Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.99662
Revised: October 25, 2024
Accepted: November 22, 2024
Published online: February 15, 2025
Processing time: 175 Days and 1.1 Hours
Perivascular epithelioid cell tumor (PEComa) of the colorectum is exceedingly rare, with only a few published reports. It presents with a wide spectrum of biological behavior, ranging from benign to malignant. The prognosis for malig
To fully characterize PEComa and standardize its diagnosis and treatment.
Patients with colorectal malignant PEComa were identified from the First Affiliated Hospital, Zhejiang University School of Medicine and People's Hospital of Anji. Cases with controversial pathology and cases lost to follow-up were excluded, leaving seven remaining cases that formed the basis of the study. We collected relevant clinicopathological, therapeutic and followup details. Disease stage and progression were assessed by contrast-enhanced computed tomography at baseline and at 3-month intervals.
The mean age was 43 years, with a range of 5 years to 73 years. The average body mass index was 21.8 ± 3.0 kg/m2, and 71.4% of cases occurred in the colon. The main symptoms of colorectal PEComas were abdominal mass and hematochezia. The most common microscopic finding of malignant behavior was infiltrative growth. Immunohistochemical analysis found that 6/7 cases were positive for HMB45, 5/7 were positive for melan-A, and 3/5 were positive for MiTF. The watch-and-wait approach to treatment was a risky option. Radical resection was preferable to systemic treatment. The median progression-free survival exceeded 38 months, longer than previously reported.
Radical or extended resection is the key to prolonged survival of malignant PEComa. More meaningful studies are urgently needed to establish the standardized diagnosis and treatment.
Core Tip: Perivascular epithelioid cell tumor (PEComa) of the colorectum is exceedingly rare, and inexperience in therapy and poor prognosis are obstacles to treatment. Here, we retrieved 7 cases censored at 3-month intervals with clinicopathological, therapeutic and follow-up details (median, 44 months). Patients benefited from R0 resection, which was superior to systemic therapy. There was a high risk of recurrence within 2 years postoperatively. The median progression-free survival exceeded 38 months, longer than previously reported. Therefore, radical or extended resection is the key to prolonging the survival of patients with colorectal malignant PEComa.
- Citation: Ma MF, Chi ZY, Zhao LJ, Zhai W, Zhong W, Wang S. Malignant perivascular epithelioid cell tumor of the colorectum: Clinicopathological characterization, diagnosis and treatment process of 7 cases. World J Gastrointest Oncol 2025; 17(2): 99662
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/99662.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.99662
Perivascular epithelioid cell tumor (PEComa) is a rare mesenchymal neoplasm composed of perivascular cells, which presents a significant diagnostic dilemma[1,2]. It does not present with any known typical clinical symptoms or signs, and the incidence is < 1 per million[3]. In 2002, the World Health Organization defined “mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells” as PEComas[4]. These include angiomyolipoma of liver and kidney, clear cell "sugar" tumor of lung, lymphangiomyomatosis, clear cell myomatosis of sickle ligament or round ligament, and not otherwise specified (PEComa-NOS). The most frequent sites of the latter subgroup are the uterus and retroperitoneum[5]. Since its first description, PEComa-NOS has been gradually reported in a wide variety of anatomical sites, including the colorectum[6-9]. As the clinicopathological features of this neoplasm may easily be confused with those of other more commonly encountered lesions, this poses difficulties in its diagnosis and treatment.
PEComa is a type of neoplasm with a range of biological behaviors, which can be classified as benign, borderline or malignant[10]. Benign means that the tumor is free of aggressive clinical behavior, such as tumor size > 5 cm, high nuclear grade, infiltrative growth, vascular invasion, necrosis, and mitotic rate > 1/50 high power field (HPF). Malignant refers to the presence of two or more aggressive features, and the rest are borderline PEComas. Schoolmeester et al[11] argued that this classification was inappropriate because patients without metastatic disease also fell into the malignant category. Metastasis of PEComas of the gastrointestinal tract occurred in occurred in 54% of women and 46% of men[12]. Clinically, malignant PEComa of the colorectum is easily misdiagnosed as adenoma, gastrointestinal stromal tumor or malignant melanoma[13]. Difficulty in diagnosis and inexperience in therapy are obstacles currently encountered. Reports of colorectal malignant PEComa remain infrequent. Furthermore, most reports have focused on diagnosis and pathology, and information on treatment and prognosis is limited.
Patients diagnosed with malignant PEComa initially presenting in the colorectum were retrieved from the First Affiliated Hospital, Zhejiang University School of Medicine and People's Hospital of Anji, China. After each case was confirmed by two pathologists, relevant clinicopathological, therapeutic and followup details were collected. Cases with controversial pathology and those lost to follow-up were excluded. Disease stage and progression were assessed by contrast-enhanced computed tomography at baseline and at 3-month intervals. The time interval for progression-free survival (PFS) starts from initial diagnosis and ends at cancer progression, relapse or metastasis. Any progression-free patient was censored at the last follow-up (June 30, 2024). Empirical survival probability was calculated via the Kaplan-Meier method. Some data and images were obtained from referring clinicians. The study was approved by the Ethics Committees of the participating hospitals.
Seven colorectal malignant PEComa cases were finally included in our study. The clinical features are summarized in Table 1. Their age at diagnosis ranged from 5 years to 73 years, with a mean of 43 years. Four patients were men and three patients were women. The average body mass index was 21.8 ± 3.0 kg/m2, including two overweight, one underweight and four healthy weight. None of the patients had features of tuberous sclerosis. One patient had high blood pressure and another had viral hepatitis; the remaining patients did not have any underlying disease. Initial presentation was abdominal mass in 3 cases (2, 5 and 6), one of which was accompanied with abdominal pain, and hematochezia in 2 cases (4 and 7). Case 3 had no clinical symptoms and PEComa was found by health screening. We found that 71.4% of PEComas occurred in the colon. In our medical records, the largest tumor had a volume of 10.5 cm × 6.5 cm × 2.5 cm.
| Case No. | Age in years | Sex | BMI in | Basic disease | Family history | TSC | Initial site | Size in cm | Clinical presentation |
| 1 | 49 | Male | 27.6 | None | None | None | Sigmoid colon | 5.5 × 4.5 × 2.5 | Difficulty in defecation |
| 2 | 73 | Female | 19.4 | Hypertension | None | None | Descending colon | 10.5 × 6.5 × 2.5 | Palpable abdominal mass |
| 3 | 30 | Male | 22.5 | None | None | None | Ascending colon | 3.0 × 2.5 × 2.0 | None2 |
| 4 | 67 | Female | 21.4 | Viral hepatitis | None | None | Rectum | 4.0 × 2.5 × 2.0 | Hematochezia |
| 5 | 5 | Female | 18.1 | None | None | None | Ascending colon | 4.0 × 3.0 × 2.5 | Abdominal mass and pain |
| 6 | 48 | Male | 24.0 | None | Carcinoma1 | None | Transverse colon | 7.7 × 6.3 × 4.0 | Palpable abdominal mass |
| 7 | 28 | Male | 19.9 | None | None | None | Middle rectum | 6.5 × 6.5 × 4.5 | Hematochezia |
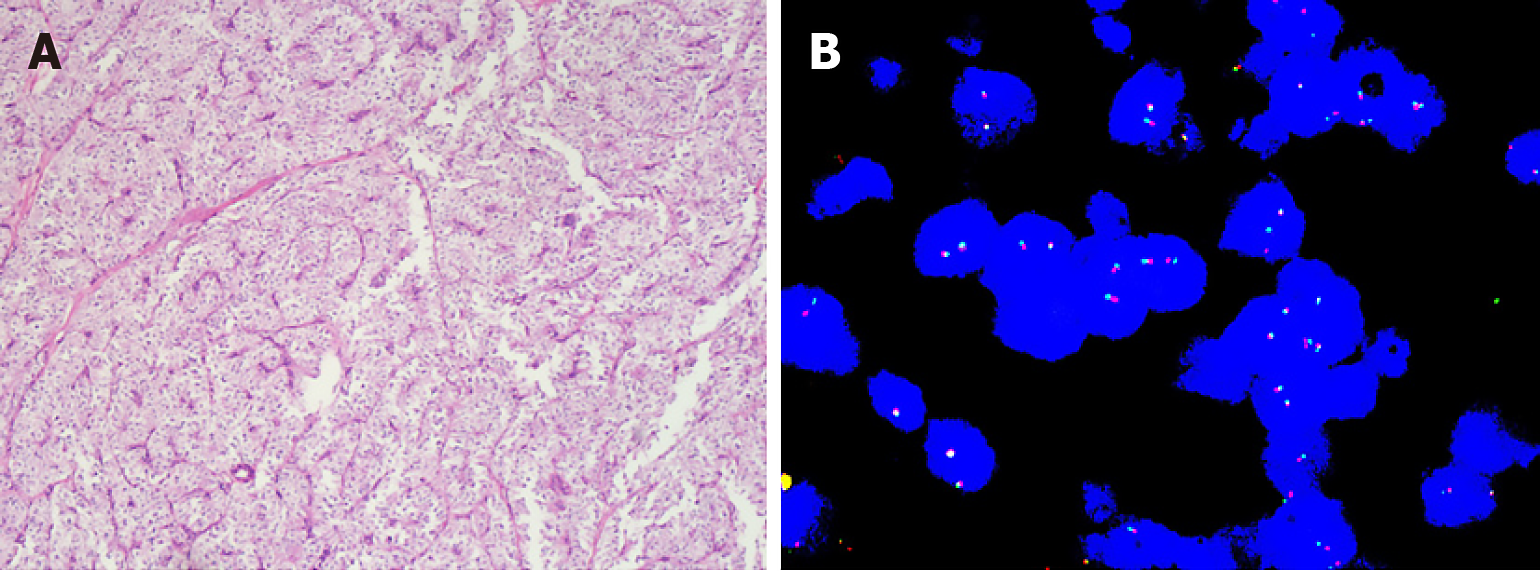
We discovered that tumor cells were surrounded by vessels with angiomyolipoma-like features. Microscopically, tumors were composed of epithelioid and spindle cells with eosinophilic, granular to clear cytoplasm (Figure 1A). Tumor cell nuclei were round, spindled or ovoid, vesicular, and usually had prominent nucleoli. The pathological results all met the malignant criteria. Details are shown in Table 2. The proportion of high nuclear grade and vascular invasion was 42.9% and 57.1%, respectively. Three were predominantly infiltrative and two had focally infiltrative edges. We also found mitosis > 1/50 HPF in 3/7 cases. Necrosis was present in only 2 cases. By immunohistochemistry, 6/7 were positive for HMB45, 5/7 for melan-A, 3/6 for SMA, 3/5 for MiTF and 3/7 for desmin. None was positive for pan-cytokeratin, and 1 case each was positive for TFE3, S100, CD117 and MyoD1. Fluorescence in situ hybridization for Ewing Sarcoma protein RNA binding protein 1 was also negative (Figure 1B).
| Findings | Positive/total | Percentage | Findings | Positive/total | Percentage |
| Tumor size > 5 cm | 4/7 | 57.1 | SMA | 3/6 | 50.0 |
| High nuclear grade | 3/7 | 42.9 | MiTF | 3/5 | 60.0 |
| Infiltrative growth | 5/7 | 71.4 | TFE3 | 1/7 | 14.3 |
| Vascular invasion | 4/7 | 57.1 | Desmin | 3/7 | 42.9 |
| Necrosis | 2/7 | 28.6 | S100 | 1/6 | 16.7 |
| Mitosis > 1/50 HPF | 3/7 | 42.9 | CD117 | 1/7 | 14.3 |
| Pan-cytokeratin | 0/5 | 0 | CD34 | 0/5 | 0 |
| HMB45 | 6/7 | 85.7 | CD10 | 0/5 | 0 |
| Melan A | 5/7 | 71.4 | Myo D1 | 1/7 | 14.3 |
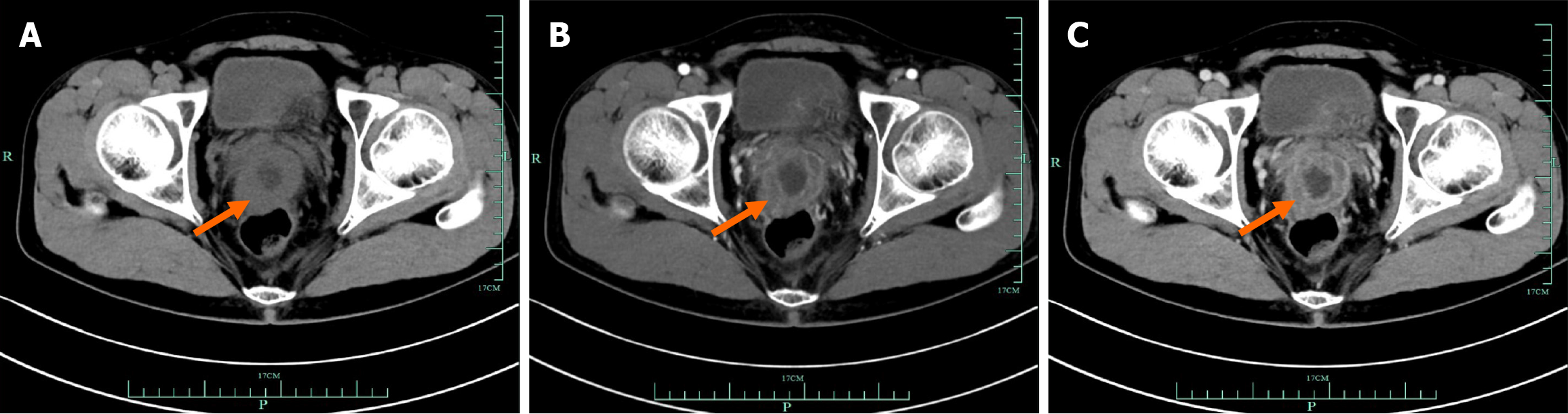
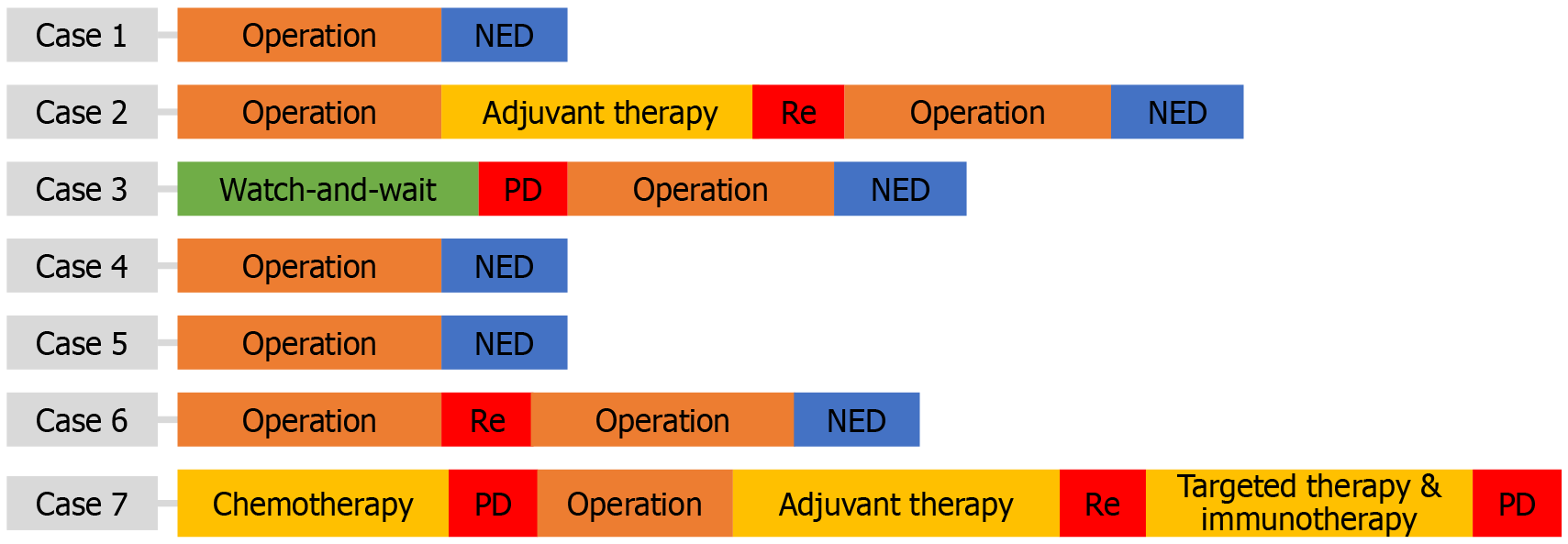
According to preoperative imaging, most tumors cannot be identified as mesenchymal sarcoma. For example, as shown in Figure 2, colorectal PEComa was difficult to distinguish from adenocarcinoma. We performed puncture biopsy to aid diagnosis of suspected PEComa. Except for case 3, which chose the watch-and-wait approach, and case 7, which received preoperative chemotherapy, the others first underwent radical resection, as shown in Figure 3. This was related to the tumor characteristics. As we anticipated, the mass of case 3 did not exhibit malignant behavior at the initial diagnosis, and the PEComa of case 7 infiltrated adjacent tissues and was difficult to perform R0 resection. Both had surgery 6 months later due to disease progression. All of the masses were confirmed to be malignant PEComas by postoperative pathology. Two of the patients underwent adjuvant therapy with the mammalian target of rapamycin (mTOR) inhibitor everolimus, but recurrence still occurred at 12 months and 21 months, respectively. For recurrence, mTOR inhibitor, tyrosine kinase inhibitor targeted therapy and immunotherapy had little effect. Nevertheless, there was no evidence of tumor after re-R0 resection in cases 2 and 6 (Figure 3).
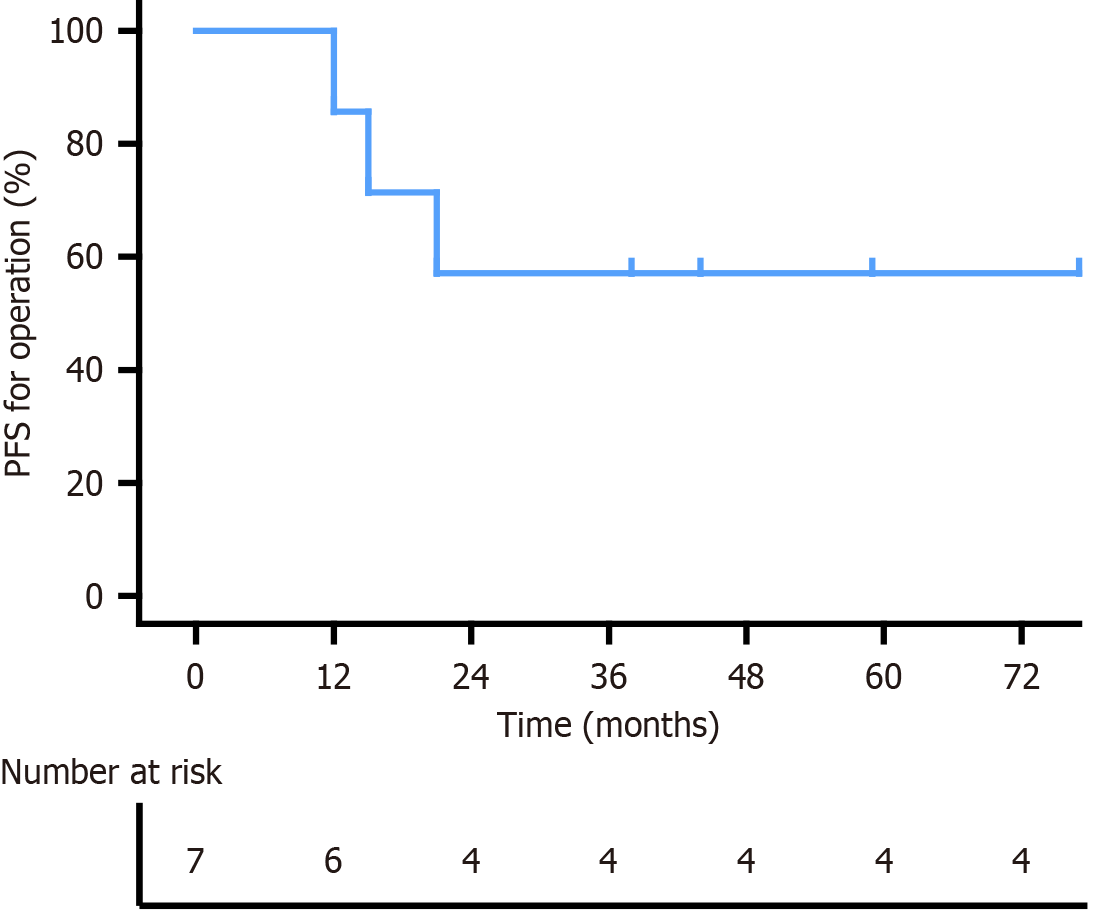
PEComa patients require rigorous follow-up. The median follow-up was 44 months, with the longest being 77 months and the shortest 28 months. Abdominal recurrence occurred in cases 2 and 6, and case 7 developed liver metastasis, retroperitoneal lymph node metastasis and pelvic implantation. After further treatments, only case 7 showed disease progression; the remaining patients had no evidence of disease. The 3-year PFS for operation was 57.1% (Figure 4). Although the median survival period has not yet been reached, it exceeded 38 months. The high-risk time for recurrence and metastasis of colorectal PEComa was within 2 years postoperatively.
Guidelines for PEComa have not yet been established. Surgical resection is the most commonly chosen therapy. For recurrence or metastasis of malignant PEComa, systemic therapy is the mainstay, but its effectiveness remains unclear. Angiomyolipoma and other tumors within the PEComa family occur at increased frequency in patients with tuberous sclerosis complex (TSC), which results from germline mutations in the tumor-suppressor genes TSC1 or TSC2[14]. Mutations in either gene provide the basis for the use of mTOR inhibitors, such as sirolimus or everolimus[15]. Colorectal PEComa-NOS seems independent of TSC, but requires further verification through more cases and genetic studies. A study by Sanfilippo et al[16] demonstrated that patients with PEComas may be responsive to gemcitabine, anthracyclines and antiangiogenic agents. In addition to surgery and systemic therapy, radiotherapy is another option worth considering[17]. There is still a lack of case reports and sharing of experience on various therapeutic effects until now. The therapy and prognosis of < 20 PEComas of the colorectum have been reported. Therefore, case reports on therapy and prognosis of malignant PEComa are urgently needed. Doyle et al[12] found that in 5 patients with colorectal malignant PEComa, the median time from diagnosis to death was only 22 months (range, 2-48 months). This was inconsistent with the results of our study, in which the median PFS was more than 38 months after radical surgery. This discrepancy may be due to differences in treatment approaches. Our center favors that radical or extended resection over current chemotherapy, targeted therapy and immunotherapy.
There is insufficient evidence to demonstrate that surgery combined with adjuvant therapy can achieve a survival benefit. The diagnostic and therapeutic procedures for 7 cases and the literature review in this study provides valuable experience and reference for subsequent patients. We report that 2 cases receiving postoperative adjuvant therapy with mTOR inhibitors experienced recurrence within 2 years. Coincidentally, Doyle et al[12] found that patients who received mTOR inhibitors still had disease progression and subsequently received doxorubicin and ifosfamide, but the clinical effect was transient. Chiang et al[18] suggested that that TSC2 mutant PEComa with a JAZF1-SUZ12 fusion might direct effective treatment. Whether to use mTOR inhibitors as first-line therapy, especially for advanced and metastatic PEComas, remains controversial. Some clinicians have reported high survival rates without therapy for up to 1 year after diagnosis[3]. We do not agree with this viewpoint because of the rapid progression in case 3 during the watch-and-wait period. Most patients with poor prognosis did not receive proper diagnosis and treatment. Different strategies were adopted in some cases with varying effects. The addition of antiestrogen treatment in female patients with advanced PEComa progressing to mTOR inhibitors, resulted in a marked clinical benefit[19]. Djerroudi et al[20] reported the first case of metastatic malignant PEComa that developed in a patient with Lynch syndrome, who showed complete response to anti-PD1 pembrolizumab. AMPECT, a phase II, single-arm, registration trial, investigated the safety and efficacy of the rapamycin inhibitor nab-sirolimus. The study showed an overall response rate of 39%, which might make nab-sirolimus an important novel drug[21]. Although there have been some reports of systematic treatment for PEComas, none of them was highly effective, consistent with our data. Therefore, these drugs should not be widely used.
In the study, the morbidity of colorectal PEComas in males and females is similar, and most cases commonly involve the colon. They exhibit variable clinical behavior, from benign to malignant. The presence of nuclear atypia, infiltrative growth, vascular invasion and mitotic activity are the strongest predictors of malignant behavior. PEComas occurring in the colorectum have been reported in small numbers, but most of them lack therapeutic and prognostic data. Difficulties in diagnosis and inexperience in treatment are responsible for poor prognosis. We believe that radical or extended resection is the key to prolonging survival. In addition, most patients do not benefit from existing drugs used for systemic therapy. More meaningful samples and key data are urgently needed to promote the standardized diagnosis and treatment of malignant colorectal PEComas.
| 1. | Cui Q, Li C, Huang T, Huang J, Chen M. Systematic analysis of perivascular epithelioid cell neoplasms in the female reproductive tract: a comprehensive review. Future Oncol. 2024;20:283-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Zhu D, Song S, Wang D, Kuang D, Cheng S, Zhou J, Zou S. Hepatic perivascular epithelioid cell tumor resembling hepatic adenoma and hepatocellular carcinoma on preoperative imaging: a case report. Front Oncol. 2024;14:1292313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Battistella E, Pomba L, Mirabella M, Gregianin M, Scapinello A, Volante M, Toniato A. Metastatic Adrenal PEComa: Case Report and Short Review of the Literature. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Son HJ, Kang DW, Kim JH, Han HY, Lee MK. Hepatic perivascular epithelioid cell tumor (PEComa): a case report with a review of literatures. Clin Mol Hepatol. 2017;23:80-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Stacchiotti S, Frezza AM, Blay JY, Baldini EH, Bonvalot S, Bovée JVMG, Callegaro D, Casali PG, Chiang RC, Demetri GD, Demicco EG, Desai J, Eriksson M, Gelderblom H, George S, Gounder MM, Gronchi A, Gupta A, Haas RL, Hayes-Jardon A, Hohenberger P, Jones KB, Jones RL, Kasper B, Kawai A, Kirsch DG, Kleinerman ES, Le Cesne A, Lim J, Chirlaque López MD, Maestro R, Marcos-Gragera R, Martin Broto J, Matsuda T, Mir O, Patel SR, Raut CP, Razak ARA, Reed DR, Rutkowski P, Sanfilippo RG, Sbaraglia M, Schaefer IM, Strauss DC, Sundby Hall K, Tap WD, Thomas DM, van der Graaf WTA, van Houdt WJ, Visser O, von Mehren M, Wagner AJ, Wilky BA, Won YJ, Fletcher CDM, Dei Tos AP, Trama A. Ultra-rare sarcomas: A consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer. 2021;127:2934-2942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 6. | Vijayanirmala P, Yadav R, Goyal S, Barwad A, Bhowmik S, Malik R, Pal S, Sharma R, Sakhuja P, Das P. Hepatic and perihepatic PEComas: A study describing a series of five rare cases. Indian J Pathol Microbiol. 2024;67:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Zhou H, Wu X. A rare case report and literature review of splenic perivascular epithelioid cell tumor. Asian J Surg. 2024;47:1414-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Rodríguez N, Pérez S, Rodríguez JN, Rodríguez J, Esquivel A, Sanabria D, Baena J. Perivascular epithelioid cell tumors (PEComas) of gynecological tract: preoperative diagnostic imaging challenge. Ultrasound Obstet Gynecol. 2024;63:286-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Chua D, Loh AH, Tan E, Seow-En I. Perivascular epithelioid cell tumour (PEComa): an unusual cause of painful defaecation. BMJ Case Rep. 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Bennett JA, Oliva E. Perivascular epithelioid cell tumors (PEComa) of the gynecologic tract. Genes Chromosomes Cancer. 2021;60:168-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Schoolmeester JK, Howitt BE, Hirsch MS, Dal Cin P, Quade BJ, Nucci MR. Perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: clinicopathologic and immunohistochemical characterization of 16 cases. Am J Surg Pathol. 2014;38:176-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Doyle LA, Hornick JL, Fletcher CD. PEComa of the gastrointestinal tract: clinicopathologic study of 35 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2013;37:1769-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Chen Z, Han S, Wu J, Xiong M, Huang Y, Chen J, Yuan Y, Peng J, Song W. A systematic review: perivascular epithelioid cell tumor of gastrointestinal tract. Medicine (Baltimore). 2016;95:e3890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Liu L, Dehner C, Grandhi N, Lyu Y, Borcherding DC, Chrisinger JSA, Zhang X, Luo J, Tao Y, Parkes A, Bui NQ, Davis EJ, Milhem MM, Monga V, Weiss M, Tine BV, Hirbe AC. The Impact of TSC-1 and -2 Mutations on Response to Therapy in Malignant PEComa: A Multicenter Retrospective Analysis. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Świtaj T, Sobiborowicz A, Teterycz P, Klimczak A, Makuła D, Wągrodzki M, Szumera-Ciećkiewicz A, Rutkowski P, Czarnecka AM. Efficacy of Sirolimus Treatment in PEComa-10 Years of Practice Perspective. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Sanfilippo R, Jones RL, Blay JY, Le Cesne A, Provenzano S, Antoniou G, Mir O, Fucà G, Fumagalli E, Bertulli R, Stacchiotti S, Brahmi M, Grosso F, Dufresne A, Hindi N, Sbaraglia M, Gronchi A, Collini P, Dei Tos AP, Casali PG. Role of Chemotherapy, VEGFR Inhibitors, and mTOR Inhibitors in Advanced Perivascular Epithelioid Cell Tumors (PEComas). Clin Cancer Res. 2019;25:5295-5300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 17. | Zagardo V, Ferini G. Is there any role for radiotherapy in the management of uterine perivascular epithelioid cell tumors (PEComas)? Arch Gynecol Obstet. 2024;310:1789-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Chiang S, Vasudevaraja V, Serrano J, Stewart CJR, Oliva E, Momeni-Boroujeni A, Jungbluth AA, Da Cruz Paula A, da Silva EM, Weigelt B, Park KJ, Soslow RA, Murali R, Ellenson LH, Benayed R, Ladanyi M, Abu-Rustum NR, Dickson MA, Cohen S, Aghajanian C, Hensley ML, Lee CH, Snuderl M, Konner JA. TSC2-mutant uterine sarcomas with JAZF1-SUZ12 fusions demonstrate hybrid features of endometrial stromal sarcoma and PEComa and are responsive to mTOR inhibition. Mod Pathol. 2022;35:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Sanfilippo R, Fabbroni C, Fucà G, Fumagalli E, Morosi C, Sbaraglia M, Gronchi A, Collini P, Dei Tos AP, Casali PG. Addition of Antiestrogen Treatment in Patients with Malignant PEComa Progressing to mTOR Inhibitors. Clin Cancer Res. 2020;26:5534-5538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Djerroudi L, Masliah-Planchon J, Brisse HJ, El Zein S, Helfre S, Tzanis D, Hamzaoui N, Bonnet C, Laurence V, Bonvalot S, Watson S. Metastatic Malignant Perivascular Epithelioid Cell Tumors With Microsatellite Instability Within Lynch Syndrome Successfully Treated With Anti-PD1 Pembrolizumab. JCO Precis Oncol. 2023;7:e2200627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Wagner AJ, Ravi V, Riedel RF, Ganjoo K, Van Tine BA, Chugh R, Cranmer L, Gordon EM, Hornick JL, Du H, Grigorian B, Schmid AN, Hou S, Harris K, Kwiatkowski DJ, Desai NP, Dickson MA. nab-Sirolimus for Patients With Malignant Perivascular Epithelioid Cell Tumors. J Clin Oncol. 2021;39:3660-3670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |