Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.98746
Revised: November 2, 2024
Accepted: December 16, 2024
Published online: March 15, 2025
Processing time: 224 Days and 3.1 Hours
For patients with advanced gastrointestinal stromal tumors (GISTs) carrying the c-kit exon 11 mutation, imatinib (IM) at a standard dosage of 400 mg per day is the preferred first-line treatment. In cases where treatment with IM fails, there is an urgent need for a more precise assessment method to determine whether to switch therapies or escalate the IM dosage. This approach will enhance clinical decision-making and optimize patient outcomes.
To investigate IM plasma concentration’s role in second-line treatment decisions for c-kit 11-mutated advanced GISTs post-IM failure.
Patients with advanced GIST harboring c-kit 11 mutation who experienced failure with IM 400 mg per day as first-line treatment at our hospital were retrospectively analyzed. Patients were categorized into a low plasma (LP) concentration group (LP group, < 1100 ng/mL) and high plasma (HP) concentration group (HP group, ≥ 1100 ng/mL). Each group was further subdivided into Group A (dose-escalation group) and Group B (drug-switch group). Baseline characteristics were compared and Kaplan-Meier curves were used to analyze the survival of patients.
Seventy-five patients were included in the analysis. For the LP group (n = 28), Group A (n = 14) had longer overall survival (OS) than Group B (n = 14) (P = 0.02). No differences were observed between the two subgroups in disease control rate (DCR), objective response rate, and progression-free survival (PFS) (P > 0.05). For the HP group (n = 47), Group B (n = 18) had a higher DCR and longer PFS than Group A (n = 29) (P = 0.008 and P = 0.03, respectively). No difference in OS was observed between the two subgroups (P > 0.05).
Increasing IM dosage for c-kit 11-mutated advanced GISTs post-IM failure may prolong OS if plasma concentration is < 1100 ng/mL. Switching tyrosine kinase inhibitors may improve DCR and PFS if ≥ 1100 ng/mL.
Core Tip: This study showed that increasing the dosage of imatinib (IM) for patients with advanced gastrointestinal stromal tumors harboring c-kit exon 11 mutation who experienced failure of first-line IM treatment can prolong overall survival when the plasma concentration is < 1100 ng/mL. Switching to another tyrosine kinase inhibitor can improve the disease control rate and achieve longer progression-free survival when the plasma concentration is ≥ 1100 ng/mL. Therefore, IM plasma concentration may guide the decision-making for second-line treatment.
- Citation: Li HT, Du YY, Huang Z, Li JJ, Zhang J. Significance of monitoring imatinib plasma concentration in second-line treatment decisions for c-kit 11 gene-mutated gastrointestinal stromal tumors. World J Gastrointest Oncol 2025; 17(3): 98746
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/98746.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.98746
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the GI tract[1]. GISTs constitute the majority of mesenchymal tumors in the digestive system, which originate from GI interstitial stem cells and exhibit multidirectional differentiation characteristics, typically consisting of undifferentiated spindle cells[2]. Mutations in genes such as c-kit or platelet-derived growth factor receptor alpha lead to continuous activation of tyrosine kinases, resulting in uncontrolled cell proliferation and differentiation, which is the key pathogenic mechanism of GIST[1,3]. GISTs can occur at any site within the GI tract, with a predilection for the stomach and small intestine[4]. These tumors are often spherical, firm in texture, with nodular or lobulated surfaces, and are richly vascularized. High-risk GISTs have a relatively high local recurrence rate, with peritoneal and liver metastases; however, lymph node metastasis is rare[5]. Most patients are diagnosed with GIST between the ages of 50 and 80, typically after GI bleeding, during surgeries for other GI conditions, or through medical imaging examinations. Only a fraction of GIST patients are diagnosed following tumor rupture or GI obstruction[6,7].
Surgery is the primary treatment for localized GIST, with imatinib (IM) recommended as adjuvant therapy post-surgery for intermediate to high-risk patients. A standard dose of IM (400 mg/day) is the preferred first-line treatment for patients with c-kit exon 11 (c-kit 11)-mutated advanced GIST (primary or recurrent unresectable). A higher dose of IM is recommended for patients with c-kit 9 mutations[8]. According to the 2022 European Society for Medical Oncology guideline, sunitinib is the recommended first-line treatment for patients with unresectable or locally advanced disease when IM treatment fails and dose escalation of IM is a second-line option[9].
Clinical studies have demonstrated that the plasma concentration of IM significantly affects its efficacy. Demetri et al[10] found that maintaining a steady-state plasma concentration above 1100 ng/mL significantly increased the objective response rate (ORR) and prolonged progression-free survival (PFS). Wu et al[11] reported that the trough level (Cmin) of IM peaked within the first 3 months of treatment and then showed a declining trend, with long-term IM use maintaining relatively stable trough plasma concentrations. Body surface area, creatinine clearance rate, surgical methods, frequency of bowel movements, and relevant genetic single nucleotide polymorphisms affect IM plasma concentration[12]. IM treatment failure for patients with c-kit 11 mutations can be attributed to acquired secondary mutations leading to resistance, as well as suboptimal plasma concentrations.
Therefore, the present study explored the relationship between the efficacy of dose escalation or drug switching and plasma concentration levels of IM. The significance of monitoring IM plasma concentration in guiding second-line treatment decisions for patients with advanced GIST harboring c-kit 11 mutation, who failed first-line IM treatment, was also investigated.
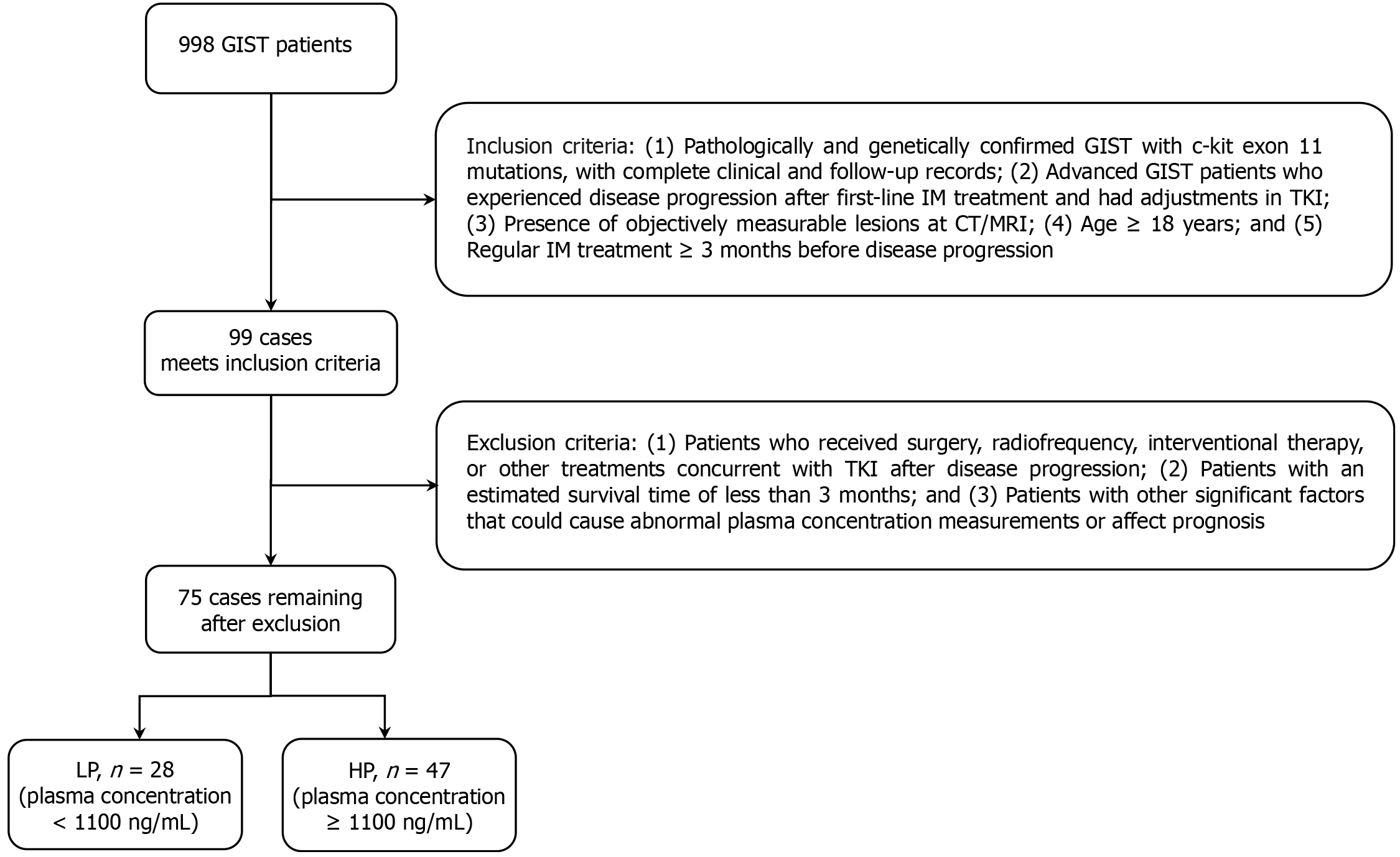
Clinicopathological data of patients with advanced c-kit 11-mutated GIST, who experienced disease progression after treatment with 400 mg/day IM at the First Affiliated Hospital of Chongqing Medical University (Yuzhong District, China) between June 2015 and June 2022, were retrospectively analyzed. Inclusion criteria were: (1) Pathologically and genetically confirmed GIST with c-kit 11 mutations, with complete clinical and follow-up records; (2) Patients with advanced GIST who experienced disease progression after first-line IM treatment and had adjustments for the tyrosine kinase inhibitor (TKI); (3) Presence of objectively measurable lesions at computed tomography/magnetic resonance imaging; (4) Patients aged ≥ 18 years; and (5) Regular IM treatment ≥ 3 months before disease progression. Exclusion criteria were: (1) Patients who received surgery, radiofrequency, interventional therapy, or other treatments concurrent with TKI after disease progression; (2) Patients with an estimated survival time of < 3 months; or (3) Patients with other significant factors that could cause abnormal plasma concentration measurements or affect prognosis. A flowchart of the patient screening process is shown in Figure 1.
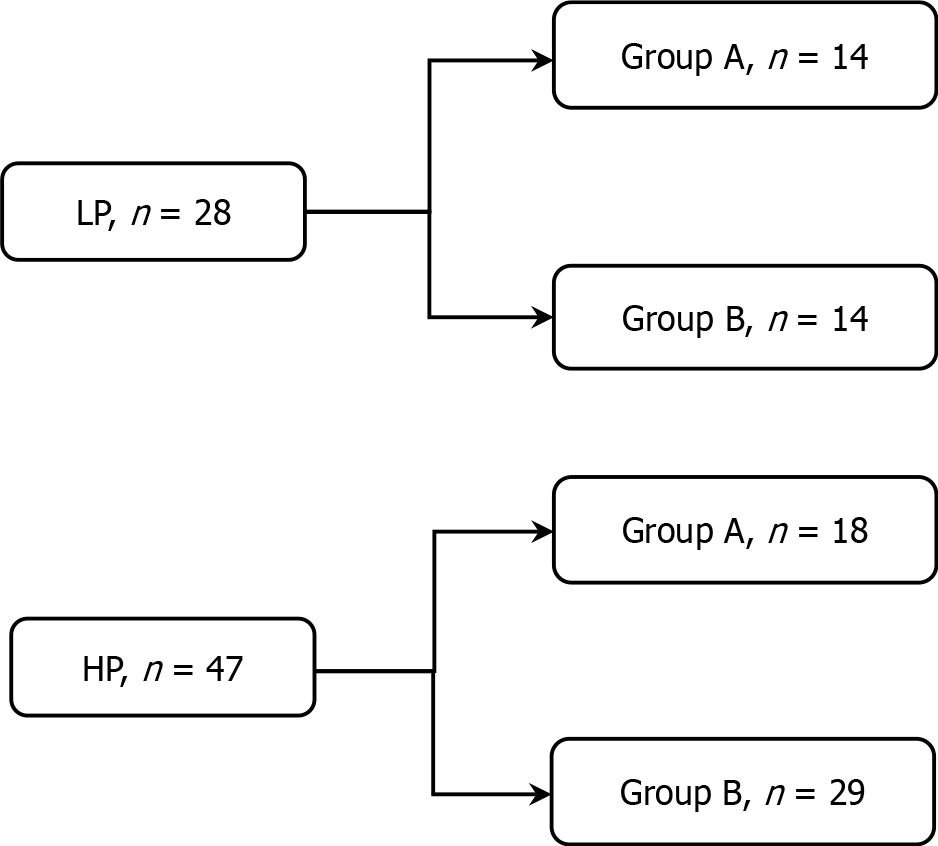
Patients were divided into a low plasma (LP) concentration group (LP group, < 1100 ng/mL; n = 28) and a high plasma (HP) concentration group (HP group, ≥ 1100 ng/mL; n = 47) based on their IM plasma concentration at the time of first-line treatment failure. Furthermore, each group was subdivided into Group A (dose-escalation group) and Group B (drug-switch group) based on whether they received an increased IM dosage (600 mg/day) or switched to another TKI. The grouping method is illustrated in Figure 2.
Previous studies have shown that the IM plasma concentration can reach relatively stable trough blood levels during long-term medication after 3 months of intake[11,13]. Therefore, the most recent measurement at the time of first-line treatment failure was considered the IM trough concentration value. High-dose IM treatment was defined as doses of 600 mg/day, and alternative medication was regarded as switching to second-line TKI. Disease control rate (DCR) was defined as the percentage of patients achieving complete response (CR), partial response (PR), or stable disease (SD). The ORR was defined as the percentage of patients achieving CR and PR after treatment change. PFS was defined as the time from inclusion to the first time a patient experienced disease progression as assessed by the imaging evaluation. Overall survival (OS) was defined as the time from inclusion to patient death resulting from any cause. Efficacy was evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1[14].
Blood samples were collected at the GIST specialized outpatient clinic of the First Affiliated Hospital of Chongqing Medical University. Patients took IM with their lunch daily, and venous blood samples were collected the following morning. All plasma concentration results were measured using the Shimadzu HPLC LC-20A system (Shimadzu, Japan).
Follow-up was conducted by attending physician’s outpatient service or via telephone. The follow-up information mainly included the patient’s survival and recurrence status, the type and dosage of TKI, and IM plasma concentration levels. Imaging examinations were conducted to determine disease progression. Follow-up was conducted at least once every 3 months, with the follow-up cut-off date being December 30, 2023.
An information database for GIST was established by collecting patient hospitalization records and outpatient follow-up information. The data mainly included the patient’s age, sex, primary site, pathological information (mitotic count, Ki-67 percentage, and National Institute of Health risk classification), genetic mutation types, treatment information (surgical margins and medication history), drug plasma concentration levels, and imaging data.
Statistical analyses were performed using SPSS 26.0 software. Normally distributed quantitative data are expressed as the mean ± standard deviation and compared using the t-test. Categorical data are expressed as numbers and percentages n (%) and compared using the χ² test or Fisher’s exact test. Kaplan-Meier survival curves were drawn for both groups, and the log-rank test was used to analyze and compare the differences in survival rates between the groups. All tests were two-sided, and P < 0.05 was considered statistically significant.
No statistically significant differences in the baseline clinical characteristics were found between the dose-escalation group and drug-switch groups (Table 1). After stratifying patients based on whether the blood drug concentration was > 1100 ng/mL, no statistically significant differences in the baseline clinical characteristics were found between the dose-escalation group and drug-switch therapy groups within each stratum (Table 2).
| Characteristics | Group A (n = 32) | Group B (n = 43) | P value |
| Age | 51 ± 16 | 53 ± 12 | 0.608 |
| Sex | 0.888 | ||
| Male | 25 (78) | 33 (77) | |
| Female | 7 (22) | 10 (23) | |
| Primary tumor site | 0.275 | ||
| Stomach | 12 (38) | 10 (23) | |
| Small intestine | 14 (44) | 19 (44) | |
| Other | 6 (19) | 14 (33) | |
| Mitotic count (/50 HPF) | 0.142 | ||
| ≤ 5 | 13 (41) | 27 (63) | |
| 5 to ≤ 10 | 10 (31) | 7 (16) | |
| > 10 | 9 (28) | 9 (21) | |
| Ki 67 | 0.189 | ||
| ≤ 10 | 16 (50) | 28 (65) | |
| > 10 | 16 (50) | 15 (35) | |
| Risk classification | > 0.9991 | ||
| Medium | 1 (3) | 1 (2) | |
| High | 31 (97) | 42 (98) | |
| Mutation type | 0.5181 | ||
| Point mutation | 6 (19) | 13 (30) | |
| Delete mutation | 25 (78) | 29 (67) | |
| Other | 1 (3) | 1 (2) | |
| Sum of largest diameter (cm) | 0.317 | ||
| ≤ 5 | 10 (31) | 12 (28) | |
| 5-10 | 10 (31) | 8 (19) | |
| > 10 | 12 (38) | 23 (53) | |
| Lesions > 3 | 0.435 | ||
| Yes | 18 (56) | 28 (65) | |
| No | 14 (44) | 15 (35) | |
| R0 resection | 0.350 | ||
| Yes | 13 (41) | 13 (30) | |
| No | 19 (59) | 30 (70) |
| Characteristics | LP (n = 28) | HP (n = 37) | ||||
| Group A (n = 14) | Group B (n = 14) | P value | Group A (n = 18) | Group B (n = 29) | P value | |
| Age | 51 ± 12 | 53 ± 12 | 0.611 | 51 ± 19 | 52 ± 13 | 0.785 |
| Sex | > 0.9991 | > 0.999 | ||||
| Male | 11 (79) | 10 (71) | 14 (78) | 23 (79) | ||
| Female | 3 (21) | 4 (29) | 4 (22) | 6 (21) | ||
| Primary tumor site | 0.3561 | 0.5981 | ||||
| Stomach | 6 (43) | 2 (14) | 6 (33) | 8 (28) | ||
| Small intestine | 5 (36) | 7 (50) | 9 (50) | 12 (41) | ||
| Other | 3 (21) | 5 (36) | 3 (17) | 9 (31) | ||
| Mitotic count (/50 HPF) | 0.1841 | 0.408 | ||||
| ≤ 5 | 7 (50) | 12 (86) | 6 (33) | 15 (52) | ||
| 5 to ≤ 10 | 4 (29) | 1 (7) | 6 (33) | 6 (21) | ||
| > 10 | 3 (21) | 1 (7) | 6 (33) | 8 (28) | ||
| Ki 67 | > 0.999 | 0.096 | ||||
| ≤ 10 | 8 (57) | 8 (57) | 8 (44) | 20 (69) | ||
| > 10 | 6 (43) | 6 (43) | 10 (56) | 9 (31) | ||
| Risk classification | > 0.9991 | > 0.9991 | ||||
| Medium | 1 (7) | 0 (0) | 0 (0) | 1 (3) | ||
| High | 13 (93) | 14 (100) | 18 (100) | 28 (97) | ||
| Mutation type | 0.2091 | > 0.9991 | ||||
| Point mutation | 2 (14) | 6 (43) | 4 (22) | 7 (24) | ||
| Delete mutation | 11 (79) | 8 (57) | 14 (78) | 21 (72) | ||
| Other | 1 (7) | 0 (0) | 0 (0) | 1 (3) | ||
| Sum of largest diameter (cm) | 0.7971 | 0.520 | ||||
| ≤ 5 | 6 (43) | 5 (36) | 4 (22) | 7 (24) | ||
| 5-10 | 4 (29) | 3 (21) | 6 (33) | 5 (17) | ||
| > 10 | 4 (29) | 6 (43) | 8 (44) | 17 (59) | ||
| Lesions > 3 | 0.450 | 0.869 | ||||
| Yes | 6 (43) | 8 (57) | 12 (67) | 20 (69) | ||
| No | 8 (57) | 6 (43) | 6 (33) | 9 (31) | ||
| R0 resection | > 0.999 | 0.236 | ||||
| Yes | 5 (36) | 5 (36) | 8 (44) | 8 (28) | ||
| No | 9 (64) | 9 (64) | 10 (56) | 21 (72) | ||
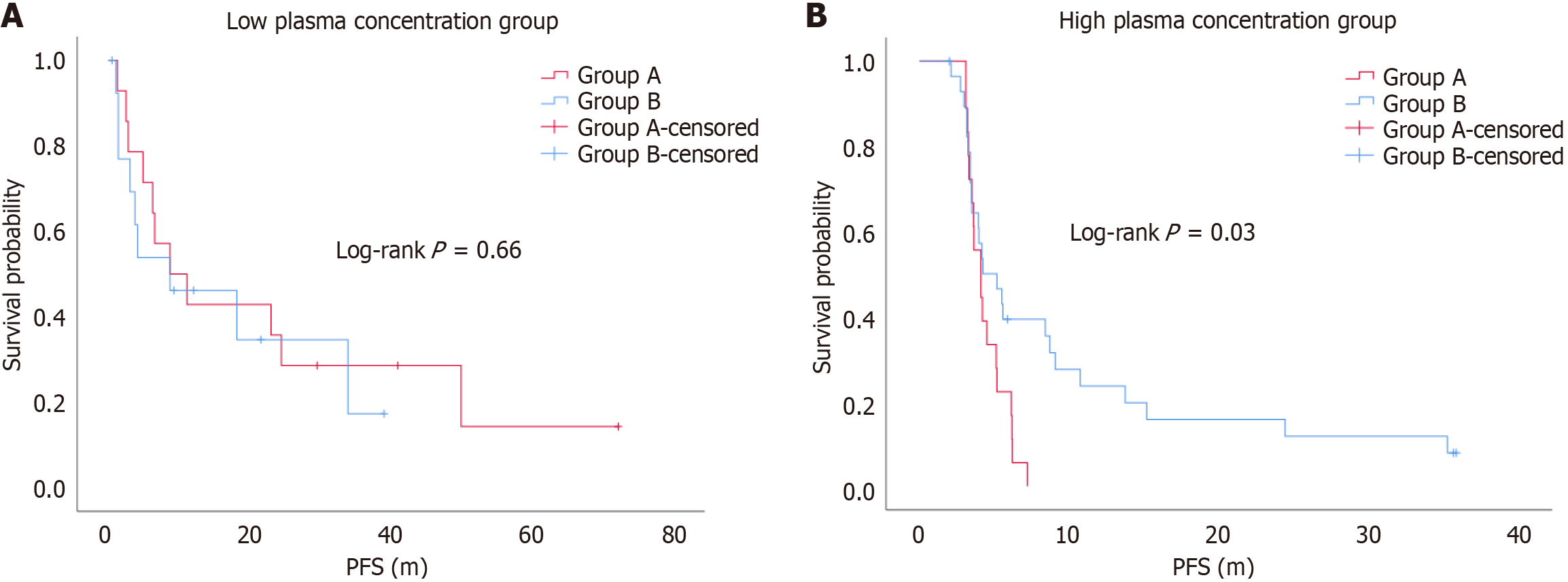
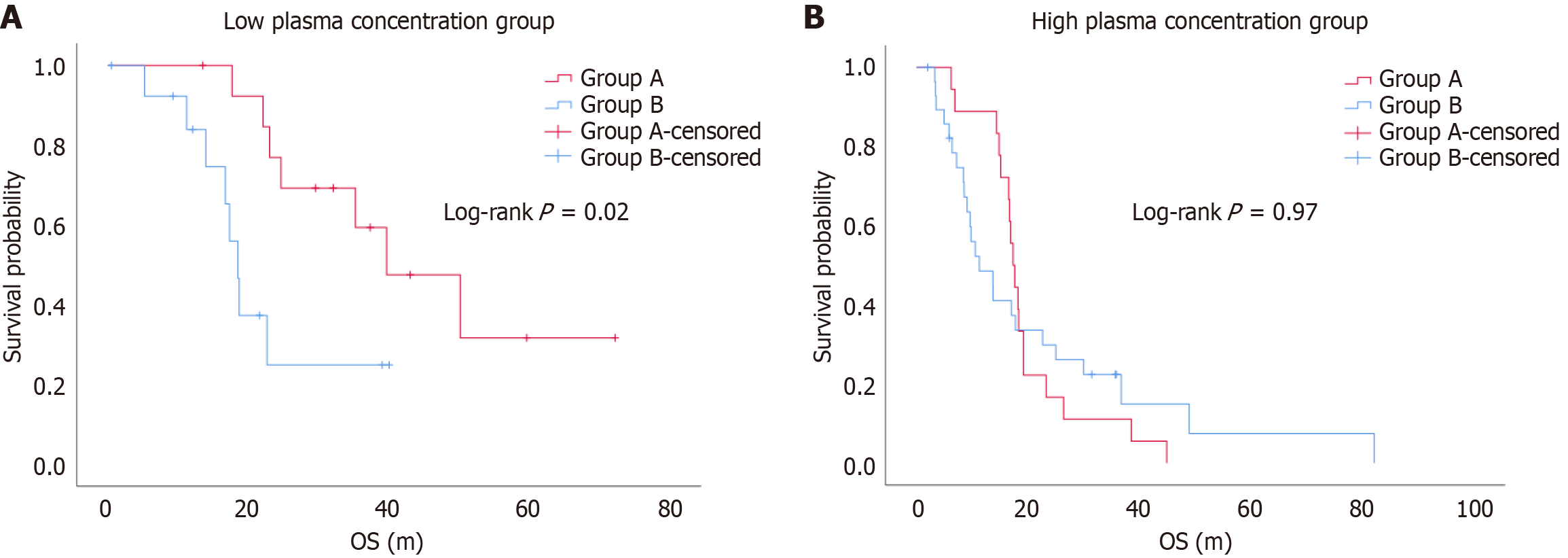
When the blood drug concentration was < 1100 ng/mL, the DCR was 71.4% and 64.3% for Groups A and B, respectively, with no statistically significant difference (P > 0.05). The ORR was 21.4% and 42.9% for Groups A and B, respectively, with no statistically significant difference (P > 0.05) (Table 3). The median PFS was 9.9 months (range 5.2-28.3 months) and 6.4 months (range 1.8-16.6 months) for Groups A and B, respectively, with no statistically significant difference (log-rank P = 0.66). The median OS was 33.7 months (range 23.4-42.2 months) and 17.0 months (range 11.4-20.8 months) for Groups A and B, respectively, with a statistically significant difference (log-rank P = 0.02) (Figure 3).
| Group | n | CR | PR | SD | PD | DCR | ORR |
| Group A | 14 | 0 (0) | 2 (14.2) | 8 (57.1) | 4 (28.5) | 10 (71.4) | 3 (21.4) |
| Group B | 14 | 0 (0) | 6 (42.8) | 3 (21.4) | 5 (35.7) | 9 (64.3) | 6 (42.9) |
| χ2 | - | - | |||||
| P value | > 0.99 | 0.42 | |||||
When the blood drug concentration was ≥ 1100 ng/mL, the DCR was 33.3% and 72.4% for Groups A and B, respectively, with a statistically significant difference (χ² = 6.94, P = 0.008). The ORR was 0% and 13.8% for Groups A and B, respectively, with no statistically significant difference (P = 0.28) (Table 4). The median PFS was 4.1 months (range 3.4-5.2 months) and 4.2 months (range 3.4-9.1 months) for Groups A and B, respectively, with a statistically significant difference (log-rank P = 0.03). The median OS was 17.5 months (range 15.5-19.2 months) and 10.5 months (range 6.4-25.0 months) for Groups A and B, respectively, with no statistically significant difference (log-rank P = 0.97) (Figure 4).
| Group | n | CR | PR | SD | PD | DCR | ORR |
| Group A | 18 | 0 (0) | 0 (0) | 6 (33.3) | 12 (66.6) | 6 (33.3) | 0 (0) |
| Group B | 29 | 0 (0) | 4 (13.7) | 17 (58.6) | 8 (27.5) | 21 (72.4) | 4 (13.8) |
| χ2 | 6.94 | - | |||||
| P value | 0.008a | 0.28 | |||||
We further analyzed the incidence of adverse reactions following dose escalation (Table 5). In the dose-escalation group, we recorded fatigue in 12 cases (37.5%), dermatitis/rash in 19 cases (59.4%), GI reactions in 27 cases (84.4%), and edema in 25 cases (78.1%). These adverse reactions were significantly different compared to pre-escalation levels (P < 0.05). Subsequently, we compared the LP group (n = 14) and the HP group (n = 18) with pre-switch therapy data. The results indicated that in the HP group, fatigue occurred in 10 cases (55.6%), dermatitis/rash in 12 cases (66.7%), muscle cramps in 7 cases (38.9%), GI reactions in 16 cases (88.9%), and edema in 17 cases (94.4%). These five adverse reactions were significantly different compared to pre-escalation levels (P < 0.05). Conversely, in the LP group, only GI reactions were recorded in 11 cases (78.6%) compared with pre-escalation levels (P < 0.05).
| Types of adverse reactions | LP (n = 14) | HP (n = 18) | ||
| Before1 | After2 | Before1 | After2 | |
| Fatigue | 0 (0.0) | 2 (14.3) | 2 (11.1) | 10 (55.6)b |
| Rash | 3 (21.4) | 7 (50.0) | 8 (44.4) | 12 (66.7) |
| Muscle spasm | 0 (0.0) | 1 (7.1) | 4 (22.2) | 7 (38.9) |
| Anemia | 5 (35.7) | 10 (71.4) | 13 (72.2) | 15 (83.3) |
| Gastrointestinal reactions | 2 (14.3) | 9 (64.3)b | 4 (22.2) | 18 (100.0)b |
| Edema | 4 (28.6) | 8 (57.1) | 10 (55.6) | 17 (94.4)a |
| Liver dysfunction | 0 (0.0) | 1 (7.1) | 2 (11.1) | 2 (11.1) |
| Granulocytopenia | 3 (21.4) | 5 (35.7) | 8 (44.4) | 11 (61.1) |
It is widely accepted that IM is the first-line treatment for both adjuvant and advanced GIST[9]. Recent studies have demonstrated a correlation between IM plasma concentrations, adverse drug effects, and clinical benefits for patients[15,16]. The B2222 study represented a significant investigation into the relationship between IM plasma levels and prognosis in patients with GIST[8]. The findings indicated that higher plasma concentrations were associated with longer PFS and highlighted the importance of achieving a plasma concentration threshold of 1100 ng/mL for the drug to effectively exert its therapeutic effects and ensure clinical benefits. These results provided a theoretical basis for monitoring IM plasma concentrations, guiding clinical decisions, and assessing prognosis. Furthermore, the efficacy of IM varies among patients with different genotypes. Patients with c-kit 11 tended to derive greater benefit from IM treatment compared to those with other mutations. However, in second-line treatment with sunitinib, patients with exon 9 mutations showed better outcomes than those with exon 11 mutations, with median PFS of 12.3 months and 7.0 months, respectively[2]. Therefore, at this critical juncture of treatment failure with IM, it is essential to determine whether to escalate the IM dose or switch to alternative targeted therapies for patients with c-kit 11. Previous studies have not incorporated plasma concentration levels as a criterion for decision-making, which this study aimed to address. We included patients with c-kit 11 and stratified them based on IM plasma concentrations at the time of disease progression. Then we compared DCRs and survival outcomes between the dose-escalation group and the drug-switch group, aiming to elucidate the significance of monitoring IM plasma concentrations in guiding second-line treatment decisions.
In our study, we found that when IM plasma concentration was < 1100 ng/mL, there were no significant differences in DCR, ORR, and PFS between the dose-escalation group and drug-switch group; however, a longer OS was observed (log-rank P = 0.02). Following dosage escalation, GI reactions increased but remained tolerable; other adverse effects, such as fatigue, edema, and leukopenia, did not show significant increases (Table 5). This suggests that in patients with disease progression and suboptimal IM plasma concentration, switching TKI should be approached with caution. This is because increasing the drug dosage can yield comparable DCR, ORR, and PFS to those of second-line TKI, allowing for regaining disease control and thereby extending the OS of patients.
When the blood concentration was ≥ 1100 ng/mL, the DCR of the dose-escalation group and the drug-switch group was 33.3% and 72.4% (P = 0.008). The PFS in the drug-switch group was longer than that in the dose-escalation group (log-rank P = 0.03). Additionally, after increasing the IM dosage, patients experienced a significant increase in adverse reactions, including fatigue, GI reactions, and edema (Table 5). This suggests that a further increase in the IM dose to raise the blood concentration may have limited efficacy improvement in patients with IM blood concentration ≥ 1100 ng/mL. However, switching to second-line drugs may achieve a higher DCR and longer PFS, which is crucial for improving the quality of life during subsequent treatment. Regarding OS, no significant difference was observed between the two groups (log-rank P = 0.97). Possible reasons included the following: (1) Although second-line TKIs may achieve a higher DCR initially, their adverse effects may be more frequent and severe than those of IM, leading to rapid health decline, hence shortening the OS; and (2) The current study excluded cases that received additional treatments such as radiofrequency ablation, interventional therapy, or surgery simultaneously with TKI treatment after IM failure. Generally, patients who achieve disease control after switching TKI may often opt for those additional treatments, which may provide better efficacy, including higher DCR and ORR and longer PFS and OS. In this study, the occurrence of fatigue, dermatitis/rash, GI reactions, and edema was significantly different between the dose-escalation group and pre-escalation group, which is consistent with previous research[17,18]. Notably, GI symptoms were significantly different across all groups, making the most pronounced adverse reaction.
This study had some limitations: (1) Secondary mutations are also a partial cause of IM treatment failure in patients with advanced GIST harboring c-kit 11 mutations. Thus, switching to sunitinib may yield better results after developing secondary mutations in c-kit 13 and c-kit 14. Since only a fraction of patients who experienced IM treatment failure underwent a re-biopsy for gene mutation detection, secondary mutations were not included in the analysis of relevant indicators. Thus, further studies should be conducted with larger clinical samples or multicenter data in the future; and (2) The threshold for an IM blood concentration of 1100 ng/mL in this study was based on the study by Demetri[10], in which patients with a Cmin below the first quartile concentration (1100 ng/mL) in the first month had shorter progression times. Other studies have reported that a Cmin threshold of 760 ng/mL is associated with longer PFS in advanced GIST[19]. However, the appropriate threshold for an IM blood concentration in the Chinese population has yet to be established. Notably, there is currently no universally accepted reference standard, necessitating further clinical research.
In summary, the present study demonstrated the following: (1) Increasing the IM dosage may achieve a longer OS in patients with advanced GIST harboring c-kit 11 mutations experiencing first-line IM treatment failure when the blood concentration is < 1100 ng/mL. Therefore, it is recommended that these patients switch to a higher dose of IM; and (2) Timely switching to second-line TKI may improve the DCR and achieve a longer PFS in this patient group when the blood concentration is ≥ 1100 ng/mL. This is meaningful for improving the quality of life in later stages. Additionally, early switching to another TKI for patients with limited progression may result in a higher proportion of opportunities for radiofrequency ablation, interventional therapy, or surgery and a longer waiting time for preoperative evaluation.
| 1. | Liu DN, Jia WW, Wang HY, Wu JH, Li CP, Hao CY. Cytoreductive surgery offers prognostic benefits in metastatic gastrointestinal stromal tumors with generalized progression following imatinib therapy: a single institute retrospective study. BMC Surg. 2023;23:189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Blay JY, Kang YK, Nishida T, von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Primers. 2021;7:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 248] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 3. | von Mehren M, Kane JM, Riedel RF, Sicklick JK, Pollack SM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, Dry S, Ganjoo KN, Gonzalez RJ, Holder A, Homsi J, Keedy V, Kelly CM, Kim E, Liebner D, McCarter M, McGarry SV, Mesko NW, Meyer C, Pappo AS, Parkes AM, Petersen IA, Poppe M, Schuetze S, Shabason J, Spraker MB, Zimel M, Bergman MA, Sundar H, Hang LE. NCCN Guidelines® Insights: Gastrointestinal Stromal Tumors, Version 2.2022. J Natl Compr Canc Netw. 2022;20:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Alvarez CS, Piazuelo MB, Fleitas-Kanonnikoff T, Ruhl J, Pérez-Fidalgo JA, Camargo MC. Incidence and Survival Outcomes of Gastrointestinal Stromal Tumors. JAMA Netw Open. 2024;7:e2428828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 5. | Mi R, Ding J, Dong Q, Jiang Z, Liu H, Liu ZH, Zhang LF, Zhang ZM. [Research progress of genes associated with gastrointestinal stromal tumors]. Zhongguo Putong Waike Zazhi. 2018;27:1341-1347. [DOI] [Full Text] |
| 6. | Alam I, Kheradmand F, Alam S, Jamil A, Wilson I, Hurley M. Laparoscopic management of acutely presenting gastrointestinal stromal tumors: a study of 9 cases and review of literature. J Laparoendosc Adv Surg Tech A. 2007;17:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Marano L, Arru GM, Piras M, Fiume S, Gemini S. Surgical management of acutely presenting gastrointestinal stromal tumors of the stomach among elderly: experience of an emergency surgery department. Int J Surg. 2014;12 Suppl 1:S145-S147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3203] [Cited by in RCA: 3110] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 9. | Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brodowicz T, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dufresne A, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Frezza AM, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Haas R, Hassan AB, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, Lopez-Pousa A, Martin-Broto J, Merimsky O, Messiou C, Miah AB, Mir O, Montemurro M, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno-Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Strauss SJ, Hall KS, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Gronchi A, Stacchiotti S; ESMO Guidelines Committee, EURACAN and GENTURIS. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 326] [Article Influence: 108.7] [Reference Citation Analysis (1)] |
| 10. | Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, Joensuu H, von Mehren M. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141-3147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 11. | Wu X, Ge Y, He X, Li J, Zhang J. Changes in imatinib plasma trough level during long-term treatment in patients with intermediate- or high-risk gastrointestinal stromal tumors: Relationship between covariates and imatinib plasma trough level. Front Surg. 2023;10:1115141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Yang ZR, Guo RJ, Niao YM, Ling HJ, Xu SJ, Wang YC, Lin T, Zhou YJ. [A study on the factors related to blood drug concentration in the treatment of gastrointestinal stromal tumors with imatinib mesylate]. Zhongliu. 2022;42: 533-541. [DOI] [Full Text] |
| 13. | Hospital Pharmacy Committee of Chinese Pharmaceutical and Association; Division of Therapeutic Drug Monitoring Chinese Pharmacological Society; Compilation Group of Chinese Expert Consensus on Therapeutic Drug Monitoring of Targeted Drugs for Gastrointestinal Stromal Tumor. [Chinese expert Consensus on therapeutic drug monitoring of targeted drugs for gastrointestinal stromal tumor]. Zhongguo Yiyuan Yaoxue Zazhi. 2021;41: 2041-2049. [DOI] [Full Text] |
| 14. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21609] [Article Influence: 1350.6] [Reference Citation Analysis (1)] |
| 15. | Precision Pharmacy Working Committee of China Pharmacist Association; Chinese Pharmacist Association Oncology Specialist Pharmacist Branch; the Writing Group of the Consensus of Chinese; Wei XH, Luan JJ, Li GH, Kong Y, Liu H, Jiang XH. [Consensus of Chinese experts on individualized medication management of imatinib for gastrointestinal stromal tumors]. Zhongguo Yaofang. 2024;35:257-270. [DOI] [Full Text] |
| 16. | Xia Y, Chen S, Luo M, Wu J, Cai S, He Y, Chen X, Zhang X. Correlations between imatinib plasma trough concentration and adverse reactions in Chinese patients with gastrointestinal stromal tumors. Cancer. 2020;126 Suppl 9:2054-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Huang FR, Sun LN, Qian Y, Zhang Q, Xu H, Wang YQ. [Effect of Different Doses of Imatinib on the Plasma Concentration and Incidence of Adverse Drug Reactions in Patients with Gastrointestinal Stromal Tumor]. Zhongguo Yaoye. 2021;30:41-44. |
| 18. | Yoo C, Ryu MH, Ryoo BY, Beck MY, Kang YK. Efficacy, safety, and pharmacokinetics of imatinib dose escalation to 800 mg/day in patients with advanced gastrointestinal stromal tumors. Invest New Drugs. 2013;31:1367-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Bouchet S, Poulette S, Titier K, Moore N, Lassalle R, Abouelfath A, Italiano A, Chevreau C, Bompas E, Collard O, Duffaud F, Rios M, Cupissol D, Adenis A, Ray-Coquard I, Bouché O, Le Cesne A, Bui B, Blay JY, Molimard M. Relationship between imatinib trough concentration and outcomes in the treatment of advanced gastrointestinal stromal tumours in a real-life setting. Eur J Cancer. 2016;57:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |