Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.101177
Revised: November 5, 2024
Accepted: December 2, 2024
Published online: February 15, 2025
Processing time: 115 Days and 0.6 Hours
Liver cancer is one of the most common malignant tumors of the digestive system, and early detection and effective treatment are crucial for improving the pro
To evaluate the clinical efficacy of Linggui Zhugan decoction combined with MWA for the treatment of primary liver cancer.
Data were collected from 164 patients with primary liver cancer who underwent MWA at our hospital between March 2019 and April 2021. Among them, 79 patients (control group) received routine treatments and 85 patients (research group) received Linggui Zhugan decoction in addition to routine treatment. The clinical efficacy, incidence of adverse reactions, and levels of serum alpha-fetoprotein (AFP), des-γ-carboxy prothrombin (DCP), AFP-L3, total bilirubin (TBil), alanine aminotransferase (ALT), CD4 cell count, CD8 cell count, and CD4/CD8 ratio were compared between the two groups, before and after treatment. The three-year recurrence rates between the two groups were compared, and independent prognostic factors for recurrence were identified.
The study results revealed that the objective response rate (ORR) in the research group was significantly higher than that in the control group (P = 0.005). After treatment, the CD4 cell count and CD4/CD8 ratio significantly increased, whereas the CD8 cell count and TBil, ALT, AFP, DCP, and AFP-L3 Levels were significantly lower in the research group than in the control group (P < 0.001). The Cox regression analysis revealed that the treatment regimen (P = 0.003), presence of cirrhosis (P = 0.019), tumor diameter (P = 0.037), Child-Pugh score (P = 0.003), pretreatment AFP level
The combination of Linggui Zhugan decoction with MWA significantly improved the clinical efficacy and long-term prognosis of patients with primary liver cancer.
Core Tip: This study revealed that the combination of Linggui Zhugan decoction with microwave ablation significantly improved clinical outcomes and prolonged disease-free survival (DFS) in patients with primary liver cancer. The independent prognostic factors identified for DFS included treatment modality, presence of cirrhosis, tumor diameter, Child-Pugh score, and pretreatment levels of alpha-fetoprotein (AFP) and AFP-L3. These findings provide valuable insights for the optimization of treatment strategies for patients with liver cancer that may improve their outcomes.
- Citation: Huang R, Cui J. Impact of Linggui Zhugan decoction on microwave ablation outcomes and recurrence in liver cancer. World J Gastrointest Oncol 2025; 17(2): 101177
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/101177.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.101177
Liver cancer is one of the most common malignancies of the digestive system. In China, it is the fourth most prevalent cancer and the second leading cause of cancer-related deaths[1,2]. Early stage liver cancer often presents with no obvious symptoms, which leads to late-stage diagnosis in many patients. According to the Barcelona Clinic Liver Cancer (BCLC) staging system, treatment mainly involves systemic antitumor therapies aimed at controlling disease progression and prolonging survival[3]. Recently, the advent of tyrosine kinase inhibitors such as sorafenib and immune checkpoint inhibitors has significantly improved the survival rates for patients with advanced liver cancer, although the medical burden remains substantial[4,5].
Identifying and monitoring high-risk populations can aid in the early detection and treatment of liver cancer, thereby improving the prognosis[6]. Clinical evidence indicates that the prognosis of liver cancer closely relates to the cancer stage. For patients diagnosed with very early and early stage BCLC, the 5-year survival rate is approximately 50%[7]. Currently, liver transplantation, surgical resection, and ablative therapies are the main treatment options for patients with stage 0/A BCLC. Although liver transplantation is theoretically the best option, as it can cure both liver tumors and underlying chronic liver disease, its widespread use is limited by donor shortages[8]. Surgical resection and local ablation are commonly used alternatives; however, the high recurrence rate after surgery due to individual variability and heterogeneity of liver cancer remains a critical factor affecting long-term prognosis[9].
Microwave ablation (MWA) is a form of local ablative therapy that uses high-frequency microwaves to generate thermal energy in target tissues, leading to apoptosis and necrosis, thereby achieving tumor ablation[10]. MWA has been widely applied for the treatment of various tumors, including thyroid, uterine fibroid, and bone tumors[11,12]. In recent years, MWA has been increasingly used for the treatment of primary liver cancer, especially small hepatocellular carcinoma (HCC)[13,14].
Linggui Zhugan decoction is a classic traditional Chinese medicine formula that was first recorded in the "Shang Han Lun". It is primarily used to treat symptoms such as fullness below the heart and qi rushing to the chest[15]. The formula consists of four herbs: Indian Buead, Cassia Twig, Largehead Atractylodes Rhizome, and Radix glycyrrhizae preparata, which work together to warm the yang, strengthen the spleen, and resolve phlegm and fluid retention. Modern pharmacological studies have further confirmed the various pharmacological actions of Linggui Zhugan decoction, and studies have also reported that Linggui Zhugan decoction has significant anti-inflammatory effects[16]. Additionally, Linggui Zhugan decoction has presented positive effects in regulating lipid metabolism, reducing blood lipid levels, and preventing lipid deposition in the liver and blood vessels, thereby reducing the risk of cardiovascular disease[17]. Recent research has explored the mechanism of Linggui Zhugan decoction in nonalcoholic steatohepatitis (NASH). Notably, Zhu et al[18] reported that Linggui Zhugan decoction significantly improved the pathological features of NASH induced by a methionine- and choline-deficient diet by partially modulating the gut microbiota and its metabolites. The effects included a reduction of hepatic steatosis and inflammatory responses and the lowering of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Given the established pathological link between NASH and primary liver cancer, where NASH is a significant risk factor, this finding suggests a potential role of Linggui Zhugan decoction in liver cancer prevention and treatment.
However, despite its beneficial effects on other liver diseases, clinical studies on Linggui Zhugan decoction in primary liver cancer remain limited; moreover, its specific mechanisms and clinical efficacy are yet to be fully determined. Therefore, in this study, we aimed to explore the potential application of Linggui Zhugan decoction in the treatment of patients with primary liver cancer, and our findings provide foundational data and theoretical support for future clinical research.
This retrospective study analyzed the data of 164 patients with primary liver cancer who underwent MWA at our hospital between March 2019 and April 2021. The study was exempt from informed consent requirement by the Ethics Committee of Baoji Central Hospital as it only involved retrospective data analysis. All patient data were anonymized, and the study was conducted in accordance with ethical standards to ensure patient privacy and confidentiality.
Inclusion criteria: (1) Patients aged 18-75 years, meeting the diagnostic criteria for primary liver cancer as outlined in the "Guidelines for Diagnosis and Treatment of Primary Liver Cancer"[19] and confirmed using imaging or pathology; (2) Patients with Child-Pugh liver function classification of A or B, BCLC stage B liver cancer, and Karnofsky Performance Score greater than 60; and (3) Expected survival time exceeding 90 days.
Exclusion criteria: (1) Patients with extrahepatic metastasis; (2) Patients with blood disorders, acute infections, coagulation disorders, or severe portal vein hypertension; (3) Patients with organ malignancies other than primary liver cancer, severe liver cirrhosis with massive ascites, or ascites complicated by infection; (4) Patients with tumors near the hepatic hilum, gallbladder, gastrointestinal tract, or tumors that are large and protrude from the liver surface; (5) Patients with immune deficiency, or a history of drug abuse; (6) Patients with ruptured tumor nodules and bleeding, or severe complications such as upper gastrointestinal bleeding; or (7) Patients currently receiving immunomodulators, unapproved traditional Chinese medicines, or other medications that may affect the study outcomes.
Based on the inclusion and exclusion criteria, 164 eligible patients were included in this study. Among them, 79 received routine hepatoprotective and enzyme-lowering treatments after MWA and were designated as the control group. The remaining 85 patients received the Linggui Zhugan decoction in addition to routine treatment and were classified as the research group.
In the control group, patients underwent MWA using an ECO-100A1 cold circulation microwave therapy system (Nanjing ECO Microwave System Engineering Co., Ltd.) with a power setting of 2450 MHz. The procedure was guided using a Toshiba ultrasound device (Japan). All patients underwent comprehensive preoperative assessments, including liver and kidney function tests, electrocardiography, computed tomography (CT), and ultrasonography. A personalized needle insertion route was planned to avoid major blood vessels, the gallbladder, and bile ducts. Informed consent was obtained from the patients and their families and MWA procedures were scheduled accordingly.
Patients fasted for 8 hours before surgery. During the procedure, routine electrocardiographic monitoring was performed, and a venous indwelling needle was established under local or intravenous anesthesia. After routine disinfection and draping, a water-cooled MWA needle was precisely inserted into the center of the lesion under CT guidance. The ablation parameters were set as follows: Power, 35-70 W, each ablation session lasting 3-7 minutes. The specific ablation mode was selected based on the lesion shape, size, and patient tolerance, with multiple overlapping or multi-needle approaches used as necessary. The ablation area extends 1 cm beyond the lesion margins. Upon completion of the ablation, the needle track was ablated while withdrawing the needle to ensure complete ablation. Following the procedure, the puncture site was disinfected and bandaged under pressure. Patients were observed for 30 minutes before returning to the ward, where they remained immobilized in a supine position for 8 hours with close monitoring of their vital signs.
Postoperative treatment included anti-infection, hemostatic, hepatoprotective, antiviral, and enzyme-lowering therapies, typically lasting 7-14 days, with adjustments based on the patient's specific condition.
In addition to the treatment regimen used in the control group, the research group received the Linggui Zhugan decoction. The formulation consisted of Indian bud (30 g), Cassia Twig (15 g), Largehead Atractylodes Rhizome (12 g), Radix glycyrrhizae preparate (8 g), Mexican Tea Herb (15 g), Grassleaf Sweelflag Rhizome (12 g), Cicada Slough (10 g), and common scouring rush herbs (9 g). The herbs were decocted in water, and the resulting liquid was divided into two doses, warmed, and consumed daily for four weeks.
In this study, we collected 5 mL of peripheral blood samples from patients before and 1 month after treatment. The samples were centrifuged at 5000 rpm for 10 minutes at a radius of 10 cm, and the supernatant was collected for subsequent testing. Before centrifugation, CD4, CD8, and CD4/CD8 ratios were detected using a Bricyte-E6 flow cytometer (Shenzhen Mindray) to analyze immune function. Alpha-fetoprotein (AFP), total bilirubin (TBil), and ALT levels were measured using a 7180 automatic biochemical analyzer (Hitachi, Japan). Des-γ-carboxy prothrombin (DCP) and AFP-L3 were detected using enzyme-linked immunosorbent assay, with kits obtained from Shanghai Enzyme-linked Biotechnology Co., Ltd.
Patients underwent enhanced liver CT or magnetic resonance imaging at least once during the first three months post-MWA to assess the clearance of tumor lesions. For the first six months, liver function, coagulation function, liver ultrasound, and AFP levels were evaluated monthly, and contrast-enhanced ultrasound was performed if necessary. At 6-24 months postoperatively, these parameters were evaluated after three months, and at every six months thereafter. Three-year follow-up data were also collected. Disease-free survival (DFS) was defined as the time from MWA treatment for liver cancer to the occurrence of new intrahepatic lesions or the last follow-up.
Postoperative imaging examinations were performed regularly, and therapeutic efficacy was evaluated using the Response Evaluation Criteria in Solid Tumors[20]. Complete response (CR) was defined as the complete necrosis of the lesion with no new lesions detected after four weeks. Partial response (PR) was defined as > 30% necrosis of the lesion compared with baseline, with no new lesions detected for four weeks. Stable disease (SD) was defined as 0%-30% necrosis of the lesion compared to baseline or an increase of < 20%. Progressive disease (PD) was defined as > 20% increase in the lesion size compared with baseline or the emergence of new lesions. Overall response rate (ORR) was defined as the sum of CR and PR divided by the total number of patients, and disease control rate (DCR) was defined as the sum of CR, PR, and SD divided by the total number of patients.
Primary outcome was to evaluate the impact of the two treatment regimens on clinical efficacy, independent prognostic factors for DFS were identified using Cox regression analysis.
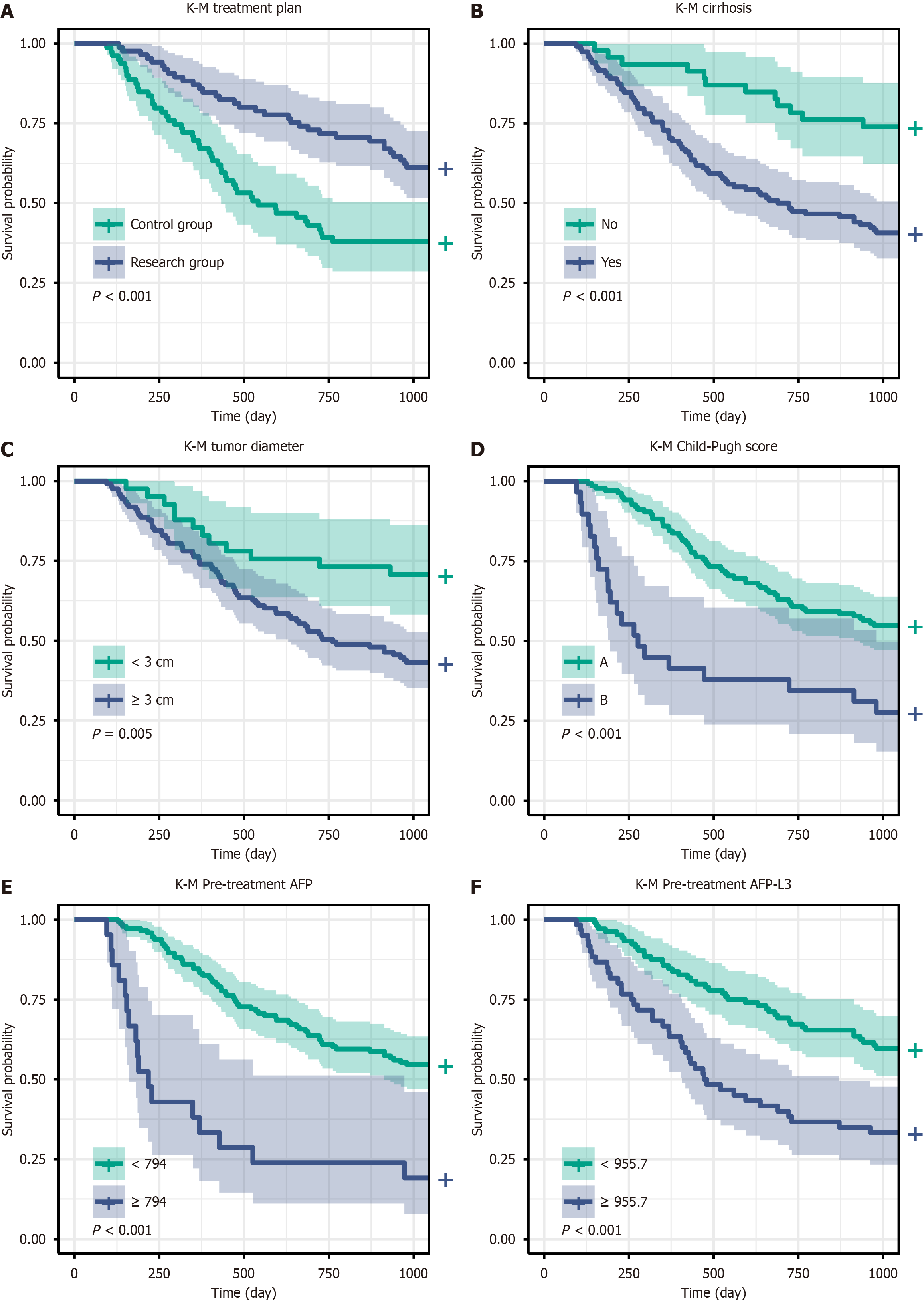
Secondary outcome was to analyze the changes in the levels of AFP, DCP, AFP-L3, TBil, ALT, CD4, CD8, and CD4/CD8 ratios before and one month after treatment. The incidence of adverse reactions during treatment was also recorded. Kaplan-Meier (K-M) survival curves were used to plot DFS curves for indicators with significant differences in univariate Cox regression analysis.
Data were analyzed using SPSS 26.0. Continuous variables were assessed for normal distribution using the K-S test, and normally distributed data were compared between groups using the independent sample t-test and within groups using the paired t-test. Non-normally distributed data were analyzed using the rank-sum test, and categorical variables were analyzed using the χ2 test. K-M survival curves were used to visually display the prognostic factors affecting 3-year DFS. Cox regression analysis was used to identify independent prognostic factors for 3-year DFS. Data visualization was performed using the ggplot2 package in R software (version 4.3.3). Statistical significance was set at P value < 0.05.
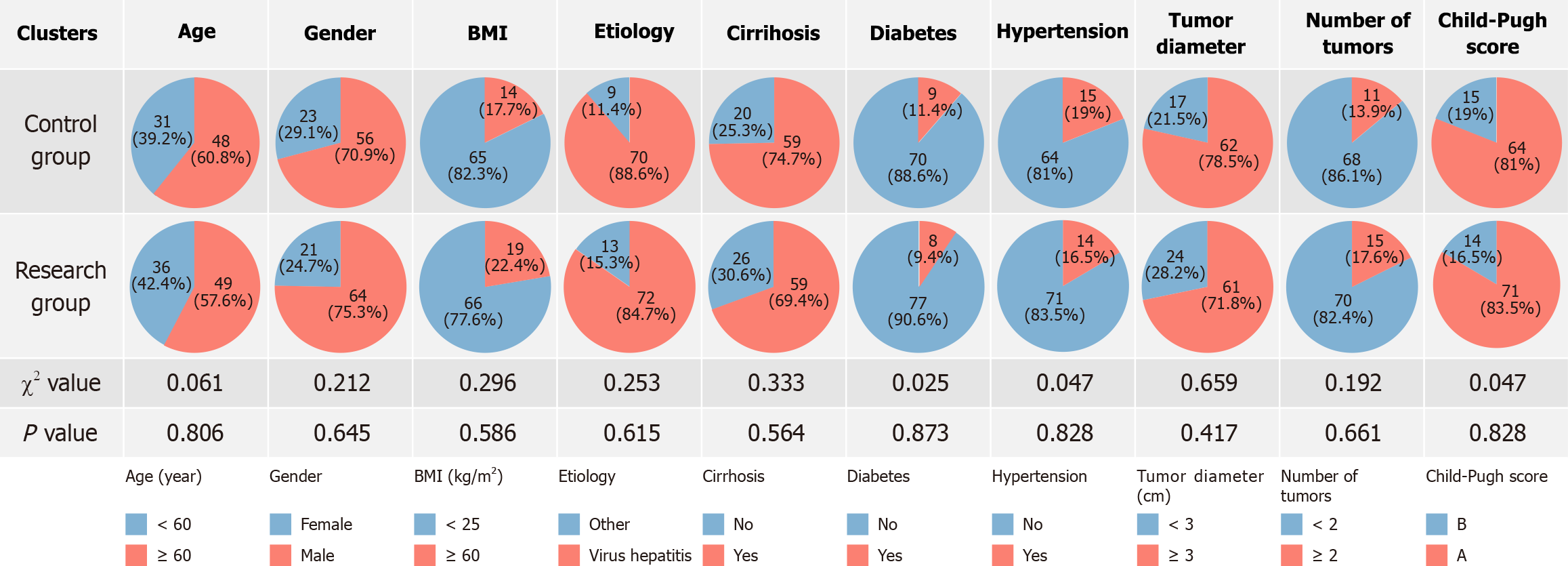
The comparison of baseline data between the two groups revealed no statistically significant differences in age, sex, body mass index, etiology, cirrhosis, diabetes, hypertension, tumor diameter, tumor number, or Child-Pugh score (P > 0.05; Figure 1).
The study results revealed significant differences in clinical efficacy between the research and control groups. The CR rate in the research group was 30.59% (n = 26), which was significantly higher than the rate of 11.39% (n = 9) in the control group, with statistical significance (χ2 = 8.988, P = 0.003). The PR rate did not differ significantly between the two groups, with the research group at 32.94% (n = 28) and the control group at 30.37% (n = 24) (P = 0.725). The SD rate in the research group was 25.88% (n = 22), which was lower than the rate of 39.24% (n = 31) in the control group, with statistical significance (χ2 = 4.638, P = 0.031). The PD rates were 10.59% (n = 9) and 18.98% (n = 15) in the research and control groups, respectively, and this difference was not statistically significant (P = 0.128). The ORR in the research group was 63.53% (n = 54), which was higher than the rate of 41.76% (n = 33) in the control group, with statistical significance (χ2 = 7.782, P = 0.005). Additionally, the DCR was 89.41% (n = 76) in the research group and 81.01% (n = 64) in the control group, and this difference was not statistically significant (P = 0.128; Table 1).
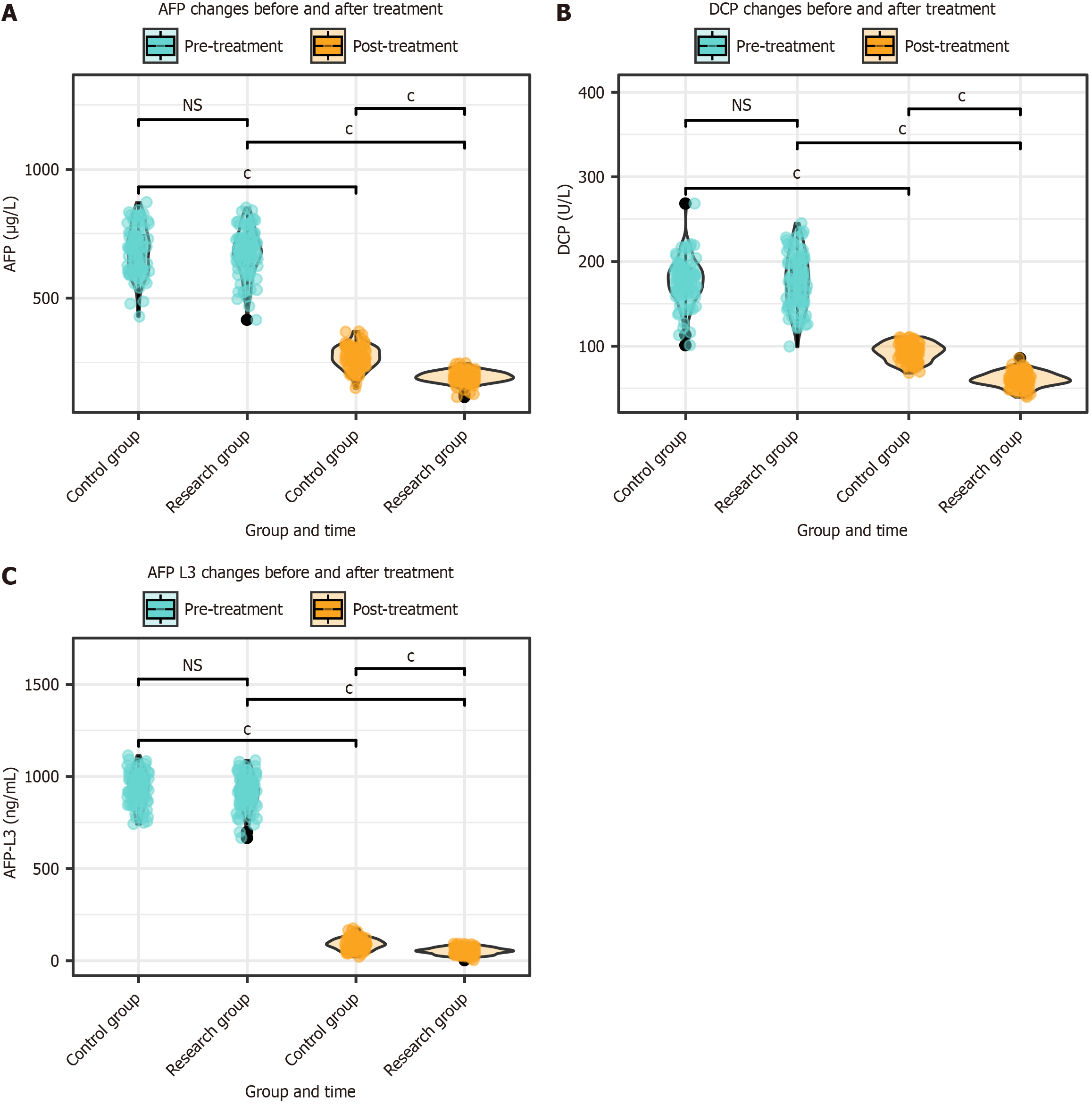
The AFP, DCP, and AFP-L3 levels were assessed in both groups before and after treatment. There were no significant differences in the AFP, DCP, or AFP-L3 levels between the two groups before treatment (P > 0.05). After treatment, the levels of AFP, DCP, and AFP-L3 in both groups were significantly lower than those before treatment (P < 0.001). Moreover, after treatment, AFP, DCP, and AFP-L3 levels were significantly lower in the research group than in the control group (P < 0.001; Figure 2).
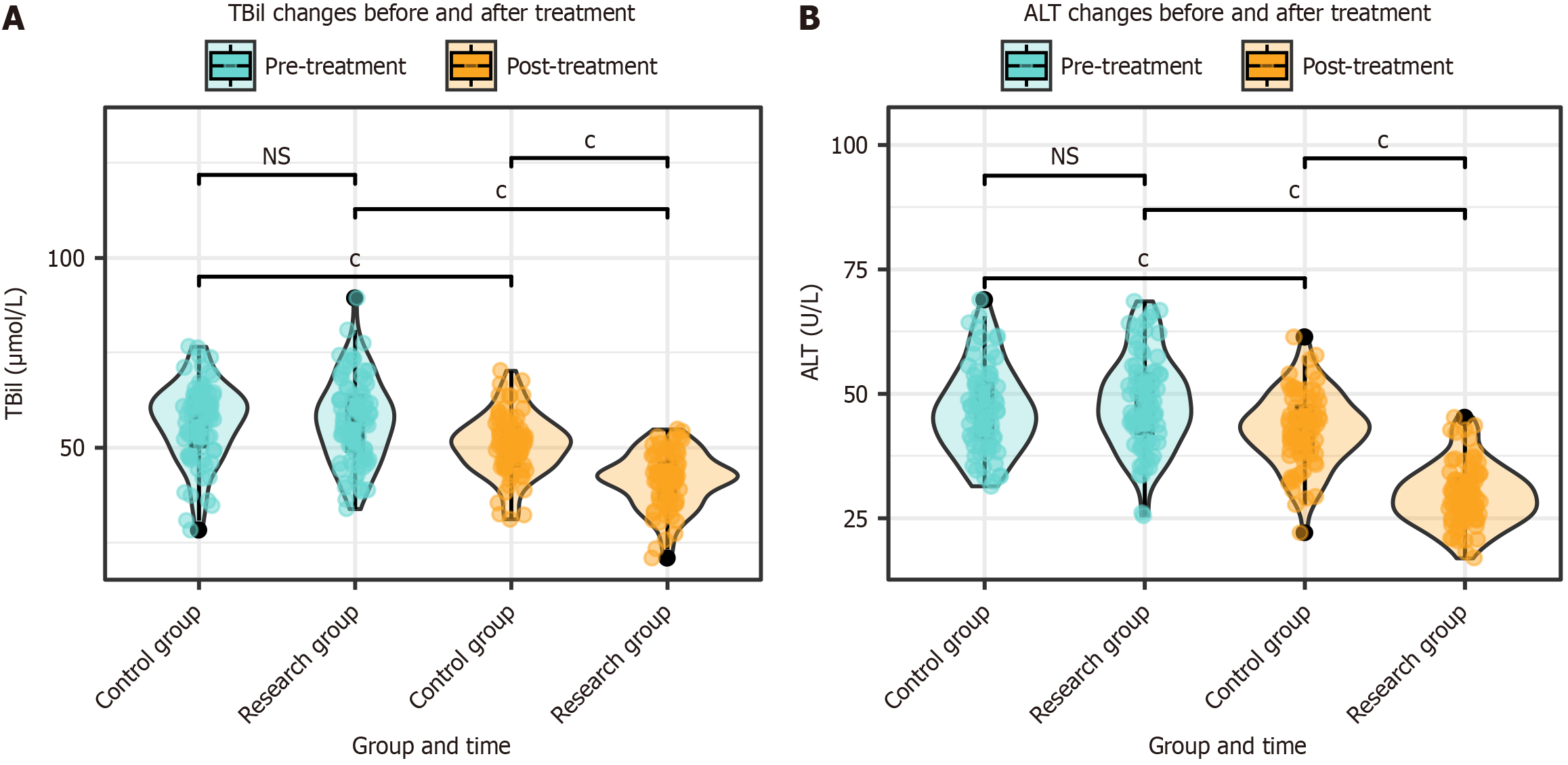
The TBil and ALT levels were evaluated in both groups before and after treatment. The results indicated no significant differences in the TBil and ALT levels between the two groups before treatment (P > 0.05). After treatment, the TBil and ALT levels decreased significantly in both groups (P < 0.001). Additionally, the TBil and ALT levels were significantly lower in the research group than in the control group after treatment (P < 0.001; Figure 3).
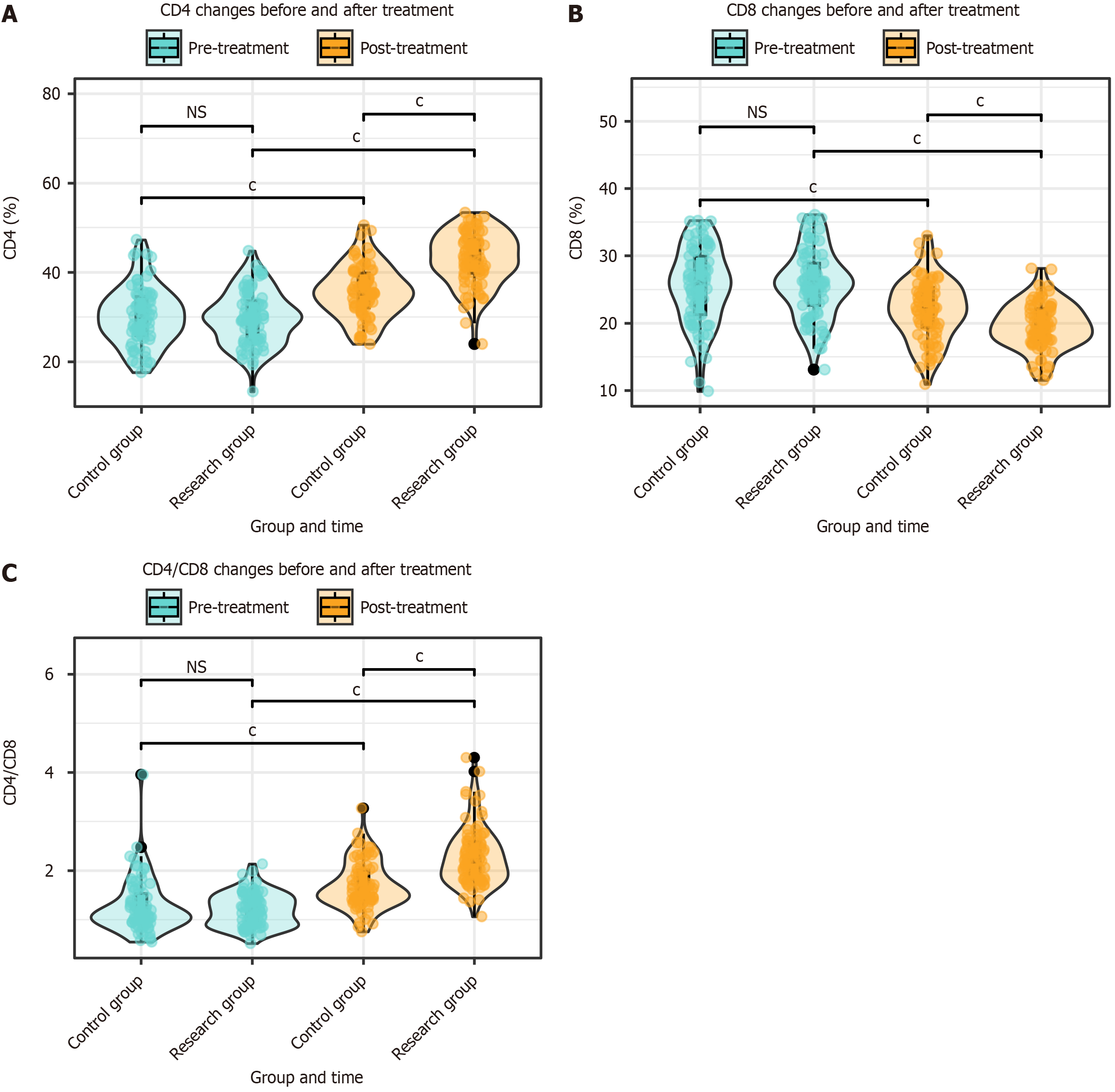
The CD4, CD8, and CD4/CD8 ratios were assessed in both groups before and after treatment. There were no significant differences in CD4, CD8, and CD4/CD8 ratios between the two groups before treatment (P > 0.05). After treatment, significant changes were observed in the CD4, CD8, and CD4/CD8 ratios in both groups (P < 0.001). Furthermore, after treatment, the CD4 and CD4/CD8 ratios in the research group were significantly higher than those in the control group, whereas the CD8 ratio was lower in the research group than in the control group (P < 0.001; Figure 4).
The study results revealed significant differences in the incidence of adverse reactions between the research and control groups. Specifically, the incidence of gastrointestinal reactions was 2.35% (n = 2) in the research group and 5.06% (n = 4) in the control group; however, this difference was not statistically significant (χ2 = 0.853, P = 0.355). The incidence of fever was 1.18% (n = 1) in the research group and 3.80% (n = 3) in the control group, and this difference was also not statistically significant (χ2 = 1.182, P = 0.276). The incidence of ascites was 2.35% (n = 2) in the research group and 6.33% (n = 5) in the control group, without statistical significance (χ2 = 1.584, P = 0.208), the incidence of hepatalgia was 3.53% (n = 3) in the research group and 5.06% (n = 4) in the control group, without statistical significance (χ2 = 0.235, P = 0.627), and the incidence of nausea and vomiting was 3.53% (n = 3) in the research group and 7.59% (n = 6) in the control group, also without statistical significance (χ2 = 1.305, P = 0.253). The overall incidence of adverse reactions was 12.94% (n = 11) in the research group, which was lower than the incidence of 27.85% (n = 22) in the control group, with statistical significance (χ2 = 5.661, P = 0.017; Table 2).
The Cox regression analysis revealed several factors that were significantly associated with the risk of recurrence. In the univariate analysis, treatment plan [hazard ratio (HR) = 2.165, 95%CI: 1.390-3.373, P < 0.001], cirrhosis (HR = 3.058, 95%CI: 1.656-5.648, P < 0.001), tumor diameter (HR = 2.342, 95%CI: 1.268-4.323, P = 0.007), Child-Pugh score (HR = 0.371, 95%CI: 0.225-0.610, P < 0.001), pre-treatment AFP levels (HR = 1.004, 95%CI: 1.002-1.007, P < 0.001), and pre-treatment AFP-L3 levels (HR = 1.005, 95%CI: 1.002-1.007, P < 0.001) were significantly associated with patient recurrence (Table 3 and Figure 5). In the multivariate analysis, treatment plan (HR = 1.957, 95%CI: 1.249-3.068, P = 0.003), cirrhosis (HR = 2.104, 95%CI: 1.127-3.927, P = 0.019), tumor diameter (HR = 1.963, 95%CI: 1.042-3.699, P = 0.037), Child-Pugh score (HR = 0.445, 95%CI: 0.260-0.761, P = 0.003), pre-treatment AFP levels (HR = 1.004, 95%CI: 1.001-1.007, P = 0.006), and pre-treatment AFP-L3 levels (HR = 1.004, 95%CI: 1.002-1.007, P = 0.002) remained independent risk factors for recurrence, which indicates their significant predictive value, even after adjusting for other variables (Table 4).
| Factor | Beta | SE | P value | HR | Lower | Upper |
| Treatment plan | 0.773 | 0.226 | 0.001b | 2.165 | 1.390 | 3.373 |
| Age | -0.131 | 0.223 | 0.558 | 0.877 | 0.566 | 1.359 |
| Sex | 0.335 | 0.262 | 0.200 | 1.398 | 0.837 | 2.336 |
| BMI | 0.111 | 0.267 | 0.677 | 1.118 | 0.662 | 1.886 |
| Etiology | -0.321 | 0.302 | 0.288 | 0.725 | 0.401 | 1.312 |
| Cirrhosis | 1.118 | 0.313 | < 0.001c | 3.058 | 1.656 | 5.648 |
| Diabetes | -0.315 | 0.395 | 0.426 | 0.730 | 0.336 | 1.585 |
| Hypertension | 0.236 | 0.272 | 0.386 | 1.266 | 0.742 | 2.160 |
| Tumor diameter | 0.851 | 0.313 | 0.007b | 2.342 | 1.268 | 4.323 |
| Number of tumors | 0.114 | 0.294 | 0.698 | 1.121 | 0.630 | 1.992 |
| Child-Pugh score | -0.992 | 0.254 | < 0.001c | 0.371 | 0.225 | 0.610 |
| Pre-treatment AFP | 0.004 | 0.001 | 0.001b | 1.004 | 1.002 | 1.007 |
| Pre-treatment DCP | 0.003 | 0.004 | 0.496 | 1.003 | 0.995 | 1.010 |
| Pre-treatment AFP-L3 | 0.005 | 0.001 | < 0.001c | 1.005 | 1.002 | 1.007 |
| Pre-treatment TBil | 0.012 | 0.011 | 0.256 | 1.012 | 0.991 | 1.034 |
| Pre-treatment ALT | -0.013 | 0.012 | 0.304 | 0.987 | 0.963 | 1.012 |
| Pre-treatment CD4 | 0.011 | 0.019 | 0.545 | 1.011 | 0.975 | 1.049 |
| Pre-treatment CD8 | -0.014 | 0.020 | 0.479 | 0.986 | 0.947 | 1.026 |
| Pre-treatment CD4/CD8 | 0.139 | 0.229 | 0.544 | 1.150 | 0.733 | 1.802 |
| Factor | Beta | SE | P value | HR | Lower | Upper |
| Treatment plan | 0.671 | 0.229 | 0.003b | 1.957 | 1.249 | 3.068 |
| Cirrhosis | 0.744 | 0.318 | 0.019a | 2.104 | 1.127 | 3.927 |
| Tumor diameter | 0.675 | 0.323 | 0.037a | 1.963 | 1.042 | 3.699 |
| Child-Pugh score | -0.809 | 0.274 | 0.003b | 0.445 | 0.260 | 0.761 |
| Pre-treatment AFP | 0.004 | 0.002 | 0.006b | 1.004 | 1.001 | 1.007 |
| Pre-treatment AFP-L3 | 0.004 | 0.001 | 0.002b | 1.004 | 1.002 | 1.007 |
In this study, we observed that the combination of Linggui Zhugan decoction with MWA revealed significant clinical efficacy in the treatment of patients with primary HCC. Compared with the control group that received MWA alone, the research group presented higher rates of CR and ORR, with significantly lower rates of SD and PD. Additionally, DFS in the research group was significantly prolonged. Previous studies have shown that combination therapies enhance the treatment outcomes. For example, Liu et al[21] reported that DEB-TACE combined with MWA improved ORR and DCR and prolonged OS and PFS compared with MWA alone in patients with early stage HCC. Similarly, Ji et al[22] reported that TACE-MWA resulted in better tumor response rates and longer PFS in patients with recurrent small HCC. Zuo et al[23] further highlighted that combining MWA with systemic therapies, such as immune checkpoint inhibitors, significantly improved survival in patients with advanced HCC. The Cox regression analysis that was performed in our study confirmed that combination therapy with Linggui Zhugan decoction, along with factors such as the presence of cirrhosis, tumor diameter, Child-Pugh score, pretreatment AFP, and AFP-L3 levels, were independent risk factors for recurrence. These results suggest that Linggui Zhugan decoction may improve patient prognosis after HCC treatment through multiple mechanisms, similar to the improved efficacy demonstrated in other studies by enhancing immune function, controlling tumor growth, and reducing recurrence.
Improvement in immune function is a critical factor for delaying liver cancer progression, and CD4+ and CD8+ T cells play key roles in the antitumor immune response. CD4+ T cells primarily enhance the immune response by secreting cytokines and assisting in the activation of other immune cells, whereas CD8+ T cells directly kill tumor cells[24]. In this study, the Linggui Zhugan decoction significantly improved the CD4/CD8 ratio, possibly by enhancing CD4+ T cell activity and suppressing excessive CD8+ T cell responses, thereby strengthening immune surveillance, reducing tumor immune evasion, and ultimately inhibiting tumor recurrence and progression. Previously, Li et al[25] demonstrated that Cassia Twig significantly inhibits cancer cell growth by inducing G2/M phase arrest, thereby inhibiting cancer cell proliferation. Additionally, Yang et al[26] previously observed that polysaccharide-functionalized oxidized graphene nanosheets from Indian Buead revealed significant effects in cancer immunotherapy, further validating the immune-regulatory role of the key components in the Linggui Zhugan decoction. This immunomodulatory effect may be an important reason for the significant increase in CR and ORR in this research group, along with the lower incidence of adverse reactions, thus reflecting its safety.
Maintaining liver function is crucial to extend the survival of patients with liver cancer, and liver function indicators such as TBil and ALT are important parameters that reflect liver cell damage and hepatic metabolic capacity[27]. Elevated TBil levels typically indicate impaired bilirubin metabolism in the liver, whereas ALT levels reflect the degree of liver cell inflammation and damage[28]. The Linggui Zhugan decoction significantly reduced TBil and ALT levels in the research group, possibly because of the hepatoprotective and enzyme-lowering effects of its components, such as the Indian Buead and Largehead Atractylodes Rhizome. These components may promote liver cell regeneration, reduce liver fibrosis, and improve detoxification, thus helping to restore and maintain liver function and thereby reducing tumor progression, due to worsening liver function[29]. Jiang et al[30] reported that the Linggui Zhugan decoction effectively delayed liver function deterioration in a nonalcoholic fatty liver disease model by inhibiting the Akt/mTOR/SREBP-1/SCD-1 signaling pathway and reducing the expression of inflammatory factors.
Tumor markers such as AFP, DCP, and AFP-L3 play important roles in liver cancer diagnosis, prognosis, and treatment. AFP is the most commonly used tumor marker for liver cancer, and elevated AFP levels are typically closely associated with the presence and progression of liver cancer[31]. DCP is an abnormal coagulation protein that is typically elevated in patients with liver cancer and is associated with tumor invasiveness and metastasis[32]. AFP-L3 is a glycoprotein isoform of AFP that is considered a sensitive indicator for predicting early liver cancer recurrence[33]. The Linggui Zhugan decoction significantly reduced AFP, DCP, and AFP-L3 levels in the research group, which suggests that this formula may inhibit tumor growth and metastasis by suppressing tumor cell proliferation and reducing the secretion of tumor markers. Notably, Cao et al[34] reported that the Linggui Zhugan decoction enhanced the chemotherapeutic effect of doxorubicin in non-alcoholic fatty liver disease-related HCC by modulating autophagy pathways. Moreover, Wang et al[35] observed that Indian Buead acid significantly inhibited the invasion and metastasis of gastric cancer cells, which further supports the role of Linggui Zhugan decoction in regulating tumor markers. This regulation of tumor markers significantly improved CR and ORR and may be one of the key mechanisms underlying the improved efficacy in patients, along with the reduced incidence of adverse reactions, thus reflecting its good tolerance and safety.
DFS is an important indicator of treatment efficacy and prognosis in patients with liver cancer and is defined as the time from the completion of surgery or other treatments to disease recurrence or the appearance of new lesions. The duration of DFS directly reflects the effectiveness of the treatment regimen and the degree of tumor control; thus, DSF is crucial for assessing the long-term survival of patients with liver cancer. In this study, the Cox regression analysis identified several independent prognostic factors that significantly affected DFS in patients with primary liver cancer, including treatment regimen, presence of cirrhosis, tumor diameter, Child-Pugh score, and pretreatment AFP and AFP-L3 levels.
The treatment regimen is an important factor that influences DFS, and the combination of the Linggui Zhugan decoction and MWA significantly prolonged DFS in patients, possibly owing to its multiple mechanisms of action, such as immune modulation, liver function protection, and tumor marker regulation. Koch et al[36] observed that an increase in AFP levels closely correlated with the risk of liver cancer recurrence, which suggests that changes in AFP levels could serve as an important reference for individualized patient monitoring strategies. Additionally, the presence of cirrhosis is considered a significant risk factor for liver cancer recurrence, as cirrhosis reflects the patient's liver function status, and may exacerbate the tumor microenvironment and promote tumor cell proliferation and metastasis, thereby shortening DFS. Norman et al[37] observed that AFP-L3 and DCP outperformed traditional AFP markers in predicting liver cancer recurrence, which further supports our conclusion that AFP-L3 is a prognostic factor for DFS. Giard et al[38] further revealed that the rate of change in AFP levels closely related to the risk of liver cancer recurrence and microvascular invasion.
An increase in tumor diameter is significantly associated with shorter DFS because larger tumors typically exhibit higher invasiveness and metastatic potential, which leads to a higher risk of recurrence after surgery or ablation. The Child-Pugh score is an important indicator of liver function status, with higher scores indicating poorer liver function reserves, which may limit patient tolerance to treatment and increase the risk of postoperative complications, thereby affecting DFS. Previously, Yamamoto et al[39] reported that DCP closely associates with tumor invasiveness, vascular invasion, and poor cell differentiation, which further explains the significant association of DCP levels with poor DFS in our study. Furthermore, Meischl et al[40] highlighted that integrating AFP and tumor size indicators could better predict patient survival after liver transplantation.
Finally, pretreatment levels of AFP and AFP-L3 have also been proven to be important predictors of DFS. Higher levels of AFP and AFP-L3 are typically associated with increased tumor burden and higher invasiveness, and patients with higher levels are more prone to recurrence after surgery and shorter DFS durations. Mehta et al[41] reported that the combined assessment of AFP-L3 and DCP levels significantly improved the prediction of liver cancer recurrence, which supports our findings. Furthermore, a systematic review by Al-Ameri et al[42] also revealed that AFP levels closely relate to survival after liver cancer recurrence, further emphasizing the importance of AFP as a prognostic indicator. In summary, these prognostic factors should receive special attention during the treatment and follow-up of liver cancer, to provide better individualized treatment plans and improve long-term survival rates.
This study identified several independent prognostic factors that influence DFS in patients with primary liver cancer through a Cox regression analysis, and the factors included treatment regimen, cirrhosis, and tumor diameter. While this information provides a reference for personalized treatment, this study has some limitations that should be noted when interpreting our findings. First, the small sample size limited the statistical power and generalizability of our results. Second, the single-center design may introduce biases in the treatment strategies and patient populations; thus, it may be difficult to generalize our findings to other settings. Finally, the short follow-up period did not allow a comprehensive assessment of long-term survival and recurrence risk. Future studies should expand their sample size, involve multicenter collaborations, and extend the follow-up period to further validate our findings in broader patient populations.
This study revealed that the combination of Linggui Zhugan decoction with MWA has significant advantages in prolonging DFS in patients with primary liver cancer. Cox regression analysis revealed that treatment regimen, presence of cirrhosis, tumor diameter, Child-Pugh score, pretreatment AFP, and AFP-L3 levels were independent prognostic factors affecting DFS.
| 1. | Yu XC, Liu JB, Tang QH, Diao X, Fan QY, Huang ZY, Tang XM, Li S, Cao YF, Ma YS, Fu D. Recent trends in the incidence and survival of stage I liver cancer: a surveillance, epidemiology, and end results analysis. Ann Med. 2022;54:2785-2795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 7677] [Article Influence: 7677.0] [Reference Citation Analysis (2)] |
| 3. | Zhang TQ, Geng ZJ, Zuo MX, Li JB, Huang JH, Huang ZL, Wu PH, Gu YK. Camrelizumab (a PD-1 inhibitor) plus apatinib (an VEGFR-2 inhibitor) and hepatic artery infusion chemotherapy for hepatocellular carcinoma in Barcelona Clinic Liver Cancer stage C (TRIPLET): a phase II study. Signal Transduct Target Ther. 2023;8:413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 4. | Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, Chen Z, Jia W, Jin Y, Guo Y, Hu X, Meng Z, Liang J, Cheng Y, Xiong J, Ren H, Yang F, Li W, Chen Y, Zeng Y, Sultanbaev A, Pazgan-Simon M, Pisetska M, Melisi D, Ponomarenko D, Osypchuk Y, Sinielnikov I, Yang TS, Liang X, Chen C, Wang L, Cheng AL, Kaseb A, Vogel A; CARES-310 Study Group. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023;402:1133-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 334] [Article Influence: 167.0] [Reference Citation Analysis (2)] |
| 5. | Kim J, Byun HK, Kim TH, Kim SI, Kim BK, Kim SU, Park JY, Kim DY, Seong J. Liver-Directed Concurrent Chemoradiotherapy versus Sorafenib in Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Cancers (Basel). 2022;14:2396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1195] [Article Influence: 398.3] [Reference Citation Analysis (41)] |
| 7. | Elhence A, Shalimar. Liver dysfunction in Barcelona Clinic Liver Cancer-2022 update: Clear as day or still in fog? J Hepatol. 2022;76:1236-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | He X, Xu S, Tang L, Ling S, Wei X, Xu X. Insights into the history and tendency of liver transplantation for liver cancer: a bibliometric-based visual analysis. Int J Surg. 2024;110:406-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Hu T, Wang Z, Yuan J. Clinical efficacy of precision liver resection for primary liver cancer. Am J Transl Res. 2024;16:897-904. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Tong Y, Cai R, Li JX, Chang DH, Wang LZ, Cai WW, Xiao YD. Liver resection versus microwave ablation for hepatocellular carcinoma in ideal candidates for ablation per Barcelona Clinic Liver Cancer staging: a propensity score matching and inverse probability of treatment weighting analysis. Aliment Pharmacol Ther. 2022;56:1602-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Farina L, Ruvio G, Shatwan R, Shalaby A, O'Halloran M, White A, Soo A, Breen D, Lowery A, Quinn AM. Histology-Validated Dielectric Characterisation of Lung Carcinoma Tissue for Microwave Thermal Ablation Applications. Cancers (Basel). 2023;15:3738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Adwan H, Vogl TJ, Balaban Ü, Nour-Eldin NA. Percutaneous Thermal Ablation Therapy of Hepatocellular Carcinoma (HCC): Microwave Ablation (MWA) versus Laser-Induced Thermotherapy (LITT). Diagnostics (Basel). 2022;12:564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Wei Y, Zhang L, Zhang S, Song M, Ji C. Laparoscopic-assisted microwave ablation in treatment of small hepatocellular carcinoma: safety and efficacy in comparison with laparoscopic hepatectomy. BMC Surg. 2024;24:138. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Cheng X, Huang J, Li WF, Zhong T, Cai LJ, Li H, Guo YB, Chen JZ. [Analysis of the effect of microwave ablation in the treatment of small liver cancer]. Zhonghua Gan Zang Bing Za Zhi. 2021;29:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Yang M, Wu H, Qian H, Li D, Xu H, Chen J, Zhong J, Wu W, Yang H, Chen X, Min X, Chen J. Linggui Zhugan decoction delays ventricular remodeling in rats with chronic heart failure after myocardial infarction through the Wnt/β-catenin signaling pathway. Phytomedicine. 2023;120:155026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Wu R, Zhao D, An R, Wang Z, Li Y, Shi B, Ni Q. Linggui Zhugan Formula Improves Glucose and Lipid Levels and Alters Gut Microbiota in High-Fat Diet-Induced Diabetic Mice. Front Physiol. 2019;10:918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Liu L, Zhao Y, Birling Y, Sun Y, Shang Q, Hu ZJ, Liu J, Liu Z. Effectiveness and safety of Linggui Zhugan decoction for the treatment of premature contraction in patients with coronary heart disease: A systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:1002378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Zhu M, Wang X, Wang K, Zhao Z, Dang Y, Ji G, Li F, Zhou W. Lingguizhugan decoction improves non-alcoholic steatohepatitis partially by modulating gut microbiota and correlated metabolites. Front Cell Infect Microbiol. 2023;13:1066053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Bureau of Medical Administration, National Health Commission of the People's Republic of China. [Standardization for diagnosis and treatment of hepatocellular carcinoma (2022 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:367-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 20. | Iannessi A, Beaumont H, Liu Y, Bertrand AS. RECIST 1.1 and lesion selection: How to deal with ambiguity at baseline? Insights Imaging. 2021;12:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Liu J, Zhang W, Lu H, Li H, Zhou X, Li J, Han X. Drug-eluting bead trans-arterial chemoembolization combined with microwave ablation therapy vs. microwave ablation alone for early stage hepatocellular carcinoma: a preliminary investigation of clinical value. J Cancer Res Clin Oncol. 2022;148:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Ji J, Yang W, Shi HB, Liu S, Zhou WZ. Transcatheter arterial chemoembolization alone versus combined with microwave ablation for recurrent small hepatocellular carcinoma after resection: a retrospective comparative study. BMC Gastroenterol. 2022;22:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Zuo MX, An C, Cao YZ, Pan JY, Xie LP, Yang XJ, Li W, Wu PH. Camrelizumab, apatinib and hepatic artery infusion chemotherapy combined with microwave ablation for advanced hepatocellular carcinoma. World J Gastrointest Oncol. 2024;16:3481-3495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 24. | Magen A, Hamon P, Fiaschi N, Soong BY, Park MD, Mattiuz R, Humblin E, Troncoso L, D'souza D, Dawson T, Kim J, Hamel S, Buckup M, Chang C, Tabachnikova A, Schwartz H, Malissen N, Lavin Y, Soares-Schanoski A, Giotti B, Hegde S, Ioannou G, Gonzalez-Kozlova E, Hennequin C, Le Berichel J, Zhao Z, Ward SC, Fiel I, Kou B, Dobosz M, Li L, Adler C, Ni M, Wei Y, Wang W, Atwal GS, Kundu K, Cygan KJ, Tsankov AM, Rahman A, Price C, Fernandez N, He J, Gupta NT, Kim-Schulze S, Gnjatic S, Kenigsberg E, Deering RP, Schwartz M, Marron TU, Thurston G, Kamphorst AO, Merad M. Intratumoral dendritic cell-CD4(+) T helper cell niches enable CD8(+) T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat Med. 2023;29:1389-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 186] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 25. | Li J, Huang HY, Lin YC, Zuo H, Tang Y, Huang HD. Cinnamomi ramulus inhibits cancer cells growth by inducing G2/M arrest. Front Pharmacol. 2023;14:1121799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Yang J, Dong X, Li B, Chen T, Yu B, Wang X, Dou X, Peng B, Hu Q. Poria cocos polysaccharide-functionalized graphene oxide nanosheet induces efficient cancer immunotherapy in mice. Front Bioeng Biotechnol. 2022;10:1050077. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Huang Y, Chen F, Li H. Effect of rapid rehabilitation nursing on inflammation and liver function after laparoscopic radical resection of primary liver cancer. Am J Transl Res. 2022;14:8156-8165. [PubMed] |
| 28. | Xiang Y, Zhang S, Cui Z, Yang Y. Exploring the effect of microecological agents on postoperative immune function in patients undergoing liver cancer surgery: a systematic review and meta-analysis. Ann Palliat Med. 2021;10:11615-11627. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Zhao Z, Yue H, Cui X. Homotherapy for Heteropathy: A Molecular Mechanism of Poria Sini Decoction for Treatment of Liver Cancer and Chronic Heart Failure. Evid Based Complement Alternat Med. 2024;2024:9958258. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Jiang H, Mao T, Liu Y, Tan X, Sun Z, Cheng Y, Han X, Zhang Y, Wang J, Shi L, Guo Y, Li J, Han H. Protective Effects and Mechanisms of Yinchen Linggui Zhugan Decoction in HFD-Induced Nonalcoholic Fatty Liver Disease Rats Based on Network Pharmacology and Experimental Verification. Front Pharmacol. 2022;13:908128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 31. | Zhao K, Zhou X, Xiao Y, Wang Y, Wen L. Research Progress in Alpha-fetoprotein-induced Immunosuppression of Liver Cancer. Mini Rev Med Chem. 2022;22:2237-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 32. | Sha M, Cao J, Xia Q. Incorporating AFP-L3 and DCP in selecting patients with hepatocellular carcinoma for liver transplantation: What are the optimal criteria? J Hepatol. 2024;80:e171-e172. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Yuan JJ, Xu YD, Li H, Guo QJ, Li GC, Chai W, Zhang ZQ, Liu RH. Magnetic Resonance Imaging and Serum AFP-L3 and GP-73 in the Diagnosis of Primary Liver Cancer. J Oncol. 2022;2022:1192368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Cao HM, Kong L, Sui GY, Wu J, Jia LQ. [Synergistic effects of Linggui Zhugan decoction regulating autophagy on doxorubicin against nonalcoholic fatty liver disease-hepatocellular carcinoma]. Zhongguo Yaofang. 2023;34:2316-2322. [DOI] [Full Text] |
| 35. | Wang H, Luo Y, Chu Z, Ni T, Ou S, Dai X, Zhang X, Liu Y. Poria Acid, Triterpenoids Extracted from Poria cocos, Inhibits the Invasion and Metastasis of Gastric Cancer Cells. Molecules. 2022;27:3629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Koch C, Bette T, Waidmann O, Filmann N, Schrecker C, Trojan J, Weiler N, Vermehren J, Schnitzbauer AA, Bechstein WO, Zeuzem S, Herrmann E, Welker MW. AFP ratio predicts HCC recurrence after liver transplantation. PLoS One. 2020;15:e0235576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Norman JS, Li PJ, Kotwani P, Shui AM, Yao F, Mehta N. AFP-L3 and DCP strongly predict early hepatocellular carcinoma recurrence after liver transplantation. J Hepatol. 2023;79:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 67] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 38. | Giard JM, Mehta N, Dodge JL, Roberts JP, Yao FY. Alpha-Fetoprotein Slope >7.5 ng/mL per Month Predicts Microvascular Invasion and Tumor Recurrence After Liver Transplantation for Hepatocellular Carcinoma. Transplantation. 2018;102:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Yamamoto K, Imamura H, Matsuyama Y, Kume Y, Ikeda H, Norman GL, Shums Z, Aoki T, Hasegawa K, Beck Y, Sugawara Y, Kokudo N. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 2010;45:1272-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 40. | Meischl T, Rasoul-Rockenschaub S, Győri G, Scheiner B, Trauner M, Soliman T, Berlakovich G, Pinter M. Alpha-fetoprotein-adjusted-to-HCC-size criteria are associated with favourable survival after liver transplantation for hepatocellular carcinoma. United European Gastroenterol J. 2021;9:209-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Mehta N, Kotwani P, Norman J, Shui A, Li PJ, Saxena V, Chan W, Yao FY. AFP-L3 and DCP are superior to AFP in predicting waitlist dropout in HCC patients: Results of a prospective study. Liver Transpl. 2023;29:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 42. | Al-Ameri A, Yu X, Zheng S. Predictors of post-recurrence survival in hepatocellular carcinoma patients following liver transplantation: Systematic review and meta-analysis. Transplant Rev (Orlando). 2022;36:100676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |