 inhibitors, nab-paclitaxel, S-1, and oxaliplatin demonstrates considerable potential in the treatment of locally advanced GC. With further clinical research and validation, this therapeutic regimen could become a more effective and safer treatment option for patients with GC and other gastrointestinal malignancies in the future.
ACKNOWLEDGEMENTS
We acknowledge the patient and her family for allowing us to publish the report. We also thank our colleagues for their work on the treatment course.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade A, Grade A, Grade C
Novelty: Grade A, Grade A, Grade A
Creativity or Innovation: Grade A, Grade B, Grade B
Scientific Significance: Grade A, Grade A, Grade B
P-Reviewer: Gugulothu D; Jiang YC S-Editor: Lin C L-Editor: A P-Editor: Zhao YQ