Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.102021
Revised: January 26, 2025
Accepted: February 18, 2025
Published online: April 15, 2025
Processing time: 171 Days and 0.9 Hours
Colon cancer is a common malignancy of the digestive tract. An estimated 1148515 new cases of colon cancer were reported in 2020 worldwide. Chronic myeloid leukemia (CML) is a malignant tumor formed by the clonal proliferation of bone marrow hematopoietic stem cells, with an annual incidence rate of 1-2 cases per 100000 people worldwide. Leukemia can be secondary to solid tumors, and vice versa. Reports on CML secondary malignant tumors account for 8.7% but CML secondary to malignancy is extremely rare. Therapy-related CML is a rare but potentially fatal adverse event of chemotherapy or radiotherapy. Herein, we report a case of CML with colon cancer and discuss this unique patient popu
A 61-year-old male patient attended our hospital due to leukocytosis for 5 days. In February 2020, the patient was diagnosed with colon cancer and underwent ra
CML in patients with colon cancer is extremely rare. Secondary hematological tumors may be multifactorial, and the exact mechanism is currently unknown. Owing to the slow progression of the disease, patients with CML show no sy
Core Tip: We report a case of chronic myelogenous leukemia secondary to colon cancer. Secondary hematological tumors may be multifactorial, and the exact mechanism is currently unknown. Owing to the slow progression of the disease, patients with chronic myelogenous leukemia show no symptoms in the early stage. However, with disease progression, obvious but non-specific symptoms may appear, including fever, anemia, bleeding tendency, and hypertrophy. Therefore, complete blood count monitoring for routine examination is recommended after cancer treatment for early detection of occult hematological tumors.
- Citation: Li XL, Li M, Yang H, Tian J, Shi ZW, Wang LZ, Song K. Chronic myelogenous leukemia secondary to colon cancer: A case report. World J Gastrointest Oncol 2025; 17(4): 102021
- URL: https://www.wjgnet.com/1948-5204/full/v17/i4/102021.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i4.102021
Colorectal cancer is the third most common cancer and the third leading cause of cancer death globally[1]. Leucovorin, fluorouracil, and oxaliplatin (FOLFOX) or leucovorin, fluorouracil, and irinotecan (FOLFIRI) plus bevacizumab have been widely used as the first-line treatment for metastatic colorectal cancer[2]. Chronic myeloid leukemia (CML) is a clonal myelodysplastic disease of pluripotent hematopoietic stem cell origin caused by the Philadelphia (Ph) chromosome. Ph is derived from a translocation t(9;22)(q34q11) that generates a BCR-ABL fusion gene and is transcribed as a protein with abnormal tyrosine kinase activity that drives aberrant white blood cell (WBC) proliferation[3]. Solid tumors such as those of the lung, thyroid, breast, and skin may occur in 3% of patients with CML. Gastrointestinal system malignancies such as colon, rectal, esophageal, and liver cancers are rarely reported in patients with CML, with only a few cases of CML secondary to colon cancer reported worldwide[4]. The origin of both concomitant malignancies is unclear. In this case report, we describe our clinical experience of a patient with Ph-positive CML accompanying colon cancer and discuss this unique patient population.
A 61-year-old male patient was diagnosed with colon cancer in February 2020 and was found to have a chronic myelogenous leukemia in June 2023.
The patient visited a Fenghuang County Human County Hospital with a complaint of hematuria on June 18, 2023, and the initial examination showed an elevated WBC count of 40 × 109/L (normal range, 4.0-10.0 × 109/L), a platelet count of 165 × 109/L and a hemoglobin level of 119 g/L. Doppler ultrasound of the abdomen (also known as color blood flow chart; the color ultrasound instrument is unified as red from the near ultrasound probe; the blood flow leaving the probe is blue; turbulence and shunt are polychromosaic) revealed a large liver and double kidney stones. Intravenous meropenem (1 g intravenous drip per 8 hours) and fluid replacement to control these symptoms. Hematuria improved following the anti-inflammation routine, while the leukocyte levels increased significantly (about 60 × 109/L). The patient consulted the hematology department of our hospital on June 20, 2023 due to leukocytosis.
In February 2020, the patient was diagnosed with colon cancer and underwent radical surgery and conventional chemotherapy with a stable condition. Past medical history also included type 2 diabetes for more than 10 years and appendectomy.
No special personal or family history.
The patient had stable vital signs, no superficial lymph node enlargement, and no tenderness or mass in the abdomen.
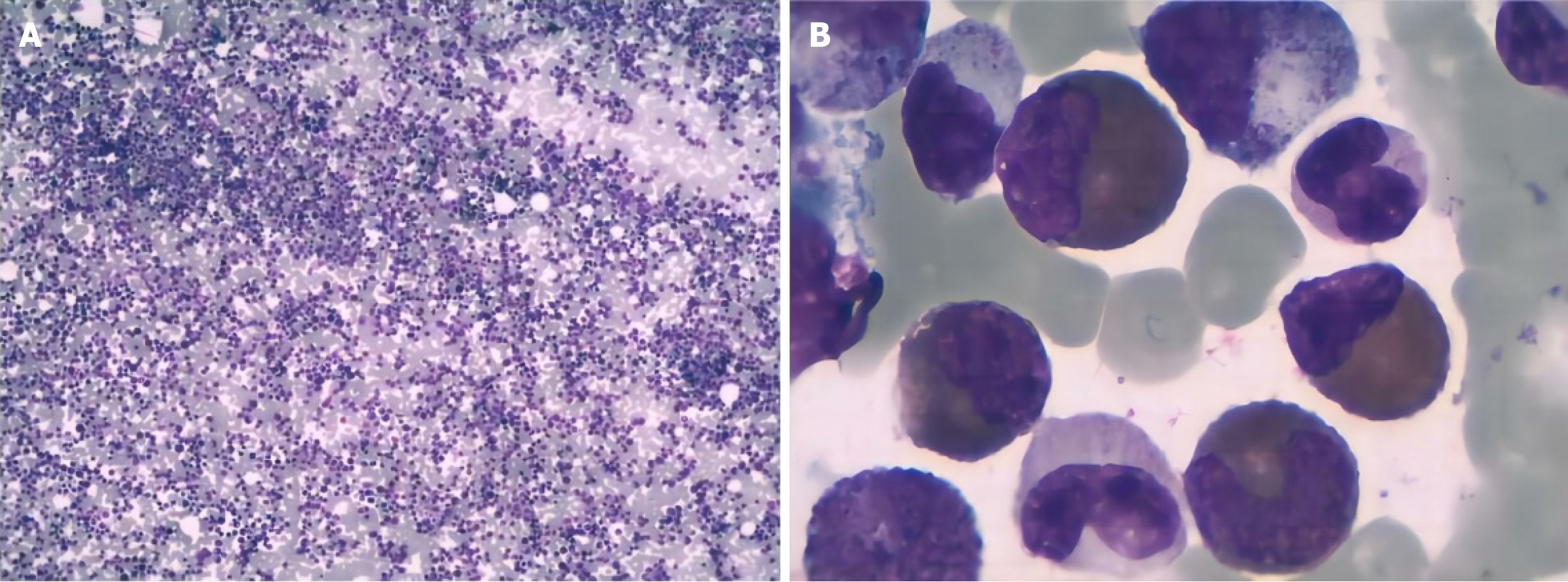
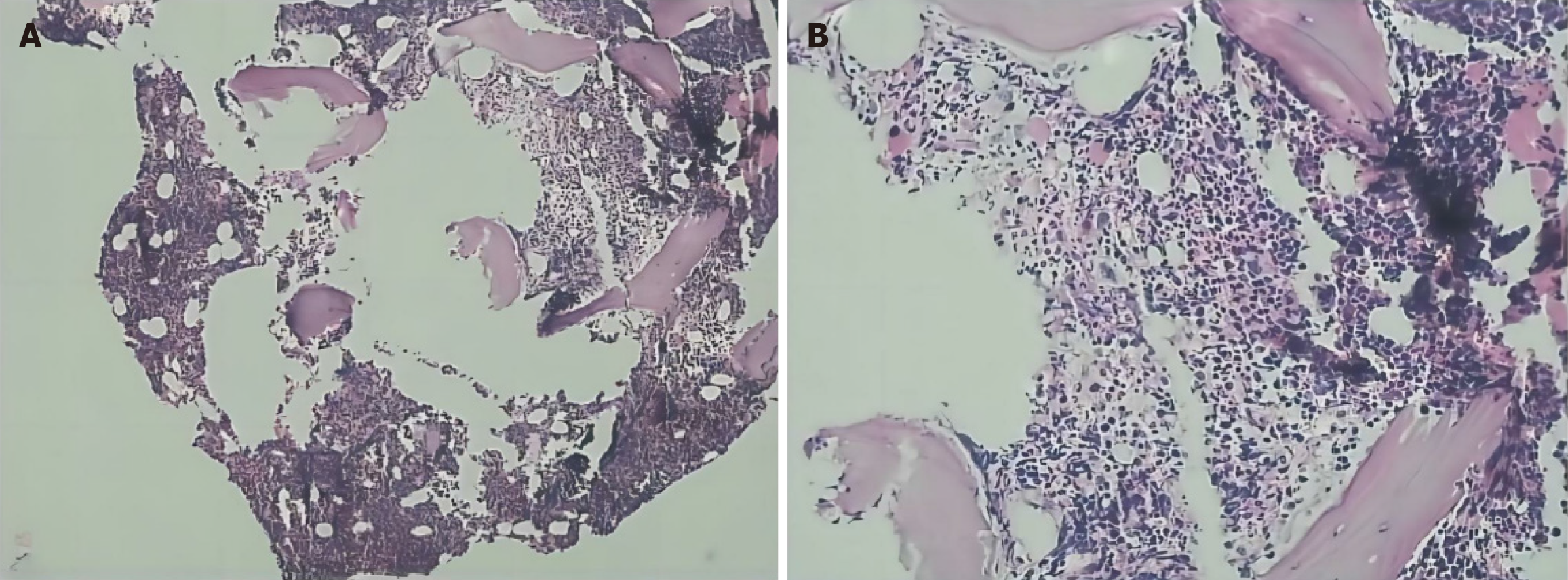
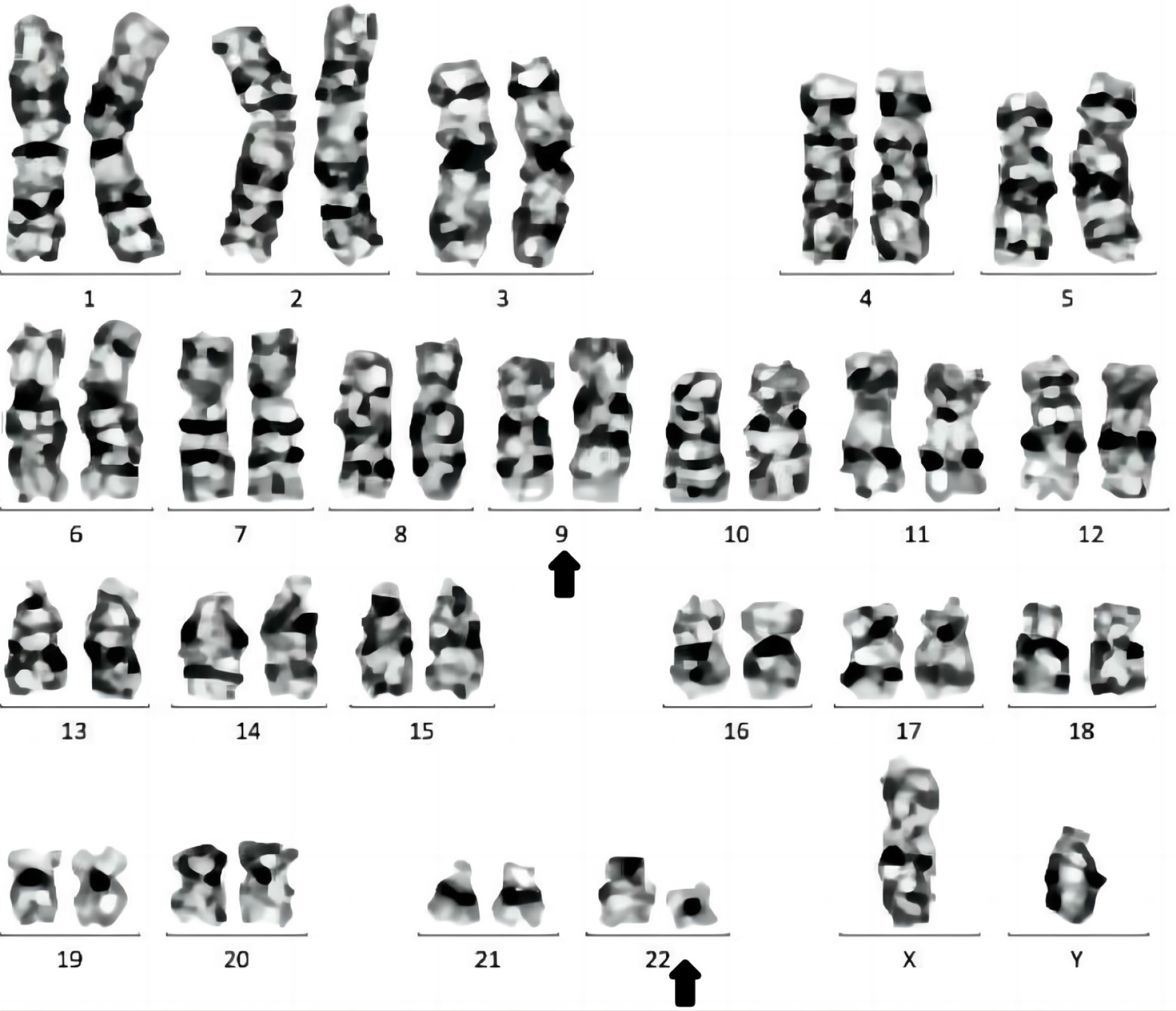
In 2021 (Hunan Provincial People’s Hospital), routine blood tests were normal. In June, 2023 (Fenghuang County Human County Hospital), the initial examination showed an elevated WBC count of 40 × 109/L (normal range, 4.0-10.0 × 109/L), a platelet count of 165 × 109/L and a hemoglobin level of 119 g/L. In June, 2023 (the First Affiliated Hospital of Jishou University), routine blood test revealed a WBC count of 78.03 × 109/L (normal range, 4.0-10.0 × 109/L), a hemoglobin level of 125 g/L (normal range, 120-160 g/L), and a platelet count of 273 × 109/L (normal range, 100-400 × 109/L). Bone marrow cytology (rapid smear of fresh bone marrow 0.1-0.2 mL was collected, and cells was performed, and further chemical staining such as peroxidase and glycogen staining) indicated hyperactive hyperplasia and an increased proportion of granulocytes, eosinophils, and basophils (Figure 1). Erythroid cell proportion was reduced, mainly those of middle and late red but the morphology of mature red cells was not abnormal. There was a decrease in the proportion of lymphocytes, specifically mature lymphocytes (Figure 2). Flow cytometry (DxFLEX, Beckman Coulter, Kaluza Software-Analysis) revealed an increased proportion of granular lines, whereby eosinophils and basophils were easily observed. Bone marrow biopsy (the tissue blocks were fixed with Bouin fluid without decalcification or molding Material bag buried. Three to five consecutive sections were sectioned for each specimen, lined separately hepatocyte growth factor, hematoxylin and eosin, and Gomori staining) showed that the bone marrow was active in nucleated cell proliferation (about 70% of the hematopoietic area), with scattered immature cells (Figure 3). Moreover, mainly middle and late juvenile erythroid cells were observed. Karyotype analysis (ISCN2020) showed Ph [t(9;22)(q34;q11)] (Figure 4). BCR::ABL1 fusion genotyping (qualitative) indicated P210 positivity (e14a2+) (two-color dual-fusion DNA probe, Beijing Jinpujia Company; Olympus-BX 51 fluorescence microscope). Peripheral blood smears showed rare plasma cells (< 10%). The blood routine review at discharge indicated a WBC count of 35.8 × 109/L, a hemoglobin level of 117 g/L, and a platelet count of 191 × 109/L.
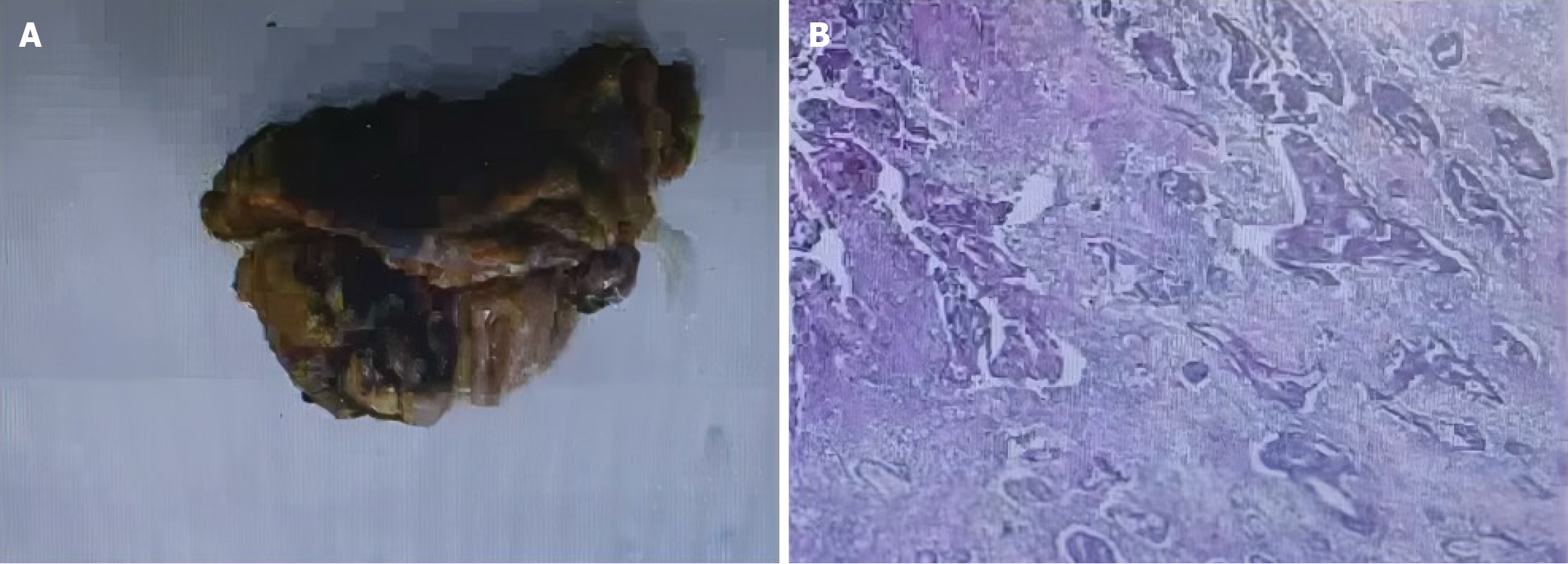
In February, 2020 (Hunan Provincial People’s Hospital), enhanced abdominal computed tomography suggested irregular thickening in the middle and upper part of the rectum, and rectal cancer was considered. Magnetic resonance imaging results showed the following: (1) Rectal (upper) carcinoma, muscle layer invasion, circumferential margin (negative), positive external vessel invasion, lower margin 71 mm from the anus, four lymph nodes in the mesenteric area, pelvic and pelvic floor muscles (no) invasion, and bilateral internal and external iliac vessel branches (no) invasion; and (2) Magnetic resonance stage: Stage cT3N2. Furthermore, results of the electronic colonoscopy showed the following: Rectal cancer (?), straight junction polyps, and colonic polyps (transverse and descending colonic polyps were removed). The pathologic results of polyps were as follows (Figure 1): Moderately differentiated tubular adenocarcinoma (rectal mass) and adenoma (transverse colon polyp). In June, 2023 (Fenghuang County Human County Hospital), Doppler ultrasound of the abdomen (also known as color blood flow chart; the color ultrasound instrument is unified as red from the near ultrasound probe; the blood flow leaving the probe is blue; turbulence and shunt are polychromosaic) revealed a large liver and double kidney stones.
The final diagnosis was CML-chronic phase (Sokal score of 0.72).
Excluding the contraindications, the first cycle of chemotherapy following the FOLFOX6 regimen was administered on February 23, 2020. Specifically, the following drugs were administered: Oxaliplatin 140 mg + fluorouracil 660 mg + leucovorin calcium 200 mg + fluorouracil 4000 mg (pumped for 48 hours), supplemented with those for liver protection and against gastric nausea. The preoperative examination identified improvement in colon cancer. Indocyanine green laparoscopic radical resection of rectal cancer and intestinal adhesiolysis were performed on March 3, 2020. After chemotherapy, the patient’s condition improved. Subsequently, four cycles of FOLFOX6 regimen chemotherapy were administered on March 12, April 18, May 5, and May 30 in 2020, with the same specific medication as stated above. Repeated colonoscopy showed a reduction in the tumor area. XELOX chemotherapy was initiated on June 13, 2020, with the following drugs: Oxaliplatin 200 mg day 1 and capecitabine 3000 mg day 1-14. Subsequently, three cycles of XELOX chemotherapy with the same specific drugs as above were administered on July 4, July 25, and August 11 in 2020. The patient visited Hunan Provincial People’s Hospital for examination, and the results showed that the patient’s condition was under control and the blood routine was normal. The patient was eventually diagnosed with CML-chronic phase (Sokal score of 0.72) and received imatinib (400 mg daily) on June 26, 2023.
After discharge, the patient was prescribed imatinib (400 mg daily) and hydroxyurea tablets (0.5 g twice a day) and visited the outpatient department for follow-up monthly. The patient visited the outpatient department of our hospital in late December 2023, and the blood routine review indicated that the counts of WBC, platelets, and hemoglobin levels were all normal.
Secondary malignancies such as non-Hodgkin’s lymphoma, myelodysplastic syndrome, and acute leukemia have been observed in some patients with cancer. As a rare complication, secondary CML accounts for approximately 2.6% of all cases of secondary leukemia. Although CML only accounts for a small portion of secondary leukemia, reports on therapy-related CML (TR-CML) have increased in recent years along with continuously updated treatment methods[4,5]. This report describes a case of CML secondary to colon cancer, and we summarize the treatment options for this unique patient population.
Among malignancies, colon cancer is the second leading cause of cancer-related mortality worldwide. Early colon cancer is typically asymptomatic, and symptoms such as abdominal distension, indigestion, change of defecation habits, abdominal pain, and anemia may be observed in the middle and late stages. Owing to the slow progression of the disease, patients with CML show no symptoms in the early stage. However, with disease progression, obvious but non-specific symptoms may appear, including fever, anemia, bleeding tendency, and hypertrophy. On the one hand, the use of chemotherapy drugs can prolong the survival time and improve the quality of life of the patients; on the other hand, it increases the risk of secondary tumor occurrence.
In most cases, patients with colon cancer show peripheral blood changes with or without clinical symptoms, which was confirmed by bone marrow puncture. This patient had undergone multiple courses of chemotherapy with capecitabine and showed no obvious discomfort after medication. Hematological changes occurred three years after chemotherapy, and bone marrow puncture and fusion gene testing confirmed CML. Existing case reports of CML secondary to colon cancer are rare, and the association between these two diseases is unclear. TR-CML cannot be made cytogenetically distinguished from de novo CML, and typical chromosomal aberrations associated with treatment-related CML have not been described[6]. Several factors may be associated with this concomitant tumor, including host-specific features, past radiotherapy and chemotherapy, epigenetic up-down regulation or exposure to environmental carcinogens. In this patient, we speculate that the possible pathogenesis of CML includes the following.
An intuitive assumption is that CML is the result of colon cancer treatment. Chemotherapy and radiotherapy may inhibit the immune system of the body, thus weakening its monitoring effect on tumor cells, and providing opportunities for the proliferation of tumor cells. Both solid tumors and leukemia require chemotherapy and (or) radiation therapy, and many studies show that chemotherapy and radiation can treat tumors or cause cancer[6]. Drugs inducing secondary leukemia include alkylating agents, topoisomerase 2 poisons (e.g., etoposide and teniposide), cyclophosphamide, and anthracyclines[7]. Etoposide is a well-known topoisomerase 2 poison. It was first reported in 1987 that etoposide can induce secondary leukemia, and the specific mechanism may involve mixed-lineage leukemia gene translocation[8,9]. Platinum compounds are effective broad-spectrum anticancer drugs widely used in the treatment of various malignant tumors. Cisplatin and carboplatin are associated with the treatment-related leukemia (TRL)[10]. With improvements in diagnosis and treatment methods, reports on oxaliplatin and TRL have increased[11,12]. Platinum drugs may kill cancer cells by forming DNA cross-links within and between chains by forming DNA adducts, thus disrupting DNA replication and transcription. As an antimetabolite, 5-fluorouracil is a pyrimidine analog that is ineffective on its own but becomes potent after conversion into 5-fluorouracil by pyrimidine nucleoside phosphorylase which is preferentially found in tumor tissues[13]. It can also simulate the functions of trona and synthetase after administration, thereby altering the activity of thymidine synthetase and causing a failure of normal DNA synthesis by inducing p53-dependent cell growth arrest and apoptosis[14]. Through cytotoxic therapy, selective pressure on hematopoietic progenitor cells carrying mutations in the tumor protein p53 pathway can lead to TRL[15,16]. Irinotecan is an agent that selectively targets the topoisomerase, and irinotecan is degraded through complex metabolic pathways[17]. Irinotecan can cause secondary leukemia[18,19] but the mechanism is unclear and needs further clarification.
Another potential hypothesis is genetic susceptibility. The incidence and prognosis of multiple primary cancers for different ethnicities vary, which may be due to genetic factors[20]. Although familial clustering of primary and secondary relative malignancies in patients with CML has not been demonstrated, further research on this topic is needed. If there is a genetic susceptibility to CML and other malignancies due to poor DNA repair, the accumulation of other malignancies in close relatives of patients with CML can be expected. Notably, host susceptibility is related to oncogenes, and the overexpression of oncogenes and the deletion of tumor suppressor genes are closely related to multiple primary cancers, such as Li-Fraumeni syndrome[21]. However, genetic instability in the host may produce atypical progenitor cells, which puts patients in a state where they are more likely to acquire a variety of diseases. During treatment, the expression of cellular molecular pathways is down-regulated, and these malignant cells gain the ability and mechanism of anti-apoptosis. When the immune response is lost or reduced, tumor cells escape immune surveillance leading to disease occurrence.
Host-specific factors are a possible explanation. When tumorigenic factors act on cells for a long time, they denature and change their functions, and some damaged cells mutate or transform due to the lack of repair function or replication errors. When human immune function is defective, these mutated or transformed cells may develop into cancer cells or cloned cells. Many multiple tumors occur in older adults, mainly due to the long-term action of carcinogens, internal molecular changes due to aging, and changes in the internal environment[3].
Because malignant tumors often reduce the immune monitoring function of cellular immunity and humoral immunity, combined with strong chemotherapy or radiotherapy commonly used for treatment, the original immune deficiency is aggravated and the chance of tumor development is greatly increased. Theoretically, weakened immune surveillance may increase the chances of pre-leukemia cloned hematopoietic stem cells escaping from checkpoint restrictions and gaining growth advantages after receiving additional genetic or epigenetic attacks[22]. In addition to chronic immune stimulation, deficiencies in the immune system lead to less effective “tumor surveillance”, or abnormal DNA repair and apoptosis in patients with cancer may contribute[20].
Chemotherapy causes translocation in bone marrow stem cells but necessitates further research[21]. The bone marrow microenvironment may be compromised in patients undergoing chemotherapy or radiation therapy. This may lead to a tendency to develop secondary tumors through damaged “soil” in favor of mutated hematopoietic clones. Leukemia is a cancer of the human hematopoietic system but its exact cause is unknown. Cellular disorders and environmental factors are certainly involved[23]. Ionizing radiation has been associated with the development of leukemia, including CML, in surviving Japanese victims of the nuclear explosion. This exposure causes DNA mutations. Ionizing radiation produces many double-stranded breaks in the G0/G1 phase of the cell cycle, and there are deviations in double-stranded break proximity spatially and temporally[5]. These features favor the production of the BCR/ABL1 oncogene. Radiation therapy triples the incidence of leukemia, with 18% of cases of CML. The incidence of leukemia appears to be related to the dose and duration of radiation therapy, and the occurrence of leukemia can be explained by radiation-induced chromosome breaks. Granulocyte colony-stimulating factor: The incidence of treatment-associated leukemia increases dramatically in patients receiving granulocyte colony-stimulating factor support and tumor-producing endogenous granulocyte colony-stimulating factor[3,11,24]. Other factors include smoking, I-131 therapy[20,25], and endocrine factors.
The accompanying disease of hematopathologic neoplasms may be multifactorial. Our patient was treated with 5-fluorouracil, oxaliplatin, and capecitabine, each of which can lead to the occurrence of a secondary tumor[7-19]. CML may be related to the previous use of chemotherapeutic agents in this patient. Unfortunately, evidence of TR-CML com
In conclusion, CML in patients with colon carcinoma is extremely rare. Secondary hematological tumors may be multifactorial, and the exact mechanism is currently unknown. Therefore, further investigation and monitoring of its potential associations are needed to identify the exact cause of secondary malignancy.
| 1. | Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1504] [Article Influence: 100.3] [Reference Citation Analysis (1)] |
| 2. | Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1720] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 3. | Maerki J, Katava G, Siegel D, Silberberg J, Bhattacharyya PK. Unusual case of simultaneous presentation of plasma cell myeloma, chronic myelogenous leukemia, and a jak2 positive myeloproliferative disorder. Case Rep Hematol. 2014;2014:738428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Kadikoylu G, Yavasoglu I, Barutca S, Meydan N, Bolaman Z. Chronic myeloid leukemia following the treatment of rectal adenocarcinoma. Med Oncol. 2008;25:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Vakili-Sadeghi M, Omranpour M. Chronic myeloid leukemia following colon cancer treatment: A case report and literature review. Caspian J Intern Med. 2013;4:739-742. [PubMed] |
| 6. | Waller CF, Fetscher S, Lange W. Treatment-related chronic myelogenous leukemia. Ann Hematol. 1999;78:341-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Zhao YX, Yang Z, Ma LB, Dang JY, Wang HY. Synchronous gastric cancer complicated with chronic myeloid leukemia (multiple primary cancers): A case report. World J Clin Cases. 2022;10:11146-11154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (0)] |
| 8. | Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35:418-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Zhang W, Gou P, Dupret JM, Chomienne C, Rodrigues-Lima F. Etoposide, an anticancer drug involved in therapy-related secondary leukemia: Enzymes at play. Transl Oncol. 2021;14:101169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 10. | Lovett BD, Strumberg D, Blair IA, Pang S, Burden DA, Megonigal MD, Rappaport EF, Rebbeck TR, Osheroff N, Pommier YG, Felix CA. Etoposide metabolites enhance DNA topoisomerase II cleavage near leukemia-associated MLL translocation breakpoints. Biochemistry. 2001;40:1159-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Travis LB, Holowaty EJ, Bergfeldt K, Lynch CF, Kohler BA, Wiklund T, Curtis RE, Hall P, Andersson M, Pukkala E, Sturgeon J, Stovall M. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med. 1999;340:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 248] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2950] [Cited by in RCA: 2822] [Article Influence: 112.9] [Reference Citation Analysis (1)] |
| 13. | Tsuzuki M, Handa K, Yamamoto K, Hasegawa A, Yamamoto Y, Watanabe M, Mizuta S, Maruyama F, Okamoto M, Emi N, Ezaki K. Chronic myeloid leukemia following chemotherapy with 5'-deoxy-5-fluorouridine for gastric cancer. Intern Med. 2008;47:1739-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS, Lamprecht TL, Shen D, Hundal J, Fulton RS, Heath S, Baty JD, Klco JM, Ding L, Mardis ER, Westervelt P, DiPersio JF, Walter MJ, Graubert TA, Ley TJ, Druley T, Link DC, Wilson RK. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518:552-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 494] [Cited by in RCA: 669] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 15. | Pinczés L, Molnár S, Telek B, Illés Á. A Case of Therapy-related Acute Myeloid Leukemia Following Treatment with 5-Fluorouracil. Cureus. 2018;10:e3769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Hartmann JT, Lipp HP. Camptothecin and podophyllotoxin derivatives: inhibitors of topoisomerase I and II - mechanisms of action, pharmacokinetics and toxicity profile. Drug Saf. 2006;29:209-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Patel S, Liu D, Caron P, Seiter K. Acute myelogenous leukemia following irinotecan-based chemotherapy for adenocarcinoma of the small intestine. Leuk Lymphoma. 2007;48:1032-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Merrouche Y, Mugneret F, Cahn JY. Secondary acute promyelocytic leukemia following irinotecan and oxaliplatin for advanced colon cancer. Ann Oncol. 2006;17:1025-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Bauduer F, Ducout L, Dastugue N, Marolleau JP. Chronic myeloid leukemia as a secondary neoplasm after anti-cancer radiotherapy: a report of three cases and a brief review of the literature. Leuk Lymphoma. 2002;43:1057-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Gunnarsson N, Höglund M, Stenke L, Wållberg-Jonsson S, Sandin F, Björkholm M, Dreimane A, Lambe M, Markevärn B, Olsson-Strömberg U, Wadenvik H, Richter J, Själander A. Increased prevalence of prior malignancies and autoimmune diseases in patients diagnosed with chronic myeloid leukemia. Leukemia. 2016;30:1562-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Swaminathan M, Bannon SA, Routbort M, Naqvi K, Kadia TM, Takahashi K, Alvarado Y, Ravandi-Kashani F, Patel KP, Champlin R, Kantarjian H, Strong L, DiNardo CD. Hematologic malignancies and Li-Fraumeni syndrome. Cold Spring Harb Mol Case Stud. 2019;5:a003210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Yang LH, Su P, Luedke C, Lu CM, Louissaint A, McCall CM, Rapisardo S, Vallangeon B, Wang E. Chronic Myeloid Leukemia Following Treatment for Primary Neoplasms or Other Medical Conditions. Am J Clin Pathol. 2018;150:246-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer. 2011;2:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Kjeldsen L. [Leukemoid reaction caused by hypernephroma primary diagnosed as chronic granulocytic leukemia]. Ugeskr Laeger. 1990;152:2862-2863. [PubMed] |
| 25. | Huang K, Xu L, Jia M, Liu W, Wang S, Han J, Li Y, Song Q, Fu Z. Second primary malignancies in cervical cancer and endometrial cancer survivors: a population-based analysis. Aging (Albany NY). 2022;14:3836-3855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |