Published online Jan 15, 2025. doi: 10.4251/wjgo.v17.i1.98725
Revised: September 30, 2024
Accepted: November 1, 2024
Published online: January 15, 2025
Processing time: 106 Days and 1.5 Hours
Gastric cancer (GC) is a prevalent malignancy with a substantial health burden and high mortality rate, despite advances in prevention, early detection, and treatment. Compared with the global average, Asia, notably China, reports disproportionately high GC incidences. The disease often progresses asymptoma
To investigate coagulation and fibrinogen products in GC tumor-node-metastasis (TNM) stage and metastasis correlation.
Retrospectively analyzed the clinical data of 148 patients with GC treated at the Civil Aviation Shanghai Hospital between December 2022 and December 2023. The associations of coagulation indices - partial thromboplastin time (APTT), prothrombin time (PT), thrombin time (TT), fibrinogen, fibrinogen degradation products (FDP), fasting blood glucose, and D-dimer (D-D) with TNM stage and distant metastasis were examined.
Prolongation of APTT, PT, and TT was significantly correlated with the GC TNM stage. Hence, abnormal coagulation system activation was closely related to disease progression. Elevated FDP and D-D were significantly associated with distant metastasis in GC (P < 0.05), suggesting that increased fibrinolytic activity contributes to increased metastatic risk.
Our Results reveal coagulation indices, FDPs as GC biomarkers, reflecting abnormal coagulation/fibrinolysis, aiding disease progression, metastasis prediction, and helping clinicians assess thrombotic risk for early intervention and personalized treatment plans.
Core Tip: Coagulation indices (associations of coagulation indices - partial thromboplastin time, prothrombin time, thrombin time) and fibrinogen degradation products (FDP and D-dimer) are significantly correlated with tumor-node-metastasis stage and distant metastasis in gastric cancer. These biomarkers are indicative of abnormal coagulation and fibrinolytic states and provide essential insights into disease progression. Understanding these associations will enhance diagnostic precision and facilitate the development of personalized treatment strategies for patients with gastric cancer.
- Citation: Shen YQ, Wei QW, Tian YR, Ling YZ, Zhang M. Coagulation indices and fibrinogen degradation products as predictive biomarkers for tumor-node-metastasis staging and metastasis in gastric cancer. World J Gastrointest Oncol 2025; 17(1): 98725
- URL: https://www.wjgnet.com/1948-5204/full/v17/i1/98725.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i1.98725
Gastric cancer (GC), a gastrointestinal cancer with a high global incidence, poses a crucial health challenge worldwide[1-3]. China is one of the countries with the highest incidence of GC globally, accounting for approximately 50% of GC cases[4]. In China, GC is the second most common cancer in men and the third most common cancer in women. Furthermore, its mortality rate is the second highest among cancer-related deaths worldwide[5].
Owing to the lack of specific clinical symptoms, signs, and highly sensitive diagnostic methods in the early stages of GC, metastatic tendencies and microscopic metastatic lesions cannot be detected in a timely manner. Hence, most patients are diagnosed at advanced or locally advanced stages, which is the optimal time to undergo radical surgery. However, advanced GC is typically accompanied by metastases from other sites, adding significant complexity and challenges to clinical treatment[6]. Therefore, early diagnosis and preoperative risk assessment are the key issues in GC management.
Clinically, it has been found that prothrombin time (PT), partial thromboplastin time (APTT), FBG, D-dimer (D-D), and antithrombin-III (AT-III) are of great significance for evaluating the risk of thrombosis in patients and guiding treatment. At present, the relationship between coagulation index and cancer progression is not clear, which may involve various pathophysiological mechanisms. Tumor cells can disrupt the dynamic balance of the coagulation-fibrinolytic system via multiple pathways, affecting coagulation and anticoagulation, thereby leading to abnormalities in coagulation functions in patients. Tumors, especially those with concurrent hyperfibrinogenemia, often exhibit a hyperfibrinolytic system and a hypercoagulable state[7]. Blood hypercoagulability is a pathological state in which blood is highly susceptible to clotting due to an imbalance in the coagulation, hemostasis, anticoagulation, and fibrinolytic systems caused by various factors[8]. The hypercoagulable state in patients with malignant tumors is multifactorial and includes direct expression of tissue factors and tumor procoagulant proteins, alterations in the fibrinolytic system, cytokine secretion, vascular endothelial growth factor release, and endothelial cell damage due to tumor-cell-blood-cell interactions[9]. Collectively, these mechanisms result in abnormal coagulation and elevated FBG levels. In this study, APTT, PT, and TT levels were significantly correlated with tumor-node-metastasis (TNM) stage and distant metastasis in patients with GC. PT primarily reflects the common pathway of the coagulation cascade and the exogenous coagulation pathway, whereas APTT primarily reflects the common pathway of the coagulation cascade and the endogenous coagulation pathway. TT primarily reflects FBG conversion to fibrin[10].
Coagulation activation may cause thrombosis and promote tumor growth and metastasis through various mechanisms. GC is often associated with varying degrees of coagulation abnormalities, including hyperfibrinolysis and hypercoagulable states[11]. The mechanisms by which GC leads to abnormal coagulation indices are multifaceted. Tumor cells release procoagulant substances, such as tissue factors and cancer procoagulants, which can directly activate the coagulation system and lead to blood coagulation. In addition, GC cells can also promote intravascular coagulation by interacting with and activating platelets and enhancing their adhesion ability. This triggers a hypercoagulable state, which not only increases the risk of thrombosis but also accelerates tumor progression and metastasis and may also affect the therapeutic strategy and prognosis. Collectively, these mechanisms result in abnormal coagulation and elevated FBG levels.
For patients with advanced GC, anticoagulant therapy may help to improve hypercoagulability, reduce tumor metastasis, and possibly improve patient survival. The study aims to delve deeper into these mechanisms and explore the specific ways in which coagulation indices and fibrinogen degradation products (FDP) interact with the TNM stage and distant metastasis. Unlike previous studies that may have focused on individual coagulation factors or limited aspects of the disease, adopting a comprehensive approach to analyze multiple coagulation indices simultaneously. This study is of great significance for optimizing diagnostic methods and developing individualized treatment plans.
A retrospective analysis was performed to select 148 patients with GC who were treated at the Civil Aviation Shanghai Hospital from December 2022 to December 2023, and general data and clinical indices of the patients were collected. The inclusion criteria were as follows: (1) Initial pathological diagnosis of primary GC; and (2) No previous history of radiotherapy. Exclusion criteria were as follows: (1) Previous history of other tumors; (2) A combination of functional coagulation diseases or the use of coagulation drugs in the last six months; and (3) A combination of multiple organ functional disorders. This study was reviewed and approved by the Institutional Review Board of the Civil Aviation Shanghai Hospital.
General data, past medical history, and clinical indicators of patients were collected. Gastroscopy was performed to examine the tumor sites, including the cardia, fundus, gastric body, gastric sinus, pylorus, and whole stomach. In the early morning of the preoperative period, 2 mL of venous blood was collected from patients on an empty stomach. Electrochemical hemagglutination analyzer (Model: OGE-101, manufacturer: Hunan Wandeshan Biotechnology Co., LTD.) for the detection of PT, APTT, TT, AT-III, D-D, FDP, and FBG. TNM staging was performed according to the eighth edition of the American Joint Committee on Cancer GC staging criteria[12]. GC was divided into stages I, II, III, and IV: T stage: Unknown (Tx), carcinoma in situ (Tis), submucosal (T1), intrinsic muscular layer (T2), plasma layer (T3), and extra-plasma membrane (T4). N stage: Unknown (Nx), none (N0), 1-2 (N1), 3-6 (N2), 7 or more (N3). M stage: Unknown (Mx), absent (M0), and present (M1).
Statistical software SPSS26.0 was used for statistical analysis. When the data were normally distributed, the mean standard deviation (mean ± SD) was used to represent the measurement data. An independent samples t-test was used for analysis between groups, whereas one-way ANOVA test was used for comparison between multiple groups. The sample size (percentage) [n (%)] indicated the counting data, and χ2 test was used for the analysis of one-way factors. Pearson's correlation analysis was used for the normally distributed data. For multivariate analyses, a logistic regression model was used to adjust for potential confounders, such as age, sex, and comorbidities. To assess the correlation between TNM stages and coagulation and FDP, as well as the correlation of distant metastasis with these factors, Pearson's correlation analysis was used. The correlation coefficients (r) and corresponding P values were calculated to determine the strength and significance of the correlations. A logistic regression model was built, including variables such as age, sex, and comorbidities, to adjust for their effects on the relationship between coagulation indices, FDP, TNM stage, and distant metastasis. The results of the multivariate analysis were presented as odds ratios (ORs) with 95%CI. All differences were considered statistically significant at P < 0.05.
In this study, 148 patients with primary GC were included and were diagnosed at the following stages: 13 with TNM stage I, 38 with stage II, 40 with stage III, 57 with stage IV, and 57 with distant metastasis, with a distant metastasis rate of 38.51%. No statistically significant difference was noted between the baseline data of patients with GC with and without distant metastases (P < 0.05; Table 1).
| Group | Metastases (n = 57) | Absence of metastases (n = 91) | t/χ2 | P value | |
| Age (years), mean ± SD | 48.61 ± 12.99 | 48.39 ± 12.16 | 0.110 | 0.912 | |
| Sex | Male | 30 (52.63) | 44 (48.35) | 0.287 | 0.591 |
| Female | 27 (47.37) | 47 (51.65) | |||
| BMI (kg/m2), mean ± SD | 22.61 ± 2.13 | 22.42 ± 2.25 | 0.236 | 0.814 | |
| A family history of malignancy | 3 (5.26) | 5 (5.49) | 0.004 | 0.952 | |
| Present with hypertension | 7 (12.28) | 12 (13.19) | 0.026 | 0.873 | |
| Hyperglycemia | 4 (7.02) | 10 (10.99) | 0.641 | 0.423 | |
| Hyperlipidemia | 5 (8.77) | 8 (8.79) | < 0.001 | 0.997 | |
| History of alcohol use | 12 (19.30) | 16 (17.58) | 0.273 | 0.601 | |
| Endoscopic tumor site | Cardia (of stomach) | 8 (14.04) | 17 (18.68) | 4.830 | 0.395 |
| Fundus of stomach | 7 (12.28) | 10 (10.99) | |||
| Body of stomach | 12 (21.05) | 16 (17.58) | |||
| Antrum of stomach | 26 (45.61) | 47 (51.65) | |||
| Pylorus | 2 (3.51) | 1 (1.10) | |||
| Whole stomach | 2 (3.51) | 0 (0.00) | |||
| 14C-urea breath test | Masculine | 48 (84.21) | 72 (79.12) | 0.592 | 0.442 |
| Feminine character | 9 (15.79) | 19 (20.88) | |||
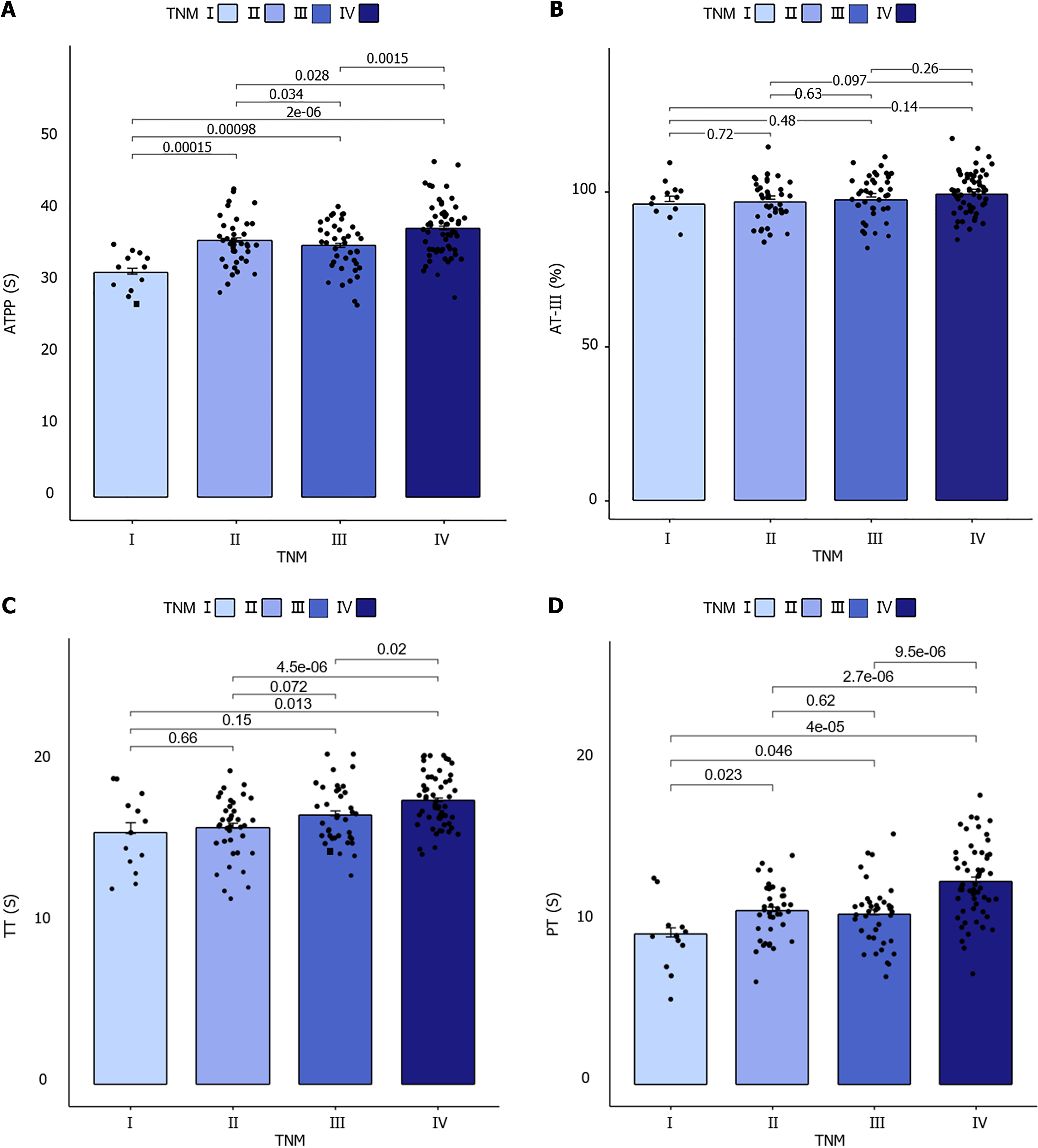
There was no significant difference in APTT and PT time between the two groups (P > 0.05). TT time did not differ significantly between patients with TNM stage I and those with TNM stages II and III (P > 0.05). In contrast, the four groups of GC patients with different TNM stages exhibited statistically significant differences in coagulation function APTT (F = 11.626, P < 0.001), PT (F = 14.733, P < 0.001), and TT time (F = 7.991, P < 0.001). No significant difference was observed between AT-III and TNM stages (F = 1.346, P = 0.736 > 0.05), as illustrated in Figure 1.
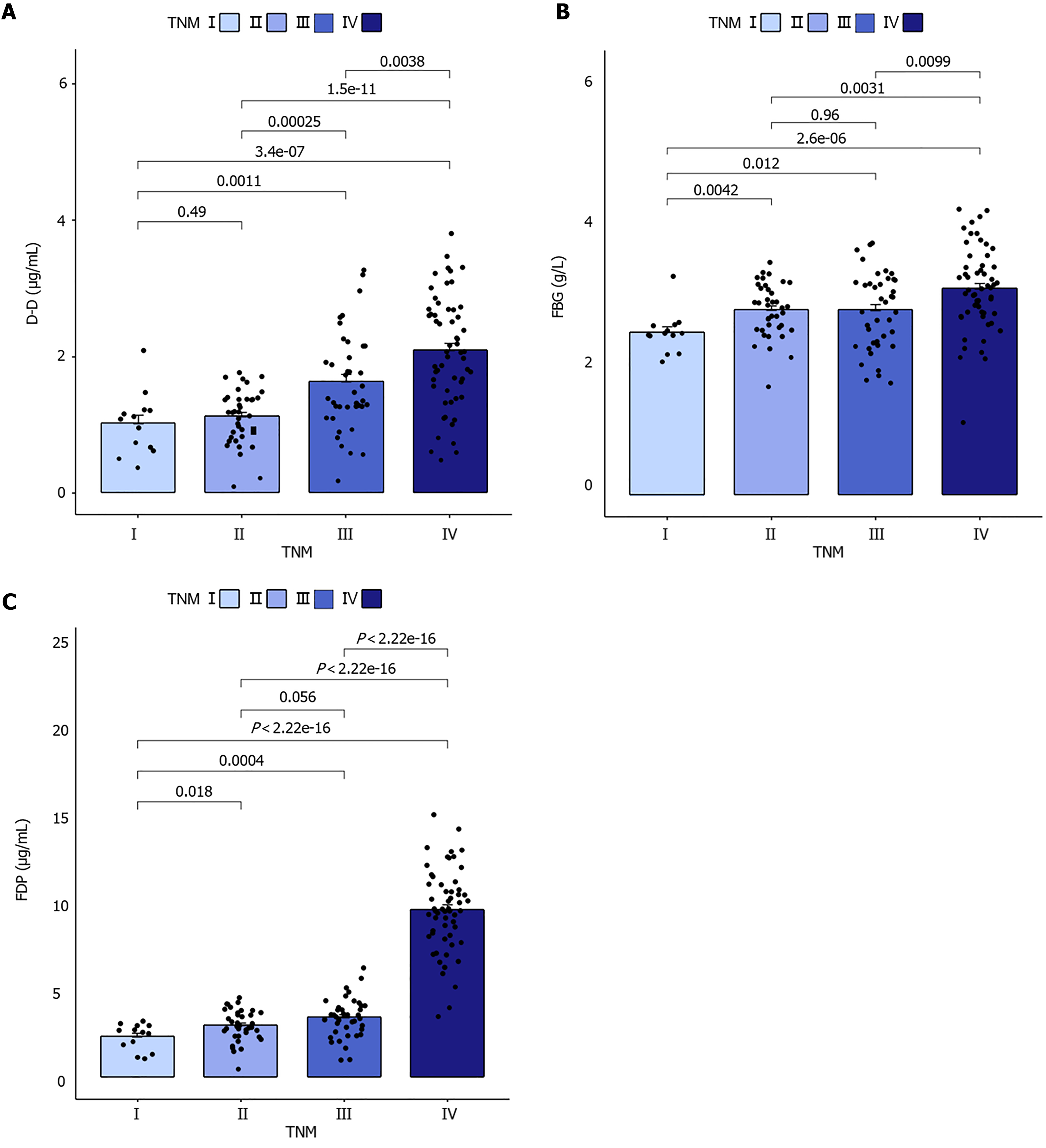
In patients with TNM stages I and II, there was no significant difference between the D-D groups (P > 0.05). Furthermore, there was no significant difference between FBG and FDP groups in patients with TNM stage II and III (P > 0.05). However, there were statistically significant differences in fibrin and FDP FBG (F = 6.892, P < 0.001), D-D (F = 19.836, P < 0.001), and FDP (F = 177.027, P < 0.001) among the four groups of GC patients with different TNM stages (P < 0.001), as shown in Figure 2.
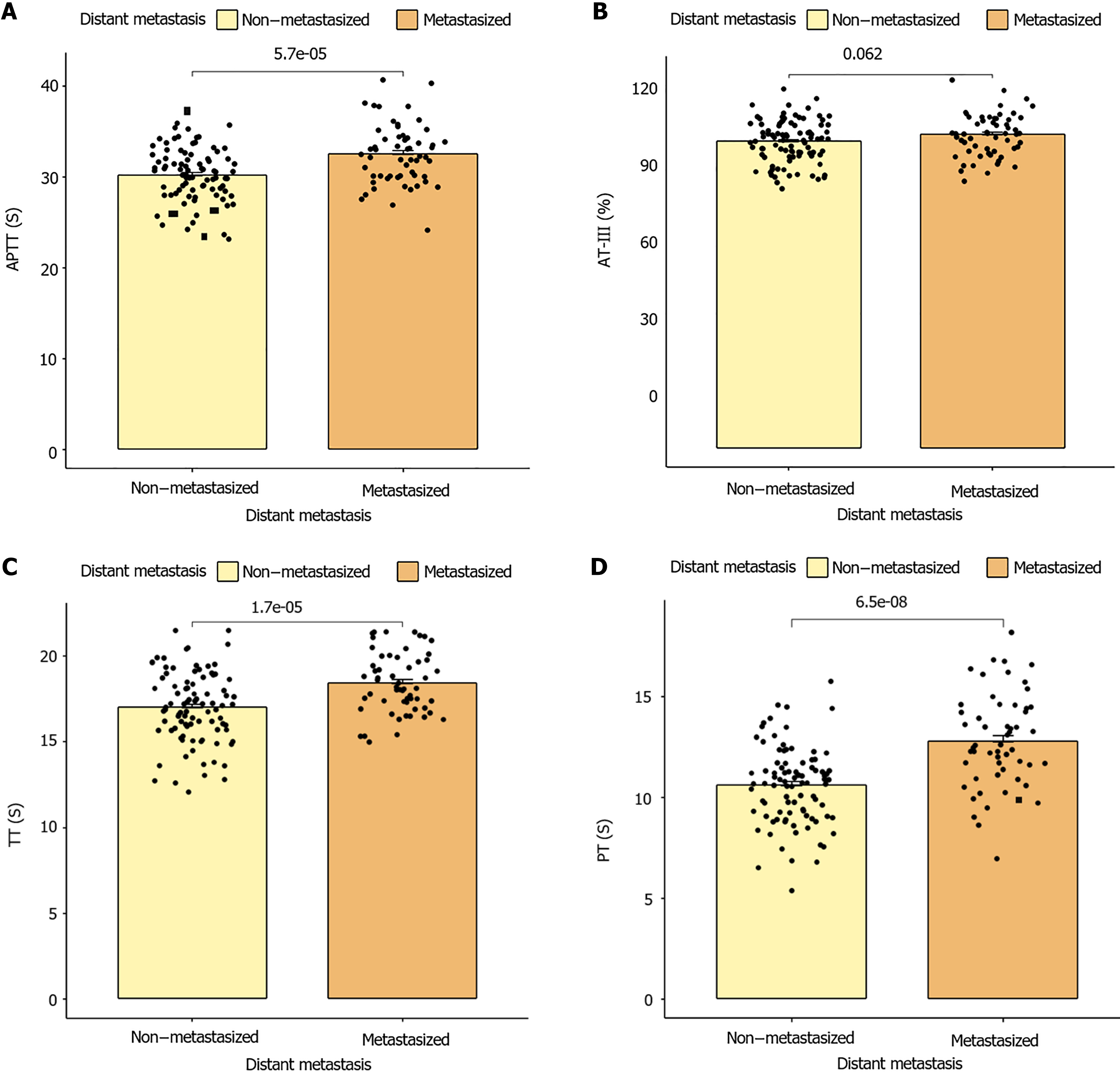
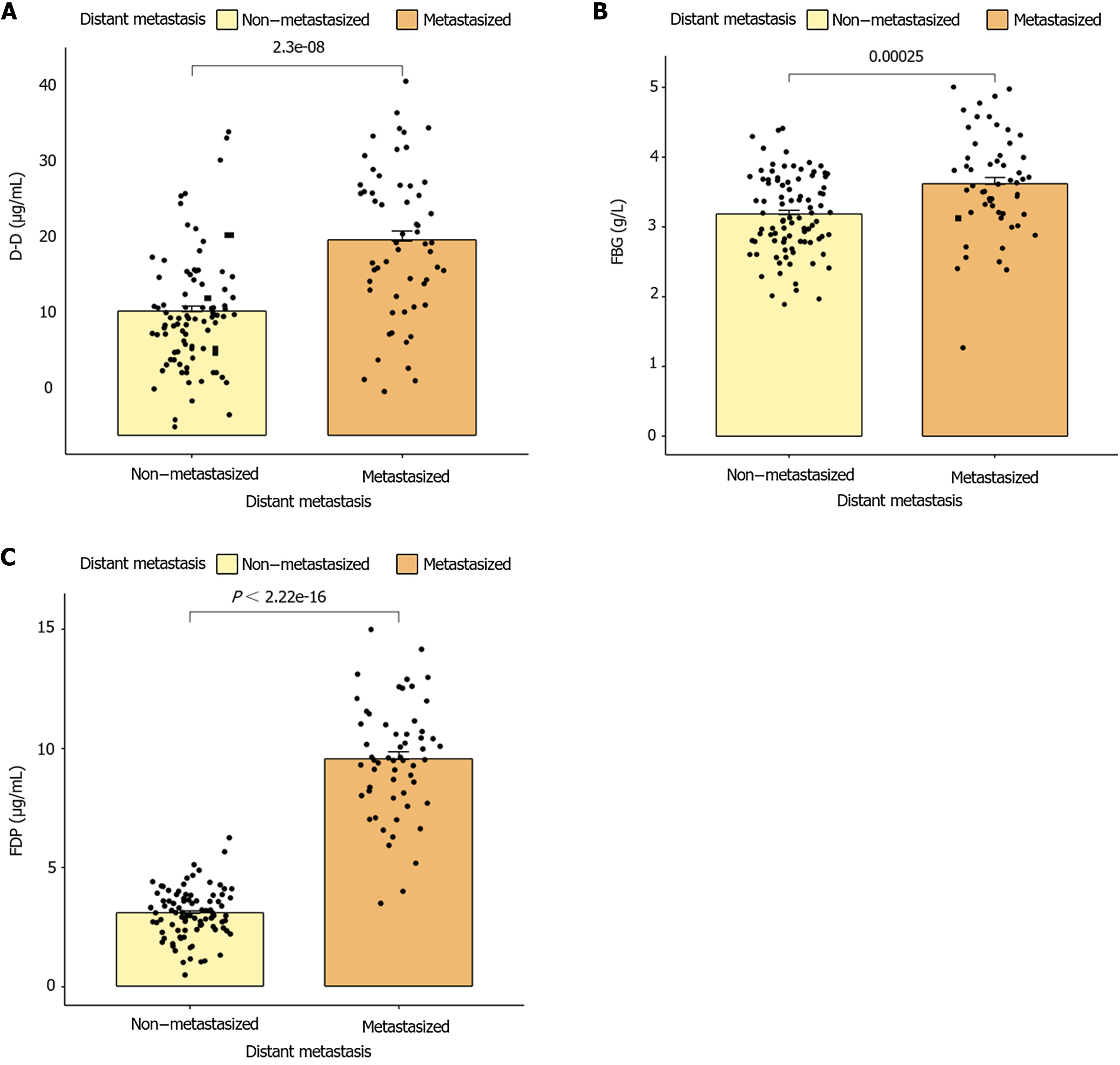
(APTT, PT, and TT time) between patients with GC at different TNM stages (P < 0.001). No significant differences were observed between AT-III and TNM stages (P = 0.050), as shown in Figure 3. However, statistically significant differences were observed in fibrin and FDP (FBG, D-D, and FDP) between groups of patients with GC with different TNM stages (P < 0.001; Figure 4).
A significant positive correlation was observed between APTT, PT, TT, FDP, D-D, FBG, and TNM in patients with GC (P < 0.001); however, no significant positive correlation was observed between AT-III and TNM (P = 0.062), as shown in Table 2.
| Index | APTT | PT | TT | AT-Ⅲ | FDP | D-D | FBG | ||||||||
| r | P value | r | P value | r | P value | r | P value | r | P value | r | P value | r | P value | ||
| TNM staging | 0.368 | < 0.001 | 0.437 | < 0.001 | 0.373 | < 0.001 | 0.162 | 0.050 | 0.780 | < 0.001 | 0.530 | < 0.001 | 0.329 | < 0.001 | |
A significant positive correlation was observed between APTT, PT, TT, FDP, D-D, and FBG and the onset of distant metastasis in patients with GC (P < 0.05), whereas no significant positive correlation was observed between AT-III and the onset of distant metastasis (P > 0.05), as shown in Table 3.
| Index | APTT | PT | TT | AT-Ⅲ | FDP | D-D | FBG | |||||||
| r | P value | r | P value | r | P value | r | P value | r | P value | r | P value | r | P value | |
| Distant metastasis of gastric cancer | 0.330 | < 0.001 | 0.451 | < 0.001 | 0.335 | < 0.001 | 0.154 | 0.062 | 0.883 | < 0.001 | 0.471 | < 0.001 | 0.316 | < 0.001 |
Clinically, APTT, PT, TT, and AT-III are commonly used coagulation indices that reflect a patient's coagulation status to a certain extent[13]. The HYPERCAN study conducted in Italy highlighted the importance of hypercoagulation screening as an innovative tool for assessing cancer risk, early diagnosis, and prognosis prediction[14]. Abnormal coagulation contributes to maintaining a hypercoagulable state in the body, increasing the risk of thrombosis, which can lead to tumor cell migration, invasion, and lymph node metastasis[15]. This not only exacerbates the condition of a patient but also increases the likelihood of complications, thereby intensifying the patient’s suffering. FDP is a collective term used for various degradation fragments and complexes formed by fibrin breakdown under the action of fibrinolytic enzymes. It is used to assess the activity of the fibrinolytic system of the body and is often measured in conjunction with D-D levels to collectively reflect the state of the coagulation and fibrinolytic systems[16]. Previous studies have found that the progression of endometrial cancer and breast cancer is related to coagulation indexes[17,18]. Research examining the correlation between the TNM stage, distant metastasis, and coagulation indices in patients with GC is limited. This study investigated the correlation of coagulation indexes and FDP with TNM stage and distant metastasis in GC patients, aiming to provide theoretical basis for clinical identification of high-risk patients. Abnormal coagulation parameters (APTT, PT, TT) and FDP were significantly associated with an increased risk of late and distant metastasis of TNM (P < 0.05). The results of this study suggest that these indicators not only reflect dysregulation of the coagulation and fibrinolysis systems but also provide insights into disease progression and prognosis in patients with GC. One possible factor is inflammation; Inflammatory processes often accompany cancer and can activate the clotting system. Tumor cells can release inflammatory cytokines that stimulate the expression of tissue factors and other pro-coagulant molecules, resulting in an imbalance of the clotting and fibrinolytic systems. At the same time, inflammation damages the endothelium of blood vessels, further promoting clotting. Another aspect to consider is the immune response; The immune system plays a complex role in the development of cancer and can interact with the clotting system. Immune cells can release mediators that affect blood clotting, and changes in the clotting system can also affect immune cell function. Hypercoagulation may impair the transport and function of immune cells, creating a favorable environment for tumor growth and metastasis.
Changes in coagulation indices, such as APTT, PT, and TT, in patients with GC reflect an imbalance in the coagulation and fibrinolytic systems. GC cells release tissue factors that activate both endogenous and exogenous coagulation pathways, leading to prolonged APTT and PT, indicating abnormal activation of the coagulation system[19]. Meanwhile, the hyperfibrinolytic system increases FDP, exacerbating blood hypercoagulability and thrombosis risk[20]. Additionally, advanced GC is often accompanied by liver function impairment, impacting coagulation factor synthesis and worsening abnormal coagulation function[21]. These changes are closely related to the clinical stage and prognosis of GC and may promote hematogenous tumor cell metastasis, constituting a high-risk state for thrombosis. Therefore, APTT, PT, and TT are crucial biomarkers that can be used to assess the risk of thrombosis in patients with GC and guide personalized treatment strategies.
Xing et al[22] demonstrated that FBG levels ≥ 3.495 g/L are an independent risk factor for primary stage I-II GC. During infiltration and metastasis, malignant tumor cells contribute to excessive tissue factor release, coagulation system activation, and thrombin generation. This process converts FBG into fibrin, thereby inducing a hypercoagulable state and increasing the risk[14].
Ji et al[23] reported elevated levels of FDP in patients with GC. Patients with a disease in stages III-IV disease exhibited higher FDP levels than those with stage I-II disease, and a combined analysis using four or six indicators showed enhanced diagnostic sensitivity and specificity, consistent with our findings. In our study, FBG, D-D, and FDP levels in patients with GC significantly correlated with TNM stage and distant metastasis. GC progression often involves abnormal activation of the coagulation system, which increases the risk of thrombosis. Elevated FBG levels indicate enhanced coagulation activity, likely due to the tumor-induced secretion of FBG activators by vascular endothelial cells, stimulating the increased synthesis of FBG and fibrin degradation products. Elevated FBG levels promote tumor cell adhesion to the vascular endothelium, facilitating metastasis[24]. Increased FDP levels may reflect hyperfibrinolytic activity, with D-D serving as a specific indicator of FBG degradation, which is crucial for assessing coagulation status and identifying hypercoagulability and thrombosis[25]. Patients with malignant tumors typically exhibit elevated fibrinolytic enzyme levels, and tumor cells secrete significant FBG activators[26]. Abnormal levels of these indicators correlate with increased tumor burden and invasiveness and are closely aligned with the pathobiological characteristics of GC and its impact on host blood environments. Therefore, FBG, D-D, and FDP serve not only as essential blood biochemical markers for coagulation abnormalities in GC patients with GC but also provide a robust biological foundation for disease staging and prognosis assessment. This study's limitations include: First, the retrospective study design may introduce inherent biases, including selective bias and the possibility of difficulty in establishing a causal relationship between abnormal coagulation and GC disease outcomes. Second, being a single-center study limits the generalization of the findings to a wider population with different demographic characteristics and treatment options. In addition, while the sample size was sufficient to detect a significant association, future studies are needed with larger, multicentre studies to verify applicability across different GC patient populations and in different healthcare Settings. Despite these limitations, the findings have important implications for future clinical practice and research. The correlation of coagulation markers and fibrin degradation product levels with TNM staging and remote metastasis observed in this study suggests that these biomarkers may be valuable prognostic indicators for GC. Integrating clotting measures with FDP measures into daily assessments helps stratify patients' risk and guide personalized treatment strategies, potentially improving patient outcomes. Future studies should focus on addressing the limitations of this study and investigating the mechanisms of coagulation abnormalities in GC patients, exploring how GC cells interact with the clotting and fibrinolysis system, paving the way for the development of targeted therapies and personalized medicine approaches. In addition, prospective multicenter studies should be conducted to validate the clinical utility of these biomarkers in different patient populations and treatment regimens.
In summary, coagulation function and indices were significantly associated with TNM staging and distant metastasis in patients with GC. In clinical practice, coagulation indicators and FDP such as APTT, PT, TT, FDP, D-D, and FBG should be monitored as part of the routine examination of GC patients to gain a comprehensive understanding of the patient's condition and identify high-risk patients with disease progression and metastasis. This allows for early intervention and personalized treatment plans, which are integrated into diagnostic algorithms to improve the accuracy of disease staging and prognosis prediction. At the same time, GC patients should reasonably plan a healthy diet and regular exercise to reduce the risk of inflammation and improve blood clotting. In the future, it could help to develop guidelines for the use of clotting biomarkers in the diagnosis and treatment of GC, ensure consistent, evidence-based approaches in different healthcare settings, and conduct additional research to verify the applicability of these biomarkers in different GC patient populations and treatment regimens, and further explore their mechanisms of action. Prospective multi-center studies are conducted to further validate its clinical efficacy, explore its combination with other clinical indicators, and develop new therapeutic targets and personalized medicines based on a deeper understanding of the mechanism of blood coagulation abnormalities to improve treatment outcomes and quality of life in patients with GC.
| 1. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2847] [Article Influence: 569.4] [Reference Citation Analysis (5)] |
| 2. | López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, Quispe L, Zevallos A, Buleje JL, Cho CE, Sarmiento M, Pinto JA, Fajardo W. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 162] [Reference Citation Analysis (3)] |
| 3. | Petryszyn P, Chapelle N, Matysiak-Budnik T. Gastric Cancer: Where Are We Heading? Dig Dis. 2020;38:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 4. | Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 409] [Reference Citation Analysis (5)] |
| 5. | Wang Z, Han W, Xue F, Zhao Y, Wu P, Chen Y, Yang C, Gu W, Jiang J. Nationwide gastric cancer prevention in China, 2021-2035: a decision analysis on effect, affordability and cost-effectiveness optimisation. Gut. 2022;71:2391-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 6. | Xu H, Li W. Early detection of gastric cancer in China: progress and opportunities. Cancer Biol Med. 2022;19:1622-1628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 7. | Zhang L, Qin S, Chen H, Hu Z, Li S. Diagnostic Values of the Prealbumin-to-Fibrinogen, Albumin-to-Fibrinogen, and Monocyte-to-Lymphocyte Ratios in Gastric Cancer. Ann Clin Lab Sci. 2021;51:385-392. [PubMed] |
| 8. | Ding P, Zheng C, Cao G, Gao Z, Lei Y, Deng P, Hou B, Li K. Combination of preoperative plasma fibrinogen and AJCC staging improves the accuracy of survival prediction for patients with stage I-II gastric cancer after curative gastrectomy. Cancer Med. 2019;8:2919-2929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 9. | Liu X, Shi B. Progress in research on the role of fibrinogen in lung cancer. Open Life Sci. 2020;15:326-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 10. | Wada H, Shiraki K, Matsumoto T, Shimpo H, Shimaoka M. Clot Waveform Analysis for Hemostatic Abnormalities. Ann Lab Med. 2023;43:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 11. | Repetto O, De Re V. Coagulation and fibrinolysis in gastric cancer. Ann N Y Acad Sci. 2017;1404:27-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 12. | Lin JX, Desiderio J, Lin JP, Wang W, Tu RH, Li P, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Zheng CH, Zhou ZW, Parisi A, Huang CM. Multicenter Validation Study of the American Joint Commission on Cancer (8th Edition) for Gastric Cancer: Proposal for a Simplified and Improved TNM Staging System. J Cancer. 2020;11:3483-3491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 13. | Suzuki T, Shimada H, Nanami T, Oshima Y, Yajima S, Ito M, Washizawa N, Kaneko H. Hyperfibrinogenemia is associated with inflammatory mediators and poor prognosis in patients with gastric cancer. Surg Today. 2016;46:1394-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 14. | Nataraj A, Govindan S, Ramani P, Subbaiah KA, Sathianarayanan S, Venkidasamy B, Thiruvengadam M, Rebezov M, Shariati MA, Lorenzo JM, Pateiro M. Antioxidant, Anti-Tumour, and Anticoagulant Activities of Polysaccharide from Calocybe indica (APK2). Antioxidants (Basel). 2022;11:1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 15. | Hosoya Y, Matsumura M, Madoiwa S, Zuiki T, Matsumoto S, Nunomiya S, Lefor A, Sata N, Yasuda Y. Acquired hemophilia A caused by factor VIII inhibitors: report of a case. Surg Today. 2013;43:670-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 16. | Chen L, Lin J, Chen Y, Yu J, Wang X. Clinicopathologic features and prognosis of 71 patients with gastric cancer and disseminated intravascular coagulation. PeerJ. 2023;11:e16527. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 17. | Wang Y, Guan W, Zhang Y, Wang Y, Shi B, Liu J, Zhang S. Using heart rate variability to evaluate the association between the autonomic nervous system and coagulation function in patients with endometrial cancer. Oncol Lett. 2024;28:499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 18. | Peng Q, Zhu J, Ren X. Thromboelastogram and coagulation function index: relevance for female breast cancer. Front Oncol. 2024;14:1342439. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 19. | Zhang L, Ye J, Luo Q, Kuang M, Mao M, Dai S, Wang X. Prediction of Poor Outcomes in Patients with Colorectal Cancer: Elevated Preoperative Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT). Cancer Manag Res. 2020;12:5373-5384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 20. | Zeng ZY, Chen XS. [Impact of hemocoagulase on coagulatory function and deep venous thrombosis after abdominal surgery]. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:353-356. [PubMed] |
| 21. | Stoencheva SS, Popov VG, Grudeva-Popova ZG, Deneva TI. Markers of activation of coagulation in cancer patients. Bratisl Lek Listy. 2023;124:29-35. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Xing X, Zhang Y, Wang Y, Li M. NLR in combination with plasma FIB and RDW is a useful predictor for the diagnosis of early gastric cancer. Asian J Surg. 2023;46:2219-2220. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 23. | Ji Y, Qin Y, Tan Q, Qiu Y, Han S, Qi X. Development of a chemiluminescence assay for tissue plasminogen activator inhibitor complex and its applicability to gastric cancer. BMC Biotechnol. 2024;24:30. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 24. | Ge W, Zheng LM, Chen G. The Combination of Seven Preoperative Markers for Predicting Patients with Gastric Cancer to Be Either Stage IV or Non-Stage IV. Gastroenterol Res Pract. 2018;2018:3450981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 25. | Zhang X, Wang X, Li W, Dang C, Diao D. Effectiveness of managing suspected metastasis using plasma D-dimer testing in gastric cancer patients. Am J Cancer Res. 2022;12:1169-1178. [PubMed] |
| 26. | Wu ZJ, Xu H, Wang R, Bu LJ, Ning J, Hao JQ, Sun GP, Ma T. Cumulative Score Based on Preoperative Fibrinogen and Pre-albumin Could Predict Long-term Survival for Patients with Resectable Gastric Cancer. J Cancer. 2019;10:6244-6251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (1)] |