Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.100908
Revised: October 30, 2024
Accepted: November 14, 2024
Published online: February 15, 2025
Processing time: 141 Days and 8 Hours
Rectal cancer is prevalent and associated with substantial morbidity and mor
To develop a nomogram prediction model for overall survival (OS) in patients with rectal cancer by leveraging a comprehensive analysis of demographic, clinicopathological, haematological, and follow-up data to identify independent prognostic factors.
We conducted a prospective cohort study in China involving rectal cancer pa
Multivariate analysis revealed independent predictors of OS, including the Karnofsky performance status, age, sex, TNM stage, chemotherapy, surgery, targeted therapy, β2-microglobulin, lactate dehydrogenase, and the neutrophil-to-lymphocyte ratio. The nomogram demonstrated a C-index of 0.80 for the training and validation cohorts, with AUC values indicating high predictive accuracy for 1-year, 3-year, and 5-year OS. The calibration curves confirmed the model's excellent agreement with the observed survival rates, and DCA revealed the superior clinical utility of the nomogram over the TNM staging system.
In this study, a novel prognostic model that accurately predicts the OS of rectal cancer patients was developed. The model exhibited excellent discriminatory and calibration capabilities, thus offering a reliable tool for health care professionals to estimate patient survival.
Core Tip: This study developed and validated a novel prognostic nomogram for rectal cancer, incorporating demographic, clinicopathological, haematological, and follow-up data. The prognostic factors include Karnofsky performance status, age, sex, TNM stage, chemotherapy, surgery, targeted therapy, β2-microglobulin, lactate dehydrogenase (LDH), and neutrophil-to-lymphocyte ratio. Compared to the TNM staging system, the nomogram demonstrated high accuracy in predicting 1-year, 3-year, and 5-year overall survival (C-index 0.80), with excellent generalizability and clinical utility. It provides a reliable tool for personalized interventions. However, further research is needed to explore the role of β2-microglobulin and LDH in the progression of rectal cancer and to validate the model.
- Citation: Liang L, Li XS, Huang ZJ, Hu ZH, Xu QJ, Yuan YL, Zhang W, Lei HK. Development and validation of a nomogram prediction model for overall survival in patients with rectal cancer. World J Gastrointest Oncol 2025; 17(2): 100908
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/100908.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.100908
Rectal cancer is the third most common tumour throughout the world and the second leading cause of cancer-related death, constituting one-third of all cases[1]. Despite an overall decline in the incidence and mortality rates of colorectal cancer, there has been a notable increase in the occurrence of rectal cancer among individuals under 50 years of age. Projections suggest a 124.2% increase in rates for those aged 20-34 years in the USA by 2030[2], and parallel trends have been noted in Europe[3,4]. With a standardized mortality rate of 10.9 per 100000, rectal cancer is the fifth leading cause of cancer-related mortality in China and ranks forty-third throughout the world[5]. China accounts for the largest global burden, contributing to 28.2% of cases and 28.1% of deaths. The increasing prevalence and mortality of colorectal cancer in China are attributed to changing lifestyles and an expanding elderly population, which poses not only a threat to public health but also a significant financial burden[6]. Given the increasing morbidity and mortality associated with rectal cancer, there is an urgent need for an improved predictive tool to assess the long-term survival of rectal cancer patients. As user-friendly statistical visualization tools, nomograms have gained widespread use in recent years for predicting disease prognosis and survival. Numerous studies in the literature have validated the successful application of nomograms in oncology prognostics[7].
Several factors, including patient sex, treatment type, and the TNM staging system, may significantly impact the overall survival (OS) of rectal cancer patients[8]. However, to date, no holistic prognostic model has been established to accurately predict the follow-up outcomes and survival of rectal cancer patients. A well-developed and precise nomogram would be highly advantageous in clinical decision-making. Our primary goal was to construct an enhanced nomogram capable of predicting the survival of individuals diagnosed with rectal cancer.
From January 1, 2018, to December 31, 2020, rectal cancer patient data were retrospectively collected from the tumour database at the Chongqing University Cancer Hospital. The inclusion criteria were as follows: (1) Patients aged 18 or older; (2) Patients with pathologically confirmed primary rectal cancer, and (3) Patients who completed primary clinical treatments at our hospital. The exclusion criteria were as follows: (1) Patients who died within 48 hours of admission; (2) Patients with other concurrent malignancies, and (3) Patients with incomplete baseline data or missing follow-up records.
A prospective cohort study collected demographic information, including sex, age, and marital status. The clinical features included the Karnofsky performance status (KPS) rating, TNM clinicopathological stage, type of pathological tissue, and several treatment approaches, including surgery, radiotherapy, chemotherapy, targeted therapy, and im
Active and passive follow-up strategies, including telephone follow-ups, patient clinic visits, and hospital examinations, were implemented. The primary outcome was OS, which was defined as the time from the diagnosis of rectal cancer to either death or the latest follow-up. The primary metrics that were assessed included survival probabilities at 1, 3, and 5 years. The follow-up period is set to conclude on May 31, 2024.
Patients who met the inclusion and exclusion criteria were randomly assigned to a training cohort (n = 1293) or a validation cohort (n = 553) at a 7:3 ratio. This process was performed via the 'caret' statistical package in R software, with fixed random seed numbers used for consistency. The nomogram model was established via the training cohort. For feature selection, we employed least absolute shrinkage and selection operator (LASSO) regression to identify the clinical features in the training cohort. The optimal parameter (λ) for LASSO regression was determined through cross-validation, with essential variables selected according to the minimum λ principle. Stepwise multivariate Cox regression was then used to further refine these variables by applying the principle of the minimum Akaike information criterion. This two-step variable screening method provided better insights into the data and reduced model complexity. The final no
Several methods were utilized to assess the model's predictive accuracy and consistency, including receiver operating characteristic (ROC) curves, calculation of the area under the curve (AUC), determination of the concordance index (C-index), evaluation of calibration curves, and decision curve analysis (DCA). The C-index and AUC of the ROC curve indicated the model's discriminatory ability. Calibration was confirmed when the calibration curve closely aligned with the diagonal line, thus signifying strong agreement between the predicted and observed outcomes. Different probability thresholds were applied to assess the nomogram's clinical net benefit via DCA. If the DCA curve consistently surpasses the reference lines across a broad range of thresholds, this signifies robust clinical utility.
The data analysis was conducted via R software version 4.2.1. To optimize and evaluate the model, we employed various R packages, including 'survival' (3.3-1), 'foreign' (0.8-82), 'rms' (6.3-1), 'timeROC' (0.4), and 'ggDCA' (5.0.1). Additionally, the R libraries 'rsconnect' (v0.8.27) and 'DynNom' (v5.0.1) were used to establish a rectal cancer nomogram web server. LASSO and multivariate Cox regression were employed to select each variable, and a nomogram model was established. The significance level was set at P < 0.05 for each two-tailed test.
A total of 1846 patients who met the inclusion and exclusion criteria were enrolled for this study. They were randomly assigned at a 7:3 ratio, resulting in 1293 patients in the training group and 553 patients in the validation group. The average age of the patients was 60.35 ± 12.62 years, with 1062 (57.53%) being male. Adenocarcinoma was the predominant pathological tissue type and was observed in 1831 patients (99.19%). The training and validation cohorts included demographic and clinical data, as detailed in Table 1. No statistically significant differences were found between the two cohorts.
| Variables | Overall (1846) | Training cohort (1293) | Validation cohort (553) | P value |
| Age1 | 60.35 ± 12.62 | 60.19 ± 12.71 | 60.72 ± 12.42 | 0.416 |
| KPS1 | 81.79 ± 8.52 | 81.80 ± 8.43 | 81.75 ± 8.72 | 0.908 |
| BMI, n (%) | ||||
| < 18.5 | 110 (5.96) | 79 (6.11) | 31 (5.61) | 0.841 |
| 18.5-23.9 | 1021 (55.31) | 719 (55.61) | 302 (54.61) | |
| 24-27.9 | 582 (31.53) | 400 (30.94) | 182 (32.91) | |
| ≥ 28 | 133 (7.20) | 95 (7.35) | 38 (6.87) | |
| Sex, n (%) | ||||
| Male | 1062 (57.53) | 731 (56.54) | 331 (59.86) | 0.204 |
| Female | 784 (42.47) | 562 (43.46) | 222 (40.14) | |
| Marita, n (%) | ||||
| Married | 1712 (92.74) | 1204 (93.12) | 508 (91.86) | 0.393 |
| Others | 134 (7.26) | 89 (6.88) | 45 (8.14) | |
| Histology, n (%) | ||||
| Adenocarcinoma | 1831 (99.19) | 1282 (99.15) | 549 (99.28) | 1.000 |
| Others | 15 (0.81) | 11 (0.85) | 4 (0.72) | |
| TNM, n (%) | ||||
| I-II | 561 (30.39) | 406 (31.40) | 155 (28.03) | 0.154 |
| III | 508 (27.52) | 361 (27.92) | 147 (26.58) | |
| IV | 777 (42.09) | 526 (40.68) | 251 (45.39) | |
| Radiation, n (%) | ||||
| No | 1702 (92.20) | 1195 (92.42) | 507 (91.68) | 0.655 |
| Yes | 144 (7.80) | 98 (7.58) | 46 (8.32) | |
| Chemotherapy, n (%) | ||||
| No | 743 (40.25) | 521 (40.29) | 222 (40.14) | 0.994 |
| Yes | 1103 (59.75) | 772 (59.71) | 331 (59.86) | |
| Surgery, n (%) | ||||
| No | 663 (35.92) | 458 (35.42) | 205 (37.07) | 0.533 |
| Yes | 1183 (64.08) | 835 (64.58) | 348 (62.93) | |
| Immunotherapy, n (%) | ||||
| No | 1805 (97.78) | 1262 (97.60) | 543 (98.19) | 0.539 |
| Yes | 41 (2.22) | 31 (2.40) | 10 (1.81) | |
| Targeted, n (%) | ||||
| No | 1453 (78.71) | 1020 (78.89) | 433 (78.30) | 0.826 |
| Yes | 393 (21.29) | 273 (21.11) | 120 (21.70) | |
| Hypertension (%) | ||||
| No | 1416 (76.71) | 986 (76.26) | 430 (77.76) | 0.523 |
| Yes | 430 (23.29) | 307 (23.74) | 123 (22.24) | |
| Diabetes, n (%) | ||||
| No | 1601 (86.73) | 1127 (87.16) | 474 (85.71) | 0.444 |
| Yes | 245 (13.27) | 166 (12.84) | 79 (14.29) | |
| WBC2 | 6.03 (4.84, 7.69) | 5.97 (4.81, 7.70) | 6.10(4.94, 7.69) | 0.570 |
| β2.microglobulin2 | 2.50 (1.90, 3.20) | 2.50 (1.87, 3.20) | 2.50 (2.00, 3.20) | 0.055 |
| LDH2 | 186.20 (159.93, 230.38) | 187.80 (161.70, 228.80) | 184.60 (158.00, 235.00) | 0.386 |
| PLR2 | 170.84 (120.59, 246.22) | 172.37 (122.60, 251.67) | 168.29 (113.39, 234.88) | 0.108 |
| NLR2 | 2.81 (1.95, 4.44) | 2.83 (1.98, 4.48) | 2.75 (1.88, 4.36) | 0.243 |
| LMR2 | 3.15 (2.09, 4.46) | 3.15 (2.10, 4.52) | 3.14 (2.09, 4.28) | 0.425 |
| AGR2 | 1.35 (1.15, 1.56) | 1.35 (1.16, 1.56) | 1.35 (1.12, 1.57) | 0.611 |
| CD4/CD82 | 1.66 (1.16, 2.31) | 1.66 (1.16, 2.35) | 1.64 (1.17, 2.24) | 0.757 |
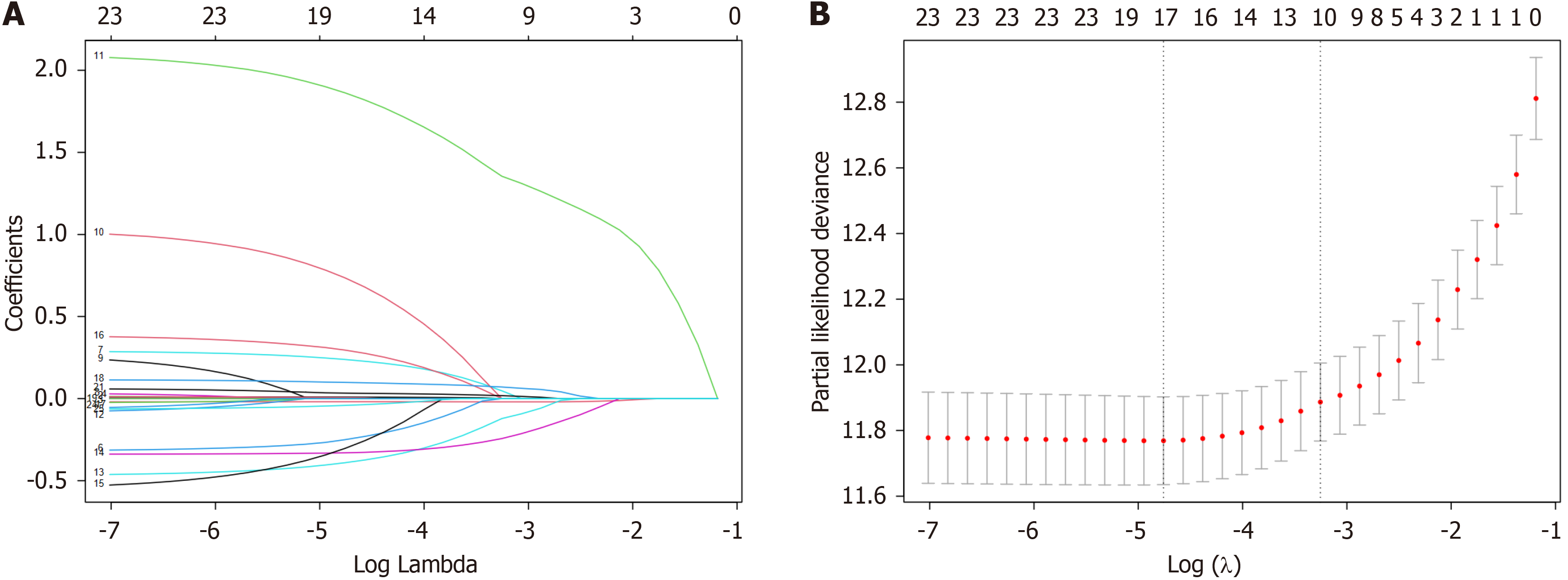
Initially, variables with potential predictive influence were selected via LASSO regression and univariate analysis. The results of the LASSO regression revealed that at a logarithmic value of the regularization coefficient λ of approximately -4.8, the model included 17 variables, which effectively minimized model bias (Figure 1). The 17 variables that were chosen via LASSO regression were subsequently incorporated into a bidirectional stepwise Cox regression model. The objective of this study was to identify the optimal combination of variables for predicting OS in patients with rectal cancer. When considering clinical expertise, age, KPS, sex, and the other 10 variables that were selected through the abovementioned steps were chosen as predictive variables for the prognosis of rectal cancer patients. These variables were the foundation for establishing the nomogram model in subsequent analyses.
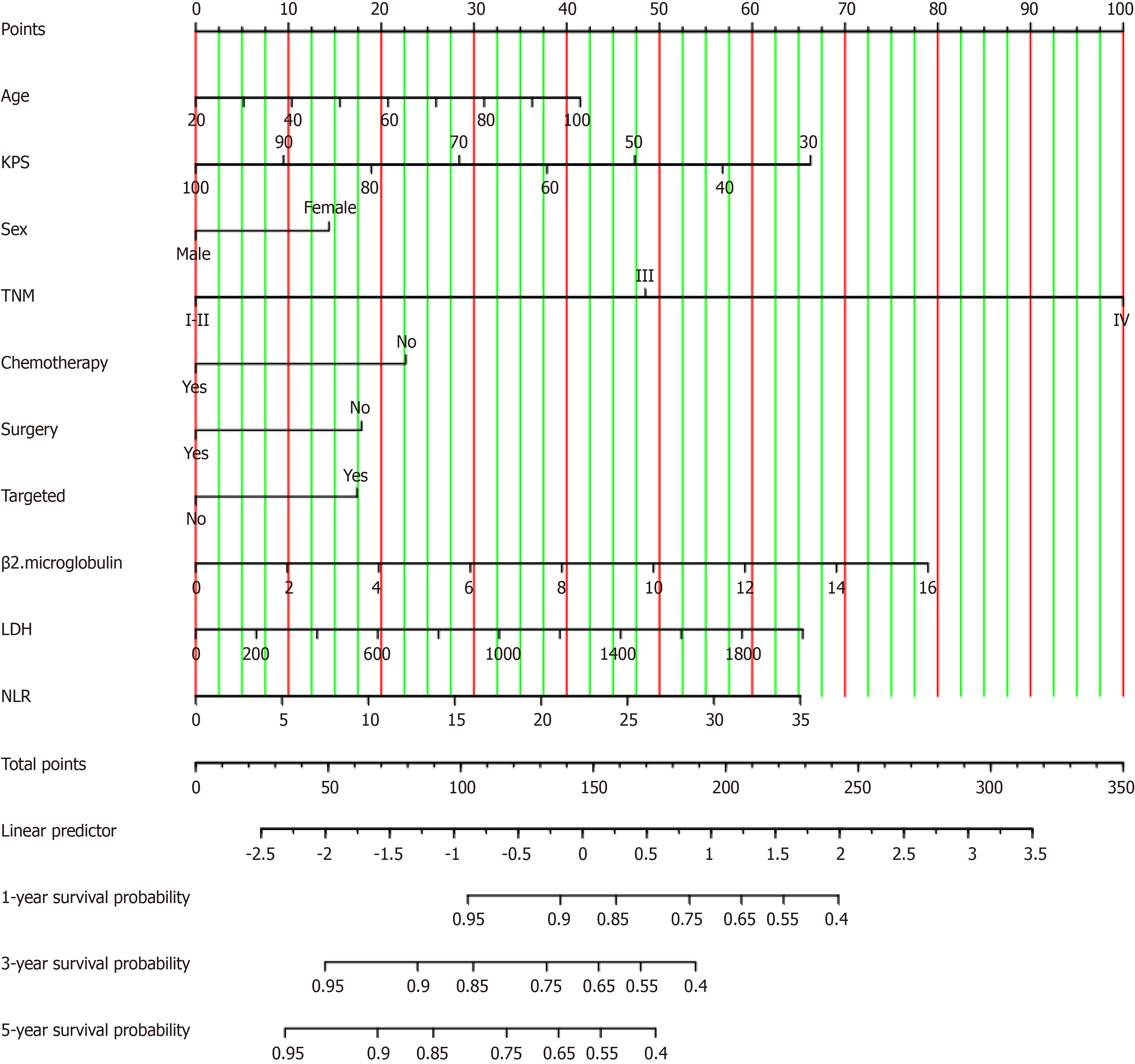
Table 2 presents the results of a Cox regression model that was developed by using the training cohort. Variables such as age, KPS, body mass index, sex, TNM stage, radiation therapy, chemotherapy, surgery, targeted therapy, and various laboratory indicators, including WBC, β2-microglobulin, LDH, PLR, NLR, AGR, and the CD4/CD8 ratio, significantly influenced the OS of patients (P < 0.05) via univariate analysis. In the multivariate analysis, age (HR = 1.01, 95%CI: 1.00-1.02), KPS (HR = 0.98, 95%CI: 0.97-0.99), sex (HR = 1.32, 95%CI: 1.10–1.58), TNM stage III (HR = 2.82, 95%CI: 1.98-4.01), stage IV (HR = 8.16, 95%CI: 5.82-11.44), chemotherapy (HR = 0.63, 95%CI: 0.50-0.79), surgical treatment (HR = 0.70, 95%CI: 0.57–0.86), targeted therapy (HR = 1.44, 95%CI: 1.13-1.84), β2-microglobulin (HR = 1.11, 95%CI: 1.03-1.18), LDH (HR = 1.02, 95%CI: 1.01-1.03), and the NLR (HR = 1.04, 95%CI: 1.02-1.07) were identified as being independent prognostic factors that independently affect the OS of rectal cancer patients.
| Variables | HR (univariable) | HR (multivariable) | Variables | HR (univariable) | HR (multivariable) |
| Age | 1.01 (1.00-1.02, P = 0.006) | 1.01 (1.00-1.02, P = 0.002) | KPS | 0.95 (0.94-0.95, P < 0.001) | 0.98 (0.97-0.99, P < 0.001) |
| BMI | Chemotherapy | ||||
| < 18.5 | ref | No | |||
| 18.5-23.9 | 0.61 (0.44-0.85, P = 0.003) | Yes | 0.64 (0.53-0.76, P < 0.001) | 0.63 (0.50-0.79, P < 0.001) | |
| 24-27.9 | 0.47 (0.33-0.67, P < 0.001) | Surgery | |||
| ≥ 28 | 0.41 (0.25-0.66, P < 0.001) | No | |||
| Sex | Yes | 0.35 (0.29-0.42, P < 0.001) | 0.70 (0.57-0.86, P = 0.001) | ||
| Male | Immunotherapy | ||||
| Female | 1.22 (1.02-1.45, P = 0.033) | 1.32 (1.10-1.58, P = 0.003) | No | ||
| Marital | Yes | 0.65 (0.31-1.38, P = 0.261) | |||
| Married | Targeted | ||||
| Others | 1.23 (0.87-1.74, P = 0.236) | No | |||
| Histology | Yes | 2.12 (1.74-2.58, P < 0.001) | 1.44 (1.13-1.84, P = 0.003) | ||
| Adenocarcinoma | WBC | 1.06 (1.03-1.09, P < 0.001) | |||
| Others | 1.03 (0.38-2.74, P = 0.961) | β2.microglobulin | 1.19 (1.13-1.26, P < 0.001) | 1.11 (1.03-1.18, P = 0.004) | |
| TNM | LDH | 1.01 (1.01-1.02, P < 0.001) | 1.02 (1.01-1.03, P < 0.001) | ||
| I-II | PLR | 1.02 (1.01-1.03, P = 0.002) | |||
| III | 2.43 (1.73-3.43, P < 0.001) | 2.82 (1.98-4.01, P < 0.001) | NLR | 1.07 (1.05-1.09, P < 0.001) | 1.04 (1.02-1.07, P = 0.001) |
| IV | 9.92 (7.32-13.44, P < 0.001) | 8.16 (5.82-11.44, P < 0.001) | LMR | 0.98 (0.97-1.00, P = 0.085) | |
| Radiation | AGR | 0.40 (0.29-0.53, P < 0.001) | |||
| No | CD4/CD8 | 0.89 (0.81-0.99, P = 0.024) | |||
| Yes | 1.67 (1.26-2.22, P < 0.001) |
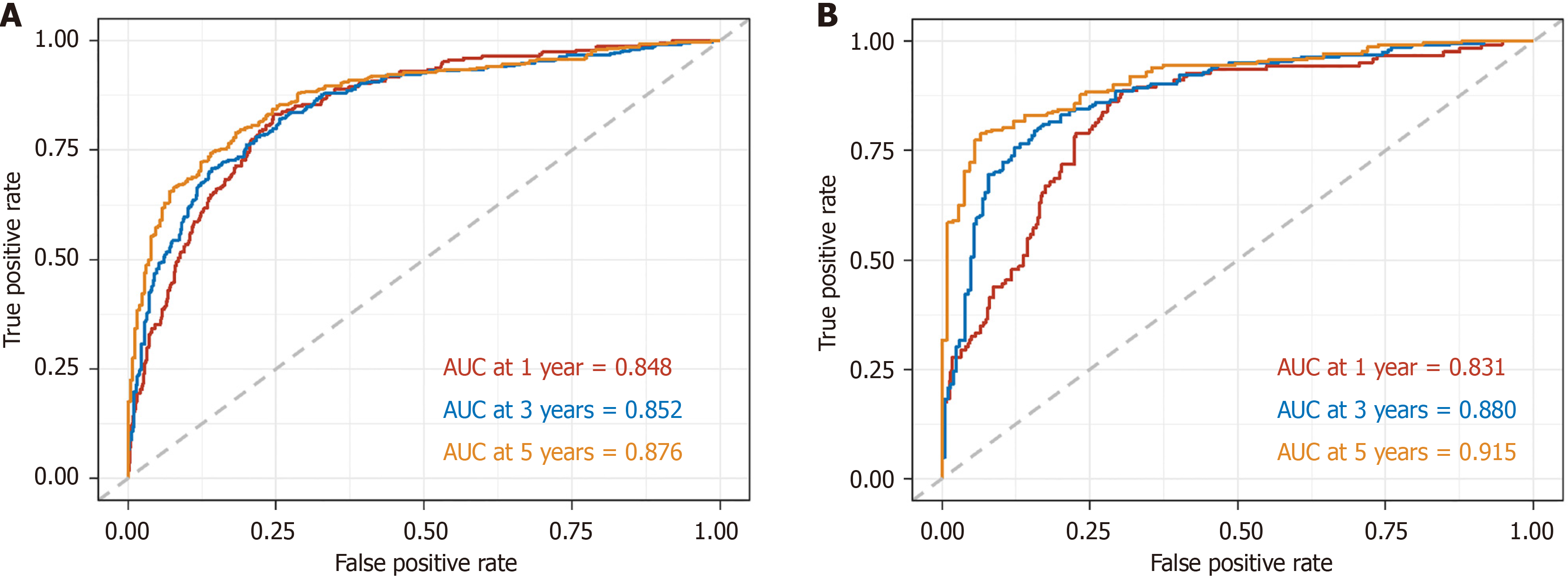
A nomogram model for predicting the 1-year, 3-year, and 5-year OS of patients with rectal cancer was developed based on the statistically significant variables obtained via the multivariate Cox regression analysis (Figure 2). The concordance indices (C-indices) for both the training and validation cohorts were 0.80 (95%CI: 0.78-0.82) and 0.80 (95%CI: 0.77-0.83), respectively. The AUC values for the training cohort at 1 year, 3 years, and 5 years were 0.85 (95%CI: 0.821-0.87), 0.85 (95%CI: 0.83-0.88), and 0.88 (95%CI: 0.85-0.90), respectively. The AUC values for the validation group were 0.83 (95%CI: 0.79-0.97), 0.88 (95%CI: 0.85-0.91), and 0.92 (95%CI: 0.88-0.95), respectively. The ROC curves are illustrated in Figure 3.
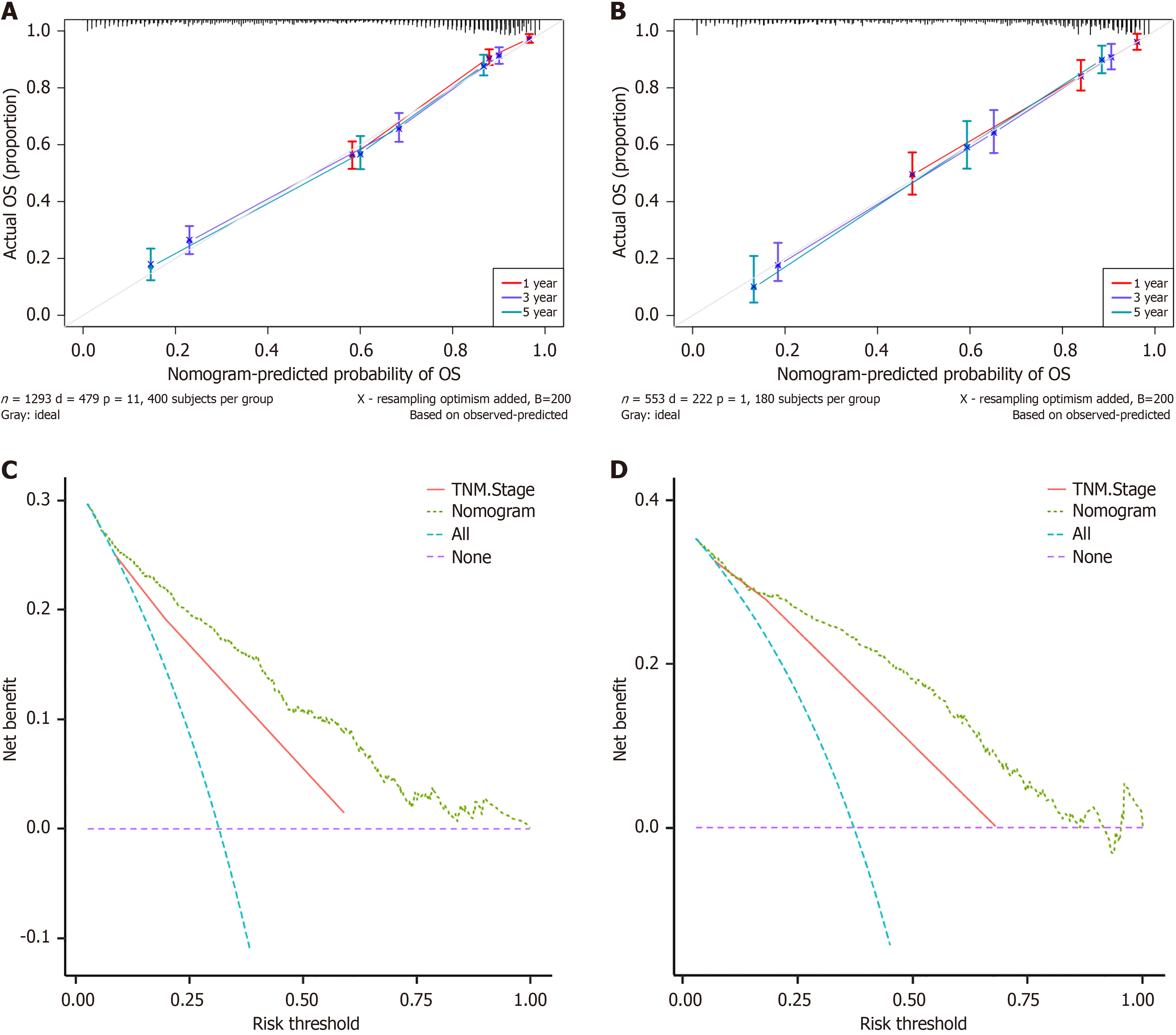
Furthermore, in both the training (Figure 4A) and validation cohorts (Figure 4B), the calibration curves for the 1-year, 3-year, and 5-year survival periods demonstrated strong concordance between the predicted outcomes of the nomogram and the observed results. The comparative performance of the nomogram and TNM staging in predicting 5-year OS was assessed via DCA. In the training cohort (Figure 4C) and validation cohorts (Figure 4D), the nomogram and TNM staging models displayed greater positive net benefit values than did the reference lines (all and none) for predicting OS, as evidenced by the data. The nomogram model consistently outperformed the TNM staging model in terms of net benefits and accuracy of clinical outcome predictions, as evidenced by the higher positioning of the DCA curve of the nomogram model than the DCA curve of the TNM staging model across a wide range. These findings suggest a notable enhancement in the nomogram's capacity to predict the 5-year OS of individuals with rectal cancer.
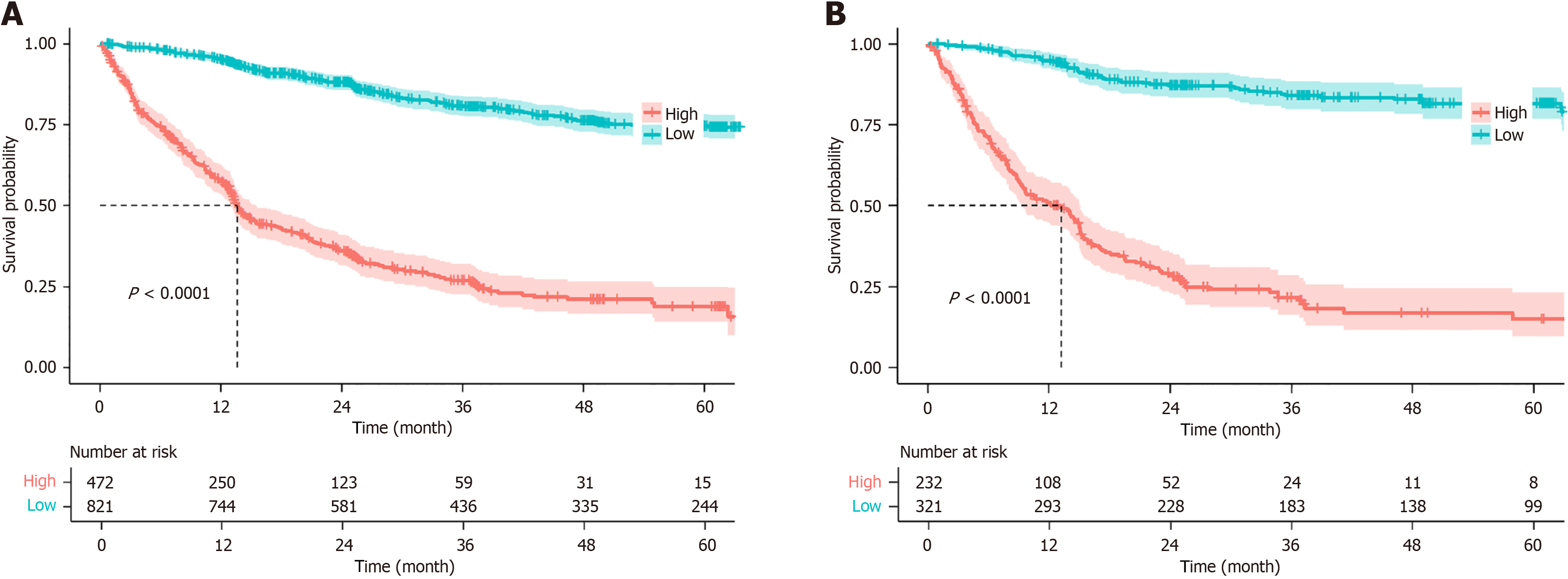
The training and validation cohorts were stratified into low-risk (prognostic risk score below the specified limit) and high-risk (prognostic risk score exceeding the specified limit) categories via the nomogram model. The numbers of patients in the high-risk and low-risk categories for both the training and validation sets were calculated. The Kaplan-Meier survival plots for OS in the training and validation sets revealed a significant difference between the two groups, thus indicating that the model has a robust ability to differentiate between high-risk and low-risk patients (P < 0.001) (Figure 5).
The nomogram predicts OS in patients with rectal cancer, considering both conventional clinical and prognostic factors (such as previous regimens of radiotherapy and chemotherapy, as well as TNM stages) and additional variables such as LDH, NLR, and β2-microglobulin levels, which are particularly significant for this patient cohort. Based on the AUC, this nomogram demonstrated superior predictive ability compared with the TNM staging system, especially at 2 and 3 years postsurgery. The model exhibits excellent calibration, discrimination, and accuracy in predicting clinical outcomes, thus providing valuable insights for clinical decision-making. The AUC values suggest that our nomogram has greater predictive accuracy than the TNM staging system. Moreover, DCA indicated that our model may provide greater benefits than the TNM system in predicting OS for rectal cancer patients.
Prior predictive models for rectal carcinoma, which have included limited tumour markers (CA199 and CEA), were based on relatively small sample sizes[8,9]. Moreover, other studies with larger sample sizes did not incorporate clinical biomarkers[10]. Liu et al[10] developed a nomogram for predicting OS in middle-aged and elderly patients with rectal adenocarcinoma, which exhibited good predictive ability and clinical utility (the C-indices for the training and validation sets were 0.763 and 0.787, respectively). However, this model was predicated on the middle-aged and elderly population aged 45 years and above, thus limiting its predictive performance for younger patients. Our model, which encompasses the entire age range, can accurately determine the prognosis of rectal cancer patients across all age groups[11].
Currently, the TNM classification is widely applied[12]. In the 9th edition of the TNM staging system, TD is recognized as being an unfavourable prognostic factor and is categorized as N1c. TD does not alter the T stage; however, in the absence of regional lymph node metastasis, TD increases the N stage from N0 to N1c[13]. Despite its reliance on disease anatomy, TNM staging remains a mainstay in routine clinical practice. Consistent with previous research, the TNM staging system is a distinct risk factor for favourable prognosis in rectal cancer patients.
A considerable amount of evidence indicates that older age is associated with mortality, particularly in the context of rectal cancer[11,13,14]. Studies have demonstrated a correlation between age and the stage of colorectal cancer, with rectal cancer patients frequently exhibiting age as a predictive and prognostic factor[15,16]. Therefore, it is essential to consider the impacts of these factors on the relationship between age and survival. Consistent with these findings, our results show that older patients (due to multiple health conditions, increased vulnerability, and a higher risk of treatment-related complications) had a greater mortality rate than their younger counterparts.
The prognostic value of performance status, as measured via the KPS, has been consistently recognized in individuals with rectal cancer[17-19]. Our study confirmed that the KPS was significantly associated with OS. Advanced age is associated with increased comorbidity and reduced immunity, thus leading to decreased functional capabilities and lower KPS. Therapeutic strategies, including sphincter-saving surgery, minimally invasive surgery, chemotherapy, and immunotherapy, have been identified as being potential factors associated with mortality[20-22]. These therapeutic factors resulted in a significant increase in the OS rate. The patients in our study who received chemotherapy, surgery, or targeted therapy had markedly improved prognoses compared with those who did not receive these treatments.
This study revealed a significant association between elevated serum β2-microglobulin levels and poor prognosis in individuals with rectal cancer, which is a finding that has not been previously reported. A case-control study by Wang et al[23] detailed the relationship between β2-microglobulin and rectal cancer. The study demonstrated that, after adjusting for baseline clinical features and laboratory parameters, β2-microglobulin was positively correlated with hospitalized rectal cancer patients (OR = 1.32, 95%CI: 1.11-1.58). The role of serum β2-microglobulin in the prediction of various lymphoproliferative disorders and hepatocellular carcinoma has been extensively studied[24-26]. β2-microglobulin, which is the light chain subunit of human leukocyte antigen-I, is crucial for tumour progression because it significantly impacts the immune microenvironment, supports tumour cell populations with stem cell-like traits, enhances migratory and invasive capabilities, stimulates growth, and acts as a signalling factor that promotes bone metastasis[27,28]. Despite its association with human tumours, the significance of β2-microglobulin in rectal cancer remains largely unexplored. Additional investigations are necessary to clarify the exact pathways through which β2-microglobulin enhances the development of rectal cancer.
LDH, which is an isoenzyme that originates in the cytoplasm and is converted to lactic acid during the terminal stage of glycolytic enzymes, is essential for the survival of both normal tissues and malignant tumour cells. LDH levels may indicate the recruitment of tumour-infiltrating cells and inflammatory cells, which can worsen the prognosis of various cancers[29]. Elevated serum LDH levels are often associated with a negative prognosis in various cancers, which is typically due to increased tumour burden and metabolism[30-32]. However, its association with outcomes in patients with rectal cancer has not been previously reported. The findings of this research indicate that increased LDH levels play a crucial role as a prognostic factor in rectal cancer. Prior research has shown that elevated LDH levels are significantly associated with poor prognosis in cancer patients and are included in the nomogram.
As a subset of NLR proteins, the NLR facilitates the function of the inflammasome, which is an effective mechanism for the host response to various pathogens. The NLR has recently demonstrated utility in facilitating clinical endeavours by enhancing the effectiveness of predicting the response to neoadjuvant chemoradiotherapy in individuals with locally advanced rectal cancer[33]. Consistent with these findings, Hamid et al. reported that the preoperative NLR in rectal cancer patients is a powerful predictor of poor prognosis. Its prognostic value is superior to that of other biological standards, such as the LMR[34]. Our results are consistent with those of previous studies, thus indicating that an elevated NLR serves as both a prognostic indicator and a predictive indicator for individuals diagnosed with rectal cancer.
In summary, the ability to predict outcomes demonstrates that we have effectively created and verified an innovative prognostic chart that is tailored for individuals with rectal cancer. This was achieved by incorporating β2-microglobulin, LDH, NLR, the TNM staging system, and clinical characteristics. Moreover, this research highlights the crucial benefit of identifying a new predictive marker (β2-microglobulin) for predicting hepatocellular carcinoma. Due to the convenience of obtaining β2-microglobulin, LDH, and NLR values from regular preoperative laboratory tests, our nomogram is expected to be useful in clinical practice. It serves as a valuable resource for making clinical decisions and administering personalized adjuvant therapy for patients with rectal cancer.
Nevertheless, certain constraints exist in our research. First, this was a single-centre study, and all of the patient data were obtained from one hospital. Therefore, the extrapolation of the nomogram model that was constructed in this study remains to be discussed. Subsequent research can involve cross-hospital collaboration to validate the model's per
Our research revealed robust correlations between increased serum β2-microglobulin levels, LDH levels, the NLR, the TNM staging system, clinical features, and positive prognosis in patients with rectal cancer. We developed a prognostic nomogram for OS in rectal cancer patients, which demonstrated high accuracy and precision. The use of this nomogram simplifies data collection and enhances predictive accuracy. This approach provides a straightforward and reliable tool for predicting survival in individuals with rectal cancer, thus potentially allowing for personalized interventions. Further investigations are needed to explore the mechanisms through which β2-microglobulin and LDH contribute to rectal cancer progression, and validation using multicentre studies is essential.
| 1. | Keller DS, Berho M, Perez RO, Wexner SD, Chand M. The multidisciplinary management of rectal cancer. Nat Rev Gastroenterol Hepatol. 2020;17:414-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4893] [Article Influence: 699.0] [Reference Citation Analysis (1)] |
| 3. | Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, Zadnik V, Pellisé M, Esteban L, Kaminski MF, Suchanek S, Ngo O, Májek O, Leja M, Kuipers EJ, Spaander MC. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68:1820-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 476] [Cited by in RCA: 532] [Article Influence: 88.7] [Reference Citation Analysis (1)] |
| 4. | Yu Z, Bai X, Zhou R, Ruan G, Guo M, Han W, Jiang S, Yang H. Differences in the incidence and mortality of digestive cancer between Global Cancer Observatory 2020 and Global Burden of Disease 2019. Int J Cancer. 2024;154:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 5. | Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 1122] [Article Influence: 187.0] [Reference Citation Analysis (1)] |
| 6. | Yang Y, Wang HY, Chen YK, Chen JJ, Song C, Gu J. Current status of surgical treatment of rectal cancer in China. Chin Med J (Engl). 2020;133:2703-2711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Miao S, Lei H, Li X, Zhou W, Wang G, Sun A, Wang Y, Wu Y. Development and validation of a risk prediction model for overall survival in patients with nasopharyngeal carcinoma: a prospective cohort study in China. Cancer Cell Int. 2022;22:360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Kim CG, Ahn JB, Jung M, Beom SH, Heo SJ, Kim JH, Kim YJ, Kim NK, Min BS, Koom WS, Kim H, Roh YH, Ma BG, Shin SJ. Preoperative Serum Carcinoembryonic Antigen Level as a Prognostic Factor for Recurrence and Survival After Curative Resection Followed by Adjuvant Chemotherapy in Stage III Colon Cancer. Ann Surg Oncol. 2017;24:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Shin JK, Kim HC, Lee WY, Yun SH, Cho YB, Huh JW, Park YA, Chun HK. High preoperative serum CA 19-9 levels can predict poor oncologic outcomes in colorectal cancer patients on propensity score analysis. Ann Surg Treat Res. 2019;96:107-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Liu H, Lv L, Qu Y, Zheng Z, Zhao J, Liu B, Zhang D, Wang H, Zhang J. Prediction of cancer-specific survival and overall survival in middle-aged and older patients with rectal adenocarcinoma using a nomogram model. Transl Oncol. 2021;14:100938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Qu Y, Liu H. Construction of a predictive model for clinical survival in male patients with non-metastatic rectal adenocarcinoma. Asian J Surg. 2023;46:132-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Hecht JR, Hoffe S, Hubbard J, Hunt S, Hussan H, Jeck W, Johung KL, Joseph N, Kirilcuk N, Krishnamurthi S, Maratt J, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Williams G, Algieri F, Gurski L, Stehman K. Anal Carcinoma, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:653-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 13. | Lauricella S, Caricato M, Mascianà G, Carannante F, Carnazza M, Bonaccorso A, Angeletti S, Ciccozzi M, Coppola R, Capolupo GT. Topographic lymph node staging system shows prognostic superiority compared to the 8th edition of AJCC TNM in gastric cancer. A western monocentric experience. Surg Oncol. 2020;34:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Gheybi K, Buckley E, Vitry A, Roder D. Associations of advanced age with comorbidity, stage and primary subsite as contributors to mortality from colorectal cancer. Front Public Health. 2023;11:1101771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Lu S, Chen Z, Peng R, Zhang Q, Wang Y, Li X, Qu R, Zhou X, Fu W, Sun T, Wang H. Prognostic effect of preoperative Controlling Nutritional Status score in patients with locally advanced rectal cancer: A two-center, retrospective study. Nutrition. 2023;112:112078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Guo Z, Li W, Wu K, Fu Y, Yan R, Zhou X. A nomogram based on the log odds of positive lymph nodes for predicting the prognosis of T1 stage rectal cancer. Am J Cancer Res. 2023;13:1498-1508. [PubMed] |
| 17. | Li W, Wang T, Zhu Y, Yu H, Ma L, Ding Y, Hong G, Lei D. Brain metastasis from colorectal cancer: Treatment, survival, and prognosis. Medicine (Baltimore). 2022;101:e30273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Xie H, Ruan G, Zhang H, Zhang Q, Ge Y, Song M, Zhang X, Lin S, Liu X, Liu Y, Zhang X, Li X, Zhang K, Yang M, Tang M, Li Z, Shi H. Association of Modified Geriatric Nutrition Risk Index and Handgrip Strength With Survival in Cancer: A Multi-Centre Cohort Study. Front Nutr. 2022;9:850138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Kato S, Miyoshi N, Fujino S, Minami S, Matsuda C, Yasui M, Ohue M, Sekido Y, Hata T, Ogino T, Takahashi H, Uemura M, Yamamoto H, Doki Y, Eguchi H. Combined Inflammation and Nutrition Factors Reinforce the Prognostic Prediction for Stage III Colorectal Cancer Patients. Anticancer Res. 2022;42:4989-4999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 20. | Dijkstra EA, Nilsson PJ, Hospers GAP, Bahadoer RR, Meershoek-Klein Kranenbarg E, Roodvoets AGH, Putter H, Berglund Å, Cervantes A, Crolla RMPH, Hendriks MP, Capdevila J, Edhemovic I, Marijnen CAM, van de Velde CJH, Glimelius B, van Etten B; Collaborative Investigators. Locoregional Failure During and After Short-course Radiotherapy Followed by Chemotherapy and Surgery Compared With Long-course Chemoradiotherapy and Surgery: A 5-Year Follow-up of the RAPIDO Trial. Ann Surg. 2023;278:e766-e772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 181] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 21. | Jin J, Tang Y, Hu C, Jiang LM, Jiang J, Li N, Liu WY, Chen SL, Li S, Lu NN, Cai Y, Li YH, Zhu Y, Cheng GH, Zhang HY, Wang X, Zhu SY, Wang J, Li GF, Yang JL, Zhang K, Chi Y, Yang L, Zhou HT, Zhou AP, Zou SM, Fang H, Wang SL, Zhang HZ, Wang XS, Wei LC, Wang WL, Liu SX, Gao YH, Li YX. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J Clin Oncol. 2022;40:1681-1692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 262] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 22. | Ni K, Zhan Y, Liu Z, Yuan Z, Wang S, Zhao XZ, Ping H, Liu Y, Wang W, Yan S, Xin R, Han Q, Zhang Q, Li G, Zhang X, Wang G, Zhang Z, Ma H, Zhang C. Survival outcomes in locally advanced dMMR rectal cancer: surgery plus adjunctive treatment vs. surgery alone. BMC Cancer. 2023;23:1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Wang H, Zheng H, Cao X, Meng P, Liu J, Zheng C, Zuo H, Wang Z, Zhang T. β2-microglobulin and colorectal cancer among inpatients: a case-control study. Sci Rep. 2023;13:12222. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Chen Y, Chen Z, Tan X, Zhang Q, Zhou Y, Yuan H, Jiang L. Role of body composition and metabolic parameters extracted from baseline (18)F-FDG PET/CT in patients with diffuse large B-cell lymphoma. Ann Hematol. 2023;102:2779-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 25. | Chen NC, Chang H, Kao HW, Ou CW, Kuo MC, Wang PN, Lin TL, Wu JH, Hung YS, Su YJ, Ong YC, Shih HJ. Beta2-microglobulin is a valuable marker and identifies a poor-prognosis subgroup among intermediate-risk patients with diffuse large B cell lymphoma. Clin Exp Med. 2023;23:3759-3766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Lin Q, Jiang Z, Mo D, Liu F, Qin Y, Liang Y, Cheng Y, Huang H, Fang M. Beta2-Microglobulin as Predictive Biomarkers in the Prognosis of Hepatocellular Carcinoma and Development of a New Nomogram. J Hepatocell Carcinoma. 2023;10:1813-1825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Li D, Zhang Q, Li L, Chen K, Yang J, Dixit D, Gimple RC, Ci S, Lu C, Hu L, Gao J, Shan D, Li Y, Zhang J, Shi Z, Gu D, Yuan W, Wu Q, Yang K, Zhao L, Qiu Z, Lv D, Gao W, Yang H, Lin F, Wang Q, Man J, Li C, Tao W, Agnihotri S, Qian X, Shi Y, You Y, Zhang N, Rich JN, Wang X. β2-Microglobulin Maintains Glioblastoma Stem Cells and Induces M2-like Polarization of Tumor-Associated Macrophages. Cancer Res. 2022;82:3321-3334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 28. | Hofbauer D, Mougiakakos D, Broggini L, Zaiss M, Büttner-Herold M, Bach C, Spriewald B, Neumann F, Bisht S, Nolting J, Zeiser R, Hamarsheh S, Eberhardt M, Vera J, Visentin C, De Luca CMG, Moda F, Haskamp S, Flamann C, Böttcher M, Bitterer K, Völkl S, Mackensen A, Ricagno S, Bruns H. β(2)-microglobulin triggers NLRP3 inflammasome activation in tumor-associated macrophages to promote multiple myeloma progression. Immunity. 2021;54:1772-1787.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 29. | Basile D, Garattini SK, Corvaja C, Montico M, Cortiula F, Pelizzari G, Gerratana L, Audisio M, Lisanti C, Fanotto V, Ongaro E, Iacono D, Cardellino GG, Foltran L, Pella N, Buonadonna A, Aprile G, Di Maio M, Fasola G, Puglisi F. The MIMIC Study: Prognostic Role and Cutoff Definition of Monocyte-to-Lymphocyte Ratio and Lactate Dehydrogenase Levels in Metastatic Colorectal Cancer. Oncologist. 2020;25:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Claps G, Faouzi S, Quidville V, Chehade F, Shen S, Vagner S, Robert C. The multiple roles of LDH in cancer. Nat Rev Clin Oncol. 2022;19:749-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 145] [Reference Citation Analysis (0)] |
| 31. | Miholjcic TBS, Halse H, Bonvalet M, Bigorgne A, Rouanne M, Dercle L, Shankar V, Marabelle A. Rationale for LDH-targeted cancer immunotherapy. Eur J Cancer. 2023;181:166-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 32. | Wu J, You K, Chen C, Zhong H, Jiang Y, Mo H, Song J, Qiu X, Liu Y. High Pretreatment LDH Predicts Poor Prognosis in Hypopharyngeal Cancer. Front Oncol. 2021;11:641682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Shi X, Zhao M, Shi B, Chen G, Yao H, Chen J, Wan D, Gu W, He S. Pretreatment blood biomarkers combined with magnetic resonance imaging predict responses to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Front Oncol. 2022;12:916840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Hamid HKS, Davis GN, Trejo-Avila M, Igwe PO, Garcia-Marín A. Prognostic and predictive value of neutrophil-to-lymphocyte ratio after curative rectal cancer resection: A systematic review and meta-analysis. Surg Oncol. 2021;37:101556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |