Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.102038
Revised: January 8, 2025
Accepted: January 21, 2025
Published online: April 15, 2025
Processing time: 170 Days and 1.3 Hours
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality worldwide. Transarterial chemoembolization (TACE) combined with percuta
To evaluate the effectiveness and safety of TACE combined with RFA compared to TACE alone in the management of primary HCC.
A comprehensive retrospective analysis was conducted at our institution from January 2020 to January 2024, involving 106 patients diagnosed with intermediate to advanced-stage HCC. Patients were divided into two groups: Those receiving TACE alone (n = 56) and those undergoing combined TACE and RFA therapy (n = 50). Treatment efficacy was assessed based on tumor response rates, serum alpha-fetoprotein (AFP) levels, and survival outcomes. Statistical analyses, including χ2 tests and Kaplan-Meier survival analysis, were performed to compare the out
The TACE + RFA group demonstrated significantly higher rates of complete response (15 vs 4, P < 0.01) and partial response (23 vs 15, P = 0.046) compared to the TACE group. Conversely, the TACE group exhibited higher rates of stable disease (25 vs 7, P < 0.01) and progressive disease (12 vs 5, P < 0.01). Serum AFP levels decreased over time in the TACE + RFA group, while they increased in the TACE group. Survival analysis revealed superior survival outcomes in the TACE + RFA group, with higher survival rates and a prolonged median survival time compared to the TACE group.
The combination of RFA with TACE could offer enhanced treatment response and prolonged survival in patients with primary HCC compared to TACE alone. These findings might support the adoption of multimodal thera
Core Tip: Our study addresses a critical aspect of hepatocellular carcinoma (HCC) treatment, comparing the outcomes of transarterial chemoembolization (TACE) alone vs the combination of TACE with percutaneous radiofrequency ablation. With a robust retrospective analysis of 106 patients, our findings highlight significant enhancements in treatment efficacy and survival rates when these therapies are combined, thus proposing a pivotal shift in therapeutic strategies for patients with unresectable HCC.
- Citation: Fei J, Qi LW, Liu Y, Shu M, Mo WQ. Comparing transarterial chemoembolization alone to combined transarterial chemoembolization and radiofrequency ablation in primary hepatocellular carcinoma treatment. World J Gastrointest Oncol 2025; 17(4): 102038
- URL: https://www.wjgnet.com/1948-5204/full/v17/i4/102038.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i4.102038
Hepatocellular carcinoma (HCC) is the predominant form of primary liver cancer, representing a substantial burden of liver disease globally. It primarily occurs in individuals with preexisting chronic liver conditions, especially those infected with hepatitis B or hepatitis C viruses[1,2]. The therapy of HCC has distinct problems owing to the disease’s complexity, which is generally identified at an advanced stage and frequently arises in the context of cirrhosis[3,4]. Transarterial chemoembolization (TACE) is a recognized palliative intervention for unresectable HCC. TACE functions by precisely administering chemotherapeutic drugs alongside embolic substances straight to the tumor via the hepatic artery, so depriving the tumor of its blood supply while enhancing the local medication concentration[5,6]. This method has demonstrated the ability to extend survival in patients with intermediate-stage HCC who are ineligible for curative treatments such surgical resection or liver transplantation[7,8].
Percutaneous radiofrequency ablation (RFA) is an efficacious method for treating HCC, especially in tumors measuring less than 3 cm in diameter. RFA employs high-frequency electrical currents to create thermal damage to the tumor, resulting in coagulative necrosis. Although RFA offers benefits because to its minimally invasive characteristics and favorable safety profile, its effectiveness is frequently constrained by the tumor’s dimensions and position, in addition to the heat-sink effect caused by nearby blood arteries[9,10]. The integration of TACE and RFA has surfaced as a promising therapeutic approach to augment therapy efficacy in patients with HCC. This method leverages the advantages of both modalities: TACE diminishes the tumor’s arterial blood flow, alleviating the heat-sink effect and thereby improving the efficacy of later RFA[11]. Furthermore, the chemoembolic drugs administered during TACE may exhibit a synergistic cytotoxic impact when coupled with the thermal damage caused by RFA[12].
We emphasize the complexities in selecting appropriate therapeutic approaches for primary vs recurrent HCC patients, where the lack of standardized guidelines for recurrent HCC treatments (e.g., repeated liver resection vs liver trans
A comprehensive retrospective analysis was performed at our hospital to evaluate the efficacy and safety of TACE in conjunction with percutaneous RFA for the treatment of primary HCC. The trial duration extended from January 2020 to January 2024, encompassing a cohort of 106 patients with intermediate to advanced-stage HCC who satisfied the inclusion criteria. All cases were validated using radiological and histological assessments. Patients were categorized into two groups according to the treatment administered: One group received TACE exclusively (TACE group), while the other group was treated with a combination of TACE and percutaneous RFA (TACE + RFA group). All procedures in this study were performed by the same dedicated medical team with a minimum of five years of clinical experience in the management of HCC. The expertise of the team ensured the standardization of treatments and contributed to the consistency and reliability of the results. Informed consent was obtained from all subjects. The study protocols were rigorously reviewed and approved by the ethics committee of the First Affiliated Hospital of Shihezi University, ensuring compliance with the Declaration of Helsinki and applicable guidelines. All methods adhered to strict ethical standards, and the study was designed, conducted, and reported with full confidentiality, with all personal identifiers removed to safeguard participant privacy.
Inclusion criteria: (1) Patients diagnosed with primary HCC using histological analysis or non-invasive criteria es
Exclusion criteria: (1) Patients exhibiting extrahepatic metastases or invasion of the principal branches of the portal or hepatic vein; (2) Prior or simultaneous cancers excluding HCC; (3) Notable cardiovascular illness, encompassing recent myocardial infarction (within the past six months), unstable angina, or poorly managed hypertension; (4) Significant coagulopathy or thrombocytopenia that contraindicates percutaneous interventions; (5) Inability to furnish informed consent or adhere to the procedure due to psychiatric disorders or socio-economic conditions; and (6) Documented allergy to contrast chemicals utilized in imaging studies that cannot be mitigated with pre-medication.
The modified Seldinger technique was employed for femoral artery catheterization to administer TACE. After a high-pressure injection of contrast agent, the digital subtraction angiography perfusion imaging system was utilized to precisely locate the hepatic tumor arteries. Super-selective catheterization of the feeding arteries was then performed for the infusion of chemotherapeutic agents, specifically 20-30 mg of pirarubicin combined with 5-15 mL of superfluid iodized oil. The frequency of TACE procedures was adjusted based on the vascular supply to the tumor, with treatments repeated every three weeks until complete deposition of the iodized oil within the lesion was achieved.
The RFA system functioned within a power range of 0-200 watts and a frequency of 480 kHz, employing a cold-circulation system and pulsed radiofrequency delivery mode. Percutaneous RFA was conducted 3 to 14 days after TACE treatment. The pre-procedural interventional examination established the tumor status to customize analgesic management, incorporating local anesthetic with 2% lidocaine at the puncture site. Utilizing computed tomography (CT) guidance, the puncture trajectory was carefully devised to circumvent bile ducts and major veins, positioning the needle through the lesion to the contralateral edge. A cooling pump circulated isotonic 0.9% saline to sustain a consistent needle-tip temperature. The treatment settings were established at a power range of 40-60 watts for a period of 10-15 minutes. Lesions were addressed with a single needle for 1-2 nodules and a dual-needle technique for 4-6 lesions; patients with lesser tolerance could have graded ablations. Post-treatment care encompassed immediate hemostasis, prophylactic anti-infection strategies, and management of any sequelae.
The treatment’s efficacy was evaluated using the World Health Organization guidelines for the objective assessment of solid tumors. Evaluations were performed two months after therapy, employing dynamic contrast-enhanced CT scans to compare tumor responses before and after treatment, alongside simultaneous evaluations of serum alpha-fetoprotein (AFP) levels.
The response criteria were delineated as follows: Complete response: Elimination of all target lesions; Partial response: A minimum 50% reduction in the aggregate diameters of target lesions, with no emergence of new lesions; Stable disease: Insufficient tumor decrease to meet the criteria for partial response and inadequate growth to be classified as progressive disease, characterized by a tumor reduction of less than 50% or an increase of less than 25%; Progressive disease: A more than 25% increase in the aggregate diameters of target lesions.
The overall response rate was determined by the total of complete and incomplete responses. Furthermore, alterations in AFP levels were systematically documented, with a decrease above 50% or a transition to negative AFP values seen as suggestive of a substantial tumor response. Liver function tests were systematically evaluated prior to and during the surgery to determine any possible effects of the therapy. Consideration was also given to the occurrence of problems, guaranteeing thorough post-treatment care and supervision.
Statistical analyses were performed utilizing statistical product and service solutions software (Version 27.0) to guarantee accuracy and dependability. Continuous variables, confirmed for normal distribution, were analyzed using independent sample t-tests, with findings presented as mean ± SD. Categorical variables were displayed as frequencies and per
The trial included 106 individuals, comprising 56 patients in the TACE group and 50 patients in the TACE + RFA group. In the TACE cohort, there were 35 males and 21 females, with a median age of 56 years (range: 39-71 years). The TACE + RFA group consisted of 31 males and 19 females, with a median age of 53 years (range: 35-73 years). Both groups demonstrated similar distributions in Child-Pugh classification, Barcelona Clinic Liver Cancer stage, AFP levels (> 200 μg/L), and tumor diameter, with statistical analyses indicating no significant differences (Table 1). The consistency in baseline characteristics guarantees the validity and reliability of subsequent efficacy evaluations comparing the two treatment methods.
| Parameter | TACE group (n = 56) | TACE + RFA group (n = 50) |
| Male | 35 | 31 |
| Female | 21 | 19 |
| Child-Pugh class A | 42 | 39 |
| Child-Pugh class B | 14 | 11 |
| BCLC stage B | 31 | 25 |
| BCLC stage C | 18 | 18 |
| BCLC stage D | 7 | 7 |
| AFP > 200 μg/L | 23 | 19 |
| Mean tumor diameter (cm) | 6.9 ± 1.08 | 7.01 ± 1.18 |
| Median age (years) | 56 | 53 |
| Age range (years) | 39-71 | 35-73 |
This study evaluated the treatment response rates of the TACE and TACE + RFA groups. The TACE + RFA cohort exhibited markedly superior rates of complete response (30.0% vs 7.1%, χ² = 19.036, P < 0.01) and partial response (46.0% vs 26.8%, χ² = 3.992, P = 0.046) in comparison to the TACE cohort. In contrast, the TACE group demonstrated elevated rates of stable disease (44.6% vs 14.0%, χ² = 9.413, P < 0.01) and progressive disease (21.4% vs 10.0%, χ² = 13.518, P < 0.01) relative to the TACE + RFA group. The results indicate that incorporating RFA with TACE may improve therapy efficacy, especially in attaining complete and partial responses, while perhaps decreasing the incidence of stable and progressing disease (Table 2).
| Factors | TACE group (n = 56) | TACE + RFA group (n = 50) | χ² value | P value |
| Complete response | 4 (7.1) | 15 (30.0) | 19.036 | < 0.01 |
| Partial response | 15 (26.8) | 23 (46.0) | 3.992 | 0.046 |
| Stable disease | 25 (44.6) | 7 (14.0) | 9.413 | < 0.01 |
| Progressive disease | 12 (21.4) | 5 (10.0) | 13.518 | < 0.01 |
This study examined the changes in serum AFP levels prior to and during therapy in individuals undergoing TACE and TACE + RFA. Both therapy groups exhibited variations in serum AFP levels throughout the trial period. Prior to the commencement of treatment, the TACE group demonstrated a mean serum AFP level of 543.4 ± 196.7 μg/L, whereas the TACE + RFA group presented a mean level of 565.2 ± 206.7 μg/L. After one year of treatment, the TACE group exhibited a notable rise in serum AFP levels to 639.1 ± 190.9 μg/L, while the TACE + RFA group showed a reduction to 475.4 ± 200.7 μg/L. The results persisted in the following years of follow-up, revealing diverse patterns between the two groups (Table 3). The findings indicate that TACE may increase serum AFP levels, however the incorporation of RFA may reduce this effect, possibly signifying a more advantageous therapy response for AFP dynamics.
| Group | Time point | Number | Serum AFP (μg/L) |
| TACE group | Before treatment | 56 | 543.4 ± 196.7 |
| After 1 year | 28 | 639.1 ± 190.9 | |
| After 2 years | 6 | 623.4 ± 234.6 | |
| After 3 years | 2 | 674.4 ± 300.2 | |
| TACE + RFA group | Before treatment | 50 | 565.2 ± 206.7 |
| After 1 year | 32 | 475.4 ± 200.7 | |
| After 2 years | 15 | 416.4 ± 229.0 | |
| After 3 years | 9 | 320.4 ± 243.5 |
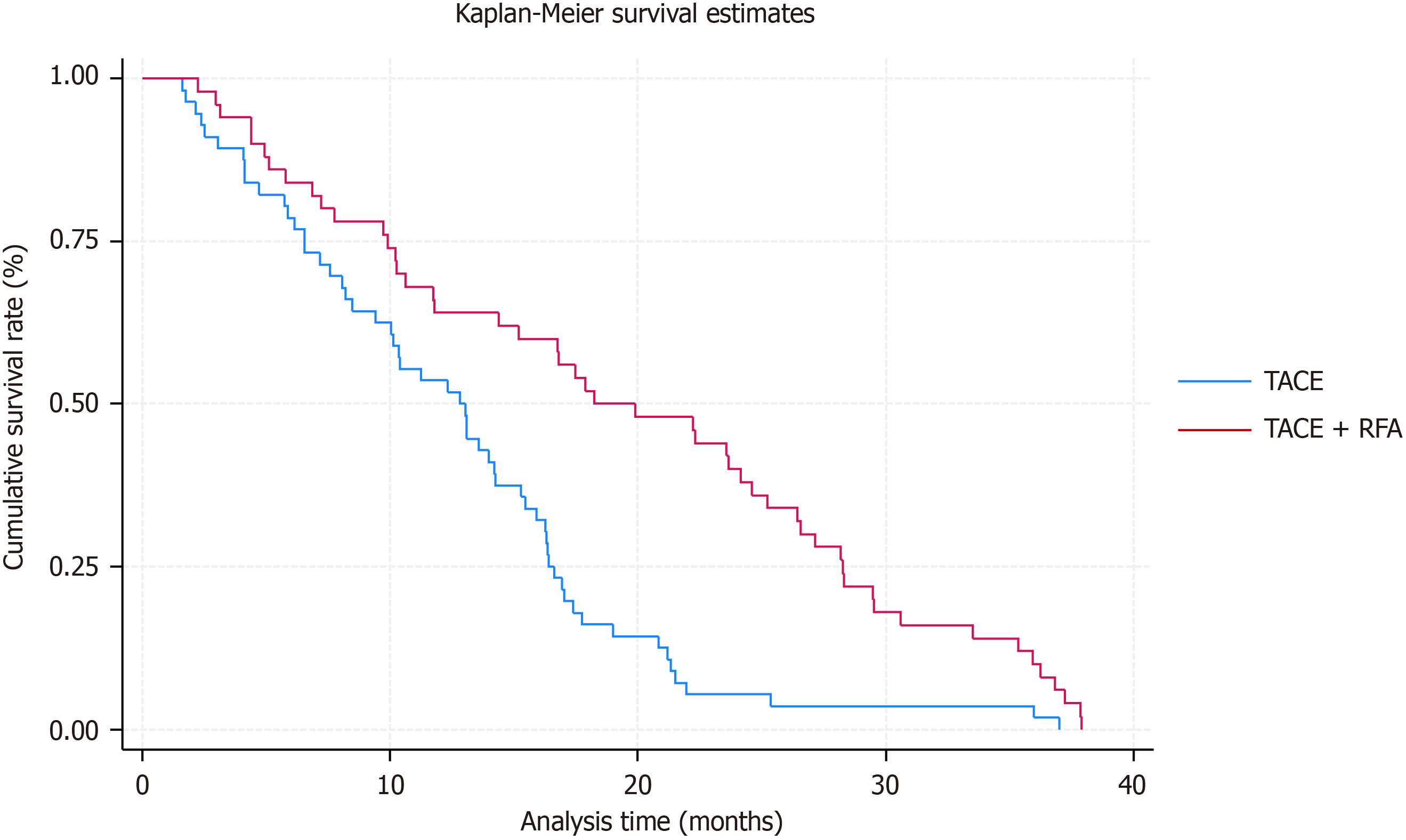
Our study’s long-term follow-up data indicated no occurrences of patients lost to follow-up in either therapy cohort. The TACE + RFA cohort demonstrated favorable survival outcomes, with a one-year survival rate of 64.0%, decreasing to 30.0% at two years and further to 18.0% at three years. Conversely, the survival rates in the TACE group exhibited a distinct pattern, with rates of 50.0%, 10.71%, and 3.57% at one, two, and three years, respectively. Subsequent analysis using Kaplan-Meier survival curves demonstrated divergent survival patterns. The median survival duration for patients in the TACE group was 13 months, whereas those in the TACE + RFA group had an extended median survival duration of 20 months, as illustrated in Figure 1. The extensive findings highlight the potential advantages of integrating RFA with TACE to enhance long-term survival rates for patients with hepatocellular cancer. The enhanced survival rates and extended median survival time noted in the TACE + RFA group underscore the significance of multimodal therapy strategies in the treatment of this cancer.
After the conclusion of TACE treatment, patients in both cohorts had differing levels of pain, fever, and diminished appetite within a week. Nonetheless, these symptoms resolved naturally without intervention. Transient modifications in liver function were recorded in both cohorts, with a marginally elevated percentage found in the combination therapy cohort (32.28%) relative to the TACE cohort (28.56%). Nevertheless, these alterations were temporary, with normalization noted within one week following treatment. Significantly, neither cohort encountered significant problems, including pneumothorax or bile duct fistula, throughout the surveillance period. The findings indicate that both TACE and combination therapy modalities are typically well-tolerated, exhibiting tolerable adverse effects and minimal incidences of severe consequences.
Primary HCC represents a considerable global health challenge, with few curative treatment alternatives accessible, especially for patients with unresectable tumors or surgical contraindications. TACE and percutaneous RFA have become primary treatment options for unresectable HCC, providing localized tumor management and perhaps extending patient survival[18,19]. The efficacy and safety of the combination of TACE and RFA in treating primary HCC require additional research[20,21]. This study sought to assess the efficacy and safety of the combination of TACE and RFA in treating primary HCC. The results demonstrated significant disparities in treatment responsiveness, changes in blood AFP levels, survival outcomes, and occurrence of adverse events between the TACE and TACE + RFA groups. This study provides novel insights into the management of intermediate- to advanced-stage HCC, where curative options are limited. We demonstrate the synergistic potential of combining TACE and percutaneous RFA, significantly enhancing tumor response and improving long-term survival. Our findings show higher complete and partial response rates, with reduced stable and progressive disease, compared to TACE alone. The combination therapy also led to better survival outcomes (median survival of 20 months vs 13 months) and more favorable serum AFP dynamics, suggesting its value as a therapeutic biomarker. The absence of major complications further supports the safety of this approach. These results highlight the importance of multimodal therapies and personalized treatment strategies, offering a more effective, tailored approach for HCC patients ineligible for curative treatments, with potential for widespread clinical application.
The justification for integrating TACE and RFA is based on their unique yet synergistic modes of action. TACE administers chemotherapeutic medicines directly to the tumor via the hepatic artery, causing tumor ischemia and necrosis while reducing systemic toxicity. RFA employs thermal energy to ablate neoplastic tissue, facilitating precise and targeted tumor eradication. Integrating various modalities may yield synergistic benefits, resulting in higher tumor response rates, enhanced local tumor control, and perhaps extended patient survival[22,23]. The elevated rates of complete and partial responses in the TACE + RFA group relative to the TACE group indicate a possible synergistic impact of integrating RFA with TACE. RFA, through the application of targeted heat energy, may augment the embolic effects of TACE, leading to increased tumor necrosis and improved therapy response. The reduction of AFP increase in the TACE + RFA group following therapy suggests a potential decrease of tumor activity and a more advantageous treatment response profile[24,25]. This is consistent with other research indicating that AFP dynamics are associated with treatment response and overall survival (OS) in HCC patients undergoing locoregional treatments.
The prolonged median survival time and enhanced survival rates seen in the TACE + RFA group highlight the potential clinical advantages of integrating RFA with TACE. The extended survival results in this cohort may be ascribed to various variables. The synergistic effect of TACE with RFA may result in more thorough tumor ablation and improved disease management, therefore postponing disease progression and extending survival[26]. Furthermore, RFA’s capacity to address residual or satellite lesions that are not suitable for TACE alone may enhance long-term outcomes[27]. Finally, the synergistic relationship between TACE and RFA in addressing both macroscopic and microscopic disease may enhance the elimination of tumor cells and decrease recurrence rates, ultimately leading to improved survival outcomes[28]. The tolerance of both treatment methods was apparent, with manageable side events and low incidences of severe consequences noted in both groups. The temporary changes in liver function noted after treatment were anticipated and presumably due to the hepatotoxic effects of the chemotherapeutic drugs utilized in TACE and the localized tissue damage caused by RFA. Nonetheless, these alterations reversed spontaneously within a few period, underscoring the ephemeral nature of treatment-induced hepatic dysfunction[29,30]. The lack of significant problems, such as pneumothorax or bile duct fistula, highlights the safety profile of both TACE and combination TACE + RFA therapy, confirming their appropriateness for clinical use in HCC treatment.
Recent studies have provided valuable insights into the treatment strategies for HCC, and a comparison with our findings offers a more comprehensive perspective on the evolving approaches to managing both primary and recurrent HCC. Chen et al’s network meta-analysis on recurrent HCC found that repeated hepatectomy and liver transplantation are superior to RFA and TACE in disease-free survival (DFS) and OS[13]. In contrast, our study focuses on primary HCC, comparing TACE alone with TACE combined with RFA, providing more precise insights into multimodal therapies for primary disease, with a stronger emphasis on the synergistic effects between TACE and RFA. Unlike Chen et al’s study, which addresses recurrent cases, our paper highlights the benefits of combining two established treatments in primary HCC management. Romano et al’s meta-analysis on early-stage HCC found that liver resection outperforms TACE in OS and DFS, though no significant difference was found between liver resection and RFA in OS[14]. Our research diverges by investigating the combined use of TACE and RFA for intermediate- to advanced-stage HCC, where surgery is often not an option, demonstrating significant survival benefits in these more advanced stages. Pan et al’s meta-analysis supports the combined use of TACE and RFA, showing improved objective response rate, disease control, and OS without significant increases in adverse events[15]. Our study corroborates these findings but extends the evidence by focusing on primary HCC with a larger cohort and robust statistical analysis. Furthermore, we emphasize personalized treatment strategies, considering liver function and comorbidities, an aspect underexplored in Pan et al’s study[15].
Several limitations of this study should be addressed in future research. Firstly, multivariate analysis should be incorporated to evaluate the impact of confounding factors such as tumor size, treatment timing, and patient comor
In conclusion, the combined treatment approach of TACE with RFA might demonstrate superior efficacy compared to TACE alone in the treatment of HCC. This combined therapy could significantly enhance survival rates and extend OS while maintaining a favorable safety profile.
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4088] [Article Influence: 584.0] [Reference Citation Analysis (6)] |
| 2. | Ganesan P, Kulik LM. Hepatocellular Carcinoma: New Developments. Clin Liver Dis. 2023;27:85-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 229] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 3. | Gilles H, Garbutt T, Landrum J. Hepatocellular Carcinoma. Crit Care Nurs Clin North Am. 2022;34:289-301. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Alawyia B, Constantinou C. Hepatocellular Carcinoma: a Narrative Review on Current Knowledge and Future Prospects. Curr Treat Options Oncol. 2023;24:711-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 57] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 5. | Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent Updates of Transarterial Chemoembolilzation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 6. | Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;21:10327-10335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 131] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (2)] |
| 7. | Vogl TJ, Zangos S, Balzer JO, Nabil M, Rao P, Eichler K, Bechstein WO, Zeuzem S, Abdelkader A. [Transarterial chemoembolization (TACE) in hepatocellular carcinoma: technique, indication and results]. Rofo. 2007;179:1113-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Hashem E, Sait S, Thomas DN, Watson C, Moeen S, Peddu P. Transarterial chemoembolisation for very early and early stage hepatocellular carcinoma: single-centre experience. Clin Radiol. 2023;78:e113-e122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Deng Q, He M, Fu C, Feng K, Ma K, Zhang L. Radiofrequency ablation in the treatment of hepatocellular carcinoma. Int J Hyperthermia. 2022;39:1052-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Nguyen N, Rode A, Trillaud H, Aubé C, Manichon AF, Hocquelet A, Paisant A, Dao T, Nahon P, Ganne-Carrié N, Blaise L, Cauchy F, Sutter O, Séror O, Nault JC. Percutaneous radiofrequency ablation for hepatocellular carcinoma developed on non-alcoholic fatty liver disease. Liver Int. 2022;42:905-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 11. | Kim JG, Cho SK, Hyun D, Shin SW, Park KB, Park HS, Choo SW, Do YS, Woo SY, Baek SY. Combined transarterial chemoembolization and radiofrequency ablation for subphrenic versus nonsubphrenic hepatocellular carcinoma: a propensity score matched study. Abdom Radiol (NY). 2021;46:5735-5745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Xu Z, Xie H, Zhou L, Chen X, Zheng S. The Combination Strategy of Transarterial Chemoembolization and Radiofrequency Ablation or Microwave Ablation against Hepatocellular Carcinoma. Anal Cell Pathol (Amst). 2019;2019:8619096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Chen JL, Chen YS, Ker CG. Network meta-analysis of the prognosis of curative treatment strategies for recurrent hepatocellular carcinoma after hepatectomy. World J Gastrointest Surg. 2023;15:258-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Romano P, Busti M, Billato I, D'Amico F, Marchegiani G, Pelizzaro F, Vitale A, Cillo U. Liver resection versus radiofrequency ablation or trans-arterial chemoembolization for early-stage (BCLC A) oligo-nodular hepatocellular carcinoma: meta-analysis. BJS Open. 2024;8. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Pan K, Wang S, Li X, Wu S. Efficacy and safety of ultrasound-guided radiofrequency ablation combined with transhepatic artery embolization chemotherapy for hepatocellular carcinoma: A meta-analysis. PLoS One. 2024;19:e0305965. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Song MJ, Bae SH, Lee JS, Lee SW, Song DS, You CR, Choi JY, Yoon SK. Combination transarterial chemoembolization and radiofrequency ablation therapy for early hepatocellular carcinoma. Korean J Intern Med. 2016;31:242-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Mu S, Chen Q, Li S, Wang D, Zhao Y, Li X, Fu W, Fan Z, Tian S, Li Z. Incomplete radiofrequency ablation following transarterial chemoembolization accelerates the progression of large hepatocellular carcinoma. J Cancer Res Ther. 2023;19:924-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Zhu AX, Salem R. Combining transarterial chemoembolization with radiofrequency ablation for hepatocellular carcinoma: one step forward? J Clin Oncol. 2013;31:406-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Chen ML, Li HL, Guo CY, Zhang H, Yuan H, Li Z, Park JH, Hu HT. Radiofrequency ablation combined with transarterial chemoembolization in treatment of hepatocellular carcinoma adjacent to the second hepatic hilus. Abdom Radiol (NY). 2022;47:423-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 618] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 21. | Kong SY, Song JJ, Jin YQ, Deng MJ, Yan JX. Hepatic arterial infusion chemotherapy versus transarterial chemoembolization for patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Acta Clin Belg. 2023;78:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Yuan W, Yang MJ, Xu J, Yan ZP, Liu R, Qu XD, Wang JH, Qian S. Radiofrequency Ablation Combined With Transarterial Chemoembolization for Specially Located Small Hepatocellular Carcinoma. Technol Cancer Res Treat. 2018;17:1533033818788529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Lee BC, Liu KL, Wu CH, Huang KW, Ho CM, Hu RH, Ho MC, Wu YM, Lee PH, Liang PC. Comparison of Radiofrequency Ablation and Transarterial Chemoembolization for Hepatocellular Carcinoma in the Caudate Lobe. Cardiovasc Intervent Radiol. 2018;41:1699-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Yang Y, Yu H, Qi L, Liu C, Feng Y, Qi J, Li J, Zhu Q. Combined radiofrequency ablation or microwave ablation with transarterial chemoembolization can increase efficiency in intermediate-stage hepatocellular carcinoma without more complication: a systematic review and meta-analysis. Int J Hyperthermia. 2022;39:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 25. | Cao S, Zou Y, Lyu T, Fan Z, Guan H, Song L, Tong X, Wang J. Long-term outcomes of combined transarterial chemoembolization and radiofrequency ablation versus RFA monotherapy for single hepatocellular carcinoma ≤3 cm: emphasis on local tumor progression. Int J Hyperthermia. 2022;39:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Chen L, Sun J, Yang X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: Current status. Cancer Lett. 2016;370:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Liu D, Liu M, Su L, Wang Y, Zhang X, Long H, Kuang M, Xie X, Lin M. Transarterial Chemoembolization Followed by Radiofrequency Ablation for Hepatocellular Carcinoma: Impact of the Time Interval between the Two Treatments on Outcome. J Vasc Interv Radiol. 2019;30:1879-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Gui CH, Baey S, D'cruz RT, Shelat VG. Trans-arterial chemoembolization + radiofrequency ablation versus surgical resection in hepatocellular carcinoma - A meta-analysis. Eur J Surg Oncol. 2020;46:763-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 29. | Chu HH, Kim JH, Yoon HK, Ko HK, Gwon DI, Kim PN, Sung KB, Ko GY, Kim SY, Park SH. Chemoembolization Combined with Radiofrequency Ablation for Medium-Sized Hepatocellular Carcinoma: A Propensity-Score Analysis. J Vasc Interv Radiol. 2019;30:1533-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Georgiades CS, Hong K, Geschwind JF. Radiofrequency ablation and chemoembolization for hepatocellular carcinoma. Cancer J. 2008;14:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |