Published online Jan 15, 2025. doi: 10.4251/wjgo.v17.i1.96267
Revised: October 16, 2024
Accepted: November 5, 2024
Published online: January 15, 2025
Processing time: 225 Days and 5.2 Hours
Hepatocellular carcinoma (HCC) is the most common form of liver cancer that has limited treatment options and a poor prognosis. Transarterial chemoembolization (TACE) is the first-line treatment for intermediate-stage HCC but can induce tumour hypoxia, thereby promoting angiogenesis. Recent studies suggested that combining TACE with anti-angiogenic therapies and immunotherapy might im
To evaluate the efficacy and safety of TACE + SL therapy in comparison to TACE + L therapy in patients with intermediate-advanced HCC.
A retrospective analysis was performed on patients with intermediate-advanced HCC who received TACE plus lenvatinib with or without sintilimab between September 2019 and September 2022. Baseline characteristics were compared, and propensity score matching was applied. Overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) were evaluated between the two groups, and adverse events were analyzed.
The study included 57 patients, with 30 in the TACE + SL group and 27 in the TACE + L group. The TACE + SL group demonstrated significantly improved median PFS and OS compared to the TACE + L group (PFS: 14.1 months vs 9.6 months, P = 0.016; OS: 22.4 months vs 14.1 months, P = 0.039), along with a higher ORR (70.0% vs 55.6%). After propensity score matching, 30 patients were included, with the TACE + SL group again showing longer median PFS and a trend toward improved OS (PFS: 14.6 months vs 9.2 months, P = 0.012; OS: 23.9 months vs 16.3 months, P = 0.063), and a higher ORR (73.3% vs 53.3%). No severe adverse events were reported.
TACE + SL demonstrated superior outcomes in terms of OS and PFS, compared to TACE + L. These findings suggest that the addition of sintilimab might enhance the therapeutic response in patients with intermediate-advanced HCC.
Core Tip: This study evaluates the efficacy and safety of combining transarterial chemoembolization (TACE) with lenvatinib and sintilimab compared to TACE with lenvatinib alone in patients with intermediate-advanced hepatocellular carcinoma. The results indicate that TACE with lenvatinib and sintilimab significantly improves overall survival (22.4 months vs 14.1 months) and progression-free survival (14.1 months vs 9.6 months), with a higher objective response rate (70% vs 55.6%). Notably, no severe adverse events were reported, suggesting that the combination therapy is effective and safe. These findings support the inclusion of sintilimab in hepatocellular carcinoma treatment regimens.
- Citation: Wu FD, Zhou HF, Yang W, Zhu D, Wu BF, Shi HB, Liu S, Zhou WZ. Transarterial chemoembolization combined with lenvatinib and sintilimab vs lenvatinib alone in intermediate-advanced hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(1): 96267
- URL: https://www.wjgnet.com/1948-5204/full/v17/i1/96267.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i1.96267
Hepatocellular carcinoma (HCC), the most prevalent form of liver cancer, is a leading cause of cancer-related mortality worldwide[1,2]. Due to its usually asymptomatic in its early stages, HCC is frequently diagnosed at advanced stages, resulting in limited therapeutic options and a poor prognosis. Transarterial chemoembolization (TACE) is the recom
This retrospective analysis included all patients with intermediate-advanced HCC treated at the First Affiliated Hospital of Nanjing Medical University who received TACE plus lenvatinib, with or without sintilimab, between September 2019 and September 2022. The study was approved by the Institutional Ethics Review Boards and conducted in accordance with the World Medical Association Declaration of Helsinki. Inclusion criteria were Barcelona Clinic Liver Cancer (BCLC) stage B or C, Child-Pugh class A5, A6, or B7, Eastern Cooperative Oncology Group performance status ≤ 1, at least one assessable lesion, and ≥ 1 cycle of TACE plus lenvatinib with/without sintilimab. Exclusion criteria included < 1 month of lenvatinib or sintilimab treatment, secondary primary malignancies, follow-up < 6 months, incomplete clinical data, or loss to follow-up. Informed consent was waived due to the retrospective nature of the study.
TACE was initiated before the administration of lenvatinib and sintilimab. A percutaneous femoral or radial artery approach was used for intubation, followed by angiography to identify the tumour-feeding artery. Chemoembolization was performed using super-selective emulsions of epirubicin and iodised oil via microcatheters, followed by the injection of gelatine sponge fragments to embolise the blood vessels supplying the tumour. Post-TACE syndrome was recorded, and liver function indicators were assessed within 1 week of each TACE treatment.
Lenvatinib was administered orally at a daily dose of 12 mg for patients weighing ≥ 60 kg or 8 mg for those weighing < 60 kg. Sintilimab was administered intravenously at a dose of 200 mg within 1 week of the initial TACE procedure, followed by subsequent doses every 3 weeks. In the event of severe adverse events (AEs), sintilimab and lenvatinib doses were adjusted through dose reduction, suspension, or discontinuation to ensure patient safety and manage treatment-related toxicities.
Patients were followed up every 6-8 weeks until the end of the study (June 30, 2023) or death. Follow-up assessments included routine physical examinations, laboratory blood tests, and contrast-enhanced computed tomography/magnetic resonance imaging scans to monitor treatment-related AEs. Tumour progression was assessed using the modified Response Evaluation Criteria in Solid Tumours. OS was defined as the time from treatment initiation to death from any cause or the most recent follow-up. Progression-free survival (PFS) was measured from treatment initiation to the date of disease progression. The ORR was calculated as the sum of complete responses and partial responses, while the disease control rate (DCR) included complete responses, partial responses, and stable disease.
All statistical analyses were performed using IBM SPSS Statistics 26 software and R 4.1.2 software. Propensity score matching (PSM) was applied to minimize selection bias and reduce the influence of potential confounding factors. Due to the small sample size, continuous variables were dichotomised to ensure comparability between the groups. Baseline variables were compared using the χ2 test or Fisher’s exact test for categorical variables, and the Student’s t-test or Mann-Whitney U test for quantitative data, as appropriate. Survival analysis was conducted using the Kaplan-Meier method, with the log-rank test used to compare survival curves between groups. Univariate Cox regression analysis was per
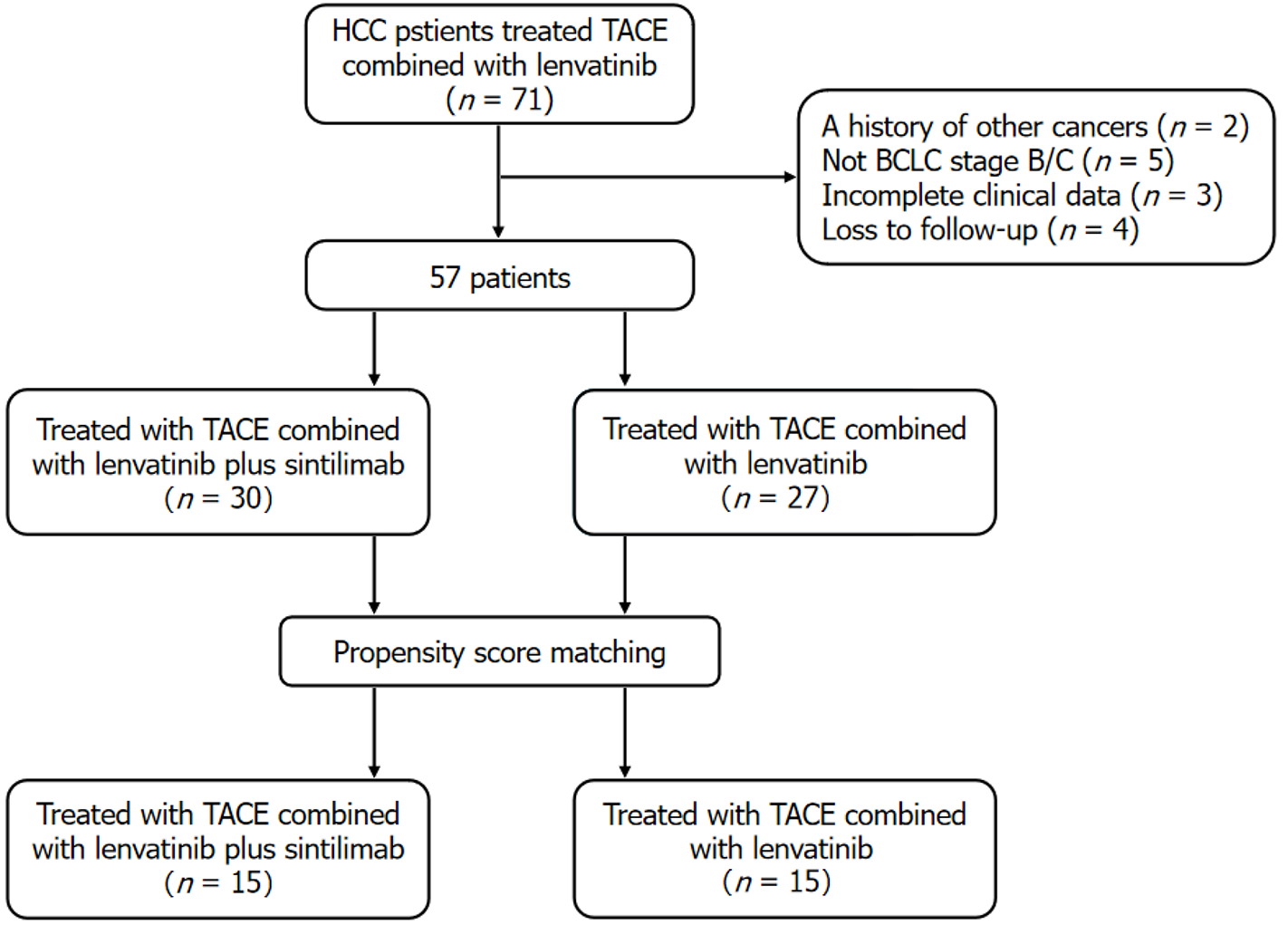
This study enrolled 57 patients with HCC who met the inclusion criteria between September 2019 and September 2022 (Figure 1). The patients were divided into two groups: The TACE + SL group (n = 30) and the TACE + L group (n = 27), with ages ranging from 37 years to 80 years. The median follow-up duration was 19.2 months for the TACE + SL group and 17.5 months for the TACE + L group. Table 1 presents the clinical characteristics of the patients. PSM at a 1:1 ratio yielded 15 matched pairs. After matching, no statistically significant differences were observed in baseline characteristics between the two groups.
| Characteristic | Before PSM | After PSM | |||||
| TACE + L | TACE + SL | P value | TACE + L | TACE + SL | P value | ||
| Gender | 1.00 | 1.00 | |||||
| Female | 3 | 3 | 2 | 1 | |||
| Male | 24 | 27 | 13 | 14 | |||
| Age (year) | 0.66 | 1.00 | |||||
| ≥ 60 | 16 | 15 | 8 | 8 | |||
| < 60 | 11 | 15 | 7 | 7 | |||
| ECOG | 1.00 | 0.43 | |||||
| 0 | 7 | 8 | 3 | 6 | |||
| 1 | 20 | 22 | 12 | 9 | |||
| Etiology | 0.49 | 0.65 | |||||
| HBV | 22 | 21 | 13 | 11 | |||
| Other | 5 | 9 | 2 | 4 | |||
| Cirrhosis | 1.00 | 1.00 | |||||
| No | 4 | 4 | 2 | 2 | |||
| Yes | 23 | 26 | 13 | 13 | |||
| Tumor number | 0.35 | 0.71 | |||||
| ≥ 3 | 11 | 17 | 6 | 8 | |||
| < 3 | 16 | 13 | 9 | 7 | |||
| Tumor size (cm) | 0.43 | 1.00 | |||||
| ≥ 7 | 8 | 13 | 4 | 5 | |||
| < 7 | 19 | 17 | 11 | 10 | |||
| PVTT | 0.05 | 1.00 | |||||
| No | 22 | 16 | 11 | 11 | |||
| Yes | 5 | 14 | 4 | 4 | |||
| Metastases | 1.00 | 0.33 | |||||
| No | 23 | 26 | 11 | 14 | |||
| Yes | 4 | 4 | 4 | 1 | |||
| BCLC | 0.03 | 1.00 | |||||
| B | 22 | 15 | 11 | 11 | |||
| C | 5 | 15 | 4 | 4 | |||
| Child-Pugh class | 1.00 | 1.00 | |||||
| A | 25 | 27 | 14 | 15 | |||
| B | 2 | 3 | 1 | 0 | |||
| AFP (ng/mL) | 0.00 | 1.00 | |||||
| ≥ 100 | 2 | 17 | 2 | 2 | |||
| < 100 | 25 | 13 | 13 | 13 | |||
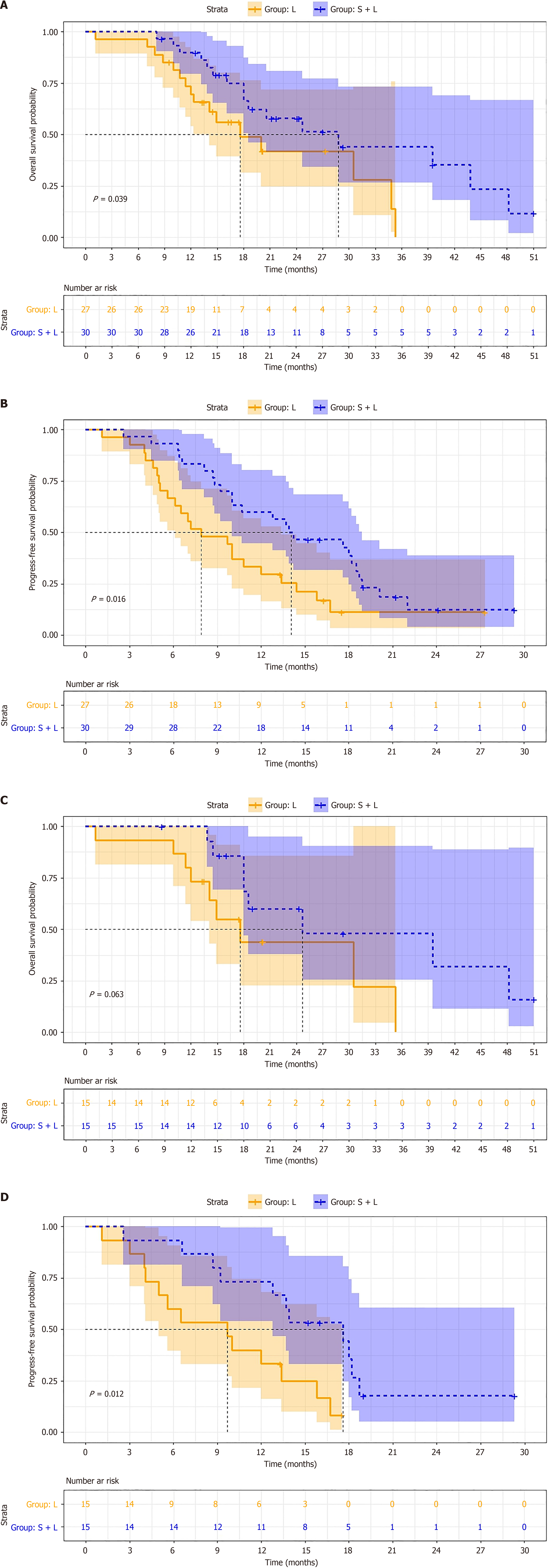
Before PSM, the median OS was 22.4 months for the TACE + SL group and 14.1 months for the TACE + L group (P = 0.039). The median PFS for the TACE + SL group was 14.1 months, compared to 9.6 months for the TACE + L group (P = 0.016) (Figure 2A and B). After PSM, the median OS for the TACE + SL group increased to 23.9 months, while it was 16.3 months for the TACE + L group (P = 0.063). The median PFS remained consistent, with 14.6 months in the TACE + SL group and 9.2 months in the TACE + L group (P = 0.012) (Figure 2C and D). The 1-year and 2-year survival rates for the TACE + SL group were 93.3% and 40.0%, respectively, compared to 80.0% and 13.3% in the TACE + L group (Table 2).
| Unmatched group | Matched group | |||
| TACE + L (n = 27) | TACE + SL (n = 30) | TACE + L (n = 15) | TACE + SL (n = 15) | |
| CR | 4 (14.8) | 6 (20.0) | 1 (6.7) | 2 (13.3) |
| PR | 11 (40.7) | 15 (50.0) | 7 (46.7) | 9 (60.0) |
| SD | 2 (7.4) | 6 (20.0) | 1 (6.7) | 3 (20.0) |
| PD | 10 (37.0) | 3 (10.0) | 6 (40.0) | 1 (6.7) |
| ORR | 15 (55.6) | 21 (70.0) | 8 (53.3) | 11 (73.3) |
| DCR | 17 (63.0) | 27 (90.0) | 9 (60.0) | 14 (93.3) |
| 1-year OS | 19 (70.4) | 26 (86.7) | 12 (80) | 14 (93.3) |
Table 3 presents the tumour responses based on modified Response Evaluation Criteria in Solid Tumours. Before PSM, three patients (10.0%) in the TACE + SL group experienced disease progression, resulting in an ORR of 70.0% and a DCR of 90.0%. In contrast, 10 patients (37.0%) in the TACE + L group experienced disease progression, yielding an ORR of 55.6% and a DCR of 63.0%. After PSM, one patient (6.7%) in the TACE + SL group experienced disease progression, with an ORR of 73.3% and a DCR of 93.3%. Conversely, six patients (40.0%) in the TACE + L group experienced disease progression, leading to an ORR of 53.3% and a DCR of 60.0%. The ORR and DCR in the TACE + SL group were consistently higher than those in the TACE + L group before and after PSM.
| Adverse events | TACE + SL (n = 30) | TACE + L (n = 27) | ||||
| Toxicity grade | / | 1/2 | 3/4 | / | 1/2 | 3/4 |
| Diarrhea | 6 (20.0) | 4 | 2 | 5 (18.5) | 3 | 2 |
| Transaminitis | 14 (46.7) | 12 | 2 | 13 (48.1) | 8 | 5 |
| Hand-foot skin reactions | 10 (33.3) | 7 | 3 | 7 (25.9) | 6 | 1 |
| Nausea with/without vomiting | 23 (76.7) | 20 | 3 | 17 (63.0) | 15 | 2 |
| Abdominal pain | 22 (73.3) | 18 | 4 | 22 (81.5) | 19 | 3 |
| Fatigue | 9 (30.0) | 6 | 3 | 7 (25.9) | 5 | 2 |
| Fever | 23 (76.7) | 20 | 3 | 17 (63.0) | 15 | 2 |
| Decreased WBC/PLT count | 7 (23.3) | 5 | 2 | 9 (33.3) | 7 | 2 |
| Thrombopenia | 8 (26.7) | 7 | 1 | 3 (11.1) | 2 | 1 |
| Decreased appetite | 5 (16.7) | 5 | 0 | 8 (30.0) | 8 | 0 |
| Hypertension | 12 (40.0) | 9 | 3 | 7 (26.0) | 4 | 3 |
| Hypothyroidism | 7 (23.3) | 6 | 1 | / | / | / |
| Proteinuria | 0 (0.0) | 0 | 0 | 1 (3.7) | 0 | 1 |
The subgroup analyses of factors associated with OS and PFS are presented in Supplementary Figure 1. The TACE + SL regimen demonstrated significantly longer PFS and OS among subgroups of patients with hepatitis B virus infection, absence of distant metastases, BCLC stage B, Child-Pugh class A, and alpha-fetoprotein (AFP) levels of ≤ 100 ng/mL.
AEs occurring during treatment were evaluated and classified according to the Common Terminology Criteria for AEs version 5.0, considering their frequency and severity (Table 2). No treatment-related deaths were reported, and all ob
This study evaluated the efficacy and safety of the TACE + SL regimen with the TACE + L regimen in treating patients with intermediate-advanced HCC. Our findings indicate that the TACE + SL regimen was associated with improved OS and PFS compared to the TACE + L regimen. These results suggest that the addition of lenvatinib and sintilimab to TACE might offer enhanced therapeutic benefits for patients with intermediate-advanced HCC. The improved outcomes observed in the TACE + SL group could be attributed to several factors. Firstly, lenvatinib, a multi-kinase inhibitor, has demonstrated efficacy as a first-line therapy for unresectable HCC[7,15,16]. Lenvatinib targets multiple angiogenic and oncogenic pathways, resulting in tumour regression and prolonged survival. By combining lenvatinib with TACE, the anti-tumour effect of TACE might be augmented, potentially inhibiting tumour progression[17-19]. Secondly, sintilimab, an anti-PD-1 monoclonal antibody, has shown promising efficacy across various cancers, including HCC[11,20]. By blocking the PD-1/programmed death ligand-1 interaction, sintilimab enhances the immune response, thereby restoring the anti-tumour immune response. When used in combination with TACE, sintilimab might further enhance immune-mediated tumour cell elimination, thereby improving overall treatment response.
Before PSM, baseline differences between the two groups, including portal vein tumour thrombosis (PVTT), BCLC stage, and AFP levels, could have influenced treatment outcomes. It is well established that PVTT, BCLC stage C, and elevated AFP levels are associated with poor prognosis in patients with HCC. Despite having a higher proportion of patients with PVTT, more advanced BCLC stages, and elevated AFP levels, the TACE + SL group exhibited longer OS and PFS compared to the TACE + L group. Subgroup analyses further suggested that the TACE + SL regimen might offer better prognostic outcomes, particularly among patients with hepatitis B virus infection, absence of distant metastases, BCLC stage B, Child-Pugh class A, and AFP ≤ 100 ng/mL. However, given the small sample size, the robustness of these subgroup results might be limited. After PSM, which balanced baseline characteristics between the two groups, no statistically significant difference in median OS was observed between the TACE + SL and TACE + L groups (P = 0.063). This lack of significance may be attributable to the small sample size and the fact that more patients in the TACE + SL group had not yet reached the endpoint due to a shorter follow-up period. However, the TACE + SL group demonstrated superior median PFS compared to the TACE + L group, suggesting the potential for longer OS in further studies. Patients with PVTT, BCLC stage C, and higher AFP levels might benefit more from the TACE + SL regimen compared to the TACE + L regimen. Therefore, large-scale clinical trials and mechanistic studies are required shortly.
The safety profile of the TACE + SL regimen was manageable, with no treatment-related deaths reported. The most common AEs reported during treatment included nausea, abdominal pain, fever, hand-foot syndrome, and hypertension. These AEs were generally mild to moderate in severity and did not significantly affect the patient’s overall well-being. These findings align with those of previous studies on combination therapies for intermediate-advanced HCC[21,22]. Appropriate management and monitoring of these AEs were implemented throughout the study to ensure patient safety and optimize treatment outcomes. This study has certain limitations. First, the retrospective design and relatively small sample size might affect the generalizations and robustness of the findings. To enhance the reliability of these results, future investigations should include larger, multicentre cohorts with extensive follow-up periods, providing more comprehensive data on the long-term efficacy and safety of the combined TACE + SL regimen and strengthening the evidence base for this therapeutic approach. Second, the focus on patients with intermediate-advanced HCC raises concerns about the applicability of the findings to individuals with early-stage disease. Future research should investigate the efficacy and safety of the TACE + SL regimen across different stages of HCC, including early-stage patients. This could involve stratifying populations by disease stage and using randomized controlled trials to draw more definitive conclusions. Addressing these limitations will contribute to a more nuanced understanding of treatment modalities for HCC in subsequent studies.
In conclusion, our study demonstrated that the TACE + SL regimen is a promising treatment option for patients with intermediate-advanced HCC. The addition of lenvatinib and sintilimab to TACE improved OS and PFS compared to TACE combined with lenvatinib alone.
The authors thank the support of Yan Shen for patient recruitment and Zhong-Ling Pei for patient data aggregation. The authors also consulted Hai-Feng Zhou on analytical methodology. The authors thank patients with intermediate-advanced hepatocellular carcinoma at the First Affiliated Hospital of Nanjing Medical University for their immense contribution to this study.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64704] [Article Influence: 16176.0] [Reference Citation Analysis (177)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13215] [Article Influence: 1468.3] [Reference Citation Analysis (3)] |
| 3. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2619] [Article Influence: 873.0] [Reference Citation Analysis (59)] |
| 4. | Zheng L, Fang S, Wu F, Chen W, Chen M, Weng Q, Wu X, Song J, Zhao Z, Ji J. Efficacy and Safety of TACE Combined With Sorafenib Plus Immune Checkpoint Inhibitors for the Treatment of Intermediate and Advanced TACE-Refractory Hepatocellular Carcinoma: A Retrospective Study. Front Mol Biosci. 2020;7:609322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 5. | Qin J, Huang Y, Zhou H, Yi S. Efficacy of Sorafenib Combined With Immunotherapy Following Transarterial Chemoembolization for Advanced Hepatocellular Carcinoma: A Propensity Score Analysis. Front Oncol. 2022;12:807102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 6. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3176] [Article Influence: 529.3] [Reference Citation Analysis (37)] |
| 7. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3832] [Article Influence: 547.4] [Reference Citation Analysis (1)] |
| 8. | Guo J, Wang S, Han Y, Jia Z, Wang R. Effects of transarterial chemoembolization on the immunological function of patients with hepatocellular carcinoma. Oncol Lett. 2021;22:554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 9. | Zhang S, Zhao Y, He L, Bo C, An Y, Li N, Ma W, Guo Y, Guo Y, Zhang C. Effect of camrelizumab plus transarterial chemoembolization on massive hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2022;46:101851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 10. | Schmidt EV. Developing combination strategies using PD-1 checkpoint inhibitors to treat cancer. Semin Immunopathol. 2019;41:21-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 11. | Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, Chen Z, Liu B, Jia W, Wu J, Wang J, Shao G, Zhang B, Shan Y, Meng Z, Wu J, Gu S, Yang W, Liu C, Shi X, Gao Z, Yin T, Cui J, Huang M, Xing B, Mao Y, Teng G, Qin Y, Wang J, Xia F, Yin G, Yang Y, Chen M, Wang Y, Zhou H, Fan J; ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 673] [Article Influence: 168.3] [Reference Citation Analysis (1)] |
| 12. | Jin ZC, Zhong BY, Chen JJ, Zhu HD, Sun JH, Yin GW, Ge NJ, Luo B, Ding WB, Li WH, Chen L, Wang YQ, Zhu XL, Yang WZ, Li HL, Teng GJ; CHANCE Investigators. Real-world efficacy and safety of TACE plus camrelizumab and apatinib in patients with HCC (CHANCE2211): a propensity score matching study. Eur Radiol. 2023;33:8669-8681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (1)] |
| 13. | Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, Zhong BY, Sun JH, Jin ZC, Chen JJ, Ge NJ, Ding WB, Li WH, Huang JH, Mu W, Gu SZ, Li JP, Zhao H, Wen SW, Lei YM, Song YS, Yuan CW, Wang WD, Huang M, Zhao W, Wu JB, Wang S, Zhu X, Han JJ, Ren WX, Lu ZM, Xing WG, Fan Y, Lin HL, Zhang ZS, Xu GH, Hu WH, Tu Q, Su HY, Zheng CS, Chen Y, Zhao XY, Fang ZT, Wang Q, Zhao JW, Xu AB, Xu J, Wu QH, Niu HZ, Wang J, Dai F, Feng DP, Li QD, Shi RS, Li JR, Yang G, Shi HB, Ji JS, Liu YE, Cai Z, Yang P, Zhao Y, Zhu XL, Lu LG, Teng GJ; CHANCE001 Investigators. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 78.5] [Reference Citation Analysis (1)] |
| 14. | Fong KY, Zhao JJ, Sultana R, Lee JJX, Lee SY, Chan SL, Yau T, Tai DWM, Sundar R, Too CW. First-Line Systemic Therapies for Advanced Hepatocellular Carcinoma: A Systematic Review and Patient-Level Network Meta-Analysis. Liver Cancer. 2023;12:7-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 15. | Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7:2641-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (1)] |
| 16. | Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A, Hoshi SS, Mimura F, Haneda T, Fukuda Y, Kamata JI, Takahashi K, Matsukura M, Wakabayashi T, Asada M, Nomoto KI, Watanabe T, Dezso Z, Yoshimatsu K, Funahashi Y, Tsuruoka A. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 377] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 17. | Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 904] [Article Influence: 90.4] [Reference Citation Analysis (1)] |
| 18. | Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018;15:310-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 473] [Article Influence: 67.6] [Reference Citation Analysis (1)] |
| 19. | Rahma OE, Hodi FS. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin Cancer Res. 2019;25:5449-5457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 342] [Article Influence: 57.0] [Reference Citation Analysis (1)] |
| 20. | Hoy SM. Sintilimab: First Global Approval. Drugs. 2019;79:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (2)] |
| 21. | Zheng Y, Xiang Y, Shi H, Lin Z, Cheng S, Zhu J. Transarterial Chemoembolization Combined with Atezolizumab Plus Bevacizumab versus Transarterial Chemoembolization Alone in Intermediate-stage Hepatocellular Carcinoma: A Multicenter Retrospective Study. J Hepatocell Carcinoma. 2024;11:1079-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 22. | Xiang YJ, Wang K, Yu HM, Li XW, Cheng YQ, Wang WJ, Feng JK, Bo MH, Qin YY, Zheng YT, Shan YF, Zhou LP, Zhai J, Cheng SQ. Transarterial chemoembolization plus a PD-1 inhibitor with or without lenvatinib for intermediate-stage hepatocellular carcinoma. Hepatol Res. 2022;52:721-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (1)] |