Published online Jan 15, 2025. doi: 10.4251/wjgo.v17.i1.100713
Revised: October 9, 2024
Accepted: October 29, 2024
Published online: January 15, 2025
Processing time: 110 Days and 2.9 Hours
In this editorial, we will discuss the article by Tang et al published in the recent issue of the World Journal of Gastrointestinal Oncology. They explored an innovative approach to enhancing gemcitabine (GEM) delivery and efficacy using human bone marrow mesenchymal stem cells (HU-BMSCs)-derived exosomes. The manufacture of GEM-loaded HU-BMSCs-derived exosomes (Exo-GEM) has been optimized. The Tang et al’s study demonstrated that Exo-GEM exhibits enhanced cytotoxicity and apoptosis-inducing effects compared to free GEM, highlighting the potential of exosome-based drug delivery systems as a more effective and targeted approach to chemotherapy in pancreatic cancer. Additional in vivo studies are required to confirm the safety and effectiveness of Exo-GEM before it can be considered for clinical use.
Core Tip: The method of loading gemcitabine into exosomes is crucial for the effectiveness of the delivery system. The study ascertained that electroporation and sonication were more efficient than incubation in delivering gemcitabine into the human bone marrow mesenchymal stem cells-derived exosomes. Exosomes gemcitabine (Exo-GEM) demonstrated potent cytotoxicity against pancreatic cancer cells by enhancing GEM-induced apoptosis. Exo-GEM offers a promising strategy for targeted therapy in pancreatic cancer by harnessing the natural advantages of exosomes, such as high biocompatibility and the ability to navigate tumor microenvironments. Exo-GEM could deliver chemotherapy more precisely to cancer cells, reducing side effects in healthy cells.
- Citation: Chew FY, Tsai CH, Chang KH, Chang YK, Chou RH, Liu YJ. Exosomes as promising frontier approaches in future cancer therapy. World J Gastrointest Oncol 2025; 17(1): 100713
- URL: https://www.wjgnet.com/1948-5204/full/v17/i1/100713.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i1.100713
Pancreatic cancer is a malignant and aggressive disease characterized by invasion, rapid progression of the disease, and resistance to treatment. The most common type of pancreatic cancer is pancreatic ductal adenocarcinoma (PDAC), which accounts for about 90% of cases[1]. Typical symptoms in pancreatic cancer patients include abdominal pain, back pain, unexplained weight loss, jaundice, lightening of stool, darkening of urine, no hunger, etc., but there are usually no obvious symptoms in its early stage[2]. Due to the lack of an accurate early diagnostic test and specific symptoms, pa
At present, the main treatments for pancreatic cancer are surgery, radiation therapy, and chemotherapy. However, only around 20% of patients with pancreatic cancer are resectable, and the remaining 80% are not amenable to surgery. Gemcitabine (GEM) is sold under the brand name Gemzar and is the standard first-line treatment for metastatic pa
Treatment efficacy is limited by strong biological barriers to drug/material transport into deep tissues, including macrophage uptake, fluid dynamics in blood vessels, and high intratumoral pressure[5]. Exosomes are natural biological nanoparticles secreted by cells (usually 30 nm-150 nm in diameter) and contain many biomolecules, including nucleic acids, proteins, and lipids. Exosomes act as novel mediators in intercellular communication, serving as messengers to mediate physiological and pathological processes[6]. Exosomes have been tested as drug delivery vehicles for cancer treatment due to their unique properties, such as size, innate stability, low immunogenicity, excellent tissue/cell penetration ability and ability to mimic synthetic liposomes in carrying various anticancer drugs/materials[7]. These nanoscale vesicles, i.e. exosomes, have garnered considerable interest in the fields of biology and medical sciences.
For example, Kamerkar et al[8] used inhibitory exosomes (iExosomes) derived from normal fibroblast-like mesench
Numerous studies have demonstrated that exosomes are potential carriers of therapeutic anti-cancer small molecules, including curcumin, paclitaxel, doxorubicin, and GEM[10-13]. Previous studies have revealed that exosomes gemcitabine (Exo-GEM) derived from tumors, M1 macrophages, and mesenchymal stem cells (MSCs) demonstrate a remarkable capacity to overcome drug resistance in pancreatic cancer[13-16]. However, the efficiency of Exo-GEM to specifically target pancreatic cancer cells has yet to be fully established. The study by Tang et al[17] in the recent issue of World Journal of Gastrointestinal Oncology has explored an innovative approach to improve GEM delivery and efficacy through the use of human bone marrow MSCs (HU-BMSCs)-derived exosomes[17].
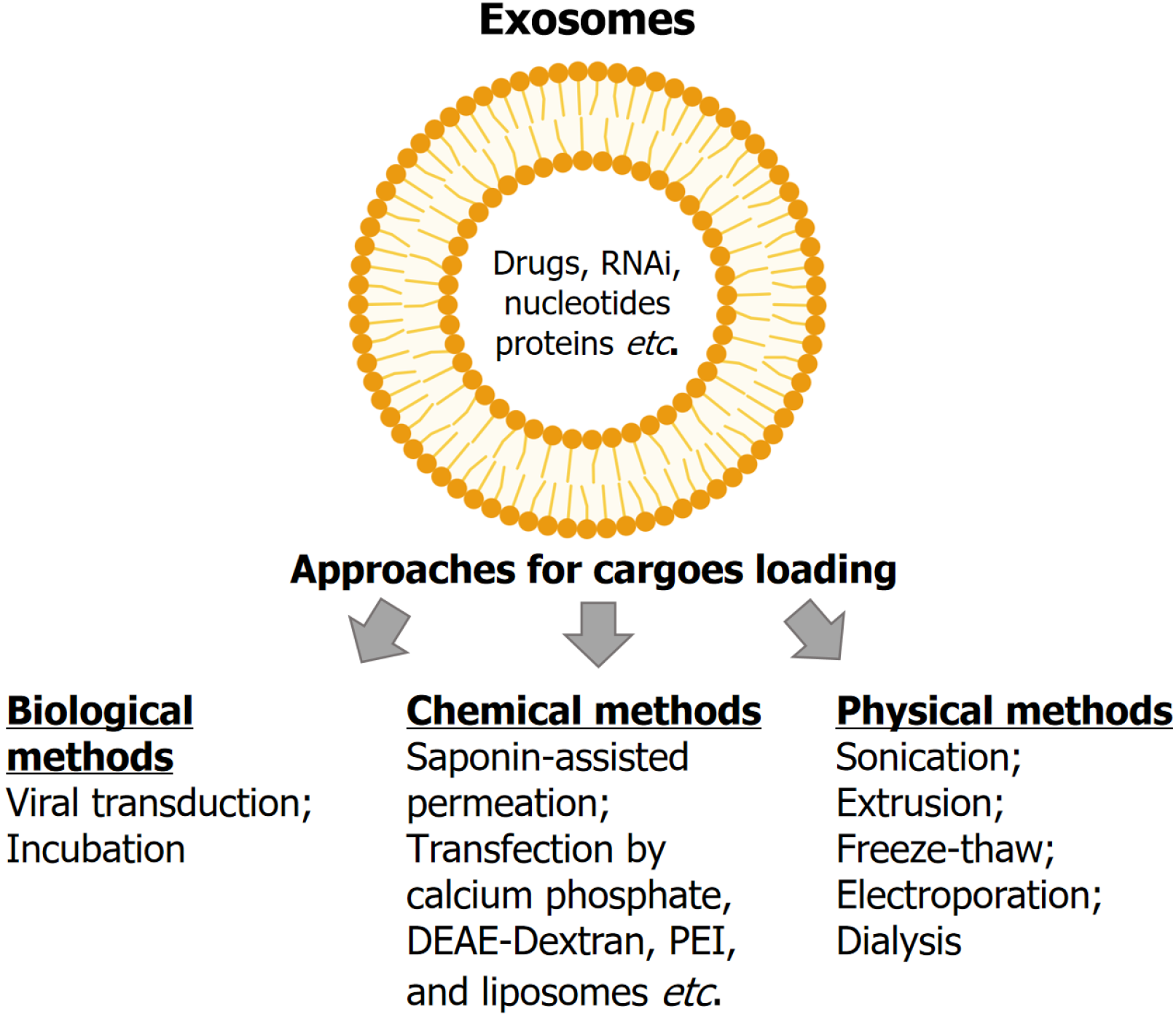
Cargo, such as drugs and materials, can be loaded into or onto exosomes via three main approaches: (1) Biological me
The recent study by Tang et al[17] compared three methods for loading GEM into the HU-BMSCs-derived exosomes: Electroporation, sonication, and incubation. Both electroporation and sonication showed significantly higher loading and encapsulation efficacy by approximately sixfold compared to incubation. Exo-GEM exhibited significantly higher cy
Conventional administration of GEM by intravenous (IV) injection is associated with well-known toxicities, including hematological, gastrointestinal, and liver-related side effects[31]. Exosomes have the potential to enhance drug targeting, reduce systemic toxicity, and provide controlled release, thereby mitigating many of the adverse effects typically as
Exo-GEM treatment significantly inhibited tumor growth and extended survival in tumor-bearing mice but caused minimal damage to normal tissues, suggesting exosomes are safe and effective carriers for the targeted delivery of GEM against pancreatic cancer[13]. Research on exosomes as therapeutic agents and drug delivery carriers has been predominantly carried out in animal models. Several clinical studies revealed no major toxicity or serious adverse events after administering dendritic cell-derived exosomes loaded with major histocompatibility complex class I or II peptides to enhance the immune response for the treatment of cancer patients[38-40]. Further clinical trials are essential to fully assess the long-term safety and efficacy of exosome-based therapies.
The study by Tang et al[17] opens new avenues for the clinical application of exosome-based drug delivery systems in the treatment of pancreatic cancer[17]. Exo-GEM could significantly impact the delivery of chemotherapy for pancreatic cancer patients. By enhancing the delivery and effectiveness of GEM, this approach may allow for lower dosages, leading to fewer side effects and an improved quality of life for pancreatic cancer patients. Additional in vivo studies are needed to validate the safety and effectiveness of Exo-GEM before it can be considered for clinical application. Several pilot clinical trials of exosome-based therapies have been launched, such as the curcumin-loaded plant exosomes for colon cancer, MSC-derived exosomes with KRASG12D siRNA (iExosomes) for metastatic pancreatic cancer, exosomes with antisense oligonucleotide-signal transducer and activator of transcription 6 (exoASO-STAT6)/(CDK-004) to target cancer-specific signaling pathways for patients with advanced hepatocellular carcinoma and patients with liver metastases, and vaccination with tumor antigen-loaded dendritic cell-derived exosomes (DEX2) for non-small cell lung cancer (Table 1).
| Study title | Conditions | Interventions | NCT number | Study status | Sponsor |
| Study investigating the ability of plant exosomes to deliver curcumin to normal and colon cancer tissue | Colon cancer | Dietary supplement: Curcumin conjugated with plant exosomes | NCT01294072 | Recruiting | University of Louisville |
| Inhibitory exosomes in treating participants with metastatic pancreas cancer with KRASG12D mutation | KRAS NP004976.2: p.G12D; Metastatic Pancreatic Adenocarcinoma; Pancreatic ductal adenocarcinoma; Stage IV pancreatic cancer | Drug: Mesenchymal stromal cells-derived exosomes with KRASG12D siRNA | NCT03608631 | Active; Not Recruiting | M.D. Anderson Cancer Center |
| A study of exoASO-STAT6 (CDK-004) in patients with advanced hepatocellular carcinoma and patients with liver metastases from either primary gastric cancer or colorectal cancer | Advanced hepatocellular carcinoma; Gastric cancer metastatic to liver; Colorectal cancer metastatic to liver | Drug: CDK-004 | NCT05375604 | Terminated | Codiak BioSciences |
| Trial of a vaccination with tumor antigen-loaded dendritic cell-derived exosomes | Non small cell lung cancer | Biological: DEX2 | NCT01159288 | Completed | Gustave Roussy, Cancer Campus, Grand Paris |
The study by Tang et al[17] marks a significant advancement in cancer therapy, particularly in the treatment of pancreatic cancer. The fabrication of Exo-GEM has been optimized. By demonstrating the enhanced cytotoxicity and apoptosis-inducing effects of Exo-GEM compared to free GEM, the research highlights the potential of exosome-based drug delivery systems as a more effective and targeted approach to chemotherapy[17]. Although further research is necessary to validate these findings in vivo and explore their clinical applications, this study establishes a strong foundation for a promising new direction in cancer treatment. The use of HU-BMSCs-derived exosomes for drug delivery could potentially address some of the major challenges in current cancer therapies, offering renewed hope for patients facing this devastating disease.
| 1. | Pishvaian MJ, Brody JR. Therapeutic Implications of Molecular Subtyping for Pancreatic Cancer. Oncology (Williston Park). 2017;31:159-166, 168. [PubMed] |
| 2. | Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 845] [Article Influence: 36.7] [Reference Citation Analysis (1)] |
| 3. | Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. BMJ. 2012;344:e2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 4. | Teague A, Lim KH, Wang-Gillam A. Advanced pancreatic adenocarcinoma: a review of current treatment strategies and developing therapies. Ther Adv Med Oncol. 2015;7:68-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 5. | Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3958] [Cited by in RCA: 4601] [Article Influence: 460.1] [Reference Citation Analysis (1)] |
| 6. | Di Bella MA. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 120] [Article Influence: 40.0] [Reference Citation Analysis (1)] |
| 7. | Chen H, Wang L, Zeng X, Schwarz H, Nanda HS, Peng X, Zhou Y. Exosomes, a New Star for Targeted Delivery. Front Cell Dev Biol. 2021;9:751079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (1)] |
| 8. | Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1167] [Cited by in RCA: 1905] [Article Influence: 238.1] [Reference Citation Analysis (1)] |
| 9. | McAndrews KM, Xiao F, Chronopoulos A, LeBleu VS, Kugeratski FG, Kalluri R. Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic Kras(G12D) in pancreatic cancer. Life Sci Alliance. 2021;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (1)] |
| 10. | Song H, Liu B, Dong B, Xu J, Zhou H, Na S, Liu Y, Pan Y, Chen F, Li L, Wang J. Exosome-Based Delivery of Natural Products in Cancer Therapy. Front Cell Dev Biol. 2021;9:650426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 11. | Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 474] [Article Influence: 47.4] [Reference Citation Analysis (1)] |
| 12. | Gomari H, Forouzandeh Moghadam M, Soleimani M, Ghavami M, Khodashenas S. Targeted delivery of doxorubicin to HER2 positive tumor models. Int J Nanomedicine. 2019;14:5679-5690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 13. | Li YJ, Wu JY, Wang JM, Hu XB, Cai JX, Xiang DX. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020;101:519-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (1)] |
| 14. | Zhao Y, Zheng Y, Zhu Y, Zhang Y, Zhu H, Liu T. M1 Macrophage-Derived Exosomes Loaded with Gemcitabine and Deferasirox against Chemoresistant Pancreatic Cancer. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 15. | Klimova D, Jakubechova J, Altanerova U, Nicodemou A, Styk J, Szemes T, Repiska V, Altaner C. Extracellular vesicles derived from dental mesenchymal stem/stromal cells with gemcitabine as a cargo have an inhibitory effect on the growth of pancreatic carcinoma cell lines in vitro. Mol Cell Probes. 2023;67:101894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (1)] |
| 16. | Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen Q, Zhang Y, Lu Y, Ding X, Jiang C. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B. 2020;10:1563-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 17. | Tang ZG, Chen TM, Lu Y, Wang Z, Wang XC, Kong Y. Human bone marrow mesenchymal stem cell-derived exosomes loaded with gemcitabine inhibit pancreatic cancer cell proliferation by enhancing apoptosis. World J Gastrointest Oncol. 2024;16:4006-4013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 18. | Shi J, Jiang X, Gao S, Zhu Y, Liu J, Gu T, Shi E. Gene-modified Exosomes Protect the Brain Against Prolonged Deep Hypothermic Circulatory Arrest. Ann Thorac Surg. 2021;111:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 19. | Brossa A, Tapparo M, Fonsato V, Papadimitriou E, Delena M, Camussi G, Bussolati B. Coincubation as miR-Loading Strategy to Improve the Anti-Tumor Effect of Stem Cell-Derived EVs. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 20. | Thakur A, Sidu RK, Zou H, Alam MK, Yang M, Lee Y. Inhibition of Glioma Cells' Proliferation by Doxorubicin-Loaded Exosomes via Microfluidics. Int J Nanomedicine. 2020;15:8331-8343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (1)] |
| 21. | Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2017;312:L110-L121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 241] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 22. | Lokossou AG, Toudic C, Nguyen PT, Elisseeff X, Vargas A, Rassart É, Lafond J, Leduc L, Bourgault S, Gilbert C, Scorza T, Tolosa J, Barbeau B. Endogenous retrovirus-encoded Syncytin-2 contributes to exosome-mediated immunosuppression of T cells†. Biol Reprod. 2020;102:185-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 23. | Zhupanyn P, Ewe A, Büch T, Malek A, Rademacher P, Müller C, Reinert A, Jaimes Y, Aigner A. Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J Control Release. 2020;319:63-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 24. | Wang H, Chen FS, Zhang ZL, Zhou HX, Ma H, Li XQ. MiR-126-3p-Enriched Extracellular Vesicles from Hypoxia-Preconditioned VSC 4.1 Neurons Attenuate Ischaemia-Reperfusion-Induced Pain Hypersensitivity by Regulating the PIK3R2-Mediated Pathway. Mol Neurobiol. 2021;58:821-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 25. | Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS, Jay SM. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell Mol Bioeng. 2016;9:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 263] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 26. | Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 550] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 27. | Hajipour H, Farzadi L, Roshangar L, Latifi Z, Kahroba H, Shahnazi V, Hamdi K, Ghasemzadeh A, Fattahi A, Nouri M. A human chorionic gonadotropin (hCG) delivery platform using engineered uterine exosomes to improve endometrial receptivity. Life Sci. 2021;275:119351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 28. | Johnsen KB, Gudbergsson JM, Skov MN, Christiansen G, Gurevich L, Moos T, Duroux M. Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology. 2016;68:2125-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 29. | Jeyaram A, Lamichhane TN, Wang S, Zou L, Dahal E, Kronstadt SM, Levy D, Parajuli B, Knudsen DR, Chao W, Jay SM. Enhanced Loading of Functional miRNA Cargo via pH Gradient Modification of Extracellular Vesicles. Mol Ther. 2020;28:975-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 30. | de Liyis BG, Nolan J, Maharjana MA. Fibroblast growth factor receptor 1-bound extracellular vesicle as novel therapy for osteoarthritis. Biomedicine (Taipei). 2022;12:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Keshishyan S, Sehdev V, Reeves D, Ray SD. Cytostatic Agents. Side Effects of Drugs Annual. 2015;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Stefańska K, Józkowiak M, Angelova Volponi A, Shibli JA, Golkar-Narenji A, Antosik P, Bukowska D, Piotrowska-Kempisty H, Mozdziak P, Dzięgiel P, Podhorska-Okołów M, Zabel M, Dyszkiewicz-Konwińska M, Kempisty B. The Role of Exosomes in Human Carcinogenesis and Cancer Therapy-Recent Findings from Molecular and Clinical Research. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 33. | Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 551] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 34. | Riau AK, Ong HS, Yam GHF, Mehta JS. Sustained Delivery System for Stem Cell-Derived Exosomes. Front Pharmacol. 2019;10:1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 35. | Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 552] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 36. | Shimizu A, Sawada K, Kobayashi M, Oi Y, Oride T, Kinose Y, Kodama M, Hashimoto K, Kimura T. Patient-Derived Exosomes as siRNA Carriers in Ovarian Cancer Treatment. Cancers (Basel). 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 723] [Cited by in RCA: 1198] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 38. | Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 763] [Cited by in RCA: 1006] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 39. | Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 678] [Cited by in RCA: 883] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 40. | Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, Ploix S, Vimond N, Peguillet I, Théry C, Lacroix L, Zoernig I, Dhodapkar K, Dhodapkar M, Viaud S, Soria JC, Reiners KS, Pogge von Strandmann E, Vély F, Rusakiewicz S, Eggermont A, Pitt JM, Zitvogel L, Chaput N. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5:e1071008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 605] [Article Influence: 60.5] [Reference Citation Analysis (0)] |