Published online Jan 15, 2025. doi: 10.4251/wjgo.v17.i1.100204
Revised: October 16, 2024
Accepted: October 29, 2024
Published online: January 15, 2025
Processing time: 124 Days and 18.9 Hours
Colorectal cancer (CRC) is a prevalent malignant neoplasm characterized by subtle early manifestations.
To investigate the correlation among serum lipid profiles, the triglyceride-glucose (TyG) index, and the atherosclerotic index (AI) in patients with CRC. Furth
A retrospective analysis encompassed 277 patients with CRC and 1034 healthy individuals.
Following propensity score matching, patients with CRC exhibited significantly reduced levels of serum triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol (LDL-C), as well as a diminished TyG index. Conversely, they displayed elevated AI levels compared to their healthy counterparts. Patients in advanced stages exhibited lower serum levels of TG, TC, and LDL-C compared to those in early stages. Patients with positive lymph node metastasis demonstrated reduced levels of TG, LDL-C, and the TyG index. Receiver operating characteristic analysis revealed that the combination of the TyG index, carcinoembryonic antigen, and carbohydrate antigen 19-9 yielded the highest positive prediction rate for CRC at 75.3%.
Preoperative serum lipid profiles exhibit a robust association with patients with CRC. The concurrent assessment of multiple serum lipids and cancer antigens effectively enhances the diagnostic accuracy for CRC.
Core Tip: Timely identification of colorectal cancer (CRC) is essential in lowering both the occurrence and death rates linked to the illness. It has been shown that serum lipids and insulin resistance (IR) markers can be useful biomarkers for detecting CRC in patients. This article retrospectively analyzed 277 patients with CRC and investigated the clinical diagnostic value of a combination of IR indicators, including the triglyceride-glucose index and atherosclerotic index, with cancer antigens as a novel diagnostic marker for CRC.
- Citation: Hu RH, Yan DY, Zhang KH, Zhang D, Cui XM, Jiang XH, Zhang S. Lipid levels and insulin resistance markers in patients with colorectal cancer: Propensity score matching analysis. World J Gastrointest Oncol 2025; 17(1): 100204
- URL: https://www.wjgnet.com/1948-5204/full/v17/i1/100204.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i1.100204
Colorectal cancer (CRC) is one of the most common malignant tumors in the world, with 1.9 million new cases per year, and ranking as the second most commonly diagnosed cancer. The prognosis of CRC critically hinges upon the cancer stage determined during diagnosis[1]. Timely detection of CRC is imperative to reduce both its incidence and mortality rates associated with the disease.
Several methods are currently being proposed and used to identify the location of tumors. These methods include the fecal occult blood test, colonoscopy, and computed tomography. Currently, the most widely used diagnostic approach for CRC is colonoscopy, which has high sensitivity and specificity for identifying polyps and cancers[2]. However, it is an invasive, high-cost, and time-consuming procedure that requires complex implementation. Blood-based biomarkers such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) have been recognized and accepted as noninvasive diagnostic procedures established to detect CRC[3].
Metabolic syndrome, consisting of impaired glucose tolerance, dyslipidemia, obesity, and hypertension, is associated with insulin resistance (IR)[4]. Dyslipidemia is an integral part of metabolic syndrome and is characterized by an alteration of serum lipids. In recent years, growing evidence has shown that serum lipids play an important role in tumor development and progression[5]. In particular, it has been shown that serum lipids can be a useful biomarker for detecting CRC in patients[6,7]. However, the limited knowledge of the relationship between serum lipids and the clinical parameters of CRC has obstructed the optimized use for serum lipids when obtaining more accurate and meaningful information for CRC diagnosis and treatment outcomes. Furthermore, there has been no research conducted using propensity score matching (PSM) to investigate the relationship between serum lipids and CRC.
Current evidence has confirmed that IR is a relevant risk factor for the morbidity and mortality of various cancers, including CRC[8,9]. Recently, the triglyceride-glucose (TyG) index and the atherosclerotic index (AI [TC−HDLC]/HDLC]), which are closely related to IR, diabetes, and metabolic syndrome, have attracted extensive attention and medical research. Previous studies have demonstrated that the TyG index may serve as a marker of CRC[7,10]. However, the role of the TyG index in cancer risk remains controversial, and its relationship has not been demonstrated in patients with CRC[11]. The AI is widely regarded as a predictor of cardiovascular disease. However, the association between AI and CRC still needs further exploration.
Therefore, we retrospectively analyzed 277 patients with CRC and investigated the clinical diagnostic value of a combination of IR indicators, including the TyG index and AI, with cancer antigens as a novel diagnostic marker for CRC.
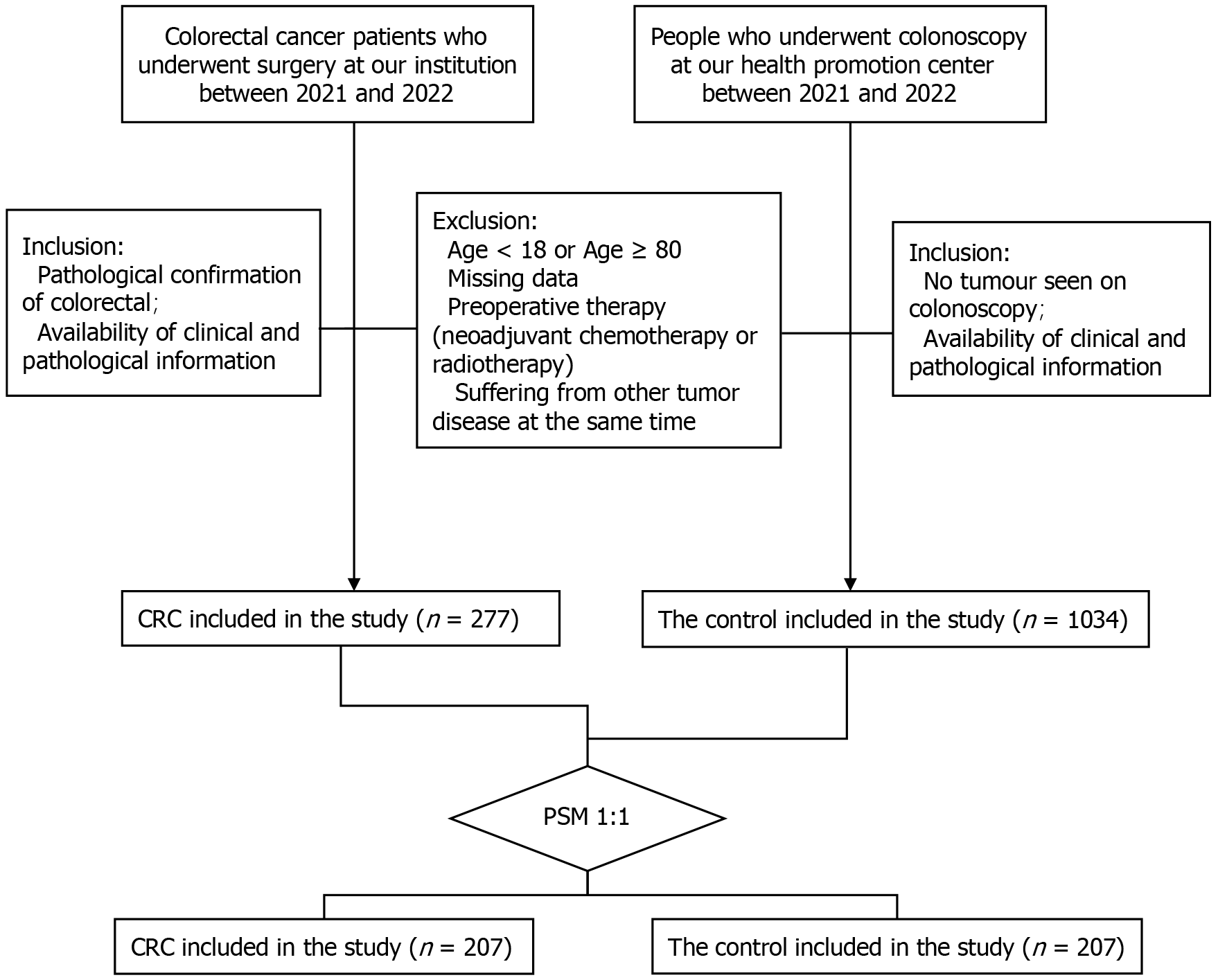
Patients with histologically confirmed CRC at the Department of Gastrointestinal Surgery of Shanghai East Hospital (Shanghai, China) between 2021 and 2022 were retrospectively reviewed. To evaluate the link between CRC and the lipid profile found in the serum, a control group was retrospectively examined from a cohort initially collected at the health promotion center. Patients had a previous history of malignancy. Participants who did not undergo lipid profile assessment in the laboratory or were lost during the follow-up period were excluded from the study. Ultimately, a total of 277 individuals diagnosed with CRC and 1034 healthy control subjects were included in the analysis (Figure 1). All participants provided written informed consent. The methods used in this study were conducted in compliance with the Declaration of Helsinki and other applicable guidelines. The study was approved by the Human Research Ethics Committee of Shanghai East Hospital.
The participants in the study were required to fill out a questionnaire that collected information regarding any current or previous illnesses, both acute and chronic, as well as any medications they were currently taking. Peripheral blood was collected for routine hematological tests and cancer antigens such as CEA and CA19-9. The clinicopathological classification of CRC was performed according to the 8th American Joint Committee on Cancer tumor-node-metastasis (TNM) staging system. The serum lipid profiles of all subjects were measured in the study. The levels of serum lipids, such as triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), were assessed utilizing the Roche Cobas e801 chemistry analyzer (Roche Diagnostics, Mannheim, Germany). The TyG index was calculated as Ln (fasting TG [mg/dL] × fasting plasma glucose [mg/dL]/2). AI was analytically calculated from (TC-HDL-C)/HDL-C.
The data are expressed as the mean ± standard deviation in cases of normal distribution, and as the median with the interquartile range for nonparametric distribution. To analyze categorical variables, χ2 or Fisher exact tests were employed, while continuous variables were assessed using the Student's t-test or one-way analysis of variance. To evaluate the discriminatory ability of the variables in predicting the incidence of CRC, a receiver operating characteristic (ROC) curve was constructed. We calculated the corresponding area under the curve, along with its 95% confidence interval (CI). The statistical software Statistical Package for the Social Sciences (version 26.0; IBM, Armonk, NY, United States) was utilized for data analysis, with statistical significance defined as P < 0.05 (two-sided). The effect of serum lipids was evaluated by one-to-one PSM to adjust for factors including sex, body mass index (BMI), coronary heart disease, cerebral infarction, and hypertension.
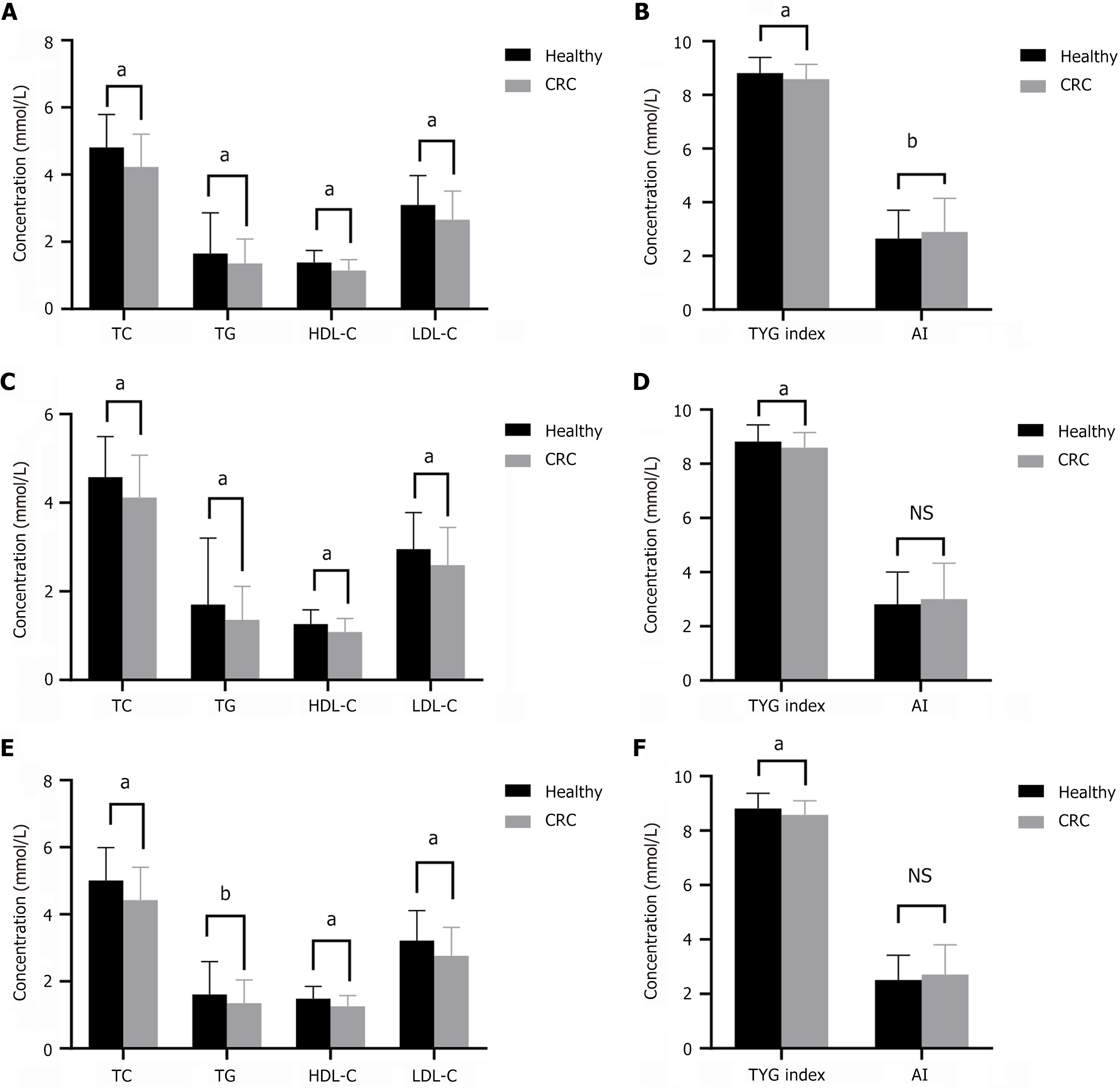
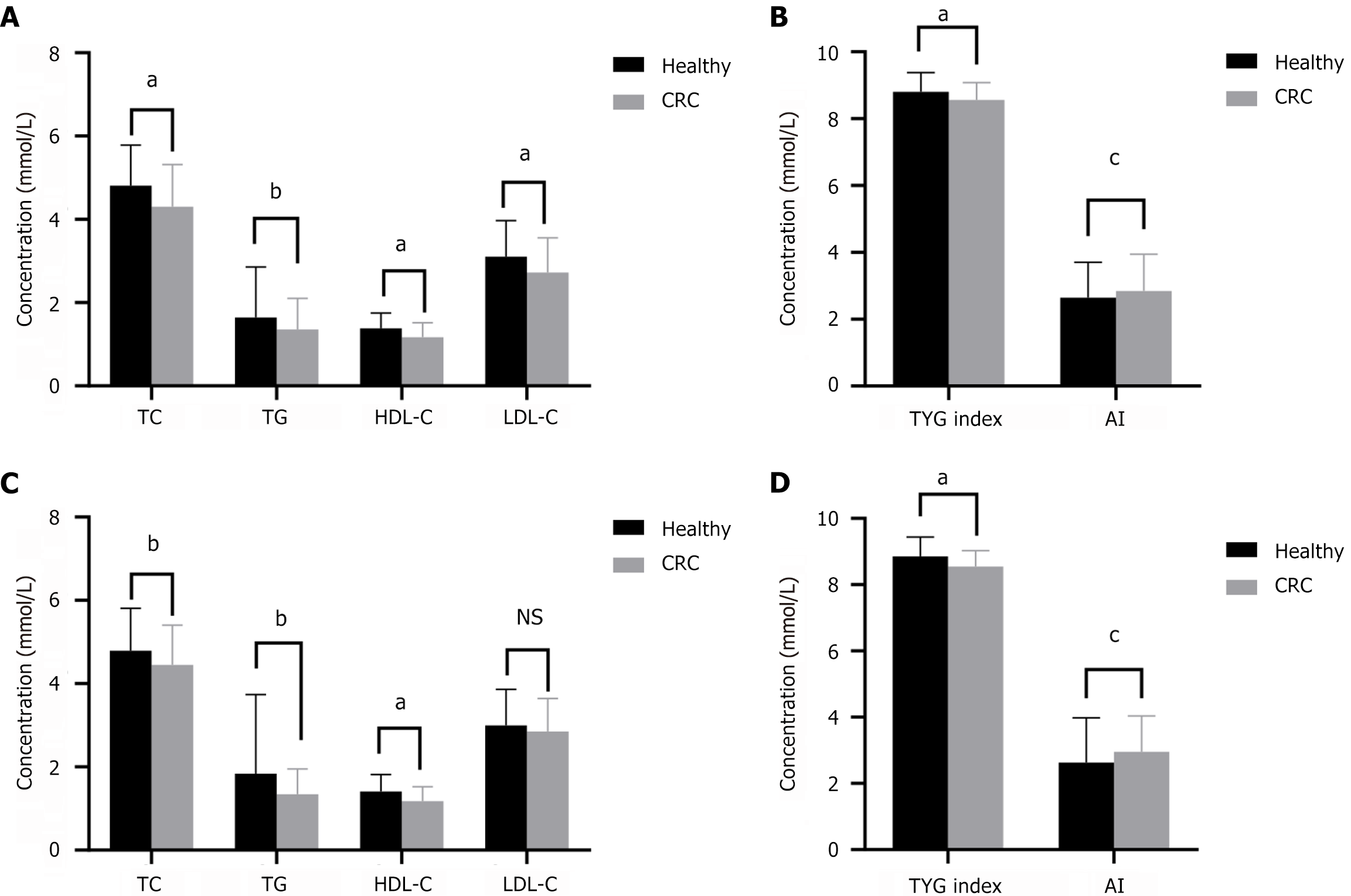
We identified 277 patients with CRC and 1034 healthy participants. Table 1 presents the fundamental data of the patients with CRC. There were significant differences between groups in terms of BMI, white blood cells, red blood cells, hemoglobin, and platelets (Table 2). The serum levels of TG, TC, HDL-C, and LDL-C, and the TyG index were significantly lower in patients with CRC than in healthy participants (P < 0.01), whereas AI was significantly higher in patients with CRC than in healthy participants (P < 0.01) (Figure 2A and B). The patients with CRC and healthy participants were subsequently divided into two subgroups depending on sex. Our results indicated that TG, TC, HDL-C, LDL-C, and TyG index were also significantly lower in both male and female patients compared with the same sex group in healthy participants (Figure 2C-F).
| Characteristics | All CRC, n = 277 | Propensity-matched CRC, n = 207 |
| Ascending colon | 55 (11.4) | 42 (8.7) |
| Transverse colon | 11 (2.3) | 8 (1.7) |
| Descending colon | 18 (3.7) | 14 (2.9) |
| Sigmoid colon | 68 (14) | 49 (10.1) |
| Rectum | 125 (25.8) | 94 (19.4) |
| T. stage | ||
| T1 | 28 (5.8) | 23 (4.8) |
| T2 | 36 (7.4) | 25 (5.2) |
| T3 | 196 (40.5) | 148 (30.6) |
| T4 | 17 (3.5) | 11 (2.3) |
| N. stage | ||
| N0 | 156 (32.2) | 119 (24.6) |
| N1 | 71 (14.7) | 53 (11) |
| N2 | 50 (10.3) | 35 (7.2) |
| M. stage | ||
| M0 | 251 (51.9) | 190 (39.3) |
| M1 | 26 (5.4) | 17 (3.5) |
| Blood vessels | ||
| - | 199 (41.1) | 150 (31) |
| + | 78 (16.1) | 57 (11.8) |
| Nerve | ||
| - | 210 (43.4) | 159 (32.9) |
| + | 67 (13.8) | 48 (9.9) |
| Size, median (IQR) | 4 (3, 5) | 4 (3, 5) |
| Differentiation | ||
| Low grade | 94 (19.4) | 69 (14.3) |
| High grade | 183 (37.8) | 138 (28.5) |
| Stage | ||
| I | 55 (11.4) | 41 (8.2) |
| II | 95 (19.6) | 72 (14.9) |
| III | 100 (20.7) | 73 (15.1) |
| IV | 27 (5.6) | 19 (3.9) |
| Characteristics | All patients | Propensity-matched patients | ||||
| Control, n = 1034 | CRC, n = 277 | P value | Controls, n = 207 | CRC, n = 207 | P value | |
| Age in years | 66.53 ± 7.41 | 66.54 ± 10.91 | 0.989 | 68.89 ± 5.53 | 65.58 ± 11.12 | 0.053 |
| Sex as male/female | 466/568 | 176/101 | < 0.001 | 96/111 | 122/85 | 0.010 |
| Hypertension in % | 10.25 | 43.68 | < 0.001 | 34.78 | 28.99 | 0.206 |
| Diabetes in % | 1.64 | 14.80 | < 0.001 | 7.73 | 5.80 | 0.434 |
| Coronary heart disease or Cerebral infarction in % | 0.48% | 12.64 | < 0.001 | 2.24 | 2.24 | 1 |
| BMI | 25.09 ± 5.25 | 23.78 ± 3.27 | < 0.001 | 24.40 ± 3.77 | 23.84 ± 3.29 | 0.106 |
| WBC as × 109/L | 6.49 ± 1.58 | 6.16 ± 1.84 | 0.003 | 6.64 ± 1.66 | 6.14 ± 1.84 | 0.004 |
| RBC as × 109/L | 4.70 ± 0.43 | 4.19 ± 0.61 | < 0.001 | 4.66 ± 0.40 | 4.21 ± 0.58 | < 0.001 |
| Hb in g/L | 143.23 ± 13.24 | 120.63 ± 25.73 | < 0.001 | 141.89 ± 12.61 | 121.47 ± 25.08 | < 0.001 |
| PLT as × 109/L | 216.94 ± 52.78 | 240.59 ± 83.72 | < 0.001 | 224.17 ± 51.69 | 240.94 ± 78.97 | 0.011 |
| ALT in U/L | 21.36 ± 7.75 | 18.50 ± 31.60 | 0.144 | 20.33 ± 7.63 | 17.65 ± 18.27 | 0.057 |
| AST in U/L | 21.40 ± 12.65 | 21.24 ± 35.77 | 0.908 | 19.36 ± 12.60 | 19.73 ± 11.93 | 0.758 |
| TC in mmol/L | 4.81 ± 0.98 | 4.23 ± 0.97 | < 0.001 | 4.80 ± 1.02 | 4.34 ± 0.94 | < 0.001 |
| TG in mmol/L | 1.65 ± 1.21 | 1.35 ± 0.73 | < 0.001 | 1.84 ± 1.87 | 1.33 ± 0.65 | < 0.001 |
| HDL-C in mmol/L | 1.38 ± 0.36 | 1.14 ± 0.32 | < 0.001 | 1.41 ± 0.41 | 1.17 ± 0.32 | < 0.001 |
| LDL-C in mmol/L | 3.10 ± 0.87 | 2.66 ± 0.85 | < 0.001 | 3.01 ± 0.86 | 2.76 ± 0.83 | 0.003 |
| UA in mmol/L | 319.84 ± 77.29 | 315.62 ± 81.37 | 0.430 | 324.75 ± 80.97 | 312.42 ± 78.70 | 0.121 |
| SCR in mmoI/L | 74.63 ± 22.89 | 76.67 ± 21.08 | 0.190 | 78.29 ± 26.10 | 73.84 ± 19.89 | 0.056 |
| CEA in ng/mL | 1.91 ± 1.51 | 35.67 ± 176.15 | 0.002 | 2.80 ± 1.54 | 26.62 ± 95.89 | 0.001 |
| CA199 in U/mL | 11.26 ± 7.64 | 176.16 ± 1013.41 | 0.009 | 12.00 ± 8.77 | 159.62 ± 929.85 | 0.027 |
| AI | 2.65 ± 1.05 | 35.67 ± 176.15 | 0.002 | 2.64 ± 1.35 | 2.89 ± 1.14 | 0.042 |
| TyG index | 8.81 ± 0.58 | 8.58 ± 0.55 | < 0.001 | 8.86 ± 0.59 | 8.55 ± 0.51 | < 0.001 |
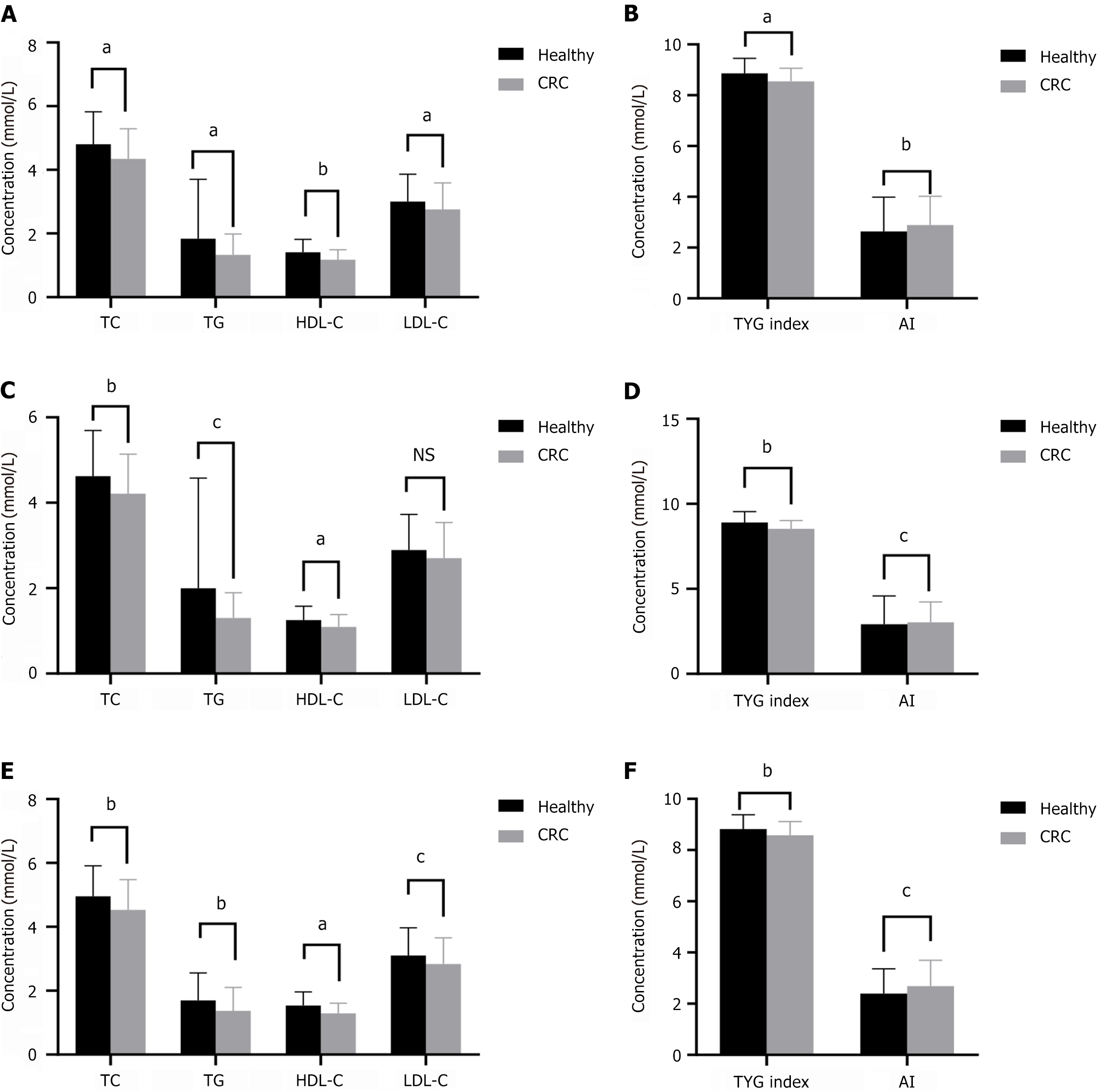
Based on these findings, we implemented PSM using a modified 1:1 ratio. We achieved successful matching of 414 participants, evenly distributed with 207 individuals in each group. Post PSM, both groups demonstrated robust balance across all variables. Analysis of matched patients showed similar results (Figure 3).
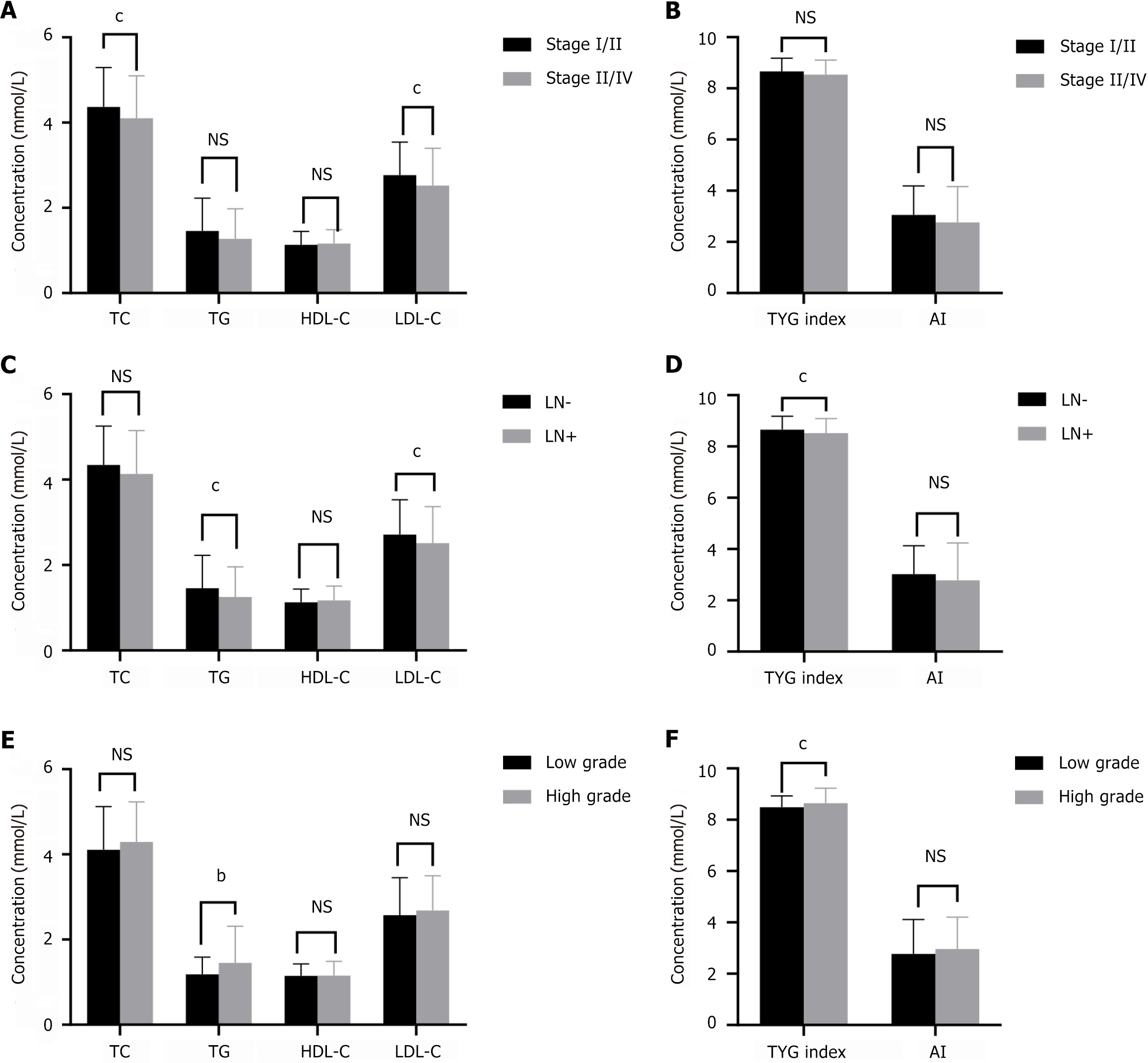
To evaluate the relationship between serum lipids and tumor stage, all patients were stratified into two groups: those with relatively early-stage tumors (stages I and II) and those with advanced-stage tumors (stages III and IV). We found that TC and LDL-C were significantly lower in patients with advanced-stage tumors (Figure 4A and B). The levels of TG and LDL-C and the TyG index in patients with CRC with lymph node metastasis were significantly lower than those in patients without lymph node metastasis (Figure 4C and D). The levels of TG and the TyG index were associated with poor differentiation in patients with CRC (Figure 4E and F).
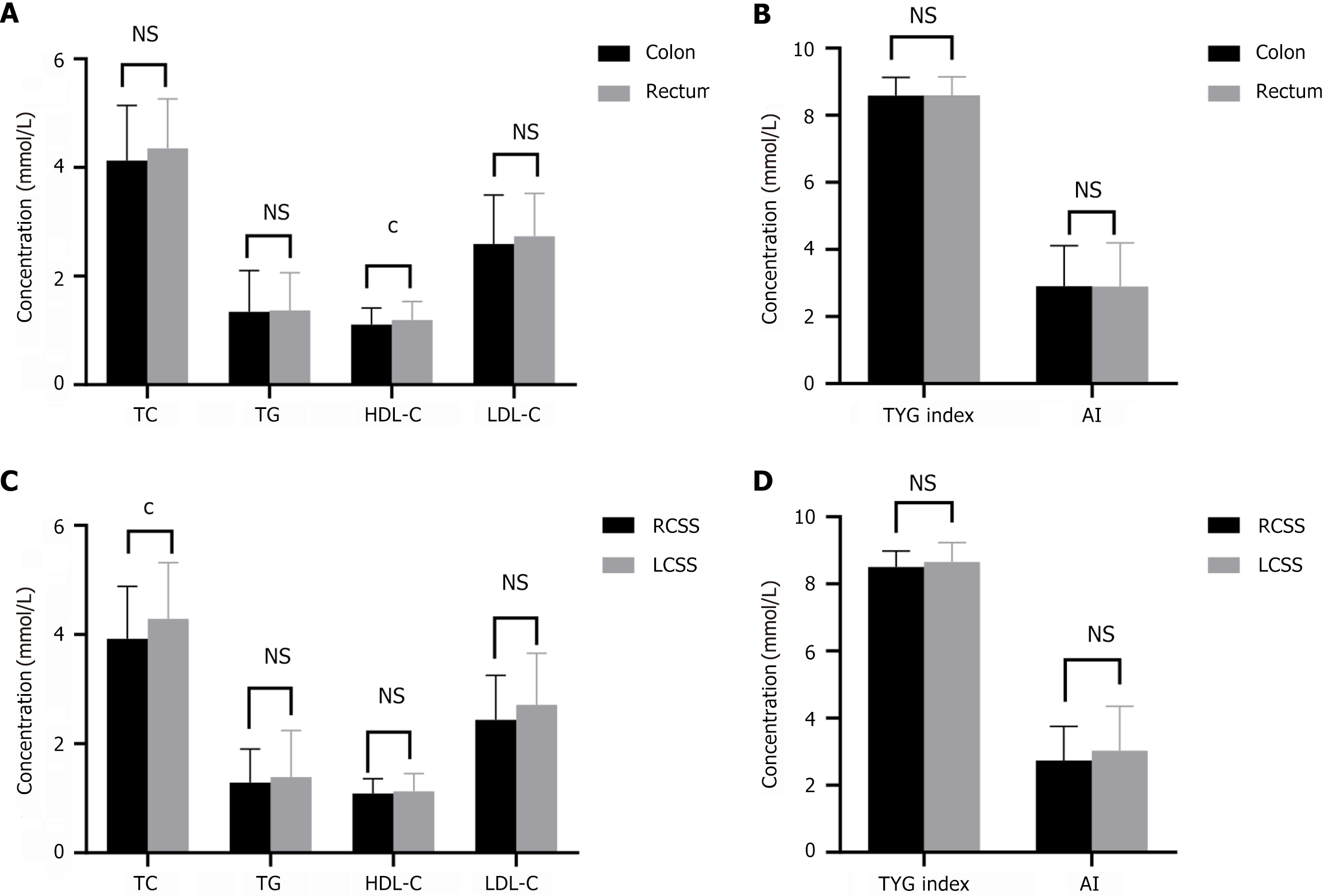
Of the 277 patients with CRC, 152 (54.9%) had CRC and 125 (45.1%) had rectal cancer. The serum HDL-C level in patients with CRC was significantly lower than that in patients with rectal cancer (P < 0.01) (Figure 5A and B). We divided patients with CRC into right-sided CRC (RSCC) and left-sided CRC (LSCC) subgroups. The serum TC level in patients with RSCC was significantly lower than that in patients with LSCC (P < 0.05) (Figure 5C and D).
Of all patients with CRC, 136 had normal preoperative serum CEA and CA19-9 levels. Serum lipid levels were associated with CRC with normal CEA and CA19-9 levels. The levels of TG, TC, HDL-C, and LDL-C and the TyG index in the CRC group were significantly lower than those in healthy participants, whereas AI was higher in the CRC group (Figure 6A and B). After PSM, analysis of matched patients showed similar results (Figure 6C and D).
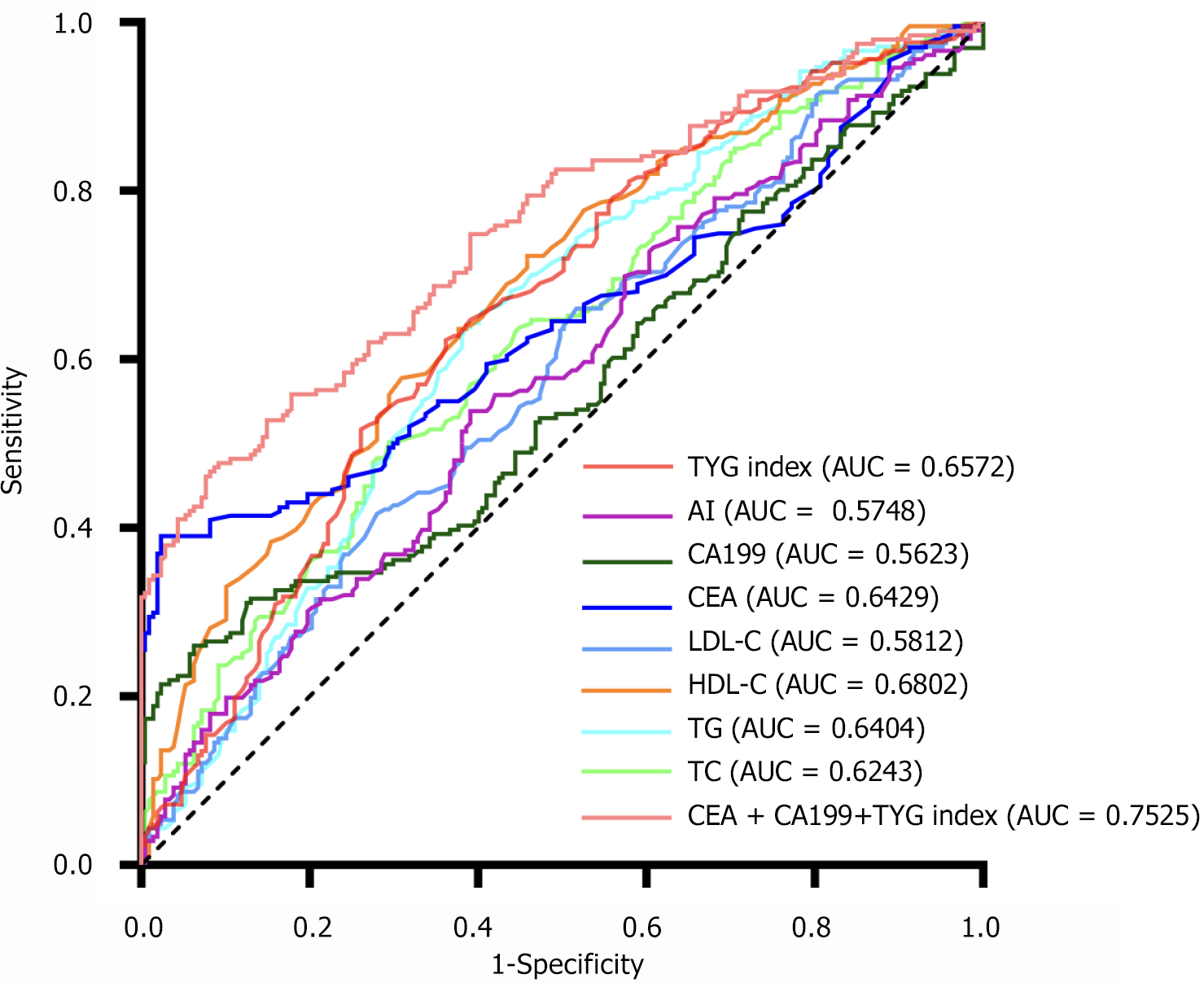
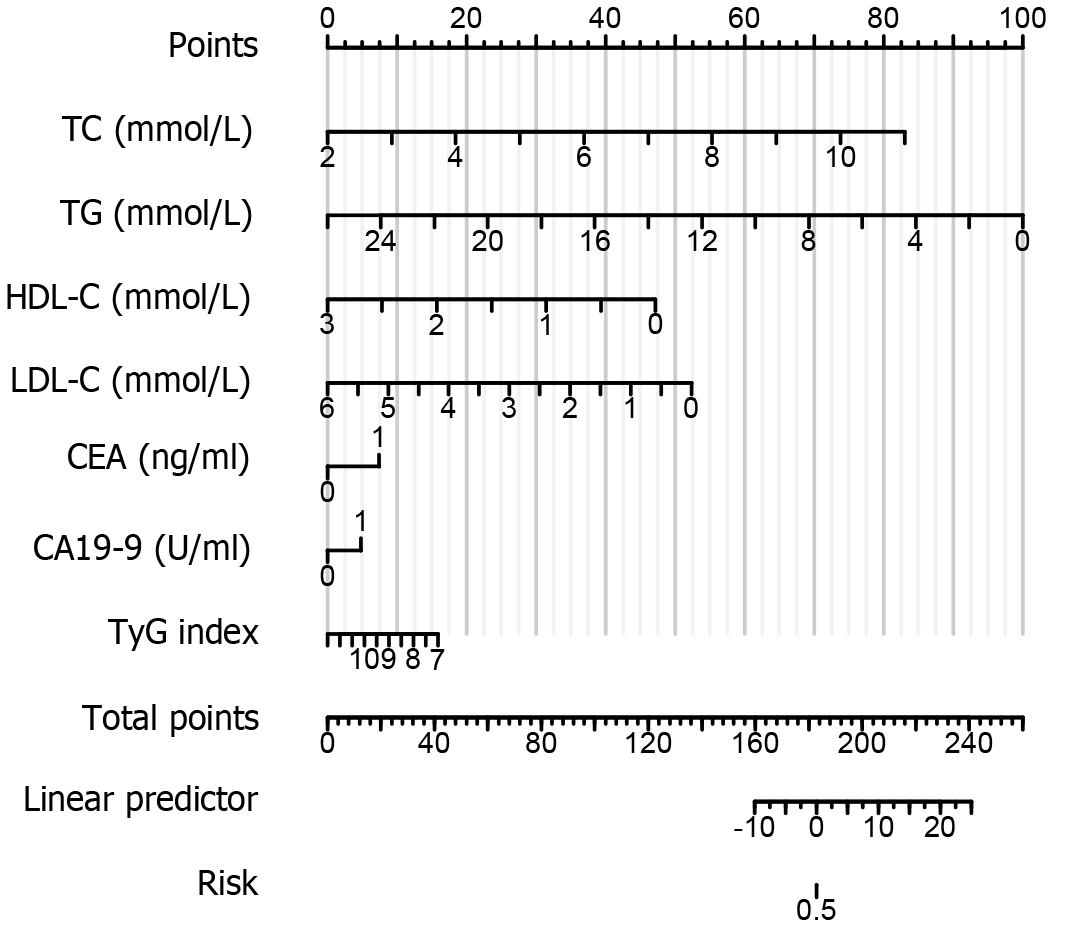
Given the abnormal rates of serum lipid levels and serum cancer antigens in patients with CRC, the TyG index, CEA, and CA19-9 were combined to examine whether the combination can enhance the diagnostic rate in patients with CRC. Our results showed that the combination of the TyG index and cancer antigens can be used as a new diagnostic marker for CRC (Figure 7). The combination of the TyG index, CEA, and CA19-9 had the highest positive prediction rate for CRC (75.3%) by ROC analysis. The remaining serum lipids and cancer antigens are shown in Table 3. Based on this candidate model, we developed a nomogram to visualize CRC diagnosis. Utilizing the patient’s blood lipids, CEA, CA19-9, and TyG index, we can predict the likelihood of the patient having CRC (Figure 8).
| Indicator | AUC | 95%CI | Cut-off value | P value |
| TC | 0.6243 | 0.5709-0.6777 | 4.325 | < 0.0001 |
| TG | 0.6406 | 0.5873-0.6938 | 1.345 | < 0.0001 |
| HDL-C | 0.6802 | 0.6292-0.7311 | 1.165 | < 0.0001 |
| LDL-C | 0.5812 | 0.5263-0.6360 | 3.035 | 0.0043 |
| CEA | 0.6429 | 0.5882-0.6976 | 5.15 | < 0.0001 |
| CA19-9 | 0.5623 | 0.5057-0.6190 | 23.225 | 0.0305 |
| AI | 0.5748 | 0.5199-0.6298 | 2.655 | 0.0085 |
| TyG index | 0.6572 | 0.6048-0.7097 | 8.635 | < 0.0001 |
| CEA + CA199 + TYG index | 0.7525 | 0.7052-0.7997 | < 0.0001 |
Identifying simple, feasible, and low-cost markers of CRC is of great significance for the treatment and prognosis of patients with CRC. However, due to the absence of distinct symptoms and restricted detection techniques, CRC is frequently misdiagnosed, resulting in the disease being detected in its advanced stages when noticeable symptoms emerge. The present retrospective study analyzed the relationship between dyslipidemia and CRC. We also explored whether the combination of serum lipids or the TyG index and AI can enhance the diagnostic rate of CRC. Of interest, our results showed that preoperative serum lipids were significantly lower in patients with CRC than in healthy participants, even after PSM. In addition, the TyG index and AI as IR markers were associated with CRC. Our study found that various factors such as lymphatic metastasis, vascular invasion, nerve infiltration, and pathologic TNM stage can impact the levels of serum lipids. Therefore, we suggest that measuring serum lipid levels, along with the TyG index and AI, can serve as biomarkers for both diagnosing CRC and predicting its characteristics such as lymph node metastasis, vascular invasion, neural infiltration, and staging. These findings highlight the potential of utilizing these biomarkers for improved understanding and management of CRC. Moreover, the use of these biomarkers can contribute to early detection and intervention strategies, ultimately enhancing outcomes for patients with CRC. Moreover, detection of the TyG index and cancer antigens can effectively improve the diagnosis of CRC.
The routine lipid panel consists of TG, TC, HDL-C, and LDL-C. Accumulating evidence suggests that lipid imbalance may contribute to CRC risk through inflammation, oxidative stress, IR, and protein function alteration[12]. A recent study suggested that serum lipids may serve as a potential marker for CRC[5,12]. However, no research has been conducted on the association of the lipid derivative TyG index and AI with patients with CRC, especially by using PSM analysis. Several studies have demonstrated the association of serum lipid levels and cardiovascular diseases. In recent years, evidence has shown that serum lipid components are associated with the occurrence and development of various types of cancer and thus can be used to evaluate cancer. However, data on the relationship of serum lipid levels with CRC are contradictory[13,14]. PSM analysis is a statistical technique designed to address confounding bias and mimic a randomized clinical trial, improving the level of evidence in studies[15]. It was found that the CRC group had lower serum lipid levels before and after PSM. It is hypothesized that the association of low serum lipids and lipoproteins with CRC results from the metabolic, competitive, and rapid consumption of nutrients by cancer cells, rather than increased levels as a protective factor.
Recent epidemiological and clinical evidence points to a strong association of IR with cancer risk[8]. The TyG index, which relies on fasting levels of TGs and glucose, is gaining recognition as a credible and straightforward proxy for IR. The TyG index is associated with an increased risk of cancers of the digestive system. Limited research has explored the correlation between patients with CRC and the TyG index. Our investigation, however, reveals noteworthy findings: The TyG index maintains a significant correlation with patients with CRC, persisting even after PSM. This study found that as the TyG index decreased, the tumor stage of CRC increased, suggesting that the TyG index may be a valuable marker for predicting CRC. ROC analysis results indicated that the area under the ROC of the TyG index was 0.657, further supporting its potential as a reliable diagnostic tool for CRC. AI is also closely related to IR, diabetes, and metabolic syndrome. Compared to previous studies, we demonstrated for the first time that both the TyG index and AI are reliable blood-based biomarkers for patients with CRC.
Tumor location plays a vital role in determining the progression of the disease, prognosis, and the appropriate management. Various factors, such as the embryonic origin of the tumor, its vascular and nervous supplies, as well as its microbiotic burden, contribute to these differences[16]. When analyzed by anatomical site, the results indicated a positive and significant association between HDL-C and rectal cancer. At a physiological level, RSCC is more likely to exhibit advanced tumor stage and increased lymphovascular invasion[17] with poor prognosis and overall survival compared with LSCC[18]. When subgroup analysis was stratified by the left and right colons, TC levels were much lower in patients with RSCC than in those with LSCC.
Over the years, researchers have pursued tumor markers capable of detecting early stages of malignant cell transformation. These biomarkers, typically proteins associated with cancer, hold promise for clinical applications in patients with cancer. Several indicators of CRC, including CEA, CA 19-9, tissue polypeptide-specific antigen, tumor-associated glycoprotein 72, and hematopoietic growth factors, are widely established and routinely used in clinical practice[19]. Nevertheless, these assessments often lack outstanding diagnostic accuracy. Individual marker analyses for disease detection and prognosis are frequently limited by the modest sensitivity and specificity of conventional medical protocols. A more effective strategy involves simultaneous evaluation of multiple markers to improve diagnostic efficacy. The tumor markers CEA and CA19-9 are widely used for real-world opportunistic cancer screening, treatment response assessment, and recurrence monitoring in CRC. However, both tumor markers do not necessarily increase in all cases; sometimes none or only one of the two tumor markers increases. It is possible that patients with advanced stages may not exhibit elevated serum levels of CEA or CA19-9. A recent study suggested that serum lipids may serve as biomarkers for CRC. Nonetheless, there is a dearth of investigations that have delved into the possibility of utilizing serum lipids to diagnose patients with CRC displaying normal levels of CEA and CA19-9[6]. Our results showed that even in patients with normal CEA and CA19-9, serum lipids, TyG index, and AI had a strong association with CRC, even after PMS.
Due to the highly heterogeneous nature of CRC, a single tumor marker is unlikely to have sufficient sensitivity or specificity for use in diagnostic tests. Moreover, combinations of two or more types of biomarkers are often used in clinical practice to improve the accuracy and efficiency of disease diagnosis. In our study, combining serum lipids and tumor markers improved clinical sensitivity for the detection of CRC and could be a more effective marker.
Our study had several limitations. Initially, it should be noted that this study was conducted retrospectively at a solitary institution, which introduces the possibility of selection bias. To adequately assess the diagnostic efficacy of serum lipids, it is imperative to conduct prospective studies spanning multiple institutions. Additionally, due to the limited number of patients with CRC, our dataset cannot be split into separate training and test sets for statistical validation. The lack of a validation cohort hampers a thorough confirmation of our findings. Moving forward, we plan to collect additional data to facilitate validation efforts. Additionally, we failed to assess treatment response or perform survival analysis based on serum lipid levels. Nevertheless, our findings convincingly demonstrate that the amalgamation of serum lipids and tumor markers holds substantial diagnostic capability for patients with CRC.
In conclusion, the results of our study suggest that the serum lipids have strong association with CRC. The detection of TyG index and cancer antigens can effectively improve the diagnosis of CRC.
| 1. | Pang X, Xu B, Lian J, Wang R, Wang X, Shao J, Tang S, Lu H. Real-world survival of colon cancer after radical surgery: A single-institutional retrospective analysis. Front Oncol. 2022;12:914076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (1)] |
| 2. | Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, Rupinski M, Dekker E, Spaander M, Bugajski M, Holme Ø, Zauber AG, Pilonis ND, Mroz A, Kuipers EJ, Shi J, Hernán MA, Adami HO, Regula J, Hoff G, Kaminski MF; NordICC Study Group. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N Engl J Med. 2022;387:1547-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 404] [Article Influence: 134.7] [Reference Citation Analysis (1)] |
| 3. | Gao Y, Wang J, Zhou Y, Sheng S, Qian SY, Huo X. Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and Ferritin as Diagnostic Markers and Factors of Clinical Parameters for Colorectal Cancer. Sci Rep. 2018;8:2732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 4. | O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 1054] [Article Influence: 105.4] [Reference Citation Analysis (1)] |
| 5. | Fang Z, He M, Song M. Serum lipid profiles and risk of colorectal cancer: a prospective cohort study in the UK Biobank. Br J Cancer. 2021;124:663-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 6. | Li T, Zhou Y, Wang J, Xiao S, Duan Y, Li C, Gao Y, An H, Tao N. Association of triglyceride-glucose index with the risk of prostate cancer: a retrospective study. PeerJ. 2023;11:e16313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 7. | Liu T, Zhang Q, Wang Y, Ma X, Zhang Q, Song M, Cao L, Shi H. Association between the TyG index and TG/HDL-C ratio as insulin resistance markers and the risk of colorectal cancer. BMC Cancer. 2022;22:1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (1)] |
| 8. | Arcidiacono B, Iiritano S, Nocera A, Possidente K, Nevolo MT, Ventura V, Foti D, Chiefari E, Brunetti A. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res. 2012;2012:789174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 396] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 9. | Yamamoto S, Nakagawa T, Matsushita Y, Kusano S, Hayashi T, Irokawa M, Aoki T, Korogi Y, Mizoue T. Visceral fat area and markers of insulin resistance in relation to colorectal neoplasia. Diabetes Care. 2010;33:184-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 10. | Li W, Liu T, Qian L, Wang Y, Ma X, Cao L, Zhang Q, Qu J. Insulin resistance and inflammation mediate the association of abdominal obesity with colorectal cancer risk. Front Endocrinol (Lausanne). 2022;13:983160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (1)] |
| 11. | Wang H, Yan F, Cui Y, Chen F, Wang G, Cui W. Association between triglyceride glucose index and risk of cancer: A meta-analysis. Front Endocrinol (Lausanne). 2022;13:1098492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (1)] |
| 12. | Yang Z, Tang H, Lu S, Sun X, Rao B. Relationship between serum lipid level and colorectal cancer: a systemic review and meta-analysis. BMJ Open. 2022;12:e052373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 13. | Chung YW, Han DS, Park YK, Son BK, Paik CH, Lee HL, Jeon YC, Sohn JH. Association of obesity, serum glucose and lipids with the risk of advanced colorectal adenoma and cancer: a case-control study in Korea. Dig Liver Dis. 2006;38:668-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 14. | Yamada K, Araki S, Tamura M, Sakai I, Takahashi Y, Kashihara H, Kono S. Relation of serum total cholesterol, serum triglycerides and fasting plasma glucose to colorectal carcinoma in situ. Int J Epidemiol. 1998;27:794-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 15. | Lonjon G, Boutron I, Trinquart L, Ahmad N, Aim F, Nizard R, Ravaud P. Comparison of treatment effect estimates from prospective nonrandomized studies with propensity score analysis and randomized controlled trials of surgical procedures. Ann Surg. 2014;259:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (1)] |
| 16. | Mukund K, Syulyukina N, Ramamoorthy S, Subramaniam S. Right and left-sided colon cancers - specificity of molecular mechanisms in tumorigenesis and progression. BMC Cancer. 2020;20:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 17. | Narayanan S, Gabriel E, Attwood K, Boland P, Nurkin S. Association of Clinicopathologic and Molecular Markers on Stage-specific Survival of Right Versus Left Colon Cancer. Clin Colorectal Cancer. 2018;17:e671-e678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 18. | Yahagi M, Okabayashi K, Hasegawa H, Tsuruta M, Kitagawa Y. The Worse Prognosis of Right-Sided Compared with Left-Sided Colon Cancers: a Systematic Review and Meta-analysis. J Gastrointest Surg. 2016;20:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 219] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 19. | Jelski W, Mroczko B. Biochemical Markers of Colorectal Cancer - Present and Future. Cancer Manag Res. 2020;12:4789-4797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (1)] |