Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.98808
Revised: October 27, 2024
Accepted: November 14, 2024
Published online: February 15, 2025
Processing time: 196 Days and 1.7 Hours
Radiation resistance limits radiotherapy efficacy in esophageal squamous cell carcinoma (ESCC). The tumor microenvironment, particularly adipocytes, plays a role in promoting cancer progression. Extracellular vesicles and microRNAs (miRNAs) regulate gene expression and hold prognostic potential for esophageal carcinoma. Elucidating radioresistance mechanisms and identifying radiosensitization targets can help enhance radiotherapy efficacy for esophageal cancer.
To investigate the potential role of miRNAs derived from adipocyte exosomes as prognostic markers for radiotherapy efficacy in ESCC.
Free adipocytes were isolated from human thoracic adipose tissue. A co-culture model of adipocytes and ESCC cells was established to observe colony formation and cell survival post-irradiation. ESCC cell apoptosis was assessed by flow cytometry. Western Blot and immunofluorescence assays were performed to evaluate DNA damage in ESCC cells post-irradiation. Adipocyte-derived exosomes were isolated by ultracentrifugation and identified by electron microscopy. A similar set of experiments was performed on ESCC cells to analyze cell survival, apoptosis, and DNA damage post-radiation exposure. Exosomes from adipose tissue and serum exosomes from ESCC patients pre- and post-radiotherapy were subjected to high-throughput miRNA-sequencing and validated using real-time quantitative polymerase chain reaction. The correlation between potential target miRNAs and the short-term prognosis of radiotherapy in ESCC was evaluated by receiver operating characteristic curve analysis.
Co-culturing adipocytes with ESCC cells enhanced radioresistance, as evidenced by increased colony formation. Adipocyte co-culture reduced ESCC cell apoptosis and DNA damage post-radiation. Adipocyte-derived exosomes similarly conferred radioresistance in ESCC cells, decreasing apoptosis and DNA damage post-irradiation. High-throughput miRNA-sequencing identified miR-660-5p in serum and adipose tissue exosomes. Patients with high expression of serum exosome miR-660-5p showed poor prognosis after radiotherapy.
Adipocyte-derived exosomal miR-660-5p is a potential biomarker for evaluating radiotherapy efficacy in ESCC.
Core Tip: This study revealed for the first time the impact of adipocyte-derived extracellular vesicle microRNAs on the radiosensitivity of esophageal squamous cell carcinoma (ESCC), providing valuable resources for studying the tumor microenvironment and ESCC, as well as predicting patient responses to radiotherapy.
- Citation: Ge YY, Xia XC, Wu AQ, Ma CY, Yu LH, Zhou JY. Identifying adipocyte-derived exosomal miRNAs as potential novel prognostic markers for radiotherapy of esophageal squamous cell carcinoma. World J Gastrointest Oncol 2025; 17(2): 98808
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/98808.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.98808
Esophageal cancer is a highly prevalent malignancy worldwide. In China, it accounts for half of all newly diagnosed cancer cases[1]. Esophageal cancer is mainly categorized into esophageal squamous cell carcinoma (ESCC) and eso
Obesity is recognized as a significant factor contributing to cancer morbidity and progression[8]. Adipocytes, the main constituents of adipose tissue, reside in the tumor microenvironment and promote the occurrence and development of various cancers. Research indicates that adipocytes facilitate tumor progression through soluble factors, such as leptin or interleukin-6, and extracellular matrix remodeling[9,10]. Cancer-associated adipocytes (CAA) accompanying tumors enhance the invasive capacity of cholangiocarcinoma by supplying free fatty acids and adipokines to tumor cells[11]. However, the impact of adipocytes in the tumor microenvironment on the radiosensitivity of ESCC and the underlying mechanisms are not well characterized in contemporary literature.
Adipose tissue functions as an endocrine organ, releasing adipocyte-specific bioactive elements that regulate various physiological and pathological processes[12]. Nevertheless, the capacity of adipocytes to remotely modulate tumor be
Exosomes, vesicle-like bodies (40-100 nm in diameter) secreted by cells, are ubiquitous in peripheral blood, urine, and other bodily fluids[13]. As extracellular messengers, they facilitate the crosstalk between tumor cells and surrounding microenvironment components. Exosomes transport various molecules such as microRNAs (miRNAs) and long non-coding RNAs (lncRNA), proteins, and lipids, influencing tumor progression, metastasis, and chemoresistance[14]. Exo
The objective of this study was to identify potential miRNAs that may mediate the promotion of ESCC radioresistance by adipocyte-derived exosomes. We first investigated the role of adipocytes and adipocyte-derived exosomes in the radioresistance of ESCC cells. Next, we employed high-throughput miRNA sequencing to investigate the changes in the expression profile of miRNAs in serum and adipose tissue-derived exosomes. Additionally, we analyzed serum exo-miR-660-5p levels in ESCC patients to assess their clinical value. Our findings indicate that adipocyte-derived exosomal miR-660-5p enhances ESCC radiation resistance, providing a potential therapeutic target to improve the radiosensitivity of ESCC.
ESCC cells KYSE30 and TE-1 were obtained from Wuhan PuNuoSai Life Technology Co. Ltd. (Wuhan, China). Cell lines were cultured in RPMI 1640 medium (Hyclone, United States), supplemented with 10% fetal bovine serum (Gibco, United States) and a 1% penicillin-streptomycin solution (Beyotime Biotechnology, Nantong, China).
Cell irradiation was performed using a linear accelerator from RAD SOURCE (Suwanee, GA, United States), delivering a dose rate of 1.15 Gy/minute at 160 kV. Clonogenic assays were conducted with absorbed doses of 0, 2, 4, 6, and 8 Gy. Apoptosis, immunofluorescence, and Western Blot experiments utilized 4 and 6 Gy.
Human thoracic adipose tissue samples and serum samples from ESCC patients were collected at The Affiliated Tumor Hospital of Nantong University, with approval from the institutional ethics committee (Ethics Research Grant 2022). The study was conducted after obtaining written informed consent from all participants. Between February 2022 and July 2022, 99 blood samples were collected. Patients were eligible for inclusion if they met the following criteria: (1) Karnofsky performance status score ≥ 70; (2) Age ≤ 75 years; (3) Inoperable cases or those who refused surgery; (4) Lesion size ≤ 10 cm, with no signs of esophageal perforation on X-ray; (5) No severe heart, liver, or kidney dysfunction with normal blood routine parameters; and (6) No severe cachexia or cardiovascular diseases and anticipated survival > 3 months. Pregnant and lactating women, patients with other concomitant malignant tumors, and those lacking follow-up data were excluded. Clinical staging adhered to the 7th edition of the American Joint Committee on Cancer Staging Manual. All patients (Table 1) received involved-field radiation therapy with a total dose of 60-66 Gy (2 Gy per fraction, 5 days per week). They also received two cycles of paclitaxel combined with platinum-based neoadjuvant chemotherapy and two cycles of adjuvant chemotherapy post-radiation. Blood samples were collected one day before treatment. Short-term efficacy evaluation included barium esophagography for primary lesions and RECIST criteria for metastatic lesions. Follow-up examinations were conducted every 3 months post-treatment until death or June 2024, consisting of physical examinations, barium swallow tests, and chest computed tomography scans.
| Characteristics | ESCC, n |
| Sex | |
| Male | 33 |
| Female | 67 |
| Age | |
| < 60 years | 21 |
| ≥ 60 years | 79 |
| Length | |
| < 5 cm | 54 |
| ≥ 5 cm | 46 |
| Therapeutic modality | |
| Radiotherapy | 58 |
| Chemoradiotherapy | 42 |
| T stage | |
| T1–2 | 52 |
| T3–4 | 48 |
| Lymph node metastasis | |
| N0–1 | 93 |
| N2–3 | 7 |
| TNM stage | |
| I-II | 75 |
| III-IV | 25 |
Thoracic adipose tissue was minced and mixed with 0.2% collagenase (Biofrox, Germany) at a 3:1 ratio in a 50 mL centrifuge tube. The mixture was shaken at 37 °C for 30 minutes and centrifuged at 1000 rpm for 5 minutes. After centrifugation, adipose tissue layers were separated: The top layer was oil, the middle layer contained adipocytes, and the bottom layer included fibrous connective tissue and collagenase solution. The middle layer of adipocytes was used for experiments. The isolated adipocytes were added to a polycarbonate membrane chamber (8 μm pore size) placed in a culture dish containing ESCC cells, establishing a co-culture model.
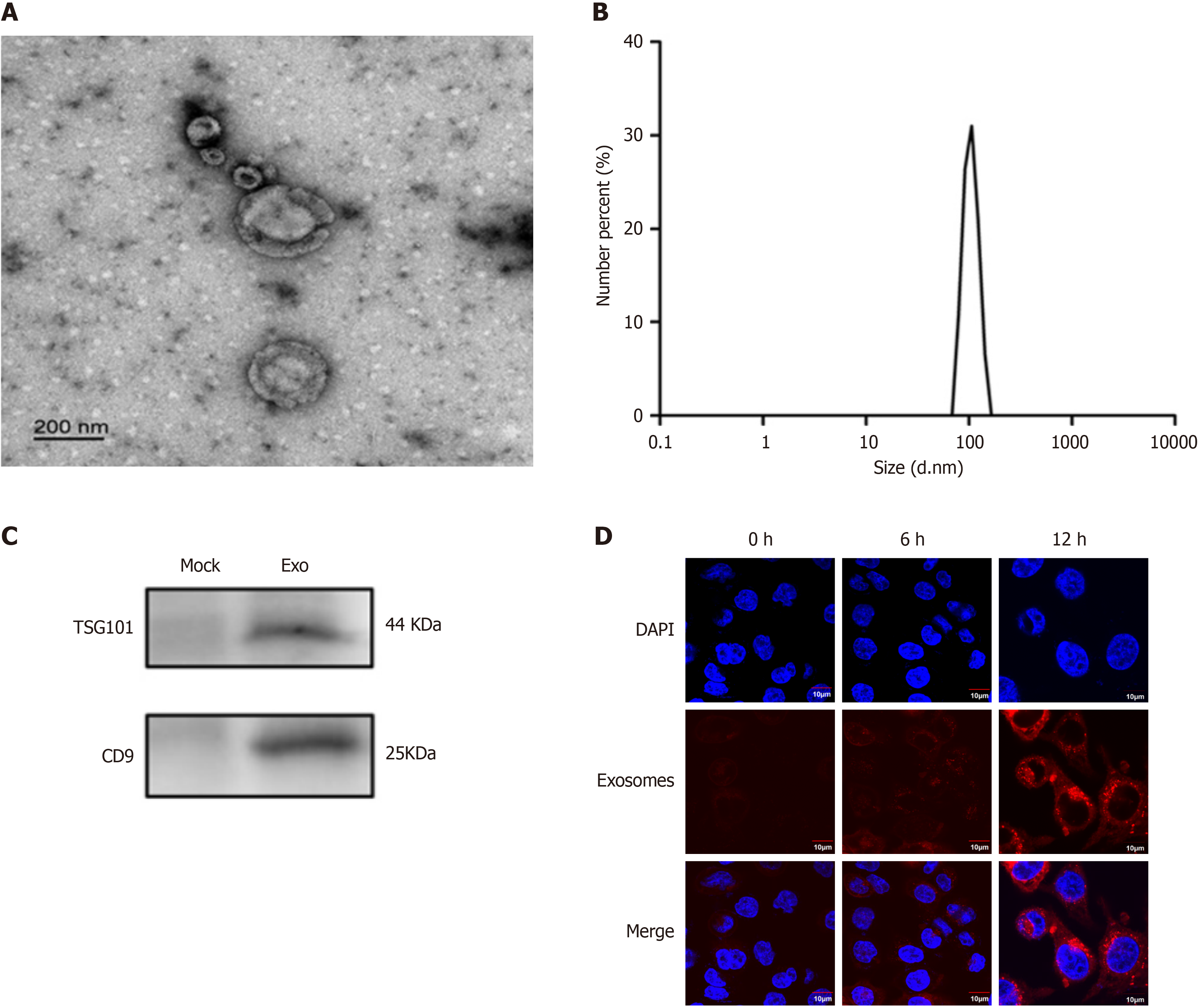
Adipocyte culture supernatant or serum was centrifuged (2000 × g, 4 °C for 10 minutes), and the upper layer was filtered (0.22 μm, Millipore, United States). The filtrate underwent ultracentrifugation (35000 rpm/210000 × g, 4 °C for 70 minutes), discarding the supernatant. The pellet was resuspended in PBS, centrifuged again (35000 rpm/210000 g, 4 °C for 70 minutes), and the supernatant was removed. The final pellet was resuspended in aseptic PE tubes. Extracellular vesicle protein concentration was measured by BCA, with a target of approximately 5 μg/μL. Samples were stored at 4 °C (short-term, approximately one week) and -80 °C (long-term, 3 months). The size distribution and concentration of EVs were analyzed using nanoparticle tracking analysis (NTA) with a ZetaView particle tracker from ParticleMetrix (Meerbusch, Germany). The structure of EVs was examined by transmission electron microscopy (TEM; JEM-1200EX, JEOL Ltd., Japan). TSG101 and CD9 were used as exosomal markers.
Exosome solutions were labeled with 2 μL of Mem Dye stock solution, incubated at 37 °C for 30 minutes, and centrifuged at 3000 × g for 5 minutes. 100 μL of PBS was added to the filter tube and centrifuged at 3000 × g for 5 minutes; these steps were repeated. Subsequently, labeled exosomes (1-50 μL) were added to cells and incubated at 37 °C, 5% CO2 for 1-24 hours. Following supernatant removal, PBS washing, and adding fresh culture medium with serum, uptake was ob
Cells in the logarithmic growth phase were digested into single-cell suspensions. Various cell numbers were seeded in six-well plates, and exposed to five dose points of 0, 2, 4, 6, and 8 Gy radiation, with three replicates per dose. After 24-hour co-culture with adipocytes and exosomes, cells were irradiated and subsequently cultured for 14 days. Cells were fixed with methanol and stained with Giemsa for 30 minutes. Colonies containing more than 50 cells were counted. Sensitizer enhancement ratios (SER) were calculated using the multi-target single-hit model: SER = D0 value of radiation alone/D0 value of radiation + treatment group. The following equation was used for the multi-target single-hit model: SF = 1 - [1 - exp (-D/D0)]N, Dq = D0logN, where SF is the cell survival fraction, D is the radiation dose (Gy), D0 is the mean lethal dose, Dq is the quasi-threshold dose, and N is the extrapolation number. Cell survival curves were plotted based on radiation dose.
ESCC cells were seeded into six-well plates in different groups and cultured in the complete medium for 24 hours. Cells were then treated under different experimental conditions: Co-cultured with adipocytes or exosomes for 24 hours followed by 6 Gy irradiation. Post-irradiation, cells were digested with trypsin for 48 hours, centrifuged to collect cell pellets, resuspended in PBS to make single-cell suspensions, centrifuged to discard the supernatant, resuspended in 1 × buffer, and stained with Annexin V 7ADD/PE (BD, United States). Then, the cells were left at room temperature in dark for 15 minutes before apoptosis detection using flow cytometry.
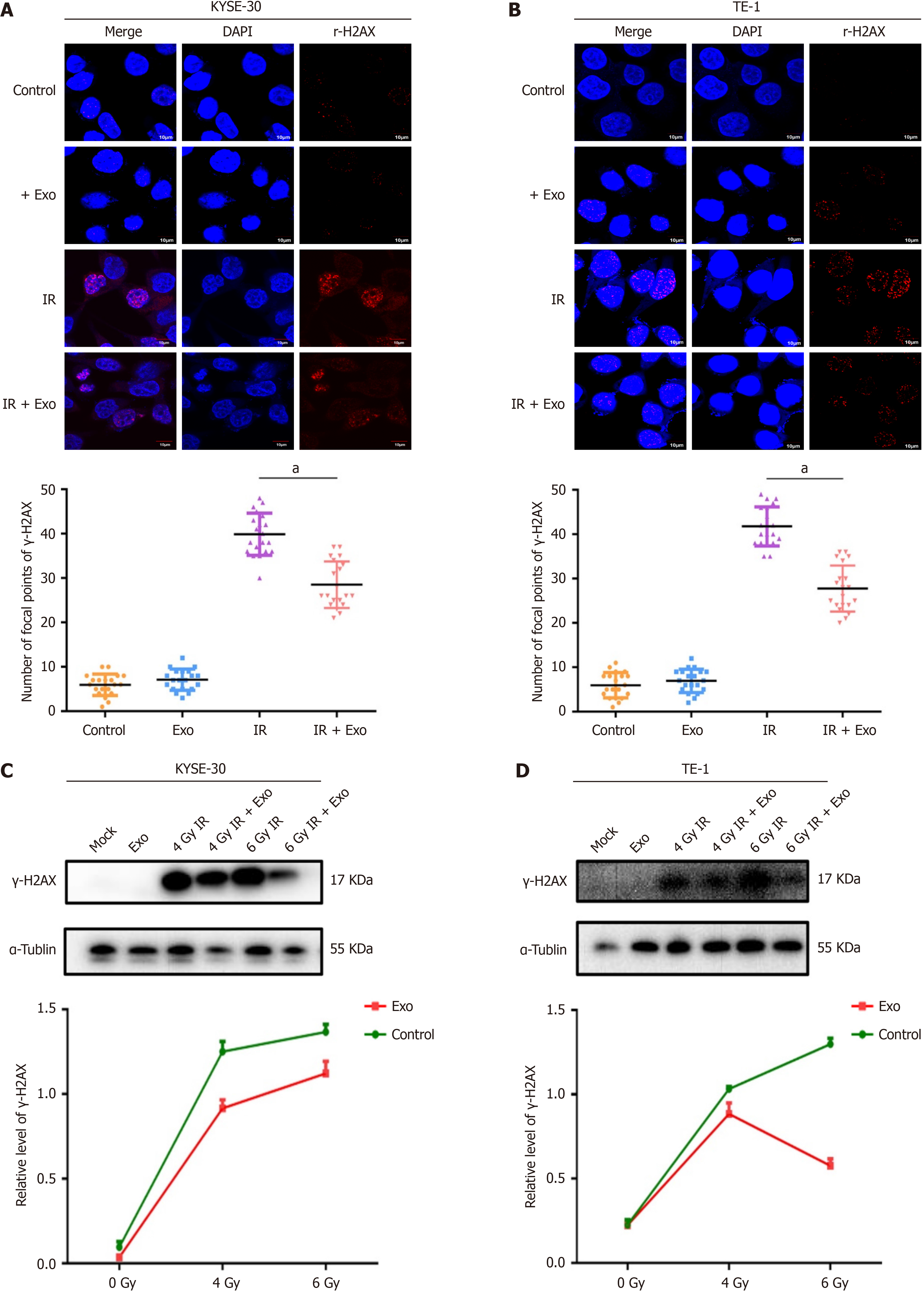
Cells were seeded in glass-bottom dishes and treated under different conditions 24 hours before irradiation. Post-irradiation (0.5 hours), cells were fixed with 4% paraformaldehyde for 10 minutes, washed gently with PBS three times, permeabilized with 1% Triton × 100 for 10 minutes, and blocked with serum at room temperature for 1 hour. After washing, cells were incubated overnight at 4 °C with mouse monoclonal anti-γH2AX antibody (1:200, Abcam, United Kingdom), followed by incubation with Cy3-labeled goat anti-mouse IgG (Beyotime, China) for 1 hour. The cells were observed using an Olympus fluorescence microscope (Olympus, Tokyo, Japan), and γH2AX foci in each cell were counted using Image Pro Plus software (Media Cybernetics). The average number of γH2AX foci per nucleus was determined from three independent experiments.
MiRNA libraries were constructed using the QIAseq miRNA Library Kit (QIAGEN, Germany). Experimental steps included 3' ligation, 5' ligation, reverse transcription, QIAseq miRNA NGS bead preparation, cDNA purification, library amplification with HT plate indices, and library amplification with tube indices. After quality control, libraries were sequenced on an Illumina HiSeq 2500 using the SE50 strategy. Cutadapt software was used to trim adapters from the ends of raw reads while retaining reads longer than 17 nucleotides. The FANse3 ultra-high-precision sequence alignment algorithm was employed to align reads obtained from each sample to reference sequences (human mature miRNAs, miRBase version 22.1). Mapping parameters were set as -E5% - indel -S14. Transcripts per million were calculated to normalize RNA sequencing depth. miRNAs with read counts ≥ 10 were considered expressed, while those with counts < 10 were considered non-expressed. For gene sequencing data, differential expression analysis was performed using the edgeR package, a statistical method based on the negative binomial distribution. miRNAs were considered differentially expressed when |logFC| > 1 and false discovery rate (FDR) < 0.01. miRNAs with logFC > 1 were considered upre
Cells from different treatment groups were lysed with RIPA buffer (Solarbio, China), and protein concentrations were determined using the BCA Protein Assay kit (Beyotime, China). After boiling denaturation with the loading buffer, SDS-PAGE electrophoresis was performed, followed by membrane transfer. The membrane was blocked with 5% BSA for 1.5 hours, then incubated overnight with primary antibody at 4 °C. After washing thrice with TBST, the membrane was incubated with secondary antibody at room temperature for 1 hour, followed by three additional washes. Chemiluminescence was used for detection, and Image J software was used for quantifying the target bands and the internal controls. The following antibodies were employed: Anti-γ-H2AX (Cell Signaling Technology, United States; 9718S, 1:1000); anti-TSG101 (Cell Signaling Technology, United States; 28405S, 1:1000); anti-CD9 (Cell Signaling Technology, United States; 98327S, 1:1000); anti-GAPDH (Cell Signaling Technology, United States; 5174S, 1:1000); anti-α-Tubulin (Cell Signaling Technology, United States; 2125S, 1:1000).
To validate the results, the selected miRNAs were subjected to real-time quantitative polymerase chain reaction (qRT-PCR) analysis. Total RNA was extracted using TRIzol reagent (Invitrogen, United States). The HyperScript' III miRNA 1st StrandcDNA Synthesis Kit (EnzyArtisan, Shanghai) was used to perform reverse transcription. Real-time PCR was conducted using NovoStart SYBR qPCR SuperMix plus (Novoprotein, Shanghai, China). The reference gene used was U6. The primers are listed in Table 2. The results are shown as relative expression levels calculated by the 2−ΔΔCt method.
| Primer | Sequences (5' to 3') |
| hsa-miR-660-5p-F | CGCGGCATACCCATTGCATATC |
| hsa-miR-660-5p-R | ATCCAGTGCAGGGTCCGAGG |
| hsa-miR-660-5p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTAT TCGCACTGGATACGACCAACTC |
| hsa-miR-378a-3p-F | AATCCGGAACTGGACTTGGAGTC |
| hsa-miR-378a-3p-R | ATCCAGTGCAGGGTCCGAGG |
| hsa-miR-378a-3p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTAT TCGCACTGGATACGACGCCTTC |
| hsa-miR-193b-3p-F | AACACGCAACTGGCCCTCAAA |
| hsa-miR-193b-3p-R | ATCCAGTGCAGGGTCCGAGG |
| hsa-miR-193b-3p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTAT TCGCACTGGATACGACAGCGGG |
| hsa-mir-30a-F | CCGCTCGTGTAAACATCCTCGA |
| hsa-mir-30a-R | ATCCAGTGCAGGGTCCGAGG |
| hsa-mir-30a-RT | GTCGTATCCAGTGCAGGGTCCGAGGTAT TCGCACTGGATACGACCTTCCA |
To assess the predictive value of differentially expressed miRNAs in ESCC radiotherapy response, receiver operating characteristic (ROC) curves were generated using MedCalc 20.218 software. Statistical significance was defined as P < 0.05.
All experiments were performed in triplicate and none of the samples were excluded from the analysis. Owing to the normal distribution of all variables, the results are presented as mean ± SD, and data analysis was performed using the Student's t-test. Statistical analysis was performed using SPSS 22.0 (Chicago, IL, United States) and GraphPad Prism 8.0 (CA, United States) software, with the significance level set at P < 0.05.
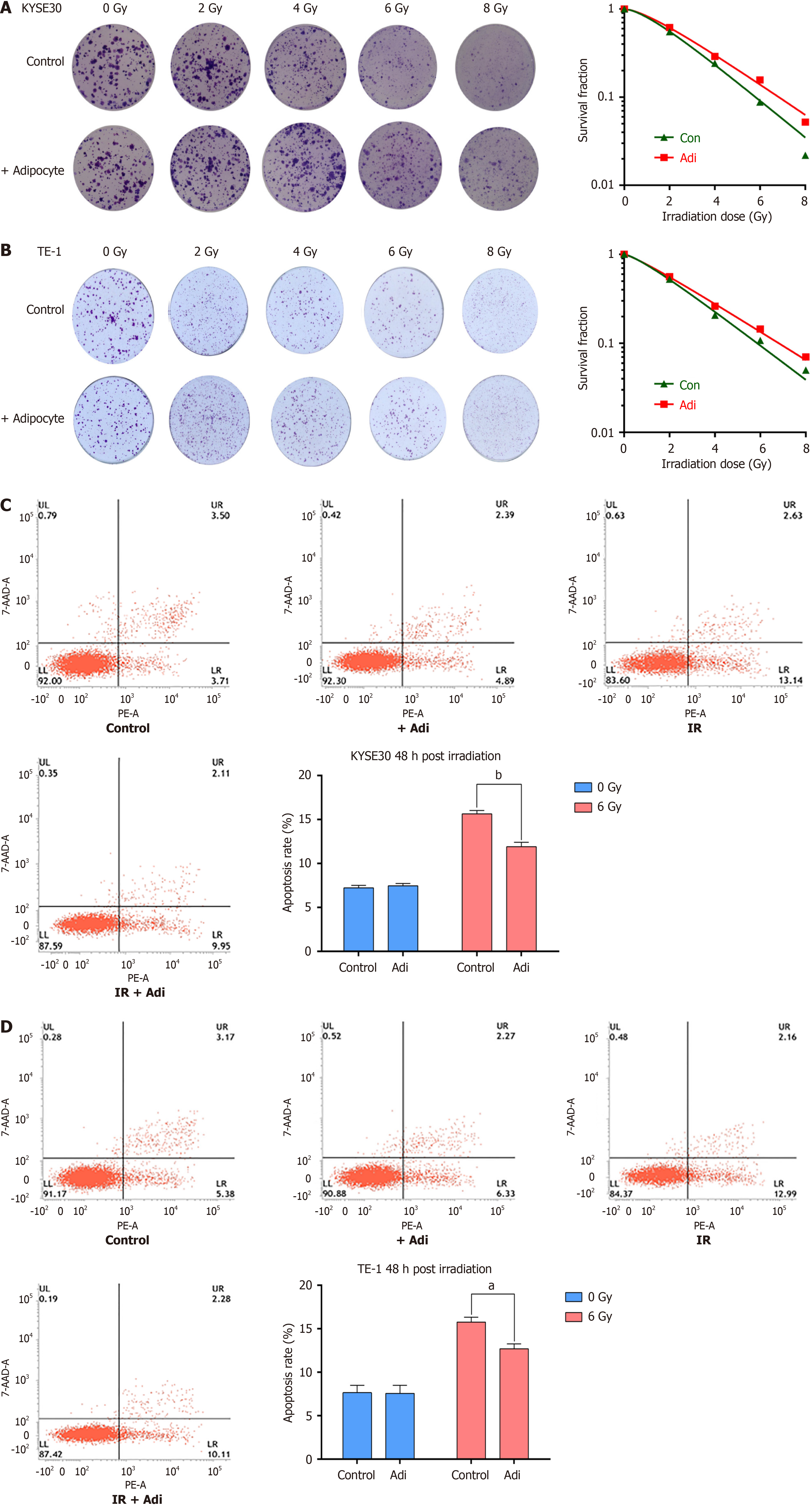
After co-culture with adipocytes and X-ray exposure, the clonogenic survival of KYSE30 (Figure 1A) and TE-1 (Figure 1B) cells was noticeably greater compared to the control group, with SER of 0.832 and 0.825, respectively (Table 3). Moreover, 48 hours post-irradiation with co-culture, the apoptotic rates of ESCC cells KYSE30 (Figure 1C) and TE-1 (Figure 1D) were significantly decreased compared to the control. These results indicate that adipocytes can inhibit Ionizing radiation-induced apoptosis and promote radioresistance, while the addition of the exosome inhibitor GW4869 in co-culture with adipocytes effectively increased the sensitivity to radiotherapy (Supplementary Figure 1).
| Cell | Group | N | Dq | D0 | SER |
| KYSE-30 | Control | 1.710 | 1.105 | 2.060 | 0.832 |
| + Adipocyte | 1.615 | 1.186 | 2.475 | ||
| Control | 1.337 | 0.705 | 2.426 | 0.883 | |
| + Exosome | 1.513 | 1.137 | 2.746 | ||
| TE-1 | Control | 1.395 | 0.745 | 2.239 | 0.825 |
| + Adipocyte | 1.248 | 0.601 | 2.713 | ||
| Control | 1.724 | 1.022 | 1.876 | 0.751 | |
| + Exosome | 1.885 | 1.584 | 2.498 |
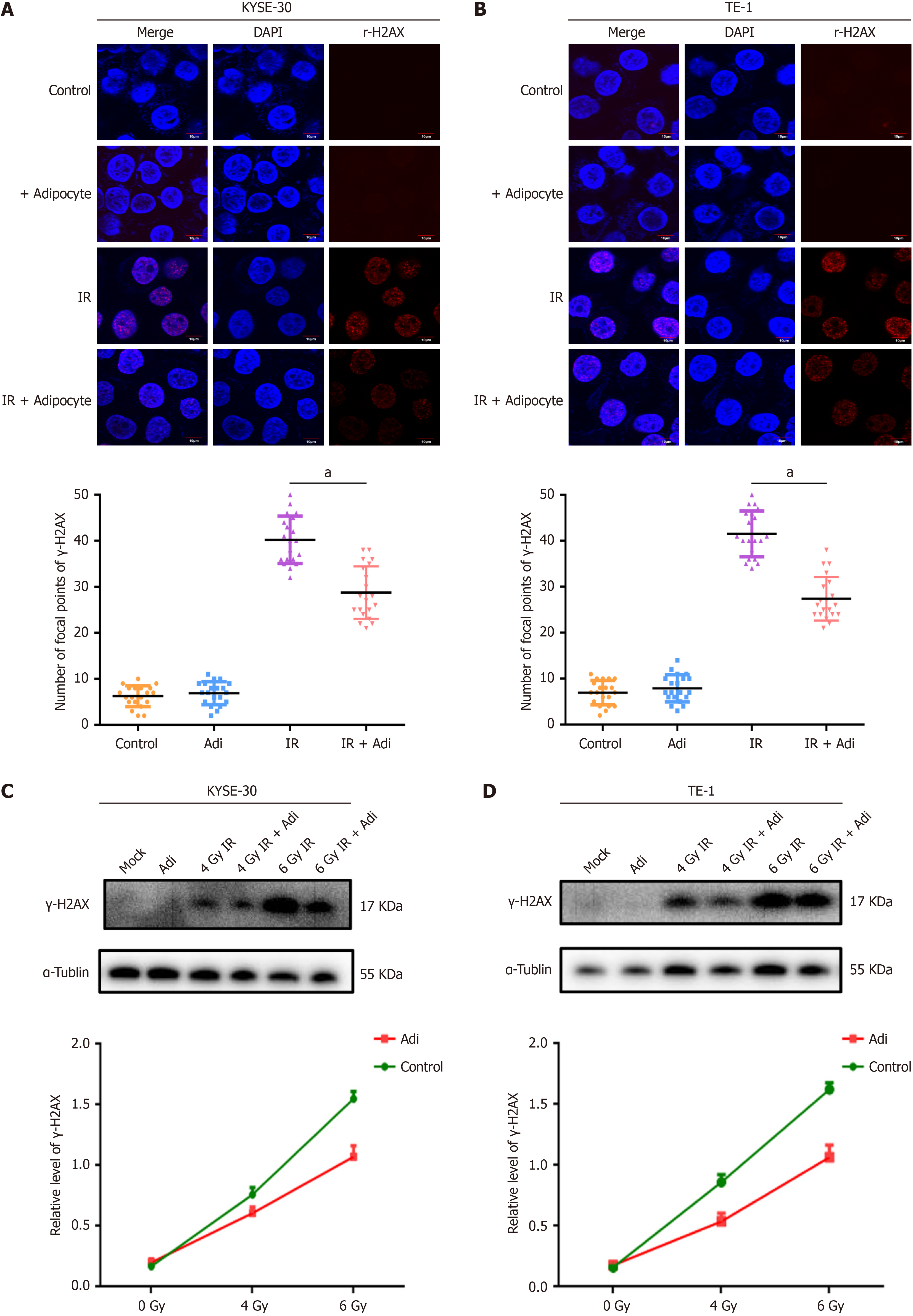
Within 24 hours before irradiation, co-culture with adipocytes significantly inhibited X-ray-induced double-strand breaks. Laser confocal microscopy revealed a reduction in γ-H2AX foci in the nuclei of KYSE30 (Figure 2A) and TE-1 (Figure 2B) cells exposed to ionizing radiation after co-culture with adipocytes. Western blot analysis indicated decreased expression of γ-H2AX protein in KYSE30 (Figure 2C) and TE-1 (Figure 2D) cells following co-culture with adipocytes, consistent with immunofluorescence findings, suggesting that adipocytes may alleviate ionizing radiation-induced DNA damage in ESCC cells. These results demonstrate that adipocytes decrease IR-induced DNA damage and promote radioresistance in ESCC.
Transmission electron microscopy revealed vesicles with a diameter of approximately 100 nm and a double-layer mem
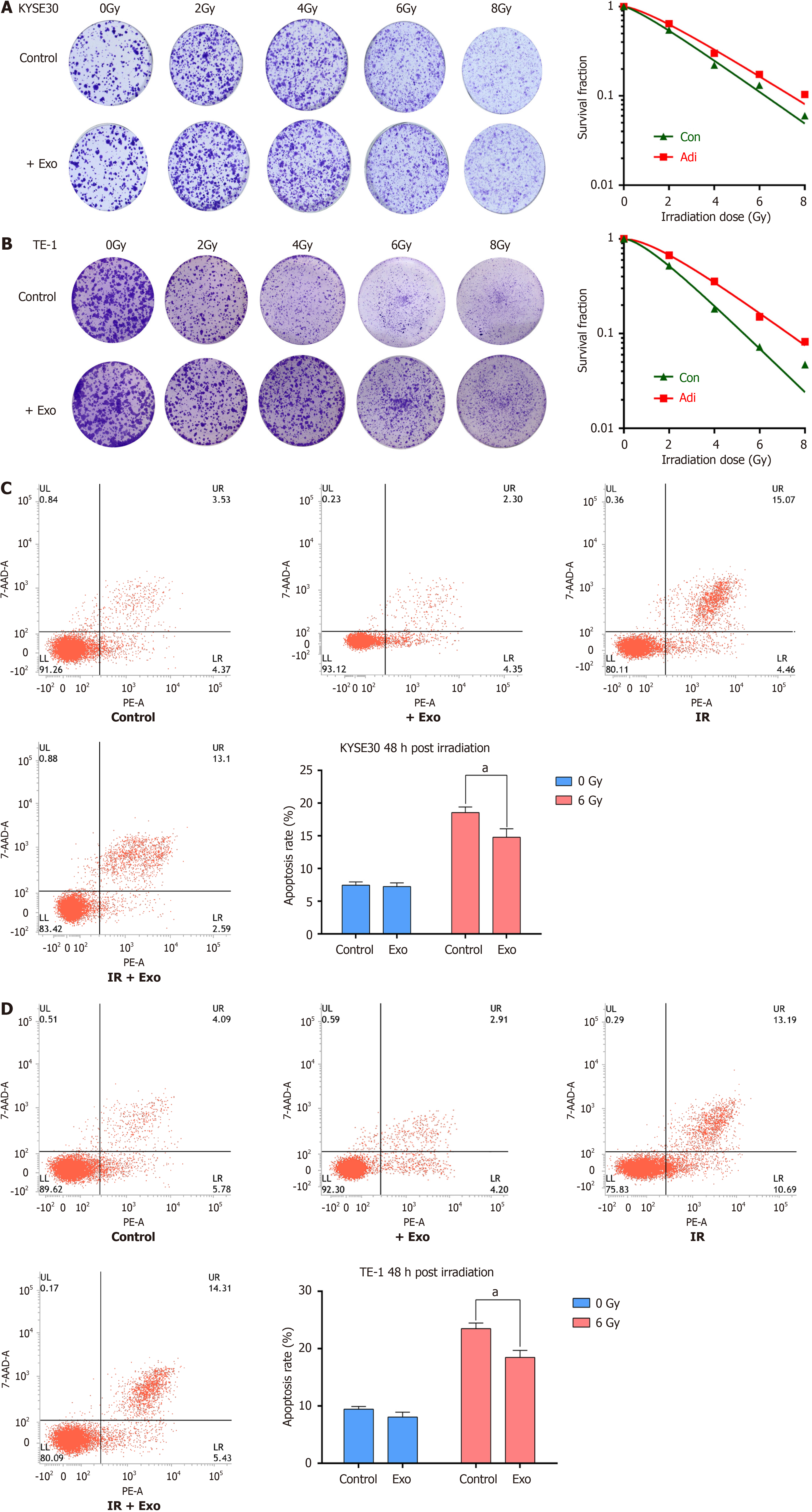
The combined impact of adipocyte-derived exosomes and X-ray irradiation on the clonogenic survival of KYSE30 (Figure 4A) and TE-1 (Figure 4B) cells was assessed. Cells treated with exosomes before irradiation exhibited higher survival rates than the control, with SER of 0.883 and 0.751, respectively (Table 3). After 48 hours of co-culture following irradiation, the apoptotic rates of KYSE30 (Figure 4C) and TE-1 (Figure 4D) cells were significantly lower in the exosome-treated groups than in irradiation-only groups. These results indicate that adipocyte-derived exosomes can inhibit ionizing radiation-induced apoptosis and promote radioresistance.
We previously demonstrated that adipocyte-derived exosomes affect the radiosensitivity of cancer cells. Here, we further investigated whether adipocyte-derived exosomes influence the dynamics of DNA damage repair. Laser confocal mi
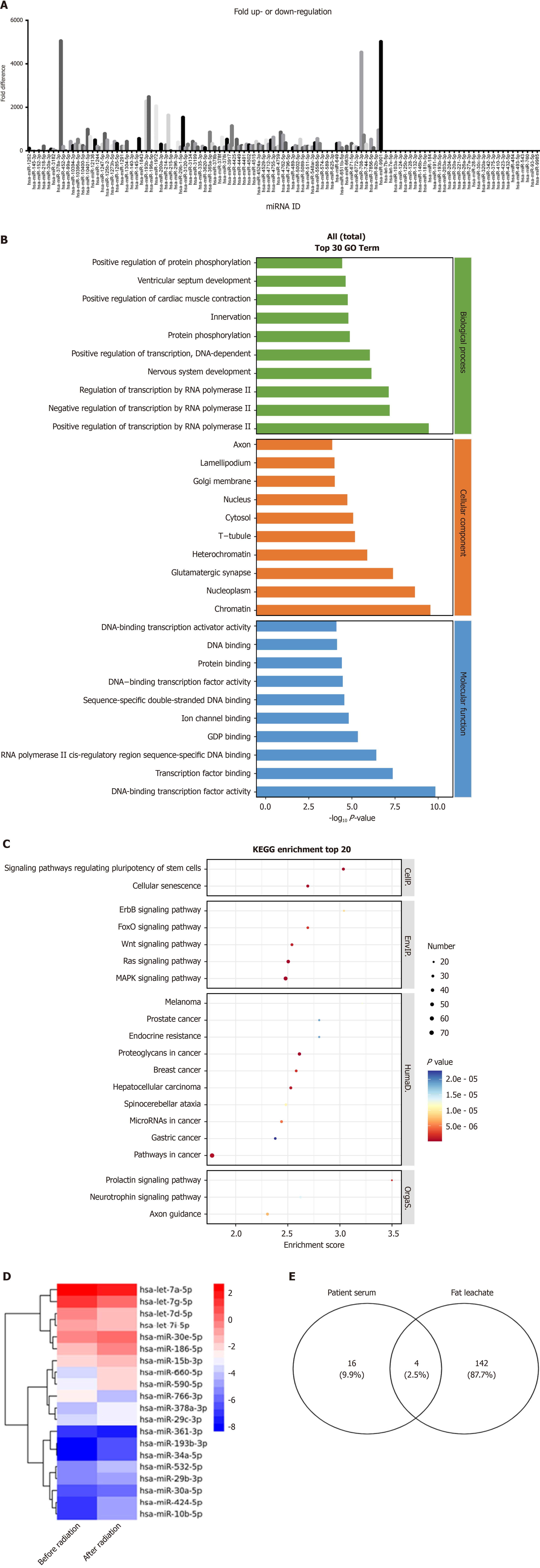
High-throughput sequencing of samples from adipocyte-derived exosomes and blank controls revealed 330 differentially expressed genes, with 219 upregulated and 111 downregulated (P < 0.05 & |log2FC| > 1) (Figure 6A). Subsequently, a comparison of high-throughput sequencing results from serum exosomes of ESCC patients before and after radiotherapy revealed 20 differentially expressed miRNAs. GO enrichment analysis of differentially expressed miRNAs in serum exosomes revealed enrichment in the Biological Process category, particularly in "positive regulation of transcription". Furthermore (Figure 6B), KEGG pathway analysis identified the top 20 entries based on log10Pvalue, highlighting several key signaling pathways associated with the target genes of these miRNAs (ErbB signaling pathway, FoxO signaling pathway, Wnt signaling pathway, Ras signaling pathway, and MAPK signaling pathway) (Figure 6C). The clustering heatmap is displayed in Figure 6D. Four intersecting miRNAs were identified: MiR-30a-5p, miR-378a-3p, miR-193b-3p, and miR-660-5p (Figure 6E).
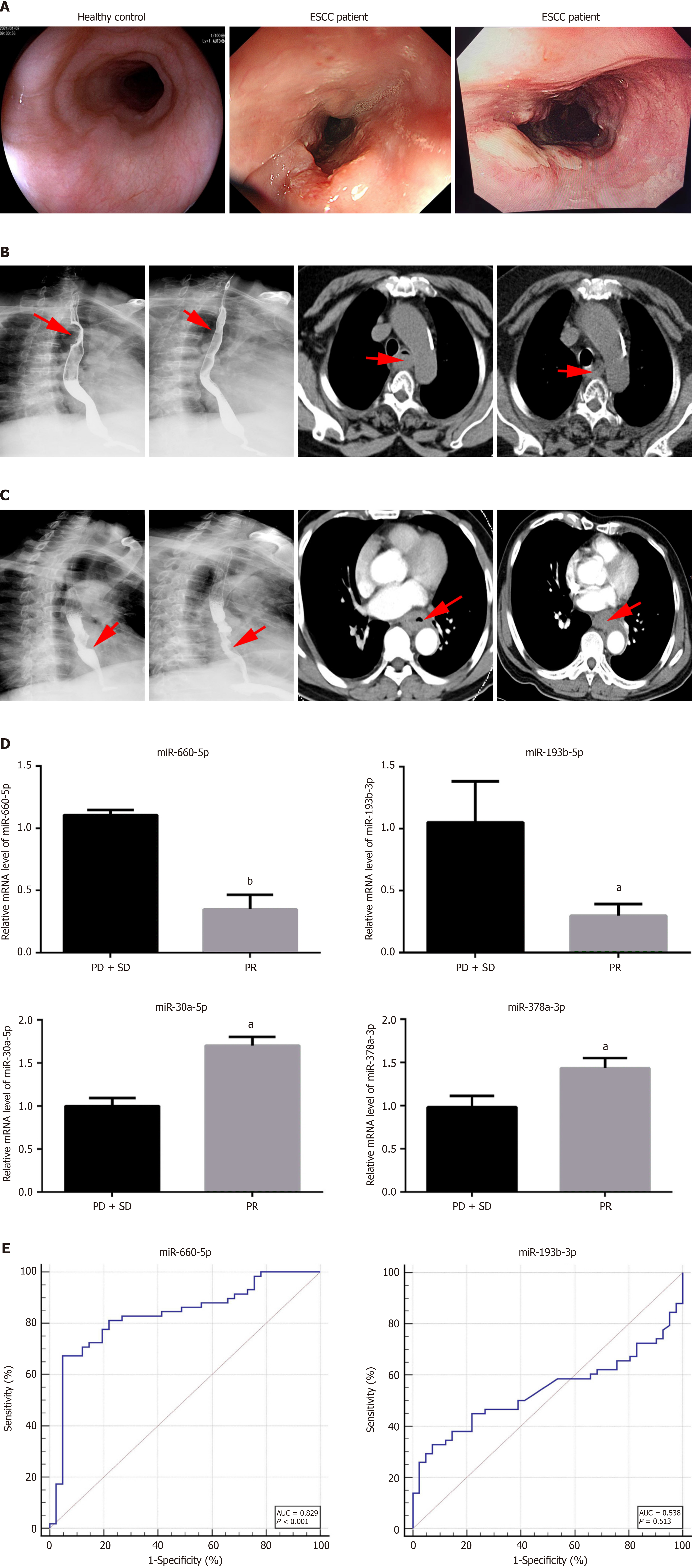
ESCC patients after radiotherapy were divided into two groups based on RECIST 1.1 criteria: 58 cases with partial response were classified as the effective radiotherapy group, while 41 cases with stable disease or progressive disease were classified as the ineffective radiotherapy group. The expression levels of the 4 selected miRNAs were validated by qRT-qPCR. Analysis revealed elevated expression levels of serum exosome miR-193b-3p and miR-660-5p in the ineffective radiotherapy group, whereas a significant decrease was observed in the effective group (P < 0.001). These findings suggested a potential role of serum exo-miR-193b-3p and exo-miR-660-5p as biomarkers for evaluating ra
The predictive capability of serum exosome miR-193b-3p and miR-660-5p in distinguishing between good and poor responders to chemo-radiotherapy was assessed using ROC curve analysis. Serum exo-miR-660-5p demonstrated strong predictive ability in differentiating responders (area under the curve: 0.829, 95%CI: 0.740-0.897, P < 0.0001; Figure 7). However, there were no significant differences in serum exo-miR-193b-3p levels between good and poor responders (Figure 7). The differences in serum exo-miR-660-5p levels between good and poor responders and its strong discriminatory power in distinguishing between the two groups suggest its potential role as a biomarker for predicting patient responses to radiotherapy.
Most contemporary research on esophageal cancer radiosensitivity is focused on functional alterations of key genes related to cancer cell proliferation, apoptosis, DNA damage, and repair[20]. The present study aimed to explore the impact and regulatory mechanisms of the tissues surrounding esophageal cancer on its radiosensitivity. Studies have shown that excessive fat accumulation, leading to obesity or overweight, is a risk factor for esophageal cancer[21]. Adipose tissue, primarily composed of adipocytes, serves as the body’s largest energy reservoir. Upon breakdown, it releases glycerol and fatty acids, providing essential sources of energy for various tissues[22]. Additionally, adipose tissue serves as a vital endocrine organ, producing and secreting important signaling molecules such as adipokines, inflammatory cytokines, and enzymes[23]. In the present study, ESCC cells co-cultured with adipocytes and exposed to high-energy X-rays showed increased resilience. Specifically, KYSE30 and TE-1 cells co-cultured with adipocytes exhibited significantly enhanced clonogenic formation rates post-irradiation compared to cells cultured alone. Additionally, apoptosis was decreased and DNA double-strand breaks in the nuclei were notably reduced in TE-1 cells co-cultured with adipocytes. These results suggest a close association between adipocytes in the tumor microenvironment and the radiation resistance of ESCC.
EVs, especially exosomes, have garnered widespread attention due to their involvement in cancer progression and their potential as diagnostic and prognostic markers. Studies have demonstrated the involvement of EVs in cancer growth, metastasis, and angiogenesis. Moreover, EVs facilitate intercellular crosstalk, particularly among adipocytes, influencing tissue microenvironments. Small EVs shed by adipocytes stimulate fatty acid oxidation and migration in melanoma cells, and these effects were enhanced in obesity[24]. EVs were first discovered in serum culture medium in 1983[25]. EVs play important roles in immune surveillance, cell apoptosis, tumor progression, and several other phy
Exosomes possess a typical lipid bilayer and can selectively enrich various proteins, miRNAs, and lncRNAs, fa
However, research on the role of exosomes in cancer is hindered by significant limitations, including the lack of validation of screened miRNAs in clinical samples and the uncertainty surrounding the biomarker potential of miRNAs in exosomes. In this study, adipose-derived exosomes were found to increase the radioresistance of ESCC cells by tran
This study demonstrates a strong link between tumor microenvironment adipocytes and ESCC radioresistance. Adi
We wish to thank Professor Yang Jiao and Dr. Yi-Ting Tang for the timely help with reviewing and revising this paper.
| 1. | Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 1120] [Article Influence: 186.7] [Reference Citation Analysis (1)] |
| 2. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2218] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 3. | Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121:1643-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15282] [Article Influence: 3056.4] [Reference Citation Analysis (4)] |
| 5. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13196] [Article Influence: 1466.2] [Reference Citation Analysis (3)] |
| 6. | Chen GZ, Zhu HC, Dai WS, Zeng XN, Luo JH, Sun XC. The mechanisms of radioresistance in esophageal squamous cell carcinoma and current strategies in radiosensitivity. J Thorac Dis. 2017;9:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Farhood B, Goradel NH, Mortezaee K, Khanlarkhani N, Salehi E, Nashtaei MS, Shabeeb D, Musa AE, Fallah H, Najafi M. Intercellular communications-redox interactions in radiation toxicity; potential targets for radiation mitigation. J Cell Commun Signal. 2019;13:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Gallagher EJ, LeRoith D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol Rev. 2015;95:727-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 538] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 9. | Andarawewa KL, Motrescu ER, Chenard MP, Gansmuller A, Stoll I, Tomasetto C, Rio MC. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 2005;65:10862-10871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Duong MN, Geneste A, Fallone F, Li X, Dumontet C, Muller C. The fat and the bad: Mature adipocytes, key actors in tumor progression and resistance. Oncotarget. 2017;8:57622-57641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 11. | Nie J, Zhang J, Wang L, Lu L, Yuan Q, An F, Zhang S, Jiao Y. Adipocytes promote cholangiocarcinoma metastasis through fatty acid binding protein 4. J Exp Clin Cancer Res. 2017;36:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018;27:68-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 344] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 13. | Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15:366-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 759] [Article Influence: 126.5] [Reference Citation Analysis (0)] |
| 14. | Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4900] [Cited by in RCA: 6141] [Article Influence: 511.8] [Reference Citation Analysis (0)] |
| 15. | Shah R, Patel T, Freedman JE. Circulating Extracellular Vesicles in Human Disease. N Engl J Med. 2018;379:958-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 521] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 16. | Gareev I, Beylerli O, Liang Y, Xiang H, Liu C, Xu X, Yuan C, Ahmad A, Yang G. The Role of MicroRNAs in Therapeutic Resistance of Malignant Primary Brain Tumors. Front Cell Dev Biol. 2021;9:740303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Xu S, Ding N, Pei H, Hu W, Wei W, Zhang X, Zhou G, Wang J. MiR-21 is involved in radiation-induced bystander effects. RNA Biol. 2014;11:1161-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Yuan W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 584] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 19. | Vader P, Breakefield XO, Wood MJ. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med. 2014;20:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 20. | Zhang H, Si J, Yue J, Ma S. The mechanisms and reversal strategies of tumor radioresistance in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2021;147:1275-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15:139-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 363] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 22. | Zhang Z, Scherer PE. Adipose tissue: The dysfunctional adipocyte - a cancer cell's best friend. Nat Rev Endocrinol. 2018;14:132-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Parra-Peralbo E, Talamillo A, Barrio R. Origin and Development of the Adipose Tissue, a Key Organ in Physiology and Disease. Front Cell Dev Biol. 2021;9:786129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Clement E, Lazar I, Attané C, Carrié L, Dauvillier S, Ducoux-Petit M, Esteve D, Menneteau T, Moutahir M, Le Gonidec S, Dalle S, Valet P, Burlet-Schiltz O, Muller C, Nieto L. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. EMBO J. 2020;39:e102525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 25. | Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1462] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 26. | Ni J, Bucci J, Malouf D, Knox M, Graham P, Li Y. Exosomes in Cancer Radioresistance. Front Oncol. 2019;9:869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Quan M, Kuang S. Exosomal Secretion of Adipose Tissue during Various Physiological States. Pharm Res. 2020;37:221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Kim H, Kim EH, Kwak G, Chi SG, Kim SH, Yang Y. Exosomes: Cell-Derived Nanoplatforms for the Delivery of Cancer Therapeutics. Int J Mol Sci. 2020;22:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 29. | Bertoli G, Cava C, Castiglioni I. MicroRNAs: New Biomarkers for Diagnosis, Prognosis, Therapy Prediction and Therapeutic Tools for Breast Cancer. Theranostics. 2015;5:1122-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 577] [Cited by in RCA: 609] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 30. | Wen J, Yang H, Liu MZ, Luo KJ, Liu H, Hu Y, Zhang X, Lai RC, Lin T, Wang HY, Fu JH. Gene expression analysis of pretreatment biopsies predicts the pathological response of esophageal squamous cell carcinomas to neo-chemoradiotherapy. Ann Oncol. 2014;25:1769-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Wen J, Luo K, Liu H, Liu S, Lin G, Hu Y, Zhang X, Wang G, Chen Y, Chen Z, Li Y, Lin T, Xie X, Liu M, Wang H, Yang H, Fu J. MiRNA Expression Analysis of Pretreatment Biopsies Predicts the Pathological Response of Esophageal Squamous Cell Carcinomas to Neoadjuvant Chemoradiotherapy. Ann Surg. 2016;263:942-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |