Published online Jan 15, 2025. doi: 10.4251/wjgo.v17.i1.99153
Revised: September 23, 2024
Accepted: October 15, 2024
Published online: January 15, 2025
Processing time: 108 Days and 1.4 Hours
Pancreatic cancer remains one of the most lethal malignancies worldwide, with a poor prognosis often attributed to late diagnosis. Understanding the correlation between pathological type and imaging features is crucial for early detection and appropriate treatment planning.
To retrospectively analyze the relationship between different pathological types of pancreatic cancer and their corresponding imaging features.
We retrospectively analyzed the data of 500 patients diagnosed with pancreatic cancer between January 2010 and December 2020 at our institution. Pathological types were determined by histopathological examination of the surgical spe
There were 320 (64%) cases of pancreatic ductal adenocarcinoma, 75 (15%) of intraductal papillary mucinous neoplasms, 50 (10%) of neuroendocrine tumors, and 55 (11%) of other rare types. Distinct imaging features were identified in each pathological type. Pancreatic ductal adenocarcinoma typically presents as a hypodense mass with poorly defined borders on computed tomography, whereas intraductal papillary mucinous neoplasms present as characteristic cystic lesions with mural nodules. Neuroendocrine tumors often appear as hypervascular lesions in contrast-enhanced imaging. Statistical analysis revealed significant correlations between specific imaging features and pathological types (P < 0.001).
This study demonstrated a strong association between the pathological types of pancreatic cancer and imaging features. These findings can enhance the accuracy of noninvasive diagnosis and guide personalized treatment approaches.
Core Tip: Understanding the correlation between the pathological types of pancreatic cancer and their corresponding imaging features is crucial for early detection and treatment planning. Different types of pancreatic cancers exhibit distinct imaging characteristics, such as hypodense masses with poorly defined borders for pancreatic ductal adenocarcinoma, cystic lesions with mural nodules for intraductal papillary mucinous neoplasm, and hypervascular lesions for neuroendocrine tumors. Utilization of a combination of computed tomography, magnetic resonance imaging, and endoscopic ultrasound can aid in an accurate diagnosis. This knowledge can significantly improve diagnostic accuracy, inform personalized treatment strategies, and potentially enhance outcomes in patients with pancreatic cancer.
- Citation: Luo YG, Wu M, Chen HG. Retrospective analysis of pathological types and imaging features in pancreatic cancer: A comprehensive study. World J Gastrointest Oncol 2025; 17(1): 99153
- URL: https://www.wjgnet.com/1948-5204/full/v17/i1/99153.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i1.99153
Pancreatic cancer is one of the most formidable challenges in oncology and is the seventh leading cause of cancer-related deaths globally[1]. Despite advancements in medical technologies and treatment modalities, the prognosis of pancreatic cancer remains poor, with a 5-year survival rate of only 9%[2]. This dismal outlook is largely attributed to late-stage diagnosis, when the cancer has often metastasized or has become locally advanced, limiting treatment options and efficacy[3].
The pancreas is a vital organ with both exocrine and endocrine functions. Various types of neoplasms can arise from the pancreas. Pancreatic ductal adenocarcinoma (PDAC) accounts for approximately 85%-90% of all pancreatic cancers, while other types such as intraductal papillary mucinous neoplasms (IPMN), neuroendocrine tumors (NET), and rarer forms constitute the remaining cases[4]. Each pathological type exhibits distinct biological behavior, clinical presentation, and response to treatment, underscoring the importance of accurate diagnosis for optimal patient management[5].
Imaging plays a pivotal role in the diagnosis, staging, and treatment planning of pancreatic cancer. Various imaging modalities, including computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS), have been used to visualize and characterize pancreatic lesions[6]. However, the interpretation of these imaging studies can be challenging owing to the complex anatomy of the pancreas and subtle differences in appearance among various pancreatic pathologies[7].
Recent studies have suggested that certain imaging features correlate with specific pathological types of pancreatic cancer. Yamada et al[8] reported that PDAC typically presents as a hypodense mass with irregular borders on CT, whereas IPMNs often appear as cystic lesions with mural nodules. Similarly, Jeon et al[9] found that NETs frequently demonstrate hypervascular enhancement on contrast-enhanced imaging. These observations suggest the potential for the noninvasive differentiation of pancreatic cancer types based on imaging characteristics.
Despite these promising findings, a comprehensive analysis of the relationship between the pathological types and imaging features of pancreatic cancer, encompassing a large patient cohort and multiple imaging modalities, is lacking. Such analysis could potentially enhance diagnostic accuracy, facilitate earlier detection, and inform personalized treatment strategies. The present study aimed to address this gap by conducting a retrospective analysis of 500 pancreatic cancer cases and correlating the pathological findings with imaging features observed on CT, MRI, and EUS.
This retrospective study was conducted at Xuanhan County People’s Hospital, a tertiary referral center for pancreatic diseases in Sichuan Province, China. The study protocol was approved by the Institutional Review Board (No. 2021-LL-092), and the requirement for informed consent was waived due to the retrospective nature of the study.
We reviewed the medical records of patients diagnosed with pancreatic cancer between January 1, 2010 and December 31, 2020. The inclusion criteria were as follows: (1) Histopathologically confirmed diagnosis of pancreatic cancer; (2) Availability of pre-treatment CT, MRI, and EUS imaging studies; and (3) Age ≥ 18 years. Exclusion criteria included: (1) History of prior pancreatic surgery or neoadjuvant therapy; (2) Presence of other concurrent malignancies; and (3) Incomplete medical records or imaging studies. A total of 500 patients met these criteria and were included in the final analysis.
All pathological specimens were obtained via surgical resection (n = 320) or image-guided biopsy (n = 180). Specimens were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned at 4 μm thickness. All sections were stained with hematoxylin and eosin. Two experienced pathologists independently reviewed all the slides. Any discrepancies were resolved by consensus. Immunohistochemical staining was performed when necessary for a definitive diagnosis. The tumors were classified according to the World Health Organization Classification of Tumors of the Digestive System, 5th Edition (2019).
CT: All CT examinations were performed using a 64-slice multi-detector CT scanner. The scanning protocol was as follows: (1) Non-contrast phase; (2) Pancreatic parenchymal phase (40 seconds after contrast injection); and (3) Portal venous phase (70 seconds after contrast injection). Contrast medium (iohexol, 350 mg/mL) was administered intravenously at a rate of 3-4 mL/s, with the total volume based on body weight (1.5 mL/kg). The scanning parameters were as follows: 120 kVp, 150-300 mAs, a slice thickness of 3 mm, and a reconstruction interval of 2 mm.
MRI: MRI was performed using a 3.0 Tesla system (Ingenia Elition 3.0T; Philips Healthcare, Eindhoven, The Netherlands) with a phased-array body coil. The protocol included: (1) T1-weighted in-phase and out-of-phase sequences; (2) T2-weighted fast spin-echo sequences; (3) Diffusion-weighted imaging (b values: 0, 500, 1000 s/mm²); and (4) Dynamic contrast-enhanced T1-weighted 3D gradient-echo sequences (pre-contrast, arterial, portal venous, and delayed phases). A gadolinium-based contrast agent (gadoteridol, 0.1 mmol/kg) was administered intravenously at a rate of 2 mL/s.
EUS: Experienced endosonographers performed EUS (> 500 pancreatic EUS procedures each) using a linear array echoendoscope (Olympus GF-UCT180; Olympus Medical Systems Corp, Tokyo, Japan). B-mode imaging and color Doppler were used to evaluate pancreatic lesions. Fine-needle aspiration was performed as clinically indicated.
Two radiologists (Luo YG and Wu M, with 10 and 12 years of experience in abdominal imaging, respectively) independently reviewed all imaging studies and were blinded to the pathological diagnosis. Any disagreements were resolved by consensus. The following imaging features were assessed: (1) Tumor location (head/uncinate, body, tail); (2) Tumor size (maximum diameter in cm); (3) Tumor margin (well-defined, ill-defined); (4) Tumor density/signal intensity (compared to normal pancreatic parenchyma); (5) Enhancement pattern; (6) Presence of cystic components; (7) Main pancreatic duct dilatation; (8) Vascular invasion; (9) Lymph node involvement; and (10) Distant metastases. Additional MRI features, such as diffusion restriction and apparent diffusion coefficient were evaluated.
Statistical analysis was performed using SPSS software (version 25.0; IBM Corp., Armonk, NY, United States). Continuous variables are expressed as mean ± SD or median (interquartile range), depending on the distribution. Categorical variables are presented as frequencies and percentages. The normality of continuous variables was assessed using the Shapiro-Wilk test. For continuous variables with normal distribution, one-way analysis of variance (ANOVA) was used to compare differences among pathological types. For non-normally distributed continuous variables, the Kruskal-Wallis test was used. Post hoc analyses were performed using Tukey’s HSD test for normally distributed variables and Dunn’s test for non-normally distributed variables. The χ2 test or Fisher’s exact test was used to compare categorical variables between the different pathological types. To identify imaging features that were significantly associated with specific pathological types, multinomial logistic regression analysis was performed. The odds ratios (ORs) with 95% confidence intervals (CIs) were calculated.
Interobserver agreement for the assessment of imaging features was evaluated using the Cohen’s kappa coefficient for categorical variables and the intraclass correlation coefficient for continuous variables. The strength of agreement was interpreted as follows: Poor (κ < 0.20), fair (κ = 0.21-0.40), moderate (κ = 0.41-0.60), substantial (κ = 0.61-0.80), and excellent (κ > 0.80). Statistical significance was set at P < 0.05 for all analyses. All statistical tests were two-tailed.
In total, 500 patients with pancreatic cancer were included in this study. The mean age of the patients was 62.7 ± 11.3 years (range: 28-89 years), with a slight male predominance (54%, n = 270). The most common symptoms were abdominal pain (68%; n = 340), weight loss (52%; n = 260), and jaundice (35%; n = 175). Table 1 summarizes the baseline characteristics of the study population.
| Characteristic | Value |
| Age (years), mean ± SD | 62.7 ± 11.3 |
| Gender, n (%) | |
| Male | 270 (54) |
| Female | 230 (46) |
| Presenting symptoms, n (%) | |
| Abdominal pain | 340 (68) |
| Weight loss | 260 (52) |
| Jaundice | 175 (35) |
| Nausea/vomiting | 125 (25) |
| Diabetes mellitus (new onset) | 75 (15) |
The distribution of pancreatic cancer types based on histopathological examination is shown in Table 2.
| Pathological type | Cases | Percentage |
| Pancreatic ductal adenocarcinoma | 320 | 64% |
| Intraductal papillary mucinous neoplasms | 75 | 15% |
| Neuroendocrine tumors | 50 | 10% |
| Other rare types | 55 | 11% |
| Acinar cell carcinoma | 20 | 4% |
| Solid pseudopapillary neoplasm | 15 | 3% |
| Mucinous cystic neoplasm | 12 | 2.4% |
| Pancreatoblastoma | 5 | 1% |
| Miscellaneous | 3 | 0.6% |
PDAC: PDAC most commonly presents as a hypodense mass on CT and hypointense on T1-weighted MRI. The key imaging features are listed in Table 3.
| Imaging feature | Percentage | Number of cases |
| Hypodense mass on CT | 92% | 294 |
| Hypointense on T1-weighted MRI | 95% | 304 |
| Ill-defined borders | 85% | 272 |
| Pancreatic duct dilatation | 78% | 250 |
| Hypoenhancement in all phases | 88% | 282 |
| Vascular invasion | 45% | 144 |
| Lymph node involvement | 60% | 192 |
| Hypoechoic mass with irregular margins on EUS | 90% | 288 |
IPMN: IPMN demonstrated distinct imaging features, as shown in Table 4. Of the 75 IPMN cases, 45 (60%) were classified as branch-duct type, 20 (26.7%) as main-duct type, and 10 (13.3%) as mixed-type. The mean size of the cystic lesions was 3.2 ± 1.5 cm (range: 1.0-7.5 cm). Mural nodules were present in 45 cases (60%), with a mean size of 5.3 ± 3.2 mm (range: 2-15 mm). Contrast enhancement of the mural nodules was observed in 41 patients (54.7%). Main pancreatic duct dilatation (> 5 mm) was noted in 49 cases (65.3%), with a mean duct diameter of 7.8 ± 3.5 mm (range: 5.5-22 mm).
| Imaging feature | Percentage | Number of cases |
| Cystic lesions communicating with pancreatic duct | 100% | 75 |
| Mural nodules | 60% | 45 |
| Main pancreatic duct dilatation | 65% | 49 |
| Enhancement of mural nodules on contrast-enhanced CT/MRI | 55% | 41 |
NET: The characteristics of the NETs are summarized in Table 5. Among the 50 NET cases, 30 (60%) were classified as G1, 15 (30%) as G2, and 5 (10%) as G3, based on the World Health Organization 2017 grading system. The mean tumor size was 2.8 ± 1.9 cm (range: 0.8-9.5 cm). Arterial-phase hyperenhancement was observed in 40 cases (80%), with the enhancement pattern becoming more heterogeneous in tumors > 2 cm in size (18/30 cases, 60%). Calcifications were present in 12 cases (24%) and were more common in G2 and G3 tumors (9/20, 45%) than in G1 tumors (3/30, 10%; P = 0.006). On MRI, NETs were typically hyperintense on T2-weighted images (85%; n = 42) and showed restricted diffusion (90%; n = 45).
| Imaging feature | Percentage | Number of cases |
| Hyperenhancement in the arterial phase | 80% | 40 |
| Well-defined borders | 90% | 45 |
| Heterogeneous enhancement in larger tumors (> 2 cm) | 60% | 30 |
| Calcifications | 25% | 12 |
| Hyperintense on T2-weighted MRI | 85% | 42 |
| Restricted diffusion on MRI | 90% | 45 |
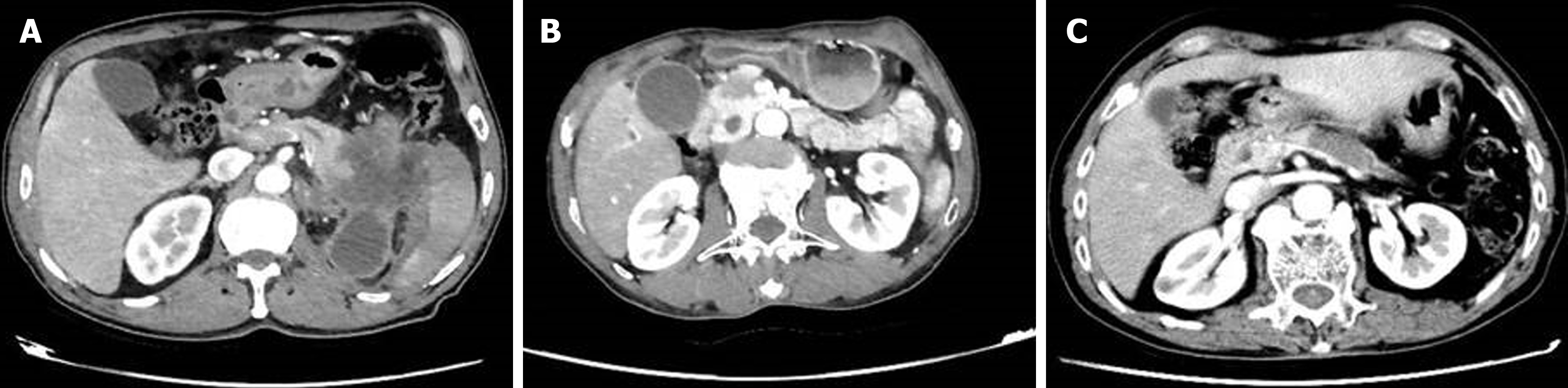
Other rare types: Imaging features varied among the rare types: Acinar cell carcinomas are large well-defined masses with heterogeneous enhancement (Figure 1A). Solid pseudopapillary neoplasms are well-encapsulated masses with heterogeneous solid and cystic components (Figure 1B). Mucinous cystic neoplasms: Unilocular or multilocular cystic lesions with enhanced septation (Figure 1C).
Multinomial logistic regression analysis revealed several imaging features that were significantly associated with specific pathological types (P < 0.001). Ill-defined borders (OR = 15.3, 95%CI: 9.2-25.4) and hypoenhancement (OR = 22.1, 95%CI: 13.5-36.2) were strongly associated with PDAC. Cystic lesions communicating with the pancreatic duct (OR = 185.7, 95%CI: 78.3-440.2) were highly predictive of IPMN. Arterial phase hyperenhancement (OR = 28.6, 95%CI: 14.9-54.8) and well-defined borders (OR = 12.4, 95%CI: 6.7-22.9) were significantly associated with NET. Table 6 summarizes the key imaging features and their associations with the pathological types.
| Imaging feature | PDAC | IPMN | NET | P value |
| Ill-defined borders | 85% | 10% | 10% | < 0.001 |
| Hypoenhancement | 88% | 5% | 15% | < 0.001 |
| Cystic components | 15% | 100% | 10% | < 0.001 |
| Arterial hyperenhancement | 5% | 15% | 80% | < 0.001 |
| Pancreatic duct dilatation | 78% | 65% | 15% | < 0.001 |
The interobserver agreement for the assessment of imaging features is shown in Table 7. Interobserver agreement was substantial to excellent for all evaluated imaging features. The highest agreement was observed for tumor size (intraclass correlation coefficient = 0.92, 95%CI: 0.90-0.94) and vascular invasion (κ = 0.88, 95%CI: 0.84-0.92). The lowest, yet still substantial, agreement was noted for enhancement pattern assessment (κ = 0.79, 95%CI: 0.75-0.83).
| Imaging feature | Agreement measure | Value | 95%CI |
| Tumor size | ICC | 0.92 | 0.90-0.94 |
| Tumor margin | κ | 0.85 | 0.81-0.89 |
| Enhancement pattern | κ | 0.79 | 0.75-0.83 |
| Vascular invasion | κ | 0.88 | 0.84-0.92 |
| Lymph node involvement | κ | 0.82 | 0.78-0.86 |
This comprehensive retrospective study of 500 pancreatic cancer cases provides valuable insights into the relationship between the pathological types and imaging features of pancreatic neoplasms. Our findings demonstrate distinct imaging characteristics associated with different pathological types, which could potentially enhance the accuracy of noninvasive diagnosis and inform treatment strategies[10].
Our results confirmed and extended previous observations regarding the imaging features of PDAC. The high frequency of ill-defined borders (85%) and hypoenhancement (88%) in our cohort aligned with that in earlier studies[11,12]. However, our study provided a more precise quantification of these features in a larger cohort. The strong association between these imaging characteristics and PDAC (OR = 15.3, ill-defined borders; OR = 22.1, hypoenhancement) suggests that these features could serve as important radiological markers of PDAC[13].
The high prevalence of pancreatic duct dilatation (78%) in PDAC in our study is particularly noteworthy. This finding supports the “double duct sign”[14] as a potential early indicator of pancreatic head tumors. Our results suggest that this sign may be more common than previously reported, emphasizing its diagnostic value. The imaging features of IPMN in our study, particularly the universal presence of cystic lesions communicating with the pancreatic duct, corroborated the findings of Lee et al[15]. However, our observation of mural nodules in 60% of the IPMN cases was higher than the 45% reported in a previous study[16]. This discrepancy may be due to the improved resolution of the current imaging techniques or differences in patient populations[17].
The strong association between cystic lesions communicating with the pancreatic duct and IPMN (OR = 185.7) underscores the diagnostic significance of this feature. This finding could aid in differentiating IPMN from other cystic pancreatic lesions, potentially reducing the need for unnecessary invasive procedures[18]. Our findings regarding NET imaging features, particularly the high frequency of arterial-phase hyperenhancement (80%) and well-defined borders (90%), were consistent with those reported by Zhang et al[19]. However, our study provided additional quantitative data on the strength of these associations (OR = 28.6; arterial hyperenhancement; OR = 12.4; well-defined borders). These data may be valuable for developing more precise imaging criteria for the diagnosis of pancreatic NETs[20].
The observation of calcification in 25% of NETs in our study is a novel finding that warrants further investigation. This feature could serve as an additional diagnostic marker for NETs, especially in cases where other imaging characteristics are equivocal. The strong association between specific imaging features and the pathological types demonstrated in this study has several important clinical implications. First, our findings could contribute to the development of more accurate imaging-based diagnostic algorithms for pancreatic cancer, potentially reducing the need for invasive diagnostic procedures, particularly for IPMN and NET. Second, the imaging features identified in our study could aid in the risk stratification of pancreatic lesions. For instance, the presence of mural nodules in IPMN has been associated with a higher risk of malignant transformation. The high detection rate of mural nodules (60%) in our study suggests that careful evaluation of this feature could improve risk assessment in patients with IPMN. Third, the ability to predict the pathological types based on imaging features can inform treatment decisions. For example, the identification of imaging features suggestive of NET might prompt the consideration of targeted therapies or more conservative management approaches than those typically used for PDAC. Finally, our findings regarding early signs of pancreatic cancer, such as the high prevalence of pancreatic duct dilatation in PDAC, could inform the development of more effective screening and surveillance protocols for high-risk individuals.
Our study has several strengths, including large sample size, comprehensive imaging evaluation using multiple modalities, and robust statistical analysis. The high inter-observer agreement for key imaging features supports the reliability and reproducibility of our findings. Our study also has several limitations. First, the retrospective nature of our study introduced potential selection bias and limited our ability to control for all confounding factors. The data collected were based on clinical records and imaging studies performed as part of routine care, which may have led to inconsistencies in imaging protocols or reporting. Second, as our findings are based on data from a single tertiary care center, generalizability to other settings or patient populations may be limited. Different institutions may have varying patient demographics, risk factors, or prevalence of pancreatic cancer subtypes, which could influence the imaging-pathology correlations we observed. Third, given the 10-year span of our study, advances in imaging technology may have influenced the detection and characterization of certain features. The evolution of CT and MRI scanners as well as improvements in imaging protocols could have affected the consistency of our imaging data over time. Fourth, we did not have access to genetic information that could have provided additional insights into the relationship between imaging features, pathological types, and underlying genetic alterations. The integration of genomic data with imaging and pathological findings is an important area for future research. Fifth, our study design did not allow for subgroup analyses of specific pancreatic cancer types, such as different PDAC variants or IPMN grades. Such analyses could potentially reveal additional patterns and refine our understanding of imaging-pathology correlations. Sixth, we did not incorporate newer imaging techniques such as PET/CT or advanced MRI sequences (e.g., diffusion-weighted imaging and dynamic contrast-enhanced MRI) in our analysis. These modalities can provide more valuable information for the characterization of pancreatic lesions. Seventh, our study lacked a longitudinal component to track the changes in imaging features over time. which could provide insights into disease progression and treatment response that were not captured in our cross-sectional analysis. Finally, although our inter-observer agreement for imaging features was high, the subjective nature of some imaging assessments could not be completely eliminated. The use of computer-aided diagnostic tools or radiomics approaches in future studies could potentially reduce this subjectivity.
Although our retrospective study provides valuable insights, we acknowledge the need for prospective studies to further validate our findings and assess their clinical impact. Future prospective, multicenter studies with standardized imaging protocols and pathological assessments would be instrumental in confirming the reliability and generalizability of the identified imaging-pathology correlations. Such studies could also evaluate the impact of these correlations on clinical decision-making, patient outcomes, and potential early detection strategies for pancreatic cancer.
An important area for future research is the longitudinal tracking of imaging features to understand disease progression and treatment responses. Such studies could provide valuable insights into the dynamic changes in imaging characteristics over time, potentially allowing earlier detection of disease progression or treatment resistance. This approach could be particularly valuable in monitoring patients with premalignant lesions, such as IPMN, or in assessing the treatment response in patients with advanced pancreatic cancer. Incorporating radiomics and artificial intelligence techniques into these longitudinal studies could further enhance our ability to detect subtle changes in imaging features that may have prognostic or predictive value.
Our comprehensive analysis of the relationship between pathological types and imaging features of pancreatic cancer provides valuable insights that could significantly impact clinical practice. By enhancing our ability to non-invasively characterize pancreatic lesions, these findings have the potential to improve early diagnosis, guide personalized treatment strategies, and ultimately improve outcomes in patients with pancreatic cancer.
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13172] [Article Influence: 1881.7] [Reference Citation Analysis (4)] |
| 2. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1520] [Article Influence: 253.3] [Reference Citation Analysis (1)] |
| 3. | Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1397] [Cited by in RCA: 1734] [Article Influence: 192.7] [Reference Citation Analysis (1)] |
| 4. | Younan G. Pancreas Solid Tumors. Surg Clin North Am. 2020;100:565-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 5. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1677] [Article Influence: 335.4] [Reference Citation Analysis (1)] |
| 6. | Rana SS. Evaluating the role of endoscopic ultrasound in pancreatitis. Expert Rev Gastroenterol Hepatol. 2022;16:953-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 7. | McKinney M, Griffin MO, Tolat PP. Multimodality Imaging for the Staging of Pancreatic Cancer. Surg Oncol Clin N Am. 2021;30:621-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 8. | Yamada Y, Mori H, Matsumoto S, Kiyosue H, Hori Y, Hongo N. Pancreatic adenocarcinoma versus chronic pancreatitis: differentiation with triple-phase helical CT. Abdom Imaging. 2010;35:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 9. | Jeon SK, Lee JM, Joo I, Lee ES, Park HJ, Jang JY, Ryu JK, Lee KB, Han JK. Nonhypervascular Pancreatic Neuroendocrine Tumors: Differential Diagnosis from Pancreatic Ductal Adenocarcinomas at MR Imaging-Retrospective Cross-sectional Study. Radiology. 2017;284:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 10. | Singhi AD, Koay EJ, Chari ST, Maitra A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology. 2019;156:2024-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 511] [Article Influence: 85.2] [Reference Citation Analysis (1)] |
| 11. | Lee SM, Katz MH, Liu L, Sundar M, Wang H, Varadhachary GR, Wolff RA, Lee JE, Maitra A, Fleming JB, Rashid A, Wang H. Validation of a Proposed Tumor Regression Grading Scheme for Pancreatic Ductal Adenocarcinoma After Neoadjuvant Therapy as a Prognostic Indicator for Survival. Am J Surg Pathol. 2016;40:1653-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 12. | Srisajjakul S, Prapaisilp P, Bangchokdee S. CT and MR features that can help to differentiate between focal chronic pancreatitis and pancreatic cancer. Radiol Med. 2020;125:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 13. | Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4541] [Cited by in RCA: 5561] [Article Influence: 617.9] [Reference Citation Analysis (3)] |
| 14. | Sinha R, Gardner T, Padala K, Greenaway JR, Joy D. Double-Duct Sign in the Clinical Context. Pancreas. 2015;44:967-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 15. | Lee JH, Kim Y, Choi JW, Kim YS. KRAS, GNAS, and RNF43 mutations in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Springerplus. 2016;5:1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 16. | Hashimoto D, Satoi S, Yamamoto T, Yamaki S, Ishida M, Hirooka S, Shibata N, Boku S, Ikeura T, Sekimoto M. Long-term outcomes of patients with multifocal intraductal papillary mucinous neoplasm following pancreatectomy. Pancreatology. 2022;22:1046-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 17. | Ohtsuka T, Fernandez-Del Castillo C, Furukawa T, Hijioka S, Jang JY, Lennon AM, Miyasaka Y, Ohno E, Salvia R, Wolfgang CL, Wood LD. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology. 2024;24:255-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 140] [Article Influence: 140.0] [Reference Citation Analysis (1)] |
| 18. | Attiyeh MA, Fernández-Del Castillo C, Al Efishat M, Eaton AA, Gönen M, Batts R, Pergolini I, Rezaee N, Lillemoe KD, Ferrone CR, Mino-Kenudson M, Weiss MJ, Cameron JL, Hruban RH, D'Angelica MI, DeMatteo RP, Kingham TP, Jarnagin WR, Wolfgang CL, Allen PJ. Development and Validation of a Multi-institutional Preoperative Nomogram for Predicting Grade of Dysplasia in Intraductal Papillary Mucinous Neoplasms (IPMNs) of the Pancreas: A Report from The Pancreatic Surgery Consortium. Ann Surg. 2018;267:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 19. | Zhang L, Sanagapalli S, Stoita A. Challenges in diagnosis of pancreatic cancer. World J Gastroenterol. 2018;24:2047-2060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 272] [Cited by in RCA: 372] [Article Influence: 53.1] [Reference Citation Analysis (12)] |
| 20. | Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 752] [Article Influence: 50.1] [Reference Citation Analysis (2)] |