Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.100152
Revised: November 3, 2024
Accepted: December 13, 2024
Published online: March 15, 2025
Processing time: 186 Days and 20.7 Hours
The incidence of colon cancer has been progressively increasing over time, whereas penile metastasis of colon cancer has remained exceedingly uncommon. Since the prognosis for colon cancer with BRAFV600E mutation is relatively unfavorable, further exploration and investigation are still required to develop treatment strategies for such rare cases.
About one year after surgery and chemotherapy, a 50-year-old patient with sigmoid colon cancer developed a mass at the base of the patient's penis, accom
Combining the BRAF/MEK-targeted therapy with cetuximab demonstrates a favorable therapeutic response in BRAFV600E-mutated colon cancer with penile metastasis.
Core Tip: Despite the increasing incidence and prevalence of colorectal cancer among malignant tumors, penile metastasis originating from colorectal cancer remains a rare occurrence. The precise mechanism underlying penile metastasis of colorectal cancer remains elusive, and its specific treatment remains a subject of controversy, particularly in patients with BRAFV600E mutation, which often signifies an unfavorable prognosis. In our case, the treatment of BRAF/MEK-targeted therapy (dabrafenib and trametinib) plus cetuximab demonstrated promising outcomes, leading to tumor regression and prolonged survival, thereby offering valuable insights for the management of analogous cases.
- Citation: Wu JC, Cheng HX, Lan QS, Xu HY, Zeng YJ, Lai W, Chu ZH. Penile metastasis from colon cancer with BRAFV600E mutation treated with BRAF/MEK-targeted therapy plus cetuximab: A case report. World J Gastrointest Oncol 2025; 17(3): 100152
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/100152.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.100152
Colorectal cancer stands as the third most frequently diagnosed and second most fatal cancer globally[1]. Colorectal cancer normally metastasizes to well-vascularized sites, such as the liver and lungs. Despite the rich vascularity and extensive circulatory communication with the surrounding tissues, penile metastasis from tumors is still very rare[2]. The primary tumor of penile metastasis is predominantly located in the bladder, prostate, and other urogenital organs (65%), followed by colorectal cancer (13%)[3]. The occurrence of metastases is frequently observed at the penile base, followed by the shaft and glans. In the majority of cases, the metastasis manifests as a solitary small nodule on the penis[4]. Penile cancer resulting from colorectal cancer often carries a grim prognosis. Literature research indicates that the median survival period following the diagnosis of penile metastasis is typically five months[5]. To address this challenge, interventions such as surgical resection and chemotherapy are employed in various studies. However, the outcomes have demonstrated limited efficacy[6]. In colorectal cancer, the BRAFV600E mutation is associated with poorly differentiated tumors, serving as a negative prognostic factor[7]. Due to poor response to standard therapies, its mortality rate is nearly twofold that of BRAF wild-type tumors[8]. Clinical studies have demonstrated that the combined treatment regimen of BRAF inhibitor and MEK inhibitor exhibits significant potential in raising the survival rate of patients harboring such mutation[9].
A 49-year-old Chinese male was admitted with a 5-day history of persistent abdominal pain, bloating, and obstipation.
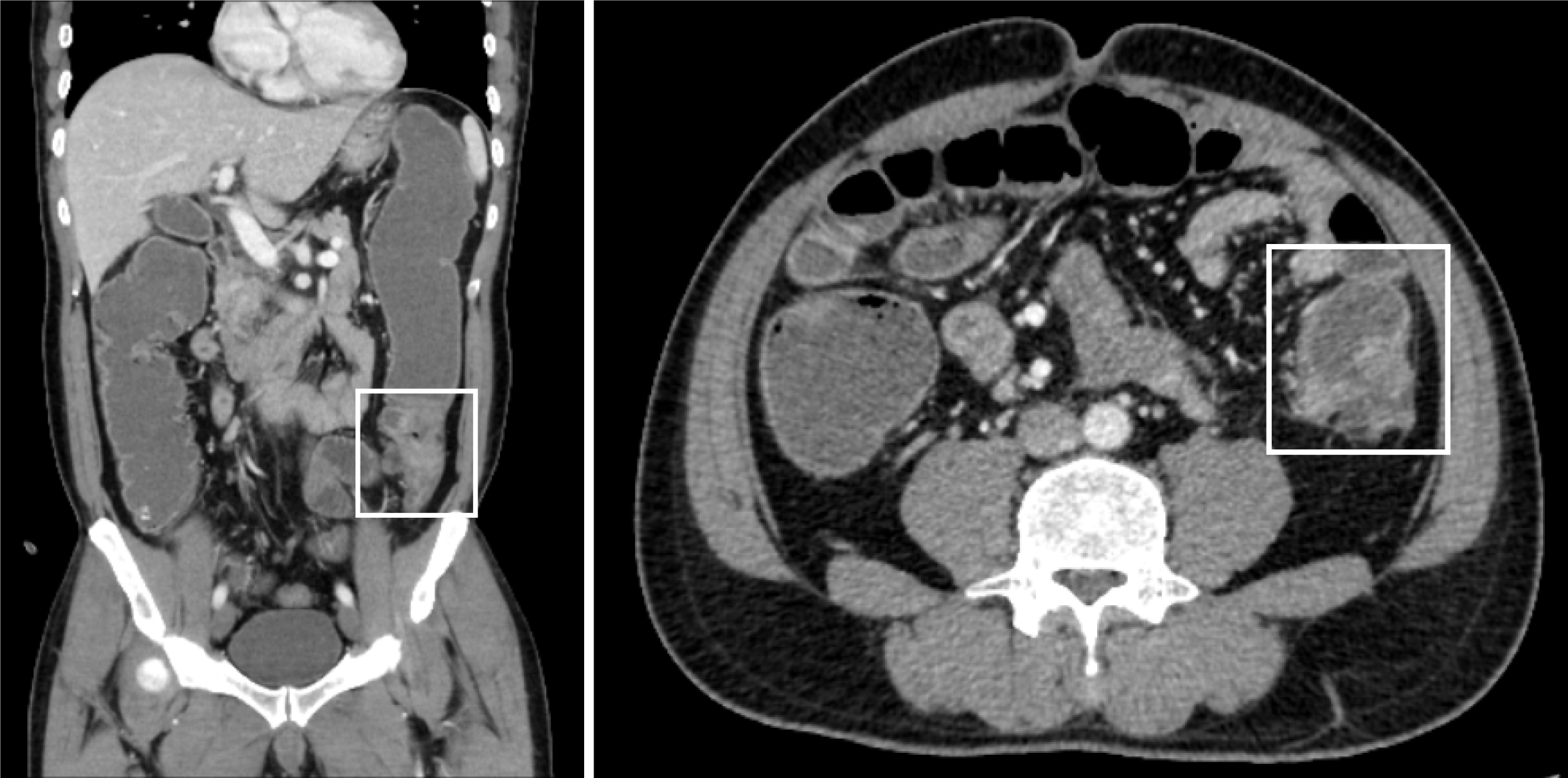
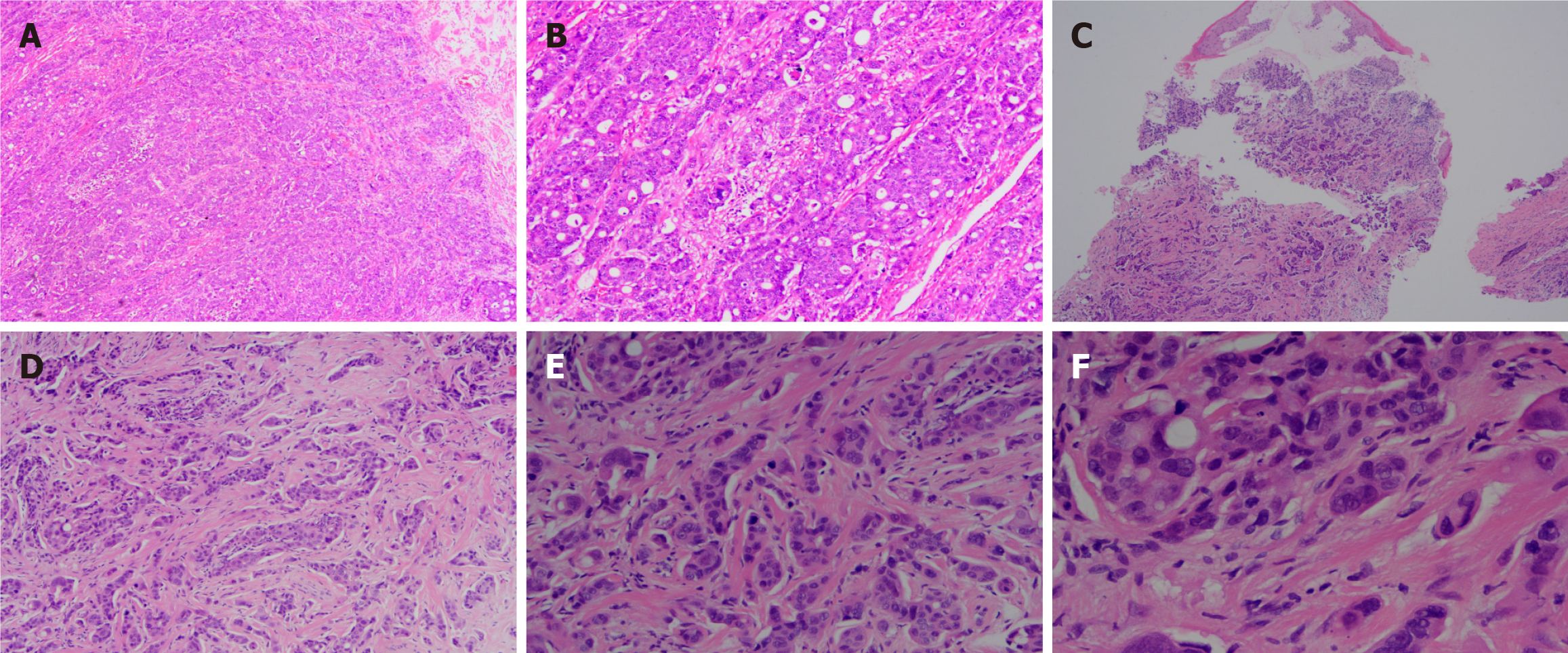
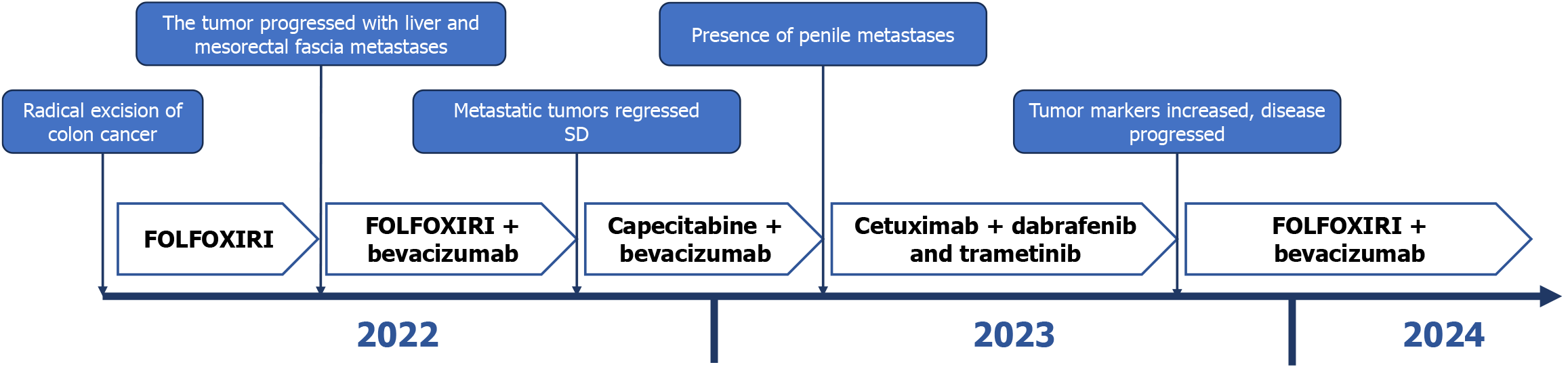
Five days after the onset of periumbilical colic, abdominal distension, and cessation of exhaust and defecation without apparent etiology, the patient sought medical attention at a local hospital. Abdominal computer tomography (CT) examination revealed thickening of the upper sigmoid colon wall, proximal bowel dilatation, and incomplete intestinal obstruction, which was considered a high possibility of colon cancer (Figure 1). The patient was referred to our hospital for treatment after the conservative therapy proved ineffective. The patient underwent a radical colon cancer resection procedure at our hospital. The surgical pathology revealed a poorly differentiated adenocarcinoma of the colon, invading and breaking through the bowel wall, where eleven out of nineteen removed lymph nodes were positive. No cancer was found in the surgical margins on both sides of the intestinal segment. The presence of tumor emboli was detected within numerous blood vessels, while no evident neural invasion was observed. The immunohistochemistry indicated Her-2 (1+), BRAF (+), and a Ki67 proliferative index of 55%. The gene high-throughput sequencing detection showed BRAFV600E mutation, APC Glu1540 alteration, and microsatellite stability (MSS) status. The disease was staged as T4a N2b M0 stage IIIC.
According to the Chinese Society of Clinical Oncology (CSCO) guidelines for the diagnosis and treatment of colorectal cancer, the patient underwent postoperative adjuvant chemotherapy of 5-FU, 2800 mg/m2, 48-hour continuous intravenous (i.v.) infusion; leucovorin, 200 mg/m2i.v.; irinotecan, 165 mg/m2; oxaliplatin, 85 mg/m2i.v. (FOLFOXIRI) for four cycles. However, subsequent metastases to the liver and mesorectal fascia were observed in the patient on the magnetic resonance imaging (MRI) scans and positron emission tomography-CT (PET-CT). The treatment regimen was subsequently switched on to FOLFOXIRI in combination with bevacizumab for eight cycles. The patient's metastases gradually diminished and his tumor markers returned to normalcy following treatment with this regimen. Consequently, the patient was transitioned to maintenance therapy of capecitabine plus bevacizumab (5 mg/kg). However, after five cycles of maintenance treatment, the patient presented with novel masses at the root of the penis and coronal sulcus. Pathological examination of the coronal sulcus mass revealed morphological similarities to that of the primary colon tumor, suggesting penile metastasis from colon cancer.
The patient had no special history of chronic diseases or infectious diseases.
The patient denied any familial history of malignancy or genetic predisposition.
The patient presented with abdominal distention accompanied by tenderness upon palpation, while the vital signs were within normal limits.
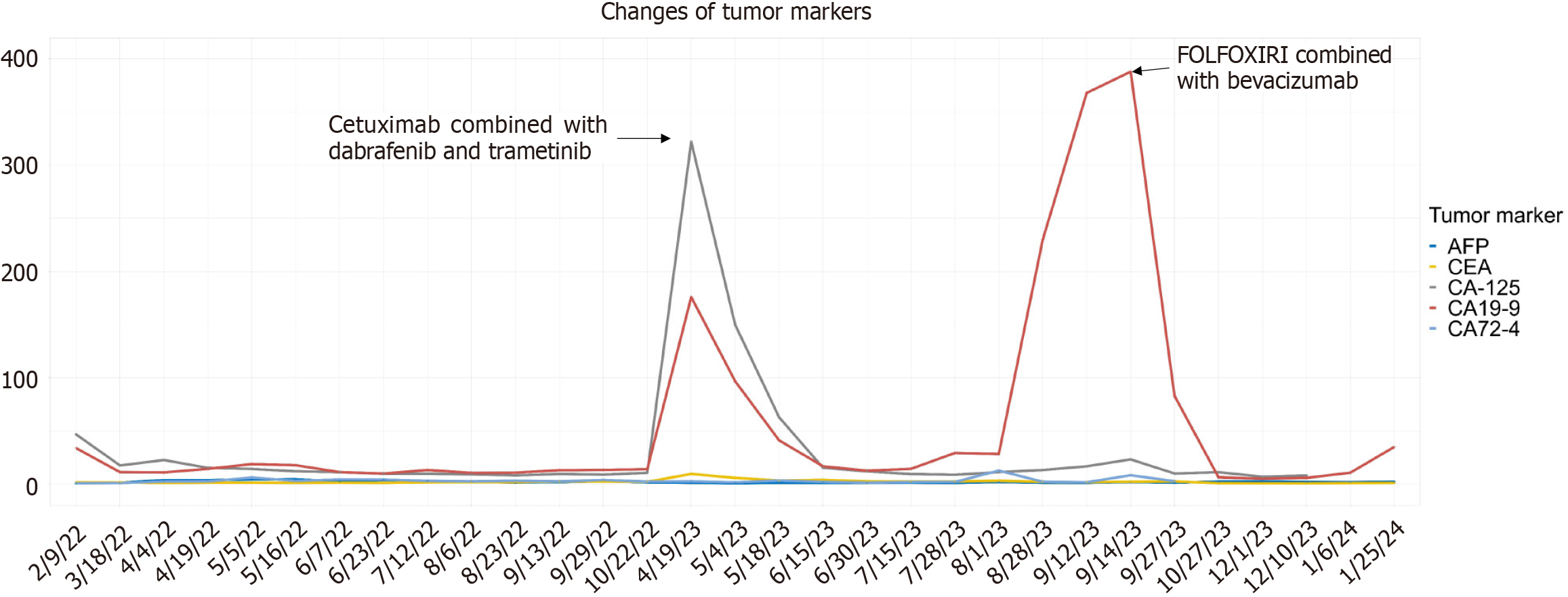
The tumor marker carbohydrate antigen-125 (CA-125) was slightly elevated on admission (46.8 U/L), followed by fluctuations in CA-125 and CA19-9 levels with the progression of the disease and implementation of the treatment regimen, while the remaining markers maintained at a low level throughout the whole process (Figure 2).
Upon initial admission, the CT scan of the whole abdomen and pelvis revealed thickening of the wall in the upper segment of the sigmoid colon, with a rough surface and a narrow lesion lumen. There were no obvious signs of metastasis in peripheral lymph nodes, liver and mesorectal fascia.
During the treatment with FOLFOXIRI, MRI and PET-CT showed the disease progression: A new nodule in the mesorectal fascia (10 mm × 10 mm) and a suspicious lesion in liver of segment IV/VIII (10 mm × 9 mm) were considered metastatic tumors.
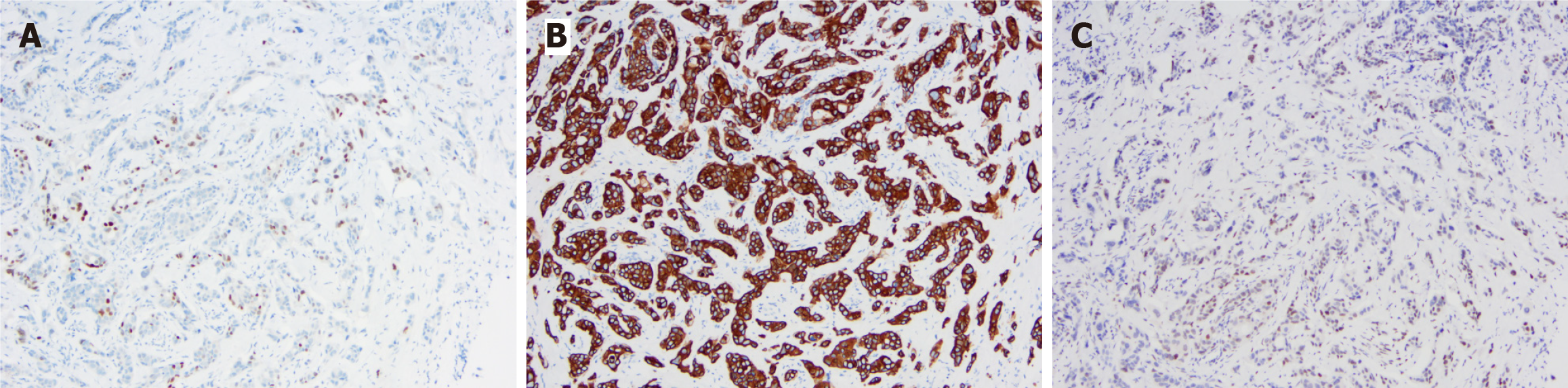
In order to further clarify the nature of the penile mass, the patient underwent local surgical resection of three masses located in the coronary sulcus. A histopathological examination of the resected masses revealed a poorly differentiated adenocarcinoma, consistent with his primary colon tumor (Figure 3). The immunohistochemical examination showed positive expression of CDX2, CK20, and SATB2, indicating a colonic origin for the penile mass (Figure 4).
Based on the patient's comprehensive medical history and pertinent diagnostic evaluations, a diagnosis of colon cancer with BRAFV600E mutation was established, accompanied by multiple metastases in the penis, liver, mesorectum, among other sites.
After initial treatment with FOLFOXIRI plus bevacizumab, the patient's tumor exhibited inadequate control and subsequently progressed and metastasized. In accordance with the CSCO guidelines for colorectal cancer diagnosis and treatment, the regimen of BRAF/MEK-targeted therapy (dabrafenib and trametinib) combined with cetuximab (dabrafenib, 150 mg twice daily; trametinib, 2 mg once daily; cetuximab 200 mg/m2 once biweekly) was devised based on the tumor's BRAFV600E mutation (Figure 5).
After the treatment of dabrafenib and trametinib plus cetuximab, the patient's serum levels of tumor markers gradually decreased to within the normal range, and all metastatic lesion exhibited varying degrees of shrinkage. Remarkably, the dimensions of the metastatic lesion at the penile base diminished substantially, decreasing from a magnitude of 1.6 cm × 1.9 cm to a mere 0.5 cm × 0.3 cm. Concurrently, the associated symptoms, including dysuria and penile pain during erection, abated progressively. However, five months later, the patient's disease exhibited further progression and there was a significant increase in tumor markers (CA19-9 388 U/mL). Subsequently, the treatment regimen of FOLFOXIRI in combination with bevacizumab was modified once again. Unfortunately, the patient was subsequently lost to follow-up.
The liver, peritoneum, lung, and bone are the most frequently observed metastatic sites in colorectal cancer. Nevertheless, there have been reports of less common sites such as the pancreas, ovary, brain, and even the oral cavity[10]. Metastasis of colorectal cancer to distant organs is often indicative of advanced tumor progression and carries a poor prognosis. After the source of metastasis is identified through immunohistochemical examination, local resection of metastatic lesions followed by systemic chemotherapy is commonly employed as a standard treatment[11].
The occurrence of penile metastasis from colorectal cancer is exceedingly rare in clinical practice, and the incidence rates differ significantly between colon cancer and rectal cancer. In reported cases of penile metastasis from colorectal cancer, the proportion of rectal cancer is significantly higher than that of colon cancer[12]. The precise mechanism underlying the metastasis of colorectal cancer to the penis remains elusive, with potential routes including retrograde venous spread, retrograde lymphatic spread, arterial spread and direct extension[13,14]. In particular, the retrograde venous spread is the most widely accepted among them. The modes of dissemination may encompass a diverse array of mechanisms. Different from the colon cancer, the low rectal cancer can drain from the middle and inferior rectal vein to the internal iliac vein. Through retrograde venous cell migration, tumor cells can be transferred to the internal pudendal vein and the dorsal penile vein system, resulting in penile metastasis of rectal cancer. However, colon cancer commonly drains via the superior and inferior mesenteric veins before eventually entering the portal vein system, thereby bypassing the penile vascular system, which necessitates further exploration of the specific route of penile metastasis in colon cancer[15,16]. Given the postoperative histopathological findings in our case, which indicated the presence of numerous vascular tumor emboli and metastatic cancer in peri-intestinal lymph nodes, it is plausible to suggest that retrograde lymphatic spread may have been one of the pathways for penile metastasis of colon cancer in this particular case[12,17]. However, the precise mechanism underlying penile metastasis remains to be further elucidated.
The most prevalent manifestations of penile metastases encompass penile pain and tenderness, the presence of a nodular mass on the penis, and the occurrence of malignant priapism[8,17]. In our case, in addition to pain during urination, we observed an intensely tender mass at the base of the patient's penis, which aligns with the characteristic symptoms associated with penile metastases. When penile metastases occur, the majority of patients exhibit concomitant metastases to other anatomical sites, including the liver, lung, and peritoneum[18]. The prognosis associated with penile metastases is generally unfavorable, characterized by a mean survival duration of merely nine months. Its treatment methods include surgical resection, radiation therapy, and chemotherapy. The surgical resection procedure involves the excision of the local mass and complete penectomy. The latter may significantly impact the social-psychological state and daily life of the patients. However, it may also enable patients to obtain greater benefits and longer survival[19]. Therefore, the choice of treatment for penile metastasis remains controversial. In addition to surgical intervention, appropriate chemotherapy and targeted therapy play a pivotal role in effectively managing micro-metastasis, suppressing tumor recurrence, and significantly prolonging patient survival. In the case we reported, the therapy of cetuximab, dabrafenib and trametinib exhibited satisfactory efficacy.
The BRAFV600E mutation occurs in roughly 8%-12% patients with metastatic colorectal cancer (mCRC), which represents a poor response to most systemic therapies and a worse prognosis[20]. BRAF controls cellular survival, replication and differentiation by encoding serine/threonine protein kinase, which plays a critical role in the RAS/RAF/MEK/ERK mitogen-activated protein kinase (MAPK) signaling pathway. When the V600E mutation occurs, BRAF kinase activity is enhanced by insertion of a negatively charged residue near the phosphorylation site in the catalytic domain, followed by phosphorylation of MEK1 and MEK2, activating ERK1 and ERK2. This process phosphorylates a variety of cell substrates involved in cell proliferation and inhibition of cell apoptosis, promoting tumor survival, growth and metastasis[21-23]. Unlike mCRC with microsatellite instability, which can benefit from immune checkpoint inhibitors such as pembrolizumab and ipilimumab + nivolumab, the mainstream treatment for mCRC with BRAFV600E mutation and MSS is still surgical resection followed by chemotherapy. Chemotherapy regimens include CAPEOX, mFOLFOX6, FOLFIRI or FOLFOXIRI combined with the anti-vascular endothelial growth factor monoclonal antibody bevacizumab. When the tumor recurred or metastasized further, BRAF and MEK inhibitors (dabrafenib and trametinib) in combination with the anti-epidermal growth factor receptor (EGFR) antibodies (cetuximab) may serve as a good option[23,24]. In contrast, single-agent BRAF inhibitors, such as dabrafenib and encorafenib, have demonstrated efficacy in BRAFV600E-mutated melanoma, thyroid cancer, and small cell lung cancer. However, they have exhibited limited therapeutic benefits in metastatic colon cancer with BRAFV600E mutation[25]. In addition, the combination of BRAF inhibitor and EGFR antibodies, such as dabrafenib + panitumumab and encorafenib + cetuximab, has demonstrated favorable outcomes in terms of both anti-tumor efficacy and overall survival[26,27]. In a clinical trial, the efficacy of dual BRAF and MEK inhibition was assessed using dabrafenib in combination with trametinib, excluding the EGFR inhibitor, among a cohort of 43 patients. Among them, five patients (12%) exhibited a positive response to the treatment regimen, with a median progression-free survival of 3.5 months. Notably, one patient achieved a complete response, maintaining the response for a duration of 36 months[28]. The lack of efficacy of single-agent BRAF inhibitor therapy in BRAFV600E mutation mCRC is due to the suppression of ERK-dependent negative feedback mediators that restrict the MAPK pathway. This may lead to the induction of RAS activity as well as the activation of RAS kinase, which generates RAF dimers that bypass activation of MAPK signaling pathway, thereby attenuating the antitumor effect. EGFR antibodies exert their best anti-tumor effect by inhibiting this process in combination with BRAF and MEK inhibitors[21,22]. When EGFR monoclonal antibodies are introduced, such as cetuximab in combination with dabrafenib, trametinib, and panitumumab, the patients' recovery rate reaches 21%, with a median progression-free survival of 4.2 months[23].
Cases of penile metastasis from colon cancer are infrequent, and several factors contribute to this rarity. The dismal prognosis associated with metastatic penile cancer often leads to a short survival period for patients, making it challenging to track certain cases. Moreover, the scarcity of this medical condition may cause surgeons to overlook its occurrence and fail to document it adequately. Additionally, patients might refrain from seeking medical attention due to symptoms such as penile pain, erectile dysfunction, or the presence of foreign objects protruding from the penis surface in order to preserve their dignity. Furthermore, reported cases also underscore instances of chemotherapy-induced penile pain and side effects like rash, which can potentially mask early signs of penile metastasis during treatment. These various factors collectively account for potential missed diagnoses.
In this case, we employed targeted mutations to modify the chemotherapy regimen, leading to rapid reduction of metastatic lesions and obviating the need for penile resection, thereby preserving the patient's quality of life. This approach not only maintained physiological function and psychological well-being but also significantly improved prognosis across multiple dimensions. Targeted therapy effectively prolonged survival period. Therefore, when penile metastasis is detected, our preferred treatment strategy would focus on preserving the penile function rather than resorting to resection, reflecting our compassionate care for the patient. These considerations enhanced patient compliance with medical advice and adherence to the treatment regimen. Taking the impact of psychosocial factors on patients' treatment into consideration proves to be highly significant and should not be underestimated.
In summary, since penile metastasis is a rare occurrence primarily originating from genitourinary organs, and colon cancer rarely serve as the primary source, further investigation into its specific mechanisms and appropriate treatment options for penile metastasis originating from colon cancer is required. The presence of BRAFV600E mutation in colorectal cancer often indicates a poor prognosis, therefore developing a targeted therapy for this specific mutation is highly important. Relevant targeted therapies on colon cancer with BRAFV600E mutation in our case effectively impeded tumor progression and extended patient survival, which can serve as a valuable reference for analogous cases.
The authors would like to thank the patient and his families for their support and cooperation.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8149] [Article Influence: 8149.0] [Reference Citation Analysis (2)] |
| 2. | Thomas A, Necchi A, Muneer A, Tobias-Machado M, Tran ATH, Van Rompuy AS, Spiess PE, Albersen M. Penile cancer. Nat Rev Dis Primers. 2021;7:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 3. | Marchionne E, Perez C, Hui A, Khachemoune A. Penile squamous cell carcinoma: a review of the literature and case report treated with Mohs micrographic surgery. An Bras Dermatol. 2017;92:95-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Cocci A, Hakenberg OW, Cai T, Nesi G, Livi L, Detti B, Minervini A, Morelli G, Carini M, Serni S, Gacci M. Prognosis of men with penile metastasis and malignant priapism: a systematic review. Oncotarget. 2018;9:2923-2930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Chaux A, Amin M, Cubilla AL, Young RH. Metastatic tumors to the penis: a report of 17 cases and review of the literature. Int J Surg Pathol. 2011;19:597-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Kimura Y, Shida D, Nasu K, Matsunaga H, Warabi M, Inoue S. Metachronous penile metastasis from rectal cancer after total pelvic exenteration. World J Gastroenterol. 2012;18:5476-5478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Li Y, Li W. BRAF mutation is associated with poor clinicopathological outcomes in colorectal cancer: A meta-analysis. Saudi J Gastroenterol. 2017;23:144-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 8. | Piringer G, Decker J, Trommet V, Kühr T, Heibl S, Dörfler K, Thaler J. Ongoing complete response after treatment cessation with dabrafenib, trametinib, and cetuximab as third-line treatment in a patient with advanced BRAF(V600E) mutated, microsatellite-stable colon cancer: A case report and literature review. Front Oncol. 2023;13:1166545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Ros J, Baraibar I, Sardo E, Mulet N, Salvà F, Argilés G, Martini G, Ciardiello D, Cuadra JL, Tabernero J, Élez E. BRAF, MEK and EGFR inhibition as treatment strategies in BRAF V600E metastatic colorectal cancer. Ther Adv Med Oncol. 2021;13:1758835921992974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Cañellas-Socias A, Sancho E, Batlle E. Mechanisms of metastatic colorectal cancer. Nat Rev Gastroenterol Hepatol. 2024;21:609-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 53] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 11. | Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. 2023;44:222-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 286] [Reference Citation Analysis (0)] |
| 12. | Tustumi F, Gerbasi LS, Pandini RV, de Araujo MNF, Seid VE, Araujo SEA. Penis metastasis in colon cancer: A case report of an unusual site of dissemination. Int J Surg Case Rep. 2023;105:108035. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Yin GL, Zhu JB, Fu CL, Ding RL, Zhang JM, Lin Q. Metachronous isolated penile metastasis from sigmoid colon adenocarcinoma: A case report. World J Clin Cases. 2022;10:11658-11664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Zhang K, Da J, Yao HJ, Zheng DC, Cai ZK, Jiang YQ, Xu MX, Wang Z. Metastatic tumors of the penis: a report of 8 cases and review of the literature. Medicine (Baltimore). 2015;94:e132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Sakorafas GH, Zouros E, Peros G. Applied vascular anatomy of the colon and rectum: clinical implications for the surgical oncologist. Surg Oncol. 2006;15:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Dwyer ME, Salgado CJ, Lightner DJ. Normal penile, scrotal, and perineal anatomy with reconstructive considerations. Semin Plast Surg. 2011;25:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Lee TG, Son SM, Kim MJ, Lee SJ. Penile metastasis in rectal cancer with pathologic complete response after neoadjuvant chemoradiotherapy: The first case report and literature review. Medicine (Baltimore). 2020;99:e21215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Powell BL, Craig JB, Muss HB. Secondary malignancies of the penis and epididymis: a case report and review of the literature. J Clin Oncol. 1985;3:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | McGuinness LA, Floyd MS Jr, Lucky M, Parr NJ. Penile metastases treated with partial glansectomy and adjuvant radiotherapy 5 years after an initial diagnosis of rectal cancer. BMJ Case Rep. 2013;2013:bcr2013200829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Martinelli E, Arnold D, Cervantes A, Stintzing S, Van Cutsem E, Tabernero J, Taieb J, Wasan H, Ciardiello F. European expert panel consensus on the clinical management of BRAF(V600E)-mutant metastatic colorectal cancer. Cancer Treat Rev. 2023;115:102541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Poulikakos PI, Sullivan RJ, Yaeger R. Molecular Pathways and Mechanisms of BRAF in Cancer Therapy. Clin Cancer Res. 2022;28:4618-4628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 22. | Strickler JH, Wu C, Bekaii-Saab T. Targeting BRAF in metastatic colorectal cancer: Maximizing molecular approaches. Cancer Treat Rev. 2017;60:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Corcoran RB, André T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, Hollebecque A, McRee AJ, Siena S, Middleton G, Muro K, Gordon MS, Tabernero J, Yaeger R, O'Dwyer PJ, Humblet Y, De Vos F, Jung AS, Brase JC, Jaeger S, Bettinger S, Mookerjee B, Rangwala F, Van Cutsem E. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAF(V600E)-Mutant Colorectal Cancer. Cancer Discov. 2018;8:428-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 446] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 24. | Tian J, Chen JH, Chao SX, Pelka K, Giannakis M, Hess J, Burke K, Jorgji V, Sindurakar P, Braverman J, Mehta A, Oka T, Huang M, Lieb D, Spurrell M, Allen JN, Abrams TA, Clark JW, Enzinger AC, Enzinger PC, Klempner SJ, McCleary NJ, Meyerhardt JA, Ryan DP, Yurgelun MB, Kanter K, Van Seventer EE, Baiev I, Chi G, Jarnagin J, Bradford WB, Wong E, Michel AG, Fetter IJ, Siravegna G, Gemma AJ, Sharpe A, Demehri S, Leary R, Campbell CD, Yilmaz O, Getz GA, Parikh AR, Hacohen N, Corcoran RB. Combined PD-1, BRAF and MEK inhibition in BRAF(V600E) colorectal cancer: a phase 2 trial. Nat Med. 2023;29:458-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 110] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 25. | Morris VK, Bekaii-Saab T. Improvements in Clinical Outcomes for BRAF(V600E) -Mutant Metastatic Colorectal Cancer. Clin Cancer Res. 2020;26:4435-4441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Grothey A, Fakih M, Tabernero J. Management of BRAF-mutant metastatic colorectal cancer: a review of treatment options and evidence-based guidelines. Ann Oncol. 2021;32:959-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 27. | Al-Salama ZT. Encorafenib: A Review in Metastatic Colorectal Cancer with a BRAF V600E Mutation. Drugs. 2021;81:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Corcoran RB, Atreya CE, Falchook GS, Kwak EL, Ryan DP, Bendell JC, Hamid O, Messersmith WA, Daud A, Kurzrock R, Pierobon M, Sun P, Cunningham E, Little S, Orford K, Motwani M, Bai Y, Patel K, Venook AP, Kopetz S. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J Clin Oncol. 2015;33:4023-4031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 415] [Article Influence: 41.5] [Reference Citation Analysis (0)] |