Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.102696
Revised: November 22, 2024
Accepted: December 25, 2024
Published online: March 15, 2025
Processing time: 111 Days and 20.1 Hours
Colorectal cancer (CRC) is the third most common cancer globally, causing over 900000 deaths annually. Risk factors include aging, diet, obesity, sedentary lifestyle, tobacco use, genetic predisposition, and inflammatory bowel disease. Despite current treatments, survival rates for advanced CRC remain low, high
To evaluate both the clinical significance and the pathological implications of the Kinesin family member 14 (KIF14) expression within CRC specimens. Addi
The expression of the KIF14 protein in CRC was analyzed using immunohistochemical staining. The integration of multicenter high-throughput data facilitated the calculation of the standardized mean difference (SMD) for KIF14 mRNA levels. The assessment of clinical and pathological impact was enhanced by analyzing combined receiver operating characteristic curves, along with measures of sensitivity, specificity, and likelihood ratios. Additionally, clustered regularly interspaced short palindromic repeats knockout screening for cell growth and single-cell sequencing were employed to validate the significance of KIF14 expression in CRC. Survival analysis established the prognostic value of KIF14 in CRC. The molecular mechanism of NC against CRC was elucidated through whole-genome sequencing and enrichment analysis, and molecular docking was utilized to explore the targeting affinity between NC and KIF14.
KIF14 was highly expressed in 208 CRC patients. Data from 17 platforms involving 2436 CRC samples and 1320 noncancerous colorectal tissue controls indicated that KIF14 expression was significantly higher in CRC samples, with an SMD of 1.92 (95%CI: 1.49-2.35). The area under the curve was 0.94 (95%CI: 0.92-0.96), with a sensitivity of 0.85 (95%CI: 0.78-0.90) and a specificity of 0.90 (95%CI: 0.85-0.93). The positive and negative likelihood ratios were 8.38 (95%CI: 5.39-13.02) and 0.17 (95%CI: 0.11-0.26), respectively. At the single-cell level, significant overexpression of KIF14 was observed in CRC cells (P < 0.001), with 35 CRC cell lines dependent on KIF14 for growth. The K-M plots demonstrated that KIF14 possesses prognostic value in CRC patients within the GSE71187 and GSE103679 datasets (P < 0.05). Binding energy calculations indicated that KIF14 is a potential target for NC (binding energy: 10.3 kcal/mol).
KIF14 promotes the growth of CRC cells and acts as an oncogenic factor, potentially serving as a therapeutic target for NC in the treatment of CRC.
Core Tip: This study evaluates the clinical and pathological significance of Kinesin family member 14 (KIF14) in colorectal cancer (CRC) and explores its interaction with nitidine chloride (NC) as a potential therapeutic target. High KIF14 expression in CRC was confirmed through immunohistochemistry and multiplatform mRNA analysis, with significant diagnostic accuracy. Clustered regularly interspaced short palindromic repeats screening and single-cell sequencing validated KIF14’s role in CRC cell growth. Survival analysis highlighted KIF14’s prognostic value. Molecular docking revealed a strong binding affinity between NC and KIF14, suggesting that KIF14 is a promising target for NC in CRC treatment.
- Citation: Qin K, Luo JY, Zeng DT, Huang WY, Li B, Li Q, Zhan YT, He RQ, Huang WJ, Chen G, Chen ZY, Chi BT, Tang YX, Tang RX, Li H. Kinesin family member 14 expression and its clinical implications in colorectal cancer. World J Gastrointest Oncol 2025; 17(3): 102696
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/102696.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.102696
Ranking as the third most prevalent cancer worldwide, colorectal cancer (CRC) presents a significant global health challenge, accounting for over 900000 deaths annually, underscoring its severity and the substantial burden it places on global health systems[1-3]. The incidence of CRC is influenced by several well-established risk factors, including aging, with a significant increase in risk after the age of 50[3]; lifestyle factors, such as dietary preferences marked by a high intake of processed meats and low fiber content[4], sedentary habits[5], obesity[6], and tobacco use[6]. Additionally, genetic predispositions and familial history further compound the risk, alongside environmental factors and pre-existing conditions, such as inflammatory bowel disease[7-10]. Current treatment strategies for CRC involve a combination of surgery, chemotherapy, and radiotherapy, which are selected based on the stage of the disease[11-13]. Despite ad
The ongoing difficulties in achieving optimal treatment outcomes reinforce the urgency of investigating the pathogenic mechanisms underlying CRC. A deeper understanding of the molecular pathways and genetic alterations involved in CRC development could lead to the identification of new therapeutic targets, enabling the creation of more effective and personalized treatment strategies. Thus, exploring the mechanisms of the disease is crucial not only for understanding CRC biology but also for pioneering novel approaches to tackle this challenging condition.
Derived from the root of Zanthoxylum nitidum, nitidine chloride (NC) is a benzophenanthridine alkaloid with notable bioactivity[14]. It is primarily known for its broad spectrum of pharmacological properties, including anti-inflammatory and antimicrobial effects, which facilitate its use in treating various non-neoplastic diseases[15,16]. In oncology, NC has garnered attention due to its potent antitumor activity and has been extensively researched for its efficacy across a spectrum of cancers, including breast, lung, prostate, gastric, and leukemia, demonstrating potential in triggering cell cycle arrest, enhancing apoptotic processes, and reducing angiogenesis[17-21]. Although NC has shown therapeutic promise in several cancers, its application in CRC treatment remains underexplored. Initial studies suggest that it may inhibit CRC cell growth, pointing to an underexplored area in the current cancer research landscape[22]. This gap reveals the urgent need for dedicated studies to explore the possible therapeutic effects of NC on CRC, potentially broadening its use in oncology.
The Kinesin family member 14 (KIF14) gene is located on chromosome 1q32.1 and encodes a motor protein critical for mitotic spindle assembly and cytokinesis[23]. KIF14 is crucial for cellular division, influencing chromosome segregation and the progression of the cell cycle[24,25]. Elevated levels of KIF14 have been documented in various cancers, such as ovarian, breast, lung, and brain. This overexpression is associated with adverse outcomes, signifying progression toward more aggressive tumor characteristics[26-29]. However, the interactions among KIF14 expression, NC, and CRC are not well understood and require thorough investigation.
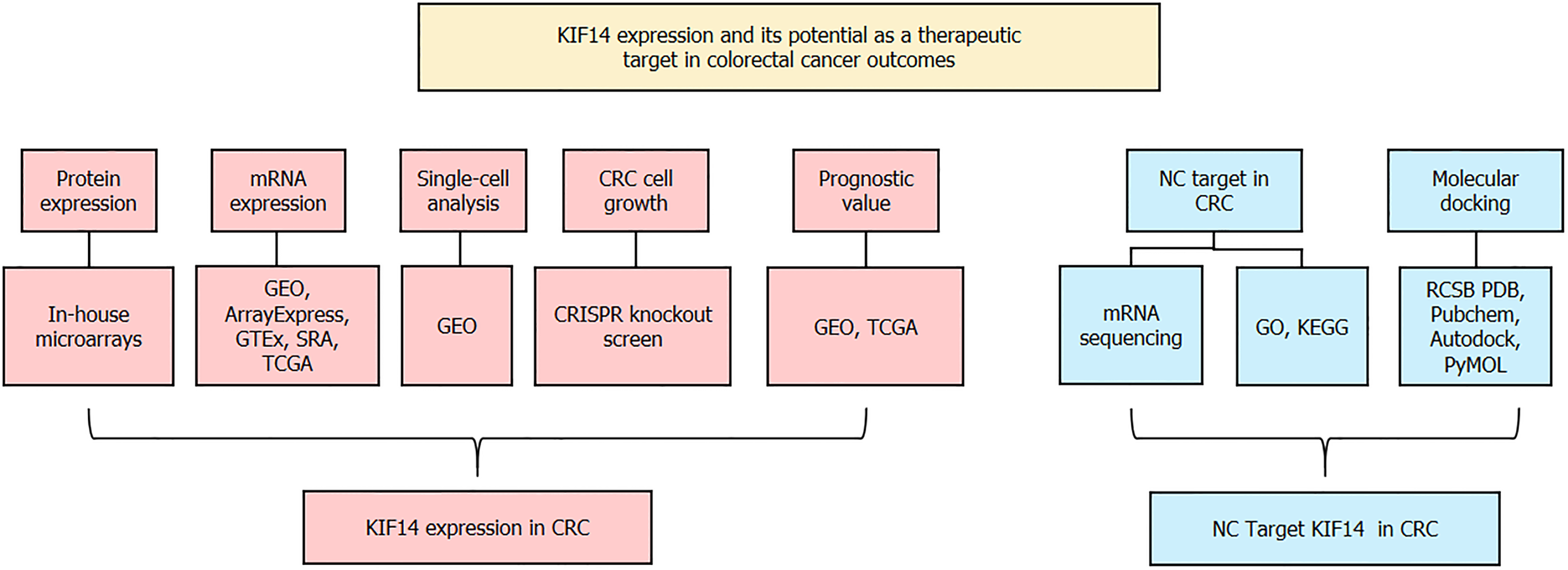
This study delineates the expression dynamics of KIF14 in CRC using immunohistochemistry (IHC) and integrates multicenter high-throughput data to derive the standardized mean difference (SMD) of KIF14 mRNA levels. Comprehensive evaluations, including sensitivity, specificity, and likelihood ratios, were conducted alongside survival analysis to establish KIF14’s prognostic significance in CRC. Clustered regularly interspaced short palindromic repeats (CRISPR) knockout screening and single-cell sequencing further authenticated the role of KIF14. The antagonistic effect of NC on CRC was explored through molecular docking and enrichment analyses to identify potential therapeutic targets.
Analyzing the differential expression of the KIF14 protein in CRC using IHC: This investigation employed tissue microarrays composed of 208 paired specimens from CRC and corresponding nonmalignant tissues to analyze the expression of the KIF14 protein. These microarrays were obtained from Yulin Red Cross Hospital, and only patients who had not undergone preoperative treatments were included. A detailed collection of clinicopathological features was assembled, encompassing data on age, sex, macroscopic type, vascular, nerve, and lymph node invasion, tumor node metastasis stage, clinical stage, and survival status. Ethical clearances were secured from Yulin Red Cross Hospital and The First Affiliated Hospital of Guangxi Medical University.
For histological evaluations, 3 μm thick sections were cut from paraffin-embedded blocks and heated at 65 °C for 30 minutes to remove the paraffin. The sections underwent dewaxing, followed by a 15-minute incubation in 3% H2O2 and subsequent rinsing in distilled water and phosphate-buffered saline (PBS). Immunostaining involved the use of a polyclonal rabbit anti-KIF14 antibody, after which the sections were washed with PBS, counterstained, dehydrated, cleared, and mounted. Adjacent noncancerous tissue sections served as positive controls, while PBS was used as the negative control.
IHC assessments were conducted on both CRC and adjacent noncancerous tissues, integrating the collected data. The stained slides were independently evaluated by two pathologists blind to the sample origins, focusing on the percentage of positively stained cells and the intensity of the staining. Cell staining positivity was assessed using a standardized scale: A score of 0 indicated no detectable expression, 1 denoted positive staining in less than 10% of cells, 2 was used for 10%-35% positivity, 3 corresponded to 36%-75% positivity, and 4 reflected greater than 75% positivity. The staining intensity was rated from 0 (no staining) to 3 (intense brown-yellow staining), with intermediate scores of 1 (faint cy
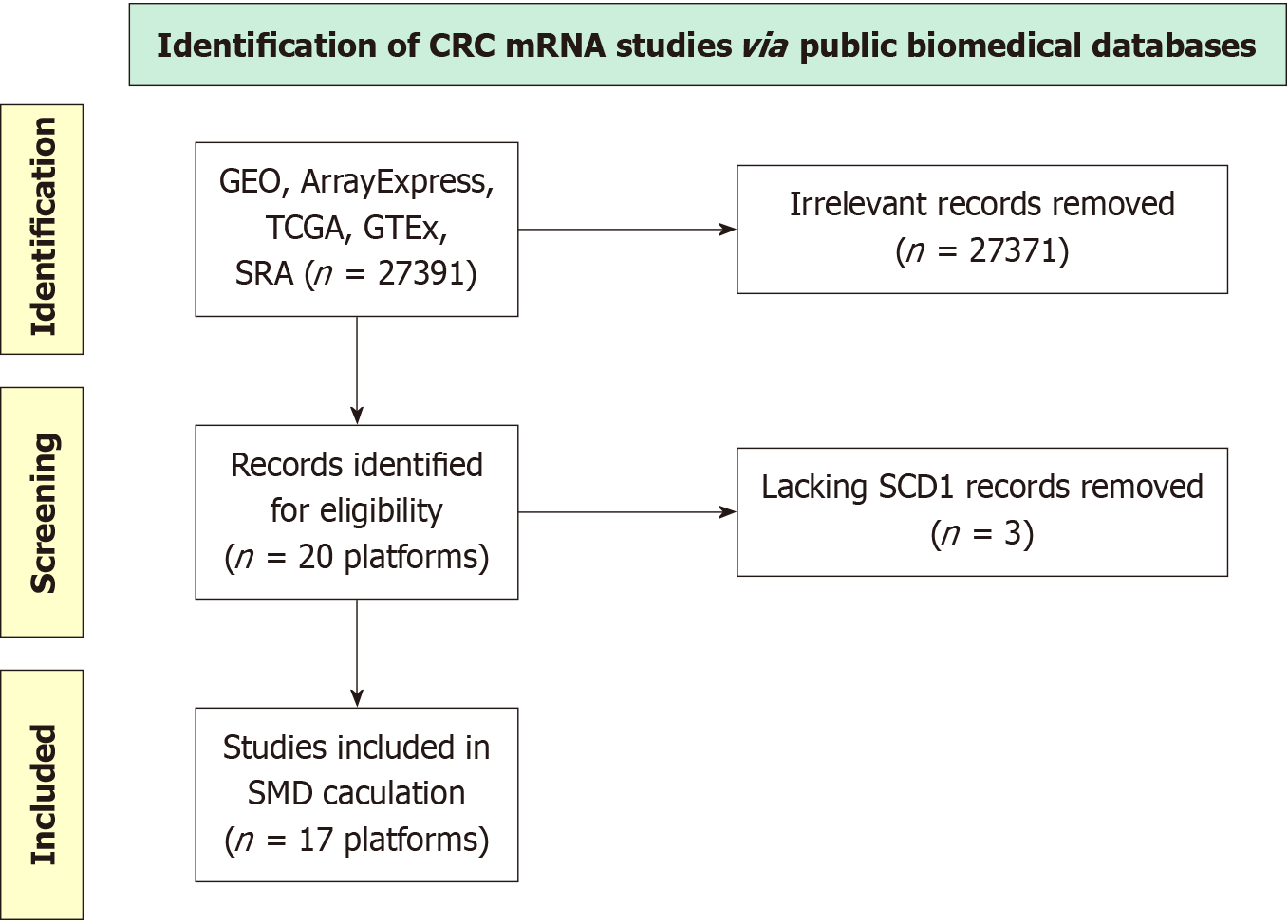
Analyzing the differential expression of KIF14 mRNA in CRC via multiple-center data: Detailed examinations were conducted to explore variations in KIF14 mRNA expression between CRC and non-CRC tissues. Data for these analyses were compiled from multiple databases, including the Gene Expression Omnibus (GEO), the Genotype-Tissue Expression project, The Cancer Genome Atlas (TCGA), ArrayExpress, and the Sequence Read Archive. The query ((colon) OR (rectal) OR (colorectal)) AND ((carcinoma) OR (cancer) OR (malignant) OR (tumor)) was utilized to retrieve the relevant data. The inclusion criteria required human samples with available KIF14 expression data and a minimum of three samples each for CRC tissues and nontumor controls. Samples that had received pretreatment interventions, such as drugs or radiation, or those that were genetically altered, were excluded. Additionally, sets with fewer than three samples, those devoid of KIF14 expression data, and samples identified as metastatic or recurrent CRC were also omitted from the study.
In the data integration phase, datasets from the same platform were combined into a single matrix. The mRNA expression data underwent normalization and were subsequently transformed to logarithmic values using the log2 (x + 1) method. To address batch effects, corrections were implemented using the limma and sva R packages.
Evaluating KIF14 expression in single-cell analysis of CRC samples: Single-cell RNA sequencing data from the GEO dataset GSE20097 were analyzed to examine the expression patterns of the KIF14 gene in individual CRC cells. The criteria for cell selection included having 200 to 2500 RNA features and mitochondrial DNA content below 5%, with the analysis conducted using the Seurat R package. The data were normalized using the NormalizeData function within Seurat. To mitigate batch effects, which are common when integrating data from different sequencing technologies, the Harmony package was employed.
Principal component analysis (PCA) was implemented using the FindVariableFeatures function, with a resolution parameter of 0.5. Dimensionality reduction was then performed using Uniform Manifold Approximation and Projection to analyze dimensions 1-20. The PCA results are depicted in a two-dimensional plot that clearly differentiates the KIF14 expression profiles between normal and CRC cells.
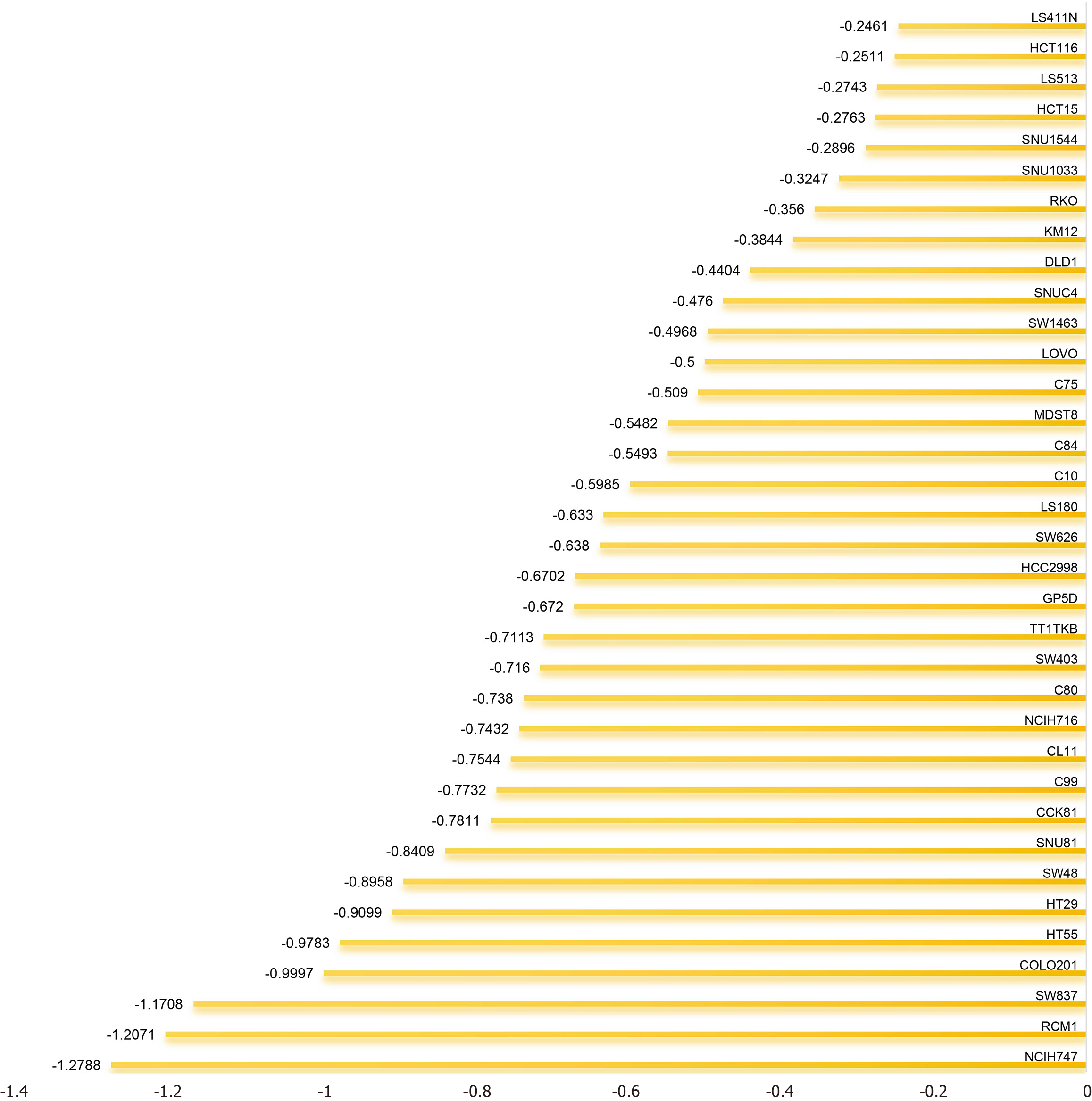
Investigating the role of KIF14 in regulating CRC cell proliferation: We utilized CRISPR knockout screening technology to elucidate the role of KIF14 in CRC cells. Dependency scores were calculated using the CERES algorithm to determine the impact of KIF14 across various CRC cell lines. Negative dependency scores indicated that KIF14 knockout impairs cell growth, underscoring its critical role in cell proliferation. Conversely, positive dependency scores, observed when KIF14 knockout resulted in increased growth, suggest that KIF14 may exert an inhibitory effect in these cell lines[33].
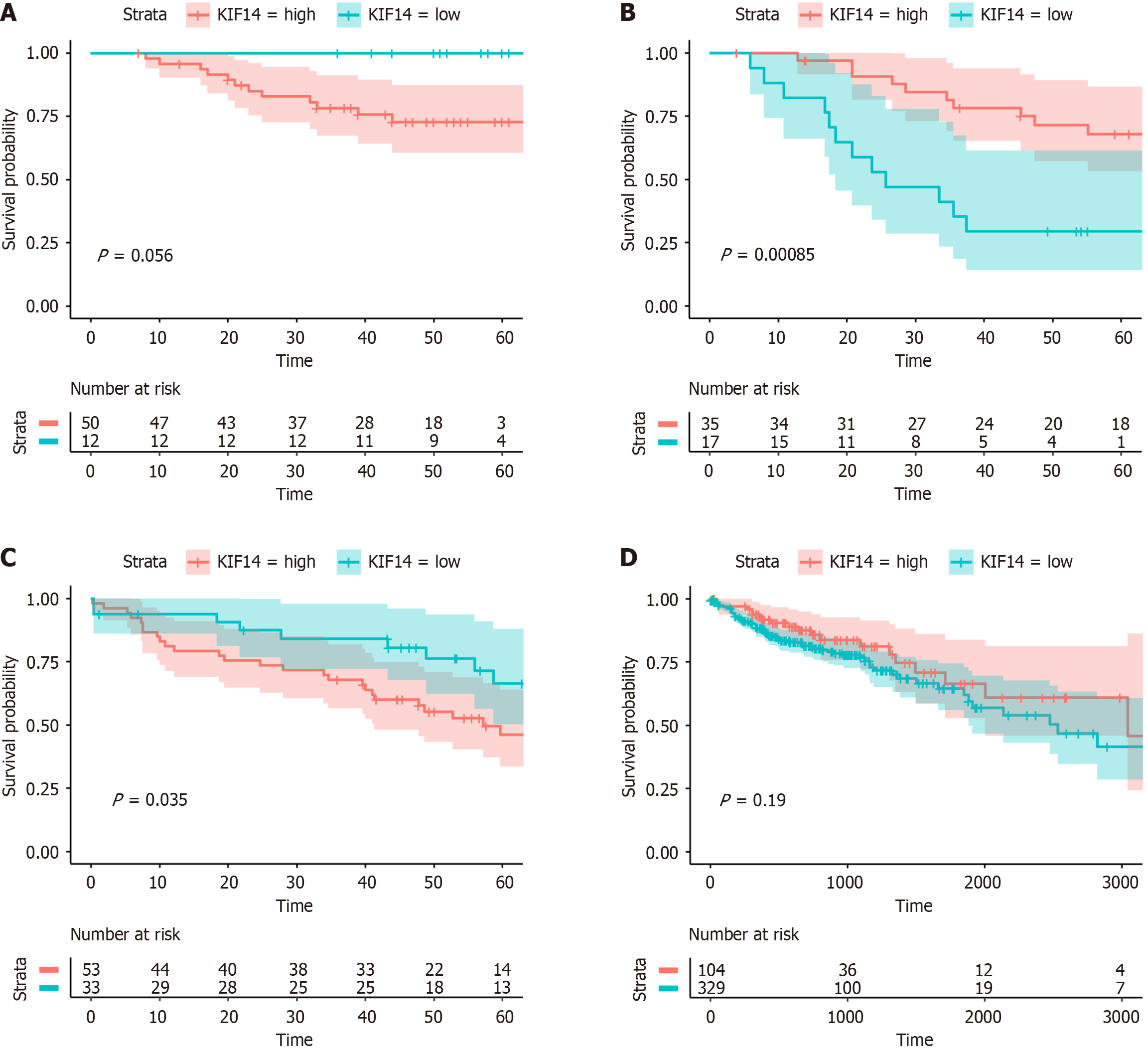
Evaluating the prognostic significance of KIF14 in CRC via survival analysis: This research assessed the prognostic significance of KIF14 expression levels in CRC patients using the survival and survminer R packages (version 4.3.1). The analysis drew upon patient data from TCGA and GEO databases. These patients were stratified into high- and low-KIF14-expression groups according to the median expression level of KIF14. Those in the upper median were classified as high-risk, whereas those below were considered low-risk. To determine the association between KIF14 expression and patient survival, the Cox proportional hazards model was employed, and differences in survival times across the CRC cohorts were analyzed using the log-rank test. Survival outcomes were visually depicted through Kaplan-Meier curves. A hazard ratio (HR) exceeding 1 and a log-rank test P value below 0.05 suggested that elevated KIF14 expression could potentially increase CRC risk.
Preparation of NC and its application in cell experiments: NC with a purity of 96%, acquired from Chengdu Herbpurify Co., Ltd., China, was initially dissolved in dimethyl sulfoxide (DMSO) sourced from Solarbio, China. This solution was further diluted with culture medium to prepare the required experimental concentrations. These concentrations were determined based on the 48-hour IC50 values obtained from an extensive dose-response analysis, ensuring distinct experimental conditions. Cells treated with DMSO alone constituted the negative control group in all experimental setups.
Maintenance of HCT116 CRC cells under optimal culture conditions: The HCT116 CRC cell line, sourced from the National Collection of Authenticated Cell Cultures and Procell Life Science & Technology Co., Ltd., was maintained in basic Roswell Park Memorial Institute 1640 Medium (1 ×) provided by Thermo Fisher, China. This medium was enriched with 10% fetal bovine serum and 1% penicillin-streptomycin solution (100 ×), both obtained from Solarbio, China. Culturing conditions were meticulously controlled at a constant temperature of 37 °C with 5% CO2 in a dedicated CO2 incubator.
Analysis of gene expression alterations post-NC treatment: For the gene expression studies, the total RNA was isolated from cells treated with the IC50 concentration of NC and the control group using the AxyPrepTM Multisource Total RNA Miniprep Kit from Axygen, China. The mRNA sequencing library was then constructed, which included steps such as mRNA purification, fragmentation, reverse transcription, adapter attachment, and PCR amplification. After library construction, sequencing was conducted on an Illumina platform.
This analysis measured the mRNA levels of annotated protein-coding genes and examined gene expression correlations within and between groups. Quantitative assessment was carried out using transcripts per kilobase million. The focus was on genes showing significant downregulation (log2 fold change < 1) with an adjusted P value less than 0.05, thereby identifying them as negatively regulated differentially expressed genes (DEGs) due to NC exposure.
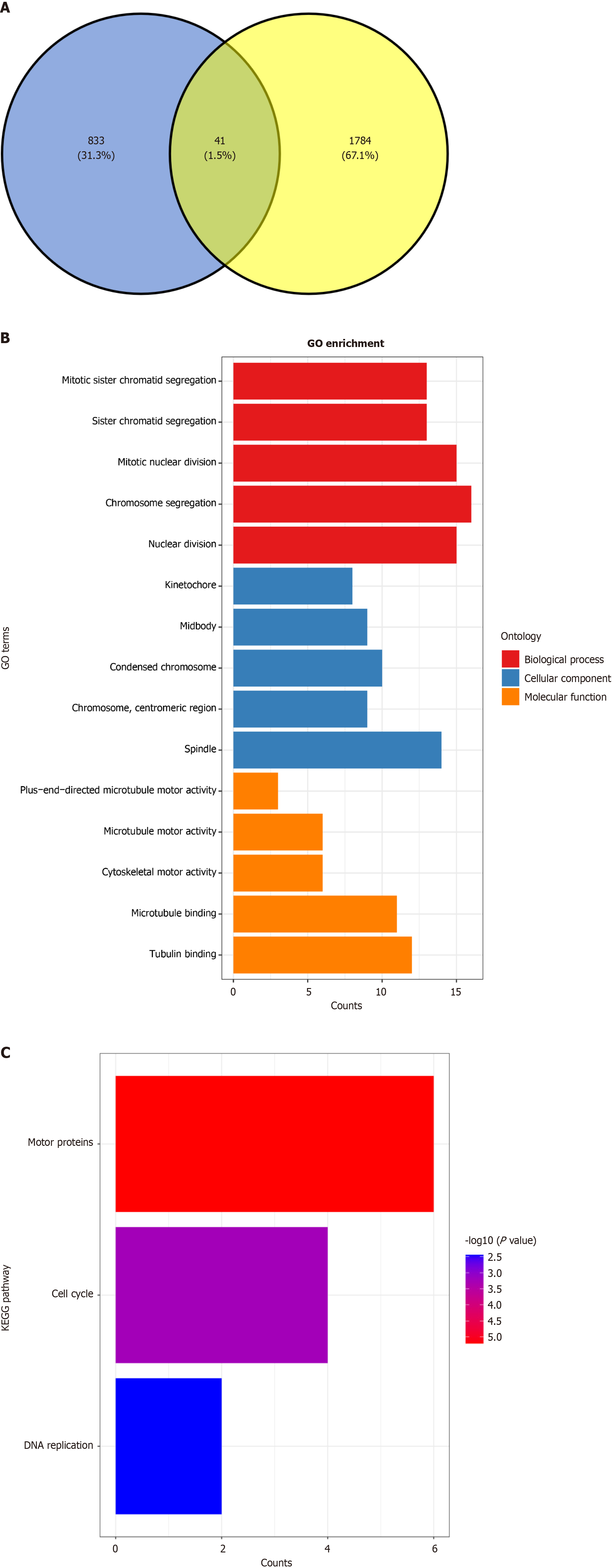
Analyzing NC targets in CRC: To explore the mechanisms through which NC affects CRC treatment, this investigation utilized Venn diagrams to pinpoint probable targets influenced by NC. Genes overexpressed in CRC were characterized based on three parameters: Presence in at least three distinct investigations, an SMD above zero, and a 95%CI not en
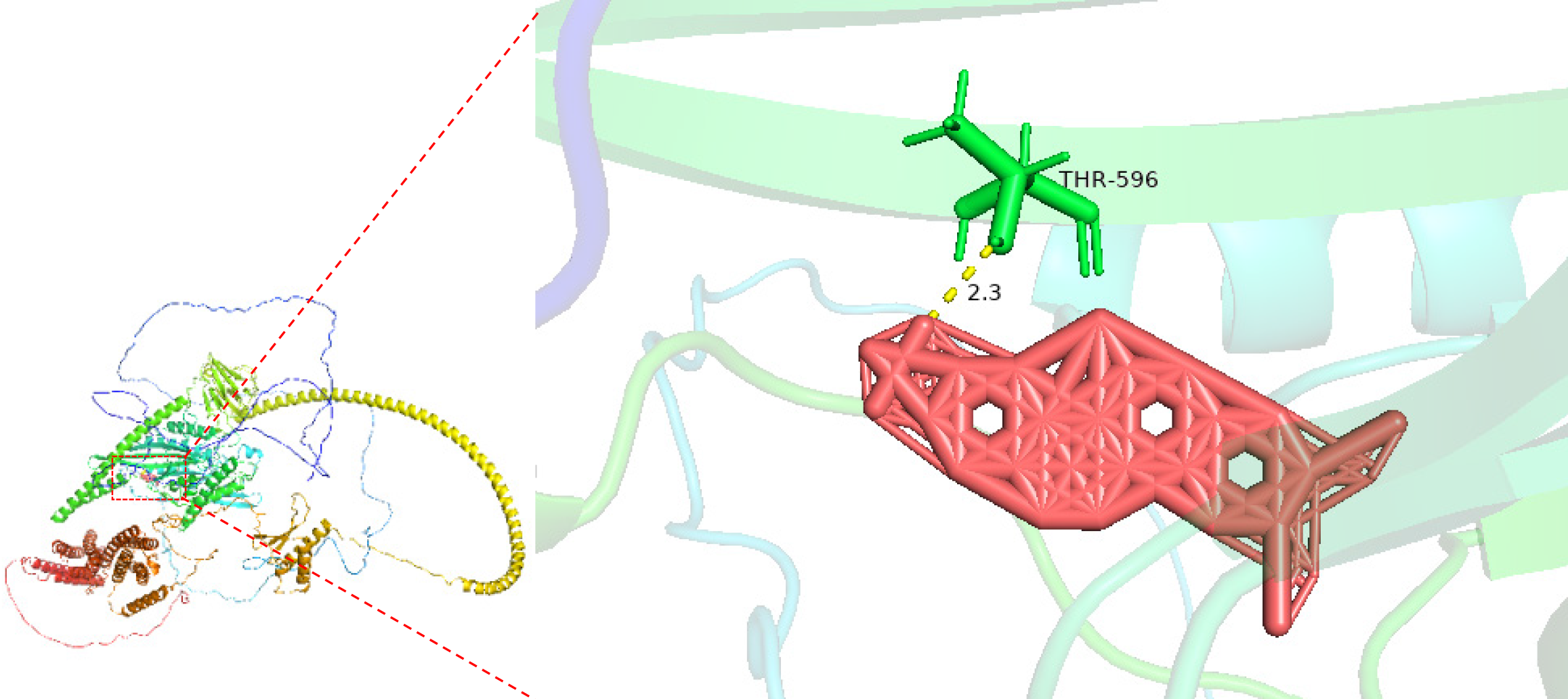
Docking targeted KIF14 in CRC using molecular methods: We explored potential CRC therapeutics by targeting KIF14 through molecular docking interactions with NC. The crystal structure of KIF14 (PDB ID: AF_AFQ15058F1) was sourced from the RCSB Protein Data Bank, with solvent molecules and cocrystallized ligands excluded from the analysis using PyMOL 2.4. The active sites for KIF14 were predicted utilizing POCASA 1.1, and the structure of NC was obtained from the PubChem database. Preparation of the KIF14 and NC structures for docking was performed using AutoDockTools. Molecular docking was conducted with AutoDock Vina 1.5.7, with a lower binding energy indicating a more stable interaction at the active site of KIF14. Visualization tools were subsequently employed to analyze this molecular in
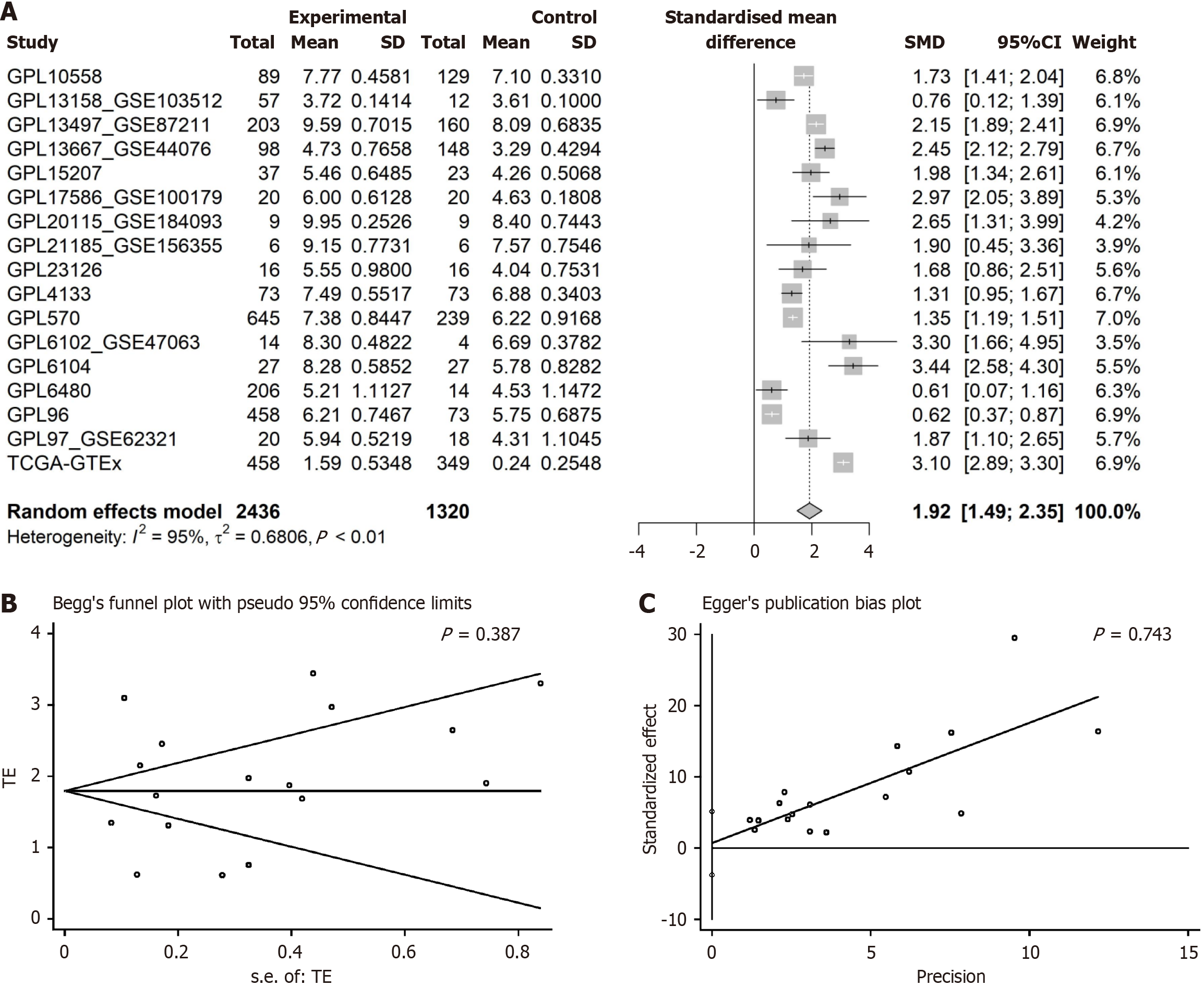
To mitigate the inherent variability and uncertainty associated with analyses based on single samples, a detailed evaluation was performed. We utilized both mRNA microarray and sequencing techniques to define the expression landscape of KIF14 in CRC tissues relative to their normal colorectal counterparts. The Wilcoxon rank-sum test, conducted using SPSS software version 22.0, was applied to quantify the expression discrepancies. These differences were visualized employing the ggplot2 package, with a threshold of P < 0.05 for determining statistical significance. Both the SMD and the 95%CI were calculated using STATA software version 12.0. The decision to use a random or fixed effects model was based on the extent of heterogeneity, assessed by the I2 statistic and χ2 tests. A random effects model was preferred if substantial heterogeneity was present (I2 > 50% and P < 0.05). Checks for publication bias were conducted using Begg’s and Egger’s tests, indicating no significant bias with P > 0.05. Additionally, the correlation between KIF14 protein levels and various clinicopathological characteristics was explored through independent t-tests or ANOVA, as dictated by the distribution characteristics of the data. The conceptual framework of this study is illustrated in Figure 1.
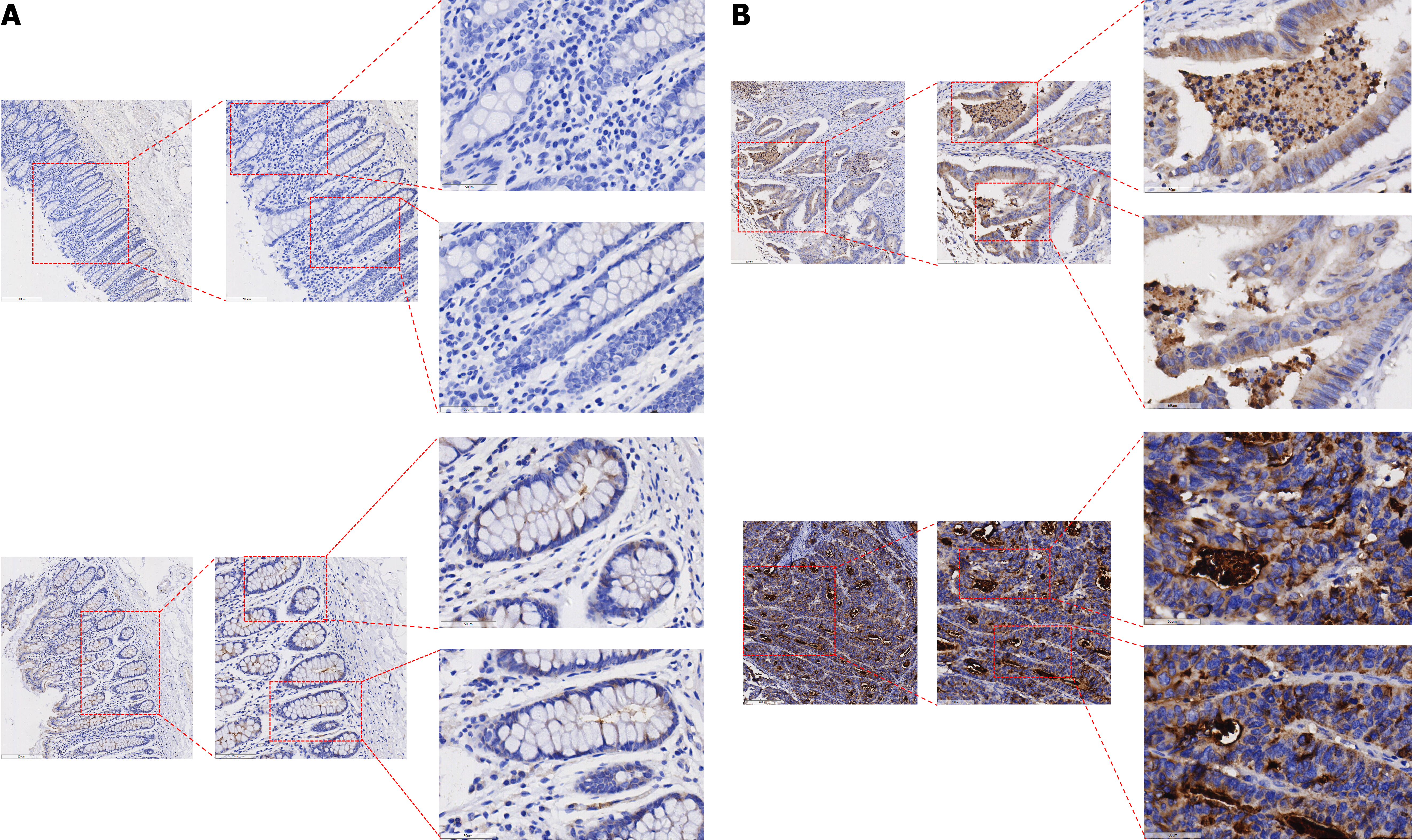
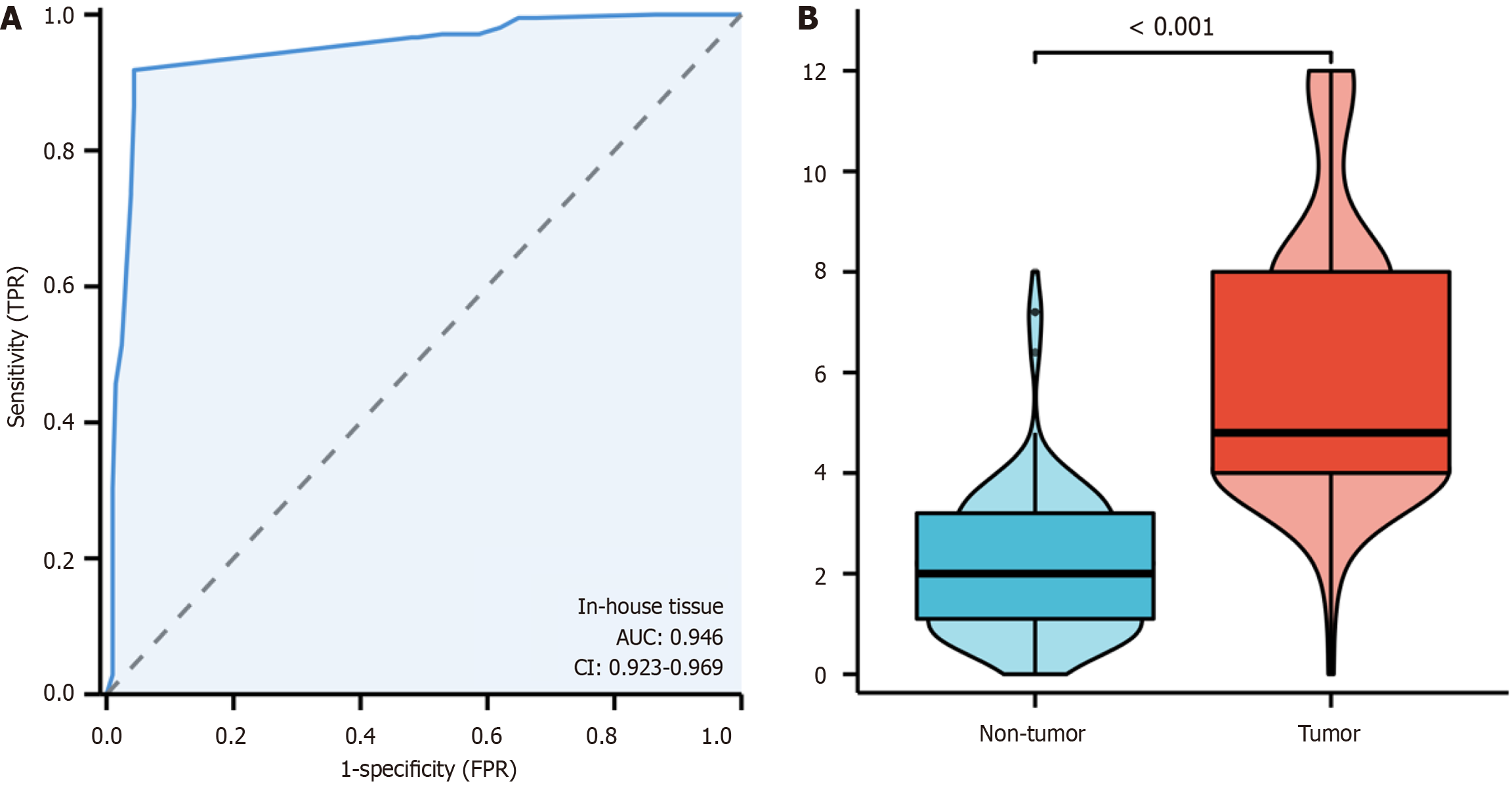
In this investigation, the levels of the KIF14 protein in CRC and adjacent noncancerous tissues were analyzed. IHC revealed only minimal to weak staining of KIF14 in noncancerous tissues (Figure 2A), whereas CRC tissues showed moderate to strong staining (Figure 2B). A quantitative evaluation of KIF14 expression in CRC was conducted using a violin plot and a receiver operating characteristic curve (ROC). The results from the ROC analysis indicated a significant upregulation of KIF14 in CRC tissues, with an area under the curve (AUC) of 0.946 (95%CI: 0.923-0.969), as detailed in Figure 3A. The findings confirmed that the expression of KIF14 in CRC tissues was significantly higher compared to noncancerous tissues (P < 0.001), as illustrated in Figure 3B. We also investigated the relationship between the KIF14 protein levels and the clinicopathological characteristics of CRC patients using independent-samples t-tests and ANOVA, which revealed no significant associations with clinicopathological parameters (P > 0.05).
Evaluating global-scale comprehensive datasets from microarray and sequencing analyses: Figure 4 illustrates the selection methodology utilized for the KIF14 mRNA dataset analysis, which covered 17 platforms. The study incor
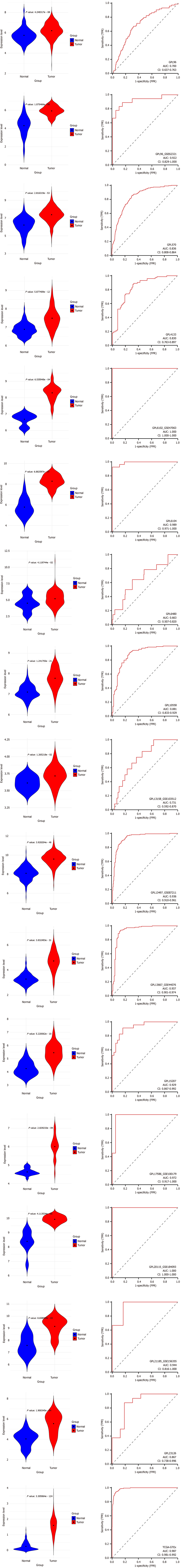
Upregulating KIF14 mRNA expression in CRC: The application of the Wilcoxon test to assess KIF14 expression levels between CRC and normal colorectal tissues indicated elevated KIF14 expression in CRC. Analysis leveraging data from various sources, utilizing a random effects model, confirmed this increase (SMD = 1.92, 95%CI: 1.49-2.35, I2 = 95%, P < 0.01), as demonstrated in Figure 5A. Furthermore, Begg’s and Egger’s tests showed an absence of publication bias (P = 0.387 and 0.743, respectively), as depicted in Figure 5B and C. The augmented expression of KIF14 mRNA was validated across the 17 platforms, indicating significant differences (P < 0.05, AUC ≥ 0.7), as shown in Figure 6.
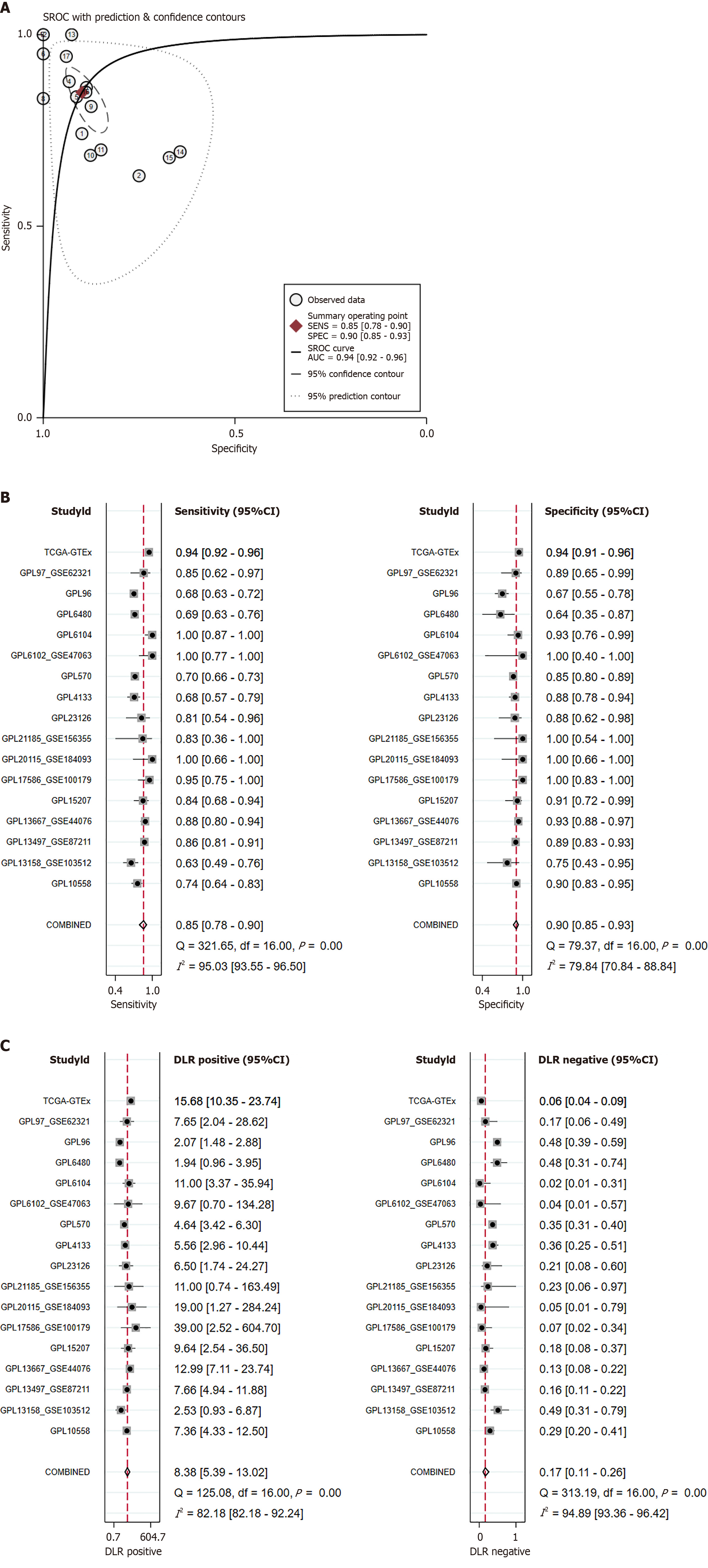
KIF14 demonstrates outstanding discriminatory potential in CRC: The ability of KIF14 to differentiate CRC from normal colorectal tissue was evaluated using the summary receiver operating characteristic (sROC) curve. The analysis revealed that the AUC for the sROC, sensitivity, and specificity were 0.94 (95%CI: 0.92-0.96), 0.85 (95%CI: 0.78-0.90), and 0.90 (95%CI: 0.85-0.93), respectively, as shown in Figure 7A and B. The positive diagnostic likelihood ratio (DLR) was recorded at 8.38 (95%CI: 5.39-13.02, I² = 82.18%), and the negative DLR was found to be 0.17 (95%CI: 0.11-0.26, I² = 94.89%), as depicted in Figure 7C. The findings from both the sROC curve and forest plot analysis affirm the significant capacity of KIF14 to accurately distinguish CRC from normal colorectal tissue.
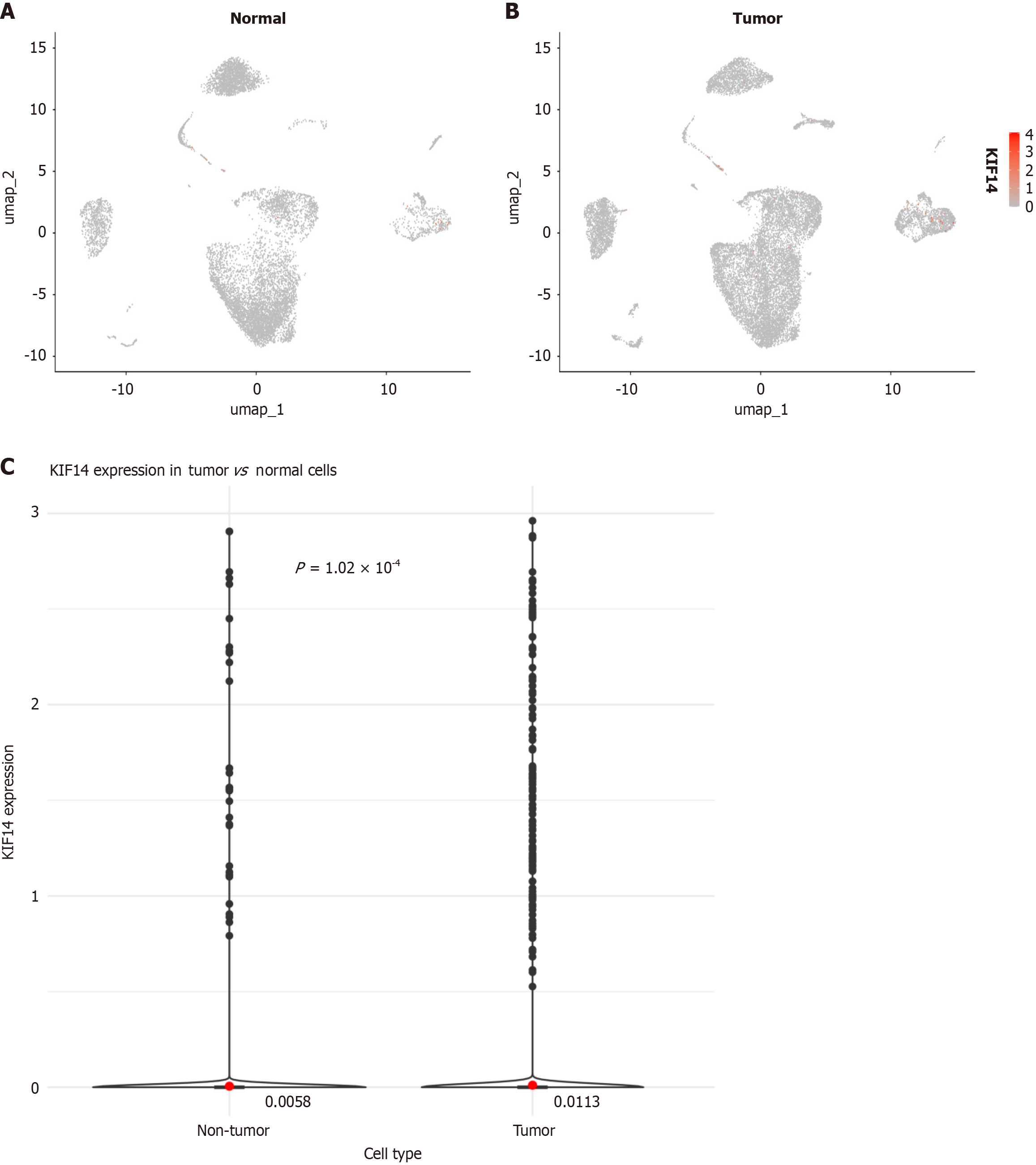
Investigating increased KIF14 expression in CRC via single-cell analysis: Following stringent quality control standards, a total of 18108 cells were extracted from CRC specimens, compared to 9901 cells from normal colorectal samples (Figure 8A and B). Additional investigations uncovered a significant increase in KIF14 expression in individual cells sourced from CRC tissues (P < 0.001), as shown in Figure 8C.
Promotion of CRC cell proliferation by the KIF14 gene: CRISPR knockout screens were utilized to examine the role of KIF14 in the proliferation of CRC cell lines, as depicted in Figure 9. Eliminating the KIF14 gene resulted in decreased proliferation rates across 35 distinct CRC cell lines, notably SW837, RCM1, and NCIH747. This suggests a critical function of KIF14 in enhancing cellular proliferation in CRC.
To investigate the association between KIF14 expression and the prognosis of CRC patients, we conducted Cox regression analysis and log-rank tests using data from TCGA, GSE12945, GSE71187, and GSE103679. K-M plots demonstrated that KIF14 possesses prognostic value in CRC patients within the GSE71187 and GSE103679 datasets (P < 0.05; Figure 10).
Research into the molecular pathways by which NC mitigates CRC has utilized both OEGs and DEGs. Our examination of CRC samples revealed 874 OEGs and 1825 DEGs associated with these analyses, with 41 genes identified as shared between these two categories. Further investigation of these overlapping genes included studies on GO and KEGG pathway enrichments, as shown in Figure 11A. The GO analysis indicated significant enrichments in biological processes (BPs), such as chromosomal segregation and nuclear division. Within the cellular component (CC) category, significant enrichments were observed in structures such as the spindle and condensed chromosomes, while the molecular function analysis showed notable activities in tubulin and microtubule binding, as detailed in Figure 11B. Additionally, the KEGG pathway analysis revealed critical pathways involving motor proteins and the cell cycle (Figure 11C).
Molecular modeling was employed to analyze the binding interactions between NC and KIF14 to investigate the inhibitory potential of NC on KIF14. It was found that NC effectively inhibits KIF14 through the formation of a hydrogen bond between them, as depicted in Figure 12.
In this study, we aimed to delineate the role of KIF14 in CRC and evaluate the viability of this gene as a therapeutic target. Our investigation provides a detailed analysis of KIF14 expression across various clinicopathological parameters and its association with CRC. We observed elevated expression levels of KIF14 in CRC tissues relative to their noncancerous counterparts.
Our comprehensive evaluation of KIF14’s role in CRC integrates data from multiple sources and a broad spectrum of samples. Furthermore, we have expanded the current understanding by demonstrating the dependency of CRC cell lines on KIF14 for growth. We also examined the prognostic significance of KIF14 by conducting survival analyses that confirmed its critical role in determining clinical outcomes for CRC patients.
In addition, our molecular docking results revealed that NC is an effective inhibitor that binds KIF14 with substantial affinity, highlighting its therapeutic potential. These comprehensive findings not only emphasize the crucial role of KIF14 in CRC progression but also set the groundwork for future therapeutic strategies targeting this essential molecule.
KIF14 is implicated in various cancer types, including breast cancer, ovarian cancer, gliomas, and CRC[29,34-36]. Collectively, these studies highlight the role of KIF14 in tumor progression and cellular proliferation. However, these findings have primarily been validated through in vitro experiments and animal models, with limited exploration in human tissue samples. In our extensive analysis across 17 distinct platforms, we noted increased expression of KIF14 in CRC tissues compared to noncancerous tissues. Our results are supported by evaluations at the mRNA and protein levels, CRISPR knockout screening, and single-cell analysis. Together, these results consistently demonstrate significant upregulation of KIF14 at the cellular level in CRC.
Previous research has explored the antitumor effects of NC, a benzophenanthridine alkaloid, in several types of cancer, including hepatocellular carcinoma, prostate cancer, and breast cancer[37-39]. Studies have demonstrated its ability to induce apoptosis and inhibit cell proliferation, highlighting its potential as a therapeutic agent in oncology[40,41]. The GO and KEGG pathway analyses from our current study add a deeper layer of understanding to the mechanistic actions of NC. The GO analysis revealed that NC significantly impacts BPs, such as chromosomal segregation and nuclear division. Such processes are essential for accurate cell division and are often dysregulated in cancer cells[42,43]. The enrichment in CCs, such as the spindle and condensed chromosomes, further supports the idea that NC targets and potentially disrupts the mitotic machinery of cells[44]. On the molecular level, our findings suggest that NC interacts with tubulin and microtubules, which are crucial for maintaining cell structure and integrity during division[45,46]. Additionally, the KEGG pathway analysis identified that NC influences pathways involving motor proteins and the cell cycle. These pathways are integral to the proper functioning of tumor cellular division and proliferation[47,48]. By engaging with motor proteins, NC can modify the dynamics of chromosome movement and segregation, potentially disrupting mitotic activities and inducing apoptosis. Therefore, this study suggests that the therapeutic efficacy of NC may be mediated through its interactions with KIF14. By targeting KIF14 and its role in cell cycle regulation and division, NC may effectively impede the progression of CRC.
Molecular docking is a pivotal computational technique in drug design that simulates the interaction between a small molecule and a protein at the atomic level[49,50]. This method helps predict the orientation and affinity of a molecule within a target protein’s active site, thereby elucidating potential inhibition mechanisms[51,52]. In our study, the interaction between NC and KIF14 highlights a potentially novel approach to targeting CRC pathways. Given KIF14’s role in cytokinesis and cellular proliferation, its inhibition could disrupt these essential processes in cancer cells. The theoretical interaction model developed here suggests a robust engagement between NC and KIF14, hinting at NC’s ability to stabilize in the binding pocket, potentially impeding KIF14’s function. This predicted interaction forms a basis for hypothesizing that NC could act as a potent inhibitor of KIF14.
In summary, the high expression of KIF14 in CRC tissues suggests its potential role as an oncogene in the pathogenesis and progression of CRC. Molecular docking between NC and KIF14 offers valuable insights for the treatment of CRC. However, this study is limited by several factors. The effects of KIF14 on the biological behavior of CRC have not yet been demonstrated through in vitro assays or animal models. Additionally, the mechanisms by which NC inhibits CRC require further exploration in both in vitro and in vivo experiments.
This study is limited by its reliance on a single cell line. Utilizing only one strain of cells may lead to results that are not generalizable across different cell types or biological contexts. This limitation calls for validation of the findings across multiple cell lines to ensure broader applicability and relevance to human CRC. Finally, larger clinical studies and additional experimental work are necessary to confirm these results.
In conclusion, this comprehensive analysis underscores the role of KIF14 as an oncogenic driver in CRC, promoting cellular proliferation and survival. Although KIF14 does not exhibit significant expression variations across different clinical and pathological contexts, it is consistently overexpressed in CRC tissues compared to noncancerous controls. This overexpression is associated with substantial prognostic potential. Consequently, these findings position KIF14 as a viable target for therapeutic intervention. Notably, NC emerges as a promising candidate, exhibiting a substantial binding affinity to KIF14, which may inhibit its oncogenic activity. Further exploration of NC’s mechanism of action against KIF14 could lead to effective targeted treatments for CRC.
We would like to thank “Guangxi Zhuang Autonomous Region Clinical Medicine Research Center for Molecular Pathology and Intelligent Pathology Precision Diagnosis” for providing technical support.
| 1. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3022] [Article Influence: 503.7] [Reference Citation Analysis (3)] |
| 2. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (2)] |
| 3. | Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 387] [Article Influence: 129.0] [Reference Citation Analysis (6)] |
| 4. | Hardt L, Mahamat-Saleh Y, Aune D, Schlesinger S. Plant-Based Diets and Cancer Prognosis: a Review of Recent Research. Curr Nutr Rep. 2022;11:695-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | An S, Park S. Association of Physical Activity and Sedentary Behavior With the Risk of Colorectal Cancer. J Korean Med Sci. 2022;37:e158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Sninsky JA, Shore BM, Lupu GV, Crockett SD. Risk Factors for Colorectal Polyps and Cancer. Gastrointest Endosc Clin N Am. 2022;32:195-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 7. | Rebuzzi F, Ulivi P, Tedaldi G. Genetic Predisposition to Colorectal Cancer: How Many and Which Genes to Test? Int J Mol Sci. 2023;24:2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 8. | Karpiński TM, Ożarowski M, Stasiewicz M. Carcinogenic microbiota and its role in colorectal cancer development. Semin Cancer Biol. 2022;86:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 9. | Feizi H, Rezaee MA, Ghotaslou R, Sadrkabir M, Jadidi-Niaragh F, Gholizadeh P, Taghizadeh S, Ghanbarov K, Yousefi M, Kafil HS. Gut Microbiota and Colorectal Cancer Risk Factors. Curr Pharm Biotechnol. 2023;24:1018-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Quaglio AEV, Grillo TG, De Oliveira ECS, Di Stasi LC, Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. 2022;28:4053-4060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 219] [Cited by in RCA: 221] [Article Influence: 73.7] [Reference Citation Analysis (115)] |
| 11. | Zhou H, Zhu L, Song J, Wang G, Li P, Li W, Luo P, Sun X, Wu J, Liu Y, Zhu S, Zhang Y. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol Cancer. 2022;21:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 153] [Article Influence: 51.0] [Reference Citation Analysis (1)] |
| 12. | Luo XJ, Zhao Q, Liu J, Zheng JB, Qiu MZ, Ju HQ, Xu RH. Novel Genetic and Epigenetic Biomarkers of Prognostic and Predictive Significance in Stage II/III Colorectal Cancer. Mol Ther. 2021;29:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 13. | Shinji S, Yamada T, Matsuda A, Sonoda H, Ohta R, Iwai T, Takeda K, Yonaga K, Masuda Y, Yoshida H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J Nippon Med Sch. 2022;89:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 14. | Lu Q, Luo S, Shi Z, Yu M, Guo W, Li C. Nitidine chloride, a benzophenanthridine alkaloid from Zanthoxylum nitidum (Roxb.) DC., exerts multiple beneficial properties, especially in tumors and inflammation-related diseases. Front Pharmacol. 2022;13:1046402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 15. | Lin C, Ge L, Tang L, He Y, Moqbel SAA, Xu K, Ma D, Zhou X, Ran J, Wu L. Nitidine Chloride Alleviates Inflammation and Cellular Senescence in Murine Osteoarthritis Through Scavenging ROS. Front Pharmacol. 2022;13:919940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Lu Q, Li C, Wu G. Insight into the inhibitory effects of Zanthoxylum nitidum against Helicobacter pylori urease and jack bean urease: Kinetics and mechanism. J Ethnopharmacol. 2020;249:112419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Sun M, Zhang N, Wang X, Li Y, Qi W, Zhang H, Li Z, Yang Q. Hedgehog pathway is involved in nitidine chloride induced inhibition of epithelial-mesenchymal transition and cancer stem cells-like properties in breast cancer cells. Cell Biosci. 2016;6:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Yu F, Tan W, Chen Z, Shen X, Mo X, Mo X, He J, Deng Z, Wang J, Luo Z, Yang J. Nitidine chloride induces caspase 3/GSDME-dependent pyroptosis by inhibting PI3K/Akt pathway in lung cancer. Chin Med. 2022;17:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 19. | Shi Y, Cao T, Sun Y, Xia J, Wang P, Ma J. Nitidine Chloride inhibits cell proliferation and invasion via downregulation of YAP expression in prostate cancer cells. Am J Transl Res. 2019;11:709-720. [PubMed] |
| 20. | Chen J, Wang J, Lin L, He L, Wu Y, Zhang L, Yi Z, Chen Y, Pang X, Liu M. Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer. Mol Cancer Ther. 2012;11:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Liu N, Li P, Zang S, Liu Q, Ma D, Sun X, Ji C. Novel agent nitidine chloride induces erythroid differentiation and apoptosis in CML cells through c-Myc-miRNAs axis. PLoS One. 2015;10:e0116880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Zhai H, Hu S, Liu T, Wang F, Wang X, Wu G, Zhang Y, Sui M, Liu H, Jiang L. Nitidine chloride inhibits proliferation and induces apoptosis in colorectal cancer cells by suppressing the ERK signaling pathway. Mol Med Rep. 2016;13:2536-2542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Ju JQ, Zhang HL, Wang Y, Hu LL, Sun SC. Kinesin KIFC3 is essential for microtubule stability and cytokinesis in oocyte meiosis. Cell Commun Signal. 2024;22:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 24. | Miyamoto I, Kasamatsu A, Yamatoji M, Nakashima D, Saito K, Higo M, Endo-Sakamoto Y, Shiiba M, Tanzawa H, Uzawa K. Kinesin family member 14 in human oral cancer: A potential biomarker for tumoral growth. Biochem Biophys Rep. 2015;3:26-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Loncar A, Rincon SA, Lera Ramirez M, Paoletti A, Tran PT. Kinesin-14 family proteins and microtubule dynamics define S. pombe mitotic and meiotic spindle assembly, and elongation. J Cell Sci. 2020;133:jcs240234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Lu X, Li G, Liu S, Wang H, Zhang Z, Chen B. Bioinformatics Analysis of KIF1A Expression and Gene Regulation Network in Ovarian Carcinoma. Int J Gen Med. 2021;14:3707-3717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Singel SM, Cornelius C, Zaganjor E, Batten K, Sarode VR, Buckley DL, Peng Y, John GB, Li HC, Sadeghi N, Wright WE, Lum L, Corson TW, Shay JW. KIF14 promotes AKT phosphorylation and contributes to chemoresistance in triple-negative breast cancer. Neoplasia. 2014;16:247-256, 256.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Wang H, Tang F, Tang P, Zhang L, Gan Q, Li Y. Noncoding RNAs-mediated overexpression of KIF14 is associated with tumor immune infiltration and unfavorable prognosis in lung adenocarcinoma. Aging (Albany NY). 2022;14:8013-8031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Xu H, Zhao G, Zhang Y, Jiang H, Wang W, Zhao D, Yu H, Qi L. Long non-coding RNA PAXIP1-AS1 facilitates cell invasion and angiogenesis of glioma by recruiting transcription factor ETS1 to upregulate KIF14 expression. J Exp Clin Cancer Res. 2019;38:486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Liu Z, Gu S, Lu T, Wu K, Li L, Dong C, Zhou Y. IFI6 depletion inhibits esophageal squamous cell carcinoma progression through reactive oxygen species accumulation via mitochondrial dysfunction and endoplasmic reticulum stress. J Exp Clin Cancer Res. 2020;39:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 31. | Li SH, Zhai GQ, He RQ, Chen G, Wang SS, Liu JL, Cheng JW, Yan HB, Huang ZG. Down-regulation and clinical significance of Sorbin and SH3 domain-containing protein 1 in bladder cancer tissues. IET Syst Biol. 2023;17:70-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 32. | Zhang W, Li GS, Gan XY, Huang ZG, He RQ, Huang H, Li DM, Tang YL, Tang D, Zou W, Liu J, Dang YW, Chen G, Zhou HF, Kong JL, Lu HP. MMP12 serves as an immune cell-related marker of disease status and prognosis in lung squamous cell carcinoma. PeerJ. 2023;11:e15598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 33. | Ho KH, Huang TW, Liu AJ, Shih CM, Chen KC. Cancer Essential Genes Stratified Lung Adenocarcinoma Patients with Distinct Survival Outcomes and Identified a Subgroup from the Terminal Respiratory Unit Type with Different Proliferative Signatures in Multiple Cohorts. Cancers (Basel). 2021;13:2128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Gerashchenko TS, Zolotaryova SY, Kiselev AM, Tashireva LA, Novikov NM, Krakhmal NV, Cherdyntseva NV, Zavyalova MV, Perelmuter VM, Denisov EV. The Activity of KIF14, Mieap, and EZR in a New Type of the Invasive Component, Torpedo-Like Structures, Predetermines the Metastatic Potential of Breast Cancer. Cancers (Basel). 2020;12:1909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Sishtla K, Pitt N, Shadmand M, O'Hare MN, Sulaiman RS, Sinn AL, Condon K, Pollok KE, Sandusky GE, Corson TW. Observations on spontaneous tumor formation in mice overexpressing mitotic kinesin Kif14. Sci Rep. 2018;8:16152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Wang ZZ, Yang J, Jiang BH, Di JB, Gao P, Peng L, Su XQ. KIF14 promotes cell proliferation via activation of Akt and is directly targeted by miR-200c in colorectal cancer. Int J Oncol. 2018;53:1939-1952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Li D, Liu S, Zhu J, Shen L, Zhang QY, Zhu H. Folic acid modified TPGS as a novel nano-micelle for delivery of nitidine chloride to improve apoptosis induction in Huh7 human hepatocellular carcinoma. BMC Pharmacol Toxicol. 2021;22:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Gulia S, Chandra P, Das A. Combating anoikis resistance: bioactive compounds transforming prostate cancer therapy. Anticancer Drugs. 2024;35:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 39. | Hao M, Liu W, Ding C, Peng X, Zhang Y, Chen H, Dong L, Liu X, Zhao Y, Chen X, Khatoon S, Zheng Y. Identification of hub genes and small molecule therapeutic drugs related to breast cancer with comprehensive bioinformatics analysis. PeerJ. 2020;8:e9946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Cui Y, Wu L, Cao R, Xu H, Xia J, Wang ZP, Ma J. Antitumor functions and mechanisms of nitidine chloride in human cancers. J Cancer. 2020;11:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 41. | Zhang J, Wu L, Lian C, Lian S, Bao S, Zhang J, Wang P, Ma J, Li Y. Nitidine chloride possesses anticancer property in lung cancer cells through activating Hippo signaling pathway. Cell Death Discov. 2020;6:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Di Bona M, Bakhoum SF. Micronuclei and Cancer. Cancer Discov. 2024;14:214-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 43. | Goh JJH, Goh CJH, Lim QW, Zhang S, Koh CG, Chiam KH. Transcriptomics indicate nuclear division and cell adhesion not recapitulated in MCF7 and MCF10A compared to luminal A breast tumours. Sci Rep. 2022;12:20902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 44. | Ou X, Lu Y, Liao L, Li D, Liu L, Liu H, Xu H. Nitidine chloride induces apoptosis in human hepatocellular carcinoma cells through a pathway involving p53, p21, Bax and Bcl-2. Oncol Rep. 2015;33:1264-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Janke C, Magiera MM. The tubulin code and its role in controlling microtubule properties and functions. Nat Rev Mol Cell Biol. 2020;21:307-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 520] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 46. | Yao RQ, Ren C, Xia ZF, Yao YM. Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles. Autophagy. 2021;17:385-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 47. | Olatunji D, Clark NM, Kelley DR. The class VIII myosin ATM1 is required for root apical meristem function. Development. 2023;150:dev201762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 48. | Wang D, Liang W, Huo D, Wang H, Wang Y, Cong C, Zhang C, Yan S, Gao M, Su X, Tan X, Zhang W, Han L, Zhang D, Feng H. SPY1 inhibits neuronal ferroptosis in amyotrophic lateral sclerosis by reducing lipid peroxidation through regulation of GCH1 and TFR1. Cell Death Differ. 2023;30:369-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 91] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 49. | Stanzione F, Giangreco I, Cole JC. Use of molecular docking computational tools in drug discovery. Prog Med Chem. 2021;60:273-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 50. | Agu PC, Afiukwa CA, Orji OU, Ezeh EM, Ofoke IH, Ogbu CO, Ugwuja EI, Aja PM. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci Rep. 2023;13:13398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 277] [Article Influence: 138.5] [Reference Citation Analysis (0)] |
| 51. | Li X, Wei S, Niu S, Ma X, Li H, Jing M, Zhao Y. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput Biol Med. 2022;144:105389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 209] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 52. | Shang L, Wang Y, Li J, Zhou F, Xiao K, Liu Y, Zhang M, Wang S, Yang S. Mechanism of Sijunzi Decoction in the treatment of colorectal cancer based on network pharmacology and experimental validation. J Ethnopharmacol. 2023;302:115876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 114] [Article Influence: 57.0] [Reference Citation Analysis (0)] |