Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.98844
Revised: November 8, 2024
Accepted: December 16, 2024
Published online: March 15, 2025
Processing time: 221 Days and 20.7 Hours
Ferroptosis is an iron-dependent programmed non-apoptotic cell death characterized by the accumulation of free iron ions and lipid peroxidation. It is asso
To elucidate the anti-tumor characteristics and potential mechanisms of CGA in inducing ferroptosis in HCC cells.
The effects of CGA on the proliferation, migration, and invasion of HCC cells were evaluated through in vitro experiments. Bioinformatics analysis combined with network pharmacology was used to study the potential targets and molecular mechanisms of CGA intervention in HCC ferroptosis. In vitro experiments were conducted to verify and explore the anti-HCC effects and mechanisms of CGA through the ferroptosis pathway.
In vitro experiments showed that CGA dose-dependently inhibited the proliferation, invasion, and migration of HCC cells. Bioinformatics analysis combined with network pharmacology revealed that the pathway of CGA inter
This study demonstrates that CGA inhibits HCC cell proliferation, migration, and invasion by inducing ferroptosis through the PTGS2/AKR1C3/GPX4 axis, suggesting its potential as a novel ferroptosis inducer or anti-HCC drug.
Core Tip: This study reveals that chlorogenic acid (CGA) dose-dependently inhibits the proliferation, invasion, and migration of hepatocellular carcinoma (HCC) cells. Through bioinformatics analysis and network pharmacology, it is found that CGA induces ferroptosis in HCC cells by regulating the prostaglandin endoperoxide synthase 2/aldo-keto reductase family 1 member C3/glutathione peroxidase 4 signaling pathway, reprogramming arachidonic acid metabolism, and causing mitochondrial damage. This research highlights the potential of CGA as a novel ferroptosis inducer or anti-HCC therapeutic.
- Citation: Wu L, Chen HY, Zhang JT, Yang RY, Wang ZB, Xue PS, Peng W, Li KX, Gao WH, Zeng PH. Chlorogenic acid induces hepatocellular carcinoma cell ferroptosis via PTGS2/AKR1C3/GPX4 axis-mediated reprogramming of arachidonic acid metabolism. World J Gastrointest Oncol 2025; 17(3): 98844
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/98844.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.98844
Primary liver cancer (PLC), with hepatocellular carcinoma (HCC) constituting 75%-85% of cases, represents a major global health threat. Despite advances in treatment modalities, including surgical resection, radiotherapy, chemotherapy, and targeted immunotherapy, the prognosis for HCC remains unfavorable, particularly in its advanced stages. The limitations of these therapeutic approaches, such as prohibitive costs, severe side effects, drug resistance, and reduced efficacy in advanced HCC, underscore the urgent need for novel therapeutic strategies and the identification of new molecular targets. According to the latest data from the International Agency for Research on Cancer of the World Health Organization, the global incidence of PLC in 2022 was 865300 cases, with an incidence rate of 4.3%, positioning it as the sixth most common malignancy worldwide. The mortality rate for PLC is 7.8%, corresponding to 757900 deaths, making it the third leading cause of cancer-related mortality[1]. HCC accounts for 75%-85% of PLC cases, making it the most common histological subtype of PLC[2]. Its highly invasive and rapidly progressive nature results in a relatively low survival rate for patients, with only a 5-year survival rate of 10.1% for advanced-stage patients and a poor overall prognosis, posing a serious threat to the health of people worldwide[3]. Due to the lack of prominent symptoms in the early stages of HCC, patients are frequently diagnosed at advanced stages, which limits the window of opportunity for effective intervention. Traditional treatment modalities, including surgery, radiotherapy, and chemotherapy, often prove ineffective in many cases. While targeted immunotherapy has shown some progress in HCC treatment, it remains costly, and its efficacy is limited, particularly in advanced stages. Moreover, the prognosis for HCC remains poor, with severe side effects, drug resistance, and limited therapeutic effectiveness in advanced disease continuing to pose significant challenges. Consequently, in-depth investigations into the pathogenesis of HCC and the development of novel therapeutic strategies hold substantial clinical relevance.
The Shi-pi-xiao-ji recipe is a traditional Chinese medicine (TCM) formula developed by our research group based on years of clinical experience. It consists of a combination of herbs and animal-derived ingredients, including Astragalus
Ferroptosis is a novel, non-typical form of programmed cell death that differs from traditional modes such as apoptosis and necrosis. The onset of ferroptosis is closely associated with disturbances in iron metabolism and elevated levels of lipid peroxidation within the cell. Key features of ferroptosis include a reduction or disappearance of mitochondrial cristae, rupture or shrinkage of the mitochondrial outer membrane, deepening of mitochondrial coloration, and maintenance of nuclear integrity without fragmentation. Additionally, ferroptosis is characterized by the rupture of the cell membrane, increased expression of lipid ROS, acyl-coenzyme A (CoA) synthetase long chain 4 (ACSL4), and lysophosphatidylcholine acyltransferase 3 (LPCAT3), and a decrease in the expression of cystine glutamate reverse transporter (xCT) and glutathione peroxidase 4 (GPX4). Studies have demonstrated that ferroptosis can inhibit tumor progression and plays a critical role in cancer therapy[9]. In the context of HCC, ferroptosis influences disease deve
Based on these observations, the present study utilized the HepG2 HCC cell line as a model to investigate the inhibitory effects of CGA on the proliferation, invasion, and metastasis of HCC cells. Additionally, the study aimed to explore the molecular mechanisms underlying CGA’s potential to reprogram fatty acid metabolism and induce ferroptosis in HCC cells. The findings of this study are anticipated to provide a scientific foundation for the use of CGA in the prevention and treatment of HCC, and contribute to the development of novel anti-HCC therapeutic agents.
Materials and antibodies: CGA (high-performance liquid chromatography ≥ 98%, product code PHR2202), erastin (HY-15763), penicillin-streptomycin solution (PB180120), Dulbecco’s modified eagle’s medium (DMEM, PM150210), 0.25% trypsin (PB180226), phosphate-buffered saline (PBS, PB180327), and fetal bovine serum (FBS, 164210) were purchased from Procell Life Science & Technology Co., Ltd (Wuhan, China). Cholecystokinin octapeptide (CCK-8) cell viability assay kit (C0038), EdU cell proliferation kit (C0071S), malondialdehyde (MDA) assay kit (S0131M), ROS assay kit (S0033M), ROS probe bicinchoninic acid (BCA) protein assay kit (P0012), lipid peroxidation MDA assay kit (S0131M), radioimmunoprecipitation assay lysis buffer (P0013B), sodium-dodecyl sulfate gel electrophoresis protein sample loading buffer (P0015 L), and phenylmethanesulfonyl fluoride protease inhibitor (K1007) were obtained from Beyotime Biotechnology Co., Ltd (Shanghai, China). Reduced glutathione (GSH) content determination kit (BC1175) and Fe2+ determination kit (BC5415) were purchased from Solarbio Science & Technology Co., Ltd (Beijing, China). Osmium tetroxide (AWI0136) was obtained from Abiowell Biotechnology Co., Ltd (Changsha, China). AA enzyme-linked immunosorbent assay (ELISA) kit (E-EL-0051) was provided by Elabscience & Technology Co., Ltd (Wuhan, China). Polymerase chain reaction (PCR) extraction kit (DP419) was purchased from Beijing Tiangen Biotech Co., Ltd, and PCR reverse transcription kit (E047) was obtained from Shanghai Jing’an Technology Co., Ltd. Anti-xCT antibody [EPR8290(2), ab175186], recombinant anti-GPX4 antibody (EPNCIR144, ab125066), recombinant anti-aldo-keto reductase family 1 member C3 (AKR1C3) antibody (EPR28747-41, ab316864), and recombinant anti-cyclooxygenase 2 (COX-2) antibody (EPR12012, ab179800) were purchased from Abcam Trading Co. Ltd (Shanghai, China). ACSL4/fatty acid-CoA ligase 4 antibody (DF12141) was obtained from Pro-Tech Biological Research Center Co (Jiangsu, China). Additionally, E-cadherin polyclonal antibody (20874-1-AP), LPCAT3 monoclonal antibody (67882-1-Ig), horseradish peroxidase (HRP)-conjugated affinity-purified goat anti-rabbit immunoglobulin G (IgG) (H+L, SA00001-2), HRP-conjugated affinity-purified goat anti-mouse IgG (H+L, SA00001-1), CoraLite 488-conjugated goat anti-mouse IgG (H+L, SA00013-1), and Coralite 488-conjugated goat anti-rabbit IgG (H+L, SA00013-2) were purchased from Proteintech Group Co., Ltd (Wuhan, China). N-cadherin polyclonal antibody (AF05410) was obtained from Aifang Biotechnology Company (Hunan, China).
Cell culture: Human hepatoma cells HepG2 were purchased from Original Cells Life Science & Technology (Wuhan, China). They were cultured in DMEM medium containing 10% FBS and 1% penicillin-streptomycin double antibiotic solution (penicillin 100 g/L and streptomycin 50 g/L) at 37 °C and 5% CO2 in a humidified incubator (Thermo Fisher Scientific). The cells were passaged every 2-3 days and used for subsequent experiments when they were in the logarithmic growth phase.
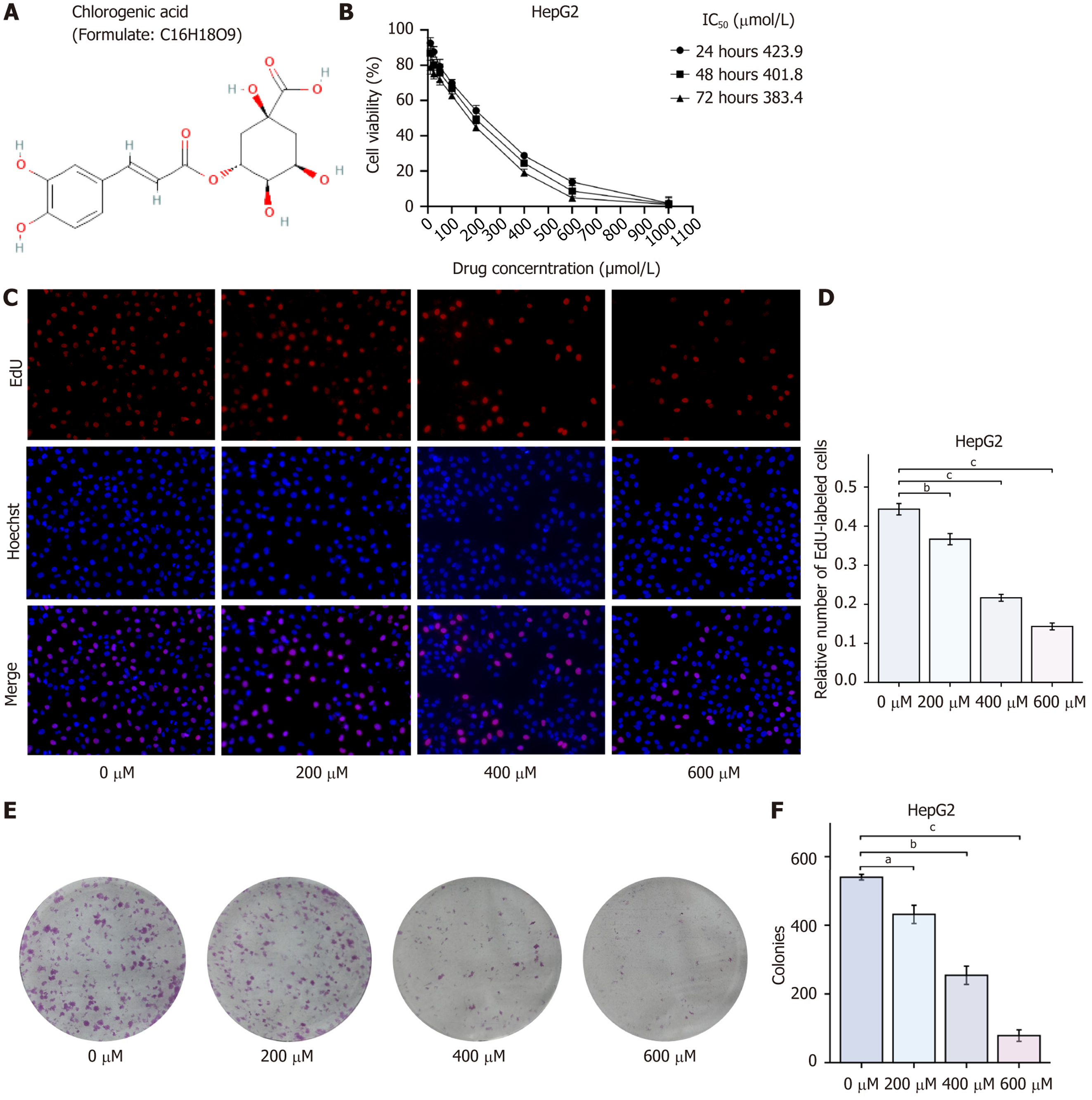
CCK-8 assay for cell viability: Logarithmically growing HepG2 cells were seeded in 96-well plates at a density of 5 × 103 cells per well. Once the cells had adhered, they were treated with increasing concentrations of CGA (0, 10, 25, 50, 100, 200, 400, 600, and 1000 μmol) and incubated at 37 °C with 5% CO2 for 24, 48, and 72 hours. After treatment, the culture medium was removed, and 10 μL of CCK-8 solution was added to each well, followed by a 30-minute incubation. Absorbance (A) was measured at 450 nm to assess cell viability. The half-maximal inhibitory concentration (IC50) was calculated using GraphPad Prism 9.0, and cell survival rate was determined using the formula: Cell survival rate = (ACGA - Ablank) / (Acontrol - Ablank) × 100%. CGA concentrations were selected based on the IC50 values obtained from the assay to ensure experimental consistency.
EdU assay for cell proliferation: Logarithmically growing HepG2 cells were seeded in 24-well plates at a density of 3 × 104 cells per well and cultured in medium containing varying concentrations of CGA (0, 200, 400, and 600 μmol) for 48 hours at 37 °C. The EdU cell proliferation assay kit was used according to the manufacturer’s instructions. After a 3-hour incubation, the culture medium was removed, and 500 μL of diluted EdU working solution was added to each well for an additional 3-hour incubation. The cells were then fixed with 4% paraformaldehyde at room temperature for 15 min, followed by three washes with PBS (3 minutes each). The fixation solution was removed, and cells were permeabilized with PBS containing 0.3% Triton X-100 for 10 minutes. After two more PBS washes (3 minutes each), Hoechst 33342 was added to stain the nuclei. Cells were observed under a fluorescence microscope, with EdU-positive proliferating cells appearing red and Hoechst-stained live cells appearing blue. The relative number of EdU-positive cells was calculated as follows: Relative number of EdU-positive cells = number of EdU-positive cells/total number of live cells.
Clone formation assay for cell proliferation: Logarithmically growing HepG2 cells were seeded in 6-well plates at a density of 600 cells per well. After 48 hours of incubation, the cells were cultured in medium containing different concentrations of CGA (0, 200, 400, and 600 μmol/L). Following 10 days of incubation, the culture medium was removed, and the cells were washed three times with PBS. Each well was then filled with 1 mL of 4% paraformaldehyde and fixed for 15 minutes. Subsequently, the cells were stained with 1 mL of 1% crystal violet solution at room temperature for 15 minutes. The plates were dried, and the staining solution was rinsed away with tap water. Colonies containing more than 50 cells were photographed with a digital camera and quantified using ImageJ.
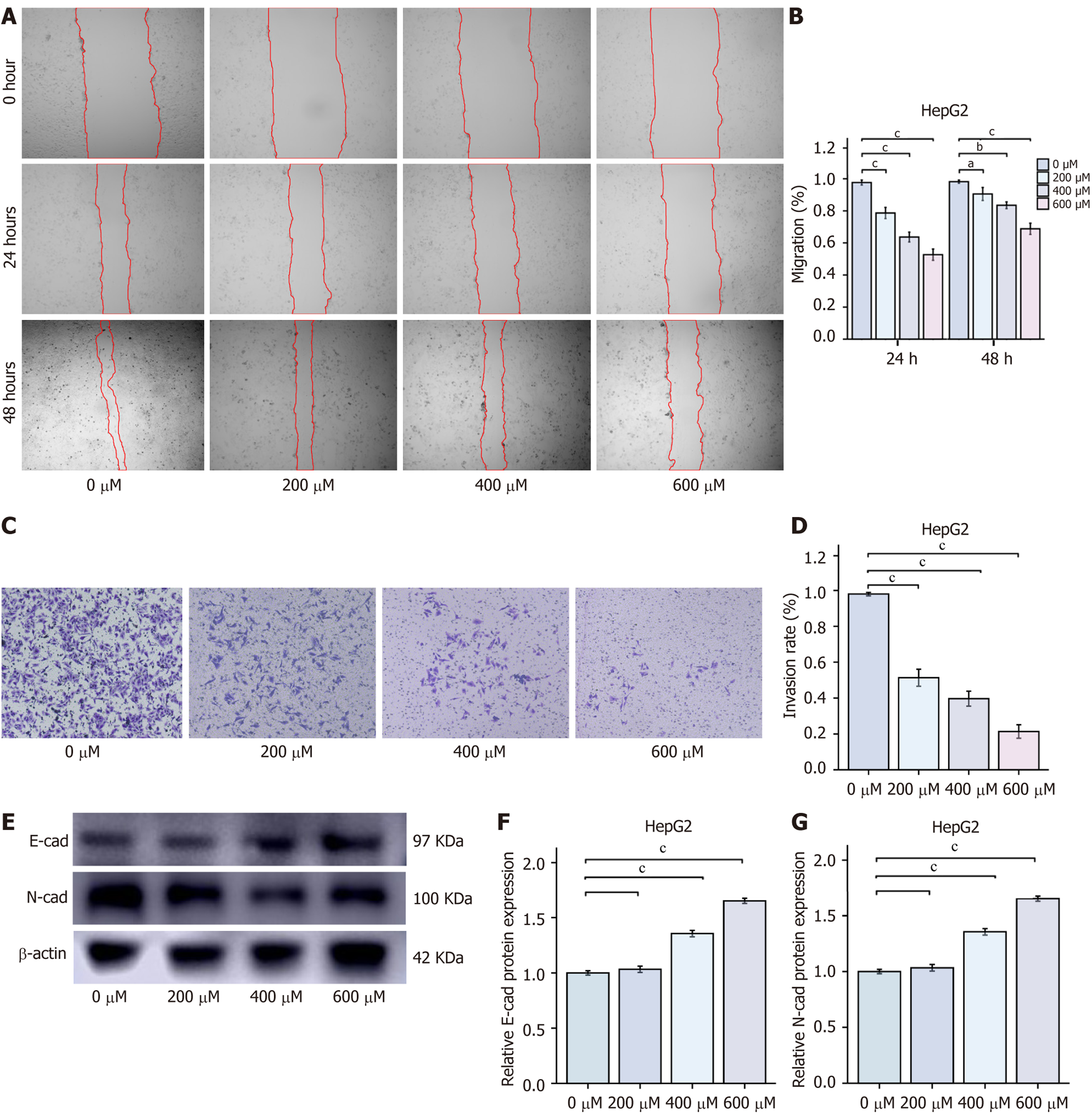
Wound healing assay for cell migration: The wound healing assay was performed to assess cell migration. Logarithmically growing HepG2 cells were cultured in 6-well plates until a confluent monolayer was established. A straight scratch was created in the center of each well using a 1 mL pipette tip. Floating cells were removed by washing with PBS, and fresh serum-free DMEM containing various concentrations of CGA (0, 200, 400, and 600 μmol/L) was added to each well. The plates were then incubated in a CO2 incubator. Wound areas were photographed at 24 hours and 48 hours, and the wound healing rate was quantified using ImageJ software according to the formula: Migration (%) = (initial wound area - final wound area)/initial wound area × 100%.
Transwell assay for cell invasion: To assess cell invasion, HepG2 cells were seeded in 24-well transwell chambers (8 μm pore size) coated with Matrigel (1:8 dilution in serum-free DMEM, 50 μL per well) and allowed to solidify at 37 °C for 4-5 hours. After washing with PBS, 200 μL of cell suspension (5 × 104 cells) in serum-free DMEM was added to the upper chamber, while the lower chamber contained 600 μL of 10% FBS as a chemoattractant. Cells were treated with CGA (0, 200, 400, and 600 μmol/L) and incubated for 48 hours in a CO2 incubator. Non-invading cells were removed, and the remaining cells were fixed with formaldehyde for 30 minutes, stained with 0.1% crystal violet for 20 minutes, and then rinsed with PBS. Invading cells were quantified using ImageJ software.
Acquisition of CGA intervention targets and their expression analysis in HCC patients: The structure of CGA was obtained from the PubChem database by searching “chlorogenic acid” and downloading its SMILES or 3-dimensional structure (.sdf format). Drug targets were sourced from PharmMapper (task ID: 240410134454), SwissTarget, and TargetNet. After removing duplicates, target names were standardized to gene names using the UniProt database. Ferroptosis-related genes (FRGs) were obtained from the FerrDb database and supplemented with genes identified from PubMed articles on ferroptosis published between January 1, 2022, and December 31, 2022. Differential analysis of GSE19665 and GSE84402 datasets from the Gene Expression Omnibus database was conducted using the limma package in R. Transcriptome data were log2-transformed and normalized, with box plots and principal components analysis used for quality assessment. Differentially expressed genes in HCC were identified with criteria of P < 0.05 and |log2 fold change| > 1. Data from CGA targets, the ferroptosis gene set, and HCC differentially expressed genes were integrated and analyzed in R to identify unique and shared targets. Visualization was performed using the ggplot2 and Venn
Prognostic analysis and nomogram construction of CGA intervention targets in HCC patients: RNAseq and clinical data from the TCGA-LIHC cohort were curated, and samples lacking clinical information were excluded. Fourteen genes were analyzed using Lasso regression to identify those most associated with HCC prognosis, with cross-validation ensuring robustness. The glmnet package in R (version 4.2.1) was used for data analysis and visualization. Genes significantly correlated with prognosis [AKR1C3, aurora kinase A (AURKA), and androgen receptor (AR)] were further analyzed using univariate and multivariate Cox regression models to calculate risk scores based on survival outcomes. The timeROC package was utilized to assess the predictive efficacy of these scores for 1-year, 3-year, and 5-year survival. Patients were stratified into high-risk and low-risk groups based on median risk scores, and survival outcomes were compared using log-rank tests and Kaplan-Meier curves.
Univariate Cox regression (P < 0.1) identified variables, including risk score, age, and tumor-node-metastasis (TNM) stage, for multivariate Cox regression to determine independent prognostic factors. Hazard ratios (HRs), 95% confidence intervals (CIs), and P values were calculated. A nomogram model was constructed using the rms and survival packages in R, integrating risk scores and clinical data to predict 1-year, 3-year, and 5-year survival. Calibration analysis was performed to validate the nomogram’s predictive performance.
Protein-protein interaction network construction and enrichment analysis: The STRING database (https://cn.string-db.org/) was used to perform protein-protein interaction analysis of the CGA intervention targets, and the network was drawn using Cytoscape 3.9.1. The clusterProfiler and org.Hs.eg.db packages in R were used to perform gene ontology and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of the CGA intervention targets, focusing on the biological processes, cellular components, molecular functions, and KEGG pathways of the CGA intervention targets, to analyze the potential mechanisms of CGA intervention in HCC ferroptosis.
Molecular docking: Molecular docking was used to evaluate the binding of CGA to intervention target proteins. The 3-dimensional molecular structure of CGA in sdf format was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/)[11]. The X240 X-ray crystal structure files of prostaglandin endoperoxide synthase 2 (PTGS2), AKR1C3, GPX4, and xCT proteins were obtained from the RCSB Protein Data Bank (http://www.rcsb.org/)[12]. The proteins were dehydrated, hydrogenated, and saved in pdb format. AutoDock 4.2.6[13] and PyMOL 2.5.2 software were used to perform docking studies between CGA and PTGS2, AKR1C3, GPX4, and xCT proteins. The grid box function of the AutoDock tool was used to define the specific pocket of CGA binding to the active component of the protein. Subsequently, molecular docking analysis was performed using the command prompt, and the results were visualized using PyMOL.
Detection of Fe2+ levels: Logarithmically growing HepG2 cells were seeded in 6-well plates at 5 × 104 cells per well and treated with varying concentrations of CGA (0, 200, 400, and 600 μmol/L) and erastin (10 μmol/L) at 37 °C for 48 hours. Erastin, a ferroptosis inducer[14], depletes GSH and inactivates GPX4, exhibiting antitumor activity[15]. Cells were then lysed on ice, ultrasonicated, and centrifuged at 12000 g for 10 minutes after adding 900 μL of extraction agent. A 300 μL aliquot of the supernatant was mixed with 150 μL of detection solution and incubated at 37 °C for 10 minutes. After a final centrifugation at 12000 g for 10 minutes, the supernatant was transferred to an ELISA plate, and absorbance at 593 nm was measured to calculate Fe2+ content based on the standard curve.
Measurement of MDA and GSH levels: Logarithmically growing HepG2 cells were seeded in 6-well plates at 5 × 104 cells per well and treated with varying concentrations of CGA (0, 200, 400, and 600 μmol/L) and erastin (10 μmol/L) at 37 °C for 48 hours. MDA and GSH levels were measured using specific assay kits. After cell lysis and centrifugation, 200 μL of the supernatant was transferred to a 96-well plate. Absorbance at 532 nm (for MDA) and 512 nm (for GSH) was recorded with a microplate reader, and relative levels were calculated based on the standard curve.
Detection of intracellular lipid ROS: Intracellular ROS levels were measured using the 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) fluorescent probe. Logarithmically growing HepG2 cells were seeded in 6-well plates at 5 × 104 cells per well and treated with CGA (0, 200, 400, and 600 μmol/L) and erastin (10 μmol/L) at 37 °C for 48 hours. After washing with PBS, cells were incubated with 10 μmol/L DCFH-DA in the dark at 37 °C for 20 minutes. Fluorescence was observed under a microscope, and ImageJ software was used to quantify ROS levels.
Transmission electron microscopy for mitochondrial ultrastructure: Transmission electron microscopy was used to examine mitochondrial ultrastructure in HepG2 cells. Cells were digested with 0.25% trypsin, fixed in 2.5% glutaraldehyde for 12 hours, and post-fixed in 1% osmium tetroxide. Samples were then dehydrated in graded epoxy propylene alcohol (30%-100%), embedded in epoxy resin, and incubated at 60 °C for 48 hours. Semi-thin sections (1.0298 mm) were stained with toluidine blue to assess section quality, followed by negative staining of ultrathin sections with 2% uranyl acetate and lead citrate for contrast enhancement. Ultrastructural images were captured using a Hitachi HT7700 transmission electron microscope.
Detection of AA content: KEGG enrichment analysis indicated that CGA-induced ferroptosis in HCC cells might involve AA metabolism. To confirm this, AA levels were measured under different treatments using an AA ELISA kit. HepG2 cells were seeded in 6-well plates at 5 × 104 cells per well, and treated with CGA (0, 200, 400, and 600 μmol/L) and erastin (10 μmol/L) for 48 hours. AA levels in the medium were then measured according to the kit instructions.
Quantitative real-time PCR detection: Logarithmically growing HepG2 cells were seeded in 6-well plates at a density of 5 × 104 cells per well. The cells were cultured in medium containing different concentrations of CGA (0, 200, 400, 600 μmol) and erastin (10 μmol) for 48 hours at 37 °C. The cells were then collected, and total RNA was extracted according to the kit instructions. After detecting the concentration and purity of RNA, reverse transcription was performed using a reverse transcription kit, and the resulting cDNA was used as the template for two-step real-time fluorescence quantitative PCR using SYBR Green. The reaction conditions were as follows: Pre-denaturation at 95 °C for 3 minutes, and 40 cycles of denaturation at 95 °C for 30 seconds, annealing at 55 °C for 30 seconds, and extension at 72 °C for 30 seconds. The primer sequences were synthesized by Shanghai Sangon Biological Engineering Co., Ltd (Table 1). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal reference, and 5 biological samples were used in each group with 3 replicates per sample. The relative expression of the target genes was calculated using the 2-ΔΔCt method to verify the effect of CGA on the mRNA expression of the key genes GPX4 and xCT involved in ferroptosis induction in HCC cells. In the AA metabolism pathway, AKR1C3 and PTGS2 play important roles. ACSL4 and LPCAT3 are two critical indicators linking lipid metabolism to ferroptosis. ACSL4, a lipid metabolism enzyme, promotes ferroptosis, while LPCAT3, an acyltransferase on the cell membrane in the Lands’ cycle, is responsible for producing C20:4 PLs that play a role in the ferroptosis process. To elucidate the molecular mechanism of CGA-induced ferroptosis in HCC and its association with AA metabolism, we also assessed the mRNA expression of PTGS2, AKR1C3, ACSL4, and LPCAT3 in response to CGA treatment within the cells.
| Gene name | Primer sequence (5’-3’) | Product length, bp |
| GPX4 | F: CCCAGTGAGGCAAGACCGAAG | 118 |
| R: GGCTCCTGCTTCCCGAACTG | ||
| xCT | F: AGGCGGAGGAACAAGCTGAAC | 138 |
| R: TGCGGACCTGAATAACTCGTCTATG | ||
| PTGS2 | F: AATCTGGCTGCGGGAACACAAC | 85 |
| R: TGTCTGGAACAACTGCTCATCACC | ||
| AKR1C3 | F: CGAGCGAGGGCGAGGAGAG | 102 |
| R: AGAAGCGGGCGGTGAATTTGTG | ||
| ACSL4 | F: CCAAGTAGACCAACGCCTTCAGAC | 154 |
| R: ATGTGTCCTTCGGTCCCAGTCC | ||
| LPCAT3 | F: ACTCTCTCAGGGTCTCTCGTTGC | 179 |
| R: AGAAGGGCTGGAGGACAGTAATGG | ||
| GAPDH | F: CTGGAGAAACCTGCCAAGTATG | 138 |
| R: GGTGGAAGAATGGGAGTTGCT |
Western blot detection: Western blot analysis was used to assess the effects of CGA on the expression levels of proteins related to tumor invasion and metastasis, such as epithelial cadherin (E-cadherin) and neural cadherin (N-cadherin), as well as the expression levels of key proteins involved in ferroptosis, including xCT and GPX4, and key proteins involved in AA metabolism, including PTGS2, AKR1C3, ACSL4, and LPCAT3. HepG2 cells treated with different concentrations of CGA (0, 200, 400, and 600 μmol) and erastin (10 μmol) were collected and total protein was extracted using radioimmunoprecipitation assay lysis buffer containing 1% phenylmethanesulfonyl fluoride. The protein concentration was measured using the BCA method, and an equal amount (10 μL) of protein was loaded onto a sodium-dodecyl sulfate gel electrophoresis gel. The proteins were then separated and transferred to a polyvinylidene fluoride membrane (Millipore, United States). The membrane was blocked with 5% skim milk at room temperature for 1 hour and then incubated with primary antibodies [E-cadherin (1:10000), N-cadherin (1:1000), anti-xCT (1:4000), anti-GPX4 (1:4000), anti-ACSL4 (1:2000), anti-LPCAT3 (1:2000), anti-PTGS2 (1:2000), anti-AKRLC3 (1:2000), and anti-β-actin (1:5000)] at 4 °C overnight. The membrane was then washed with Tris-buffered saline with Tween (10 minutes each) three times and incubated with HRP-conjugated secondary antibody (1:5000) for 60 minutes. The membrane was then washed with Tris-buffered saline with Tween three times and visualized using a chemiluminescence detection system (enhanced chemiluminescence). The relative protein expression levels were quantified using ImageJ software.
All statistical analyses were performed using the stats (version 4.2.1) and car (version 3.1-0) packages in R software (version 4.2.1). All data are expressed as the mean ± SD. One-way analysis of variance was used to compare the differences between groups. If the data satisfied the assumptions of normality and homogeneity of variance, one-way analysis of variance was performed first, followed by the Tukey post-hoc test. However, if the assumptions of homo
Inhibition of HCC cell proliferation by CGA: The effect of CGA on HepG2 cell viability was assessed using the CCK-8 assay. Compared with the control group, the survival rate of HepG2 cells decreased with increasing CGA concentration, showing statistically significant differences (P < 0.01). This suggests that CGA significantly inhibited the proliferation of HepG2 cells. The IC50 of CGA for inhibiting HepG2 cell viability was determined using GraphPad Prism 9.0, with IC50 values at 24 hours, 48 hours, and 72 hours recorded as 423.9 μmol/L, 401.08 μmol/L, and 383.4 μmol/L, respectively. For subsequent experiments, a concentration near the IC50 (i.e., 400 μmol/L) was selected for 48 hours of CGA treatment.
The inhibitory effect of CGA on HepG2 cell proliferation was further analyzed using the EdU assay. Compared with the control group, the number of EdU-labeled proliferating cells significantly decreased after 48 hours of treatment with 200, 400, and 600 μmol/L CGA, with statistically significant differences (P < 0.001). This confirmed that CGA dose-dependently inhibited HepG2 cell proliferation, consistent with the CCK-8 assay results. The long-term inhibitory effect of CGA on HepG2 cell proliferation was further evaluated through the clone formation assay. Compared with the control group, the number of colonies formed by HepG2 cells significantly decreased after 10 days of treatment with 200, 400, and 600 μmol/L CGA (P < 0.001), indicating that CGA dose-dependently inhibited HepG2 cell proliferation (Figure 1).
Inhibition of HCC cell migration and invasion by CGA: To further assess the effects of CGA on the migration and invasion abilities of HepG2 cells, wound healing and transwell assays were performed. CGA significantly inhibited the migration and invasion of HepG2 cells. As CGA concentration increased, the number of cells migrating to the wound area and the number of cells passing through the transwell chamber membrane both significantly decreased. These data suggest that CGA effectively reduces the migration and invasion capabilities of HepG2 cells. E-cadherin and N-cadherin are key cadherins involved in cell-cell adhesion and tissue development. In tumor cells, the upregulation of N-cadherin is associated with the downregulation of E-cadherin, a phenomenon referred to as the “cadherin switch”. In cancer cells, the loss of E-cadherin and the concurrent upregulation of N-cadherin are related to tumor invasion and metastasis. Therefore, Western blot analysis was used to measure E-cadherin and N-cadherin protein levels. Compared with the control group, CGA dose-dependently increased E-cadherin expression while inhibiting N-cadherin expression. These findings confirmed that CGA effectively inhibits the migration and invasion of HepG2 cells in vitro (Figure 2).
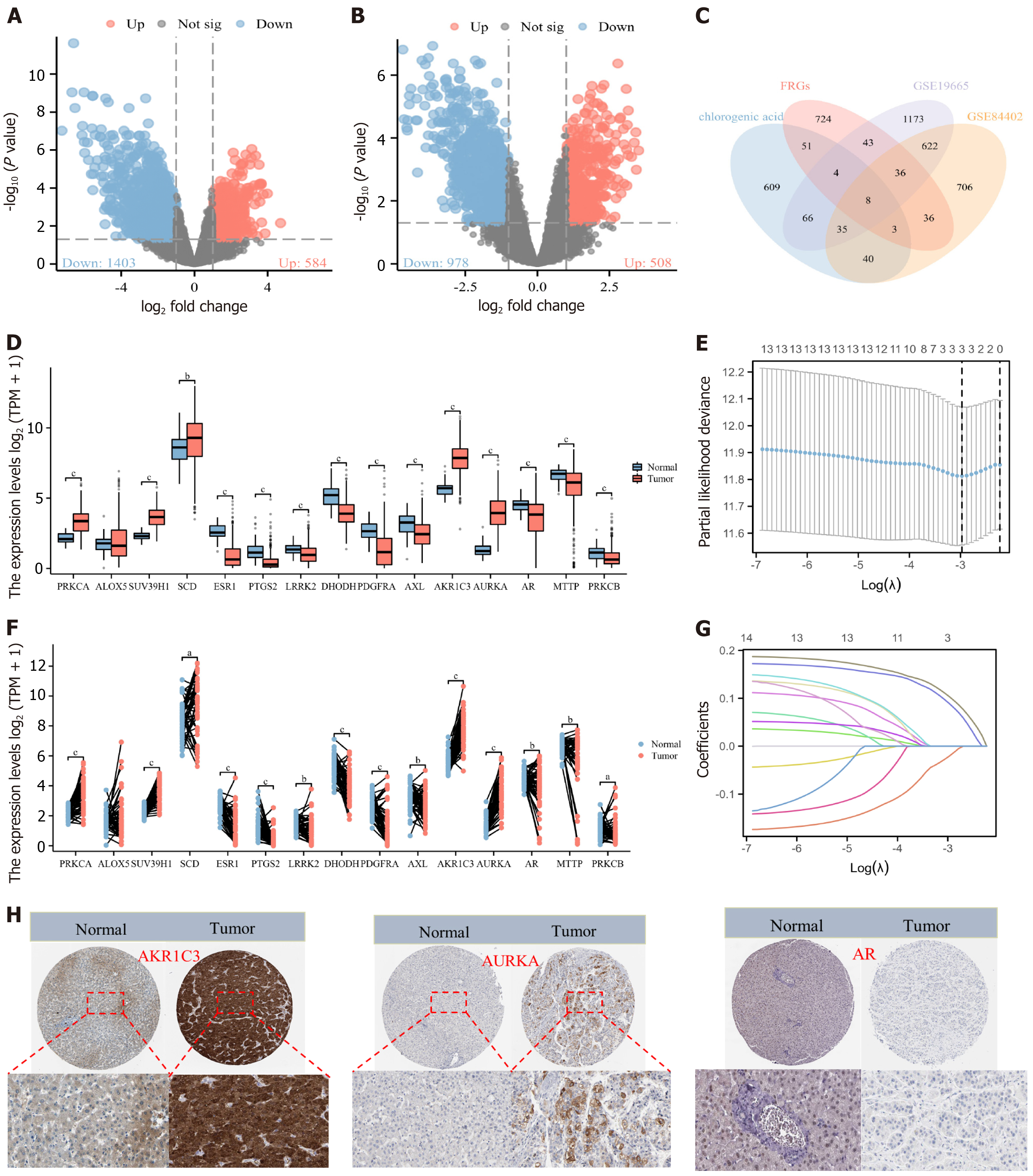
Acquisition and expression analysis of CGA intervention targets in HCC patients: A total of 299 targets (e.g., secreted aspartyl proteinase 4, neuraminidase 1H, and acyl-CoA oxidase 1) were identified from PharmMapper, 100 targets (e.g., AKR1B1, AKR1B10, and MMP13) from SwissTarget, and 623 targets (e.g., AKT1, corticotropin releasing hormone receptor 1, and steroid 5 alpha-reductase 2) from TargetNet. The UniProt database was used to convert all targets to gene names. Of the 299 PharmMapper targets, 18 had no corresponding gene names, and among the 623 TargetNet targets, five had no corresponding gene names. After removing duplicates, a total of 816 potential CGA targets were obtained. From the FerrDb database, 629 ferroptosis-promoting genes (e.g., ribosomal protein L8, iron responsive element binding protein 2, and adenosine triphosphate synthase membrane subunit C locus 3), 727 ferroptosis-inhibiting genes [e.g., solute carrier family 7 member 11 (SLC7A11), GPX4, and AKR1C1], and 125 ferroptosis-involved genes (e.g., dual specificity phos
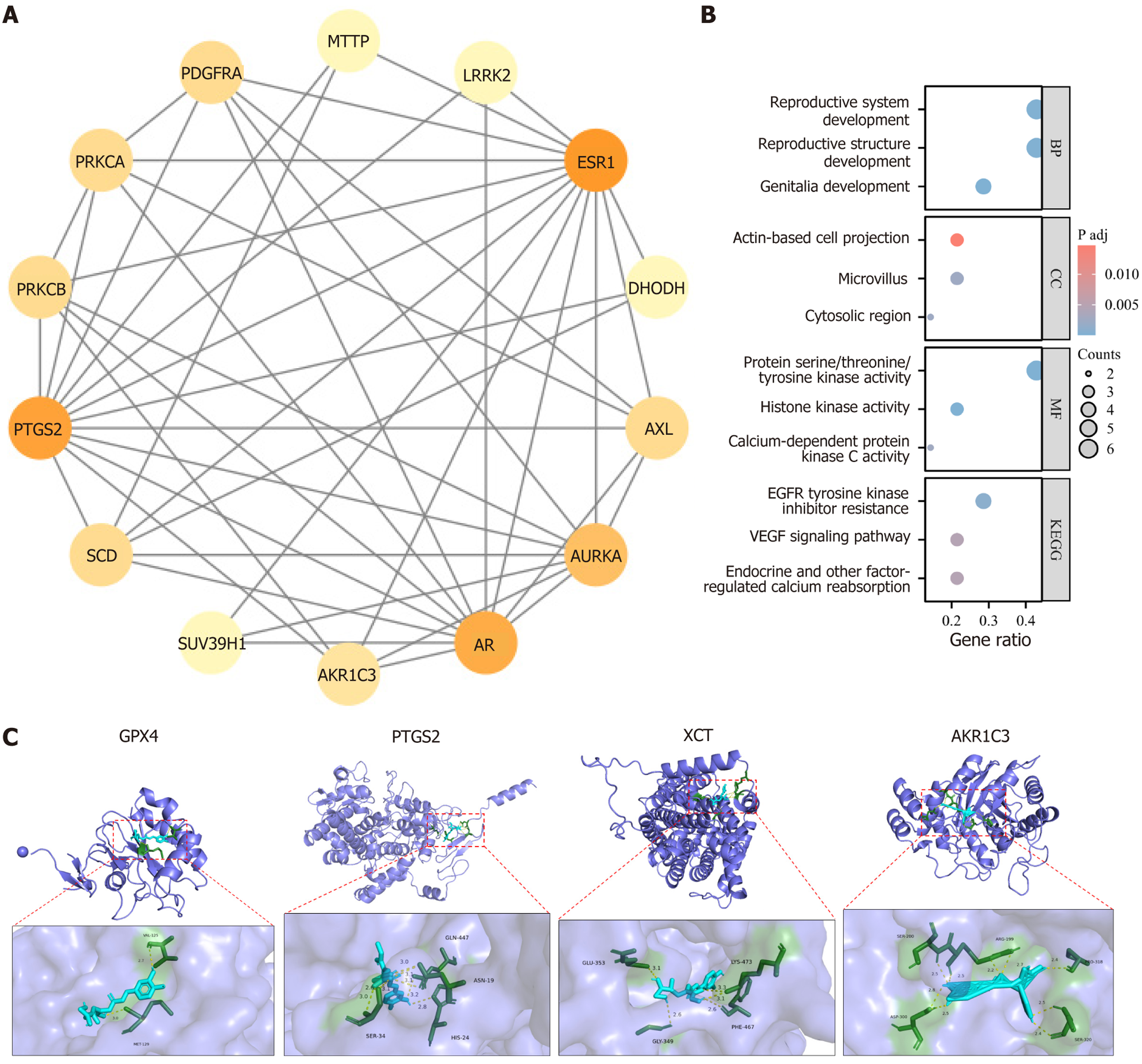
Differential analysis was performed using the limma package, with a screening threshold of P < 0.05 and |log2 fold change| > 1. In the GSE19665 dataset, 1987 differential genes were identified, of which 1403 were downregulated and 584 were upregulated in HCC samples. In the GSE84402 dataset, 1486 differential genes were identified, with 978 downregulated and 508 upregulated. R was used to analyze unique and overlapping targets among the groups, and visualization was achieved using ggplot2 and VennDiagram packages, identifying 15 CGA intervention targets: Protein kinase C alpha, arachidonate 5-lipoxygenase (ALOX5), suppressor of variegation 3-9 1, stearoyl-CoA desaturase, estrogen receptor 1 (ESR1), PTGS2, leucine rich repeat kinase 2, dihydroorotate dehydrogenase, platelet derived growth factor receptor alpha, AXL, AKR1C3, AURKA, AR, microsomal triglyceride transfer protein, and protein kinase C beta.
Expression differences of these 15 genes in the TCGA-LIHC cohort were evaluated using paired and non-paired sample tests. Among them, 14 genes showed differential expression in HCC tissues. Nine genes (ESR1, PTGS2, leucine rich repeat kinase 2, dihydroorotate dehydrogenase, platelet derived growth factor receptor alpha, AXL, AR, microsomal triglyceride transfer protein, and protein kinase C beta) were downregulated in HCC tissues (P < 0.05), while five genes (protein kinase C alpha, suppressor of variegation 3-9 1, stearoyl-CoA desaturase, AKR1C3, and AURKA) were upregulated (P < 0.05). The expression of ALOX5 did not differ in HCC tissues and was therefore excluded (Figure 3).
Prognostic analysis and nomogram construction of CGA intervention targets in HCC patients: Among the 14 CGA intervention targets, Lasso regression with ten-fold cross-validation identified three genes - AKR1C3, AURKA, and AR - as having the highest correlation with CGA-induced ferroptosis and HCC patient prognosis. Analysis yielded variable lambda values, maximum likelihood estimates, and C-index, with results visualized. Immunohistochemistry data from the HPA database indicated that AR was lowly expressed in HCC tissues, while AKR1C3 and AURKA were highly expressed, consistent with TCGA dataset results.
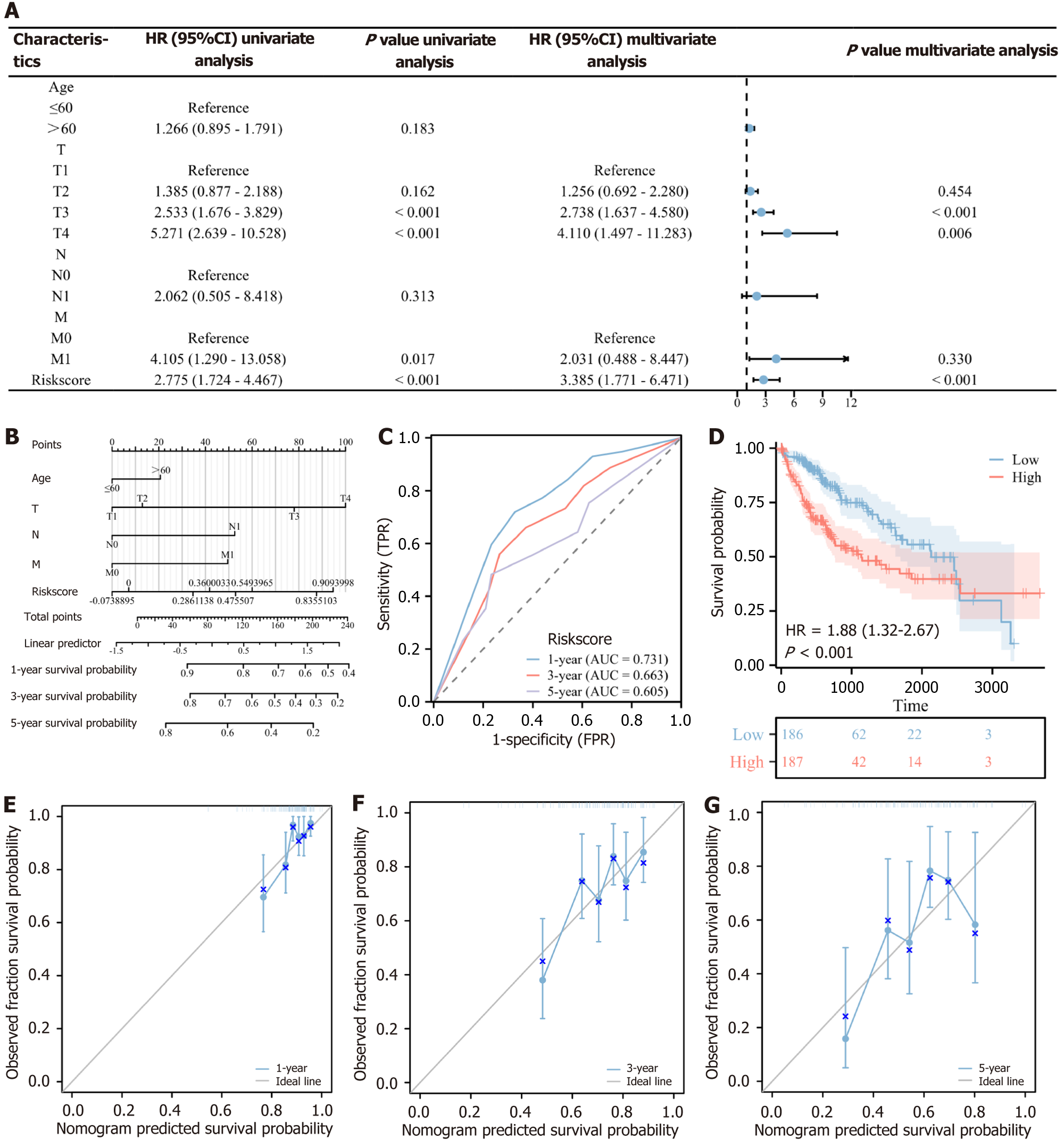
Univariate and multivariate Cox regression analyses were conducted on AKR1C3, AURKA, and AR using overall survival as the dependent variable. A Cox risk model was developed, calculating the correlation coefficient and risk scores based on the expression of these three genes. This 3-gene risk scoring system’s prognostic efficacy was evaluated using the time-receiver operating characteristic curve for 1-year, 3-year, and 5-year survival, demonstrating predictive value. Based on the median risk score, patients in the TCGA-LIHC cohort were classified into high-risk and low-risk groups, with 186 patients in the high-risk group and 187 in the low-risk group. The high-risk group showed poorer survival (HR = 1.88, 95%CI: 1.32-2.67, P < 0.001), indicating that the 3-gene risk score effectively predicts prognosis.
Univariate and multivariate Cox regression analyses were also performed on the risk score and clinical information of HCC patients. Univariate analysis identified TNM stage and risk score as significant prognostic factors (P < 0.1). Multivariate analysis revealed that T3 stage (HR = 2.738, 95%CI: 1.637-4.580, P < 0.001), T4 stage (HR = 4.110, 95%CI: 1.497-11.283, P = 0.006), and risk score (HR = 3.385, 95%CI: 1.771-6.471, P < 0.001) were independent prognostic factors, as shown in a forest plot. A nomogram incorporating age, TNM stage, and risk score was constructed to predict 1-year, 3-year, and 5-year survival. The nomogram’s C-index was 0.722 (95%CI: 0.691-0.752), indicating good predictive accuracy. Calibration analysis demonstrated that the nomogram’s predictions for 1-year, 3-year, and 5-year survival were consistent with observed outcomes, supporting the model’s prognostic validity (Figure 4).
Network pharmacology research of CGA intervention targets: Protein-protein interaction analysis revealed 65 interactions among the 14 targets, with the strongest interactions observed between AR, PTGS2, ESR1, and AURKA. Gene ontology and KEGG enrichment analyses were performed with a significance threshold of P < 0.05, and select results were visualized. Key biological processes for CGA intervention targets included reproductive system development, reproductive structure development, rhythmic processes, cell response to external stimuli, and serine phosphorylation. Major cellular components included microvilli, actin-based cell projections, and cytoplasmic regions. Key molecular functions involved histone kinase activity, protein serine/threonine/tyrosine kinase activity, calcium-dependent protein kinase C activity, and protein kinase C activity.
KEGG analysis indicated that CGA’s anti-HCC effects primarily occur through pathways such as epidermal growth factor receptor tyrosine kinase inhibitor resistance, endocrine regulation of calcium reabsorption, AA metabolism signaling, and chemical carcinogenesis-receptor activation, suggesting CGA’s therapeutic potential in HCC (Figure 5A and B). KEGG enrichment analysis further suggested that CGA-induced ferroptosis in liver cancer cells might be linked to AA metabolism. PTGS2 catalyzes the conversion of AA to prostaglandin H2 (PGH2), which is subsequently converted into various bioactive prostaglandins, including PGE2, enhancing intracellular GSH consumption and increasing ferroptosis susceptibility.
AKR1C3 catalyzes the conversion of AA to PGH2 and is often overexpressed in tumors, including HCC. Studies indicate that AKR1C3 suppresses ferroptosis in HCC via the yes-associated protein (YAP)/SLC7A11 signaling pathway. Specifically, AKR1C3 knockdown increases HCC cell sensitivity to ferroptosis inducers, acting through YAP nuclear translocation regulation, which influences xCT expression (the cystine transporter), thereby modulating intracellular iron levels and ferroptosis occurrence. Thus, CGA inhibits AKR1C3 expression and promotes PTGS2 expression, regulating AA metabolism, increasing cellular AA content, and inducing ferroptosis.
Molecular docking: To clarify the mechanism by which CGA induces ferroptosis in HepG2 cells, the effect of CGA on the PTGS2/AKR1C3/GPX4 antioxidant pathway was examined based on KEGG enrichment analysis results. PTGS2 and AKR1C3 are key enzymes in the AA metabolism pathway, while GPX4 is a crucial negative regulator of ferroptosis. The results showed that CGA had strong binding affinities with PTGS2, AKR1C3, GPX4, and xCT. Specifically, the binding energy between CGA and PTGS2 was 9.2, with CGA forming hydrogen bonds with His-24 (2.8 Å), Gln-477 (3.2 Å), Asn-19 (3.0, 3.1, 3.1 Å), and Ser-34 (2.6, 3.0, 3.1 Å). Similarly, the binding energy between CGA and AKR1C3 was 8.8, with hydrogen bonds forming at Ser-320 (2.4, 2.5 Å), Pro-318 (2.4 Å), Arg-199 (2.2, 2.7 Å), Ser-200 (2.5, 2.5 Å), and Asp-300 (2.5, 2.8 Å). For GPX4, the binding energy was 8.1, with hydrogen bonds forming with Met-129 (3.0 Å) and Val-125 (2.7 Å). Additionally, CGA displayed a binding energy of 8.6 with xCT, forming hydrogen bonds with Glu-353 (3.1 Å), Gly-349 (2.6 Å), Phe-467 (2.6 Å), and Lys-473 (3.1, 3.3 Å). These findings indicate that CGA is directly associated with the PTGS2/AKR1C3/GPX4 pathway (Figure 5C). Molecular docking results confirmed the strong binding affinity between CGA and PTGS2, AKR1C3, GPX4, and xCT, supporting a direct association between CGA and the PTGS2/AKR1C3/GPX4 pathway. Consequently, PTGS2/AKR1C3/GPX4 was selected as a key target for further investigation.
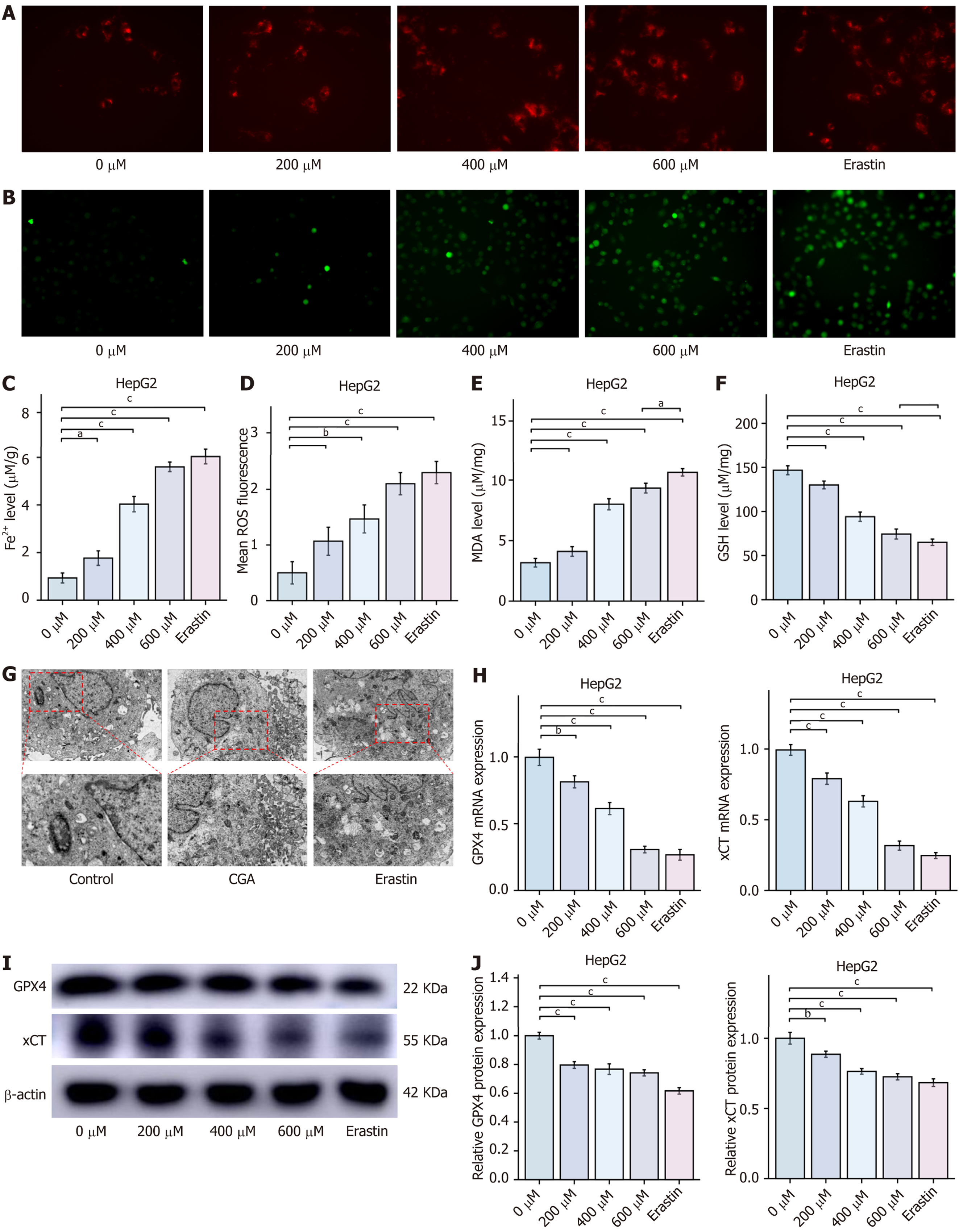
CGA induces ferroptosis in HCC cells: Bioinformatics and network pharmacology analyses revealed that the CGA-mediated ferroptosis pathway in HCC cells is primarily enriched in the PTGS2/AKR1C3/GPX4 signaling pathway. Unlike apoptosis and necrosis, ferroptosis is marked by iron-dependent lipid peroxidation, with the PTGS2/AKR1C3/GPX4 axis playing a pivotal role in its development. This suggests that targeting the PTGS2/AKR1C3/GPX4 axis to induce ferroptosis in HCC cells could provide a novel therapeutic approach for HCC treatment. Ferroptosis is an iron-dependent lipid peroxidation-dependent cell death, and the Ferro Orange fluorescent probe was used to detect Fe2+ levels in live cells, producing an irreversible orange fluorescence upon interaction with Fe2+. Additionally, the DCFH-DA probe was used to measure intracellular ROS levels, while MDA and GSH kits were employed to quantify intracellular lipid peroxidation and reduced glutathione, respectively, to comprehensively assess intracellular Fe2+ and lipid peroxidation levels. GPX4 and xCT serve as key negative regulators of ferroptosis in HCC cells, and Western blot analysis of their expression provides insight into the degree of ferroptosis in HepG2 cells.
This study assessed whether CGA affects ferroptosis in HCC cells by treating HepG2 cells with different concentrations of CGA and erastin. Compared with the control group, treatment with CGA (400 and 600 μmol/L) and erastin (10 μmol/L) significantly increased intracellular Fe2+ levels in HepG2 cells. Measurements of MDA and GSH levels indicated that treatment with 400 and 600 μmol/L CGA and erastin led to an increase in MDA and a decrease in GSH levels. Further analysis revealed that CGA and erastin significantly elevated intracellular lipid ROS levels in HepG2 cells, suggesting that high doses of CGA can induce lipid peroxidation, comparable to the effects of the ferroptosis inducer erastin.
Quantitative real-time PCR (RT-qPCR) analysis demonstrated that CGA (200, 400, and 600 μmol/L) and erastin (10 μmol/L) significantly inhibited GPX4 and xCT mRNA expression levels compared with the control group (P < 0.05). Western blot results confirmed that CGA (200, 400, and 600 μmol/L) and erastin (10 μmol/L) significantly reduced GPX4 and xCT protein expression in HepG2 cells (P < 0.05), indicating that CGA induces ferroptosis in HCC cells.
To determine whether CGA-induced ferroptosis in HepG2 cells is associated with mitochondrial structural and functional changes, mitochondrial ultrastructure was examined via transmission electron microscopy. After 48 hours of treatment with CGA (200, 400, and 600 μmol/L) and erastin (10 μmol/L), HepG2 cells displayed increased mitochondrial density, reduced mitochondrial volume, increased double membrane density, diminished mitochondrial cristae, and ruptured outer mitochondrial membranes. These observations suggest that CGA induces mitochondrial damage in HCC cells (Figure 6).
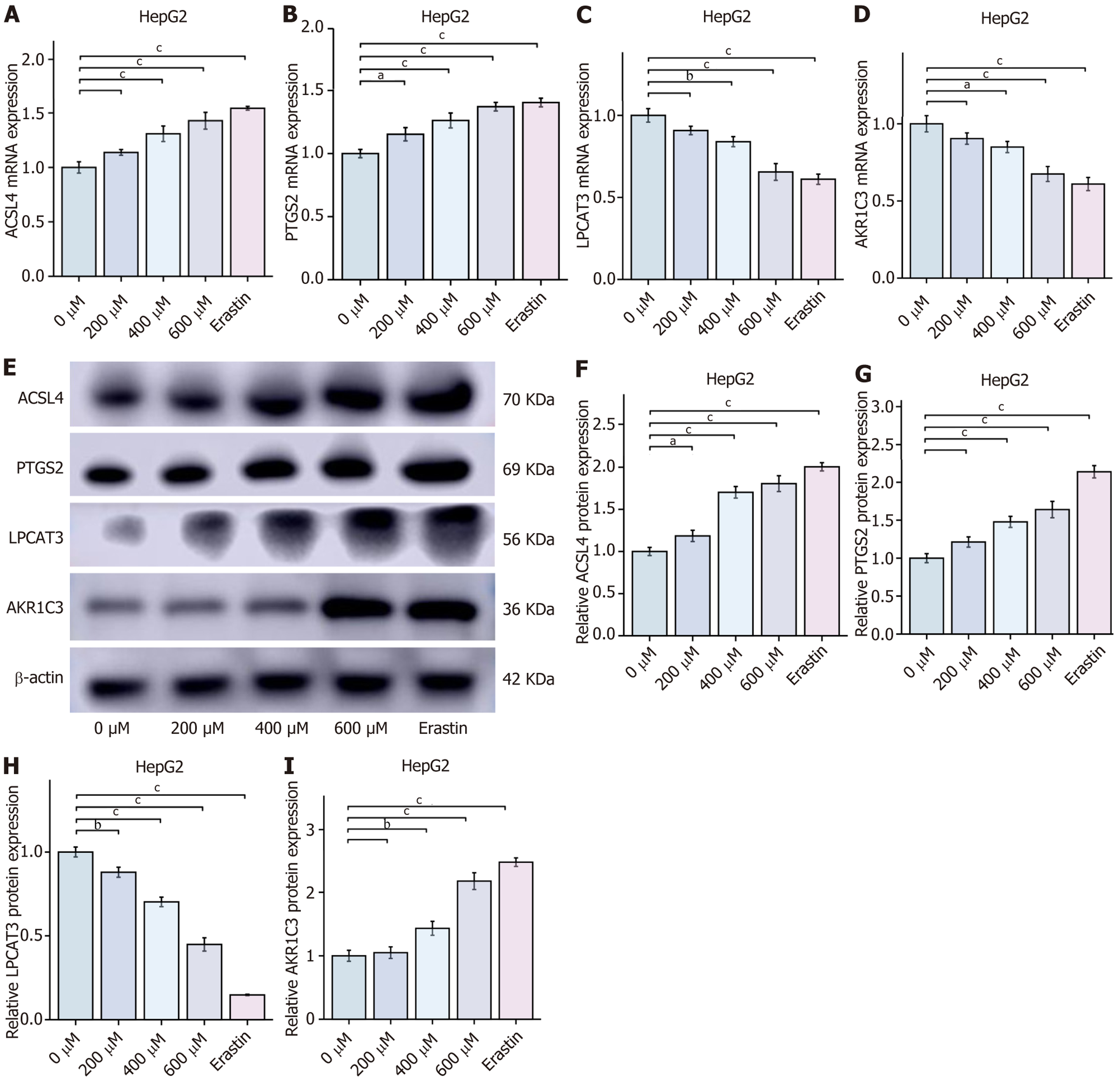
CGA regulates PTGS2/AKR1C3/GPX4 pathway to reprogram AA metabolism and induce ferroptosis in HCC cells: KEGG enrichment analysis indicated that the mechanism of CGA-induced ferroptosis in HCC cells might involve AA metabolism, with PTGS2 and AKR1C3 as key components. To validate this, intracellular AA levels were measured in cells treated with CGA (0, 200, 400, and 600 μmol/L) and erastin (10 μmol/L) using an ELISA kit. Intracellular AA content increased with CGA concentration, with levels in cells treated with high-dose CGA comparable to those in cells treated with the ferroptosis inducer erastin. To further elucidate the molecular mechanism of CGA-induced ferroptosis in HCC cells, the effects of CGA on PTGS2, AKR1C3, ACSL4, and LPCAT3 mRNA and protein expression were analyzed. RT-qPCR results demonstrated that, compared with the control group, CGA (400 and 600 μmol/L) and erastin (10 μmol/L) significantly inhibited AKR1C3 mRNA expression, while significantly promoting PTGS2, ACSL4, and LPCAT3 mRNA expression (P < 0.05). Western blot analysis showed that CGA dose-dependently inhibited AKR1C3 protein expression and promoted PTGS2, ACSL4, and LPCAT3 protein expression, suggesting that CGA may regulate the PTGS2/AKR1C3/GPX4 pathway, upregulate ACSL4 and LPCAT3, reprogram AA metabolism, and induce ferroptosis in HepG2 cells. These findings further support that CGA-induced ferroptosis in HCC is linked to the reprogramming of AA metabolism through the PTGS2/AKR1C3/GPX4 pathway (Figure 7).
According to global cancer statistics in 2022, PLC is the third leading cause of cancer-related deaths worldwide, accounting for 7.8% of cancer-related mortalities and posing a serious threat to human health. HCC represents approximately 75%-85% of PLC cases, with more than 70% of newly diagnosed HCC patients being in intermediate or advanced stages. These patients often miss the opportunity for curative surgical treatment and face a poor prognosis. The median survival time for advanced HCC is only 5.7 months, with a 5-year survival rate of just 10.1%. Currently, surgery, ablation, liver transplantation, radiotherapy, and pharmacotherapy are the most effective treatments for HCC, but their efficacy remains suboptimal[11]. Therefore, finding safe and effective therapeutic agents for HCC remains an urgent clinical challenge.
CGA is abundant in Eucommia ulmoides (Du Zhong), honeysuckle, green coffee beans, potatoes, apples, and tea, and is one of the most prevalent polyphenolic compounds found in 61 traditional Chinese medicinal plants and in the diet[12,13]. It has various pharmacological effects, such as anti-oxidation, anti-bacterial, anti-viral, anti-tumor, and immunomodulation[14,15], and has wide applications in the medical field[16]. Since the discovery of CGA’s inhibitory effect on tumors in the 1980s[17], more and more studies have been conducted on its inhibitory effects on different types of tumors and the underlying mechanisms. Huang et al[18] demonstrated that CGA has a significant inhibitory effect on HCC, lung cancer, and glioma, and its mechanism is not direct cell killing, but rather the induction of tumor cell differentiation through the influence of tumor cells, indicating that CGA has the potential to become a safe and effective tumor differentiation inducer. Sapio et al[19] reported that CGA can effectively inhibit osteosarcoma, and its mechanism is to inhibit the phosphorylation of signal transduction and transcription activator protein 3, and it is suggested that when CGA is combined with the inhibitor of extracellular signal-regulated kinase 1/2 PD98059, it can significantly enhance the anti-tumor effect.
CGA has been proven in multiple studies to significantly inhibit the proliferation of HCC cells, such as HepG2[6]. Yan et al[5] demonstrated that CGA may inhibit the proliferation of HCC cells by inhibiting the activity of the mitogen-activated protein kinase/ERK signaling pathway and induce a significant arrest of the HepG2 cell cycle in the S phase. Liu et al[20] conducted in vitro and in vivo experiments and found that CGA may inhibit the proliferation of HepG2 cells by downregulating the protein expression of DNA methyltransferase 1 and significantly upregulating the protein expression of p53 and p21, as well as inhibiting the activity of ERK1/2. Jiang et al[21] demonstrated that CGA may inhibit the proliferation of hepatoma cells Huh-7 by significantly inhibiting the non-canonical nuclear factor kappa B signaling pathway and upregulating the expression of BH3-only protein B cell lymphoma-2 binding component 3 in the B cell lymphoma-2 family, thereby activating the mitochondrial apoptosis pathway. Zhang et al[22] conducted a study on the effects of caffeic acid and CGA on the progression of rat HCC induced by diethylnitrosamine using metabolomics and gut microbiota, and found that treatment with CGA could reduce pathological changes and markers of liver injury in liver tissue and restore 28 different gut metabolites to normal, thereby regulating the structure of the gut microbiota. Based on the above, we believe that CGA is expected to be an effective drug for the treatment of HCC. As a natural product, CGA has the advantages of high safety and low toxicity, and is expected to become a new choice for HCC treatment.
In line with previous studies, our study confirmed that CGA significantly inhibits the proliferation, migration, and invasion of HepG2 cells. Then, we consider the mechanism by which the drug exerts its inhibitory effect. Ferroptosis is an iron-dependent, lipid peroxidation-driven, specific, and regulated cell death mode, driven by the accumulation of lipid ROS. It is closely related to the occurrence and development of HCC[23]. In the process of HCC occurrence and development, the mechanism of ferroptosis is very complex, involving the action of many proteins and molecular pathways. Among them, the regulation of the system Xc- and GPX4 signaling pathways is closely related to the occurrence of ferroptosis. System Xc- is composed of SLC7A11 (xCT) and SLC3A2, a cystine/glutamate antiporter that participates in the synthesis of GSH. GPX4 uses GSH as a substrate to reduce lipid ROS to normal lipids, preventing the accumulation of lipid ROS and inhibiting the occurrence of ferroptosis. When the activity of system Xc- and GPX4 is inhibited, the accumulation of lipid ROS in the cell increases, leading to the induction of ferroptosis. Ferroptosis has important applications in the treatment of HCC. Studies have shown that ferroptosis can be used as a target for the diagnosis, prevention, and treatment of HCC[24]. By regulating ferroptosis, the progression of liver diseases can be affected, providing new strategies and targets for the treatment of HCC. Ferroptosis is a novel programmed cell death that differs from traditional cell death modes such as apoptosis and necrosis. However, ferroptosis and other cell death mechanisms overlap to some extent. For example, apoptosis may also occur during ferroptosis, and ferroptosis may also accompany apoptosis. The interplay and regulation between ferroptosis and other cell death mechanisms need to be further explored.
Based on the above findings, our study employed bioinformatics and network pharmacology to identify 14 potential CGA targets in HCC ferroptosis. Further analysis revealed a 3-gene prognostic model for HCC patients. Network pharmacology highlighted the PTGS2/AKR1C3/GPX4 pathway as a key mediator, implicating AA metabolism. Mole
AA exists in cell membrane PLs, especially in liver tissue, and is an important precursor for the synthesis of prostaglandins and other bioactive lipids. The generation of certain prostaglandins through the COX pathway by AA can promote the consumption of intracellular GSH, thereby increasing the susceptibility of cells to ferroptosis[25]. Tumor cells with high levels of reactive ROS are prone to undergo lipid peroxidation reactions with aliphatic double bonds rich in the biofilm of the biological lipid membrane, resulting in the accumulation of lipid ROS and its metabolites, leading to cell membrane rupture and the occurrence of ferroptosis. There is also evidence that AA increases the incorporation of AA into oleic acid/palmitoleate C16/C18 fatty acids by T cell killing-induced interferon γ, and synergistically induces tumor cell ACSL4-dependent ferroptosis[26]. In our study, CGA may regulate the PTGS2/AKR1C3/GPX4 pathway, upregulate the expression of ACSL4 and LPCAT3, reprogram AA metabolism, and induce ferroptosis in HepG2 cells. Moreover, AA metabolism is closely related to ferroptosis. AA metabolism produces lipid peroxidation products, such as PGE2, which can activate cell signaling pathways, such as the nuclear factor kappa B signaling pathway, thereby inhibiting ferroptosis. At the same time, lipid peroxidation products can also act as second messengers in cells, participate in intracellular signal transduction, and regulate cell growth, differentiation, apoptosis and other processes. PTGS2, also known as COX-2, is one of the key enzymes in AA metabolism. The overexpression of a few genes/ proteins is considered a biomarker of ferroptosis. The PTGS2 gene encodes the COX-2 enzyme that catalyzes the conversion of AA to PGH2 in the body[27], and PGH2 is further converted into various bioactive prostaglandins, such as PGE2, PGD2, PGF2α, PGI2, and thromboxane A2, which is the rate-limiting step in the synthesis of all prostaglandins. These COX metabolites may also directly participate in lipid peroxidation reactions and promote the occurrence of ferroptosis[28]. AKR1C3, also known as PGF2α reductase, is an aldo-keto reductase that plays an important role in AA metabolism. It can reduce the ring oxide of AA to convert AA to PGH2, which is a key step in the prostaglandin biosynthesis pathway. PGH2 can then be further converted into various bioactive prostaglandins, such as PGE2, PGD2, PGF2α, and PGI2, which play important roles in regulating inflammation, pain, blood pressure, and platelet aggregation. AKR1C3 is often overexpressed in various tumors, including HCC. Studies have shown that AKR1C3 suppresses the occurrence of ferroptosis in HCC by regulating the YAP/SLC7A11 signaling pathway[29]. Specifically, knocking down AKR1C3 enhances the sensitivity of HCC cells to ferroptosis inducers. This action mechanism involves the regulation of AKR1C3 on YAP nuclear translocation, which then affects the expression of the cystine transporter xCT, ultimately affecting intracellular iron levels and influencing the occurrence of ferroptosis.
When ferroptosis occurs, the mitochondrial cristae decrease or disappear, the mitochondrial outer membrane ruptures or shrinks, the mitochondrial color deepens, the iron-dependent cell nucleus does not rupture, the cell membrane ruptures, and lipid ROS, ACSL4, LPCAT3, and the lipoxygenase family genes (ALOX5, ALOX12, and ALOX15) are upregulated, which are associated with the survival prognosis of HCC patients. AA is an important ω-6 series polyunsaturated fatty acid in the fatty acid metabolism, with four carbon double bonds in the carbon chain, which are prone to lipid peroxidation reactions with ROS. In the upstream of lipid peroxidation, ACSL4 and LPCAT3 are key fatty acid metabolism enzymes[30,31]. ACSL4 preferentially recognizes AA and adrenaline and catalyzes the binding of long-chain free fatty acid with CoA, and then LPCAT3 catalyzes the acylation of AA to form PL. ACSL4 and LPCAT3 are the “bridge” molecules that reprogram AA metabolism to induce ferroptosis[30].
We confirmed that CGA induces ferroptosis in HCC cells by assessing Fe2+ levels, lipid peroxidation, and GSH. CGA dose-dependently increased lipid peroxidation, decreased GPX4 and xCT expression, and damaged mitochondria. AA content also increased with CGA treatment, suggesting involvement of AA metabolism. RT-qPCR and Western blot confirmed that CGA upregulates PTGS2, ACSL4, and LPCAT3 while downregulating AKR1C3. These results demon
Nonetheless, there are certain limitations to this study. The research mainly used in vitro cell models, and lacked in vivo experimental verification. In addition, this study only studied the effect of CGA on the proliferation, migration, invasion, and ferroptosis of HCC cells, and did not study the effect of CGA on apoptosis, necrosis, and other cell death modes of HCC cells. Moreover, this study only studied the effect of CGA on the PTGS2/AKR1C3/GPX4 axis, and did not study the effect of CGA on other signaling pathways. Therefore, our research group will further explore the molecular mechanism of CGA in the intervention of liver cancer, and further carry out in vivo and in vitro or clinical research to improve the subsequent research.
CGA could inhibit the proliferation, migration, and invasion of HCC cells in a dose and time dependent manner. Bioinformatics analysis showed that CGA could induce ferroptosis in HCC, and its mechanism of action may be related to AA metabolism, especially the key role of PTGS2 and AKR1C3 in the process. Our in vitro experiments verified the significant effect of CGA in inducing ferroptosis. CGA dose-dependently increased Fe2+ levels in HepG2 cells, decreased GSH levels, increased MDA and ROS content, increased AA content, and significantly inhibited the mRNA and protein expression of GPX4, xCT, and AKR1C3, while promoting the mRNA and protein expression of PTGS2, ACSL4, and LPCAT3. These findings further confirmed the key role of ferroptosis in the inhibition of HCC by CGA and supported the relationship between the induction of ferroptosis by CGA in HCC and the reprogramming of AA metabolism through the PTGS2/AKR1C3/GPX4 pathway. The findings of this study provide a new perspective and strategy for the treatment of HCC.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8144] [Article Influence: 8144.0] [Reference Citation Analysis (2)] |
| 2. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1342] [Article Influence: 335.5] [Reference Citation Analysis (1)] |
| 3. | Mao JJ, Pillai GG, Andrade CJ, Ligibel JA, Basu P, Cohen L, Khan IA, Mustian KM, Puthiyedath R, Dhiman KS, Lao L, Ghelman R, Cáceres Guido P, Lopez G, Gallego-Perez DF, Salicrup LA. Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA Cancer J Clin. 2022;72:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 199] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 4. | Liu SL, Peng BJ, Zhong YL, Liu YL, Song Z, Wang Z. Effect of 5-caffeoylquinic acid on the NF-κB signaling pathway, peroxisome proliferator-activated receptor gamma 2, and macrophage infiltration in high-fat diet-fed Sprague-Dawley rat adipose tissue. Food Funct. 2015;6:2779-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Yan Y, Liu N, Hou N, Dong L, Li J. Chlorogenic acid inhibits hepatocellular carcinoma in vitro and in vivo. J Nutr Biochem. 2017;46:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Granado-Serrano AB, Martín MA, Izquierdo-Pulido M, Goya L, Bravo L, Ramos S. Molecular mechanisms of (-)-epicatechin and chlorogenic acid on the regulation of the apoptotic and survival/proliferation pathways in a human hepatoma cell line. J Agric Food Chem. 2007;55:2020-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Qi G, Liu Z, Fan R, Yin Z, Mi Y, Ren B, Liu X. Athyrium multidentatum (Doll.) Ching extract induce apoptosis via mitochondrial dysfunction and oxidative stress in HepG2 cells. Sci Rep. 2017;7:2275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Yan Y, Li J, Han J, Hou N, Song Y, Dong L. Chlorogenic acid enhances the effects of 5-fluorouracil in human hepatocellular carcinoma cells through the inhibition of extracellular signal-regulated kinases. Anticancer Drugs. 2015;26:540-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4608] [Cited by in RCA: 4973] [Article Influence: 621.6] [Reference Citation Analysis (0)] |
| 10. | Chen J, Li X, Ge C, Min J, Wang F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022;29:467-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 399] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 11. | Bruix J, Colombo M. Hepatocellular carcinoma: current state of the art in diagnosis and treatment. Best Pract Res Clin Gastroenterol. 2014;28:751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Lepelley M, Cheminade G, Tremillon N, Simkin A, Caillet V, Mccarthy J. Chlorogenic acid synthesis in coffee: An analysis of CGA content and real-time RT-PCR expression of HCT, HQT, C3H1, and CCoAOMT1 genes during grain development in C. canephora. Plant Sci. 2007;172:978-996. [DOI] [Full Text] |
| 13. | Duarte GS, Pereira AA, Farah A. Chlorogenic acids and other relevant compounds in Brazilian coffees processed by semi-dry and wet post-harvesting methods. Food Chem. 2010;118:851-855. [RCA] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Bagdas D, Gul Z, Meade JA, Cam B, Cinkilic N, Gurun MS. Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation. Curr Neuropharmacol. 2020;18:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 15. | Li Y, Wang Q, Yao X, Li Y. Induction of CYP3A4 and MDR1 gene expression by baicalin, baicalein, chlorogenic acid, and ginsenoside Rf through constitutive androstane receptor- and pregnane X receptor-mediated pathways. Eur J Pharmacol. 2010;640:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Santana-Gálvez J, Cisneros-Zevallos L, Jacobo-Velázquez DA. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 407] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 17. | Huang MT, Smart RC, Wong CQ, Conney AH. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48:5941-5946. [PubMed] |
| 18. | Huang S, Wang LL, Xue NN, Li C, Guo HH, Ren TK, Zhan Y, Li WB, Zhang J, Chen XG, Han YX, Zhang JL, Jiang JD. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics. 2019;9:6745-6763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 19. | Sapio L, Salzillo A, Illiano M, Ragone A, Spina A, Chiosi E, Pacifico S, Catauro M, Naviglio S. Chlorogenic acid activates ERK1/2 and inhibits proliferation of osteosarcoma cells. J Cell Physiol. 2020;235:3741-3752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Feng Y, Li Y, Hu Y, Zhang Q, Huang Y, Shi K, Ran C, Hou J, Zhou G, Wang X. Chlorogenic Acid Decreases Malignant Characteristics of Hepatocellular Carcinoma Cells by Inhibiting DNMT1 Expression. Front Pharmacol. 2020;11:867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Jiang Y, Nan H, Shi N, Hao W, Dong J, Chen H. Chlorogenic acid inhibits proliferation in human hepatoma cells by suppressing noncanonical NF-κB signaling pathway and triggering mitochondrial apoptosis. Mol Biol Rep. 2021;48:2351-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Zhang Z, Wang D, Qiao S, Wu X, Cao S, Wang L, Su X, Li L. Metabolic and microbial signatures in rat hepatocellular carcinoma treated with caffeic acid and chlorogenic acid. Sci Rep. 2017;7:4508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Xu Y, Xing Z, Abdalla Ibrahim Suliman R, Liu Z, Tang F. Ferroptosis in liver cancer: a key role of post-translational modifications. Front Immunol. 2024;15:1375589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 24. | Ajoolabady A, Tang D, Kroemer G, Ren J. Ferroptosis in hepatocellular carcinoma: mechanisms and targeted therapy. Br J Cancer. 2023;128:190-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 84] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 25. | Wang B, Wu L, Chen J, Dong L, Chen C, Wen Z, Hu J, Fleming I, Wang DW. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct Target Ther. 2021;6:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 667] [Article Influence: 166.8] [Reference Citation Analysis (0)] |
| 26. | Liao P, Wang W, Wang W, Kryczek I, Li X, Bian Y, Sell A, Wei S, Grove S, Johnson JK, Kennedy PD, Gijón M, Shah YM, Zou W. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022;40:365-378.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 510] [Article Influence: 170.0] [Reference Citation Analysis (0)] |
| 27. | Kamal MV, Damerla RR, Dikhit PS, Kumar NA. Prostaglandin-endoperoxide synthase 2 (PTGS2) gene expression and its association with genes regulating the VEGF signaling pathway in head and neck squamous cell carcinoma. J Oral Biol Craniofac Res. 2023;13:567-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Zhang Y, Liu Y, Sun J, Zhang W, Guo Z, Ma Q. Arachidonic acid metabolism in health and disease. MedComm (2020). 2023;4:e363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 76] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 29. | Chen J, Zhang J, Tian W, Ge C, Su Y, Li J, Tian H. AKR1C3 suppresses ferroptosis in hepatocellular carcinoma through regulation of YAP/SLC7A11 signaling pathway. Mol Carcinog. 2023;62:833-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayır H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1637] [Cited by in RCA: 2003] [Article Influence: 250.4] [Reference Citation Analysis (0)] |
| 31. | Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trümbach D, Mao G, Qu F, Bayir H, Füllekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 2712] [Article Influence: 301.3] [Reference Citation Analysis (0)] |