Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.92437
Revised: September 30, 2024
Accepted: November 22, 2024
Published online: February 15, 2025
Processing time: 358 Days and 14.7 Hours
Although transmembrane protein 106C (TMEM106C) has been elucidated to be overexpressed in cancers, its underlying mechanisms have not yet been fully understood.
To investigate the expression levels and molecular mechanisms of TMEM106C across 34 different cancer types, including liver hepatocellular carcinoma (LIHC).
We analyzed TMEM106C expression patterns in pan-cancers using microenvironment cell populations counter to evaluate its association with the tumor mi
TMEM106C was significantly overexpressed in 27 different cancer types and presaged poor prognosis in four of these types, including LIHC. Across pan-cancers, TMEM106C was inversely correlated to the abundances of immune and stromal cells. Furthermore, TMEM106C was significantly linked to cell cycle and DNA replication pathways in pan-cancers. ChIP-seq analysis predicted CCCTC-binding factor as a pivotal transcriptional factor targeting the TMEM106C gene in pan-cancers. Integrated analysis showed that TMEM106C was upregulated in 4657 LIHC compared with 3652 normal liver tissue [combined standardized mean difference = 1.31 (1.09, 1.52)]. In-house LIHC samples verified the expression status of TMEM106C. Higher TMEM106C expression signified worse survival conditions in LIHC patients treated with sorafenib, a tyrosine kinase inhibitor (TKI). Co-expressed analysis revealed that TMEM106C were significantly enriched in the cell cycle pathway. Knockout experiments demonstrated that TMEM106C plays a crucial role in LIHC cell proliferation, migration, and invasion, with cell cycle arrest occurring at the DNA synthesis phase, and increased apoptosis. Notably, TMEM106C upregulation was attenuated by NC treatment. Finally, TMEM106C expression levels were significantly correlated with the drug sensitivity of anti-hepatocellular carcinoma agents, including JNJ-42756493, a TKI agent.
Overexpressed TMEM106C was predicted as an oncogene in pan-cancers, which may serve as a promising therapeutic target for various cancers, including LIHC. Targeting TMEM106C could potentially offer a novel direction in overcoming TKI resistance specifically in LIHC. Future research directions include in-depth experimental validation and exploration of TMEM106C’s role in other cancer types.
Core Tip: Herein, we conducted the first study to investigate the expression implications, immune associations, and transcriptional regulatory mechanisms of transmembrane protein 106C (TMEM106C) across 34 cancer types, including liver hepatocellular carcinoma (LIHC). Our multicenter study represents the largest investigation to date of the expression pattern and biological functions of TMEM106C in LIHC. Of particular note, our study discovered the inhibitory effect of nitidine chloride on TMEM106C overexpression, which has not been reported previously.
- Citation: Li JD, He RQ, Dang YW, Huang ZG, Xiong DD, Zhang L, Du XF, Chen G. Unveiling expression patterns, mechanisms, and therapeutic opportunities of transmembrane protein 106C: From pan-cancers to hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(2): 92437
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/92437.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.92437
Liver hepatocellular carcinoma (LIHC) accounts for nearly 85 percent of liver cancers and has emerged as the third leading cause of cancer-related death globally[1]. The predominant cause of LIHC is now recognized to be non-alcoholic fatty liver disease, although other risk factors such as hepatitis and aflatoxin exposure may also contribute to hepatocarcinogenesis[2-4]. However, the precise etiology of LIHC remains unclear. While tyrosine kinase inhibitors (TKIs) have become a cornerstone of first-line LIHC treatment, the issue of TKI drug resistance poses a significant challenge for most patients[5]. Although immune checkpoint blockers have shown promise in treating advanced LIHC, their widespread use is hampered by economic constraints, safety considerations, and suboptimal therapeutic efficacy[6]. There is, therefore, an imperative for research to uncover the molecular underpinnings of LIHC and to develop more effective, personalized treatment strategies.
Recently, a growing body of research has reported the multifaceted roles of transmembrane proteins in the development of cancer[7,8]. These proteins, which form the membranes of the endoplasmic reticulum, play a crucial role in regulating cellular apoptosis and autophagy[9]. Among them, transmembrane protein 106C (TMEM106C) has emerged as a fascinating candidate, as it has been found to be overexpressed in LIHC[10]. However, the expression levels and clinical significance of TMEM106C in other types of cancer remain largely unexplored. Furthermore, the molecular mechanisms underlying the involvement of TMEM106C in various cancers have not been fully elucidated and require thorough investigation. Additionally, the potential therapeutic value of targeting TMEM106C in the treatment of cancers, particularly LIHC, has yet to be studied. As such, further research in this area is warranted to uncover the potential of TMEM106C as a therapeutic target for cancer treatment.
This study aimed to assess TMEM106C’s expression and molecular mechanisms across 34 cancer types, with a focus on LIHC. We sought to determine the biological functions of TMEM106C in LIHC and to evaluate its therapeutic potential. Our findings suggest that TMEM106C may have important clinical implications for the development of novel therapeutic strategies.
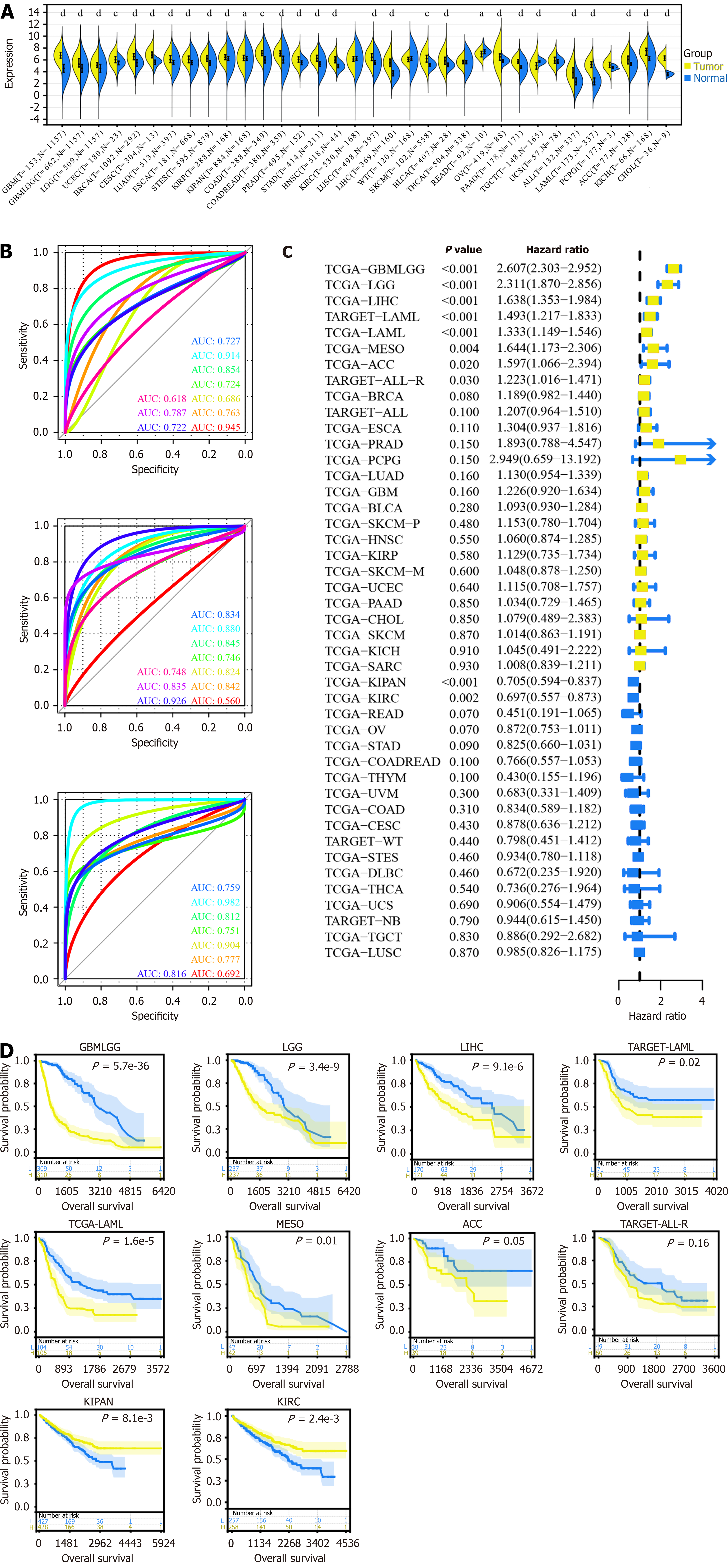
The mRNA expression level of TMEM106C was estimated across 34 different cancer types using the normalized pan-cancer RNA-sequencing (RNA-seq) by expectation maximization gene transcript per million expression profile from University of California, Santa Cruz Xena[11]. Receiver operating characteristic curves were utilized to demonstrate the ability of TMEM106C in distinguishing between cancer and normal samples. Furthermore, Kaplan-Meier and univariate Cox analyses were conducted to appraise the prognostic value of TMEM106C in pan-cancers.
The infiltration levels of immune and stromal cells in the tumor microenvironment (TME) were calculated across 33 different cancer types using the microenvironment cell populations counter algorithm[12]. Spearman correlation coefficients (SCC) were employed to evaluate the association between TMEM106C expression and TME.
Gene set enrichment analysis (GSEA) was performed across 33 different cancer types to investigate the pathways involving TMEM106C[13]. The significantly enriched pathways were ranked in pan-cancers to identify the most aggregated signals associated with TMEM106C.
The regulatory potential (RP) score was calculated to predict the key transcriptional factor in the chromosome region one kilobase upstream of the TMEM106C gene (chr12: 47962596–47968878)[14]. Chromatin immunoprecipitation followed by sequencing (ChIP-seq) analysis was utilized to capture the binding peaks of the transcriptional factor. The expressional correlation between the transcriptional factor and TMEM106C was estimated using correlation coefficients.
In-house LIHC tissue specimens: The present study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (No. 2021-KY-Guoji-054). A total of 248 LIHC and corresponding para-carcinoma paraffin-embedded tissue samples were collected from the Guangxi Medical University Cancer Hospital. The sample size was chosen based on the precedent set by the Japan project of the international cancer genome consortium (ICGC)[15,16], which has been widely recognized for its rigorous methodology and comprehensive analysis of LIHC samples. Inclusion criteria for the clinical specimens were as follows: (1) A confirmed diagnosis of LIHC established via pathological examination; and (2) The presence of ample tissue samples to facilitate quantitative polymerase chain reaction (qPCR) and immunohistochemistry (IHC) staining analyses. Informed consent was obtained from all participants involved in the study.
QPCR assay: Total RNA was extracted from both LIHC and para-carcinoma tissue specimens using the Qiagen RNeasy FFPE Kit. The quantification of TMEM106C and actin beta (ACTB) was conducted using the Applied Biosystems PCR7900 system. The forward sequence of the TMEM106C primer was 5’-AGGGGACAGGCTACATTCCA-3’, with the reverse sequence as 5’-ACCACCAAACCAGATGCCAG-3’. For the internal reference gene, ACTB, the primer sequences were 5’-CAGGCACCAGGGCGTGAT-3’ (forward) and 5’-TAGCAACGTACATGGCTGGG-3’ (reverse). Relative expressions were determined using the 2-ΔCt method[17].
Immunohistochemical staining: TMEM106C protein was stained using a two-step MaxVision IHC method. The primary antibody employed was anti-TMEM106C (Gene Tex Co., Ltd., GTX45991), while the MaxVision TM kit was utilized as the secondary antibody. The experimental procedures and result interpretation closely followed those detailed in a prior study[18].
Preprocessing of microarrays and RNA-seq data: LIHC mRNA matrices were screened out from different centers [i.e., the cancer genome atlas, ArrayExpress, gene expression omnibus (GEO), genotype-tissue expression project, and ICGC]. Rigorous inclusion criteria were applied to ensure the quality and relevance of the data: (1) Only human organisms were considered; (2) The disease had to be LIHC; (3) Tissue specimens were preferred; and (4) mRNA profiles were the specific data type sought. To maintain data integrity, duplicated specimens and those with insufficient control specimens (< 3) were excluded from the analysis. By intersecting the included LIHC mRNA profiles, platform matrices were generated based on the corresponding GEO platforms. After integrating the datasets, we precisely estimated and eliminated any batch effects using the empirical Bayes method ComBat[19], as implemented in the limma-voom and sva packages.
In-silico expression analysis of TMEM106C: To assess the comprehensive expression level of TMEM106C, standardized mean difference (SMD) were calculated in global LIHC expression matrices. The discriminatory ability of TMEM106C in LIHC patients was evaluated using summary receiver operating characteristic (sROC) curve. Additionally, the prognostic value of TMEM106C was assessed using Kaplan-Meier analysis in different LIHC subgroups[20].
Cell culture: SMMC-7721 and Huh7 LIHC cell lines were purchased from the national infrastructure of cell line resource. The inoculation and culture conditions of the LIHC cell lines followed the established protocols as described in previous research[21].
Clustered regularly interspaced short palindromic repeats vector construction: Clustered regularly interspaced short palindromic repeats (CRISPR-Cas9) gene editing technology was utilized to construct TMEM106C-knockout cells. Plasmid 62988 (addgene), as formally reported[22], was utilized for the construction of two vectors targeting TMEM106C, following the official protocol. The specific guide RNA sequences for TMEM106C were as follows: (1) Second exon: Upstream 5’-CACCGTTCTTGCTTTCGCCTGCAGG-3’, downstream 5’-AAACCCTGCAGGCGAAAGCAAGAA-3’; and (2) Fourth exon: Upstream 5’-CACCGTCAGTCCTTGTGGATGATGA-3’, downstream 5’-AAACTCATCATCCACAAGGACTGA-3’.
CRISPR-Cas9 vector transfection: LIHC cells (5 × 105 cells/well) were seeded in 6-well plates 24 hours prior to transfection. Subsequently, transient transfection was conducted using Lipofectamine™ 3000 transfection reagent (ThermoFisher Co., Ltd., L3000150) following the official protocol. Briefly, one μg each of the two plasmids and 4 μL of lipofectamine 3000 were added to the serum-free Opti-MEM medium (Gilbco Co., Ltd.) in an Eppendorf tube. Subsequently, 100 μL of Opti-MEM medium (Gilbco Co., Ltd.) was added to 6 μL lipofectamine 3000 in another Eppendorf tube, which was then gently mixed. After resting at 25 °C for five minutes, the diluted solution was poured into two Eppendorf tubes and mixed. The tubes were left to stand for 20 minutes at 25 °C before being added to the LIHC cells. Following transfection for 24 hours, the LIHC cells were screened in the complete medium supplemented with 2 μg/mL of puromycin for 48 hours. The medium was replaced by the complete medium without puromycin after a selection period of 48 hours.
Knockout efficacy verification: The stable knockout efficiency of TMEM106C was assessed using qPCR. The primers used for TMEM106C amplification were as follows: Upstream 5’-AGGGGACAGGCTACATTCCA-3’, and downstream 5’-ACCACCAAACCAGATGCCAG-3’. The relative mRNA expression values of TMEM106C were determined using a 2-ΔΔCt algorithm.
Proliferation assay: LIHC cells were inoculated in 96-well plates with a density of 2000 cells/well. After incubating for 24 hours, the LIHC cells were subjected to analysis every six hours over a period of five days using a cell counting kit-8 (Boster Co., Ltd.).
Cellular migration and invasion assays: The migration and invasion abilities of TMEM106C-knockout LIHC cells were evaluated using 24-well trans well chambers (Corning Co., Ltd., Costar 3422). The experimental protocols employed were similar to those described in previous studies[21,22].
Cell cycle and cellular apoptosis detection: A total of 5 × 105 and 1 × 105 LIHC cells were harvested to perform cell cycle and apoptosis analysis using a flow cytometer. The detailed operational procedures are consistent with those outlined in previous studies[21,22].
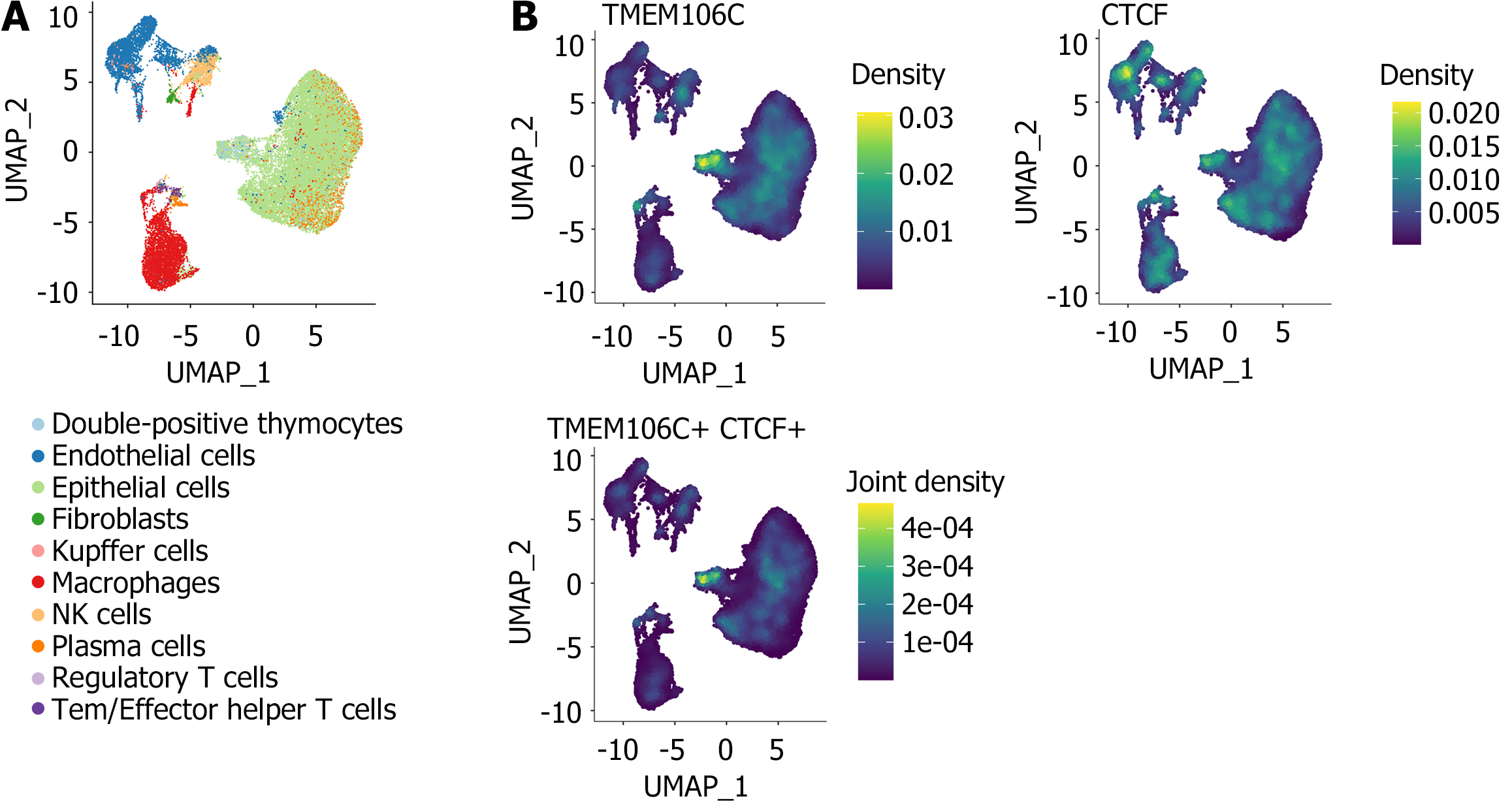
Potential co-expression mechanisms of TMEM106C: In order to investigate the molecular pathways associated with TMEM106C in LIHC, we identified LIHC overexpressed genes (OEGs) through analysis of normalized platform matrices (SMD > 0, and P < 0.05). Furthermore, genes correlated with TMEM106C were determined by calculating SCC using the normalized platform matrices (SCC ≥ 0.3, and P < 0.05). We obtained TMEM106C co-expressed genes by overlapping LIHC OEGs with TMEM106C correlated genes. Subsequently, functional annotation was performed using clusterProfiler[13]. Finally, to confirm the co-expression of TMEM106C and its upstream transcriptional factor, we analyzed single-cell RNA-seq data from GSE112271[23].
Nude mice xenograft model of LIHC: Our research team has previously demonstrated the anti-LIHC effects of traditional Chinese medicine, nitidine chloride (NC), through in vitro and in vivo experiments[24,25]. In the present study, we established an LIHC xenograft model by subcutaneously inoculating 107 SMMC7721 cells into the right axilla of six-week-old male and female nude mice. When the xenograft diameter reached 15 mm to 30 mm, the nude mice were randomly assigned to an untreated group (no treatment), an NC-treated group (7.0 mg/kg), and a negative control group (normal saline). The route of administration was intraperitoneal injection, once a day for a total of 14 days. The harvested LIHC xenografts were stored at -80 °C, and RNA profiles of two LIHC xenografts and three controls were obtained. We analyzed the expressional changes of TMEM106C in LIHC xenografts using an empirical Bayesian method with the Limma-voom package. All experimental protocols were conducted in accordance with the ethical regulations of the Ethics Committee First Affiliated Hospital of Guangxi Medical University (NO. 2021-KY-Guoji-054).
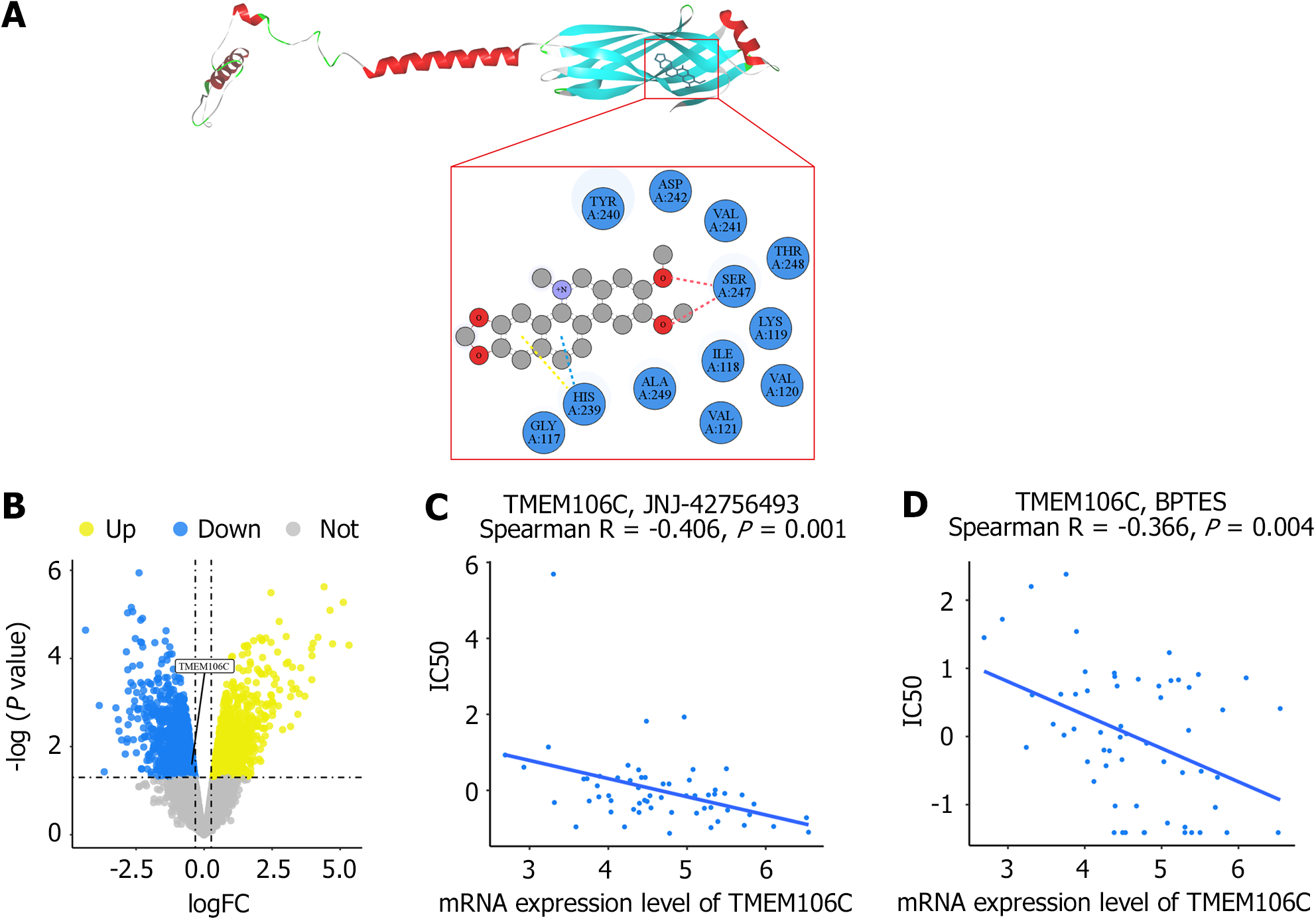
Molecular docking simulation: To investigate the pharmacological mechanisms underlying the therapeutic effects of NC in LIHC treatment, we employed AlphaFold to predict the crystal structure of the TMEM106C protein receptor[26]. Subsequently, molecular docking was performed using the NC ligand to investigate the binding interactions between TMEM106C and NC[27]. The results were visualized using discovery studio visualizer, allowing for a comprehensive analysis of the TMEM106C-NC complex and its molecular interactions[28]. Furthermore, to identify potential anti-cancer drugs targeting TMEM106C, we conducted Spearman correlation analysis in CellMiner[29]. This analysis facilitated the prediction of candidate compounds with a high correlation to TMEM106C expression, thus suggesting their potential efficacy in LIHC treatment.
The expression status of TMEM106C was compared between two groups using a paired or unpaired Wilcoxon test. To address the potential heterogeneity, a randomized-effect model was utilized when calculating the SMD. Publication bias was assessed using a Begg’s test. The discriminatory capability of TMEM106C was evaluated using the area under the sROC curve (AUC), with an AUC ≥ 0.70 indicating potential discriminative ability. Statistical significance was determined at P < 0.05 (two-tailed).
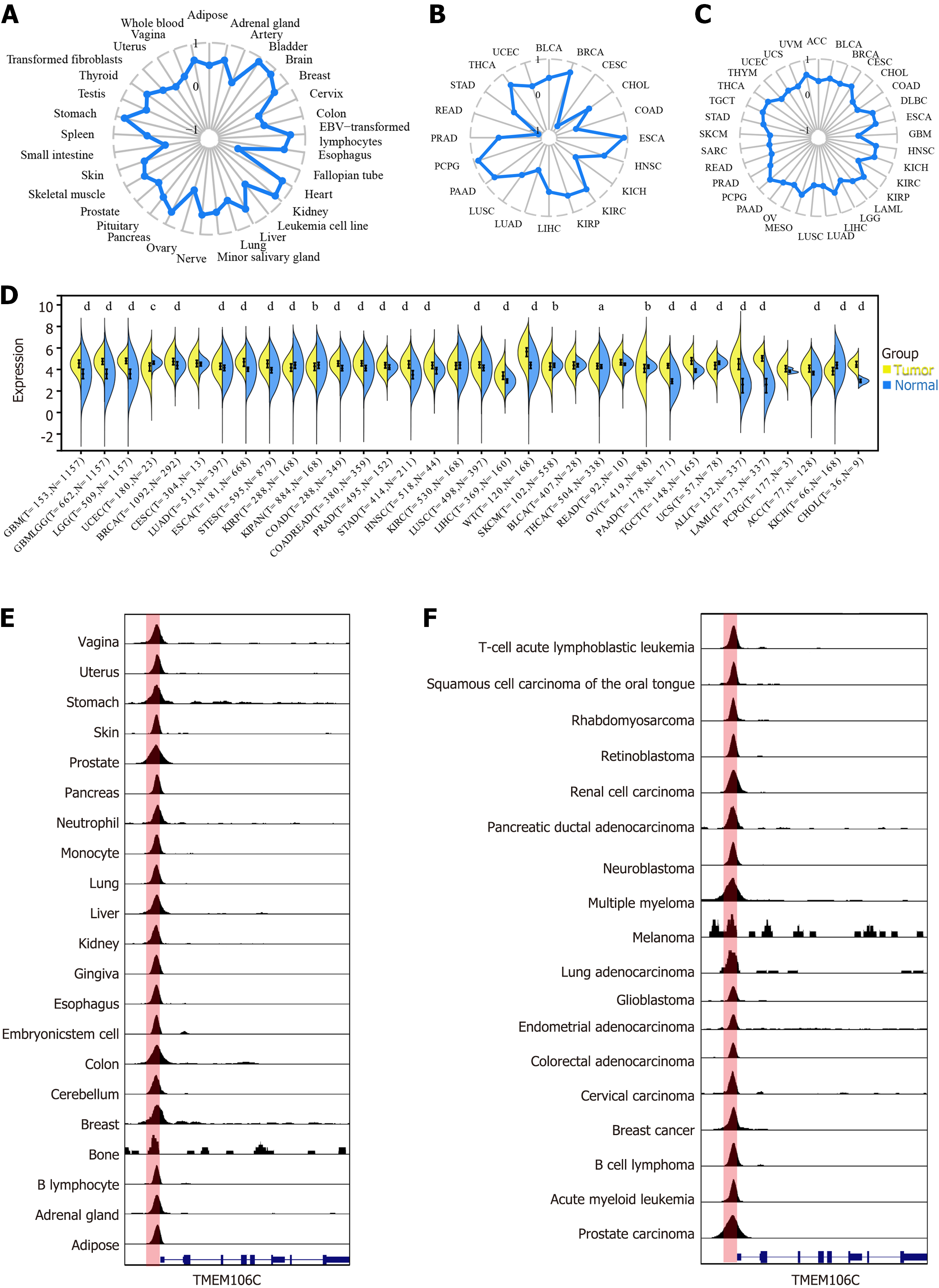
TMEM106C mRNA was significantly overexpressed in 27 different cancer types, including LIHC, while downregulated in rectum adenocarcinoma and testicular germ cell tumors (Figure 1A). TMEM106C mRNA exhibited moderate or strong discriminatory ability in differentiating cancers from the normal controls (Figure 1B). Moreover, elevated TMEM106C expression could be used to predict poor prognosis in five different cancer types, including LIHC, while indicating survival advantages in kidney renal clear cell carcinoma and pan-kidney cohort (Figure 1C and D).
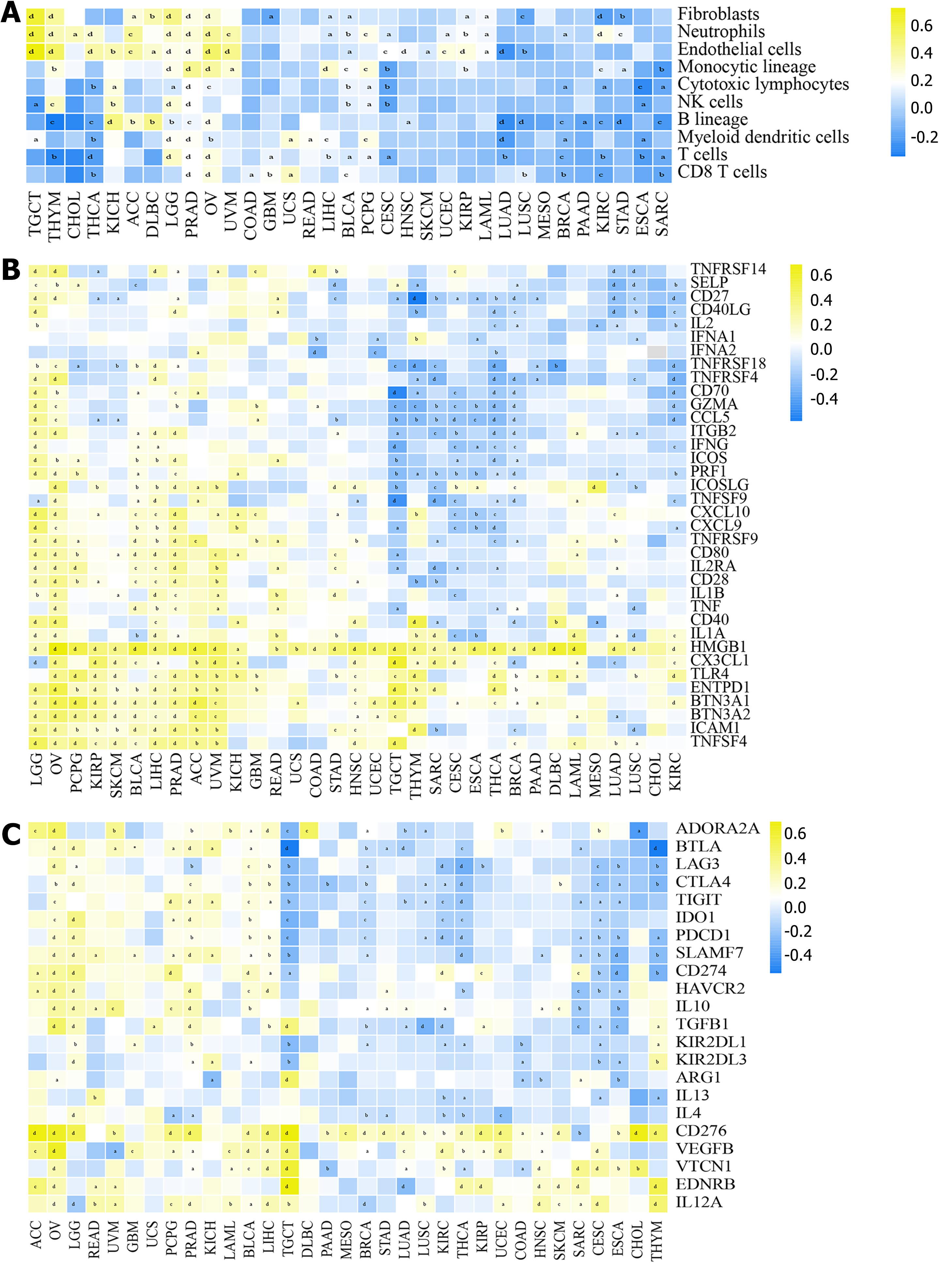
It was found that TMEM106C expression level was inversely correlated to T cell and B lineage cell infiltrations across different cancer types. However, there was a positive association between TMEM106C expression and infiltrations of neutrophils, endothelial cells, and fibroblasts (Figure 2A). Moreover, our analysis revealed a significant correlation between TMEM106C and immune-related genes, such as butyrophilin subfamily 3 member A1, cluster of differentiation 276, high mobility group box 1, and tumor necrosis factor superfamily members (Figure 2B and C). Notably, TMEM106C expression was significantly correlated to monocytic lineage infiltrations in LIHC (SCC = 0.312, P < 0.0001).
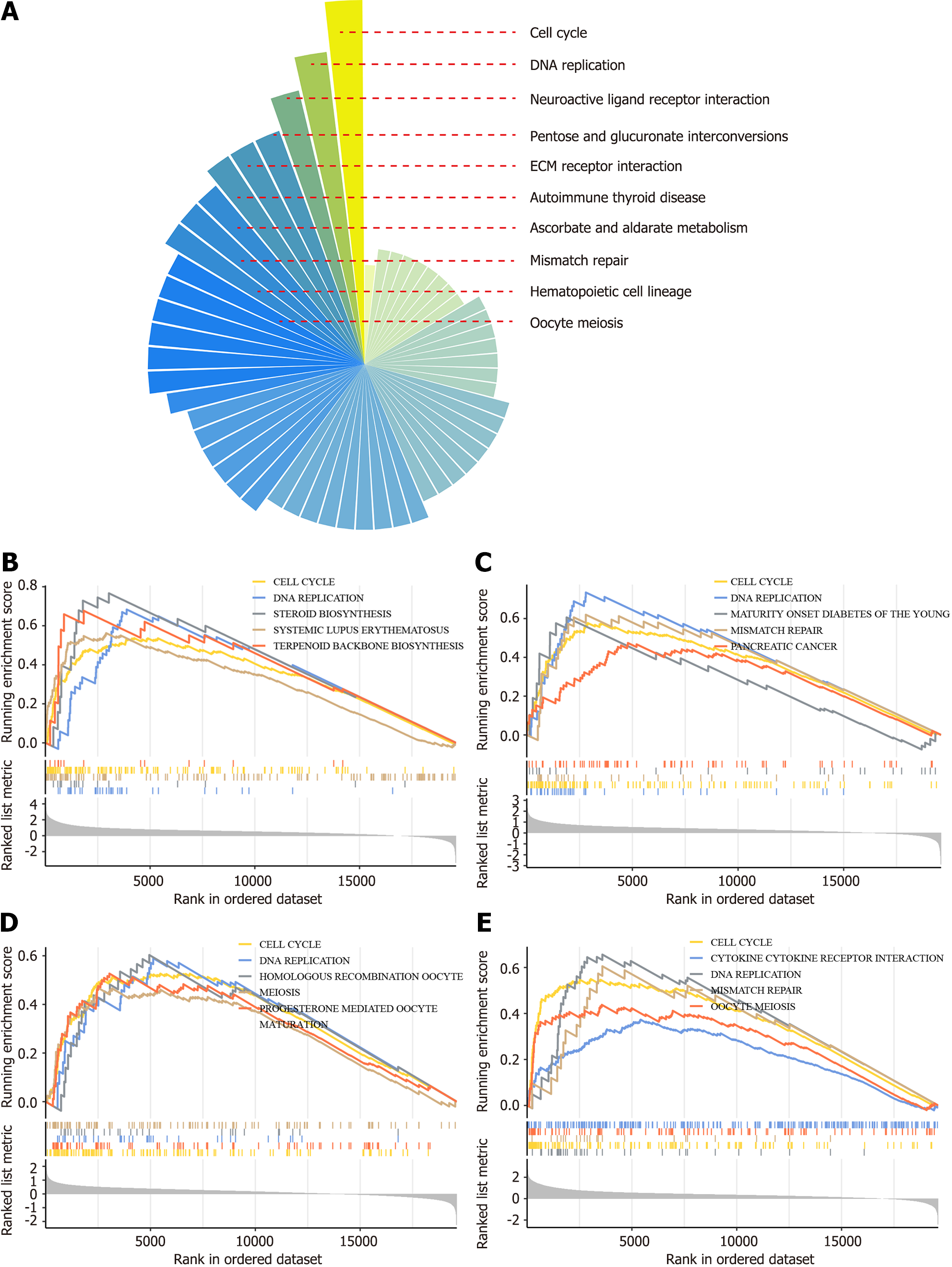
The potential molecular functions of TMEM106C was investigated in 33 different cancer types. GSEA analysis of TMEM106C revealed that the cell cycle was the most commonly enriched pathway (Figure 3), followed by DNA replication and neuroactive ligand receptor interaction pathways.
To investigate the potential mechanism underlying TMEM106C overexpression in cancers, the transcriptional factor targeting TMEM106C was predicted. We identified CCCTC-binding factor (CTCF) with a RP score of 0.98 as a potent transcriptional activator regulating TMEM106C overexpression in pan-cancers, including LIHC. Consistent with the hypothesis, we confirmed a significant positive correlation between CTCF and TMEM106C expression levels (Figure 4A-D). Furthermore, CTCF ChIP-seq results indicated a strong binding intensity in the promoter region of TMEM106C (Figure 4E and F).
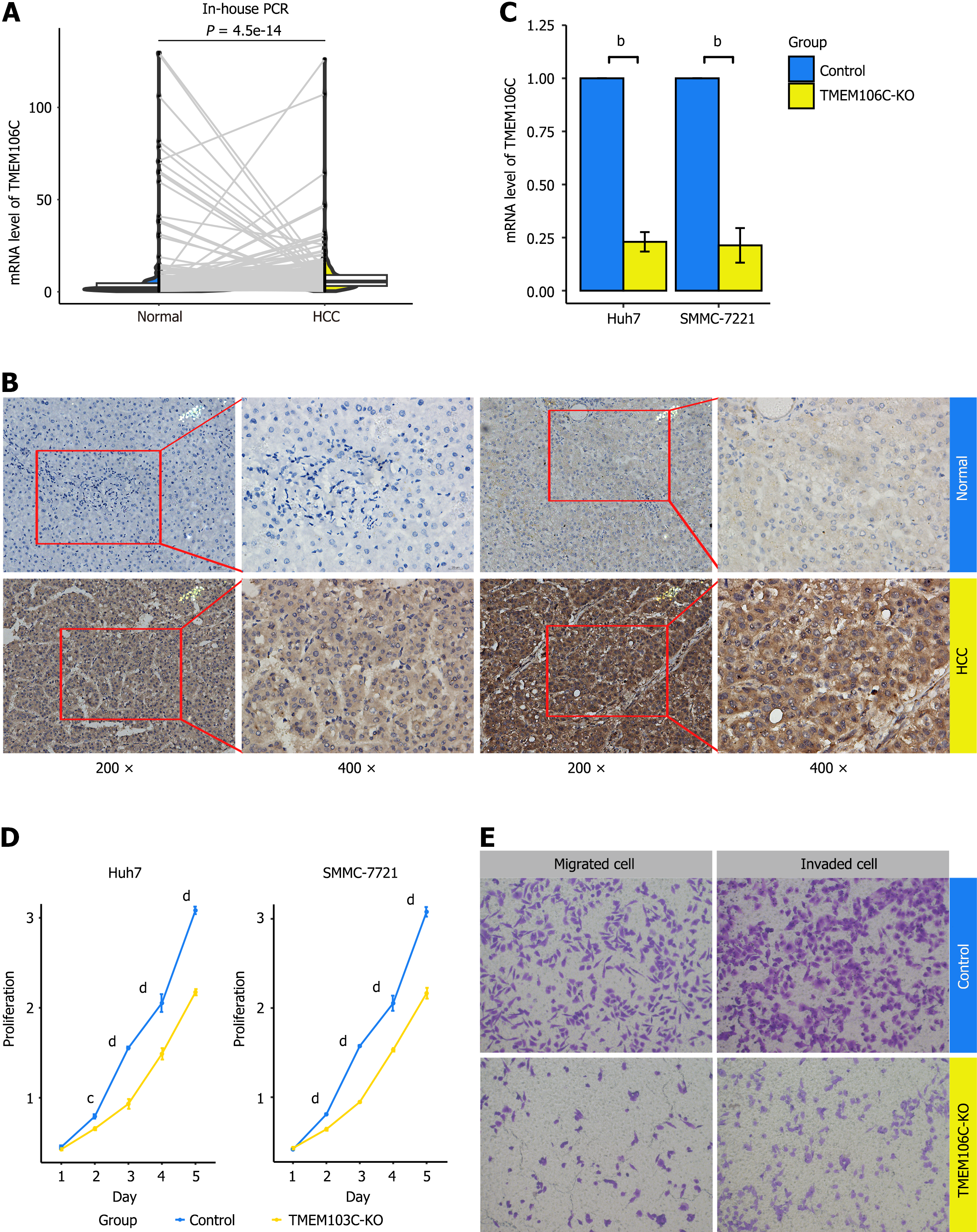
Increased expression of TMEM106C in LIHC tissue: The overexpression of TMEM106C were confirmed using in-house qPCR and IHC experiments. In comparison to 248 paired para-carcinoma tissue, LIHC tissue exhibited a significantly higher mRNA expression level of TMEM106C (Figure 5A). IHC analysis revealed strong protein staining for TMEM106C in the cytoplasm and membranes of LIHC cells, while normal liver cells showed negative staining (Figure 5B).
Furthermore, the overexpression of TMEM106C mRNA was validated across a total of 107 LIHC gene microarrays and RNA-seq datasets, encompassing 40 research platforms (Supplementary Table 1). TMEM106C expression levels were significantly elevated in 4657 LIHC samples compared to 3652 non-LIHC samples (Supplementary Figure 1A). Additionally, the overexpressed TMEM106C demonstrated a moderate capability to differentiate LIHC from non-LIHC tissue, with an AUC value of 0.88, sensitivity of 77.76%, and specificity of 87.15% (Supplementary Figure 1B). Importantly, no publication bias was detected in the analysis (Supplementary Figure 1C).
Inhibited proliferation, migration, and invasion abilities of LIHC cells: The knockout efficiency of TMEM106C was verified using qPCR (Figure 5C). Following the knockout of TMEM106C, there was a significant inhibition in the proliferation abilities of Huh7 and SMMC-7221 cells (Figure 5D). Moreover, the knockout of TMEM106C resulted in a decrease in both migrated and invaded cells (Figure 5E).
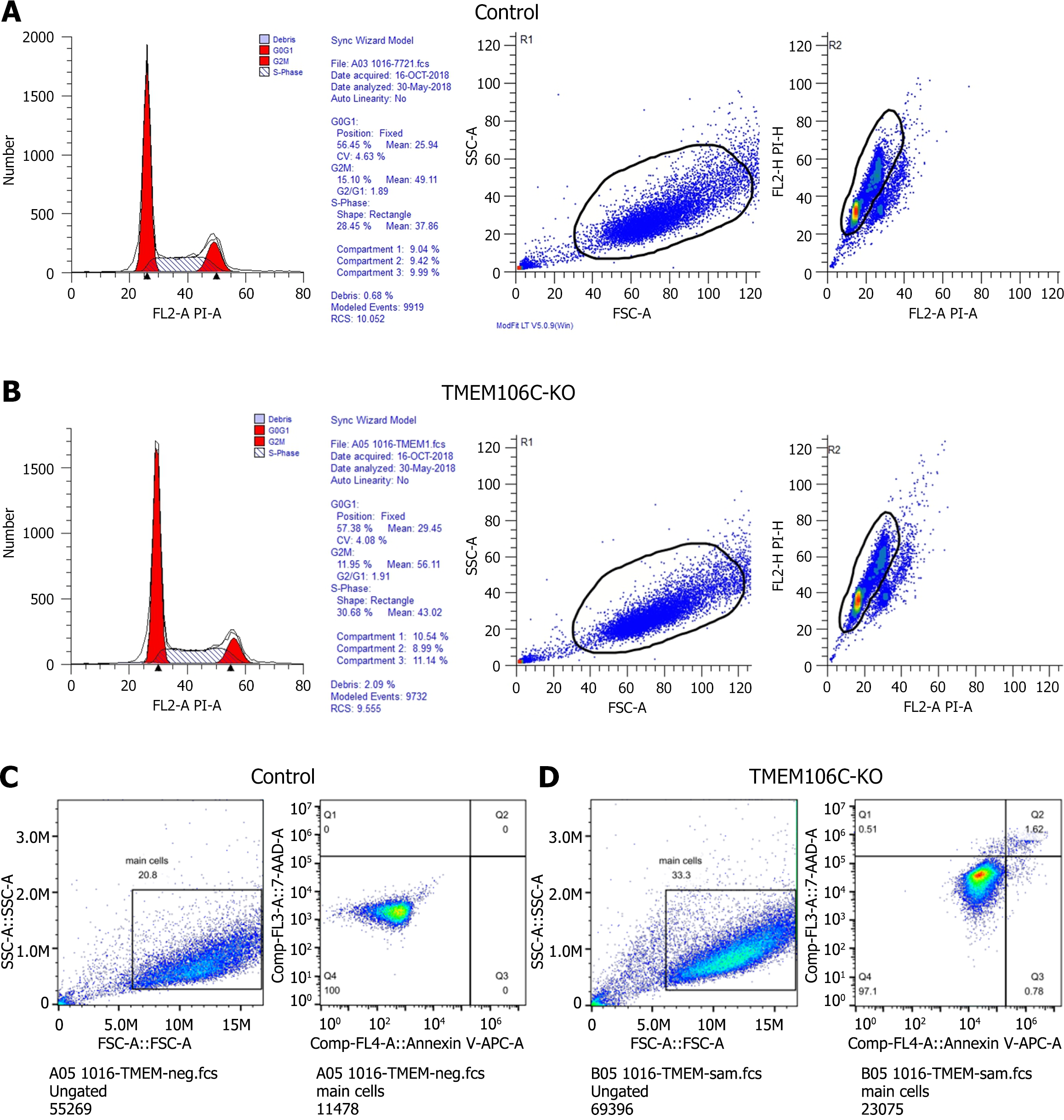
Arrested cell cycle and enhanced cell apoptosis of LIHC cells: After the knockout of TMEM106C, the cell cycle of SMMC-7221 cells was arrested at the DNA synthesis phase (Figure 6A and B). Furthermore, the knockout of TMEM106C led to an elevation in both the early and late apoptotic rate of SMMC-7221 cells (Figure 6C and D).
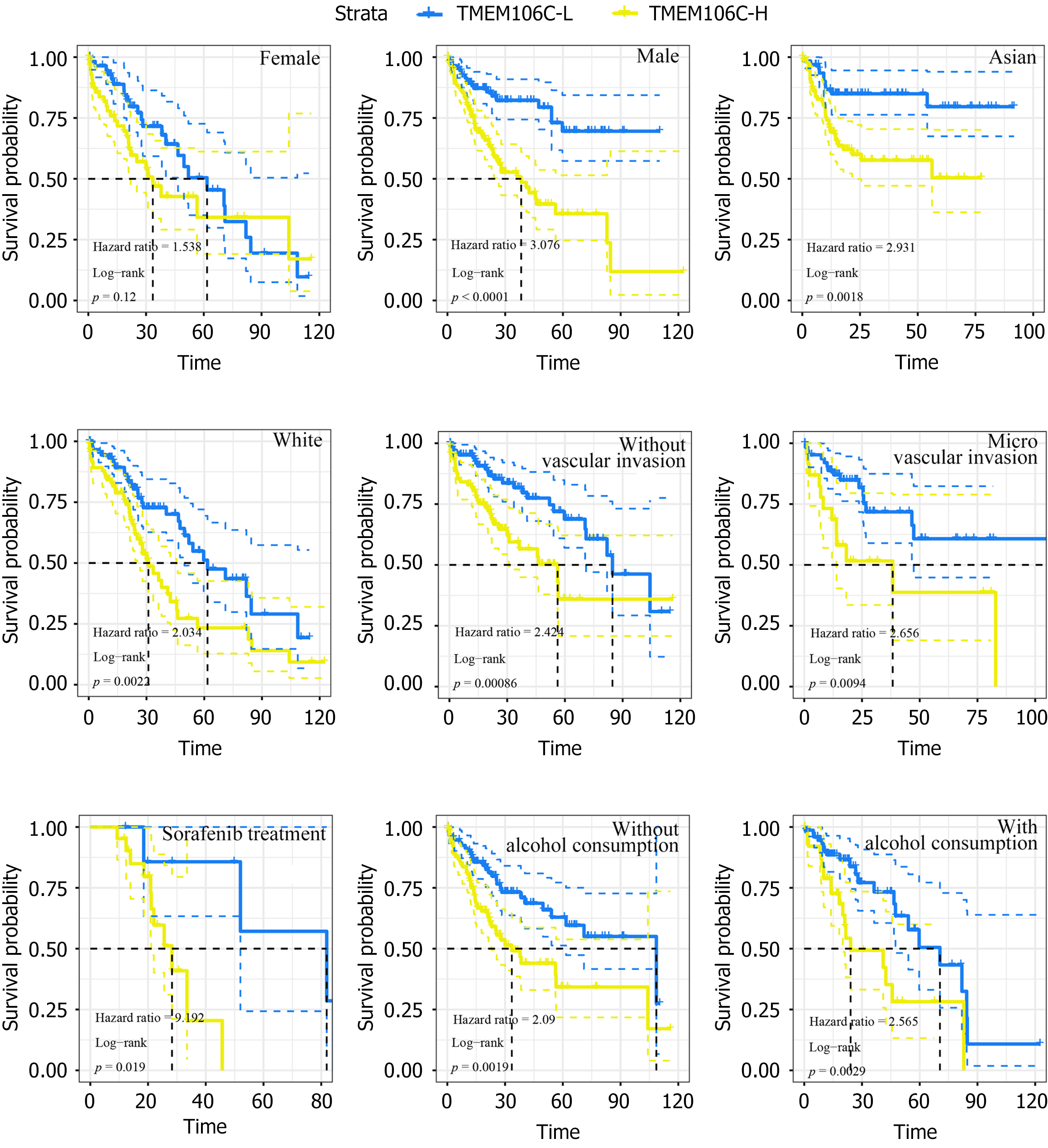
Promising prognostic implications of TMEM106C in LIHC patients: The prognostic value of TMEM106C mRNA was investigated in various LIHC cohorts. Although no significant association was observed in female LIHC patients, overexpression of TMEM106C was found to be correlated to a poor prognosis in male LIHC patients, as well as in patients of Asian, White ethnicity or those receiving sorafenib treatment (Figure 7). Moreover, high expression of TMEM106C indicated a poor prognosis of LIHC patients in both alcoholism group and non-alcoholism group, vascular invasion group and non-vascular invasion group.
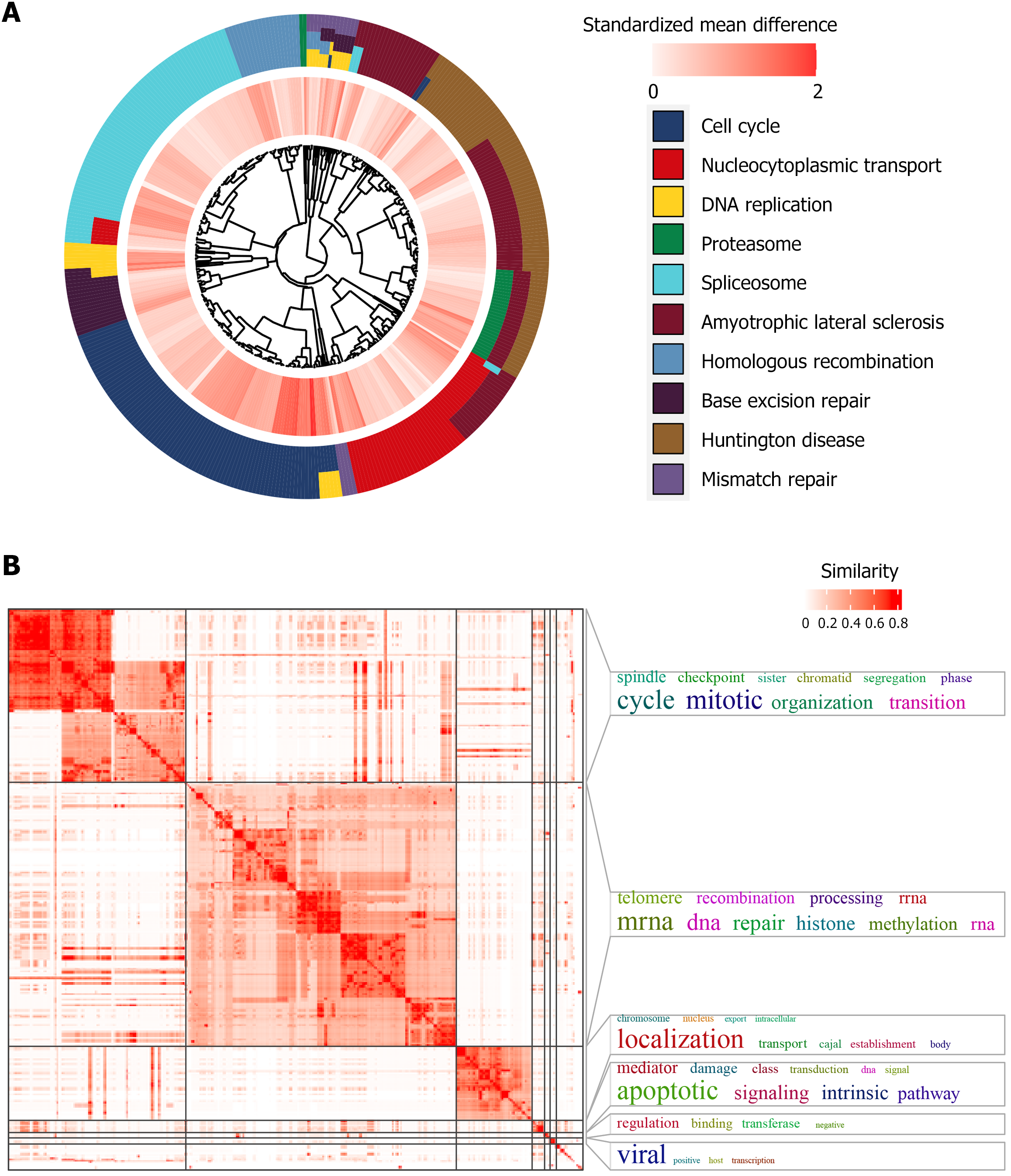
Prospective molecular mechanisms of TMEM106C in LIHC progression: TMEM106C co-expressed genes demonstrated significant enrichment in key pathways, including the cell cycle, nucleocytoplasmic transport, and DNA replication (Figure 8A). Additionally, our analysis revealed that the two most prominently enriched functional modules were mitotic cell cycle phase transition and histone methylation (Figure 8B). Similarly, when considering Reactome pathway analysis, we observed a significant activation of cell cycle-related processes, such as mitotic metaphase and anaphase, cell cycle checkpoints, DNA synthesis, mitotic G1 phase, and G1/S transition, among others (Supplementary Figure 2).
Among intricate co-expression networks, CTCF plays an important role in the transcriptional activation of TMEM106C. We confirmed that the mRNA expression levels of CTCF were markedly higher in 5219 LIHC tissue samples compared to 4094 non-LIHC tissue samples (Supplementary Figure 3A). Our analysis did not indicate any publication bias (Supplementary Figure 3B). The overexpression of CTCF showed a moderate discriminatory ability to distinguish between LIHC and non-LIHC tissue (Supplementary Figure 3C). Importantly, we observed a significant co-expression between CTCF and TMEM106C in single cells of LIHC (Figure 9 and Supplementary Figure 4).
In-silico molecular docking analysis revealed a strong binding infinity between the NC molecule and the TMEM106C protein. The vina score was determined to be -6.3, indicating a robust interaction. The binding was facilitated by conventional hydrogen bonds, pi-donor hydrogen bonds, and pi-pi T-shaped molecular interactions (Figure 10A). In the nude mice LIHC xenograft model, treatment with NC significantly counteracted the upregulation of TMEM106C (Figure 10B). Furthermore, there was a significant association between the mRNA expression level of TMEM106C and drug sensitivity to anti-hepatocellular carcinoma agents, such as JNJ-42756493 (Erdafitinib), a multi-target TKI, and bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl) ethyl sulfide (BPTES), a glutaminase inhibitor (Figure 10C and D).
LIHC is a lethal malignancy in the digestive system, notorious for its poor prognosis, especially in advanced stages[30-32]. TMEM106C has been shown to promote carcinogenesis[33], but its underlying mechanisms require further investigation. To address this gap, we conducted the first study to investigate the expression implications, immune associations, and transcriptional regulatory mechanisms of TMEM106C across 34 cancer types, including LIHC. Our multicenter study represents the largest investigation to date of the expression pattern and biological functions of TMEM106C in LIHC. Notably, our study discovered the inhibitory effect of NC on TMEM106C overexpression, a finding that has not been reported previously.
Analyses of the TME and gene set enrichment reveal that the overexpression of TMEM106C negatively correlates with immune infiltrations and is linked to enhanced cell cycle pathway activities, possibly regulated by the CTCF transcriptional factor. However, the precise functions of TMEM106C in cancer development remain unclear[10,34,35]. Our study provides a novel direction for understanding the oncogenic role of TMEM106C across various cancers.
To confirm the role of TMEM106C in LIHC, we conducted the largest multicenter study to date, incorporating gene microarrays, bulk RNA-seq, single-cell RNA-seq, qPCR, and IHC experiments. We collected a total of 107 mRNA profiles and conducted a comparative analysis of 4657 LIHC tissues and 3652 non-LIHC tissues from 14 countries, including China, France, Germany, Japan, Singapore, South Korea, and the United States. Our study confirmed the upregulation of TMEM106C at both mRNA and protein levels using in-house qPCR and IHC results, providing robust evidence that supports previous reports[10,34,35]. Moreover, our findings demonstrated that TMEM106C plays an oncogenic role in pan-cancers, including LIHC. We established two TMEM106C-knockout LIHC cell lines (Huh7 and SMCC-7221) using the CRISPR-Cas9 gene editing system. Consistently, the knockout of TMEM106C significantly inhibited the proliferation, migration, and invasion abilities of LIHC cells, suggesting that TMEM106C may promote their malignant behavior. TMEM106C overexpression was also found to promote cell cycle progression and inhibit apoptosis in SMMC-7221 cells. Therefore, overexpressed TMEM106C may contribute to the survival and metastasis of LIHC cells, leading to disease progression.
Mechanistic investigations of TMEM106C have shed light on its functional enrichment in the cell cycle pathway. Our functional enrichment analysis revealed strong activity in cell cycle related signaling pathways across 27 cancer types, including LIHC. Intriguingly, our in-house in vitro experiments demonstrated that TMEM106C promotes cell cycle progression in LIHC cells. The co-expression network of TMEM106C consistently revealed increased activity during the phase transitions of the cell cycle pathway. Notably, the CTCF transcriptional factor is predicted to positively regulate TMEM106C activities in promoting the cell cycle pathway. CTCF, which comprises 11 flexible zinc finger domains, has been shown to upregulate the expression of forkhead box protein M1, thereby promoting proliferation and metastasis in LIHC[36]. In our study, we observed that CTCF specifically binds to the upstream promoter region of TMEM106C in pan-cancers. A significant correlation was found between CTCF and TMEM106C expression in pan-cancer tissue samples, as well as in LIHC tissue and single cells. Based on these findings, it is reasonable to hypothesize that TMEM106C may be transcriptionally activated by CTCF, leading to uncontrolled cell cycle progression and LIHC deterioration. However, further experimental evidence is required to support this hypothesis.
Considering the prospective molecular mechanisms of TMEM106C and the cell cycle in LIHC development, we explored potential therapeutic targets for LIHC. NC treatment significantly inhibited the overexpression of TMEM106C. In-silico molecular docking analysis confirmed a strong binding affinity between TMEM106C and NC. The integration of computational techniques such as AlphaFold and molecular docking provided valuable insights into the pharmacological mechanisms of NC and its potential as a novel anti-cancer therapy for LIHC. Furthermore, we identified Erdafitinib and BPTES as potential therapeutic agents associated with TMEM106C expression. In particular, Erdafitinib, a fibroblast growth factor receptor TKI, has exhibited potent effects in inhibiting the growth of LIHC cells[37]. These findings lay the foundation for further investigations and the design of targeted therapeutic strategies.
Although our study concentrates on LIHC, the potential role of TMEM106C in non-liver cancers should not be overlooked. Future research should aim to characterize the expression and functional roles of TMEM106C across a broader spectrum of cancers to assess its viability as a universal therapeutic target. It is important to recognize the preliminary nature of our findings, which derived from in vitro and computational analyses. The clinical relevance of TMEM106C in non-liver cancers requires validation through larger-scale studies and clinical trials. In summary, TMEM106C is a promising target for LIHC and possibly other cancers. Further research is needed to fully understand its therapeutic potential and translate these findings into clinical practice.
We recognize the limitations of our study, particularly the lack of clinical validation in human subjects and the reliance on animal models. While our findings suggest a significant role for TMEM106C in various cancers, including LIHC, further clinical studies are needed to confirm these observations. Additionally, although the overexpression of TMEM106C was observed across different cancer types, future research should focus on a broader range of cancer types to fully understand its implications. Regarding the CTCF-TMEM106C axis, our study provides preliminary evidence of its role in LIHC progression. However, we agree that additional experimental evidence is required to establish a clear mechanistic link. Future studies should integrate both in vitro and in vivo models to establish a comprehensive understanding of this regulatory axis. We are committed to advancing this research area and believe that addressing these limitations will be crucial for the development of targeted therapies.
This study highlights the potential role of overexpressed TMEM106C as an oncogene in pan-cancers, including LIHC. Targeting TMEM106C may offer a novel direction in overcoming TKI resistance specifically in LIHC. Our study provides novel insights into the molecular mechanisms underlying cancer development and suggests a potential avenue for the development of targeted therapies in the future. Further research and clinical studies are warranted to validate the therapeutic potential of targeting TMEM106C in a broader range of cancer types, including LIHC.
We sincerely appreciate the technical support from Guangxi Key Laboratory of Medical Pathology.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8084] [Article Influence: 8084.0] [Reference Citation Analysis (2)] |
| 2. | Yang Y, Cai J, Yang X, Wang K, Sun K, Yang Z, Zhang L, Yang L, Gu C, Huang X, Wang Z, Zhu X. Dysregulated m6A modification promotes lipogenesis and development of non-alcoholic fatty liver disease and hepatocellular carcinoma. Mol Ther. 2022;30:2342-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 3. | Huang DQ, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023;20:37-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 262] [Article Influence: 131.0] [Reference Citation Analysis (0)] |
| 4. | Toh MR, Wong EYT, Wong SH, Ng AWT, Loo LH, Chow PK, Ngeow J. Global Epidemiology and Genetics of Hepatocellular Carcinoma. Gastroenterology. 2023;164:766-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 269] [Reference Citation Analysis (0)] |
| 5. | Yang Y, Li S, Wang Y, Zhao Y, Li Q. Protein tyrosine kinase inhibitor resistance in malignant tumors: molecular mechanisms and future perspective. Signal Transduct Target Ther. 2022;7:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 133] [Reference Citation Analysis (0)] |
| 6. | Tan Z, Chiu MS, Yang X, Yue M, Cheung TT, Zhou D, Wang Y, Chan AW, Yan CW, Kwan KY, Wong YC, Li X, Zhou J, To KF, Zhu J, Lo CM, Cheng AS, Chan SL, Liu L, Song YQ, Man K, Chen Z. Isoformic PD-1-mediated immunosuppression underlies resistance to PD-1 blockade in hepatocellular carcinoma patients. Gut. 2023;72:1568-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Du Y, Zeng X, Yu W, Xie W. A transmembrane protein family gene signature for overall survival prediction in osteosarcoma. Front Genet. 2022;13:937300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 8. | Fu Q, Wu X, Lu Z, Chang Y, Jin Q, Jin T, Zhang M. TMEM205 induces TAM/M2 polarization to promote cisplatin resistance in gastric cancer. Gastric Cancer. 2024;27:998-1015. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Zhang TM, Liao L, Yang SY, Huang MY, Zhang YL, Deng L, Hu SY, Yang F, Zhang FL, Shao ZM, Li DQ. TOLLIP-mediated autophagic degradation pathway links the VCP-TMEM63A-DERL1 signaling axis to triple-negative breast cancer progression. Autophagy. 2023;19:805-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 10. | Luo X, Han G, Lu R, Guan S, Wang Y, Ju L, Chen L, Shao J, Bian Z. Transmembrane protein 106C promotes the development of hepatocellular carcinoma. J Cell Biochem. 2020;121:4484-4495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Goldman MJ, Craft B, Hastie M, Repečka K, McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, Zhu J, Haussler D. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38:675-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2228] [Cited by in RCA: 2566] [Article Influence: 513.2] [Reference Citation Analysis (0)] |
| 12. | Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, Selves J, Laurent-Puig P, Sautès-Fridman C, Fridman WH, de Reyniès A. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 987] [Cited by in RCA: 2238] [Article Influence: 248.7] [Reference Citation Analysis (0)] |
| 13. | Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, Feng T, Zhou L, Tang W, Zhan L, Fu X, Liu S, Bo X, Yu G. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb). 2021;2:100141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 4752] [Article Influence: 1188.0] [Reference Citation Analysis (0)] |
| 14. | Zheng R, Wan C, Mei S, Qin Q, Wu Q, Sun H, Chen CH, Brown M, Zhang X, Meyer CA, Liu XS. Cistrome Data Browser: expanded datasets and new tools for gene regulatory analysis. Nucleic Acids Res. 2019;47:D729-D735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 574] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Chen D, Ang B, Deng X, Li B, Bai Y, Zhang Y. A necroptosis-regulated model from single-cell analysis that predicts survival and identifies the Pivotal role of MAGEA6 in hepatocellular carcinoma. Heliyon. 2024;10:e37711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Xu S, Zhang Y, Yang Y, Dong K, Zhang H, Luo C, Liu SM. A m(6)A regulators-related classifier for prognosis and tumor microenvironment characterization in hepatocellular carcinoma. Front Immunol. 2024;15:1374465. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Best LG, Azure C, Martell K, Tsosie KS, Voels B. Unactivated leukocyte expression of C-reactive protein is minimal and not dependent on rs1205 genotype. Sci Rep. 2021;11:5691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Yu Y, Liu J, Yu N, Zhang Y, Zhang S, Li T, Gao Y, Lu G, Zhang J, Guo X. IgG4 immunohistochemistry in Riedel's thyroiditis and the recommended criteria for diagnosis: A case series and literature review. Clin Endocrinol (Oxf). 2021;94:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Rohr M, Beardsley J, Nakkina SP, Zhu X, Aljabban J, Hadley D, Altomare D. A merged microarray meta-dataset for transcriptionally profiling colorectal neoplasm formation and progression. Sci Data. 2021;8:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Győrffy B. Discovery and ranking of the most robust prognostic biomarkers in serous ovarian cancer. Geroscience. 2023;45:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 230] [Reference Citation Analysis (0)] |
| 21. | Pan J, Chao NX, Zhang YY, Huang TM, Chen CX, Qin QH, Guo JH, Huang RS, Luo GR. Upregulating KTN1 promotes Hepatocellular Carcinoma progression. J Cancer. 2021;12:4791-4809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | He RQ, Li JD, Du XF, Dang YW, Yang LJ, Huang ZG, Liu LM, Liao LF, Yang H, Chen G. LPCAT1 overexpression promotes the progression of hepatocellular carcinoma. Cancer Cell Int. 2021;21:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Losic B, Craig AJ, Villacorta-Martin C, Martins-Filho SN, Akers N, Chen X, Ahsen ME, von Felden J, Labgaa I, DʹAvola D, Allette K, Lira SA, Furtado GC, Garcia-Lezana T, Restrepo P, Stueck A, Ward SC, Fiel MI, Hiotis SP, Gunasekaran G, Sia D, Schadt EE, Sebra R, Schwartz M, Llovet JM, Thung S, Stolovitzky G, Villanueva A. Intratumoral heterogeneity and clonal evolution in liver cancer. Nat Commun. 2020;11:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 257] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 24. | Gao L, Xiong DD, He RQ, Lai ZF, Liu LM, Huang ZG, Yang X, Wu HY, Yang LH, Ma J, Li SH, Lin P, Yang H, Luo DZ, Chen G, Dang YW. Identifying TF-miRNA-mRNA regulatory modules in nitidine chloride treated HCC xenograft of nude mice. Am J Transl Res. 2019;11:7503-7522. [PubMed] |
| 25. | Liu LM, Xiong DD, Lin P, Yang H, Dang YW, Chen G. DNA topoisomerase 1 and 2A function as oncogenes in liver cancer and may be direct targets of nitidine chloride. Int J Oncol. 2018;53:1897-1912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24593] [Cited by in RCA: 22533] [Article Influence: 5633.3] [Reference Citation Analysis (0)] |
| 27. | Liu Y, Yang X, Gan J, Chen S, Xiao ZX, Cao Y. CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022;50:W159-W164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 566] [Article Influence: 188.7] [Reference Citation Analysis (0)] |
| 28. | Azfaralariff A, Farahfaiqah F, Shahid M, Sanusi SA, Law D, Mohd Isa AR, Muhamad M, Tsui TT, Fazry S. Marantodes pumilum: Systematic computational approach to identify their therapeutic potential and effectiveness. J Ethnopharmacol. 2022;283:114751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Reinhold WC, Wilson K, Elloumi F, Bradwell KR, Ceribelli M, Varma S, Wang Y, Duveau D, Menon N, Trepel J, Zhang X, Klumpp-Thomas C, Micheal S, Shinn P, Luna A, Thomas C, Pommier Y. CellMinerCDB: NCATS Is a Web-Based Portal Integrating Public Cancer Cell Line Databases for Pharmacogenomic Explorations. Cancer Res. 2023;83:1941-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Sartoris R, Gregory J, Dioguardi Burgio M, Ronot M, Vilgrain V. HCC advances in diagnosis and prognosis: Digital and Imaging. Liver Int. 2021;41 Suppl 1:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Huang S, Li D, Zhuang L, Sun L, Wu J. A meta-analysis of the efficacy and safety of adjuvant sorafenib for hepatocellular carcinoma after resection. World J Surg Oncol. 2021;19:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Fancellu A, Sanna V, Scognamillo F, Feo CF, Vidili G, Nigri G, Porcu A. Surgical treatment of hepatocellular carcinoma in the era of COVID-19 pandemic: A comprehensive review of current recommendations. World J Clin Cases. 2021;9:3517-3530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Chen D, Lou Y, Lu J, Fan X, Zhu Q, Sun H. Characterization of the Clinical Significance and Immunological Landscapes of a Novel TMEMs Signature in Hepatocellular Carcinoma and the Contribution of TMEM201 to Hepatocarcinogenesis. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Duan J, Qian Y, Fu X, Chen M, Liu K, Liu H, Yang J, Liu C, Chang Y. TMEM106C contributes to the malignant characteristics and poor prognosis of hepatocellular carcinoma. Aging (Albany NY). 2021;13:5585-5606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Jiang C, Li F, Yang M, Duan J, Lai J, Sun S, Fan S. LINC00238 inhibits hepatic carcinoma progression by activating TMEM106C-mediated apoptosis pathway. Mol Med Rep. 2021;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Zhang B, Zhang Y, Zou X, Chan AW, Zhang R, Lee TK, Liu H, Lau EY, Ho NP, Lai PB, Cheung YS, To KF, Wong HK, Choy KW, Keng VW, Chow LM, Chan KK, Cheng AS, Ko BC. The CCCTC-binding factor (CTCF)-forkhead box protein M1 axis regulates tumour growth and metastasis in hepatocellular carcinoma. J Pathol. 2017;243:418-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Tao Z, Cui Y, Xu X, Han T. FGFR redundancy limits the efficacy of FGFR4-selective inhibitors in hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2022;119:e2208844119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |