Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.100954
Revised: October 30, 2024
Accepted: December 2, 2024
Published online: February 15, 2025
Processing time: 140 Days and 1.2 Hours
Colorectal cancer (CRC) is the third most prevalent form of cancer worldwide. Among patients with CRC, colorectal liver metastasis (CRLM) is the foremost direct contributor to mortality. In recent years, immunotherapy has swiftly risen to prominence as a vital approach for treating a range of solid tumors, including CRC. We present a unique case of a patient suffering from CRLM, with the goal of offering an insightful example and relevant references for the treatment of CRLM.
We report a patient who experienced liver metastasis after undergoing successful surgical removal of CRC, with the postoperative pathological stage identified as pT4N2aM0. The patient has been receiving a combination treatment of Western and Traditional Chinese Medicine. Regular assessments of the patient’s condition have been conducted, encompassing evaluations of serum carcinoembryonic antigen levels, carbohydrate antigen 199, and observations of the tongue complexion and its coating. The patient achieved clinical remission after anti-programmed death-1 immunotherapy when various systemic therapies failed. Since the diagnosis of CRLM, the patient has survived for more than 6 years, surpassing the expected survival time for those with advanced CRC.
This case illustrates the considerable promise of anti-programmed death-1 immunotherapy in managing CRLM, especially in scenarios of drug resistance and disease progression.
Core Tip: The patient described in this report has survived for more than 6 years since the diagnosis of colorectal liver metastasis. The patient achieved clinical remission after anti-programmed death-1 (PD-1) immunotherapy when different systemic therapies failed. This case demonstrates the promising potential of holistic integrative medicine, combining anti-PD-1 immunotherapy with complementary and alternative treatments, for managing advanced malignancies amid drug resistance and disease progression.
- Citation: Guo TH, Hong SW, Zhu WJ, Hui YF, Qiu WL, Wu Y, Li X, Ke F, Li L, Cheng HB. Anti-programmed death-1 immunotherapy-promising treatment for metastatic colorectal cancer: A case report. World J Gastrointest Oncol 2025; 17(2): 100954
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/100954.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.100954
Globally, colorectal cancer (CRC) ranks the third among the cancers with the highest incidence and mortality rates[1]. Colorectal liver metastasis (CRLM) markedly reduces life expectancy and is the primary direct mortality factor in affected individuals[2]. The 5-year survival rate for patients with CRLM is less than 50%[3]. Traditional treatments such as surgery, chemotherapy, and radiotherapy, all come with their own set of constraints[4]. The national comprehensive cancer network guidelines suggest the use of bevacizumab in conjunction with chemotherapy for patients with CRC that may be surgically removed[5,6].
However, the emergence of drug resistance and disease progression narrows the therapeutic avenues. In such contexts, immunotherapy has swiftly ascended as a principal strategy against numerous solid tumors in recent years[7]. By blocking programmed death-1 (PD-1) with antibodies, its binding to programmed cell death ligand 1 and programmed cell death ligand 2 is hindered, which in turn disrupts their subsequent pathways and reactivates the T cells’ ability to combat tumors[8]. This method ranks as one of the most effective among clinically utilized checkpoint inhibitors[9].
We here report a special case of a CRLM patient treated with anti-PD-1 immunotherapy, who has survived for more than 6 years since the diagnosis of CRLM. Anti-PD-1 immunotherapy, as an innovative therapy, could provide a fresh strategy in the treatment of CRLM.
The patient was a 53-year-old male. He was admitted to our hospital for further treatment after colon cancer surgery 26 days ago.
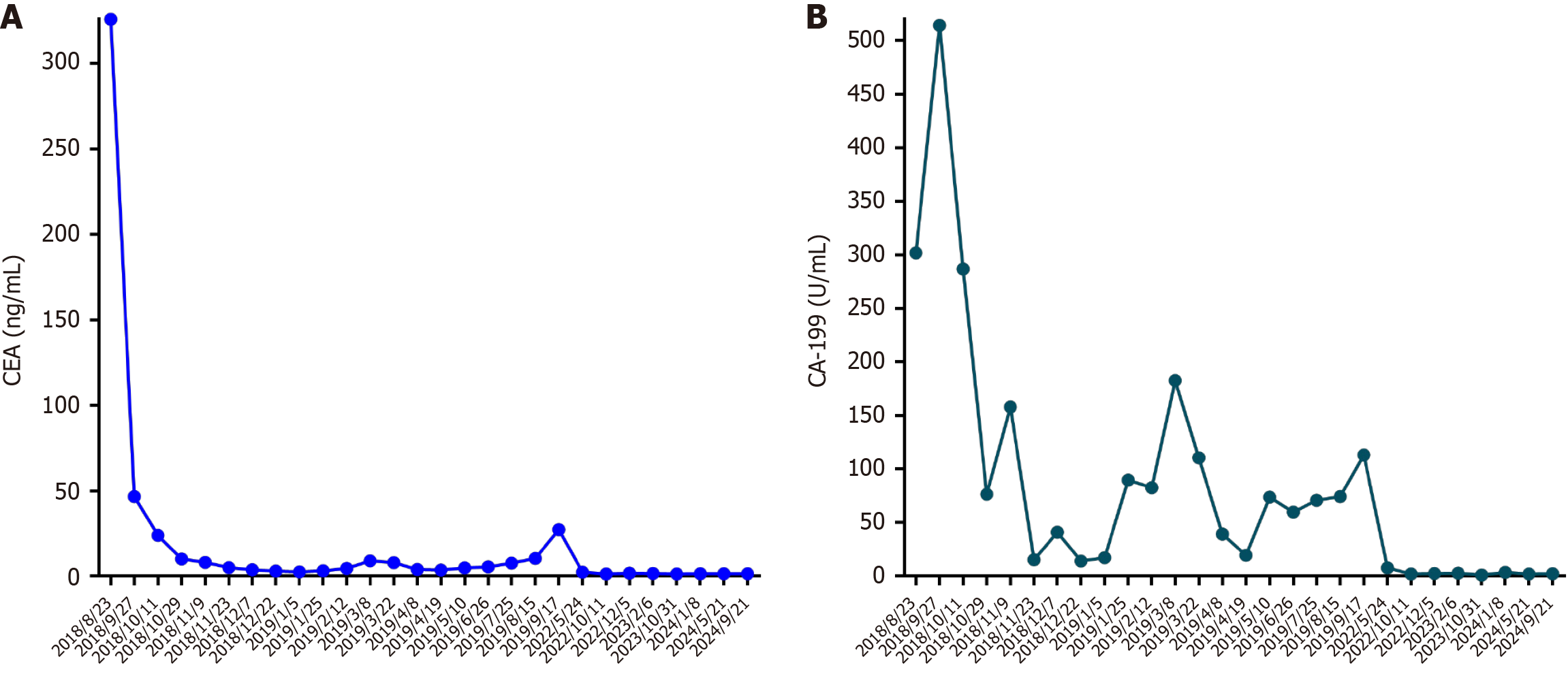
Before admission to our hospital, the patient exhibited increased tumor markers: Serum carcinoembryonic antigen (CEA): 325.87 ng/mL, carbohydrate antigen (CA) 242: > 220 U/mL, and CA-199: 301.80 U/mL (Figure 1), during a routine health examination on August 23, 2018. Subsequently, on August 27, 2018, the patient underwent an abdominal computed tomography (CT) scan at Nanjing First Hospital. The scan revealed a lesion in the ileocecal region suggestive of CRC, accompanied by several enlarged peripheral lymph nodes; appendix thickening; emphysema adjacent to the right superior lobar septum; a pulmonary bulla; a nodule in the lower right lung; atherosclerosis; fatty liver; enhanced nodules in the left liver lobe indicative of hemangioma; and minor pelvic effusion. The following day, a gastrointestinal endo
The patient denied any medical history of chronic illnesses or infection.
The patient denied notable family history of cancer.
The patient was 185 cm tall and weighed 90 kg. His physical examination was unremarkable except for a longitudinal incision approximately 12 cm long in the middle of the right abdomen, which healed well.
Serum tumor markers, including CEA and CA-199, were above the normal ranges (Figure 1). Other laboratory test results, including routine blood tests, blood biochemistry and coagulation function, were unremarkable.
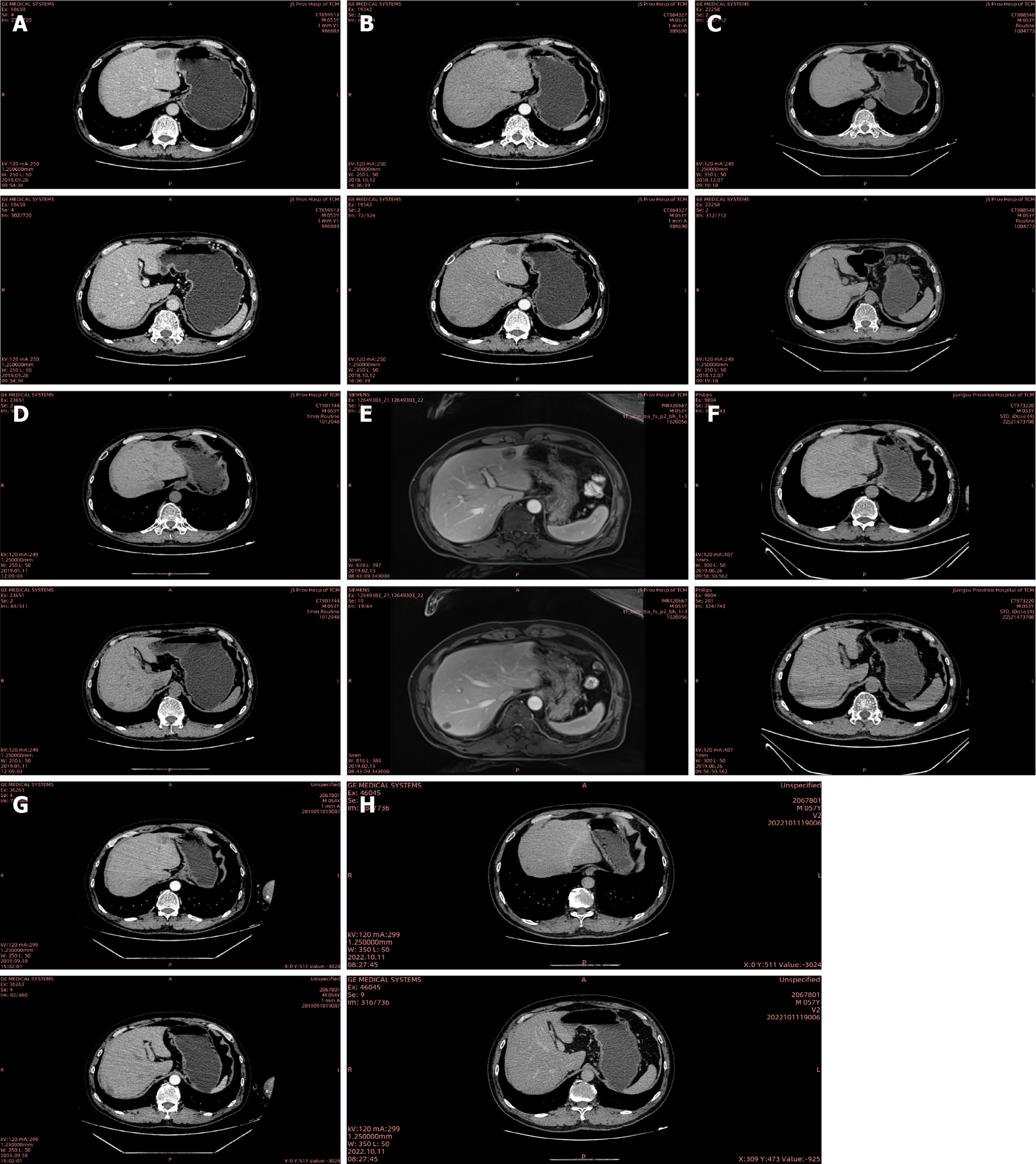
Chest and abdominal CT (Figure 3A) showed the change after radical resection of right colon cancer; a high possibility of metastasis in the left lateral lobe and right posterior lobe of the liver; minor pelvic effusion; pulmonary emphysema adjacent to the right superior lobar septum; a ground-glass nodule in the right lower lung; coronary atherosclerosis; thoracolumbar degeneration (September 28, 2018).
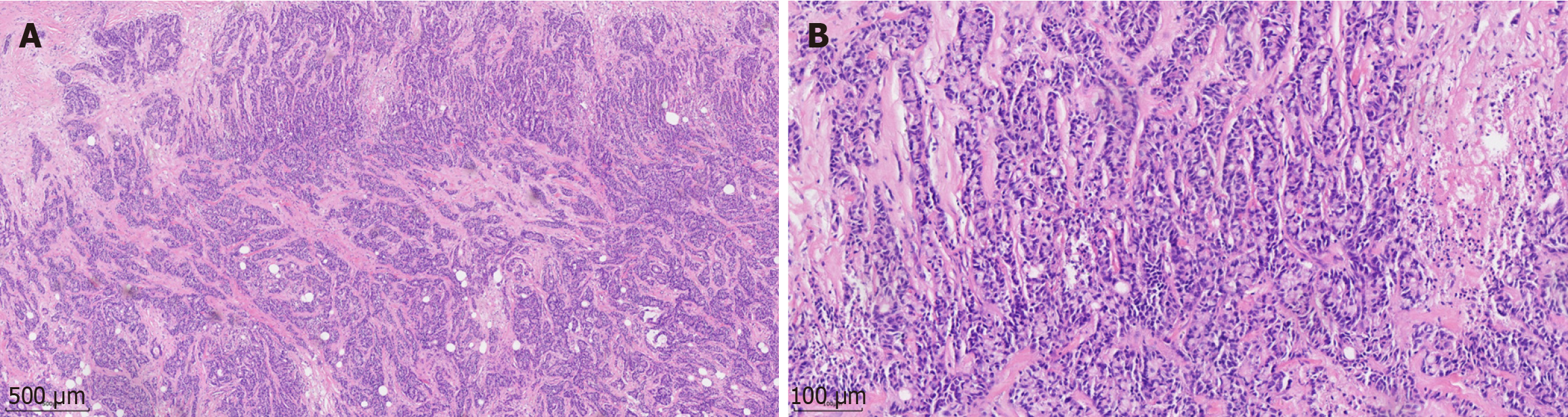
The patient was diagnosed with CRC and CRLM based on the pathological examination and imaging examinations.
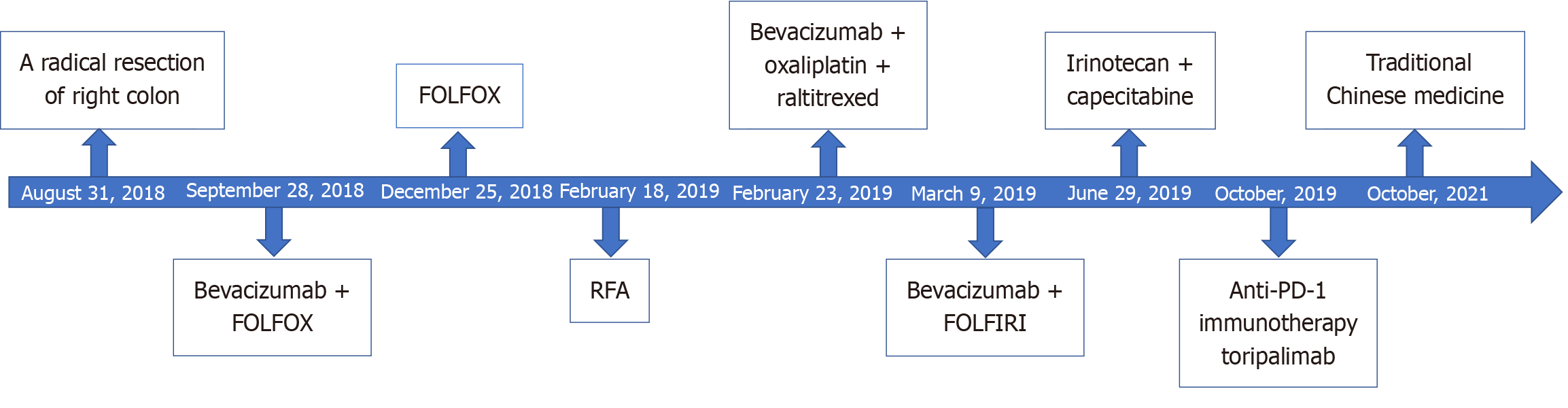
The patient received bevacizumab combined with FOLFOX treatment [bevacizumab 300 mg day 1 + oxaliplatin 150 mg day 1 + calcium folinate 200 mg day 1 + fluorouracil (5-FU) 0.5 g day 1, 5-FU 2.0 g continuous intravenous infusion (civ) 46 hours] on September 28, October 30, November 13, November 27, and December 11, 2018. The liver metastases were shown to be smaller than before from October 12, 2018 to December 07, 2018 (Figure 3B and C).
The patient intended to undergo surgical treatment. It was recommended by the Department of Surgical Oncology that bevacizumab should be stopped 4 weeks before surgery. The patient received FOLFOX treatment (oxaliplatin 150 mg day 1 + calcium folinate 200 mg day 1 + 5-FU 0.5 g day 1, 5-FU 2.0 g civ 46 hours) on December 25, 2018, January 10, and January 26, 2019. On January 11, 2019, abdominal CT (Figure 3D) indicated a slight progression (small lymph nodes around the operative area similar to CT findings on December 07, 2018; the liver metastases were slightly larger compared with CT findings on December 07, 2018). The patient was admitted to the Department of Surgical Oncology for preoperative assessment on February 11, 2019. On February 13, 2019, abdominal magnetic resonance imaging (Figure 3E) showed a high possibility of multiple intrahepatic metastatic tumors; thus, metastases could not be excluded. Department of Surgical Oncology suggested that the surgical indication was still uncertain. It was recommended that radiofrequency ablation (RFA) of the liver metastases should be performed by the Intervention Therapy Department. On February 18, 2019, the patient underwent RFA of the liver metastases, with 2 targets in total.
The patient was subsequently admitted to our department for further treatment. On February 23, 2019, the patient received targeted therapy combined with chemotherapy (bevacizumab 300 mg day 1 + oxaliplatin 150 mg day 1 + raltitrexed 5 mg day 1). The tumor markers were higher than before (CEA: 9.01 ng/mL, CA-242: 103.60 U/mL, CA-199: 182.57 U/mL) (Figure 1) on March 9, 2019. Treatment was changed to bevacizumab combined with FOLFIRI (bevaci
The disease continued to progress rapidly after first-, second- and third-line treatments. The patient sought further evaluation at Jiangsu Cancer Hospital, where next-generation sequencing revealed the tumor’s microsatellite instability-high status. Subsequently, the patient received anti-PD-1 immunotherapy (toripalimab 240 mg day 1) from October 2019 to October 2021. The tumor markers were normal when reassessed at Jiangsu Cancer Hospital on May 24, 2022. On October 10, 2022, the patient was admitted to our department for further re-examination. Chest and abdominal CT (Figure 3H) indicated a clinical cure of CRLM [change after radical resection of right colon cancer; review after treatment of liver metastasis, multiple mild enhanced liver lesions shown in the previous film were not seen this time; small cystic focus in the liver; multiple enlarged lymph nodes in the liver hilum and the soft tissue mass in front of the right psoas major muscle shown in the previous film was not seen this time; multiple nodules in both lungs; slight fibrosis of the right lower lung; coronary arteriosclerosis; thoracolumbar degeneration; mild instability of lumbar vertebral bodies 2 and 3; patch shadow on L3 vertebral body (October 11, 2022)]. Over the past year to six months ago, the patient gained 15 kg, with no significant weight changes observed in the past six months. Chest and abdominal CT indicated a stable condition on February 6, 2023; October 31, 2023; May 21, 2024 and September 20, 2024. The treatments received by the patient and the subsequent outcomes are listed in Table 1.
| Time period | Treatment and dose | Results |
| From September 28, 2018 to December 11, 2018 | Bevacizumab + FOLFOX treatment (bevacizumab 300 mg day 1 + oxaliplatin 150 mg day 1 + calcium folinate 200 mg day 1 + fluorouracil 0.5 g day 1, fluorouracil 2.0 g civ 46 hours) | Stable disease |
| From December 25, 2018 to January 26, 2019 | FOLFOX treatment (oxaliplatin 150 mg day 1 + calcium folinate 200 mg day 1 + fluorouracil 0.5 g day 1, fluorouracil 2.0 g civ 46 hour) | Progressive disease |
| February 18, 2019 | RFA | Progressive disease |
| February 23, 2019 | Bevacizumab 300mg day 1 + oxaliplatin 150 mg day 1 + raltitrexed 5 mg day 1 | Progressive disease |
| From March 9, 2019 to May 11, 2019 | Bevacizumab + FOLFIRI treatment (bevacizumab 300 mg day 1 + irinotecan 200 mg day 1 + calcium folinate 200 mg day 1 + fluorouracil 0.5 g day 1, fluorouracil 2.0 g civ 46 hours) | Progressive disease |
| From June 29, 2019 to September 19, 2019 | Irinotecan 200 mg day 1 + capecitabine 1.5 g day 1-day 14 | Progressive disease |
| From October 2019 to October 2021 | Anti-PD-1 immunotherapy (toripalimab 240 mg day 1) | Stable disease |
| From October 2021 to September 2024 | Single Traditional Chinese Medicine treatment | Stable disease |
CRLM significantly impacts the prognosis of patients with CRC[10]. We report the special case of a patient with CRLM (Figure 4). The disease continued to progress rapidly following first-, second- and third-line treatments. The patient failed different systemic therapies, such as chemotherapy, targeted therapy and interventional therapy. Subsequently, the patient underwent anti-PD-1 immunotherapy (toripalimab 240 mg day 1) after the development of multiple metastases of CRC. Surprisingly, he achieved clinical remission, as confirmed by tumor markers and CT scans. Researches showed that anti-PD-1 immunotherapy exhibits anti-tumor efficacy in metastatic CRC[11,12].
The patient has survived for more than 6 years since the diagnosis of CRLM, which is a long course of CRC disease. At present, there is a lack of treatment methods for CRLM. This patient’s clinical cure demonstrates the significant potential of anti-PD-1 immunotherapy for treating CRLM. It is essential to follow up patients to observe their outcome and prognosis, as this can contribute to improving clinical diagnosis and treatment strategies. However, this can be particularly challenging, especially in China, where the high patient volume and low staff-patient ratio often lead to difficulties in doctor-patient communication. Clinicians are burdened with heavy workloads and have limited time to conduct thorough follow-ups with each patient. In China, patients often compare hospitals and select the one they prefer. Conse
The present patient has consistently undergone Traditional Chinese Medicine (TCM) treatment in our department. Modified Liujunzi Decoction was administered and was adjusted based on the patient’s symptoms at different times. The patient’s condition, including complexion and tongue coating, was constantly assessed. Patients with Chinese back
We report a rare case where the patient’s overall survival has been more than 6 years, even though CRLM occurred. This case demonstrates the promising potential of holistic integrative medicine, combining anti-PD-1 immunotherapy with complementary and alternative treatments, for managing advanced malignancies amid drug resistance and disease progression.
The authors thank the patient for his kind consent for the anonymized publication of this case report and accompanying images.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 63668] [Article Influence: 15917.0] [Reference Citation Analysis (174)] |
| 2. | Lin N, Li J, Yao X, Zhang X, Liu G, Zhang Z, Weng S. Prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer liver metastasis: A meta-analysis of results from multivariate analysis. Int J Surg. 2022;107:106959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Wang Y, Ma LY, Yin XP, Gao BL. Radiomics and Radiogenomics in Evaluation of Colorectal Cancer Liver Metastasis. Front Oncol. 2021;11:689509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, Kramer J, Siegel RL. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 1515] [Article Influence: 505.0] [Reference Citation Analysis (0)] |
| 5. | Messersmith WA. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J Natl Compr Canc Netw. 2019;17:599-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 6. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 927] [Article Influence: 231.8] [Reference Citation Analysis (16)] |
| 7. | Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA Jr. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1198] [Article Influence: 199.7] [Reference Citation Analysis (0)] |
| 8. | Patsoukis N, Wang Q, Strauss L, Boussiotis VA. Revisiting the PD-1 pathway. Sci Adv. 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 342] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 9. | He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 835] [Article Influence: 167.0] [Reference Citation Analysis (0)] |
| 10. | Dai W, Wu J, Peng X, Hou W, Huang H, Cheng Q, Liu Z, Luyten W, Schoofs L, Zhou J, Liu S. CDK12 orchestrates super-enhancer-associated CCDC137 transcription to direct hepatic metastasis in colorectal cancer. Clin Transl Med. 2022;12:e1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38:2053-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 520] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 12. | Yu W, Tao Q, Zhang Y, Yi F, Feng L. Efficacy and Safety of Regorafenib Combined with Toripalimab in the Third-Line and beyond Treatment of Advanced Colorectal Cancer. J Oncol. 2021;2021:9959946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Lou Y, Wang J, Yu C, Shen W. Research Status and Molecular Mechanism of the Traditional Chinese Medicine and Antitumor Therapy Combined Strategy Based on Tumor Microenvironment. Front Immunol. 2020;11:609705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 14. | Kong MY, Li LY, Lou YM, Chi HY, Wu JJ. Chinese herbal medicines for prevention and treatment of colorectal cancer: From molecular mechanisms to potential clinical applications. J Integr Med. 2020;18:369-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Sang T, Qiu W, Li W, Zhou H, Chen H, Zhou H. The Relationship between Prevention and Treatment of Colorectal Cancer and Cancerous Toxin Pathogenesis Theory Basing on Gut Microbiota. Evid Based Complement Alternat Med. 2020;2020:7162545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Wang S, Fu JL, Hao HF, Jiao YN, Li PP, Han SY. Metabolic reprogramming by Traditional Chinese Medicine and its role in effective cancer therapy. Pharmacol Res. 2021;170:105728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 17. | Yu CT, Chen T, Lu S, Hu W, Zhang Q, Tan J, Sun D, Li L, Sun X, Xu C, Lai Y, Fan M, Shen Z, Shen W, Cheng H. Identification of Significant Modules and Targets of Xian-Lian-Jie-Du Decoction Based on the Analysis of Transcriptomics, Proteomics and Single-Cell Transcriptomics in Colorectal Tumor. J Inflamm Res. 2022;15:1483-1499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Fan D. Holistic integrative medicine: toward a new era of medical advancement. Front Med. 2017;11:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |