Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.103328
Revised: December 26, 2024
Accepted: January 14, 2025
Published online: March 15, 2025
Processing time: 91 Days and 7.2 Hours
Breast cancer (BC) metastasis to the gastrointestinal tract is uncommon, colonic metastasis from BC (CMBC) is even rarer.
This report describes a 44-year-old female patient with metastatic triple-negative BC in the ascending colon who underwent laparoscopic radical right hemico
CMBC should be highly cautious in patients with a previous history of BC, especially triple-negative BC, and further examination to aid in diagnosis.
Core Tip: This report describes a 44-year-old female patient with metastatic ductal breast cancer (BC) in the ascending colon who underwent laparoscopic radical right hemicolectomy. Colonic metastasis from BC (CMBC) is extremely rare. The case is presented along with a literature review. The results of our study remaindered that CMBC should be highly cautious in patients with a previous history of breast carcinoma, especially triple negative BC. Attention should be paid to the gastrointestinal symptoms during follow-up for BC. A detailed examination including immunohistochemical analysis of endoscopic biopsies prior to surgery should be performed.
- Citation: Wang BQ, Fang XG, Xiang BH, Wang XJ, Cao M, Zhuang CL, Liu ZC, Wang Z. Metastasizing to the colon from triple-negative breast cancer: A case report and review of literature. World J Gastrointest Oncol 2025; 17(3): 103328
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/103328.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.103328
Breast cancer (BC) is the most common malignancy among women, with nearly 2090000 new cases diagnosed each year[1]. Triple-negative BC (TNBC) accounts for 10%–20% of all cases. TNBC is a subtype of breast tumor, accounting for 10%–20% of all cases, and it lacks the expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER-2). Compared to hormone-sensitive subtypes, TNBC has a higher recurrence rate, an increased risk of visceral metastasis, and a poorer prognosis[2]. TNBC also tends to be more aggressive and difficult to diagnose by conventional imaging[3]. Though BC is the second leading cause of mortality in women, recent advancements in systemic treatments, such as surgical resection, chemotherapy, radiotherapy, and hormonal therapy, have remarkably improved its prognosis compared to other types of cancer[4]. Approximately 5%-10% of diagnosed BCs may metastasize, and at least 20%-50% of patients experience metastasis at some point during their lifetime[5]. The common sites for BC metastasis include the lungs, bones, liver, and brain, and metastasis of BC to the gastrointestinal (GI) tract is relatively rare[6]. In some cases of GI metastasis from BC, it predominantly affects the stomach or small intestine[7,8]. However, few cases of colonic metastasis from BC (CMBC) have been reported[9], let alone its pathology and effective treatment options.
To elucidate the potential progression of CMBC and develop effective therapeutic strategies for treating CMBC, we reported a case of a 44-year-old woman with colonic metastasis detected during screening colonoscopy, who underwent radical mastectomy for BC one year ago. This case report is expected to provide valuable insights into the treatment of CMBC and similar diseases.
A 44-year-old female patient was admitted to the Department of Gastroenterology at our hospital because of mild abdominal distension and intermittent abdominal dull pain in the right lower abdomen for one month.
The patient did not present with hematochezia, melena, changes in bowel habits, or any other concomitant symptoms. The patient did not complain of nausea, vomiting and fever. Also, she did not present any symptoms of obstruction such as stopped anal exhaust and defecation. However, the patient's body weight decreased by about 3 kg in the past three months.
The patient was admitted to the Department of Breast Surgery at our hospital in July 2020 due to enlarged cervical and axillary lymph nodes. Following diagnosis with TNBC, she received six cycles of preoperative neoadjuvant chemotherapy consisting of epirubicin, docetaxel, and cyclophosphamide until October 2020. According to clinical pathological staging, TNBC was classified as cT2N3M0 (stage IIIC)[10]. After chemotherapy, significant shrinkage was observed in both the breast mass and supraclavicular area, but no apparent reduction in the size of axillary lymph nodes was observed. In November 2020, under general anesthesia, a modified radical mastectomy procedure was performed on her left breast as a palliative radical treatment to suppress the metastasis of BC. Following the modified radical mastectomy, the patient received capecitabine maintenance therapy until November 2021. Radiotherapy to the left breast was completed from December 2020 to February 2021. The patient underwent regular follow-up examinations periodically from February to December 2021.
The patient had no personal history and family history of cancer.
Abdominal examination revealed slight tenderness in the right lower abdomen.
From February to December 2021, laboratory data showed that there were no elevation in the levels of carcinoembryonic antigen (CEA) and cancer antigen (CA) 19-9. In January 2022, the patient had a hemoglobin level of 124 g/L, a hematocrit level of 38.3%, a mean corpuscular volume level of 98.7 FL, an aspartate aminotransferase level of 27.9 U/L, an alanine transaminase level of 13.7 U/L, and an alkaline phosphatase level of 74.9 U/L. Moreover, the patient had a CA-153 level of 36.9 U/mL (reference range: 0-26.4 U/mL), a CA-125 level of 41.8 U/mL (reference range: 0-35 U/mL), and a CEA level of 7.88 U/mL (reference range: 0.0-5.2 ng/mL).
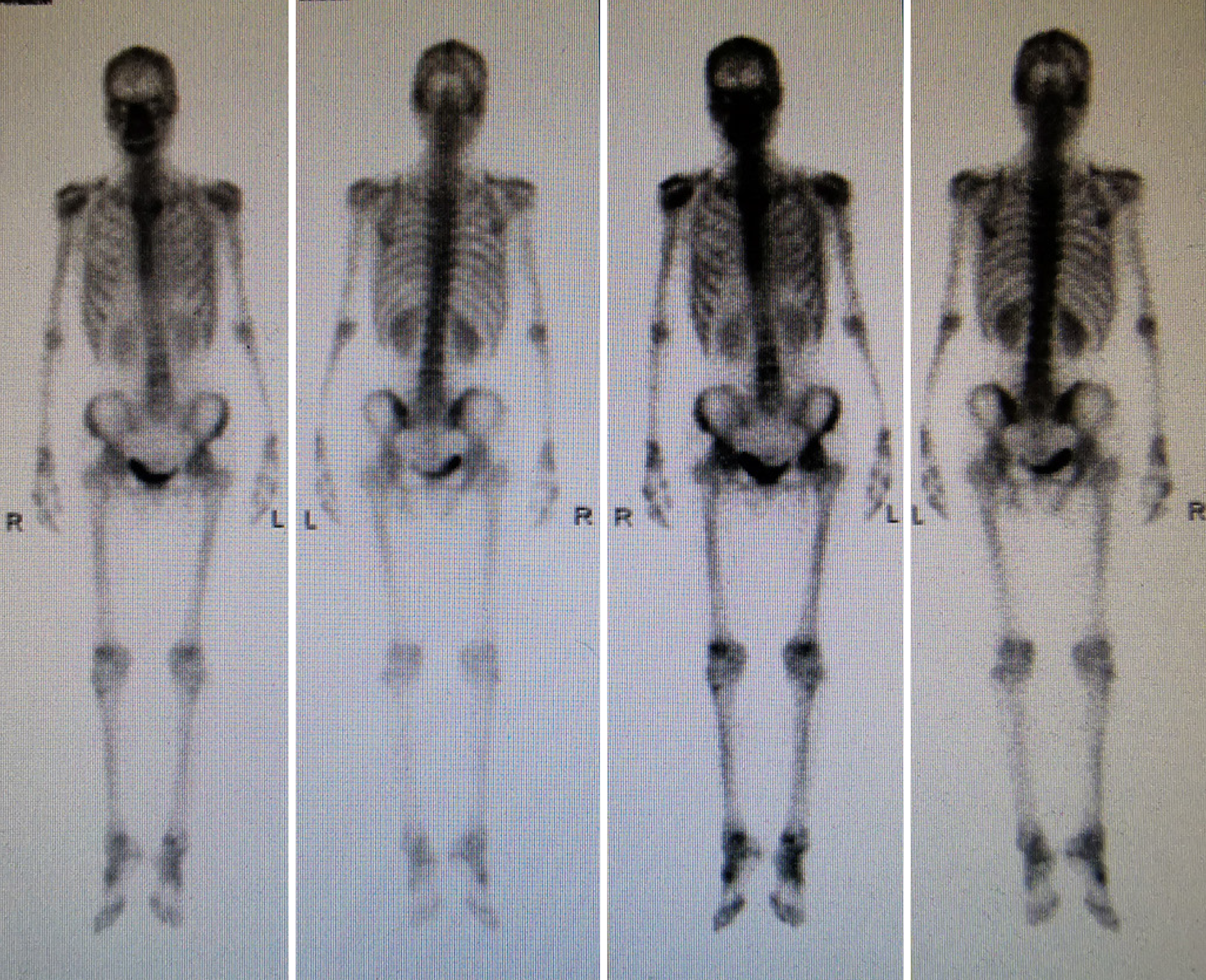
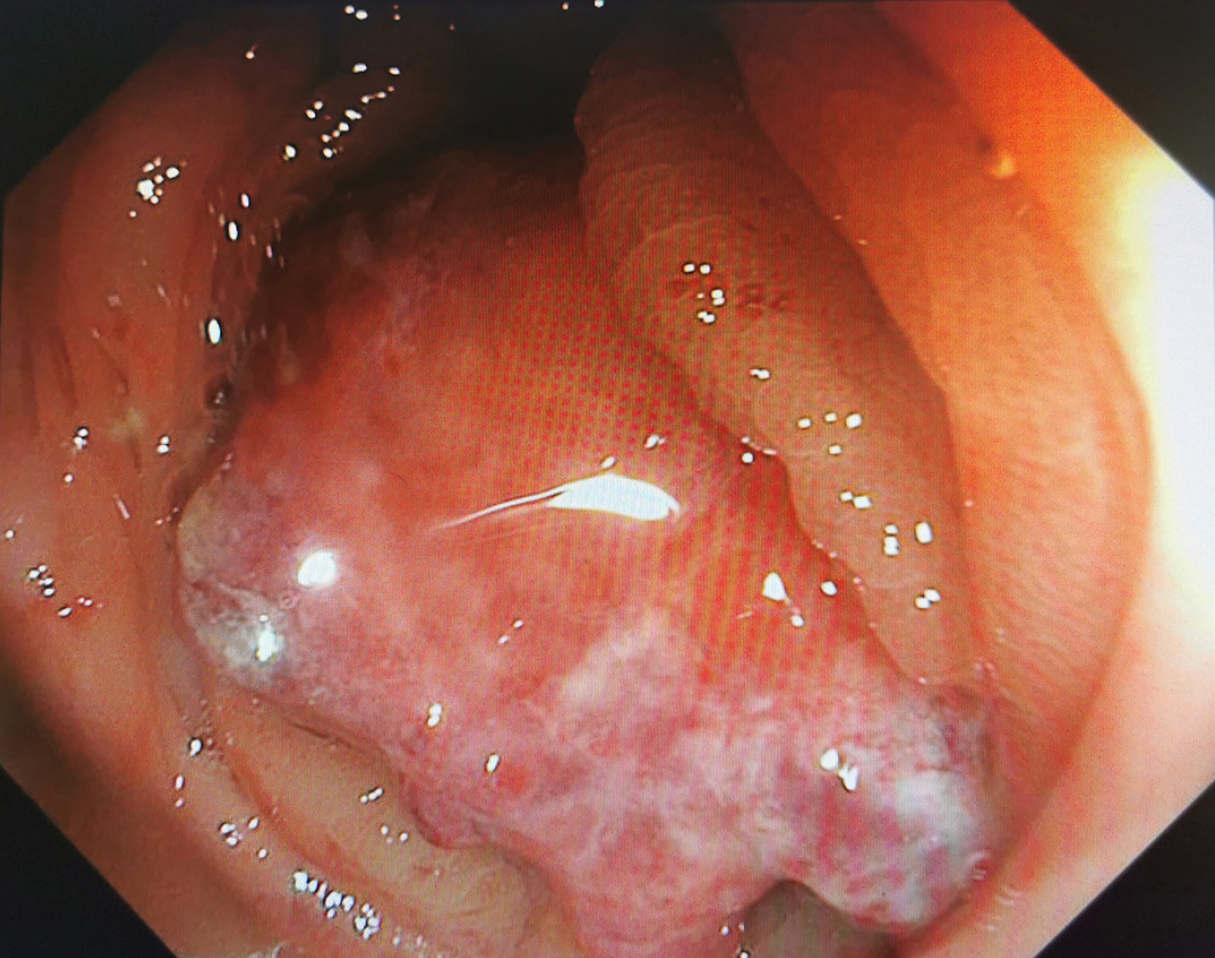
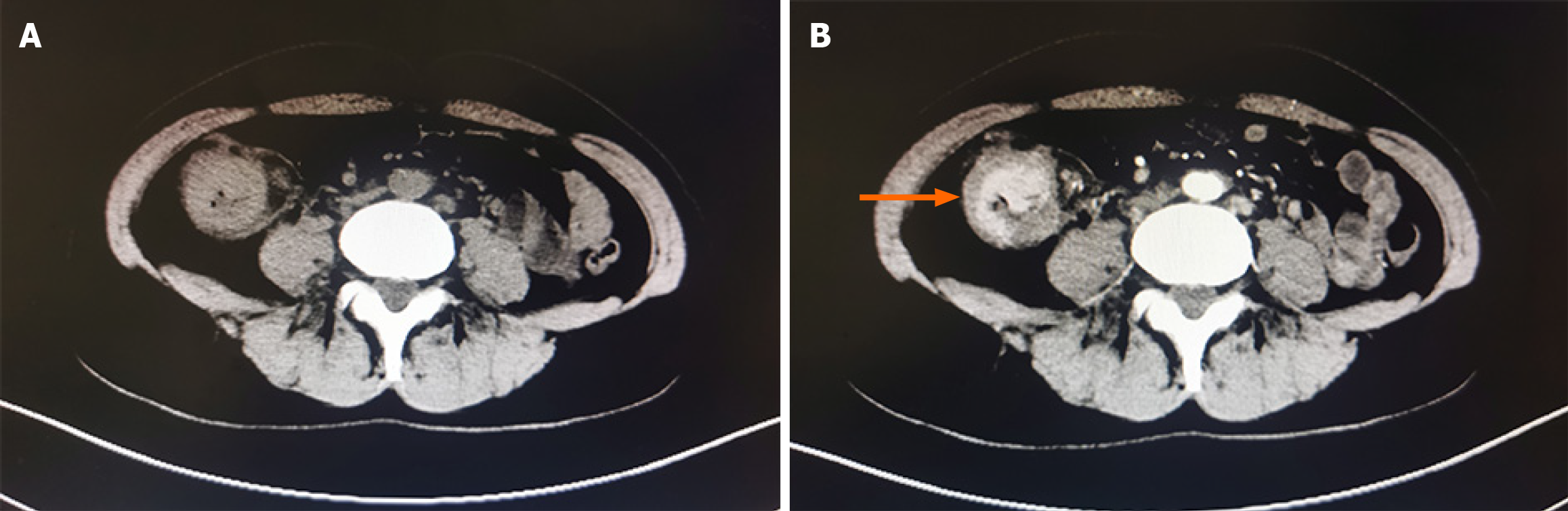
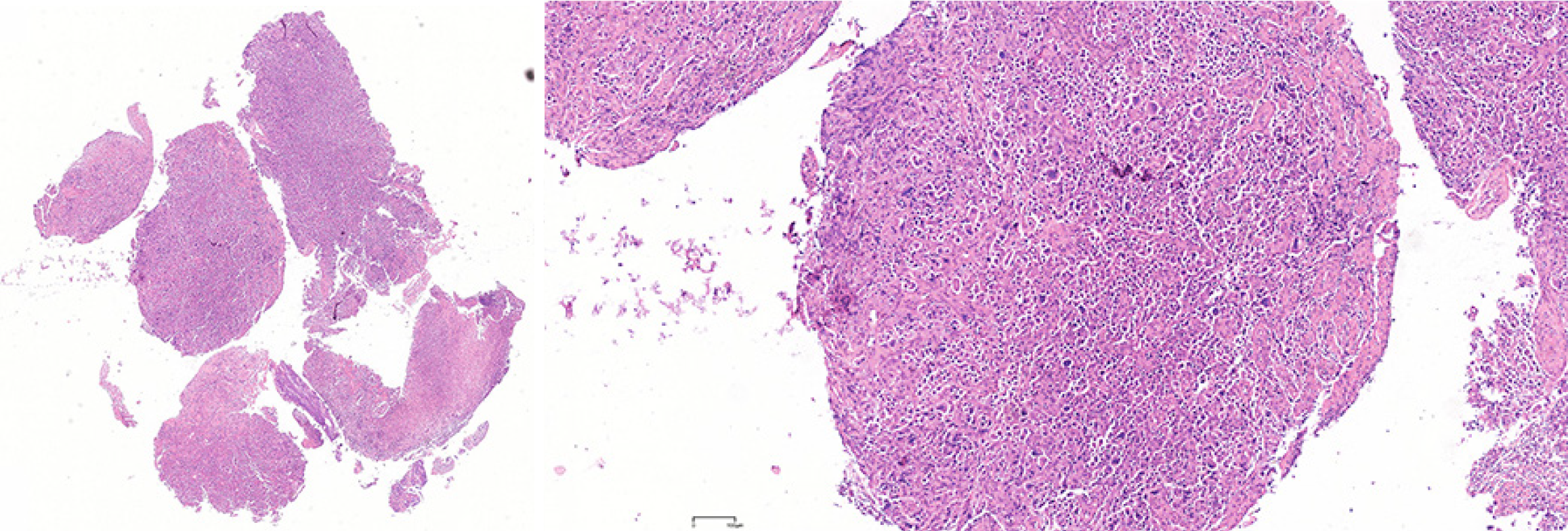
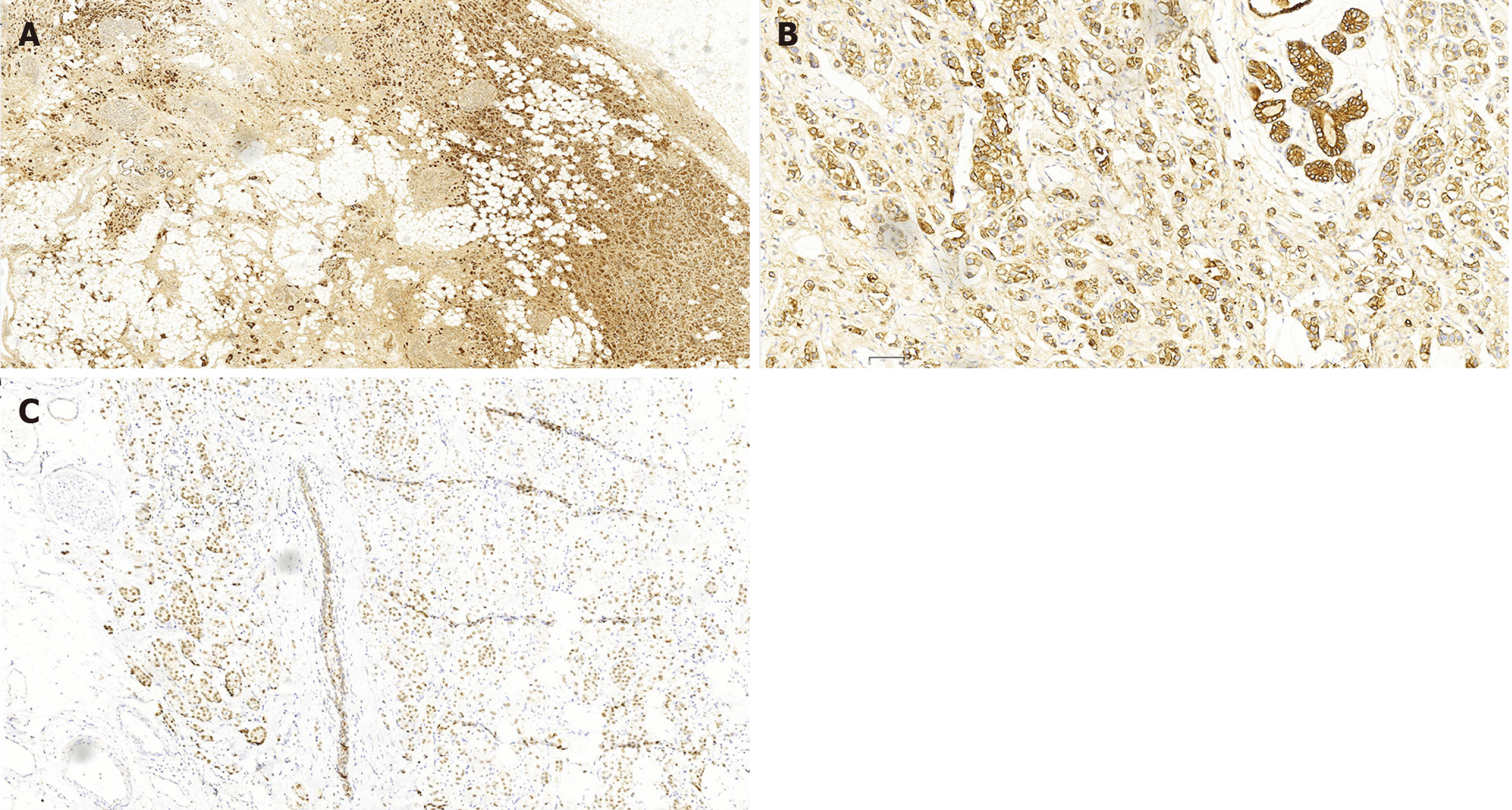
In July 2020, ultrasound (US) evaluation detected solid nodules measuring approximately 12 mm × 6 mm × 10 mm in the left breast, which was classified as Breast Imaging Reporting and Data System: 5, as well as metastatic involvement of the left supraclavicular and axillary lymph nodes. The patient received an US-guided puncture biopsy for left breast nodules and affected lymph nodes in the axilla and supraclavicular region. Pathological examination confirmed invasive carcinoma in the left breast nodules, as well as metastasis to axillary and supraclavicular lymph nodes. Immunohistochemical analysis showed negative results for HER-2, PR, and ER. However, the ki-67 index was found to be 20%. In November 2020, postoperative pathological examination of mastectomy confirmed invasive ductal carcinoma (IDC) without involvement of either the nipple or basal margins. Forty-five of 46 examined axillary lymph nodes were positive. The immunohistochemical staining of endoscopic biopsies demonstrated that this case was positive for GATA-binding protein 3 (GATA-3), gross cystic disease fluid protein 15 (GCDFP-15), E-cadherin, S-100, and cytokeratin (CK) 7, and ki-67 (10%), but negative for PR, ER, HER-2, and CK14. A positron-emission tomography scan conducted in June 2021 revealed no evidence of metastasis in the GI tract and other parts of the body (Figure 1). In January 2022, only 15 months after the modified radical mastectomy, colonoscopy unveiled a fragile mass measuring approximately 4 cm × 5 cm in size within the ascending colon that occupied the entire intestinal lumen (Figure 2). Furthermore, multiple small polyps were detected during colonoscopy in the descending colon and rectum. The contrast-enhanced computed tomography (CT) scan confirmed that adenocarcinoma was present in the ascending colon (Figure 3), which was further verified by pathological examination of endoscopic biopsies (Figure 4). Postoperative immunohistochemical staining of the resected ascending colon specimen showed that the tumor was positive for GCDFP15, GATA3, mammaglobin, E-cadherin, CK7, CK20, and CEA; and the ki-67 index was 50%. However, ER and PR were negative (Figure 5).
We examined the excised BC specimen and found that it had striking pathological similarities with the resected ascending colon specimen. As mentioned above, the BC specimen was negative for PR, ER, and HER-2. Interestingly, both breast and colon specimens were positive for GCDFP-15, GATA-3, and CK7 (Figure 6). Taken together, these results confirmed colonic metastasis from TNBC.
In July 2020, following diagnosis with TNBC, we first selected consisting of epirubicin, docetaxel, and cyclophosphamide other than endocrine to perform preoperative chemotherapy. Until October 2020, after 6 cycles, the tumor was found to be significantly reduced, while the axillary metastatic lymph nodes were not significantly reduced. In November 2020, under general anesthesia, a modified radical mastectomy procedure was performed on her left breast as a palliative radical treatment to suppress the metastasis of BC. Following the modified radical mastectomy, in view of resistance to anthracyclines, capecitabine chemotherapy was chosen until November 2021. Unfortunately, capecitabine was stopped after 4 cycles because of hand-foot syndrome. Radiotherapy to the left breast was completed from December 2020 to February 2021. In January 2022, only 15 months after modified radical mastectomy, the patient underwent laparoscopic radical right hemicolectomy for right colon cancer.
The patient experienced an uneventful postoperative course, with a satisfactory recovery, and was discharged 11 days after surgery. One month later after discharge, the follow-up examination revealed that the levels of postoperative tumor markers were elevated, with CA-15-3 increasing from 36.9 U/mL to 52.4 U/mL and CA 125 from 41.8 U/mL to 58.6 U/mL. Therefore, chemotherapy was recommenced utilizing albumin-bound paclitaxel and carboplatin. Table 1 shows the timeline of the patient’s clinical history.
| Timeline | Diagnosis and treatment process | Result |
| July 2020 | Ultrasound-guided puncture biopsy and pathological examination | Diagnosed with triple-negative breast cancer |
| Until October 2020 | Six cycles of preoperative neoadjuvant chemotherapy | Significant shrinkage of the breast mass and supraclavicular area; no apparent reduction in size for the axillary lymph nodes |
| November 2020 | A modified radical mastectomy procedure | Postoperative pathology: Invasive ductal carcinoma, 45 of 46 examined axillary lymph nodes (+), gross cystic disease fluid protein 15 (+), GATA-binding protein 3 (+), E-cadherin (+), CK7 (+), S-100 (+), and ki-67 (10%); estrogen receptor, human epidermal growth factor receptor 2 (-), progesterone receptor (-) and CK14 (-) |
| November 2020 to November 2021 | Chemotherapy consisting of capecitabine and radiotherapy | CEA 4.3 ng/mL and CA 19-9 25.1 U/mL |
| February 2021 to December 2021 | Follow-up | No observed elevation in CEA and CA 19-9 tumor markers, and no evidence of metastatic disease with positron emission tomography/CT |
| February 2022 | Colonoscopy, laboratory investigations, CT scan and biopsy outcomes | A comprehensive preoperative diagnosis with ascending colon cancer |
| February 2022 | Laparoscopic radical right hemicolectomy, morphological and immunohistochemical examination | Diagnosed with colonic metastasis from breast cancer |
BC is the most prevalent cancer among women under 50 years of age, accounting for 24% of newly diagnosed cases[11]. GI metastasis from BC is relatively rare, occurring in about 3.4%-4.5% of patients[12,13]. In comparison, CMBC is even rarer. A retrospective study conducted by McLemore et al[14] identified only 24 cases of CMBC out of 12001 BC patients between 1985 and 2000. IDC is the most common invasive BC. Borst and Ingold[15] reported that among 2605 cases of metastatic BC (MBC), including 359 Lobular carcinomas and 2246 ductal carcinomas, only a small proportion (4.7%) have metastasized to the GI. GI metastasis from BC is predominantly associated with invasive lobular carcinoma, while IDC has been reported in limited studies[16]. In this case, the histologic type of primary BC was also IDC.
By using the combined terms "Breast Cancer/carcinoma" with "Colonic Metastasis", "colon cancer", and "sigmoid" in PubMed, we obtained only 22 case reports of CMBC (Table 2)[9,17-38]. Among these reports, there was an interval of 2 years or more between the resection of BC and the surgical resection of CMBC. However, our present case is unique as it involves a patient with both IDC and advanced-stage (stage IIIC) TNBC. Consequently, the patient was treated with adjuvant systemic therapy. Despite this treatment, colonic metastasis occurred just 15 months after mastectomy and merely 3 months following chemotherapy cessation. To make matters worse, positron emission tomography (PET)/CT scans during close follow-up did not reveal any clear pathological findings; yet, 8 months later, an impending obstructive colonic lesion was discovered. Based on the available literature, our case recorded the shortest interval of time between surgical resection of BC and colonic metastasis.
| Ref. | Age | Original diagnosis (triple-negative breast cancer, Y/N) | Time since mastectomy (years) | Adjuvant treatment after mastectomy | Preoperative symptoms | Colonic metastasis detection by positron emission tomography/computed tomography (Y/N) | Preoperative diagnosis of colonic metastasis from breast cancer (Y/N) |
| Kamitani et al[17], 1987 | 56 | ILC (N) | 7 | ND | Abdominal pain | ND | N |
| Babb and Trollope[18], 2001 | 72 | ND (N) | 18 | Radiotherapy; hormonal therapy | Asymptomafic | ND | N |
| Vaidya et al[19], 2002 | 56 | IDC (ND) | 5 | Radiotherapy; hormonal therapy | Change in bowel habit and weight loss | ND | Y |
| Zhou et al[20], 2012 | 54 | IDC (N) | 6 | Chemotherapy; radiotherapy; hormonal therapy | Asymptomafic | ND | Y |
| Maekawa et al[21], 2012 | 54 | IDC (N) | 16 | Chemotherapy; hormonal therapy | Asymptomafic | Y | Y |
| Nikkar-Esfahani et al[22], 2013 | 63 | ILC (ND) | 17 | ND | Mild abdominal distension | ND | N |
| Motos-Micó et al[23], 2014 | 69 | ILC (ND) | 18 | Chemotherapy | Abdominal pain, nausea, vomiting | ND | N |
| Kim and Moon[24], 2015 | 46 | ND | 2 | Chemotherapy; radiotherapy | Intermittent hematochezia | Y | Y |
| Andriola et al[25], 2015 | 63 | IDC and ILC (N) | 23 | Chemotherapy | A moderate abdominal pain | Y | Y |
| Gizzi et al[26], 2015 | 72 | ILC (N) | 11 | Chemotherapy; radiotherapy; hormonal therapy | Abdominal pain and diarrhoea | Y | Y |
| Jeong et al[27], 2016 | 58 | Inflammatory breast cancer (N) | 4 | Chemotherapy | Obstructive symptoms | N | Y |
| Aihara et al[28], 2017 | 64 | ND (ND) | ND | ND | ND | ND | Y |
| Tsujimura et al[29], 2017 | 51 | ILC (N) | ND | ND | Abdominal pain, vomiting and diarrhea | Y | Y |
| Horimoto et al[30], 2018 | 47 | IDC and ILC (N) | 5 | Chemotherapy; hormonal therapy | Asymptomatic | No | N |
| Falco et al[31], 2018 | 67 | ILC (N) | 14 | Chemotherapy; hormonal therapy | Asymptomatic | Y | Y |
| Schellenberg et al[32], 2018 | 69 | IDC (N) | 5 | Chemotherapy; radiotherapy; hormonal therapy | Asymptomatic | N | Y |
| Jones et al[33], 2019 | 55 | IDC (N) | 4 | Hormonal therapy | Nausea, vomiting and abdominal pain | Y | N |
| Bering et al[34], 2020 | 67 | ILC (N) | ND | Chemotherapy; hormonal therapy | Change in bowel habit | N | N |
| Sarfraz et al[35], 2020 | 60 | ILC (N) | 5 | Hormonal therapy | Abdominal pain, distension, and vomiting | ND | Y |
| Higley et al[36], 2020 | 74 | ILC (Y) | 20 | Radiotherapy; hormonal therapy | Decreased stool caliber and constipation | ND | N |
| Chen et al[37], 2021 | 62 | ND (N) | 9 | ND | Abdominal pain, bloating | ND | N |
| Inoue et al[9], 2022 | 63 | ILC (N) | 15 | Chemotherapy hormonal therapy | Asymptomatic | Y | N |
| Botto et al[38], 2023 | 59 | ILC(N) | 2 | Chemotherapy; radiotherapy; hormonal therapy | Asymptomafic | N | Y |
| This case | 44 | IDC (Y) | 1 | Chemotherapy | Abdominal pain and distension | N | N |
CMBC often presents without symptoms or with non-specific symptoms. Based on our literature review, potential symptoms may include abdominal pain, diarrhea, bowel obstruction, or asymptom. If patients have a history of BC and new GI symptoms, consideration should be given to the possibility of CMBC[39]. In this particular case, the patient experienced mild abdominal distension and intermittent dull pain in the right lower abdomen for one month. It is challenging to clinically and radiologically differentiate CMBC from primary colon cancer. Radiographic imaging techniques such as conventional contrast-enhanced CT can aid in diagnosis. However, radiological features like nodular or circumferential thickening with narrowing can easily be mistaken for primary colonic tumors or inflammatory colonic diseases-similar to what occurred in this case. PET-CT is another commonly used radiological imaging method. According to the literature, PET-CT is not recommended for diagnosing primary BC due to its limited sensitivity in detecting small tumors (0–10 mm). However, it is suitable for detecting distant metastases with higher specificity and sensitivity relative to conventional imaging[40]. It has been reported that PET-CT exhibits a sensitivity of 80% to 100% and a specificity of 75% to 100%[41]. In this case, however, the PET-CT scan did not reveal a significant uptake of fluorodeoxyglucose in the ascending colon during follow-up. Only 8 months after the PET-CT scan, an impending obstructive colonic lesion was detected by colonoscopy in the patient. There could be two possible explanations for this situation. First, there might have been no tumor present at the time of the initial PET-CT scan, and the tumor might develop within eight months. Second, it is plausible that the tumor was too small to be detected by PET since PET had lower sensitivity towards metastatic masses. Nevertheless, there are also many cases where metastatic lesions are found by PET examination (Table 2). In addition to PET-CT, another important preoperative examination used in diagnosing CMBC is endoscopy biopsy. During endoscopic examinations, macroscopic appearance may appear normal which differs from primary colon cancer[42]. The pathological findings may show necrotic tissues and no carcinoma due to technical limitations in identifying tumors[42]. Therefore, obtaining multiple deeper biopsies penetrating the serous side of the colon is crucial for accurately diagnosing metastatic breast carcinoma. In our case, colonoscopy revealed a brittle mass and intestinal lumen stenosis in the ascending colon, and it was difficult to distinguish between primary and metastatic lesions. The pathological biopsy also confirmed the diagnosis of poorly differentiated adenocarcinoma in the ascending colon. Unfortunately, however, due to nonspecific symptoms and radiographic and endoscopic features resembling primary colon cancer, we were unable to make an accurate preoperative diagnosis. Although the accuracy of comprehensive multidisciplinary diagnostic methods is limited, cooperation between different departments should be enhanced to integrate more information and improve their diagnostic approaches to ensure timely and accurate identification of such metastases.
When pathologists have difficulty in histological diagnosis, they should take into account the history of BC and immunohistochemical indicators of BC-related indicators. In challenging cases, immunohistochemistry may be the only diagnostic tool for accurately diagnosing CMBC. The breast-specific markers commonly used for tumors with unknown primary origins include GCDFP-15, mammaglobin, GATA-3, and CK7[43,44]. As a glycoprotein originally identified in human breast gross cyst fluids, GCDFP-15 has been widely used as a marker for apocrine differentiation due to its relatively high specificity and sensitivity[45]. Around 20% of patients with MBC show high GCDFP-15 levels in plasma[46], and 50% or higher of cases express GCDFP-15[47]. Another study shows that immunohistochemical analysis of mammaglobin is superior to GCDFP-15 in identifying tumors of breast origin. Mammaglobin is positive in a about 84% of MBCs are positive for mammaglobin[48]. Mammaglobin is highly expressed in primary BCs, however, it exists at low levels in healthy breast tissues and has not been detected in non-breast tumors and other tissues, suggesting that it is suitable for diagnosing BC[49-51]. GATA-3 is another effective marker for detecting BC metastasis, and its expression in metastatic lesions shows high concordance with that in primary lesions[52,53]. Positive staining for CK7 (+) suggests that the primary site of metastases may be the breast, however, CK7 is usually negative in colorectal cancer[54]. Because these markers are neither fully specific nor sensitive, recognizing the clinical context and tumor location, along with the specific markers for other organs, is key to accurate diagnosis[55]. In our case, immunohistochemistry showed that excised right colon cancer specimens were positive for GCDFP-15, GATA-3, mammaglobin, and CK7, which are similar to the results of resected BC specimens. Hence, a diagnosis of CMBC was made.
The progression of TNBC is poorly understood, which may involve epigenetic and genetic alterations, intravasation through the basement membrane, extravasation into distal tissues, survival in the circulation, angiogenesis, and tumor-stroma interactions[56]. Cancer metastasis is closely associated with genetic instability of primary tumor cells, immune invasion, and the surrounding microenvironment. Surgical interventions or chemotherapeutic treatments are often ineffective against dormant cells that can reactivate and rapidly form new tumors. As one of the major cytoskeletal components, actin is involved in almost all steps of metastatic spread. Efforts have been made to find ways to treat cancer by interfering with actin and its regulatory factors. For example, low-frequency rotating magnetic fields can hinder BC metastasis by disrupting F-actin[57]. In addition, the epithelial-mesenchymal transition, angiogenesis, cell migration, and anoikis resistance are important factors in the metastatic progression of cancer cells. lncRNA-related orphan receptor (ROR) is a non-coding RNA, and it can inhibit p53 translation by directly interacting with the heterogeneous ribonuclear protein I. The primary function of mlncRNA-ROR is to maintain embryonic stem cells through induced pluripotent stem cells, and it can also play a role in carcinogenesis. It’s reported that receptor-ROR functions as a competitive endogenous RNA for miR205 in human breast epithelial cells, thereby affecting epithelial-mesenchymal transition[58]. TNBC contains high levels of ROR, which affects miR145 and its target adenosine diphosphate ribosylation factor 6, possibly leading to BC invasion and metastasis. However, because CMBC cases are reported as individual cases, it is difficult to conduct large-scale systematic studies to decipher the specific mechanism of CMBC.
Many factors can affect the treatment of MBC, such as patient age, tumor burden, tumor characteristics, hormone receptor status, previous treatments, metastatic sites, and tumor-related symptoms. The conventional treatments for MBC include chemotherapy, radiation therapy, surgery, hormonal therapy, and immunotherapy. Recently, targeted therapy and gene therapy have also been developed in treating MBC. In addition, some new methods for modern cancer treatment can provide a promising strategy. Several new herbal compounds have been reported to improve chemotherapy, hormonal therapy and gene therapy in cancer treatment[58]. Other studies have also enhanced anti-tumor effects through improved delivery methods, such as nanotechnology combined with sustained release patterns to address drug solubility and low bioavailability[59]. Photodynamic therapy and ferroptosis have mutually reinforcing effects with high anti-tumor efficacy and safety. Cen et al[60] prepared thermally responsive nanoparticles by combining the ultraminiature palladium ruthenium alloy and Ru(II) with thermally responsive phase change materials and co-encapsulating them with polypyridyl-complex.
Generally, following the initial surgical treatment, combining multiple types of treatment is recommended. Surgery can resect the primary tumor, thereby reducing tumor burden and improving overall survival. Radiotherapy is often used after surgery, however, it is preferred to be combined with other treatments because of high resistance when tumor sizes are larger than 1 cm[61]. For MBC patients who have symptomatic or hormone receptor-negative disease (i.e., resistant to endocrine therapy), chemotherapy is considered the first treatment option. Anthracyclines (like epirubicin) are commonly used antibiotics in treating MBC, and they perform anti-tumor functions through various mechanisms[62]. The common combinations of anthracyclines include cyclophosphamide 5-fluorouracil plus epirubicin or doxorubicin or doxorubicin/epirubicin plus cyclophosphamide. Capecitabine is used in patients who are resistant to anthracyclines and taxanes[63]. Capecitabine is a new generation of fluorouracil-based compounds used in cancer therapy. In cells, thymidine phosphorylase can convert capecitabine to 5-fluorouracil. Because the activity of thymidine phosphorylase is significantly higher in tumor tissues than in normal tissues, capecitabine has higher activity in tumors, especially in the hypoxic regions that are resistant to chemotherapy, with relatively lower toxicity[64]. Several studies have confirmed the efficacy of capecitabine maintenance chemotherapy for patients with MBC, but the incidence of hand-foot syndrome and GI adverse reactions is high[65-67].
The prognosis of TNBC is relatively poor, and the patients cannot receive endocrine therapy or HER-2-targeted therapy. In our case, we first selected the combined regimen of TEC to perform preoperative chemotherapy. After 6 cycles, the tumor size was significantly reduced, while the axillary metastatic lymph nodes showed no significant reduction. Therefore, given the resistance to anthracyclines, capecitabine was used after mastectomy. Unfortunately, capecitabine was stopped after 4 cycles because of hand-foot syndrome. In our case, the patient presented with a single lesion localized to the ascending colon, so surgery is the best option.
In recent years, the survival of BC has substantially improved. However, the prognosis is still poor for GI metastasis from BC, and the average survival time from detecting GI metastases is about 1 year[68]. Prognosis is affected by the characteristics of primary tumors, such as their grade and size, lymph node status, and the levels of ER, PR and HER-2. Recently, several specific genes and proteins associated with BC have been identified, but they are less useful in predicting the prognosis of metastatic cancers because they are more related to primary tumors, rather than distant metastases[69,70]. Combining multiple factors has a more significant prognostic value than individual factors.
Based on these discussions above and the changing characteristics of our patient, this patient's clinical course is aligned with typical prognostic expectations.
This case of CMBC indicates that clinicians and researchers should be highly cautious when treating patients with late-stage MBCs, particularly TNBC, even at an unusual site like the colon. Especially, when patients also present with GI symptoms, along with the possible recurrence of the tumor indicated by laboratory tests and imaging examination, it is necessary to conduct an endoscopic examination for any suspicious lesions and perform immunohistochemical examinations of the biopsies. Additionally, pathologists should take into account the history of BC and immunohistochemical analysis of BC-related indicators. We suggest that future endoscopists focus on the mucosal manifestations of CMBC and increase their experience in this area. Additionally, models of CMBC can be established to study the pathways and mechanisms of metastasis, providing theoretical support for future research.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55820] [Article Influence: 7974.3] [Reference Citation Analysis (132)] |
| 2. | Shi J, Liu F, Song Y. Progress: Targeted Therapy, Immunotherapy, and New Chemotherapy Strategies in Advanced Triple-Negative Breast Cancer. Cancer Manag Res. 2020;12:9375-9387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009;9 Suppl 2:S73-S81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 487] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 4. | Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, Marcos-Gragera R, Stiller C, Azevedo e Silva G, Chen WQ, Ogunbiyi OJ, Rachet B, Soeberg MJ, You H, Matsuda T, Bielska-Lasota M, Storm H, Tucker TC, Coleman MP; CONCORD Working Group. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385:977-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1738] [Cited by in RCA: 1735] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 5. | Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E; ESMO Guidelines Working Group. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii11-vii19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 361] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 6. | Ambroggi M, Stroppa EM, Mordenti P, Biasini C, Zangrandi A, Michieletti E, Belloni E, Cavanna L. Metastatic breast cancer to the gastrointestinal tract: report of five cases and review of the literature. Int J Breast Cancer. 2012;2012:439023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Tanaka K, Yabuuchi Y, Yamashita D, Inokuma T. Breast cancer metastasis to the stomach mimicking early gastric cancer. JGH Open. 2023;7:667-668. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Jiao X, Zhai MM, Xing FZ, Wang XL. Simultaneously metastatic cholangiocarcinoma and small intestine cancer from breast cancer misdiagnosed as primary cholangiocarcinoma: A case report. World J Clin Cases. 2023;11:4446-4453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Inoue H, Arita T, Kuriu Y, Shimizu H, Kiuchi J, Yamamoto Y, Konishi H, Morimura R, Shiozaki A, Ikoma H, Kubota T, Fujiwara H, Okamoto K, Otsuji E. Colonic Metastasis from Breast Cancer: A Case Report and Review of the Literature. In Vivo. 2022;36:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Giuliano AE, Edge SB, Hortobagyi GN. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann Surg Oncol. 2018;25:1783-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 236] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 11. | Venkitaraman R. Triple-negative/basal-like breast cancer: clinical, pathologic and molecular features. Expert Rev Anticancer Ther. 2010;10:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Schwarz RE, Klimstra DS, Turnbull AD. Metastatic breast cancer masquerading as gastrointestinal primary. Am J Gastroenterol. 1998;93:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Gifaldi AS, Petros JG, Wolfe GR. Metastatic breast carcinoma presenting as persistent diarrhea. J Surg Oncol. 1992;51:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | McLemore EC, Pockaj BA, Reynolds C, Gray RJ, Hernandez JL, Grant CS, Donohue JH. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol. 2005;12:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery. 1993;114:637-41; discussion 641. [PubMed] |
| 16. | Montagna E, Pirola S, Maisonneuve P, De Roberto G, Cancello G, Palazzo A, Viale G, Colleoni M. Lobular Metastatic Breast Cancer Patients With Gastrointestinal Involvement: Features and Outcomes. Clin Breast Cancer. 2018;18:e401-e405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Kamitani S, Fuchimoto S, Naomoto Y, Gohchi A, Matsuda T, Orita K. A case of colonic metastasis of breast cancer positive for estrogen receptor. Hiroshima J Med Sci. 1987;36:163-167. [PubMed] |
| 18. | Babb RR, Trollope M. Colonic metastases from breast cancer. Surg Endosc. 2001;15:530. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Vaidya JS, Mukhtar H, Bryan R. Colonic metastasis from a breast cancer--a case report and a few questions. Eur J Surg Oncol. 2002;28:463-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Zhou XC, Zhou H, Ye YH, Zhang XF, Jiang Y. Invasive ductal breast cancer metastatic to the sigmoid colon. World J Surg Oncol. 2012;10:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Maekawa H, Fujikawa T, Tanaka A. Successful laparoscopic investigation and resection of solitary colonic metastasis from breast cancer (with video). BMJ Case Rep. 2012;2012:bcr2012007187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Nikkar-Esfahani A, Kumar BG, Aitken D, Wilson RG. Metastatic breast carcinoma presenting as a sigmoid stricture: report of a case and review of the literature. Case Rep Gastroenterol. 2013;7:106-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Motos-Micó J, Ferrer-Márquez M, Belda-Lozano R, Reina-Duarte Á, Rosado-Cobián R. Metastasis of lobular breast carcinoma in the sigmoid colon. Rev Esp Enferm Dig. 2014;106:366-367. [PubMed] |
| 24. | Kim HW, Moon DH. Sigmoid colon metastasis from metaplastic breast carcinoma mimicking primary sigmoid colon cancer. Rev Esp Med Nucl Imagen Mol. 2015;34:211-212. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Andriola V, Piscitelli D, De Fazio M, Altomare DF. Massive colonic metastasis from breast cancer 23 years after mastectomy. Int J Colorectal Dis. 2015;30:427-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Gizzi G, Santini D, Guido A, Fuccio L. Single colonic metastasis from breast cancer 11 years after mastectomy. BMJ Case Rep. 2015;2015:bcr2015211193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Jeong W, Trembath DG, Baron TH. Endoscopic ultrasound-guided fine needle biopsy through the interstices of a colonic stent for the diagnosis of metastatic breast cancer using a forward-viewing linear echoendoscope. Endoscopy. 2016;48 Suppl 1:E283-E284. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Aihara H, Drage MG, Agoston A, Thompson CC. Metastatic Breast Cancer Mimicking a Solitary Small Colonic Erosion. Am J Gastroenterol. 2017;112:677. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Tsujimura K, Teruya T, Kiyuna M, Higa K, Higa J, Iha K, Chinen K, Asato M, Takushi Y, Ota M, Dakeshita E, Nakachi A, Gakiya A, Shiroma H. Colonic metastasis from breast carcinoma: a case report. World J Surg Oncol. 2017;15:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Horimoto Y, Hirashima T, Arakawa A, Miura H, Saito M. Metastatic colonic and gastric polyps from breast cancer resembling hyperplastic polyps. Surg Case Rep. 2018;4:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Falco G, Mele S, Zizzo M, Di Grezia G, Cecinato P, Besutti G, Coiro S, Gatta G, Vacondio R, Ferrari G. Colonic metastasis from breast carcinoma detection by CESM and PET/CT: A case report. Medicine (Baltimore). 2018;97:e10888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Schellenberg AE, Wood ML, Baniak N, Hayes P. Metastatic ductal carcinoma of the breast to colonic mucosa. BMJ Case Rep. 2018;2018:bcr2018224216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Jones A, Kocher MR, Justice A, Navarro F. Colonic metastasis from infiltrating ductal breast carcinoma in a male patient: A case report. Int J Surg Case Rep. 2019;54:34-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Bering J, Ryan M, Gurudu SR. Breast Cancer Metastasis Presenting as Colonic Polyps. ACG Case Rep J. 2020;7:e00411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 35. | Sarfraz H, Chen D, Muhsen IN, Schwartz MR, Ogbonna M. Breast Cancer Metastasis Masquerading as a Primary Gynecological / Colonic Malignancy: A Rare Diagnostic Conundrum. Cureus. 2020;12:e7806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Higley C, Hsu A, Park BU, Pang M. Back to Basics: History and Physical Examination Uncover Colonic Metastasis in a Patient With Remote History of Breast Cancer. ACG Case Rep J. 2020;7:e00494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 37. | Chen W, Jiang C, Qian Q, Ding Z. A rare case of late colonic metastasis and subsequent liver metastasis from breast cancer. Asian J Surg. 2021;44:778-779. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Botto I, Moiteiro Cruz R, Noronha Ferreira C, Valente AI, Carrilho-Ribeiro L, Tato-Marinho R, Ferreira C, Correia L. Simultaneous Gastric and Colonic Metastasis of Breast Cancer. ACG Case Rep J. 2023;10:e01168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Mistrangelo M, Cassoni P, Mistrangelo M, Castellano I, Codognotto E, Sapino A, Lamanna G, Cravero F, Bianco L, Fora G, Sandrucci S. Obstructive colon metastases from lobular breast cancer: report of a case and review of the literature. Tumori. 2011;97:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Constantinidou A, Martin A, Sharma B, Johnston SR. Positron emission tomography/computed tomography in the management of recurrent/metastatic breast cancer: a large retrospective study from the Royal Marsden Hospital. Ann Oncol. 2011;22:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Warning K, Hildebrandt MG, Kristensen B, Ewertz M. Utility of 18FDG-PET/CT in breast cancer diagnostics--a systematic review. Dan Med Bull. 2011;58:A4289. [PubMed] |
| 42. | Okido M, Seo M, Hamada Y, Kurihara S, Matsumoto K, Konomi H, Kato M, Ichimiya H. Metastatic breast carcinoma simulating linitis plastica of the colon: report of a case. Surg Today. 2011;41:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | O'Connell FP, Wang HH, Odze RD. Utility of immunohistochemistry in distinguishing primary adenocarcinomas from metastatic breast carcinomas in the gastrointestinal tract. Arch Pathol Lab Med. 2005;129:338-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Théraux J, Bretagnol F, Guedj N, Cazals-Hatem D, Panis Y. Colorectal breast carcinoma metastasis diagnosed as an obstructive colonic primary tumor. A case report and review of the literature. Gastroenterol Clin Biol. 2009;33:1114-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Chia SY, Thike AA, Cheok PY, Tan PH. Utility of mammaglobin and gross cystic disease fluid protein-15 (GCDFP-15) in confirming a breast origin for recurrent tumors. Breast. 2010;19:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Haagensen DE Jr, Dilley WG, Mazoujian G, Wells SA Jr. Review of GCDFP-15. An apocrine marker protein. Ann N Y Acad Sci. 1990;586:161-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Mazoujian G, Bodian C, Haagensen DE Jr, Haagensen CD. Expression of GCDFP-15 in breast carcinomas. Relationship to pathologic and clinical factors. Cancer. 1989;63:2156-2161. [PubMed] [DOI] [Full Text] |
| 48. | Han JH, Kang Y, Shin HC, Kim HS, Kang YM, Kim YB, Oh SY. Mammaglobin expression in lymph nodes is an important marker of metastatic breast carcinoma. Arch Pathol Lab Med. 2003;127:1330-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Al-Joudi FS, Kaid FA, Ishak I, Mohamed N, Osman K, Alias IZ. Expression of human mammaglobin and clinicopathologic correlations in breast cancer: the findings in Malaysia. Indian J Pathol Microbiol. 2011;54:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Rehman F, Nagi AH, Hussain M. Immunohistochemical expression and correlation of mammaglobin with the grading system of breast carcinoma. Indian J Pathol Microbiol. 2010;53:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Raica M, Cîmpean AM, Meche A, Alexa A, Suciu C, Mureşan A. Analysis of the immunohistochemical expression of mammaglobin A in primary breast carcinoma and lymph node metastasis. Rom J Morphol Embryol. 2009;50:341-347. [PubMed] |
| 52. | Ni YB, Tsang JYS, Shao MM, Chan SK, Cheung SY, Tong J, To KF, Tse GM. GATA-3 is superior to GCDFP-15 and mammaglobin to identify primary and metastatic breast cancer. Breast Cancer Res Treat. 2018;169:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | Liu H, Shi J, Wilkerson ML, Lin F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol. 2012;138:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 54. | Dum D, Menz A, Völkel C, De Wispelaere N, Hinsch A, Gorbokon N, Lennartz M, Luebke AM, Hube-Magg C, Kluth M, Fraune C, Möller K, Bernreuther C, Lebok P, Clauditz TS, Jacobsen F, Sauter G, Uhlig R, Wilczak W, Steurer S, Minner S, Marx AH, Simon R, Burandt E, Krech T. Cytokeratin 7 and cytokeratin 20 expression in cancer: A tissue microarray study on 15,424 cancers. Exp Mol Pathol. 2022;126:104762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 55. | Gown AM, Fulton RS, Kandalaft PL. Markers of metastatic carcinoma of breast origin. Histopathology. 2016;68:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Neophytou C, Boutsikos P, Papageorgis P. Molecular Mechanisms and Emerging Therapeutic Targets of Triple-Negative Breast Cancer Metastasis. Front Oncol. 2018;8:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 57. | Ji X, Tian X, Feng S, Zhang L, Wang J, Guo R, Zhu Y, Yu X, Zhang Y, Du H, Zablotskii V, Zhang X. Intermittent F-actin Perturbations by Magnetic Fields Inhibit Breast Cancer Metastasis. Research (Wash D C). 2023;6:0080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 58. | Hou P, Zhao Y, Li Z, Yao R, Ma M, Gao Y, Zhao L, Zhang Y, Huang B, Lu J. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 2014;5:e1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 59. | Khafaga AF, Shamma RN, Abdeen A, Barakat AM, Noreldin AE, Elzoghby AO, Sallam MA. Celecoxib repurposing in cancer therapy: molecular mechanisms and nanomedicine-based delivery technologies. Nanomedicine (Lond). 2021;16:1691-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Cen J, Huang Y, Liu J, Liu Y. Thermo-responsive palladium-ruthenium nanozyme synergistic photodynamic therapy for metastatic breast cancer management. J Mater Chem B. 2022;10:10027-10041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 61. | Murphy CT, Li T, Wang LS, Obeid EI, Bleicher RJ, Eastwick G, Johnson ME, Hayes SB, Weiss SE, Anderson PR. Comparison of Adjuvant Radiation Therapy Alone Versus Radiation Therapy and Endocrine Therapy in Elderly Women With Early-Stage, Hormone Receptor-Positive Breast Cancer Treated With Breast-Conserving Surgery. Clin Breast Cancer. 2015;15:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Yun S, Vincelette ND, Abraham I. Cardioprotective role of β-blockers and angiotensin antagonists in early-onset anthracyclines-induced cardiotoxicity in adult patients: a systematic review and meta-analysis. Postgrad Med J. 2015;91:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Karachaliou N, Kouroussis Ch, Papakotoulas P, Kalbakis K, Tryfonidis K, Vardakis N, Poppis E, Georgoulias V, Mavroudis D. A multicenter phase II trial of docetaxel plus gemcitabine as salvage treatment in anthracycline- and taxane-pretreated patients with metastatic breast cancer. Cancer Chemother Pharmacol. 2012;69:1345-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 64. | Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 930] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 65. | Surmeli ZG, Varol U, Cakar B, Degirmenci M, Arslan C, Piskin GD, Zengel B, Karaca B, Sanli UA, Uslu R. Capecitabine maintenance therapy following docetaxel/capecitabine combination treatment in patients with metastatic breast cancer. Oncol Lett. 2015;10:2598-2602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Segura-González M, Quintana-Quintana M. Systemic treatment with capecitabine as maintenance therapy in patients with recurring or metastatic breast cancer: experience in the Oncology Hospital, National Medical Center Siglo XXI, Mexican Social Security Institute. Med Oncol. 2015;32:93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Si W, Zhu YY, Li Y, Gao P, Han C, You JH, Linghu RX, Jiao SC, Yang JL. Capecitabine maintenance therapy in patients with recurrent or metastatic breast cancer. Braz J Med Biol Res. 2013;46:1074-1081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 68. | Bolzacchini E, Nigro O, Inversini D, Giordano M, Maconi G. Intestinal metastasis from breast cancer: Presentation, treatment and survival from a systematic literature review. World J Clin Oncol. 2021;12:382-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Rudland PS, Platt-Higgins A, El-Tanani M, De Silva Rudland S, Barraclough R, Winstanley JH, Howitt R, West CR. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 2002;62:3417-3427. [PubMed] |
| 70. | Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, van de Wouw AJ, Peters FP, van Riel JM, Peters NA, de Boer M, Peer PG, Tjan-Heijnen VC. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer. 2015;112:1445-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |