Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.102804
Revised: December 24, 2024
Accepted: January 15, 2025
Published online: March 15, 2025
Processing time: 107 Days and 5.9 Hours
Gastric neuroendocrine carcinomas (NECs) exhibit aggressive features, such as rapid growth, higher rate of metastasis, and a generally unfavorable prognosis compared to gastric adenocarcinoma. As a result, therapeutic options for NECs remain limited, contributing to the poor prognosis of patients. Immunotherapy has emerged as a promising treatment strategy and demonstrated the potential to partially improve the survival and prognosis of patients with NECs. Nevertheless, the unique clinical response termed pseudoprogression (PsP) has garnered considerable attention in the context of immunotherapy.
Presented here is a case of NEC recurrence five and a half months after radical gastric surgery. The 45-year-old male patient underwent combination treatment involving a PD-1 blocker and tyrosine kinase inhibitors and encountered two instances of PsP during treatment. The patient ultimately achieved a durable treatment response without altering his treatment regimens, resulting in a substantial therapeutic benefit.
This case report aimed to provide the authors’ experience with the diagnosis of PsP and treatment strategies for PsP in ongoing immunotherapy.
Core Tip: Neuroendocrine carcinomas (NECs) are generally considered highly malignant, characterized by rapid growth, a high metastasis rate, and poor prognosis, and their malignancy is much higher than that of gastric adenocarcinoma. Immune checkpoint inhibitors are a new type of immunotherapy drugs used in cancer treatment. However, the differences in clinical response and drug resistance pose significant challenges in immunotherapy. This article aims to report an extremely rare case of NEC, in which two significant pseudoprogression (PsP) occurred during treatment with PD-1 inhibitors combined with tyrosine kinase inhibitors. More rarely, this case had two PsP in the same organ under the same treatment plan. Besides, we mainly introduced the existing research on the possible mechanisms, timepoint, clinical manifestations, biomarkers, and medical imaging techniques associated with PsP.
- Citation: Mou YH, Zhang J, Shen H, Yu J, Han L, Li H, Li QF. Multiple pseudoprogressions during ongoing immunotherapy-based treatment of advanced gastric neuroendocrine carcinoma: A case report and review of literature. World J Gastrointest Oncol 2025; 17(3): 102804
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/102804.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.102804
Enterochromaffin cell-derived neuroendocrine neoplasms (NENs) were first described by Oberdorfer in 1907. Gastric NENs used to be an uncommon malignant entity, accounting for only about 0.1%-0.6% of all gastric cancers[1], but their incidence rate has increased almost 15-fold over time, likely due to advances in diagnostic techniques, technology, and increased endoscopic examinations in the population, allowing more cases to be identified[2]. In 2010, the World Health Organization classified NENs into grade 1 or 2 neuroendocrine tumors and grade 3 gastric neuroendocrine carcinomas (NECs), histologically according to the Ki-67 labeling index[3]. Generally considered to be highly malignant, NECs are characterized by a rapid growth, high metastasis rate, and unfavorable prognosis compared to other gastric malignant pathologies. Consequently, early diagnosis remains elusive for most cases, often leading to presentation at an advanced stage and/or with multiple organ metastases. Median survival has been reported to range from 7 to 46 months, with vascular and lymphatic invasion observed in 76.9%-81.5% and 73%-92.3% of cases, respectively[4].
Presently, surgical resection has typically been chosen as the primary therapeutic modality for individuals diagnosed with a localized NEC. However, in cases where surgical intervention is impractical due to late stage presentation or in metastatic diseases, alternative treatment modalities have become essential. The predominant approaches in such cases include the use of kinase inhibitors, somatostatin analogs, and the mTOR. In particular, platinum-based chemotherapy is recommended as the first-line curative option for NECs[5,6]. Unfortunately, the efficacy of these therapeutics often falls short in achieving sustained control over metastatic invasion in NECs.
In recent years, the landscape of cancer therapeutics has undergone a transformative shift with the advent of immunotherapy. Immunotherapeutic interventions, specifically the application of immune checkpoints, have shown remarkable success across various cancer types[7]. Consequently, a new paradigm in the treatment of NECs has emerged, focusing on the integration of immune checkpoint inhibitors (ICIs) for immunotherapy. This paradigm shift reflects a promising avenue to improve treatment outcomes and address the persistent challenges associated with metastatic control in NEC cases.
Immunotherapy represents an innovative therapeutic strategy that has achieved unprecedented clinical efficacy in the treatment of various types of advanced cancers. However, within the immunotherapy landscape, an unconventional response termed pseudoprogression (PsP) has emerged as a significant concern[8]. Initially documented in melanoma patients treated with ipilimumab, PsP is characterized by atypical manifestations, such as tumor enlargement or new lesion development in the absence of corresponding clinical deterioration, followed by tumor shrinkage or disease stabilization during the course of immunotherapy[8,9]. The early recognition of PsP is crucial for patient management, as it can prevent a premature discontinuation of potentially effective treatments and influence the selection and management of optimal sequential therapies. This case report presents a patient with NEC, who experienced two significant episodes of PsP during treatment with a combination of PD-1 inhibitors and tyrosine kinase inhibitors. Additionally, a literature review was conducted to further look into the pathogenesis and management of immunotherapy-related PsP.
Intermittent upper abdominal pain lasting over 3 months.
The patient experienced upper abdominal pain three months prior without any obvious cause, but did not experience any additional discomfort, such as bloating, bloody stools, vomiting, or belching. During this period, no special treatment was given. Later, as the pain persisted, the patient decided to seek further diagnosis and treatment, and hence visited our department of gastrointestinal surgery.
No underlying diseases in the past.
No family history of cancer.
Left upper abdominal tenderness, no other special symptoms.
No obvious abnormalities.
A comprehensive computed tomography (CT) scan unveiled thickening of the antral and pyloric walls accompanied by smooth serous membranes. Enlarged lymph nodes were identified in the upper and lower pyloric regions, along the lesser curvature of the stomach, and within hepatoduodenal ligaments, exhibiting characteristic features of malignancy.
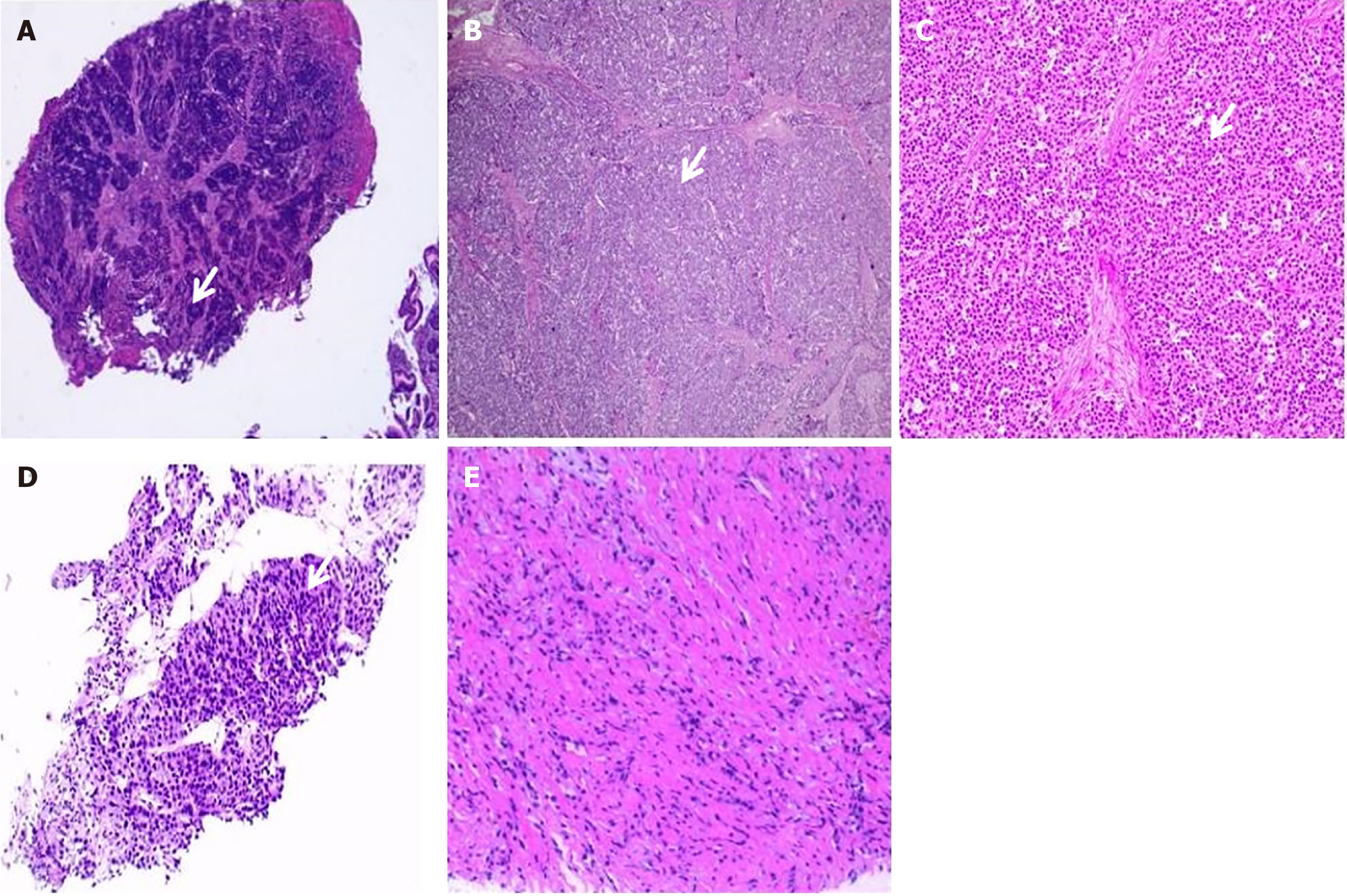
Gastroscopic examination revealed the presence of a substantial antral ulcer. Subsequent biopsy pathology confirmed the diagnosis of NEC arising from the gastric antrum (Figure 1A). Further immunohistochemical analysis revealed the following results: HER-2 (0), PCK (+), Syn (+), Ki-67 (80%+), CK18 (+), P63 (-), TTF-1 (-), P40 (-), CgA (-), and CD56 (-).
Clinical staging classified the tumor as pT3N1M0 III.
The patient underwent laparoscopic radical distal gastrectomy coupled with enterolysis on October 31, 2021. Intraoperative exploration revealed an absence of metastatic lymph nodes in certain key areas, including the liver, transverse colon, mesenteric root, omentum majus, abdominal wall, and pelvic cavity. The spleen showed no evidence of swollen enlargement, and no nodules were observed. A solid, serosa-penetrating tumor measuring approximately 4 cm × 5 cm was identified in the lesser curvature of the antrum.
Postoperative pathological analysis confirmed the diagnosis of NEC of the stomach, which was an ulcerative type, characterized by necrosis and involving the full thickness of the gastric wall extending into the subserosal fibrous adipose tissue (pT3) (Figure 1B and C). Lymph node examination revealed cancer metastasis in 1 out of 64 lymph nodes (1/43 in the lesser curvature and 0/21 in the greater curvature). After surgery, the patient experienced complications, including duodenal fistula and intra-abdominal hemorrhage, which were effectively managed through symptomatic treatments, resulting in improved clinical condition. Postoperative pathologic staging classified the tumor as pT3N1M0 III. Subsequently, the patient underwent four cycles of adjuvant chemotherapy with a specific regimen of etoposide (0.16 g on days 1-3) and cisplatin (40 mg on days 1-3).
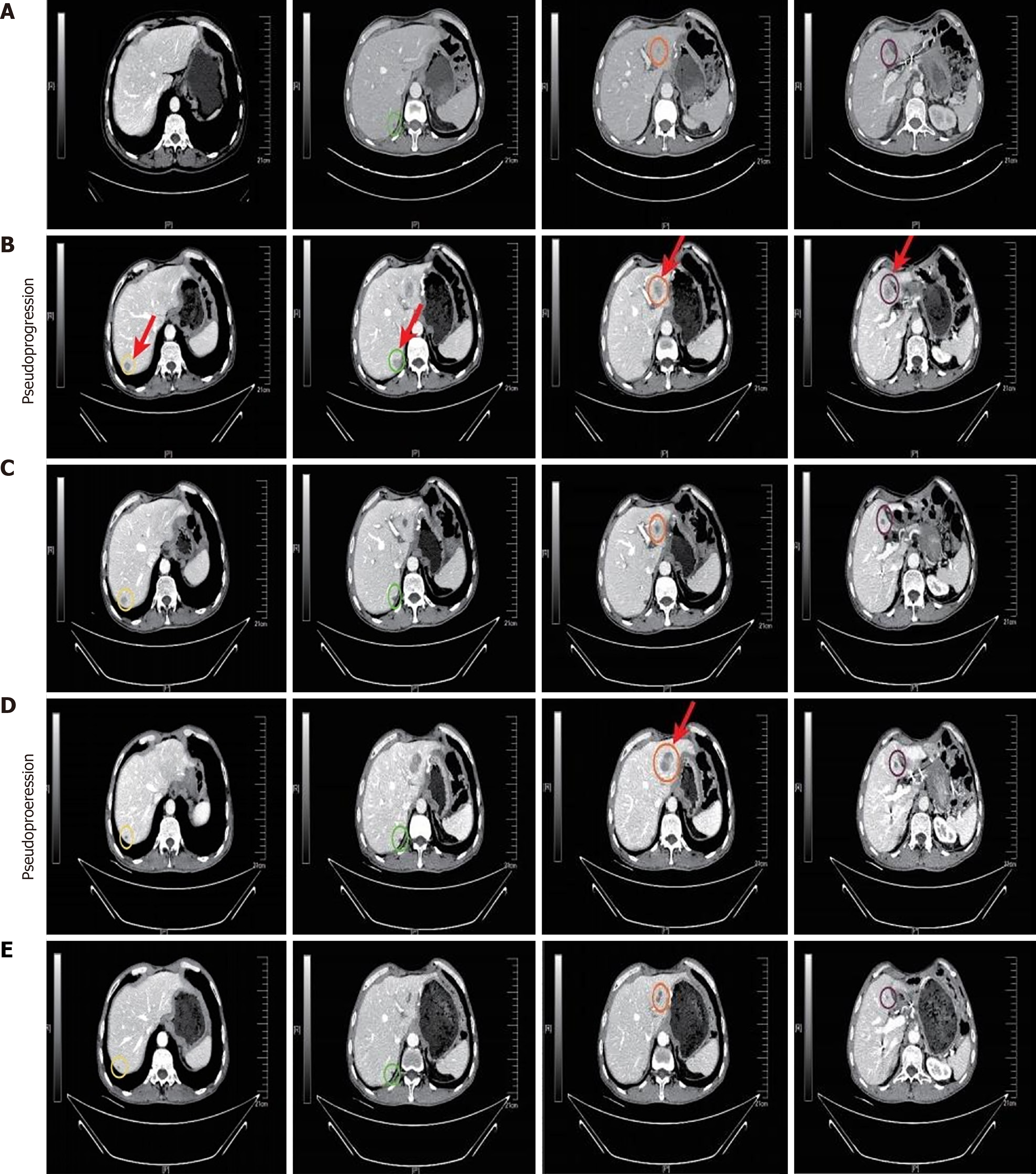
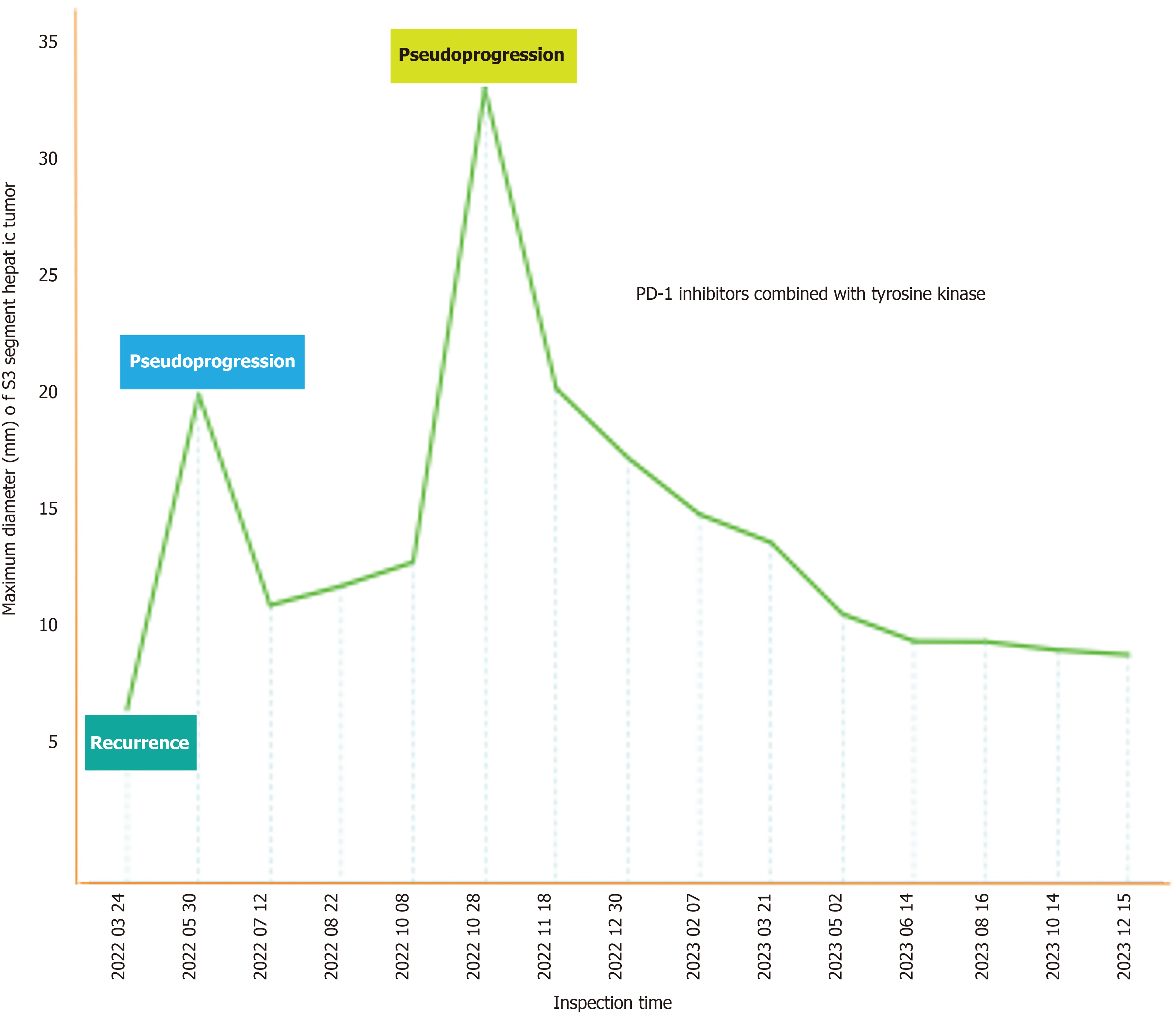
On March 15, 2022, a repeat CT scan revealed the presence of multiple nodular shadows in the liver, a phenomenon not seen on the preceding scan (Figure 2A). Fully communicate with imaging doctors and believe that the newly added liver lesions are tumor recurrence. The patient was definitively diagnosed with tumor recurrence and staged as rT0N0M1. Despite the option of chemotherapy, the patient opted against it and initiated a regiment of two cycles of teprotumumab (240 mg q3w) in combination with sulfatinib (300 mg/day) on April 19 and May 11, 2022. A follow-up CT scan on May 30, 2022 showed multiple metastatic lesions in the liver, indicating significant progression since the previous scan (Figure 2B). According to the iRECIST guideline, for patients whose clinical condition remains stable without deterioration, close imaging follow-up and continued immunotherapy are recommended. Notably, the patient here did not manifest any overt symptoms of discomfort. Considering the possibility of PsP, and after extensive communication with the patient and his family members, the decision was made to continue the current treatment of teprotumumab plus sulfatinib at the previously administered dose. Follow-up examinations after two additional cycles of anti-tumor treatment demonstrated a significant reduction in the size of multiple metastatic lesions within the liver (Figure 2C). Consequently, the anti-cancer immunotherapy regimen was maintained without further modification. To the best of our knowledge, this case marks the first documented instance of PsP during immunotherapy, with no similar case found to have been reported in the current literature.
During the scheduled follow-up visit on October 28, 2022, a significant change in the patient’s medical status was noticed. The CT scan revealed significant enlargements of certain lesions within the liver compared to previous assessments (Figure 2D). Given the rare occurrence of PsP associated with immunotherapy in this patient, a liver mass biopsy was undertaken to evaluate cancer progression. Pathological examination of the biopsy specimen revealed hepatic tissue with marked vascular fibrous tissue proliferation, and lymphocyte as well as plasma cell infiltration in selected areas, and the sporadic presence of atypical cells (Figure 1D and E). Due to the absence of overt discomfort symptoms in the patient, the occurrence of another PsP episode was considered likely. The pre-existing regimen of teprotumumab plus sulfatinib was continued. Following a single cycle of anti-cancer immunotherapy, a continued reduction in the size of the liver lesions was observed (Figure 2E), thereby further confirming our hypothesis that the patient had experienced a second PsP episode during the treatment process.
The most recent patient follow-up was conducted on December 15, 2023 and documented a sustained diminution in the sizes of the liver lesions without any significant manifestation of discomfort symptoms (Figure 3). Importantly, the Eastern Cooperative Oncology Group score remained at the optimal level of 0. This comprehensive clinical update underscores the continued efficacy and tolerability of teprotumumab plus sulfatinib as anti-cancer immunotherapy for managing the patient’s condition.
NECs represent a diverse group of malignancies arising from neuroendocrine cells that are widely distributed throughout the body, contributing to an increasing incidence rate[10]. NECs have an inherently heterogeneous nature characterized by their invasive biological behavior, which often results in metastasis to lymph nodes and various organs. ICIs have emerged as a groundbreaking treatment modality, with ICIs representing a novel category of anti-cancer drugs. The intricacies associated with clinical responses and the development of drug resistance present challenges in the field of immunotherapy. This article reports a singularly rare case of NEC exhibiting PsP during treatment with a combination of ICI and tyrosine kinase inhibitors, shedding light on the nuanced dynamics of this unconventional clinical response.
Immunotherapy represents a revolutionary paradigm shift in the therapeutic approach to tumors. In contrast to systematic chemotherapy or targeted therapy, immunotherapy introduces unique response patterns, including a prolonged duration of response, dissociated response, hyperprogression, and PsP[11]. The accurate assessment of PsP poses a significant challenge to clinicians. One potential mechanism underlying PsP may be related to the indirect effects of post-immunotherapy and delayed adaptive immune responses, which may allow or even promote progressive tumor growth until effective immune responses are reached[12,13]. Another plausible mechanism is the infiltration of lymphocytes into tumors induced by ICIs, triggering local tissue response. This cascade includes the release of antigens from decreased tumor cells, increased infiltration of inflammatory cells, edema, and abnormal vascular permeability, culminating in the striking enlargement of lesions[14,15]. In the context of our specific case, biopsies performed on the patient with PsP revealed the presence of fibrotic tissue in addition to lymphoid and plasma cell infiltration, with a remarkable absence of tumor-specific cells. This complex analysis sheds light on the multifaceted mechanisms at play during PsP and underscores the complexity inherent in its accurate evaluation.
A comprehensive review of PsP in various tumor types leaves the full spectrum of its incidence incompletely delineated, but it appears to be a rare occurrence. A careful review of the limited literature available underscores the prevalence of PsP in various cancers subjected to ICI therapy. The observed incidence rates include 4.6%-9.3% for me
Through literature search, 6 reports were found in the "PubMed" databas, totaling 11 cases of PsP of gastrointestinal tumors, comprising 7 cases of gastric cancer, 3 cases of colon cancer, and 1 case of a gastrointestinal stromal tumor[18,23-27]. The characteristics of these 11 cases are shown in Table 1. The most common manifestation of PsP in gastrointestinal tumors is new liver lesions, followed by lymph nodes and primary lesions. To the best of our knowledge, there are no reported cases of PsP associated with NEC patients undergoing immunotherapy treatment. In our new presented case, the NEC patient presented with PsP manifested as new liver lesions. Therefore, this case may help to better describe the frequency of this relatively rare tumor response in NEC and could help to shed light on the potential developmental patterns.
| Ref. | Cancer type | Pathological type | Age | Treatment | Time of pseudoprogression occurrence | Location of pseudoprogression |
| Zhang et al[18] | Gastric cancer | Adenocarcinoma | 65 | Oripalimab + Apatinib | 5 months | Postoperative recurrence of gastric cancer and continuous increase in the tumor near the pancreatic body |
| Satoyoshi et al[24] | Gastric cancer | Well-differentiated adenocarcinoma | 85 | Nivolumab | Three cycles of nivolumab (6 weeks later) | Enlargement of liver metastasis |
| Michalarea et al[23] | Gastric antrum | Poorly differentiated adenocarcinoma | 55 | PD-1 Ab+ VEGFR2 Ab | 2 cycles (8 weeks) | New nodal groups |
| Michalarea et al[23] | Gastric cancer | Adenocarcinoma | 54 | PD-1 Ab+ VEGFR2 Ab | 2 cycles | New liver metastases |
| Michalarea et al[23] | Gastroesophageal cancer | Adenocarcinoma | 64 | PD-1 Ab+ VEGFR2 Ab | First imaging assessment | New liver metastases |
| Michalarea et al[23] | Gastroesophageal cancer | Poorly differentiated adenocarcinoma | 64 | PD-1 Ab | 2 cycles | New liver metastases |
| Michalarea et al[23] | Gastroesophageal cancer | Poorly differentiated adenocarcinoma | 61 | PD-1 Ab | 2 months of treatment | New carinal, pretracheal, and retroperitoneal |
| Michalarea et al[23] | Rectal cancer | Adenocarcinoma | 31 | PD-1 Ab + MEK inhibitor | 6 months of treatment | The lung metastases relapsed |
| Tassinari et al[25] | Duodenal GIST | - | 78 | Imatinib | 1 month | Metabolic progression of the liver lesion Enlarged right hepatic lobe surface metastasis and mesenteric metastasis, along with numerous new |
| Chae et al[26] | Colorectal cancer | Adenocarcinoma | 61 | PD-L1 Ab + OX40 agonist | Week ten | Liver metastases and new periportal lymphadenopathy |
| Hutchings et al[27] | Cecum cancer | Moderately differentiated adenocarcinoma | 70 | Pembrolizumab | Two cycles of pembrolizumab | Postoperative recurrence of adenocarcinoma of the cecum |
Assessment of the tumor response in a classical radiological image of liver lesions after immunotherapy still poses significant challenges. PsP is almost always associated with a decrease in the solid tumor components. On the other hand, true progression usually involves an increase in solid tumors, unless the patient has cystic metastases, such as mucinous malignancies[28]. In many clinical cases, the imaging criteria for determining PsP are still uncertain and requires the availability of easily applicable non-invasive criteria and biomarkers.
PsP manifests in a variety of ways, with tumor enlargement or the appearance of new lesions being the most common. Rare presentations include pleural and pericardial perfusion, intestinal perforation, and brain progression, as well as lung tumor cavitation[29-33]. According to previous case reports, the majority of PsP patients do not experience any clinical deterioration, and in some cases may even show an improvement in tumor-associated symptoms. Nevertheless, there are atypical cases in which symptomatic PsP is evident, including conditions such as superior vena cava syndrome, respiratory dysfunction, hemoptysis, and systemic deterioration[17,29,34-36]. In the present case, the patient was clinically diagnosed as developing immunotherapy-associated PsP, without the manifestation of additional symptoms of discomfort. This nuanced analysis specifically emphasizes the complex spectrum of PsP presentations and highlights the importance of recognizing its diverse manifestations to enable an accurate clinical evaluation.
The definition of the exact timing of PsP remains an area of disagreement. PsP can manifest either in an early phase, within the first 12 weeks of immunotherapy treatment, or in a delayed manner, occurring after 12 weeks of treatment[16,17,29]. Studies by Hodi et al[37] and Fujimoto et al[38] suggested a higher prevalence of early PsP. Interestingly, PsP may occur even after the discontinuation of immunotherapy. In our specific case, PsP manifested successively at approximately 6 weeks and 27 weeks after the start of immunotherapy, adding a distinct temporal dimension to its occurrence. The absence of overt clinical symptoms led to the consideration of PsP, justifying the continuation of immunotherapy. Subsequent imaging follow-up supported this clinical perspective.
Findings from Wolchok et al[39] indicated that 9.7% of patients receiving ipilimumab had clinical reactions misclassified as progressive disease according to WHO criteria. The accurate identification and assessment of PsP are increasingly important, as the traditional RECIST guidelines have proven inadequate in distinguishing true progression from PsP. To address this limitation, Seymour et al[35] proposed the immune-based RECIST (iRECIST) in 2017, which introduced the concept of immune unconfirmed progressive disease (iUPD) for newly identified tumor lesions, requiring subsequent confirmation of progression. This guideline allows physicians to continue treatment beyond initial progression until a confirmed diagnosis is made, typically 4 to 8 weeks after initial assessment[13]. Note, in some literature, iUPD is used interchangeably with PsP[17]. If patients with iUPD continue to receive the original treatment plan and lesions continue to enlarge or new lesions appear, reaching the RECIST criteria, it is then classified as immune confirmed progressive disease, indicating true disease progression. However, if the tumor shrinks or stabilizes during immunotherapy, it is classified as PsP. Despite its utility, iRECIST has certain limitations, such as not considering serum markers and metabolic activity. Although widely used in clinical trials, its application in different clinical contexts requires caution due to the lack of a definitive consensus.
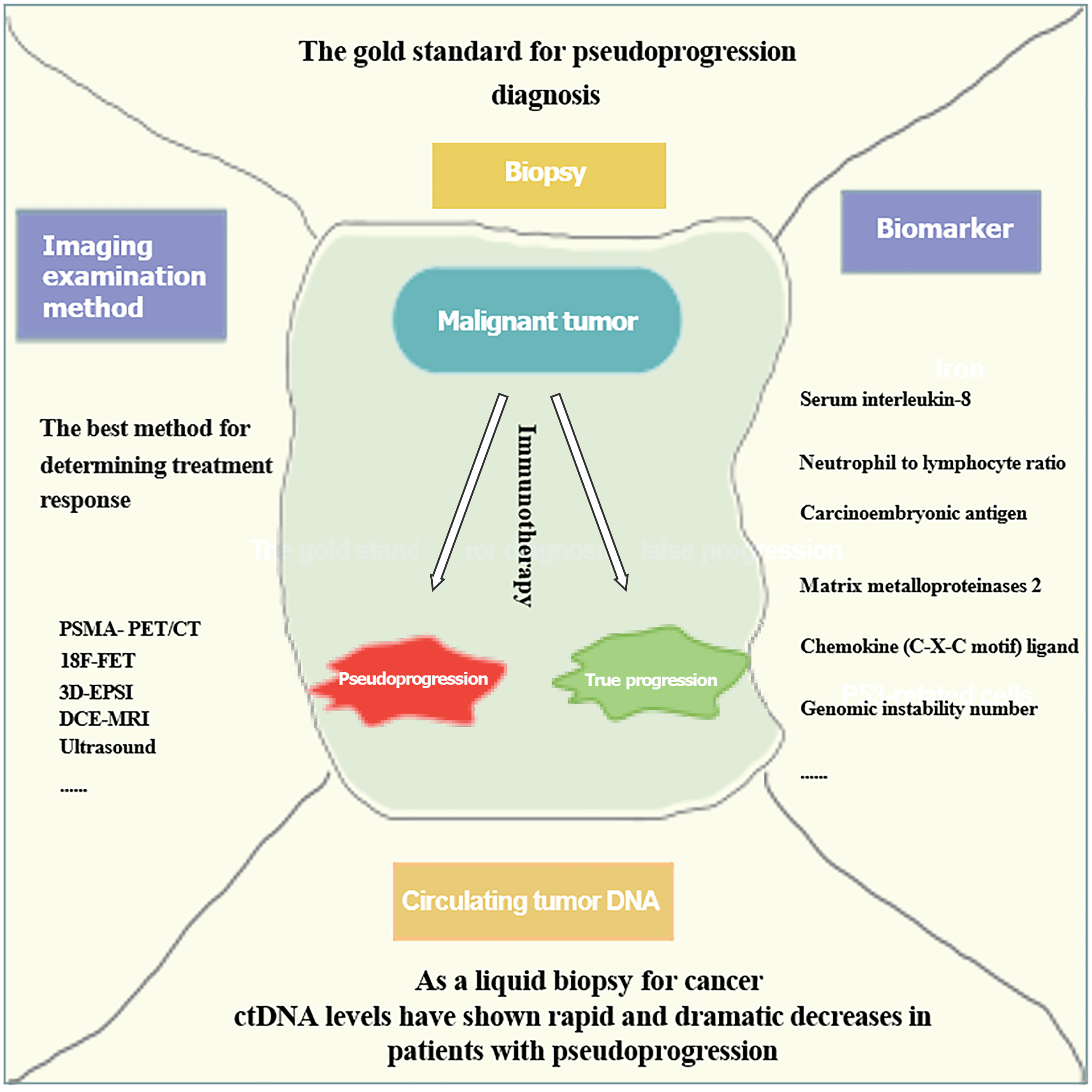
It is imperative to acknowledge that histopathological examination remains the gold standard for confirming PsP, although its accuracy may be compromised by inherent tumor heterogeneity[14]. Additionally, while puncture biopsy offers a pathological perspective, it is inherently invasive and limited in its ability to provide a comprehensive view of the entire tumor landscape. In clinical practice, a holistic assessment of disease progression requires the integration of blood-based markers, clinical presentation, imaging modalities, and biopsy findings. Therefore, we have established criteria for the confirmation of PsP that incorporate advanced medical imaging techniques and biomarkers (Figure 4).
Costa et al[40] demonstrated the use of radiotracers in aiding diagnosis, reporting that the robust radiotracer activity observed in new lesions and enlarged tumors by PSMA-PET/CT in prostate cancer patients could indicate PsP. Galldiks et al[41] evaluated 18F-FET, and found it had an accuracy of at least 85% in distinguishing true progression from PsP in glioblastoma. Verma et al[42] reported that 3D-EPSI had a sensitivity of 94% and a specificity of 87% in differentiating true progression from PsP in glioblastoma. Additionally, Chuang et al[43] and Umemura et al[44] suggested that DCE-MRI is promising for identifying PsP. Imafuku et al[45] highlighted the potential of ultrasound to detect patterns of decreased tumor blood flow for PsP identification[46].
Increasing evidence suggests that circulating tumor DNA (ctDNA) levels can predict disease recurrence, drug resistance, and therapeutic response, and may also serve as a promising indicator for prognostic prediction and the identification of PsP[47]. Lee et al[48] demonstrated that low levels of ctDNA could predict PsP with 100% specificity (95%CI: 60%-100%) and 90% sensitivity (95%CI: 68%-99%). Sanmamed et al[49] noticed that the serum level of IL-8 steadily decreased during PsP-driven disease progression, suggesting IL-8 could be a useful marker to differentiate PsP from true progressive disease (TPD). Kiriu et al[50] proposed that the neutrophil-to-lymphocyte ratio (NLR) has diagnostic potential for PsP, with PsP patients having a significantly lower NLR before and after immunotherapy compared to TPD patients. Surprisingly, Tanizaki et al[51] found that carcinoembryonic antigen (CEA) levels might aid in the identification of PsP, as they observed a decrease in CEA levels under immunotherapy. Moreover, Matsuo et al[52] and Jensen et al[53] suggested that monitoring the plasma levels of MMP2, CXCL2, and GIN may help differentiate PsP from true disease progression. Additionally, while certain imaging modalities and biomarkers have proven to be useful in the diagnosis of PsP, they remain controversial and require further in-depth research. Therefore, a deeper understanding of the molecular mechanisms underlying PsP and an exploration of the predicative factors are essential to improve diagnostic accuracy.
Zhou et al[29] performed a comprehensive analysis and showed that nearly all PD-1/PD-L1/CTLA-4 inhibitors within established therapeutic modalities can induce PsP. However, the extent to which the incidence of PsP varies among different immunotherapies requires further investigation. One important observation from a review suggested that the incidence of PsP was higher in melanoma when two immunotherapeutic agents were used compared to single-drug immunotherapy[16]. In particular, Andtbacka et al[54] confirmed an increased likelihood of PsP following human intratumoral immunotherapy, with up to 48% of melanoma patients experiencing PsP following intratumoral injections of talimogene laherparepvec. In addition, Trommer-Nestler et al[55] pointed out that the proportion of PsP in patients with brain metastasis who received radiotherapy was significantly lower than in those who received immunotherapy combined with radiotherapy.
Recent reports have described cases of PsP associated with ICIs combined with cytotoxic drugs, antiangiogenic agents, and chemotherapy[21,22,56-59]. However, the impact of combination therapy on the incidence and characteristics of PsP during ICI treatment remains uncertain. Further investigation is thus warranted to elucidate the potential influence of various immunotherapeutic agents and combination therapies on the incidence and nature of PsP, thereby contributing to a more nuanced understanding of this complex phenomenon in the context of immunotherapy.
Our case report has several limitations to note. First, we did not conduct comprehensive large-scale next-generation sequencing panels to explore the genomic characteristics of this tumor. Second, the follow-up period for this patient was relatively short. Lastly, we did not perform dynamic monitoring of the tumor markers in the patient.
This study presents a thorough report of a case of NEC with two episodes of PsP in the liver following an identical treatment regimen involving immunotherapy combined with tyrosine kinase inhibitors. Underscoring the rarity and unique nature of PsP in tumor responses, accurate assessment of this atypical response is critical to guide clinical decision-making and treatment strategies. Furthermore, assessing PsP in the field of immunotherapy remains fraught with multiple possibilities and uncertainties, emphasizing the need for continued exploration and a more detailed understanding of PsP.
| 1. | Matsubayashi H, Takagaki S, Otsubo T, Iiri T, Kobayashi Y, Yokota T, Shichijo K, Tada T, Satoh K, Iwafuchi M. Advanced gastric glandular-endocrine cell carcinoma with 1-year survival after gastrectomy. Gastric Cancer. 2000;3:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Yang Z, Wang W, Lu J, Pan G, Pan Z, Chen Q, Liu W, Zhao Y. Gastric Neuroendocrine Tumors (G-Nets): Incidence, Prognosis and Recent Trend Toward Improved Survival. Cell Physiol Biochem. 2018;45:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Köseoğlu H, Duzenli T, Sezikli M. Gastric neuroendocrine neoplasms: A review. World J Clin Cases. 2021;9:7973-7985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Iwamoto M, Gotoda T, Noda Y, Esaki M, Moriyama M, Yoshida N, Takayama T, Kobayashi H, Masuda S. Gastric Neuroendocrine Carcinoma with Rapid Progression. Intern Med. 2020;59:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Borga C, Businello G, Murgioni S, Bergamo F, Martini C, De Carlo E, Trevellin E, Vettor R, Fassan M. Treatment personalization in gastrointestinal neuroendocrine tumors. Curr Treat Options Oncol. 2021;22:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Raphael MJ, Chan DL, Law C, Singh S. Principles of diagnosis and management of neuroendocrine tumours. CMAJ. 2017;189:E398-E404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Maggio I, Manuzzi L, Lamberti G, Ricci AD, Tober N, Campana D. Landscape and Future Perspectives of Immunotherapy in Neuroendocrine Neoplasia. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Frelaut M, du Rusquec P, de Moura A, Le Tourneau C, Borcoman E. Pseudoprogression and Hyperprogression as New Forms of Response to Immunotherapy. BioDrugs. 2020;34:463-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | Di Giacomo AM, Danielli R, Guidoboni M, Calabrò L, Carlucci D, Miracco C, Volterrani L, Mazzei MA, Biagioli M, Altomonte M, Maio M. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 10. | Clift AK, Kidd M, Bodei L, Toumpanakis C, Baum RP, Oberg K, Modlin IM, Frilling A. Neuroendocrine Neoplasms of the Small Bowel and Pancreas. Neuroendocrinology. 2020;110:444-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, Goel S, Bedard P, Le Tourneau C. Novel patterns of response under immunotherapy. Ann Oncol. 2019;30:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 375] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 12. | Gainor JF, Longo DL, Chabner BA. Pharmacodynamic biomarkers: falling short of the mark? Clin Cancer Res. 2014;20:2587-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Berz AM, Dromain C, Vietti-Violi N, Boughdad S, Duran R. Tumor response assessment on imaging following immunotherapy. Front Oncol. 2022;12:982983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Chen MY, Zeng YC. Pseudoprogression in lung cancer patients treated with immunotherapy. Crit Rev Oncol Hematol. 2022;169:103531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015;33:3541-3543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 675] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 16. | Billan S, Kaidar-Person O, Gil Z. Treatment after progression in the era of immunotherapy. Lancet Oncol. 2020;21:e463-e476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 17. | Waxman ES, Lee Gerber D. Pseudoprogression and Immunotherapy Phenomena. J Adv Pract Oncol. 2020;11:723-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Zhang L, Tian T, Zhang Y, Yu S, Chen F, Qiao L, Hu P, Zhang J. Pseudoprogression during immunotherapy for gastric adenocarcinoma: A case report and literature review. J Cancer Res Ther. 2023;19:144-149. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Coudert V, Robin YM, Tessier W, Forestier A, Penel N. Two cases of rare late onset life-threatening pseudoprogression with immune check point inhibitors in advanced cancer patients - a case report. Acta Oncol. 2021;60:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Zhu W, Wu L, Wu J, Lin S, Fang C, Zhang H. Pseudoprogression Disease in a Patient with Small Cell Lung Cancer on Immune Checkpoint Inhibitor Therapy. Cancer Manag Res. 2023;15:905-911. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Odagiri N, Tamori A, Kotani K, Motoyama H, Kawamura E, Hagihara A, Fujii H, Uchida-Kobayashi S, Enomoto M, Kawada N. A case of hepatocellular carcinoma with "pseudoprogression" followed by complete response to atezolizumab plus bevacizumab. Clin J Gastroenterol. 2023;16:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 22. | Matsuki R, Okano N, Hasui N, Kawaguchi S, Momose H, Kitahama K, Nagahama K, Kogure M, Suzuki Y, Nagashima F, Shibahara J, Mori H, Sakamoto Y. Atezolizumab and Bevacizumab Combination Therapy and Sequential Conversion Hepatectomy for Advanced Fibrolamellar Hepatocellular Carcinoma Presenting Pseudoprogression. Liver Cancer. 2023;12:180-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Michalarea V, Fontana E, Garces AI, Williams A, Smyth EC, Picchia S, Rao S, Chau I, Cunningham D, Bali MA. Pseudoprogression on treatment with immune-checkpoint inhibitors in patients with gastrointestinal malignancies: Case series and short literature review. Curr Probl Cancer. 2019;43:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Satoyoshi R, Muto O, Masuda A, Kotanagi K, Kichiraku T, Kudoh K, Sawada T, Miyazawa H, Kotanagi H. A case of gastric cancer with delayed onset of tumor reduction effect by nivolumab therapy. Clin J Gastroenterol. 2019;12:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Tassinari E, Conci N, Battisti G, Porta F, Di Scioscio V, Pirini MG, de Biase D, Nigro MC, Iezza M, Castagnetti F, Lovato L, Fanti S, Pantaleo MA, Nannini M. Metabolic pseudoprogression in a patient with metastatic KIT exon 11 GIST after 1 month of first-line imatinib: a case report. Front Oncol. 2023;13:1310452. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Chae YK, Wang S, Nimeiri H, Kalyan A, Giles FJ. Pseudoprogression in microsatellite instability-high colorectal cancer during treatment with combination T cell mediated immunotherapy: a case report and literature review. Oncotarget. 2017;8:57889-57897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Hutchings K, Al Zaki A, Bhadkamkar N, Willis J. Symptomatic pseudoprogression in metastatic colorectal cancer. BMJ Case Rep. 2024;17. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Shinagare AB, Jagannathan JP, Krajewski KM, Ramaiya NH. Liver metastases in the era of molecular targeted therapy: new faces of treatment response. AJR Am J Roentgenol. 2013;201:W15-W28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Zhou L, Zhang M, Li R, Xue J, Lu Y. Pseudoprogression and hyperprogression in lung cancer: a comprehensive review of literature. J Cancer Res Clin Oncol. 2020;146:3269-3279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Song P, Zhang J, Shang C, Zhang L. Curative effect assessment of immunotherapy for non-small cell lung cancer: The "blind area" of Immune Response Evaluation Criteria in Solid Tumors (iRECIST). Thorac Cancer. 2019;10:587-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Kim HK, Baek SW, Jeong Y, Yang Y, Kwon J, Han HS, An JY, Woo CG, Lee OJ, Lee TG, Lee KH. Pseudoprogression presenting as intestinal perforation in non-small cell lung cancer treated with anti-PD-1: A case report. Mol Clin Oncol. 2019;11:132-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Melian M, Lorente D, Aparici F, Juan O. Lung brain metastasis pseudoprogression after nivolumab and ipilimumab combination treatment. Thorac Cancer. 2018;9:1770-1773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Masuhiro K, Shiroyama T, Nagatomo I, Kumanogoh A. Unique Case of Pseudoprogression Manifesting as Lung Cavitation After Pembrolizumab Treatment. J Thorac Oncol. 2019;14:e108-e109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Wang Q, Gao J, Wu X. Pseudoprogression and hyperprogression after checkpoint blockade. Int Immunopharmacol. 2018;58:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 35. | Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, Hodi FS, Therasse P, Hoekstra OS, Shankar LK, Wolchok JD, Ballinger M, Caramella C, de Vries EGE; RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143-e152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1521] [Cited by in RCA: 1696] [Article Influence: 212.0] [Reference Citation Analysis (0)] |
| 36. | Vrankar M, Unk M. Immune RECIST criteria and symptomatic pseudoprogression in non-small cell lung cancer patients treated with immunotherapy. Radiol Oncol. 2018;52:365-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, Joshua AM, Hersey P, Dronca R, Joseph R, Hille D, Xue D, Li XN, Kang SP, Ebbinghaus S, Perrone A, Wolchok JD. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol. 2016;34:1510-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 582] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 38. | Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Hata T, Kim YH, Tomii K, Ishida T, Hirabayashi M, Hara S, Ishitoko M, Fukuda Y, Hwang MH, Sakai N, Fukui M, Nakaji H, Morita M, Mio T, Yasuda T, Sugita T, Hirai T. Pseudoprogression in Previously Treated Patients with Non-Small Cell Lung Cancer Who Received Nivolumab Monotherapy. J Thorac Oncol. 2019;14:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 39. | Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412-7420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2311] [Cited by in RCA: 2455] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 40. | Costa LB, Queiroz MA, Barbosa FG, Nunes RF, Marin JFG, Dzik C, Buchpiguel CA. Pseudoprogression on PSMA PET imaging of a mCRPC patient under anti-PD1 treatment. Eur J Nucl Med Mol Imaging. 2019;46:1576-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Galldiks N, Kocher M, Langen KJ. Pseudoprogression after glioma therapy: an update. Expert Rev Neurother. 2017;17:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Verma G, Chawla S, Mohan S, Wang S, Nasrallah M, Sheriff S, Desai A, Brem S, O'Rourke DM, Wolf RL, Maudsley AA, Poptani H. Three-dimensional echo planar spectroscopic imaging for differentiation of true progression from pseudoprogression in patients with glioblastoma. NMR Biomed. 2019;32:e4042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Chuang MT, Liu YS, Tsai YS, Chen YC, Wang CK. Differentiating Radiation-Induced Necrosis from Recurrent Brain Tumor Using MR Perfusion and Spectroscopy: A Meta-Analysis. PLoS One. 2016;11:e0141438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 44. | Umemura Y, Wang D, Peck KK, Flynn J, Zhang Z, Fatovic R, Anderson ES, Beal K, Shoushtari AN, Kaley T, Young RJ. DCE-MRI perfusion predicts pseudoprogression in metastatic melanoma treated with immunotherapy. J Neurooncol. 2020;146:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Imafuku K, Hata H, Kitamura S, Yanagi T, Shimizu H. Ultrasonographic findings can identify 'pseudoprogression' under nivolumab therapy. Br J Dermatol. 2017;177:1726-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Ma Y, Wang Q, Dong Q, Zhan L, Zhang J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am J Cancer Res. 2019;9:1546-1553. [PubMed] |
| 47. | Ricciuti B, Jones G, Severgnini M, Alessi JV, Recondo G, Lawrence M, Forshew T, Lydon C, Nishino M, Cheng M, Awad M. Early plasma circulating tumor DNA (ctDNA) changes predict response to first-line pembrolizumab-based therapy in non-small cell lung cancer (NSCLC). J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 48. | Lee JH, Long GV, Menzies AM, Lo S, Guminski A, Whitbourne K, Peranec M, Scolyer R, Kefford RF, Rizos H, Carlino MS. Association Between Circulating Tumor DNA and Pseudoprogression in Patients With Metastatic Melanoma Treated With Anti-Programmed Cell Death 1 Antibodies. JAMA Oncol. 2018;4:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 49. | Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, Oñate C, Perez G, Alfaro C, Martín-Algarra S, Andueza MP, Gurpide A, Morgado M, Wang J, Bacchiocchi A, Halaban R, Kluger H, Chen L, Sznol M, Melero I. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol. 2017;28:1988-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 346] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 50. | Kiriu T, Yamamoto M, Nagano T, Hazama D, Sekiya R, Katsurada M, Katsurada N, Tachihara M, Kobayashi K, Nishimura Y. Pseudo-Progression and the Neutrophil-to-Lymphocyte Ratio in Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors: A Case-Control Study. Onco Targets Ther. 2019;12:10559-10568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Tanizaki J, Hayashi H, Kimura M, Tanaka K, Takeda M, Shimizu S, Ito A, Nakagawa K. Report of two cases of pseudoprogression in patients with non-small cell lung cancer treated with nivolumab-including histological analysis of one case after tumor regression. Lung Cancer. 2016;102:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 52. | Matsuo N, Azuma K, Hattori S, Ohtake J, Kawahara A, Ishii H, Tokito T, Yamada K, Shibata Y, Shimokawaji T, Kondo T, Kato T, Saito H, Yamada K, Sasada T, Hoshino T. Association between soluble immune mediators and tumor responses in patients with nonsmall cell lung cancer treated with anti-PD-1 inhibitor. Int J Cancer. 2019;144:1170-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Jensen TJ, Goodman AM, Kato S, Ellison CK, Daniels GA, Kim L, Nakashe P, McCarthy E, Mazloom AR, McLennan G, Grosu DS, Ehrich M, Kurzrock R. Genome-Wide Sequencing of Cell-Free DNA Identifies Copy-Number Alterations That Can Be Used for Monitoring Response to Immunotherapy in Cancer Patients. Mol Cancer Ther. 2019;18:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 54. | Andtbacka RH, Ross M, Puzanov I, Milhem M, Collichio F, Delman KA, Amatruda T, Zager JS, Cranmer L, Hsueh E, Chen L, Shilkrut M, Kaufman HL. Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial. Ann Surg Oncol. 2016;23:4169-4177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 55. | Trommer-Nestler M, Marnitz S, Kocher M, Rueß D, Schlaak M, Theurich S, von Bergwelt-Baildon M, Morgenthaler J, Jablonska K, Celik E, Ruge MI, Baues C. Robotic Stereotactic Radiosurgery in Melanoma Patients with Brain Metastases under Simultaneous Anti-PD-1 Treatment. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Zhang X, Huang H, Han L, Li T, Wang Z, Gao Q. Advanced Renal-Cell Carcinoma Pseudoprogression After Combined Immunotherapy: Case Report and Literature Review. Front Oncol. 2021;11:640447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 57. | Yang Z, Zhang G, Sun Q, Liu M, Shao J, Jiao S. Case Report: Pseudoprogression With Nivolumab and Bevacizumab Followed by Recurrent Immune-Related Pneumonitis in Urothelial Carcinoma With Lung Metastasis. Front Oncol. 2020;10:611810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Gonugunta AS, von Itzstein MS, Gerber DE. Pseudoprogression in advanced non-small cell lung cancer treated with combination chemoimmunotherapy: a case report. J Med Case Rep. 2022;16:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Zhao L, Yang Y, Li W, Li T, Han L, Lin H, Gao Q. Pseudoprogression: an indicator for cure in combined immunotherapy? Immunotherapy. 2019;11:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |