INTRODUCTION
Gallbladder cancer (GBC) is a rare but invasive malignant tumor, accompanied by high mortality (median 5-year overall survival of 18%)[1]. In its early stage, GBC often remains asymptomatic, thus leading to frequent negligence. Based on the GLOBOCAN 2018 data, 43% of GBCs are diagnosed with concurrent liver and lymph node metastases, which eliminates the chance for curative surgical resection[2].More than half of the GBC patients suffer from tumor recurrence after undergoing radical surgery[3].
Traditionally, gemcitabine and cisplatin (GC) are widely regarded as being first-line therapies for locally advanced biliary tract cancer (BTC), but their effectiveness is limited[4].
In recent years, the emergence of immunotherapy has revolutionized the treatment approach for solid tumors, thus leading to substantial improvements in patient survival. The success of the double-blind phase III TOPAZ-1 study, which has explored the effects of a combined regimen of gemcitabine, cisplatin, and durvalumab (GC-D) in combination with GC, has marked the beginning of a new era in immunotherapy for BTC[5]. Research has indicated that patients who are given GC-D have notably increased median overall survival (OS; 12.8 vs 11.5 months), 24-month OS rate (24.9% vs 10.4%), and progression-free survival (7.2 vs 5.7 months), with comparable adverse events being reported[6]. This triple therapy regimen is recommended as first-line treatment for BTC, according to the 2023 National Comprehensive Cancer Network guidelines. However, compared with other types of BTC, GBC has distinct biological characteristics, including unique genetic mutations and a more immunosuppressive microenvironment, which contributes to its aggressive nature and poor response to standard therapies[7,8]. Unlike cholangiocarcinoma or pancreatic cancer, GBC is frequently diagnosed at advanced stages because of its asymptomatic nature, thus leading to a more dismal prognosis. Given the unique biological characteristics and clinical behavior of GBC, investigations of the treatment responses within this specific context are crucial. Moreover an understanding of these nuances can provide insights into optimizing therapeutic strategies and improving patient outcomes.
Herein, we report a case in which GC-D was significantly effective for locally advanced GBC, with a clinical complete response (CR) eventually achieved. This case is particularly noteworthy, as it highlights the potential for improved outcomes in a patient population that is typically characterized by poor prognosis, thus contributing valuable insights into the treatment landscape for GBC.
CASE PRESENTATION
Chief complaints
A 68-year-old man presented with right upper abdominal pain that has persisted for 7 days.
History of present illness
Seven days earlier, the patient experienced intermittent mild pain in the right upper abdomen, which was tolerable and lasted for several hours before subsiding on its own. There were no accompanying symptoms such as fever, nausea, vomiting, or diarrhea. The patient’s abdominal pain worsened after meals, prompting the patient to seek medical attention at a local hospital, where an ultrasound examination indicated cholecystitis.
History of past illness
No significant past medical history was reported.
Personal and family history
The patient's personal and family medical histories were unremarkable.
Physical examination
Physical examination revealed tenderness in the right upper quadrant, with no signs of jaundice or masses.
Laboratory examinations
Laboratory examinations revealed a total white blood cell count of 12.32 × 109/L, with a neutrophil percentage of 80.3%. Additionally, the CA19-9 level was 28.7 U/mL, which was slightly elevated above normal limits and other routine blood tests were within normal limits.
Imaging examinations
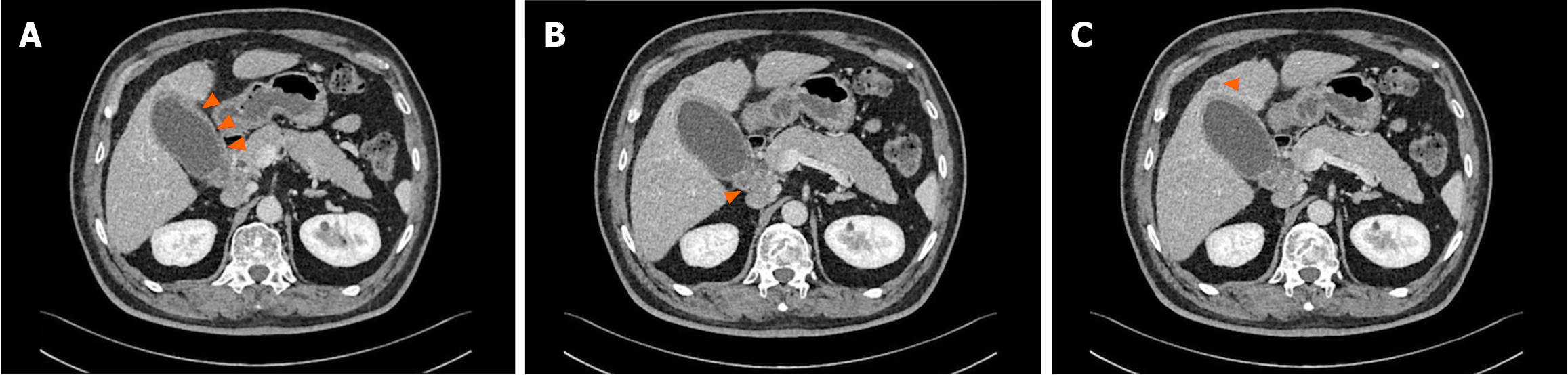
Multiphasic contrast-enhanced computed tomography (MCCT) indicated gallbladder enlargement, wall thickening, and potential cholecystitis, in addition to swollen hepatoduodenal mesenteric lymph nodes and a lower density lesion in the S4 segment of the liver, thus suggesting possible metastasis (Figure 1).
Figure 1 Multiphasic contrast-enhanced computed tomography findings.
A: Gallbladder enlargement and wall thickening; B: Swollen hepatoduodenal mesenteric lymph nodes; C: A slightly lower density lesion.
FINAL DIAGNOSIS
The preoperative diagnosis was unclear, thus raising the possibility of either cholecystitis or GBC.
TREATMENT
Given the unclear preoperative diagnosis, laparoscopic examination was performed. Thickening and hardening of the cystic duct were intraoperatively observed. A cholecystectomy was initially performed, and the proximal end of the cystic duct was sent for intraoperative frozen sectioning, which revealed the presence of tumor cells accompanied by necrosis. Further exploration revealed the presence of multiple enlarged lymph nodes at the hepatic hilum, with some nodes fused together, as well as involvement of the lower segment of the common bile duct. If radical resection had been performed, the performance of hepatopancreatoduodenectomy would have entailed significant surgical trauma and extensive resection, thus possibly resulting in a variety of postoperative complications. After communicating the corresponding risks with the patient’s family, they refused to have the patient undergo radical surgical resection and opted for later systemic treatment instead, thus eliminating the need for the operation. The patient received GC-D therapy (gemcitabine: 1000 mg/m2, days 1 and 8; cisplatin: 25 mg/m2, days 1 and 8; durvalumab: 1500mg, days 1; 1 course for 21 days).
OUTCOME AND FOLLOW-UP
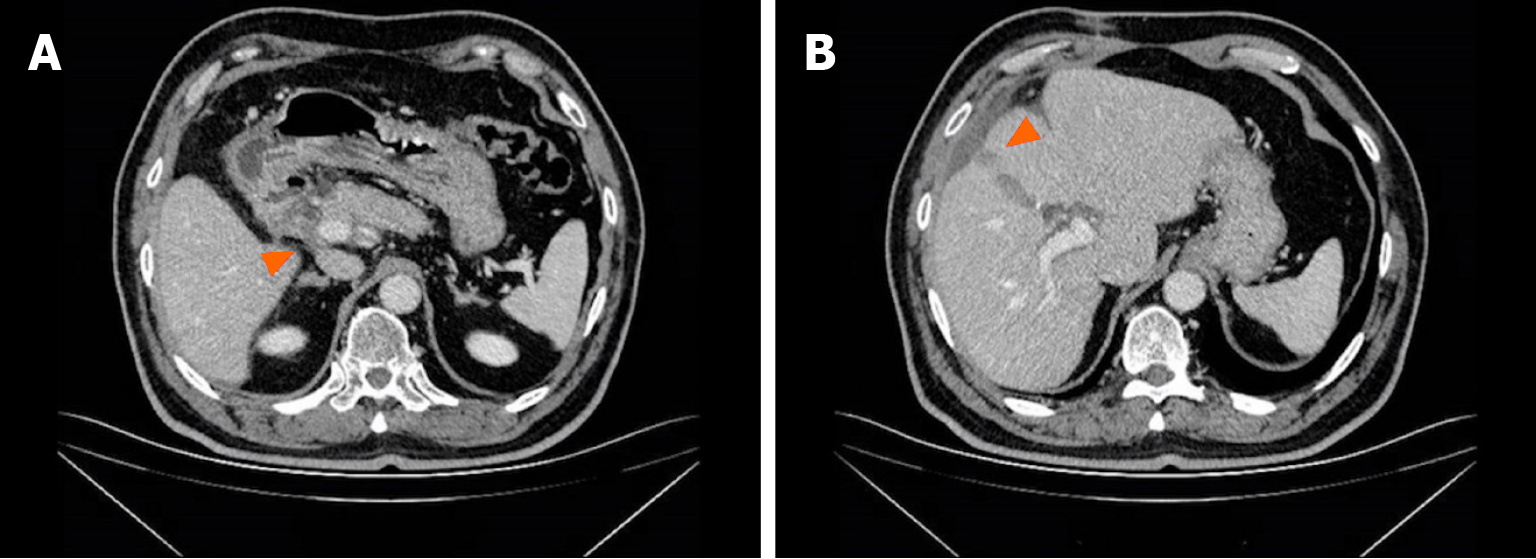
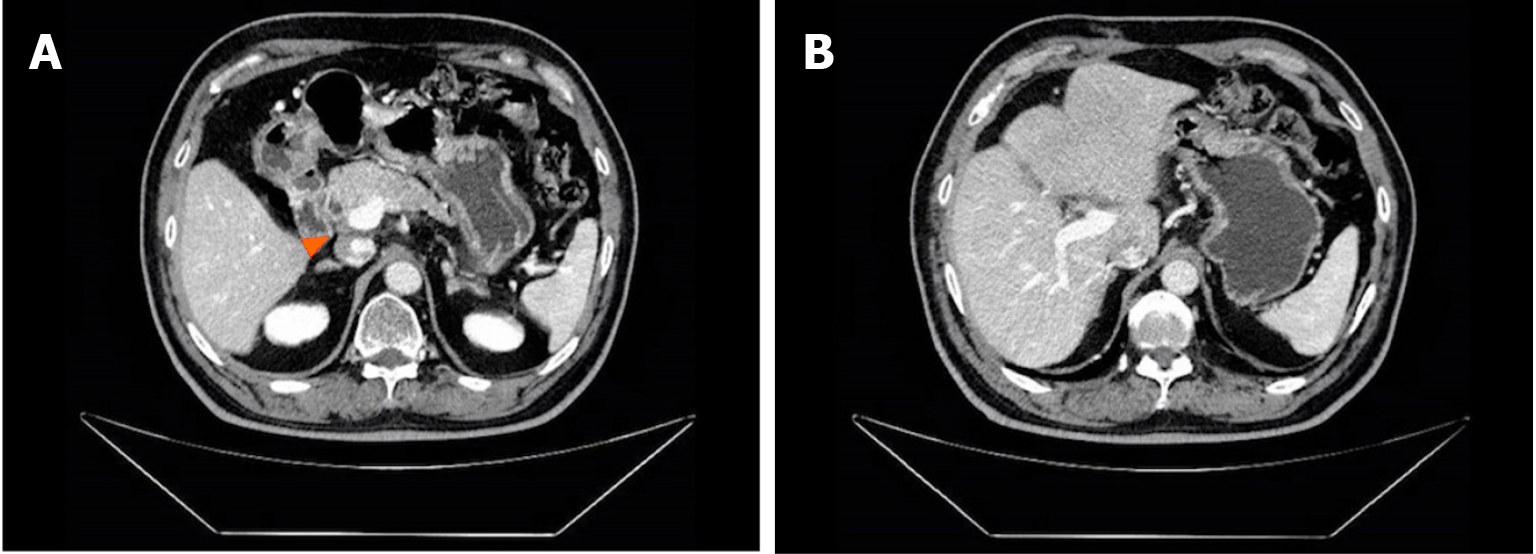
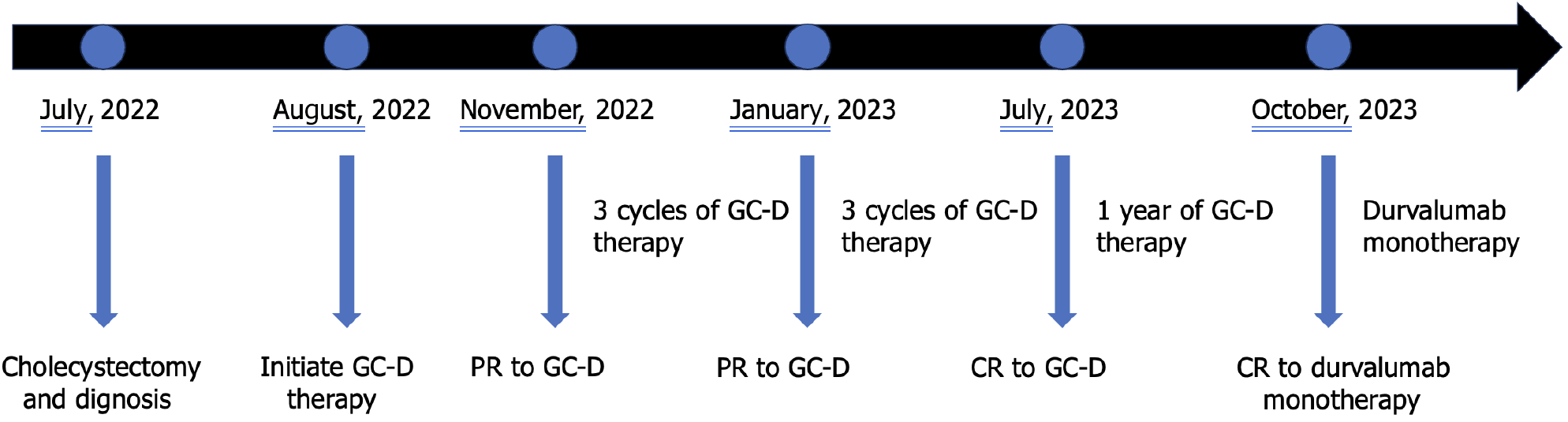
The patient experienced tolerable toxicities of reversible grade 2 nausea and fatigue. These symptoms occurred in approximately 60% of the treatment cycles and were effectively managed with tropisetron prior to chemotherapy. After 3 cycles of therapy, the CA19-9 level decreased to 11.4 U/mL. MCCT revealed a reduction in the number of hepatoduodenal mesenteric lymph nodes and a lower-density lesion in the S4 segment of the liver (Figure 2). Due to the positive outcomes, the patient proceeded with the ongoing treatment regimen. Six months after the start of GC-D therapy, the tumor marker level remained relatively low. MCCT revealed a significant reduction in the number of hepatoduodenal mesenteric lymph nodes and the disappearance of lower- density lesions in the S4 segment of the liver (Figure 3). An objective response of partial response (PR) was assessed. As a result of the favourable treatment effects, the patient was able to continue with the initial therapeutic regimen despite experiencing increased nausea and fatigue two days after chemotherapy. One year after beginning therapy, MCCT revealed the disappearance of the hepatoduodenal mesenteric lymph nodes, with his tumor marker levels remaining within normal range. A clinical CR was assessed. When considering the adverse effects of chemotherapy, the patient chose maintenance therapy with durvalumab monotherapy. The entire clinical course is displayed in Figure 4. He returned to his normal daily life, and the ultimate goal of treatment, which was to prolong the patient’s quality of life, was achieved. Given the significant CR, we plan to conduct follow-up studies to track the patient's long-term survival, recurrence rates, and overall quality of life over several years, to assess the durability of the GC-D regimen for GBC patients.
Figure 2 Multiphasic contrast-enhanced computed tomography findings after 3 cycles of therapy.
A: The shrunken hepatoduodenal mesenteric lymph nodes; B: The shrunken lower density lesion.
Figure 3 Multiphasic contrast-enhanced computed tomography findings after 6 months of therapy.
A: The significantly shrunken hepatoduodenal mesenteric lymph nodes; B: The disappeared lower density lesion.
Figure 4 The timeline of the entire clinical course.
GC-D: Gemcitabine, cisplatin, and durvalumab; PR: Partial response; CR: Complete response.
DISCUSSION
GBC is a highly malignant tumor. Due to its insensitivity to chemotherapy, its therapeutic efficacy is limited[9-11]. Despite ongoing efforts and exploration, progress in treating GBC has been limited over a prolonged period of time[12-14]. TOPAZ-1 represents a silver lining that illuminates the path for treating TBC[5]. However, the extent to which GBC patients can derive any survival benefits from triple treatment remains uncertain. This aspect has rarely been addressed in previous studies. In this study, a triple combined regimen of gemcitabine, cisplatin and durvalumab achieved a clinical CR in a patient with locally advanced GBC, which is an encouraging outcome. Consequently, the present case demonstrates considerable clinical value.
Given the atypical clinical presentation of early-stage GBC, it is challenging to determine the presence of the tumor through preoperative examinations. Intraoperative laparoscopy and frozen section biopsy serve as effective modalities for elucidating the histopathological characteristics of tumors[15-17]. In this study, the malignancy of the gallbladder tumor was intraoperatively confirmed through laparoscopic exploration and frozen section biopsy following cholecystectomy. Furthermore, the tumor was assessed as being in a locally advanced stage, thus rendering curative resection infeasible. Hepatopancreatoduodenectomy may potentially achieve curative resection of the tumor, but it is associated with significant trauma to the patient and the possibility of severe postoperative complications[18,19]. Therefore, full respect should be given to the viewpoints of patients’ family members, while actively engaging in communication to avert potential doctor-patient discord.
Multiple studies have suggested that immune checkpoint inhibitors (ICIs) have a profound impact on the treatment of BTC. In a previous phase 1 trial of nivolumab treatment alone or in combination with cisplatin plus gemcitabine for unresectable BTC, Ueno et al[20] reported that 40% of patients achieved a PR in the combination therapy group, whereas no patient achieved a CR. Findings from another research endeavor involving pembrolizumab in advanced biliary adenocarcinoma demonstrated an objective response rate (ORR) of 5.8% (with all 6 out of 104 patients displaying a PR)[21]. A real-world study investigating the treatment of advanced biliary tract tumors with the combination of durvalumab and the GC regimen revealed an ORR of 34.5%, with a CR rate of 4.8% and a PR rate of 29.6%[22]. Unfortunately, none of the abovementioned studies conducted subgroup analyses specifically for GBC. Therefore, further research to validate the efficacy of the combination of durvalumab and the GC regimen in the context of GBC is warranted.
The mechanism underlying the combination of chemotherapy and immunotherapy in GBC remains a complex and debated topic. Programmed death-1 (PD-1) protein is a checkpoint protein that, when engaged, inhibits T-cell activation and proliferation[23]. In GBC, tumor cells can upregulate PD-1 expression in response to chemotherapy, thus promoting an immune-evasive environment. This upregulation may hinder the effectiveness of immunotherapy by reducing the activity of cytotoxic T cells against tumor cells[24]. However, the combination of chemotherapy and immunotherapy can counteract this effect, thus restoring T-cell function and enhancing antitumor immunity[25]. Additionally, GBC is characterized by a unique microenvironment that is often immunosuppressive. Chemotherapy may induce changes in the tumor microenvironment, thus potentially decreasing the levels of immunosuppressive factors and thereby enhancing the efficacy of ICIs. This synergistic effect may improve patient outcomes by promoting a more favorable immune response against the tumor[26]. Furthermore, chemotherapy can induce cellular stress responses that increase the expression of tumor-associated antigens on cancer cells, thereby enhancing antigen presentation. Improved antigen presentation can lead to increased recognition and attack by the immune system, particularly when combined with PD-1 blockade[27,28]. This dual approach may help to overcome the tumor’s mechanisms of immune evasion. Overall, the combination of chemotherapy and immunotherapy may create a synergistic effect in which chemotherapy sensitizes tumors to immune attack. By inducing immunogenic cell death, chemotherapy may enhance the effectiveness of subsequent immunotherapy, thus leading to better overall responses[23,29]. This mechanism could justify further exploration of combination regimens in clinical trials.
The most commonly observed adverse events associated with GC treatment include fatigue, anemia, neutropenia, thrombocytopenia, and other conditions[9,11,22,30]. In our study, only mild nausea and fatigue were observed, with no additional immune-related adverse events being identified. This discrepancy could be attributed to several individual patient factors, such as age, overall health status, and genetic variations that influence drug metabolism. Additionally, the specific dosing regimen may have played a role in mitigating these adverse effects, thus suggesting a potential avenue for future research into optimizing treatment protocols to increase tolerability.
Some studies have shown that curative resection is performed after effective conversion therapy for initially unresectable GBC[31-33]. With the advent of the immunotherapy era, a series of questions have arisen. For example, there is a question regarding if surgery is still warranted for patients achieving CR through immunotherapy combined with chemotherapy. Additionally, there are questions regarding the extent of targeted resection, as well as the optimal timing for surgery. These inquiries all necessitate further research efforts to offer definitive answers.
This case provides critical insights into the potential efficacy of the GC-D regimen for GBC patients, particularly in achieving a clinically CR. These findings encourage clinicians to consider the role of immunotherapy in GBC treatment protocols. Furthermore, these findings underscore the need for larger-scale clinical trials to confirm these findings and to explore the optimal integration of immunotherapy in the management of GBC.
CONCLUSION
The combination of GC chemotherapy with durvalumab has proven to be an effective treatment approach for advanced GBC, with manageable adverse events. Further research is warranted to substantiate the effectiveness of the combined regimen in the context of GBC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Rusman RD S-Editor: Lin C L-Editor: A P-Editor: Zhang XD