Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.100724
Revised: November 1, 2024
Accepted: December 4, 2024
Published online: February 15, 2025
Processing time: 146 Days and 21.5 Hours
The 5-year survival rate for patients with pancreatic cancer (PC) is 4%-12%. Sur
Histopathological analysis of a 53-year-old female PC patient who underwent Whipple surgery revealed poorly differentiated tumor cells infiltrating nerves, lymphatics, and blood vessels. The patient received two different first-line chemo
Immunotherapy and targeted therapy have the potential to increase both the quality of life and survival time of PC patients, particularly those whose tumor progression is not effectively controlled by chemotherapy alone. Never
Core Tip: Pancreatic cancer (PC) is among the most lethal malignancies worldwide, with minimal advancements in treatment over the past three decades. We report the case of a patient with highly malignant PC who demonstrated a poor response to chemotherapy. However, immunotherapy and targeted therapy extended the patient's progression-free survival and improved her quality of life. Immunotherapy and targeted therapy may offer new hope for PC patients.
- Citation: Luo YH, He T, Lin L, Wang RQ, Cai HX, Hu W. Advanced pancreatic cancer treated with camrelizumab combined with apatinib: A case report. World J Gastrointest Oncol 2025; 17(2): 100724
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/100724.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.100724
Pancreatic cancer (PC) is recognized as one of the most lethal malignancies worldwide. According to cancer statistics for China and the United States in 2022, as reported by Xing et al[1], PC represents 2.8% of all newly diagnosed cases and accounts for 4.1% of all cancer-related fatalities among 34 distinct types of cancer. The incidence of PC is increasing by 0.5% to 1.0% annually, and it is projected to become the second leading cause of cancer-related deaths by 2030[2]. Surgery is considered the optimal treatment option for PC. However, unfortunately, only approximately 15%-20% of patients with PC are eligible for pancreatic resection. This is because PC often presents with nonspecific symptoms in its early stages, resulting in a diagnosis of locally advanced stage (30%-35%) or metastatic disease (50%-55%) in the majority of patients[1]. The first-line chemotherapy regimens for PC encompass two primary options. The first regimen consists of gem
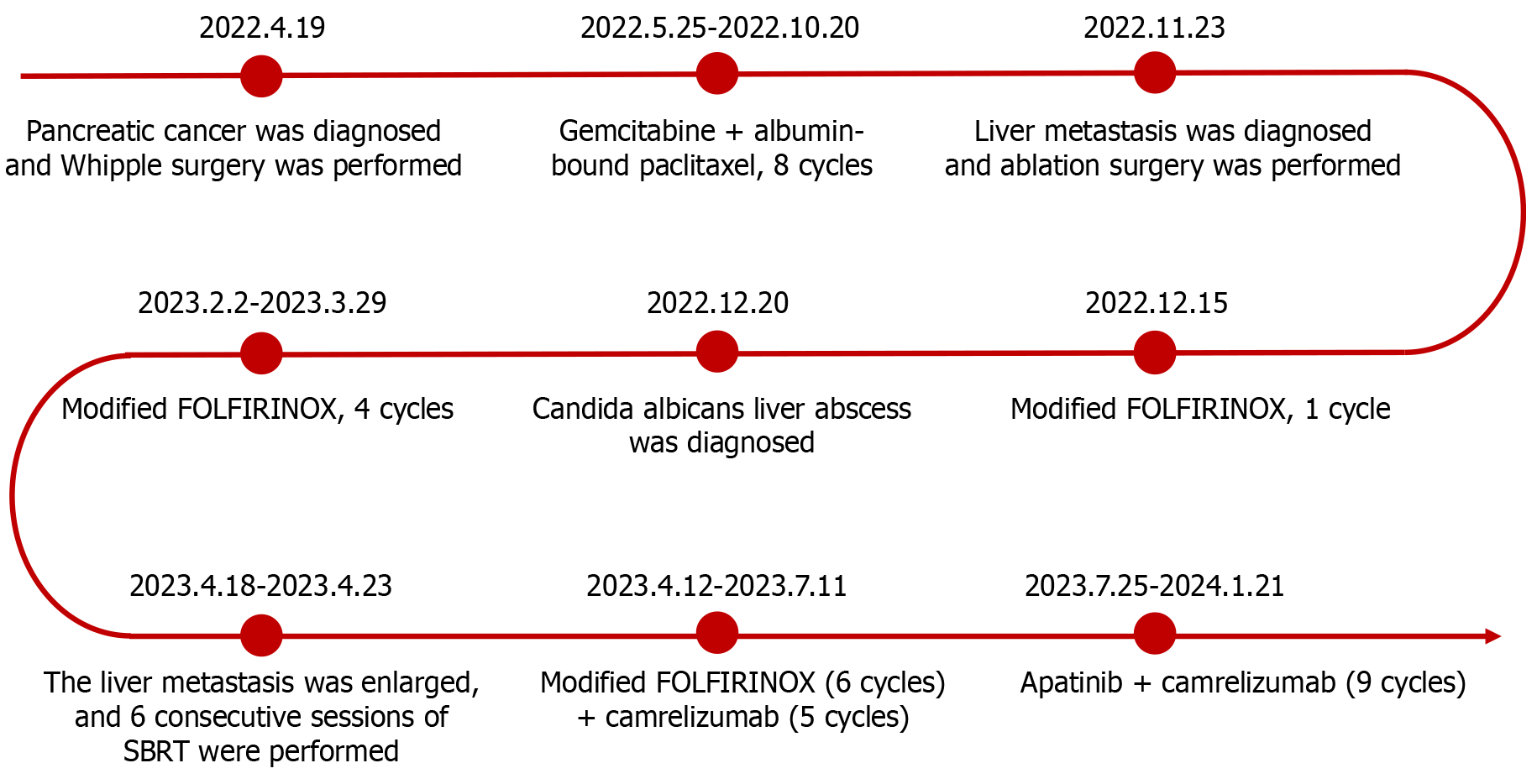
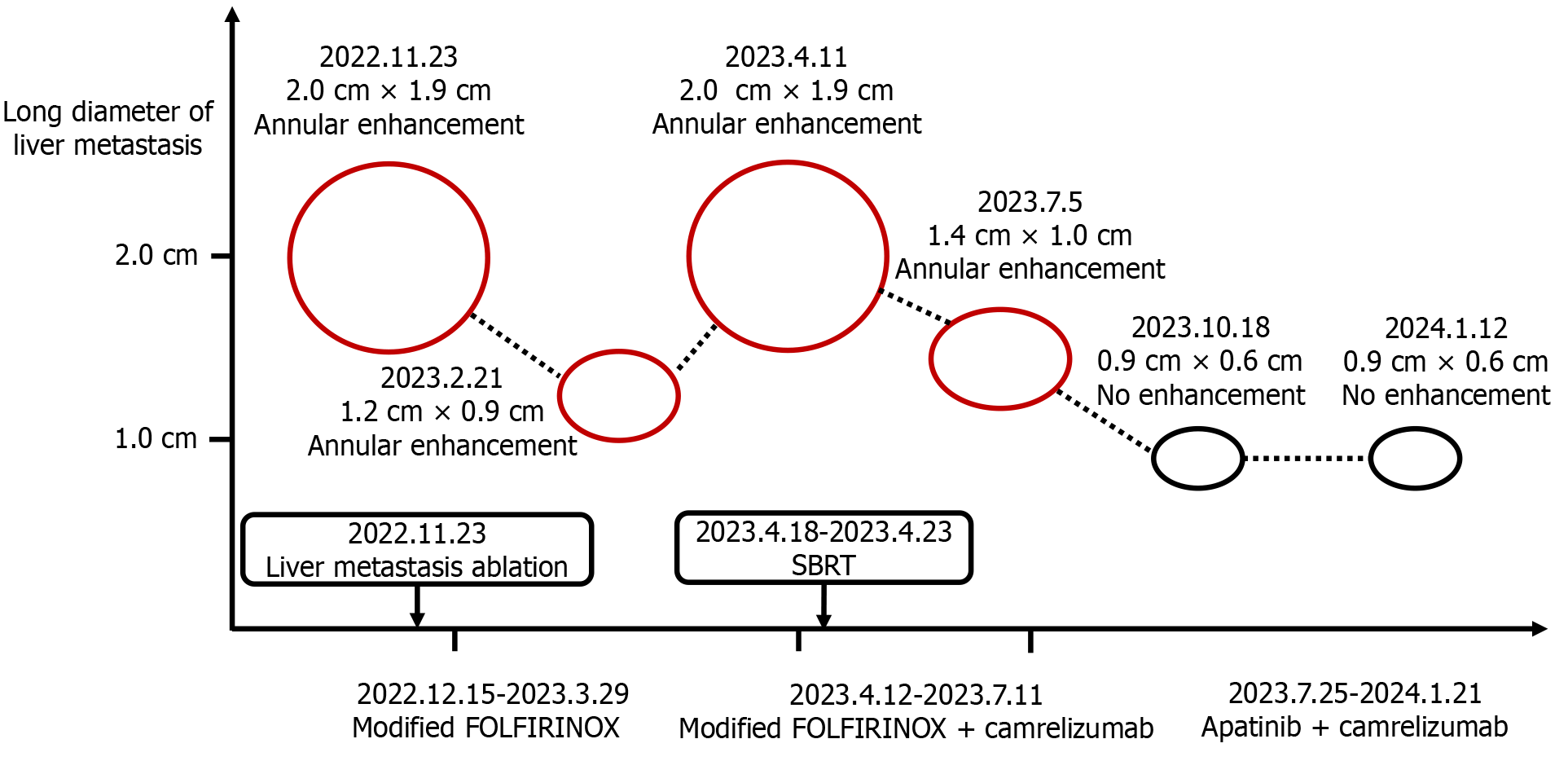
This case report describes a patient with highly aggressive PC who had multiple concurrent adverse prognostic factors, rendering disease progression challenging to control solely with chemotherapy. The combination of immunotherapy and targeted therapy not only extended her progression-free survival (PFS) but also significantly improved her quality of life, ultimately yielding an overall survival (OS) of 2 years. The anticancer regimens administered to the patient at different stages of the disease are depicted in Figure 1, while the imaging changes associated with the liver metastasis are illustra
A 53-year-old female patient was diagnosed with PC in April 2022 and was found to have a liver metastasis in November 2022.
In April 2022, the patient was incidentally diagnosed with PC during hospitalization for a right hand injury. At that time, she did not exhibit any symptoms such as abdominal pain or jaundice. Enhanced abdominal magnetic resonance imaging (MRI) revealed pancreatic head tumor with a diameter of approximately 2.2 cm × 2.0 cm, with no evidence of metastasis. The carbohydrate antigen (CA) 19-9 level was 45.3 U/mL (normal range ≤ 30 U/mL), while the carcinoembryonic antigen (CEA) level was within normal limits. She subsequently underwent Whipple surgery at West China Hospital of Sichuan University. Histopathological analysis revealed pancreatic adenocarcinoma with mucinous adenoma (comprising approximately 5%-10%, some of which exhibited signet ring cell carcinoma morphology), graded as G3/poorly differentiated. The tumor cells infiltrated nerves, lymphatics, and blood vessels, with no tumor cells detected at the resection margins. She was diagnosed with PC at stage T2N1M0. Genetic testing revealed mutations in the KRAS and TP53 genes, whereas no mutations were detected in mismatch repair genes or HER2. After surgery, the CA19-9 level decreased to within the normal range. The patient subsequently received chemotherapy consisting of gemcitabine in combination with albumin-bound paclitaxel at the hospital. From May to October 2022, the patient completed a total of 8 cycles of this chemotherapy regimen. During the entire treatment period, the patient experienced bone marrow suppression, gastrointestinal reac
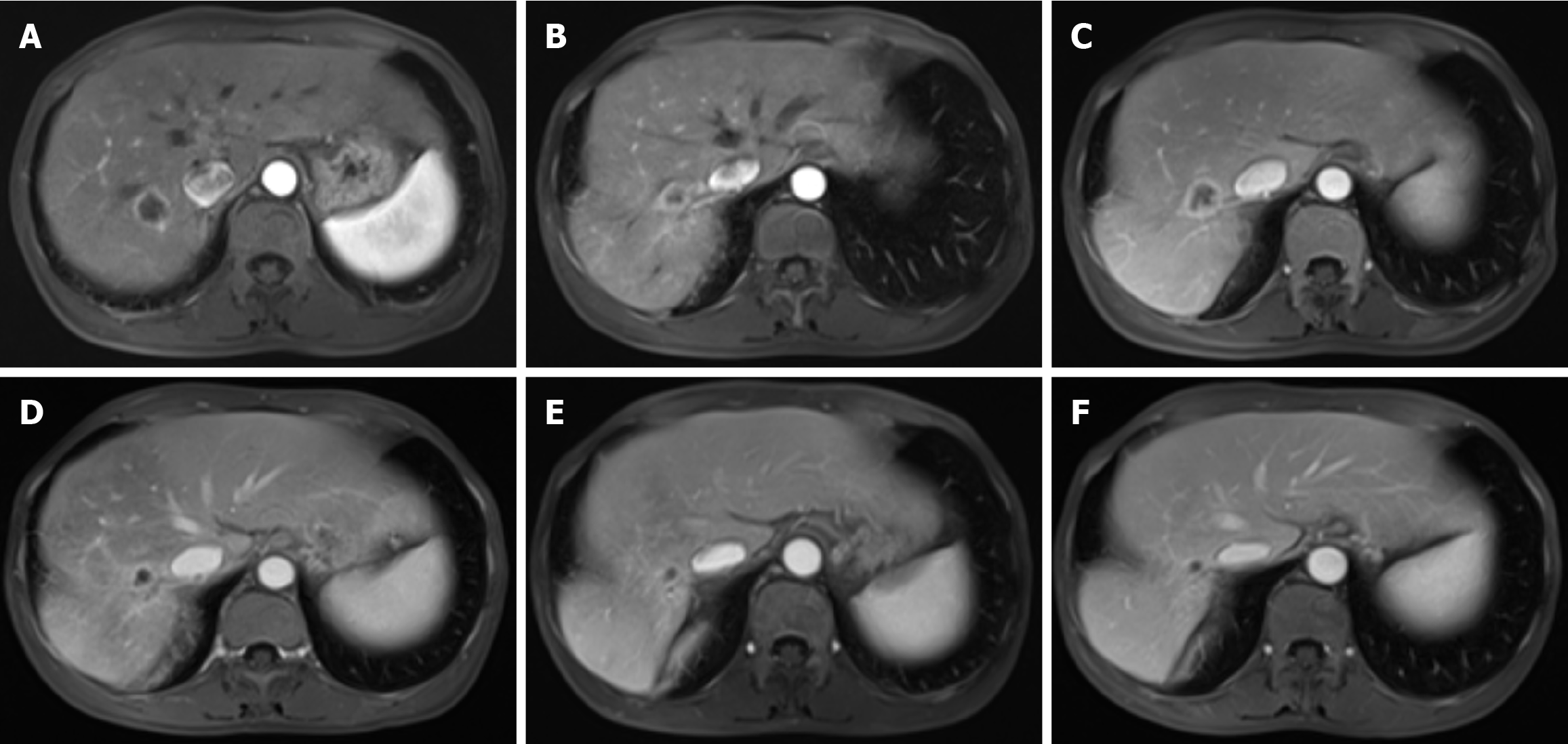
One month after the completion of chemotherapy, the patient's CEA level increased to 9.39 ng/mL (normal range ≤ 5 ng/mL), whereas her CA19-9 level remained within the normal limit. A follow-up enhanced abdominal MRI revealed a nodular lesion in the right lobe of the liver, showing enhancement and a maximum cross-sectional diameter of approximately 2.0 cm × 1.9 cm (Figure 3A). We organized a multidisciplinary consultation for the patient and subsequently performed computed tomography (CT)-guided ablation surgery to treat the liver metastasis. In December 2022, the patient was admitted to the hospital for chemotherapy. CEA and CA19-9 levels were within normal ranges. The patient underwent chemotherapy with modified FOLFIRINOX. Following chemotherapy, the patient gradually experienced fever, abdominal pain, and decreased appetite. Abdominal CT and real-time polymerase chain reaction confirmed the presence of a liver abscess and coronavirus disease 2019. The patient subsequently underwent CT-guided percutaneous catheter drainage to treat the liver abscess. The culture of the pus revealed Candida albicans. The patient recovered after receiving a 5-week course of fluconazole and azvudine treatment. In February 2023, follow-up enhanced MRI revealed a reduction in the diameter of the liver metastatic lesion to 1.2 cm × 0.9 cm with enhancement (Figure 3B). She continued to undergo a modified FOLFIRINOX regimen. During the treatment, the patient experienced bone marrow suppression, gastrointestinal reactions, and elevated liver enzymes. However, the numbness in both feet decreased, and the patient’s hair began to regenerate gradually.
The patient was readmitted to the hospital for chemotherapy.
She had no other known medical conditions.
Her father died of an unknown cancer at the age of 49, and her uncle died of PC at the age of 68.
The patient's vital signs were stable, and her skin and sclera showed no signs of jaundice. There was no tenderness or palpable masses in the abdomen.
The results of the routine blood, renal function, coagulation, and CA 19-9 assays were all within the normal ranges. However, the CEA level increased to 9.89 ng/mL (normal range ≤ 5 ng/mL). Additionally, there was a mild elevation in liver transaminase levels.
Enhanced abdominal MRI revealed a ring-enhancing lesion in the right lobe of the liver, with a maximum cross-sectional diameter of approximately 2.0 cm × 1.9 cm, indicating the recurrence of the ablated liver metastasis (Figure 3C).
Advanced PC.
After recurrence of the liver metastasis was detected, the patient underwent 6 consecutive sessions of stereotactic body radiotherapy targeting the metastasis (GTV 10Gy). The patient subsequently continued modified FOLFIRINOX chemo
In July 2023, contrast-enhanced MRI revealed an enhancing nodular lesion in the right lobe of the liver, with a maximum cross-sectional diameter of approximately 1.4 cm × 1.0 cm (Figure 3D). The patient refused to undergo chemo
In January 2024, the patient gradually began to experience intermittent abdominal pain at night. The CEA level was 8.7 ng/mL (normal range ≤ 5 ng/mL), and the CA19-9 Level was 22.1 U/mL (normal range ≤ 30 U/mL). A follow-up enhanced MRI revealed no recurrence of PC, no changes in the liver metastasis, and no other metastases (Figure 3F). Gastrointestinal endoscopy ruled out immune-mediated enteritis, and mesenteric CT angiography excluded mesenteric thrombosis. Initially, the patient managed the abdominal pain with oral loxoprofen, but the pain progressively worsened. Oral morphine or oxycodone resulted in persistent constipation and vomiting. The patient unilaterally discontinued the use of camrelizumab and apatinib, and steadfastly refused any form of anticancer therapy. In February 2024, the patient was hospitalized at a local hospital and diagnosed with recurrent PC. The cancer had metastasized extensively to the abdominal lymph nodes, liver, and multiple sites in both lungs (as reported by the patient's family; no imaging or relevant reports were provided). She died at home 1 month after discharge.
PC is among the most lethal malignancies worldwide. The incidence of PC is increasing by 0.5% to 1.0% annually, and it is projected to become the second leading cause of cancer-related deaths by 2030[2]. Surgery remains the most effective treatment for PC. However, PC often presents with an insidious onset, and by the time patients experience symptoms such as abdominal pain and jaundice, the disease has typically progressed to an advanced stage. Consequently, only approximately 15%-20% of PC patients are eligible for surgical intervention. Moreover, even among those who undergo pancreatic resection, approximately 60% will experience recurrence or metastasis within one year after surgery[1]. For patients who have undergone surgery for pancreatic ductal adenocarcinoma, the 5-year OS rate is approximately 20%-25%[4]. Overall, the 5-year OS rate for PC patients is only 4%-12%[1,2]. Patients with PC who have positive resection margins, poorly differentiated tumors, large tumors size, lymph node involvement, and KRAS and TP53 mutations have a lower 5-year survival rate[5]. Consequently, there is an urgent need to identify effective treatments for PC.
Currently, there are two first-line chemotherapy regimens for PC: Gemcitabine in combination with albumin-bound paclitaxel, and either FOLFIRINOX or its modified version[3]. The study revealed that, for patients with resected pancrea
In the past 30 years, despite extensive research by medical experts, there has been minimal improvement in the effectiveness of PC treatment compared with other types of cancer. However, recent studies have demonstrated the potential of immunotherapy to improve the prognosis of PC patients. The tumor microenvironment induces high expression of programmed cell death protein 1 (PD-1) on infiltrating T cells, whereas tumor cells express high levels of the PD-1 ligands, PD-L1 and PD-L2. PD-L1 binds to PD-1 on T cells, inhibiting their proliferation and activation, which results in T cells being unable to kill tumor cells. To overcome this, camrelizumab, a humanized monoclonal antibody, specifically binds to PD-1 and blocks its interaction with PD-L1. This allows T cells to regain their ability to fight against tumor immune responses[9]. In the present case, the patient experienced a recurrence of liver metastasis despite undergoing modified FOLFIRINOX chemotherapy for 4 months. However, the tumor was subsequently controlled by the administration of camrelizumab. After more than a year of chemotherapy, the patient expressed a reluctance to continue with further treatments. Consequently, we were compelled to seek a new anti-cancer regimen for this patient. Published studies have demonstrated that immunotherapy and targeted therapy can prolong PFS and OS in patients with PC. However, some trials have failed to observe any beneficial effects of either monotherapy or combination therapy in PC patients. Additionally, others have reported severe side effects, including bone marrow suppression and immune enteritis. These outcomes are significantly influenced by individual patient differences and the specific drugs used[10]. The aggressiveness of PC is based mainly on its ability to form extensive new blood vessels. Apatinib, a targeted antiangiogenic drug, inhibits the tyrosine kinase receptor VEGFR2, thereby reducing tumor angiogenesis. A published study has reported that combination therapy with camrelizumab and apatinib altered the tumor microenvironment in several ways. Specifically, it increases the infiltration and activation of CD8+ cytotoxic T cells. Additionally, this combination therapy alters the M1/M2 ratio of tumor-associated macrophages and decreases the infiltration of T regulatory cells and the presence of chemokine receptor 2-positive monocytes in cancer tissues. Dual anti-PD-1/VEGFR-2 treatment has a durable vascular strengthening effect in hepatocellular carcinoma, which reduces drug resistance to either treatment alone and improves OS[11]. Numerous studies have demonstrated that the combination of camrelizumab and apatinib offers promising efficacy and manageable safety in patients with hepatocellular carcinoma. Additionally, apatinib is effectively inhibits the reactive capillary proliferation induced by camrelizumab[11,12]. After discussion with the patient and her family, the anticancer regimen was modified to incorporate camrelizumab and apatinib. Patient treated with camrelizumab in combination with modified FOLFIRINOX or camrelizumab in combination with apatinib experienced a prolonged PFS and improved quality of life. Unfortunately, the patient unilaterally discontinued the anticancer regimen because of the pain caused by cancer and the adverse effects of opioid analgesics. Furthermore, the patient refused any further anticancer therapeutic regimens. The OS was 2 years.
Immunotherapy and targeted therapy have the potential to increase both the quality of life and survival time of PC patients, particularly those whose tumor progression is not effectively controlled by chemotherapy alone. Nevertheless, further clinical trials are necessary to validate these findings.
Grammar consulting and writing assistance were kindly provided by Ying Liu.
| 1. | Xing L, Lv L, Ren J, Yu H, Zhao X, Kong X, Xiang H, Tao X, Dong D. Advances in targeted therapy for pancreatic cancer. Biomed Pharmacother. 2023;168:115717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021;326:851-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 1083] [Article Influence: 270.8] [Reference Citation Analysis (0)] |
| 3. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2115] [Article Influence: 151.1] [Reference Citation Analysis (3)] |
| 4. | Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh Rde W, Collisson E, Schwartz L, Frankel W, Martin R, Conway W, Truty M, Kindler H, Lowy AM, Bekaii-Saab T, Philip P, Talamonti M, Cardin D, LoConte N, Shen P, Hoffman JP, Venook AP. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 2016;151:e161137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 360] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 5. | Kaushik AC, Wang YJ, Wang X, Wei DQ. Irinotecan and vandetanib create synergies for treatment of pancreatic cancer patients with concomitant TP53 and KRAS mutations. Brief Bioinform. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1945] [Article Influence: 277.9] [Reference Citation Analysis (0)] |
| 7. | Wainberg ZA, Melisi D, Macarulla T, Pazo Cid R, Chandana SR, De La Fouchardière C, Dean A, Kiss I, Lee WJ, Goetze TO, Van Cutsem E, Paulson AS, Bekaii-Saab T, Pant S, Hubner RA, Xiao Z, Chen H, Benzaghou F, O'Reilly EM. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, phase 3 trial. Lancet. 2023;402:1272-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 174] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 8. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5637] [Article Influence: 402.6] [Reference Citation Analysis (1)] |
| 9. | Guo Y, Wang R, Shi J, Yang C, Ma P, Min J, Zhao T, Hua L, Song Y, Li J, Su H. Machine learning-based integration develops a metabolism-derived consensus model for improving immunotherapy in pancreatic cancer. J Immunother Cancer. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Cao D, Song Q, Li J, Jiang Y, Wang Z, Lu S. Opportunities and challenges in targeted therapy and immunotherapy for pancreatic cancer. Expert Rev Mol Med. 2021;23:e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, Kikuchi H, Mamessier E, Aoki S, Ramjiawan RR, Ochiai H, Bardeesy N, Huang P, Cobbold M, Zhu AX, Jain RK, Duda DG. Dual Programmed Death Receptor-1 and Vascular Endothelial Growth Factor Receptor-2 Blockade Promotes Vascular Normalization and Enhances Antitumor Immune Responses in Hepatocellular Carcinoma. Hepatology. 2020;71:1247-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 300] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 12. | Xia Y, Tang W, Qian X, Li X, Cheng F, Wang K, Zhang F, Zhang C, Li D, Song J, Zhang H, Zhao J, Yao A, Wu X, Wu C, Ji G, Liu X, Zhu F, Qin L, Xiao X, Deng Z, Kong X, Li S, Yu Y, Xi W, Deng W, Qi C, Liu H, Pu L, Wang P, Wang X. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |