Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.101123
Revised: January 1, 2025
Accepted: January 21, 2025
Published online: April 15, 2025
Processing time: 202 Days and 1.8 Hours
Esophageal squamous cell carcinoma (ESCC) is often managed with surgery, which is the first-line treatment option for stage I–III lesions. However, definitive chemoradiotherapy (dCRT) is associated with a recurrence rate of 30% in stage I ESCC and higher rates in advanced-staged lesions. However, several patients prefer dCRT because their general condition is poor. Salvage therapies, including esophagectomy and endoscopic resection [endoscopic submucosal dissection (ESD)/endoscopic mucosal resection], are important for residual or recurrent tumors that develop after dCRT. Esophagectomy can have curative potential. However, it has high complication and mortality rates. Therefore, ESD is a safer alternative.
A Japanese man in his 70s was concurrently diagnosed with right hypopha
This case emphasizes that ESD can be successfully utilized as a salvage treatment for ESCC after chemoradiotherapy for otolaryngological cancers.
Core Tip: The current case highlights the successful application of endoscopic submucosal dissection (ESD) as a salvage therapy for esophageal squamous cell carcinoma after chemoradiotherapy for pharyngeal cancer. This case underscores the potential of ESD to achieve curative resection in patients with complex malignancies and emphasizes the importance of further research to validate its efficacy and safety in similar contexts.
- Citation: Tachibana S, Moriichi K, Takahashi K, Sato M, Kobayashi Y, Sugiyama Y, Sasaki T, Sakatani A, Ando K, Ueno N, Kashima S, Tanabe H, Fujiya M. Curative endoscopic submucosal dissection for esophageal squamous cell carcinoma after chemoradiotherapy for pharyngeal cancer: A case report. World J Gastrointest Oncol 2025; 17(4): 101123
- URL: https://www.wjgnet.com/1948-5204/full/v17/i4/101123.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i4.101123
Patients with stage I–III esophageal squamous cell carcinoma (ESCC) are candidates for surgery, which is the first-line treatment. However, several patients, even those with lesions in operable stages, avoid surgery because their general condition is poor. This then leads to the adoption of definitive chemoradiotherapy (dCRT). However, the prevalence rate of local residual recurrence after dCRT is approximately 30% in stage I lesions[1] and is higher in lesions in more advanced stages[2]. These details are considered important because the prognosis of patients with ESCC who exhibit local failure is poor.
Salvage therapies, such as esophagectomy and endoscopic resection [endoscopic submucosal dissection (ESD)/endoscopic mucosal resection (EMR)], are essential for treating local recurrence or residual tumors after dCRT in patients with ESCC. Esophagectomy has curative potential. However, it has high complication (65.1%) and mortality (7.9%) rates[3]. In contrast, ESD is less invasive and has favorable outcomes[4]. Recent studies support the feasibility of salvage ESD for local failure after dCRT. Based on this finding, salvage ESD has acceptable long-term outcomes and is a potentially safer, curative option in cautiously selected cases[5].
ESCC is associated with synchronous and metachronous malignancies[6]. The prevalence rate of squamous cell carcinoma of the head and neck is 6.7%[7]. In cases of ESCC concomitant with otolaryngological cancer, the treatment of otolaryngological cancer is often prioritized. However, several patients avoid surgery due to the abovementioned reason and receive nonsurgical therapies, such as radiation therapy and chemoradiotherapy (CRT). The contribution of CRT for concomitant primary cancers on the feasibility of salvage therapy in patients with ESCC remains unknown. There are no reports showing the achievement of complete resection via salvage endoscopic resection after CRT for otolaryngological cancer. Herein, we report a patient with ESCC who underwent ESD and achieved complete resection after CRT for otolaryngological cancer.
A Japanese man in his 70s was referred to our institution due to pharyngeal discomfort lasting for 6 months.
He did not present with comorbidities.
He had no significant medical and personal or family history.
He consumed 75 g of alcohol daily, and he had a Brinkman Index of 1260, which indicated heavy smoking and a high risk of smoking-related diseases.
Physical examination did not show any remarkable findings.
The patient’s serum tumor markers, including squamous cell carcinoma antigen and cytokeratin 19 fragment levels, were within the normal range (Table 1).
| Complete blood count | Biochemistry test | Blood urea nitrogen | 12.2 | mg/dL | ||||
| White blood cell | 6700 | /μL | Total protein | 7.3 | g/dL | Creatinine | 00.74 | mg/dL |
| Red blood cell | 396 | × 104/μL | Albumin | 3.8 | g/dL | Estimated glomerular filtration rate | 80.3 | |
| Hemoglobin | 13.3 | g/dL | Aspartate aminotransferase | 35 | U/L | Na | 143 | mEq/L |
| Mean corpuscular volume | 99.7 | fL | Alanine aminotransferase | 14 | U/L | K | 4.0 | mEq/L |
| Platelet | 26.9 | × 104/μL | Lactate dehydrogenase | 285 | U/L | Cl | 107 | mEq/L |
| Alkaline phosphatase | 37 | U/L | Ca | 9.0 | mg/dL | |||
| γGTP | 17 | U/L | ||||||
| Coagulation test | Total cholesterol | 221 | mg/dL | Tumor marker | ||||
| Prothrombin time-international normalized ratio | 1.02 | Triglyceride | 77 | mg/dL | Somatic cell count | 1.3 | ng/mL | |
| Activated partial thromboplastin time | 27.6 | Second | C reactive protein | < 0.10 | mg/dL | Cytokeratin fragment antigen | 1.36 | ng/mL |
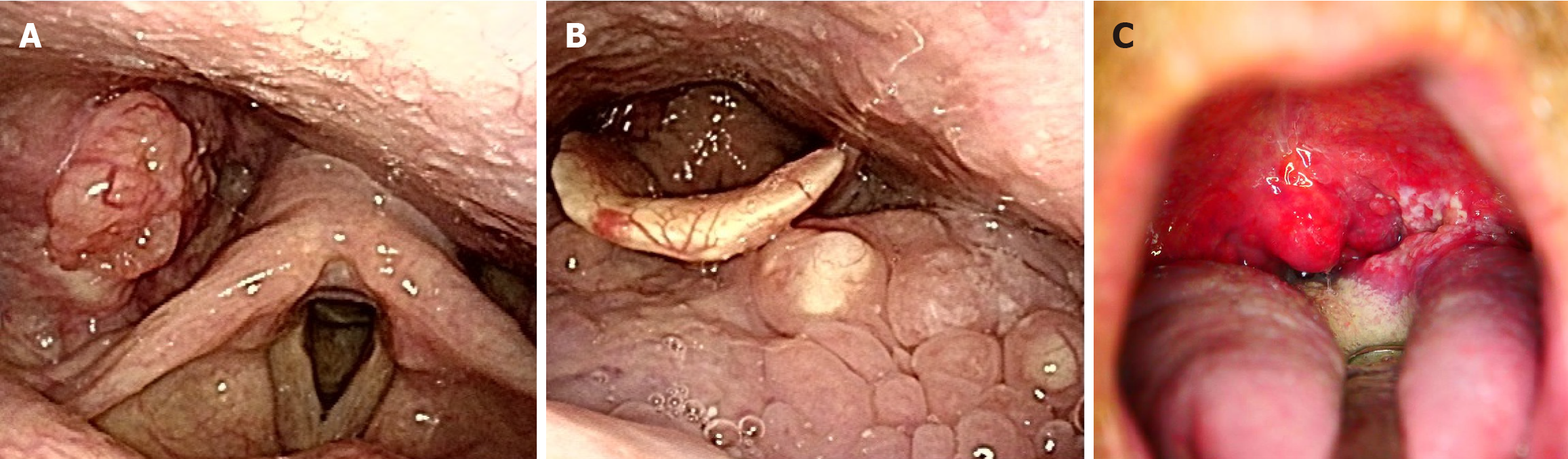
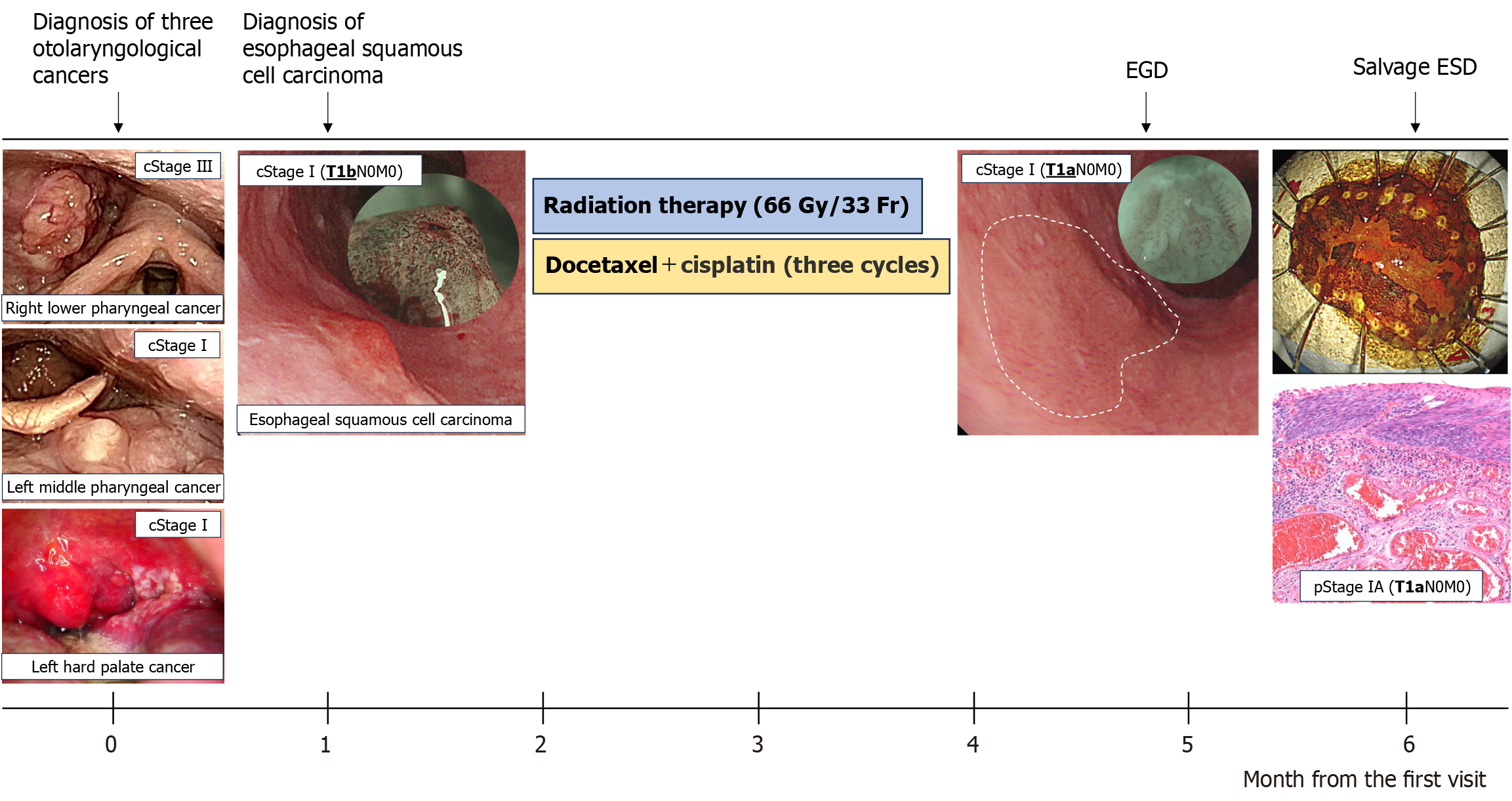
An otolaryngological examination was performed, and results revealed three types of otolaryngological cancers: (1) Right lower pharyngeal cancer (T2N1M0, cStage Ⅲ); (2) Left middle pharyngeal cancer (T1N0M0, cStage I); and (3) Left hard palate cancer (T1N0M0, cStage I) (Figure 1). Gastroenterological examination was performed prior to chemoradiotherapy for otolaryngological cancers to screen for synchronous cancers in the gastrointestinal tract. Esophagogastroduodenoscopy (EGD) revealed a reddish 0-Is+IIb lesion, measuring 20 mm, in the upper thoracic esophagus (23 cm from the incisors) via white light imaging. Narrow-band imaging showed type B2 vessels in the elevated area. For further assessment, endoscopic ultrasonography was performed. Results revealed thinning of the submucosal layer (the fifth layer), which suggested an invasion depth of SM2. A biopsy specimen taken from the elevated lesion was examined, and the findings showed a moderately differentiated squamous cell carcinoma.
Based on the abovementioned findings, the esophageal lesion was diagnosed as ESCC (T1bN0M0, cStage I) (Figure 2). In this case, ESCC was concomitantly present with pharyngeal cancers, including stage III right lower pharyngeal cancer (T2N1M0), as well as stage I left middle pharyngeal cancer (T1N0M0) and stage I left hard palate cancer (T1N0M0).
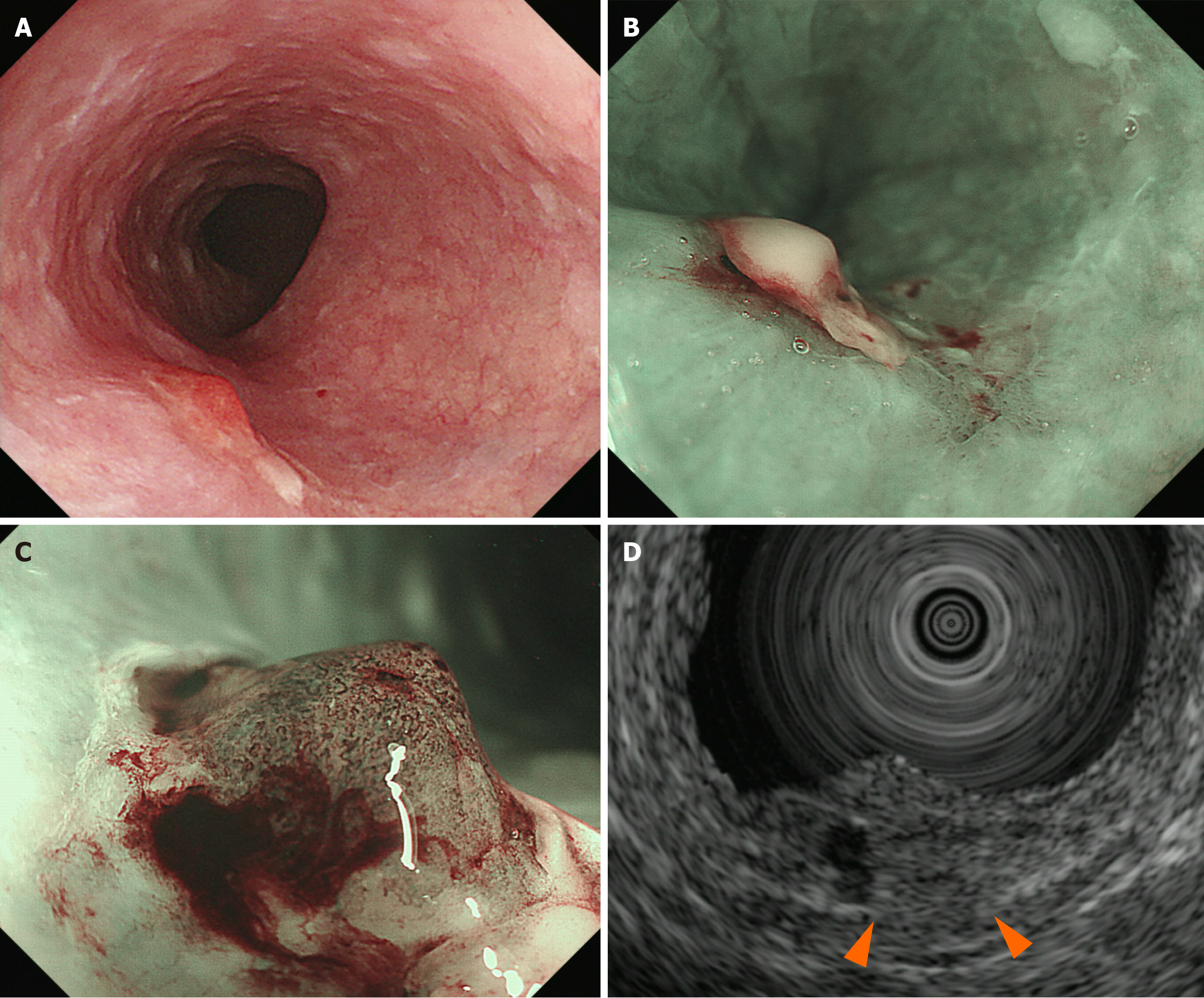
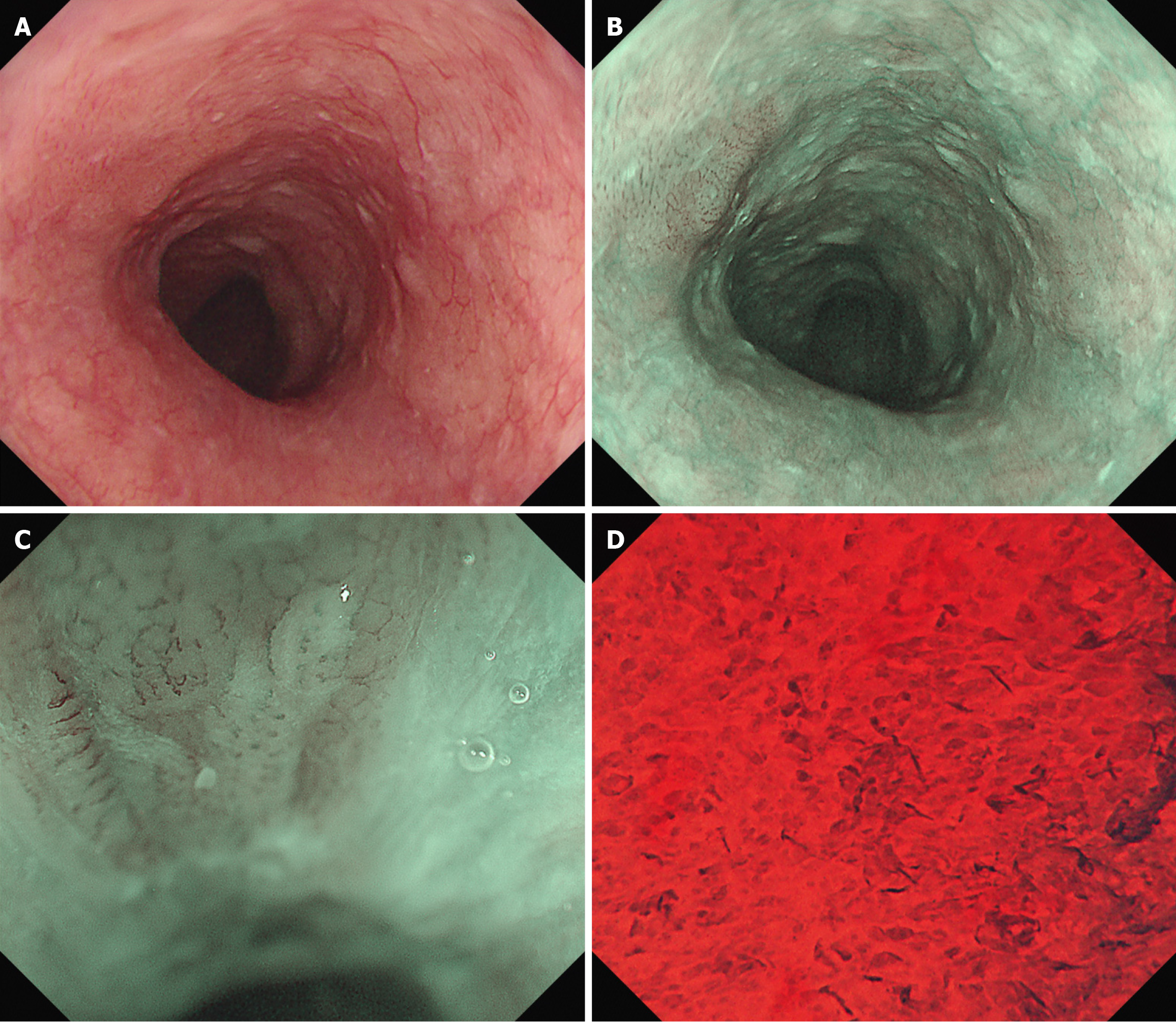
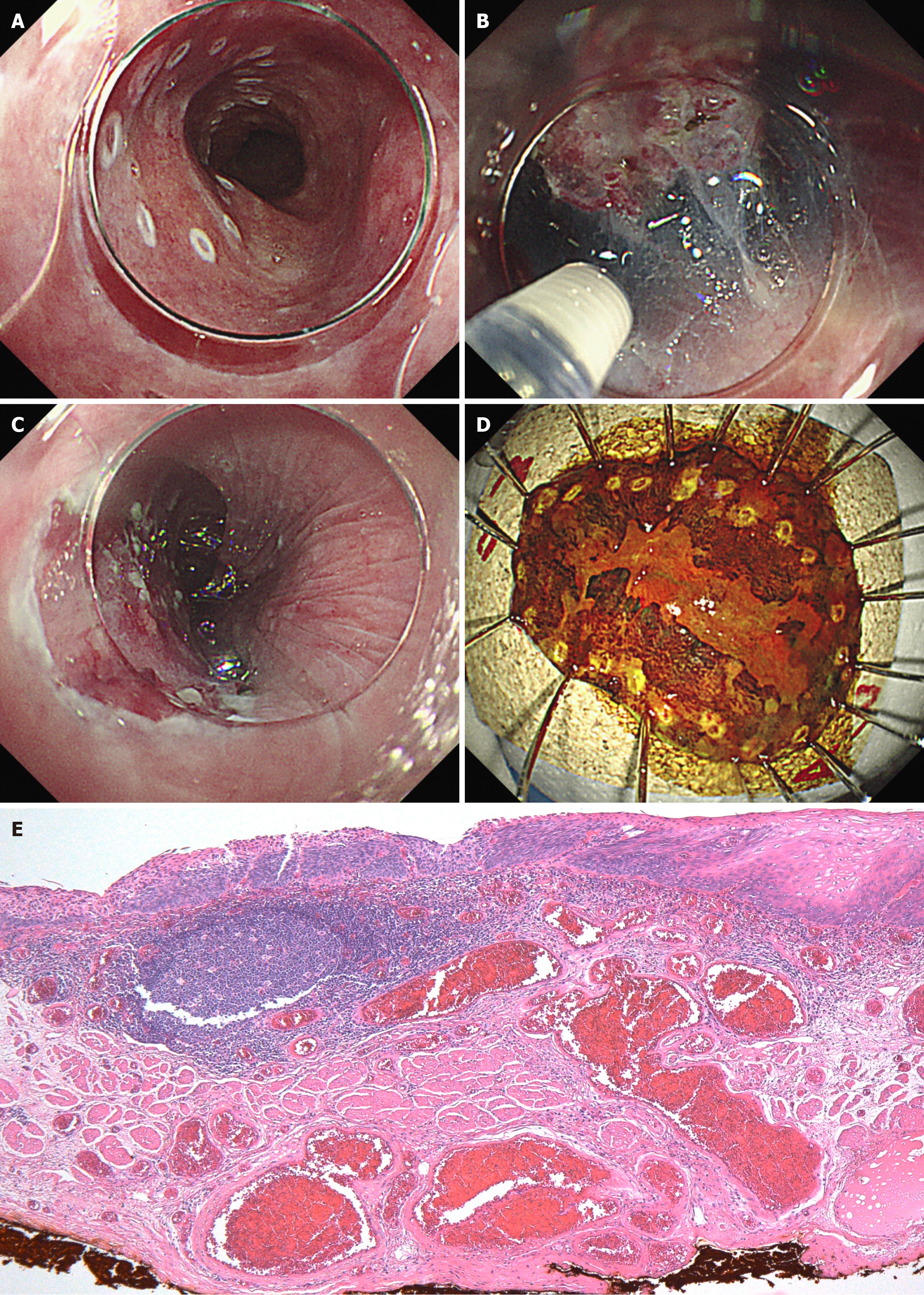
The treatment for advanced-stage pharyngeal cancers was prioritized after a discussion with the otolaryngology department (Figure 3). The patient received chemoradiotherapy in the otolaryngology department. The treatment included three cycles of docetaxel (DTX) and cisplatin (CDDP) (1st course: DTX 79 mg on day 1, CDDP 24 mg on days 2–5; 2nd and 3rd courses: DTX 78 mg on day 1 and CDDP 23 mg on days 2–5) and radiation therapy (with a dose of 66 Gy) to the neck area but excluding the thoracic area, which is where the esophageal cancer was located. Two months after completing the chemoradiotherapy, EGD revealed morphological changes in the esophageal lesion. The lesion initially comprised elevated and flat areas before the treatment. However, the elevated area had been flattened. Consequently, the lesion transformed into a 0-Ⅱb lesion. Narrow-band imaging revealed the disappearance of type B2 vessels and only detected type B1 vessels and a small avascular area, indicating the possibility of shallowing the invasion depth to epithelium or lamina propria mucosa (LPM). Endocytoscopy showed complete loss of cellular structure with a significant increase in cellular density (classified as EC3) (Figure 4). Computed tomography scan did not reveal the presence of lymph nodes or distant metastases. Based on the abovementioned findings, the esophageal lesion was diagnosed as ESCC (T1aN0M0, cStage I). In addition, as the lesion was outside the radiation field, it was not affected by fibrosis caused by radiotherapy. Thus, curative resection was achievable via ESD. After obtaining informed consent, ESD was performed to resect the esophageal lesion (Figure 3). Fibrosis was not observed in the submucosal layer during the procedure, and the lesion was completely resected without significant complications (Figure 5). Pathological analysis revealed a moderately differentiated squamous cell carcinoma, and the invasion depth of the cancer was limited to the LPM. Both vertical and horizontal margins were negative, and there was no lymphovascular invasion (Figure 5). These findings completely met the criteria for curative resection, which include invasion depth limited to the LPM, margin negativity, and absence of lymphovascular invasion. The final diagnosis was confirmed as pStage IA ESCC (T1aN0M0).
At 22 months post-ESD, there were no signs of pharyngeal cancer, hard palate cancer, or esophageal cancer recurrence.
To the best of our knowledge, this is the first case involving a patient with ESCC who successfully underwent salvage ESD after chemoradiotherapy for otolaryngological cancer. ESCC is frequently associated with multiple primary cancers. Based on a previous study on patients with ESCC who developed second primary malignancies, 35% presented with head and neck cancers, 20% with gastric cancers, and 14% with lung cancers[8]. This finding indicated that these cancers are common in patients with ESCC. The factors affecting the successful endoscopic treatment for esophageal cancer after dCRT for otolaryngological cancer in the current case still remain unclear. However, several possible reasons should be considered.
In otolaryngological cancers, the histological type is commonly squamous cell carcinoma. These cancers, along with ESCC, are examples of field cancerization[9]. Field cancerization is the concept that multiple primary cancers occur either simultaneously or metachronously in the head and neck region and the esophagus, with a high incidence in patients with similar backgrounds and risk factors. Consequently, the same drugs, such as CDDP, 5-fluorouracil, and DTX, were administered as standard chemotherapeutic agents in patients with otolaryngological cancers or ESCC. Thus, chemothe
To cure patients, several salvage therapies, including esophagectomy, lymphadenectomy, metastasectomy, endoscopic resection, and photodynamic therapy, were administered[15] because recurrence or residual tumors after dCRT are complex. Previous studies have shown that the incidence rate of local failure after dCRT in patients with ESCC patients is 19%–34%[16-19]. The prognosis of patients with local failure is poor. Among these salvage therapies, esophagectomy is a curative approach. However, 59% of the patients who underwent salvage esophagectomy developed postoperative complications, and the prevalence rates of pulmonary complications and anastomotic leakage were 30% and 37%, respectively. The mortality rate in these patients was 7.4%, indicating that salvage esophagectomy carries a high surgical risk[20].
Endoscopic resection as a salvage treatment for patients with local recurrence or residual ESCC has been found to be effective. The JCOG 9906 phase II trial evaluated the safety of dCRT for patients with stage II/III ESCC[18]. Of 76 patients who enrolled in the study, 26 presented with residual disease or locoregional recurrence without distant metastasis after dCRT. In total, 14 of 26 patients, including 11 patients who underwent esophagectomy and 3 patients who received endoscopic treatment, had salvage therapy. The details of the three patients who underwent endoscopic treatment are as follows: (1) One patient received argon plasma coagulation but later developed mediastinal lymph node metastasis; (2) Another patient died of an otolaryngological surgery-related complication after EMR; and (3) The third patient survived for > 5 years. Yano et al[16] reported that they performed salvage EMR on 21 patients with ESCC and 10 patients without recurrence. The median overall survival time was 46 months, and the 5-year survival rate was 49.1%. None of the patients developed severe complications associated with EMR. Another study[21] investigated the efficacy of salvage EMR in 11 patients with ESCC who received dCRT. Results showed that salvage EMR could be performed safely, and 6 of 11 patients did not present with recurrence. The median overall survival time was 38.9 months, and the 5-year survival rate was 41.6%. Al-Kaabi et al[22] summarized nine previous reports (eight reports regarding ESCC and one about esophageal adenocarcinoma) in which salvage endoscopic treatment was implemented after dCRT. The baseline characteristics of ESCC are as follows: (1) The total number of patients with ESCC was 225; and (2) The clinical stages were stage I (n = 111), stage II (n = 54), stage III (n = 33), and stage IV (n = 27). The number of each clinical T, N, and M stages were as follows: (1) T1/T2/T3/T4; (2) 112/27/52/17; (3) N0/N1/N2/N3; (4) 113/59/4/0; and (5) M0/M1, 68/4, respectively. However, not all papers provided detailed data. The prevalence rate of stage I or II cancer was > 70%. The recurrence rate, 3-year overall survival rate, and 5-year overall survival rate were 16%–59%, 56%–74%, and 30%–53%, respectively. In one of the eight reports regarding ESCC, the prevalence rate of adverse events, such as bleeding and stricture, was 18.9%. However, seven reports showed no major adverse events. Salvage endoscopic resection is a safe, minimally invasive, and promising option for patients with ESCC who received dCRT. In recent years, the data regarding salvage endoscopic treatment has been accumulated, and they are gradually raising awareness about its efficacy.
This study was based on a single case, thereby making it difficult to generalize the results. In addition, data on immune changes and the underlying mechanisms after chemoradiotherapy remain insufficient.
This case represents a unique example in which ESCC was unintentionally downgraded after chemoradiotherapy for otolaryngological cancers, resulting in the successful implementation of salvage ESD. The outcome is promising. Nevertheless, further research should be performed to validate the study findings in larger patient populations. Expanding studies on the efficacy, safety, and underlying mechanisms, including potential immunogenic responses and the abscopal effect, can help establish evidence-based guidelines and improve treatment strategies in similar cases.
| 1. | Kato K, Ito Y, Nozaki I, Daiko H, Kojima T, Yano M, Ueno M, Nakagawa S, Takagi M, Tsunoda S, Abe T, Nakamura T, Okada M, Toh Y, Shibuya Y, Yamamoto S, Katayama H, Nakamura K, Kitagawa Y; Japan Esophageal Oncology Group of the Japan Clinical Oncology Group. Parallel-Group Controlled Trial of Surgery Versus Chemoradiotherapy in Patients With Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology. 2021;161:1878-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1959] [Article Influence: 163.3] [Reference Citation Analysis (5)] |
| 3. | Watanabe M, Mine S, Nishida K, Yamada K, Shigaki H, Matsumoto A, Sano T. Salvage Esophagectomy After Definitive Chemoradiotherapy for Patients with Esophageal Squamous Cell Carcinoma: Who Really Benefits from this High-Risk Surgery? Ann Surg Oncol. 2015;22:4438-4444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Fajardo R, Abbas AE, Petrov RV, Bakhos CT. Salvage Esophagectomy. Surg Clin North Am. 2021;101:467-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Shiota J, Yamaguchi N, Isomoto H, Taniguchi Y, Matsushima K, Akazawa Y, Nakao K. Longterm prognosis and comprehensive endoscopic treatment strategy for esophageal cancer, including salvage endoscopic treatment after chemoradiation therapy. Exp Ther Med. 2023;25:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Chen JY, Zhang SS, Fu XY, Wen J, Yang H, Zhang YJ, Fu JH, Liu QW. The characteristics and prognostic significance of esophageal squamous cell carcinoma with synchronous multiple lesions: over 10-year experience. Esophagus. 2021;18:851-860. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Kumagai Y, Kawano T, Nakajima Y, Nagai K, Inoue H, Nara S, Iwai T. Multiple primary cancers associated with esophageal carcinoma. Surg Today. 2001;31:872-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Mitani S, Kato K, Daiko H, Ito Y, Nozaki I, Kojima T, Yano M, Nakagawa S, Ueno M, Watanabe M, Tsunoda S, Abe T, Kadowaki S, Kadota T, Sasaki K, Machida R, Kitagawa Y. Second primary malignancies in patients with clinical T1bN0 esophageal squamous cell carcinoma after definitive therapies: supplementary analysis of the JCOG trial: JCOG0502. J Gastroenterol. 2022;57:455-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 9. | SLAUGHTER DP, SOUTHWICK HW, SMEJKAL W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 10. | Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 852] [Article Influence: 121.7] [Reference Citation Analysis (0)] |
| 11. | Biswas R, Jindel R, Halder A, Sen K, Kabasi A. Abscopal effect of radiation in metastatic esophageal carcinoma: fourth reported case. Int Cancer Conf J. 2023;12:200-204. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Thuss-Patience P, Stein A. Immunotherapy in Squamous Cell Cancer of the Esophagus. Curr Oncol. 2022;29:2461-2471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Nagaki Y, Motoyama S, Sato Y, Wakita A, Fujita H, Kemuriyama K, Sasamori R, Nozaki S, Nomura K, Minamiya Y. Neoadjuvant Chemoradiotherapy Upregulates the Immunogenicity of Cold to Hot Tumors in Esophageal Cancer Patients. Ann Surg Open. 2024;5:e385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Hino C, Lee EW, Yang GY. Harnessing the abscopal effect for gastrointestinal malignancies in the era of immunotherapy. J Gastrointest Oncol. 2023;14:1613-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 15. | Yagi K, Toriumi T, Aikou S, Yamashita H, Seto Y. Salvage treatment after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Ann Gastroenterol Surg. 2021;5:436-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Yano T, Muto M, Hattori S, Minashi K, Onozawa M, Nihei K, Ishikura S, Ohtsu A, Yoshida S. Long-term results of salvage endoscopic mucosal resection in patients with local failure after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy. 2008;40:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Kawaguchi G, Sasamoto R, Abe E, Ohta A, Sato H, Tanaka K, Maruyama K, Kaizu M, Ayukawa F, Yamana N, Liu J, Takeuchi M, Kobayashi M, Aoyama H. The effectiveness of endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer. Radiat Oncol. 2015;10:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Kato K, Muro K, Minashi K, Ohtsu A, Ishikura S, Boku N, Takiuchi H, Komatsu Y, Miyata Y, Fukuda H; Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group (JCOG). Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys. 2011;81:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 19. | Koide Y, Kodaira T, Tachibana H, Tomita N, Makita C, Itoh M, Abe T, Muro K, Tajika M, Niwa Y, Itoh Y, Naganawa S. Clinical outcome of definitive radiation therapy for superficial esophageal cancer. Jpn J Clin Oncol. 2017;47:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Morita M, Kumashiro R, Hisamatsu Y, Nakanishi R, Egashira A, Saeki H, Oki E, Ohga T, Kakeji Y, Tsujitani S, Yamanaka T, Maehara Y. Clinical significance of salvage esophagectomy for remnant or recurrent cancer following definitive chemoradiotherapy. J Gastroenterol. 2011;46:1284-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Makazu M, Kato K, Takisawa H, Yoshinaga S, Oda I, Saito Y, Mayahara H, Ito Y, Itami J, Hamaguchi T, Yamada Y, Shimada Y. Feasibility of endoscopic mucosal resection as salvage treatment for patients with local failure after definitive chemoradiotherapy for stage IB, II, and III esophageal squamous cell cancer. Dis Esophagus. 2014;27:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Al-Kaabi A, Schoon EJ, Deprez PH, Seewald S, Groth S, Giovannini M, Braden B, Berr F, Lemmers A, Hoare J, Bhandari P, van der Post RS, Verhoeven RHA, Siersema PD. Salvage endoscopic resection after definitive chemoradiotherapy for esophageal cancer: a Western experience. Gastrointest Endosc. 2021;93:888-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |