Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.103450
Revised: December 20, 2024
Accepted: January 14, 2025
Published online: March 15, 2025
Processing time: 84 Days and 0.6 Hours
Esophageal squamous-cell carcinoma (ESCC) is a highly aggressive cancer, predominantly affecting populations in Eastern Asia and parts of Africa. Its pathogenesis is influenced by both genetic and environmental factors. Despite recent therapeutic advances, survival rates remain dismal, underscoring an urgent need for novel therapeutic targets.
To investigate the role of hypoxia-inducible factor 1-alpha (HIF1A) in the progression of ESCC and its impact on the metabolic enzyme lactate dehydrogenase A (LDHA), which is crucial for the glycolytic pathway in hypoxic tumor environments.
Utilizing transcriptomic data from multiple public databases, we analyzed differential gene expression and conducted gene ontology and transcription factor network analyses. The regulatory impact of HIF1A on LDHA was specifically examined through integrative analysis with HIF1A ChIP-seq data and confirmed via siRNA-mediated knockdown experiments in ESCC cell lines.
Our findings reveal a significant upregulation of HIF1A in ESCC tissues, associated with poor prognosis. HIF1A directly regulates LDHA, enhancing glycolysis under hypoxic conditions and contributing to tumor aggressiveness. Knockdown of HIF1A in cell lines not only reduced LDHA expression but also altered key pathways related to cell cycle and apoptosis.
The critical role of the HIF1A-LDHA axis in ESCC highlights its potential as a therapeutic target, underscoring the need for future clinical trials to validate the efficacy of HIF1A inhibitors in enhancing treatment outcomes.
Core Tip: This study reveals that hypoxia-inducible factor 1-alpha (HIF1A) significantly influences the progression of esophageal squamous-cell carcinoma (ESCC) by regulating lactate dehydrogenase A (LDHA), which is crucial for glycolysis in hypoxic tumor environments. Our findings highlight the HIF1A-LDHA axis as a key contributor to metabolic reprogramming and tumor aggressiveness, presenting a potential target for therapeutic intervention. This insight could guide the development of HIF1A inhibitors to improve treatment outcomes in ESCC.
- Citation: Chen X, Liu HY, Zhou WB, Zhang LL, Huang J, Bao DW. Hypoxia-inducible factor 1-alpha and lactate dehydrogenase-A axis in metabolic changes and aggression in esophageal squamous-cell carcinoma. World J Gastrointest Oncol 2025; 17(3): 103450
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/103450.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.103450
Esophageal squamous-cell carcinoma (ESCC) represents one of the most prevalent and lethal malignancies worldwide, characterized by its rapid progression and poor prognosis. The incidence of ESCC varies geographically, with the highest rates observed in Eastern Asia and parts of Africa, a pattern that suggests both genetic and environmental contributors to its etiology[1-3]. Despite advancements in therapeutic strategies, the overall survival rate for ESCC remains dismal, highlighting an urgent need for novel therapeutic targets and a deeper understanding of its molecular underpinnings[4].
The pathogenesis of ESCC is a multifactorial process influenced by genetic, epigenetic, and environmental factors. Among these, the role of transcription factors (TFs) in regulating gene expression patterns critical for cancer development and progression has garnered considerable interest. TFs such as p53, NF-κB, and AP-1 have been extensively studied for their roles in ESCC[5,6]. Hypoxia-inducible factor 1-alpha (HIF1A), a master regulator of cellular response to hypoxia, has emerged as a significant player in the oncogenic process, particularly in solid tumors where hypoxic conditions are common. HIF1A is a subunit of the TF complex HIF-1, which is stabilized under hypoxic conditions and initiates the transcription of various genes involved in crucial aspects of cancer biology, including angiogenesis, metabolism, cell survival, and invasion[7-9]. HIF1A has been reported in some studies of ESCC; however, its downstream regulatory factors and role in the progression of ESCC remain largely unknown[10]. Lactate dehydrogenase A (LDHA) is an essential enzyme that catalyzes the final step of glycolysis, playing a pivotal role in enhancing glycolytic efficiency in tumor cells while reducing their dependence on oxygen. LDHA is highly expressed in various cancer cells and is recognized as a biomarker for multiple malignant tumors, including lymphoma, prostate cancer, renal cell carcinoma, melanoma, and papillary thyroid cancer[11,12]. However, the physiological implications of LDHA-mediated metabolic reprogramming in ESCC have yet to be elucidated[13].
Here, our research identified an upregulation of HIF1A across multiple ESCC transcriptomic databases, with patients exhibiting high HIF1A expression showing poorer prognosis. By integrating transcriptomic analysis with data from HIF1A ChIP-seq databases, we discovered that HIF1A specifically regulates LDHA-a key enzyme in the glycolytic pathway. This suggests a crucial role for HIF1A in adapting tumor cells to their hypoxic microenvironment, which may not only affect tumor survival but could also contribute to the aggressive nature of the disease. Our findings extend the understanding of HIF1A's involvement in cancer progression by elucidating its regulation of glycolysis through LDHA.
The processing of bulk RNA-seq data (GSE23400, GSE17351, GSE20347)[14-16] commenced with the initial preprocessing of the raw sequencing outputs. Initially, the quality of raw sequence reads was assessed using the FastQC tool, which screens for and excludes reads below a specific Phred quality threshold. This preliminary step ensures the exclusion of substandard reads from the analysis. Subsequently, sequence reads were further refined by removing adapter sequences and regions of low complexity with the aid of Trimmomatic software, thus retaining only high-quality reads for further analysis. Following quality refinement, the remaining high-quality reads were aligned to the human reference genome (hg19) utilizing the HISAT2 aligner, selected for its efficiency in processing extensive datasets and its precision in mapping reads against the genome. After alignment, the quantification of gene expression was carried out using HTSeq-count, which assigns and counts reads to their respective genes based on the alignment data. To address potential biases arising from variations in library size and gene length, which could skew the interpretation of raw read counts, normalization was performed using the DESeq2 software. This step is critical as DESeq2 adjusts read counts to facilitate accurate comparisons of gene expression levels across different samples, ensuring that subsequent differential expression analysis accurately reflects true biological variations rather than methodological discrepancies.
To identify genes with significant changes in expression under varying experimental conditions, we conducted differential expression analysis. This process entails applying a statistical model to the normalized count data. Specifically, we employed the negative binomial model via the DESeq2 software, which is adept at handling count-based expression data. During this analysis, genes were categorized as differentially expressed genes (DEGs) if they met specific criteria: An absolute log2 fold change greater than 0.25 and an adjusted p-value less than 0.05. These thresholds ensure that only genes with statistically significant and biologically relevant changes in expression are considered as DEGs.
For the identification and functional categorization of genes based on their biological processes, cellular components, and molecular functions, we utilized the Metascape web-based platform (http://metascape.org). This analysis facilitates the interpretation of gene sets within the context of known biological responses and pathways.
To analyze TF regulatory networks, we used RcisTarget to identify TF binding motifs enriched near our genes of interest. The input gene sets for this analysis were derived from our initial differential expression studies, identifying genes significantly altered in ESCC compared to normal tissues. Using these gene sets, we applied RcisTarget to scan for TF binding motifs within the genomic regions proximal to the upregulated and downregulated genes. RcisTarget assesses motif enrichment and calculates scores to quantify the potential regulatory impact of various TFs on these gene sets. The enrichment scores are crucial for identifying which TFs are most likely to influence the expression patterns observed in ESCC. After identifying enriched motifs and associated TFs, the data were used to construct detailed TF regulatory networks. These networks map the predicted interactions between TFs and their target genes, highlighting potential regulatory pathways activated or repressed in ESCC. For visualization of these regulatory networks, we employed Cytoscape, which facilitated the graphical representation of the connections and interactions within the network, enhancing our ability to interpret and present the complex interactions at play.
Human participant project in this research was approved by the Institutional Ethics Committee of Huaian Hospital of Huaian City (KY-2022-014-01). Informed consent from each patient has been obtained by the principal investigator. The principal investigator has confirmed these participants meet all inclusion criteria after obtained written informed consent.
Between 2022 and 2024, biopsy samples from patients diagnosed with ESCC were collected at the Department of Radiation Oncology, Huaian Hospital, Huaian City. The cohort consisted of 21 individuals (14 males and 7 females), aged between 28 and 62 years, all of whom are Han Chinese locals. These patients had not received any surgical interventions prior to the biopsy. Histological examinations were conducted to confirm ESCC in the collected samples, which were immediately preserved in liquid nitrogen for future analysis.
The human esophageal squamous carcinoma cell lines KYSE-150 was provided by the Chinese Academy of Sciences Cell Bank Type Culture Collection (Shanghai, China). SiHIF1A and si-non-target-control (siNTC) primers were synthesized by Qsingke Biological Company (Shanghai, China). 50nM siRNA was transfected with Lipofectamine™ 3000 transfection reagent (Invitrogen™, L3000150) in Opti-MEM medium (cat. no. 31985070, Thermo Fisher Scientific) for 12 hours before replacing fresh medium. Cells were sampled for analysis two days after intervention. The LDHA promoter was cloned from the total DNA of KYSE-150 cells and constructed into the pGL3 vector with a rapid DNA ligation kit (beyotime, D7001M). Site-directed mutations within the promoter were generated using a Fast MultiSite Mutagenesis System (Transgen, Cat. No# FM201).
The tumor tissue sections (5 μm) were subjected to antigen retrieval, blocked with 5% donkey serum for 1 hour, and incubated with primary antibody (rabbit anti-HIF1α, 1: 200 dilution, abcam, ab92498) overnight at 4 °C and then with HRP-conjugated IgG (Boster, Wuhan, China) for 1 hour. Further, the sections were exposed to DAB (Boster) for development. After counterstained with hematoxylin, the sections were dehydrated and subjected to microscopic examination after mounting.
Frozen samples underwent physical homogenization, followed by total RNA extracted and cDNA synthesis using the high-capacity reverse transcription kit (Vazyme). Subsequently, the qPCR reactions were conducted using the Bio-rad platform and assays were performed on ABI QuantStudio 5 (Applied Biosystems, Thermo-Fisher).
Data are statistically analyzed using the GraphPad Prism 8.0 software. Results are presented as the mean ± SEM. Statistical analyses were performed using a two-tailed Student’s t-test to compare the differences between different groups. P values < 0.05 was considered statistically significant.
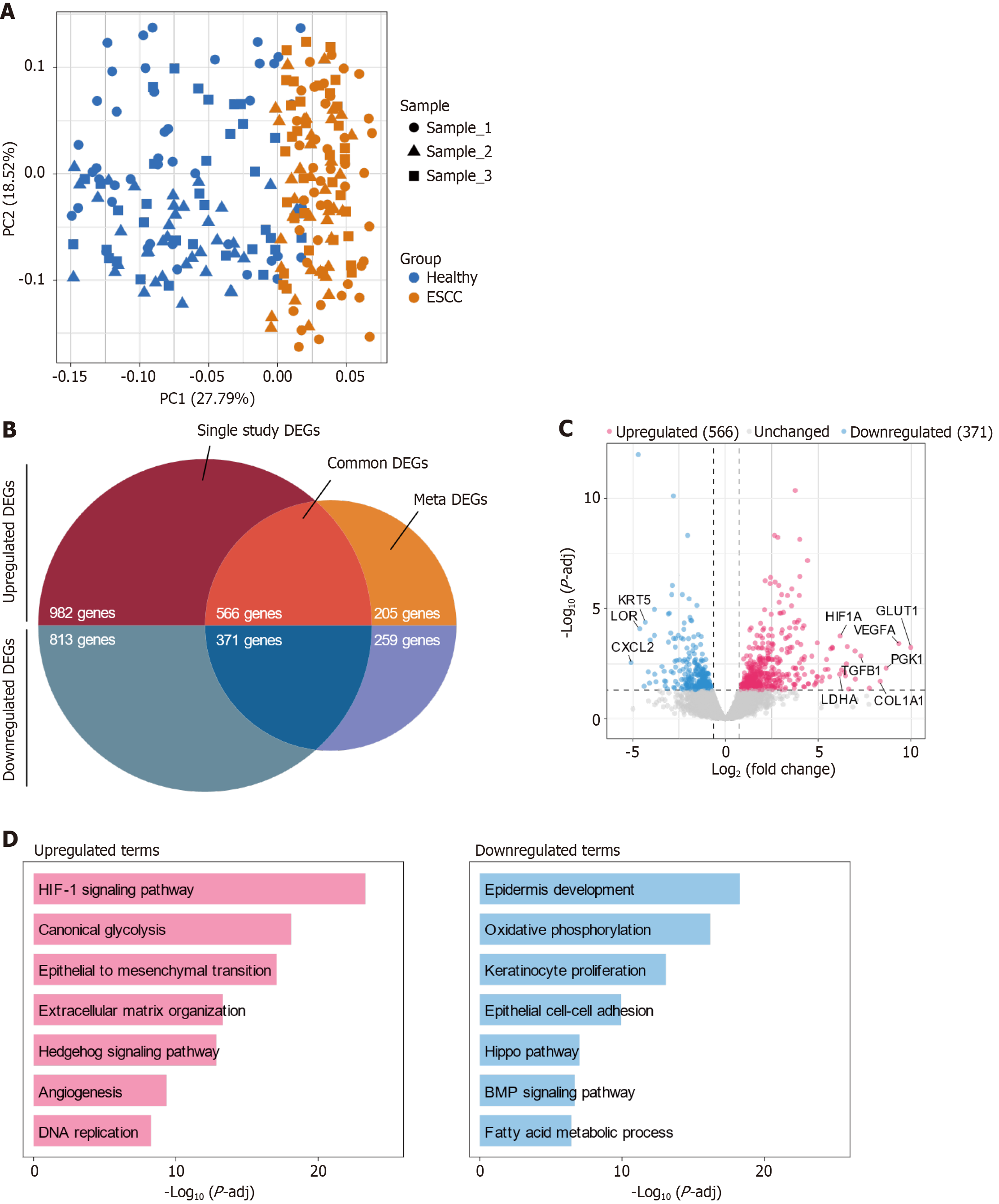
In this study, we leveraged principal component analysis (PCA) to explore the transcriptional landscape of ESCC relative to non-cancerous tissue. Our data set included 115 samples from each group, sourced from multiple public databases. The PCA revealed a distinct separation between the ESCC and healthy groups, with minimal batch effects, underscoring the robustness of our data collection and preprocessing methods (Figure 1A).
Further analysis identified significant differences in gene expression between the two groups. A total of 566 genes were consistently upregulated, while 371 were downregulated across the samples (Figure 1B). This meta-analysis reinforces the validity of these DEGs as critical players in ESCC pathogenesis.
Among the upregulated genes, notable ones included GLUT1, PGK1, VEGFA, COL1A1, and TGFB1. The upregulation of GLUT1 and PGK1 is indicative of enhanced glycolytic activity, a hallmark of cancer cell adaptation to hypoxic conditions and a mechanism for sustained energy production. Elevated levels of VEGFA contribute to angiogenesis, facilitating tumor growth and metastasis. The increased expression of COL1A1 and TGFB1 is associated with remodeling of the tumor microenvironment and extracellular matrix, processes that are crucial for tumor invasiveness and metastatic potential (Figure 1C). Conversely, the most significantly downregulated genes included KRT5, LOR, and CXCL2. These genes are generally involved in maintaining cellular integrity, signaling, and inflammatory responses. Their downregulation may play roles in tumor immune evasion and disruption of tissue architecture.
Our gene ontology analysis highlighted several key pathways differentially regulated in ESCC (Figure 1D). In our comprehensive analysis of ESCC, the transcriptomic data revealed significant modulation of pathways that underscore the tumor's complex adaptation to its microenvironment and altered cellular metabolism. The upregulation of the HIF-1 signaling pathway reflects the cellular response to hypoxia, enhancing survival, angiogenesis, and metabolic reprogramming to sustain rapid tumor growth under oxygen-deprived conditions. Similarly, the increase in canonical glycolysis, or the Warburg effect, is characteristic of cancer cells prioritizing energy production through glycolysis over oxidative phosphorylation, even in the presence of oxygen. This metabolic shift provides the necessary ATP and metabolic intermediates for supporting rapid proliferation. The activation of the epithelial to mesenchymal transition (EMT) pathway, extracellular matrix organization, and Hedgehog signaling are integral to promoting tumor invasion and metastasis, facilitating changes in cellular adhesion, migration capabilities, and the structural dynamics of the tumor microenvironment. Additionally, the upregulation of angiogenesis supports these processes by ensuring an adequate blood supply, which is crucial for tumor growth and the delivery of nutrients. Conversely, the downregulation of pathways such as epidermis development, oxidative phosphorylation, and keratinocyte proliferation indicates a significant deviation from normal epithelial functions and cellular energetics, which typically favor differentiation and barrier function over proliferation. The suppression of epithelial cell-cell adhesion and the Hippo pathway, which are critical for contact inhibition and growth control, further enables uncontrolled cellular proliferation and evasion of growth suppressive signals. Similarly, reductions in Bone Morphogenetic Protein (BMP) signaling and fatty acid metabolism reflect a reprogramming of signaling cascades and metabolic pathways that normally restrain cancer progression but have been subverted to support tumor development and survival. Together, these changes in upregulated and downregulated pathways provide a comprehensive view of the molecular reorganization occurring in ESCC, offering insights into potential therapeutic targets that could disrupt these critical adaptations and mechanisms of disease progression.
Together, these findings illustrate the complex reorganization of gene expression and cellular pathways in ESCC, highlighting potential targets for therapeutic intervention and providing a deeper understanding of the disease's molecular basis.
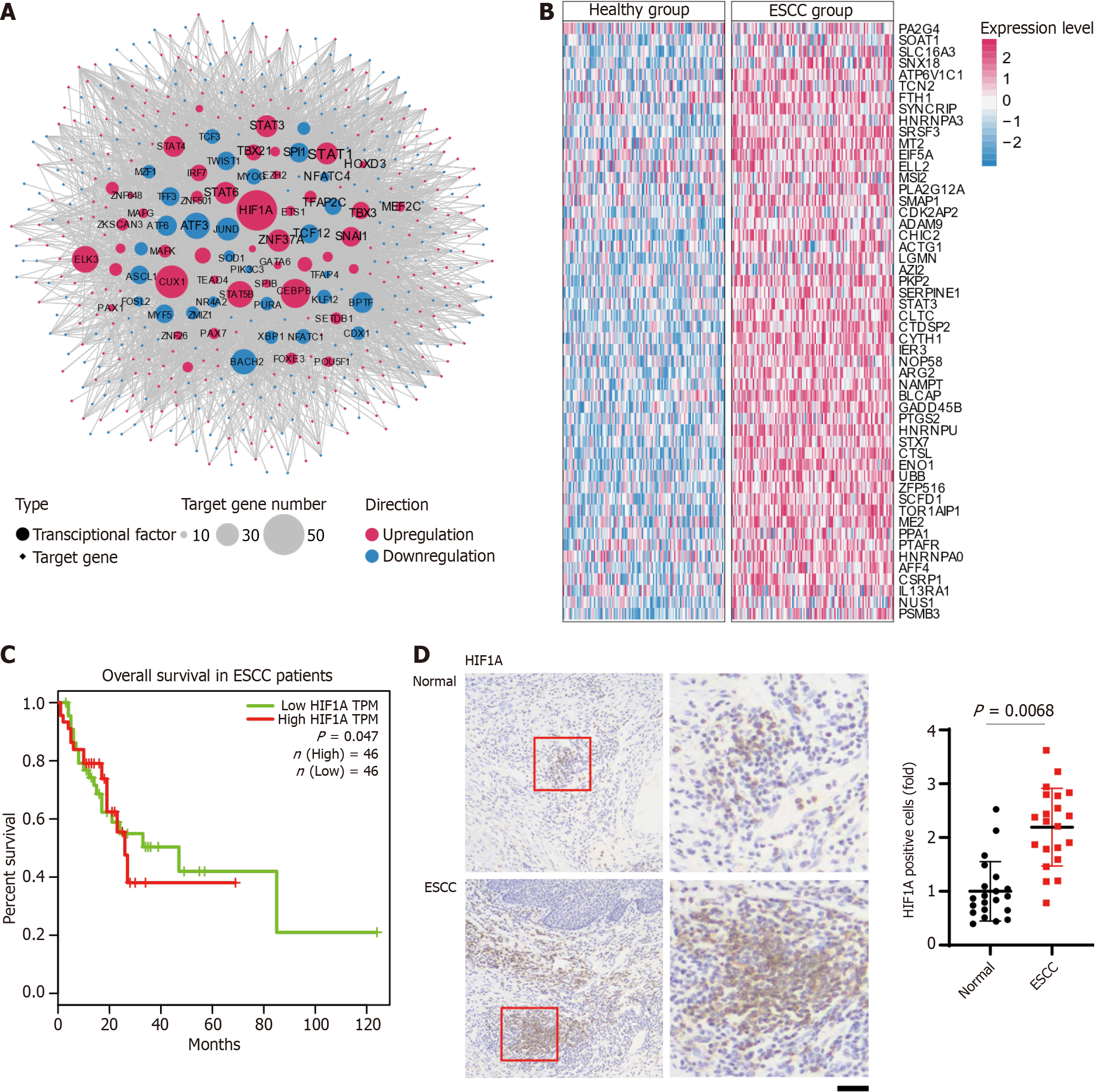
Building on our initial findings, we next examined the TF regulatory networks. Our analysis identified significant alterations with 31 TFs found to be upregulated and 43 downregulated in ESCC, reflecting crucial changes in transcriptional control mechanisms. Prominently upregulated TFs, including HIF1A, CUX1, STAT5B, CEBPB, ELK1, and STAT6, are pivotal in regulating numerous target genes, demonstrating their crucial roles in cancer progression (Figure 2A).
HIF1A emerges as a key regulator, controlling 52 target genes and playing an essential role in adapting to the hypoxic tumor environment, a common challenge in solid tumors that profoundly impacts tumor progression and resistance to therapy. Its extensive regulatory network supports vital processes such as angiogenesis, glucose metabolism, and cellular survival, which are crucial for the aggressive behavior of ESCC. Moreover, TFs such as CUX1 and STAT5B also exhibit extensive regulatory effects. CUX1 is linked to cell cycle progression and DNA repair, potentially contributing to unchecked proliferation characteristic of cancerous tissues. STAT5B, involved in cytokine signaling and immune response modulation, may foster an environment that suppresses immune surveillance and promotes inflammatory responses aiding tumor growth.
In contrast, the downregulation of TFs such as ATF3, JUND, BACH2, and TCF12, typically associated with apoptosis and cellular stress responses, reflects a strategic adaptation by the tumor to support its growth and survival under adverse conditions. ATF3 is known for its role in regulating DNA damage responses and apoptosis, and its downregulation may help tumor cells evade programmed cell death. JUND, part of the AP-1 complex, often contributes to cellular proliferation and survival; its reduction could disrupt normal cell cycle control mechanisms, facilitating unchecked cellular growth. BACH2 plays a role in regulating oxidative stress and the immune response, and its lowered expression may reduce the tumor's susceptibility to immune-mediated destruction. Lastly, TCF12, involved in developmental processes and cellular differentiation, when downregulated, may contribute to the loss of cell identity and enhanced dedifferentiation, commonly observed in aggressive tumors.
The differential expression patterns between healthy and ESCC tissues are further analyzed, emphasizing the significant impact of these transcriptional changes (Figure 2B). Notably, genes like VEGFA and GLUT1, upregulated by HIF1A, are key contributors to ESCC pathology. VEGFA promotes vascular growth, essential for tumor sustenance, while GLUT1 enhances glucose uptake, meeting the increased metabolic demands of ESCC cells. The Kaplan-Meier survival analysis demonstrated a statistically significant difference in overall survival between ESCC patients with high and low expression levels of HIF1A. Patients with high HIF1A transcript per million exhibited decreased survival over the 120-month follow-up period (Figure 2C). The survival curves indicate that high HIF1A expression may be associated with poorer prognosis in ESCC patients. Immunohistochemical staining revealed a higher percentage of HIF1A-positive cells were observed in ESCC tissues compared to normal controls. Quantitative analysis confirmed a significant increase in the number of HIF1A-positive cells in ESCC, suggesting a potential role of HIF1A in the pathogenesis of ESCC (Figure 2D).
These insights into the transcriptional regulation within ESCC clarify the complexity of gene expression control and identify potential therapeutic targets. By pinpointing the specific roles these TFs play in ESCC's molecular pathology, targeted interventions can be designed to disrupt these regulatory networks, offering new avenues for treatment and potentially halting disease progression.
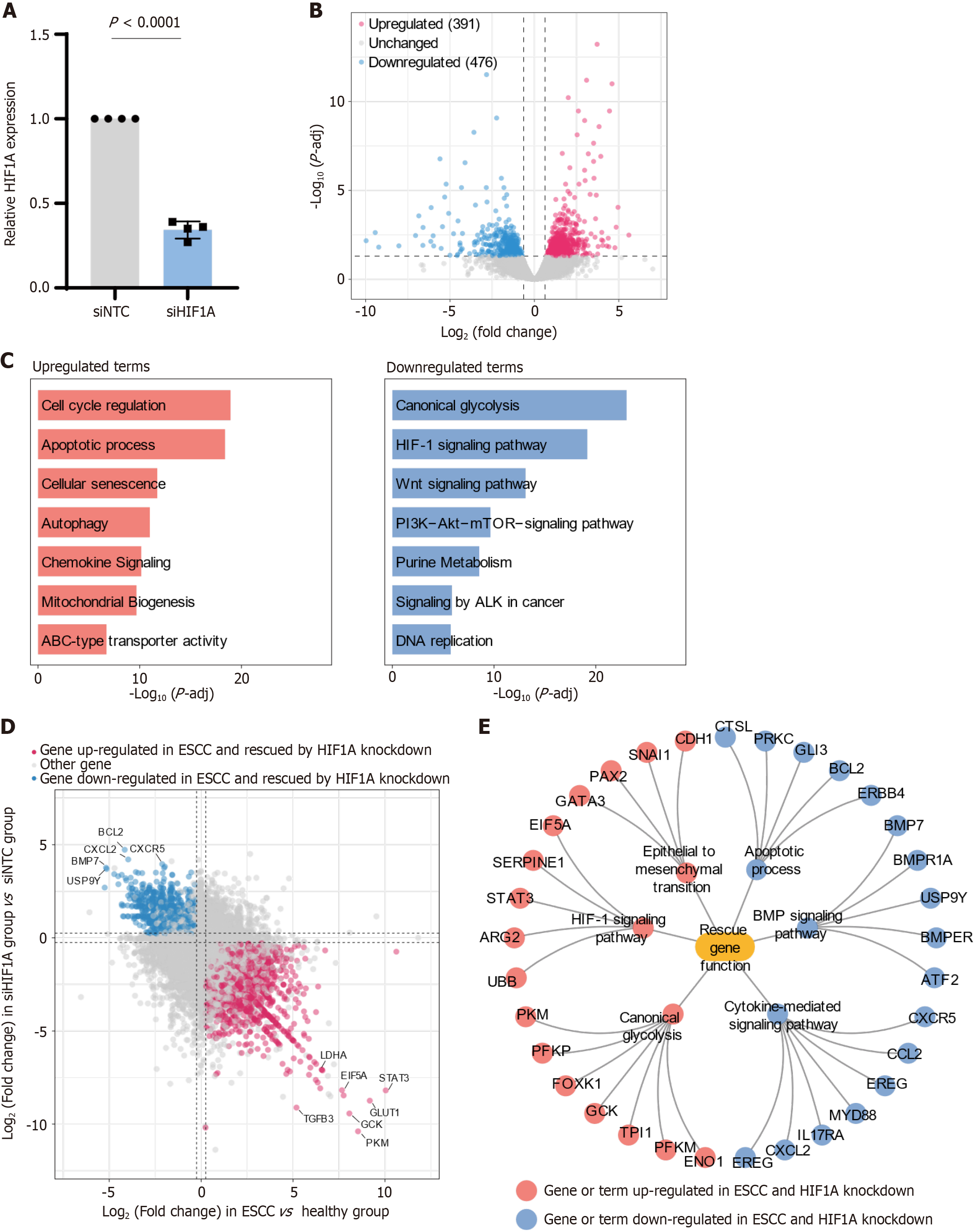
Continuing from our exploration of TF networks in ESCC, we conducted targeted experiments to knock down HIF1A in ESCC cell lines, aiming to elucidate its pivotal role in gene regulation and pathway modulation within the tumor environment. Our initial confirmation of the knockdown's efficacy showed a significant decrease in HIF1A expression levels in the siRNA-treated cells compared to siNTC treated cells, as evidenced by reduced mRNA levels (Figure 3A).
Subsequent differential gene expression analysis following HIF1A knockdown identified a substantial number of affected genes, with 391 upregulated and 476 downregulated, suggesting a broad regulatory role of HIF1A in ESCC (Figure 3B). This extensive modulation of gene expression underscores HIF1A’s critical function in maintaining the malignant phenotype of ESCC cells.
The pathway analysis of these DEGs post-knockdown provided deeper insights into the cellular processes and biological functions influenced by HIF1A (Figure 3C). Notably, pathways associated with cell cycle regulation were significantly upregulated, indicating a potential reduction in cancer cell proliferation as HIF1A usually promotes progression through the cell cycle checkpoints. Additionally, the enhancement of pathways related to the apoptotic process suggests a reactivation of programmed cell death mechanisms in cancer cells, which are often inhibited in ESCC to promote survival. Increased activity in cellular senescence pathways could indicate a move towards a less aggressive cellular state, potentially inhibiting the proliferation and spread of cancer cells.
On the other hand, the downregulation of key metabolic and signaling pathways such as canonical glycolysis points to a disruption in the metabolic adaptations cancer cells make in response to hypoxic conditions, which are typically mediated by HIF1A to enhance survival and growth under such stress. The observed reduction in HIF-1 signaling pathway activity aligns with diminished cellular responses to hypoxia, potentially affecting tumor angiogenesis and growth dynamics. Furthermore, a decrease in Wnt signaling pathway activity, vital for maintaining cellular proliferation and stemness, may impair the cancer cells' ability to invade and metastasize.
We also conducted a comparative analysis by integrating the effects of HIF1A knockdown with changes observed in gene expression during the progression of ESCC. This analysis revealed a subset of "rescue genes"-genes whose dysregulated expression in ESCC was reversed upon HIF1A knockdown. Specifically, 231 genes previously found upregulated in ESCC were effectively 'rescued' or downregulated following HIF1A knockdown, while 112 genes downregulated in ESCC were upregulated upon knockdown, highlighting HIF1A’s extensive involvement in gene regulation in cancer (Figure 3D).
Furthermore, a detailed network analysis (Figure 3E) was performed to elucidate the impact on several critical pathways involved in ESCC pathology. The results from this analysis provide insights into how the modulation of HIF1A influences specific cellular mechanisms, particularly through the regulation of rescue genes, and the potential therapeutic implications of reversing these pathways. The knockdown of HIF1A significantly impacts the EMT process, which is essential for cancer cell invasion and metastasis. By reducing the expression of EMT-promoting genes, the HIF1A knockdown likely impedes the cells' ability to invade and form metastases, suggesting a decrease in tumor aggressiveness and potentially enhancing the effectiveness of treatments aimed at containing the tumor within the primary site. Additionally, HIF1A typically acts to suppress apoptosis in tumor cells, aiding their survival. The downregulation of HIF1A reactivates apoptotic pathways, leading to increased cancer cell death. This reactivation not only limits tumor growth but also may improve the responsiveness of ESCC cells to chemotherapeutic agents that induce apoptosis. The BMP signaling pathway, involved in cellular differentiation and the maintenance of cancer cell stemness, is also disrupted by HIF1A knockdown. This disruption could lead to reduced self-renewal capabilities and potentially induce differentiation, diminishing the population of cancer stem cells, which are key drivers of tumorigenesis and recurrence, thus potentially leading to improved patient outcomes. Furthermore, cancer cells typically rely on glycolysis for energy production even under aerobic conditions, a phenomenon known as the Warburg effect, which is enhanced by HIF1A. The knockdown of HIF1A likely results in decreased glycolytic activity by modulating critical rescue genes, compelling cancer cells to revert to oxidative phosphorylation. This metabolic shift can reduce the energy supply necessary for rapid cancer cell proliferation, potentially slowing tumor growth and making cells more susceptible to metabolic pathway-targeted treatments. Lastly, HIF1A is crucial in regulating the immune environment by modulating cytokine production and secretion. Its knockdown might decrease the production of pro-inflammatory cytokines, including those regulated through rescue genes, that support tumor growth and survival, enhancing the potential efficacy of immunotherapeutic strategies by altering the tumor microenvironment to be less conducive to cancer progression.
These findings demonstrate HIF1A's profound influence on the molecular dynamics of ESCC and underscore its potential as a valuable therapeutic target. By disrupting HIF1A expression, it might be possible to significantly alter the course of ESCC, providing new opportunities for developing effective treatment strategies that could improve patient outcomes in this aggressive cancer type.
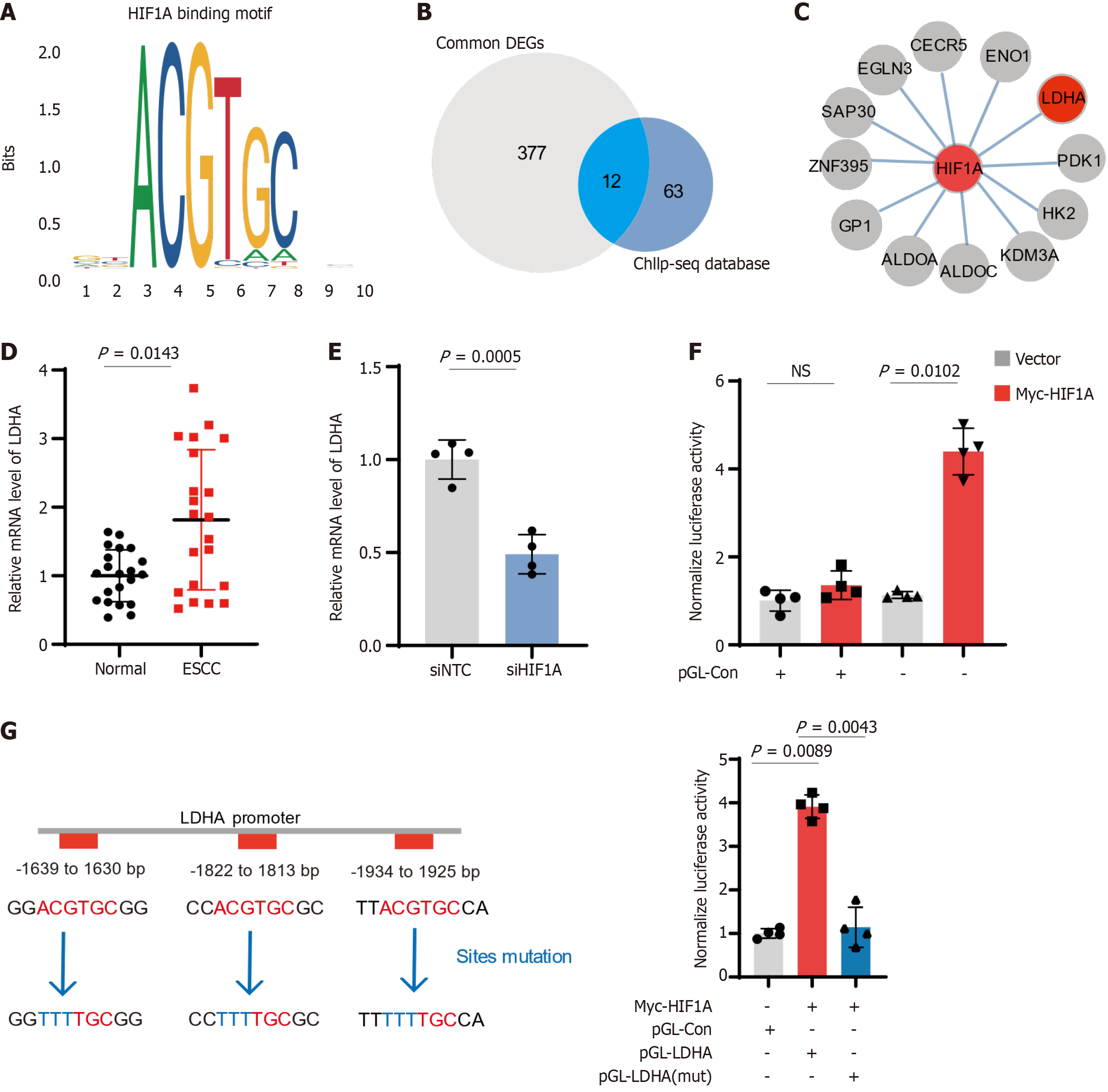
Considering that the role of HIF1A in modulating cellular functions in ESCC has been reported in several studies, we aimed to further explore the downstream factors regulated by HIF1A in ESCC and elucidate the molecular mechanisms underlying its role in tumor progression. By overlapping DEGs from public databases with those identified following HIF1A knockdown, we identified common DEGs during the development of ESCC. Furthermore, using clustering analysis based on the HIF1A binding motif and public HIF1A ChIP-seq databases[17,18], we identified 12 common downstream genes, including LDHA and PDK1 (Figure 4A-C). LDHA, a key enzyme in the glycolytic pathway, has not been previously reported to be regulated by HIF1A in ESCC. We analyzed LDHA expression in ESCC patients and siHIF1A cell lines and found that LDHA is upregulated in ESCC and downregulated following HIF1A knockdown (Figure 4D and E), highlighting HIF1A's control over LDHA expression. Luciferase assays confirmed the functional impact of HIF1A on LDHA promoter activity (Figure 4F), where HIF1A enhanced promoter activity, and mutations at the HIF1A binding sites abolished this effect (Figure 4G). Overall, these results confirm the critical role of HIF1A in regulating glycolytic metabolism in ESCC through LDHA, potentially contributing to the metabolic reprogramming associated with cancer progression.
Our study elucidates the pivotal role of HIF1A in the pathogenesis of ESCC, particularly highlighting its influence on cellular adaptation to hypoxic conditions and metabolic reprogramming. The upregulation of HIF1A identified across multiple ESCC transcriptomes correlates with poor prognosis, underlining its potential as a prognostic biomarker and a therapeutic target. By integrating transcriptomic data with HIF1A ChIP-seq analysis[17,18], we have demonstrated that HIF1A directly regulates LDHA, a key enzyme in glycolysis, thus linking hypoxic response to metabolic adaptations essential for tumor survival and progression.
The regulation of LDHA by HIF1A not only supports the Warburg effect—an increase in glycolysis in the presence of oxygen-but also facilitates the aggressive phenotype of ESCC by enhancing energy production, which is crucial for rapid tumor growth and sustenance under hypoxic tumor microenvironments. This metabolic shift is likely a survival strategy by tumor cells to meet the increased demands of proliferation and invasiveness[19,20]. Our findings suggest that targeting HIF1A-mediated pathways could disrupt these adaptations, potentially stalling tumor progression and reducing metastatic potential.
The influence of HIF1A extends beyond metabolic regulation, impacting pathways involved in angiogenesis and the EMT, both of which are critical for tumor invasiveness and metastasis[21]. Our results indicate that HIF1A enhances the expression of genes associated with EMT and angiogenesis, thus facilitating a microenvironment conducive to ESCC progression. Therapeutically targeting HIF1A could therefore not only impair tumor metabolic reprogramming but also inhibit other pathways essential for tumor growth and spread.
Furthermore, our study highlights the complex interaction between HIF1A and its downstream targets, which could inform the development of multi-targeted therapeutic strategies. For instance, the simultaneous targeting of HIF1A and LDHA could be more effective given their roles in sustaining the metabolic and invasive properties of tumor cells. This approach could be particularly advantageous in overcoming the redundancies of cellular pathways that often lead to therapy resistance. The identification of HIF1A's role in ESCC also prompts further investigation into its interaction with other TFs and signaling pathways within the tumor microenvironment. Future studies could explore the potential synergistic effects of HIF1A inhibition combined with other therapeutic modalities, such as chemotherapy, radiation, or immunotherapy. Additionally, exploring the differential roles of HIF1A in ESCC subtypes or in response to various microenvironmental stresses could uncover more refined therapeutic targets.
While our study has elucidated the role of the HIF1A-LDHA regulatory axis in the context of ESCC, it is imperative to recognize the limitations inherent in our current approach. First, further studies are needed to fully decipher the HIF1A-LDHA regulation within the cellular milieu. Understanding the intricate interactions and feedback mechanisms that modulate this axis could reveal additional targets for therapeutic intervention and provide a more comprehensive map of tumor biology. Moreover, our study primarily utilizes in vitro and transcriptomic data to infer the function of the HIF1A-LDHA axis[21]. Consequently, in vivo studies, particularly using mouse models, are crucial to confirm these findings and to provide a clearer picture of how this regulatory axis influences tumor progression in a more complex biological system[22]. Such studies could also facilitate the evaluation of potential therapeutic strategies in a pre-clinical setting, offering insights into the physiological and systemic effects of targeting this pathway. Additionally, recent studies have highlighted the potential of protease inhibitors, such as Carfilzomib, to modulate LDHA-mediated metabolic reprogramming[23]. These findings suggest that inhibitors targeting the HIF1A-LDHA pathway could be particularly effective. Investigating these inhibitors could open up new avenues for treatment, potentially leading to the development of more effective therapeutic strategies that exploit the metabolic vulnerabilities of cancer cells.
In conclusion, our research underscores the crucial role of HIF1A-LDHA axis in ESCC, significantly influencing tumor behavior and therapeutic resistance. Targeting HIF1A, especially alongside other pathways, offers a promising strategy to enhance treatment efficacy, warranting further clinical exploration.
The HIF1A-LDHA axis is critical for the metabolic reprogramming and progression of ESCC, representing a potential target for therapeutic intervention.
The authors would like to thank all staff and volunteers who contributed to this study.
| 1. | Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol. 2021;18:432-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 193] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 2. | Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154:360-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1149] [Article Influence: 164.1] [Reference Citation Analysis (1)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15261] [Article Influence: 3052.2] [Reference Citation Analysis (4)] |
| 4. | Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 996] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 5. | Tu Y, Tan F, Zhou J, Pan J. Pristimerin targeting NF-κB pathway inhibits proliferation, migration, and invasion in esophageal squamous cell carcinoma cells. Cell Biochem Funct. 2018;36:228-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Xia T, Meng L, Xu G, Sun H, Chen H. TRIM33 promotes glycolysis through regulating P53 K48-linked ubiquitination to promote esophageal squamous cell carcinoma growth. Cell Death Dis. 2024;15:740. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Wang S, Hu H, Li X. A systematic study of HIF1A cofactors in hypoxic cancer cells. Sci Rep. 2022;12:18962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4764] [Cited by in RCA: 5010] [Article Influence: 227.7] [Reference Citation Analysis (0)] |
| 9. | Qannita RA, Alalami AI, Harb AA, Aleidi SM, Taneera J, Abu-Gharbieh E, El-Huneidi W, Saleh MA, Alzoubi KH, Semreen MH, Hudaib M, Bustanji Y. Targeting Hypoxia-Inducible Factor-1 (HIF-1) in Cancer: Emerging Therapeutic Strategies and Pathway Regulation. Pharmaceuticals (Basel). 2024;17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 10. | Hu X, Lin J, Jiang M, He X, Wang K, Wang W, Hu C, Shen Z, He Z, Lin H, Wu D, Wang M. HIF-1α Promotes the Metastasis of Esophageal Squamous Cell Carcinoma by Targeting SP1. J Cancer. 2020;11:229-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Feng Y, Xiong Y, Qiao T, Li X, Jia L, Han Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018;7:6124-6136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 453] [Cited by in RCA: 429] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 12. | Yao F, Zhao T, Zhong C, Zhu J, Zhao H. LDHA is necessary for the tumorigenicity of esophageal squamous cell carcinoma. Tumour Biol. 2013;34:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2023] [Cited by in RCA: 2436] [Article Influence: 174.0] [Reference Citation Analysis (0)] |
| 14. | Hu N, Clifford RJ, Yang HH, Wang C, Goldstein AM, Ding T, Taylor PR, Lee MP. Genome wide analysis of DNA copy number neutral loss of heterozygosity (CNNLOH) and its relation to gene expression in esophageal squamous cell carcinoma. BMC Genomics. 2010;11:576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | Hyland PL, Zhang H, Yang Q, Yang HH, Hu N, Lin SW, Su H, Wang L, Wang C, Ding T, Fan JH, Qiao YL, Sung H, Wheeler W, Giffen C, Burdett L, Wang Z, Lee MP, Chanock SJ, Dawsey SM, Freedman ND, Abnet CC, Goldstein AM, Yu K, Taylor PR. Pathway, in silico and tissue-specific expression quantitative analyses of oesophageal squamous cell carcinoma genome-wide association studies data. Int J Epidemiol. 2016;45:206-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Lee JJ, Natsuizaka M, Ohashi S, Wong GS, Takaoka M, Michaylira CZ, Budo D, Tobias JW, Kanai M, Shirakawa Y, Naomoto Y, Klein-Szanto AJ, Haase VH, Nakagawa H. Hypoxia activates the cyclooxygenase-2-prostaglandin E synthase axis. Carcinogenesis. 2010;31:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Rouillard AD, Gundersen GW, Fernandez NF, Wang Z, Monteiro CD, McDermott MG, Ma'ayan A. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database (Oxford). 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1032] [Cited by in RCA: 1027] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 18. | Zhang Q, Liu W, Zhang HM, Xie GY, Miao YR, Xia M, Guo AY. hTFtarget: A Comprehensive Database for Regulations of Human Transcription Factors and Their Targets. Genomics Proteomics Bioinformatics. 2020;18:120-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 302] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 19. | Cui XG, Han ZT, He SH, Wu XD, Chen TR, Shao CH, Chen DL, Su N, Chen YM, Wang T, Wang J, Song DW, Yan WJ, Yang XH, Liu T, Wei HF, Xiao J. HIF1/2α mediates hypoxia-induced LDHA expression in human pancreatic cancer cells. Oncotarget. 2017;8:24840-24852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 20. | Zhou Y, Huang Y, Hu K, Zhang Z, Yang J, Wang Z. HIF1A activates the transcription of lncRNA RAET1K to modulate hypoxia-induced glycolysis in hepatocellular carcinoma cells via miR-100-5p. Cell Death Dis. 2020;11:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 21. | Tam SY, Wu VWC, Law HKW. Hypoxia-Induced Epithelial-Mesenchymal Transition in Cancers: HIF-1α and Beyond. Front Oncol. 2020;10:486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 200] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 22. | Si X, Su X, Lin W, Xu J, Huang W, Chen F, Huang Z, Lin J, Chen Z. Circ_ZNF778_006 promoted ESCC progression by upregulating HIF-1α expression via sponging miR-18b-5p. Sci Rep. 2023;13:19363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Chen L, Shi H, Zhang W, Zhu Y, Chen H, Wu Z, Qi H, Liu J, Zhong M, Shi X, Wang T, Li Q. Carfilzomib suppressed LDHA-mediated metabolic reprogramming by targeting ATF3 in esophageal squamous cell carcinoma. Biochem Pharmacol. 2024;219:115939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |