Published online Sep 15, 2024. doi: 10.4251/wjgo.v16.i9.4028
Revised: July 3, 2024
Accepted: July 19, 2024
Published online: September 15, 2024
Processing time: 100 Days and 22.7 Hours
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal (GI) tract, and cases of GISTs tend to be of the dis
We report two cases of rare GISTs in the same family: A male patient with the V561D mutation in exon 12 of the PDGFRA gene, who has been taking the tar
Different mutation types of familial GISTs in the same family are very rare, thus it is very important to make the correct diagnosis and treatment strategies according to the results of molecular detection for the management of familial GISTs.
Core Tip: Familial gastrointestinal stromal tumors (GISTs) include primary familial GISTs with predominantly KIT/PDGF
- Citation: Wang XK, Shen LF, Yang X, Su H, Wu T, Tao PX, Lv HY, Yao TH, Yi L, Gu YH. Two different mutational types of familial gastrointestinal stromal tumors: Two case reports. World J Gastrointest Oncol 2024; 16(9): 4028-4036
- URL: https://www.wjgnet.com/1948-5204/full/v16/i9/4028.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i9.4028
Gastrointestinal stromal tumors (GISTs), are the most common mesenchymal tumors of the gastrointestinal (GI) tract, and originate from GI pacemaker cells [interstitial cells of Cajal (ICC)] or related stem cells[1]. The biological behavior of GISTs range from benign to malignant, with an incidence rate of 10-15 cases/million each year globally, and they are seen predominantly in middle-aged and elderly people, with the median age of onset being in the 60 years, and with an even distribution in males and females[2]. Tumor sites are most commonly found in the stomach (60%-70%), followed by the small intestine (20%-25%), colon and rectum (5%), and esophagus (< 5%)[3]. The majority of GISTs are caused by KIT mutations (present in 75%-80% of cases) and platelet-derived growth factor receptor A (PDGFRA) mutations (present in approximately 10% of cases)[4], with the remaining 10%-15% of GISTs being referred to as wild-type (wt) GISTs. In GISTs, KIT and PDGFRA mutations are mutually exclusive[5], with KIT mutations generally clustered in exons 11 and 9, while PDGFRA mutations are generally clustered in exons 12, 14 and 18[6]. Targeted chemotherapy based on the relevant mutation sites of KIT/PDGFRA has become essential for the treatment of patients with GISTs.
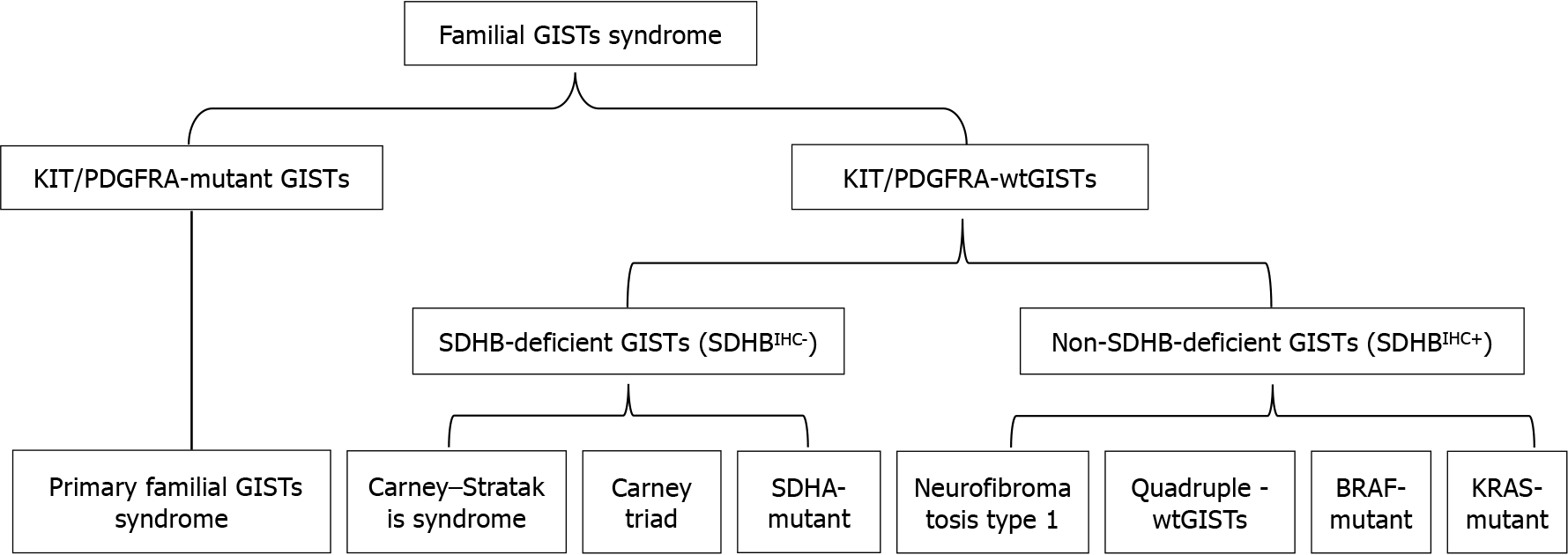
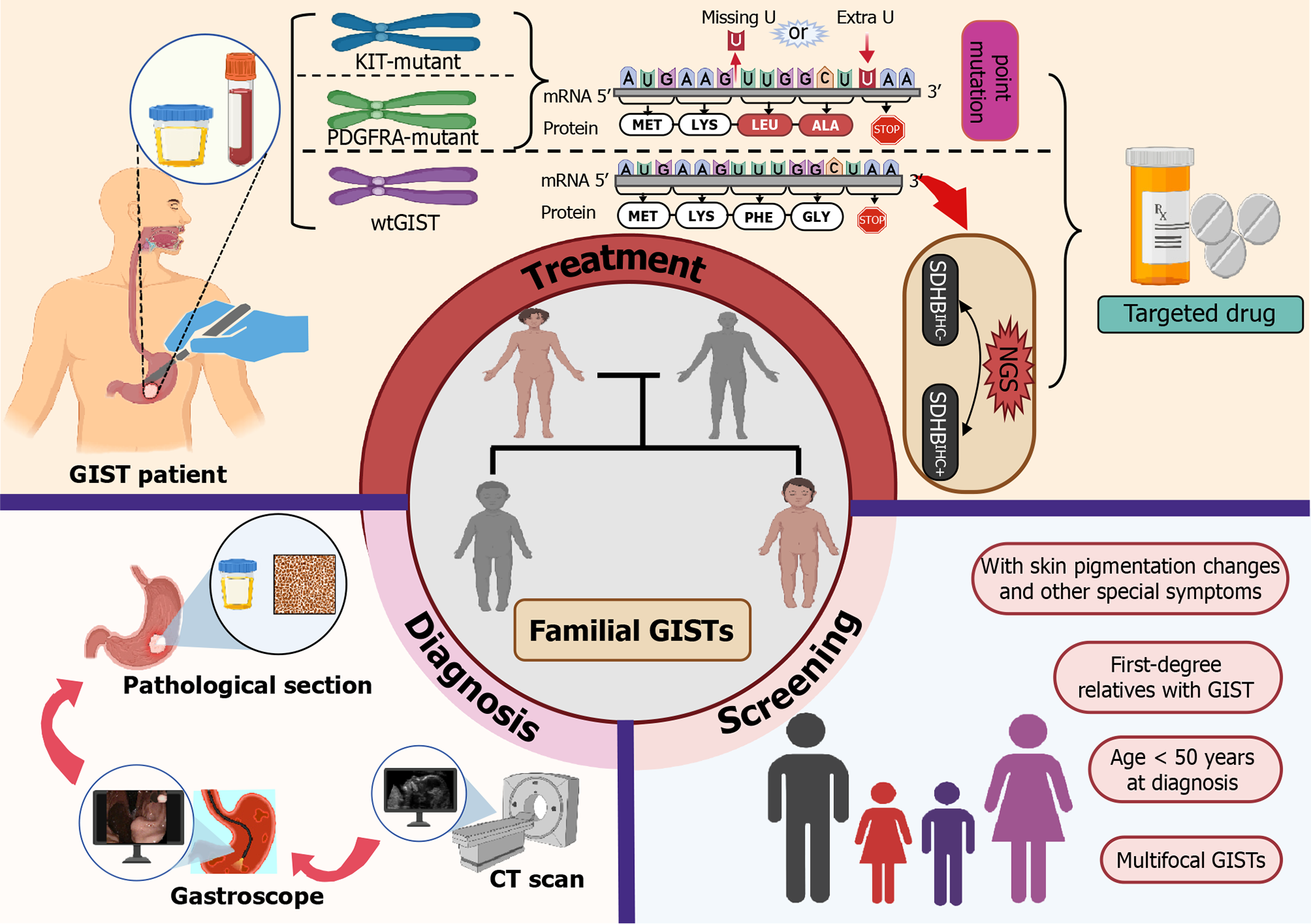
Familial GISTs mainly include primary familial GISTs with predominantly KIT/PDGFRA mutations and wtGISTs, of which wtGISTs with predominantly succinate dehydrogenase complex subunit B (SDHB) defects can be divided into SDHB-deficient and non-SDHB-deficient types. The former includes Carney-Stratakis syndrome (CSS), Carney triad, SDHA mutation and some disseminated GISTs, and the latter includes neurofibromatosis type 1 (NF1)-associated, BRAF mutant, KRAS-mutant, and quadruple wtGISTs (without SDH, NF1, BRAF, and RAS-associated mutations)[7-10] (Figure 1). Among them, the SDHB-deficient type is generally prevalent in young women (37 ± 14 years old), presenting as lobar and/or multifocal tumors in the gastric portion, which usually have a slow clinical course and are often accompanied by lymph node metastasis[11]. It has been demonstrated that negative SDHB immunohistochemical staining (SDHBIHC-) is of great significance in the diagnosis of CSS and Carney’s triad, and diagnosis of the first two conditions should be preferred if SDHB immunostaining is not present in GISTs[12]. SDHBIHC- GIST occurs in young patients, mostly in the stomach, with unremarkable clinical symptoms, and patients have a better overall survival (OS); whereas NF1-GIST generally involves the small intestine and presents as a multifocal spindle cell tumor[11].
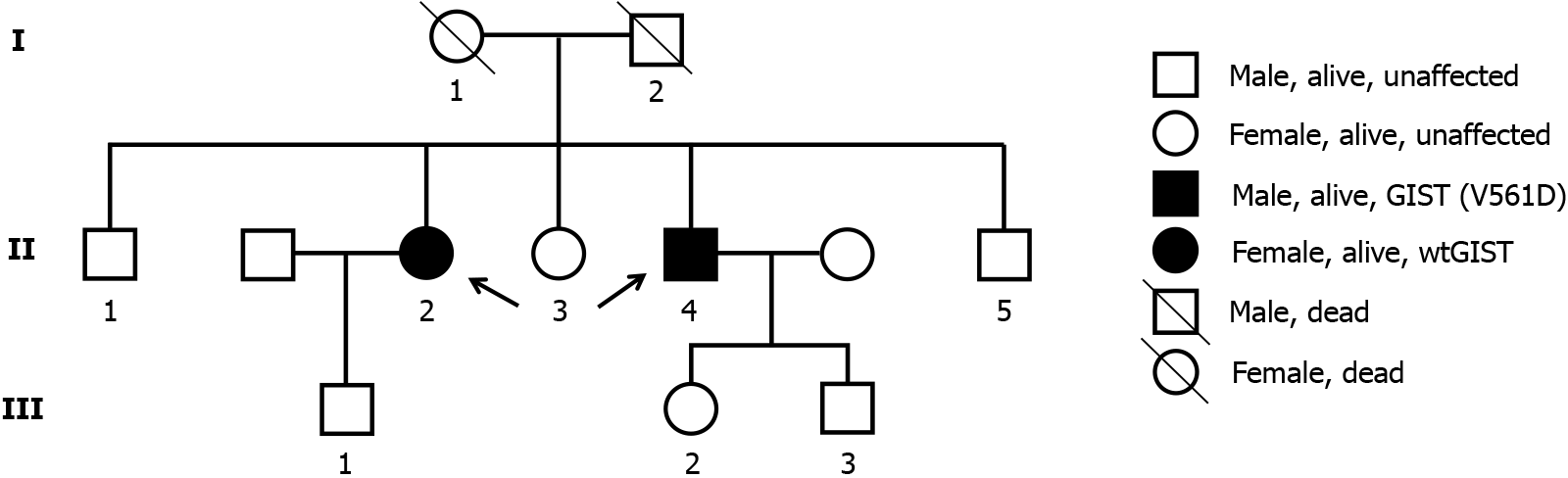
Most GISTs are sporadic and only about 5% of patients with GISTs have one of the familial GISTs syndromes, and sporadic cases of GISTs are indistinguishable from familial GISTs on phenotypic, histologic or molecular grounds alone[13]. However, compared to sporadic GISTs, familial GISTs usually present as multifocal tumors and have an earlier age of onset (40-50 years)[7], as in the female patient in the present study, who presented with multifocal lesions in the stomach and showed different risk grades. In addition, familial GISTs have specific clinical manifestations such as skin pigmentation, mastocytosis, large hands and inflammatory fibrous polyps in addition to common symptoms such as GI bleeding, abdominal distension and abdominal pain[13]; however, the above mentioned manifestations were not demonstrated in our two patients. Here, we report two cases of familial GISTs in a multitumor family (Figure 2), in which the first patient was diagnosed with gastric lesions with multiple wtGISTs (II-2), and following our investigation of the patient’s family history, her younger brother was also diagnosed with GIST (II-4) in the presence of the PDGFRA (V561D) mutation one year earlier (Figure 2). Both patients received adjuvant therapy with imatinib after surgical resection of the tumor and had a good prognosis.
A 61-year-old female patient (elder sister) was admitted to hospital for 3 months due to intermittent abdominal pain and abdominal distension for 2 years and aggravation with loss of appetite. The 49-year-old male patient (younger brother) was admitted to hospital mainly due to intermittent abdominal distension, diarrhea with black stool for more than 3 months, and aggravation with vomiting for 2 weeks.
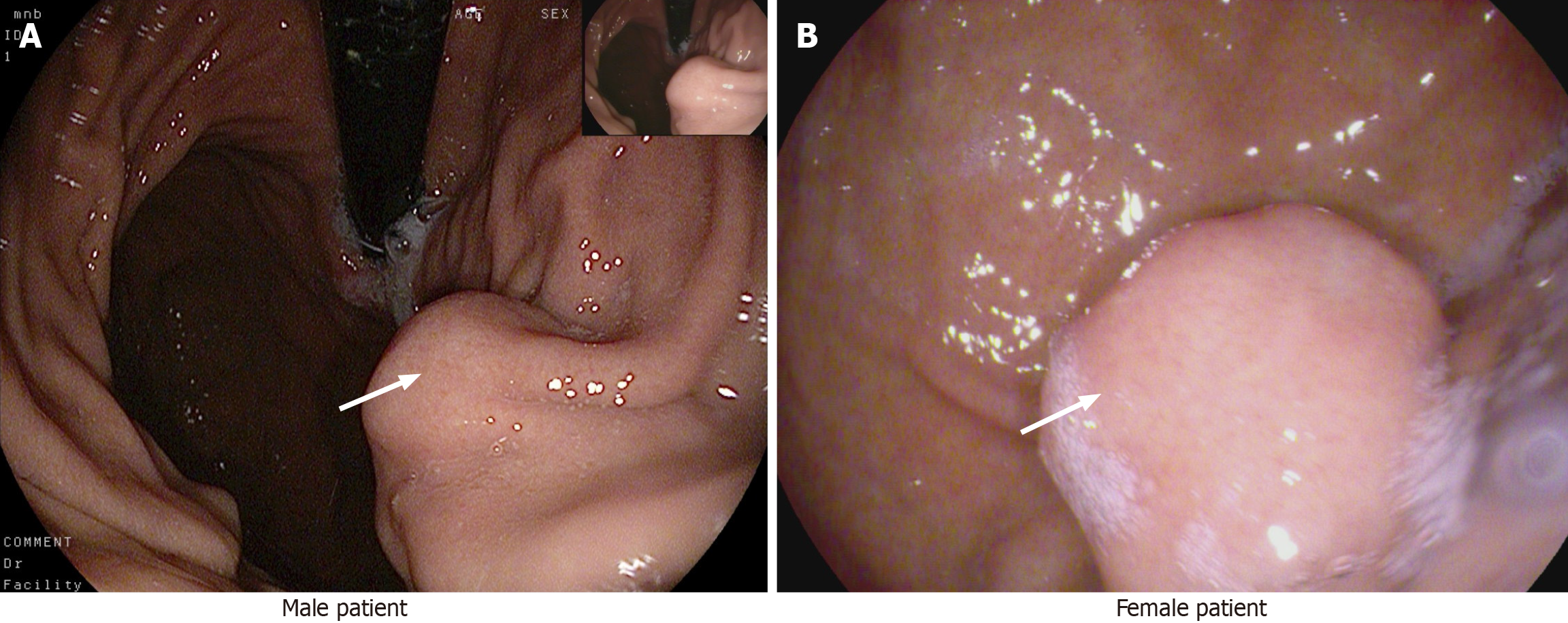
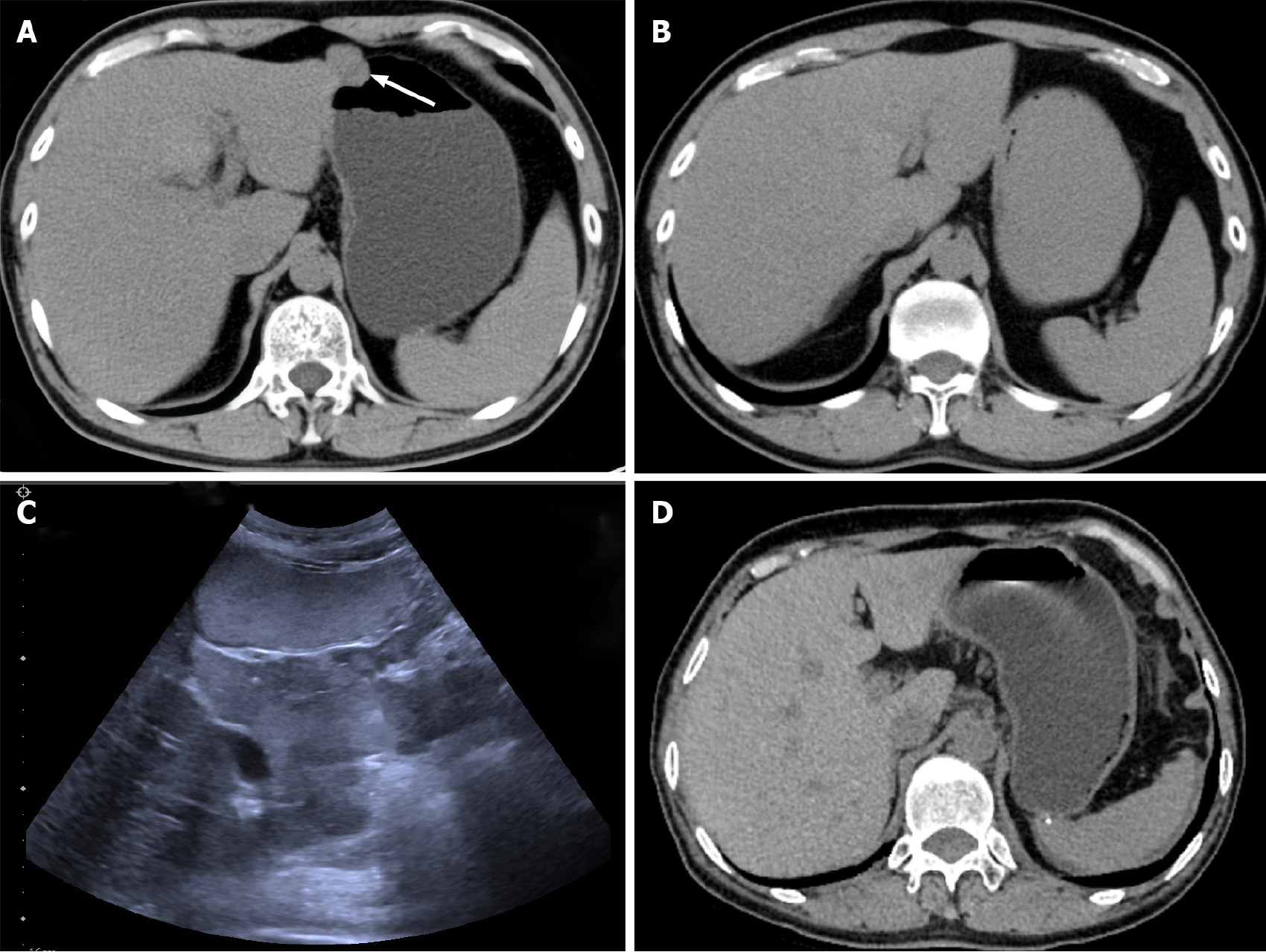
After admission, both patients were examined by electronic gastroscopy and protruding lesions in the gastric mucosa were observed (Figure 3).
Both patients had no other relevant medical history.
Both patients denied a family history of other malignant tumors.
Physical examinations showed no significant abnormalities and vital signs were stable in both patients.
Serum tumor markers including carcinoembryonic antigen, cancer antigen (CA) 199, CA125, CA724, and CA153 were within the normal range in both patients.
Endoscopic ultrasonography findings in the male patient showed the presence of an elevated hypoechoic mucosal lesion originating from the lamina propria on the greater curvature side of the gastric body, with a hyperechoic nodule visible in the center, measuring 3.0 cm × 2.0 cm, with a cross-sectional size of 21.6 mm × 18.7 mm.
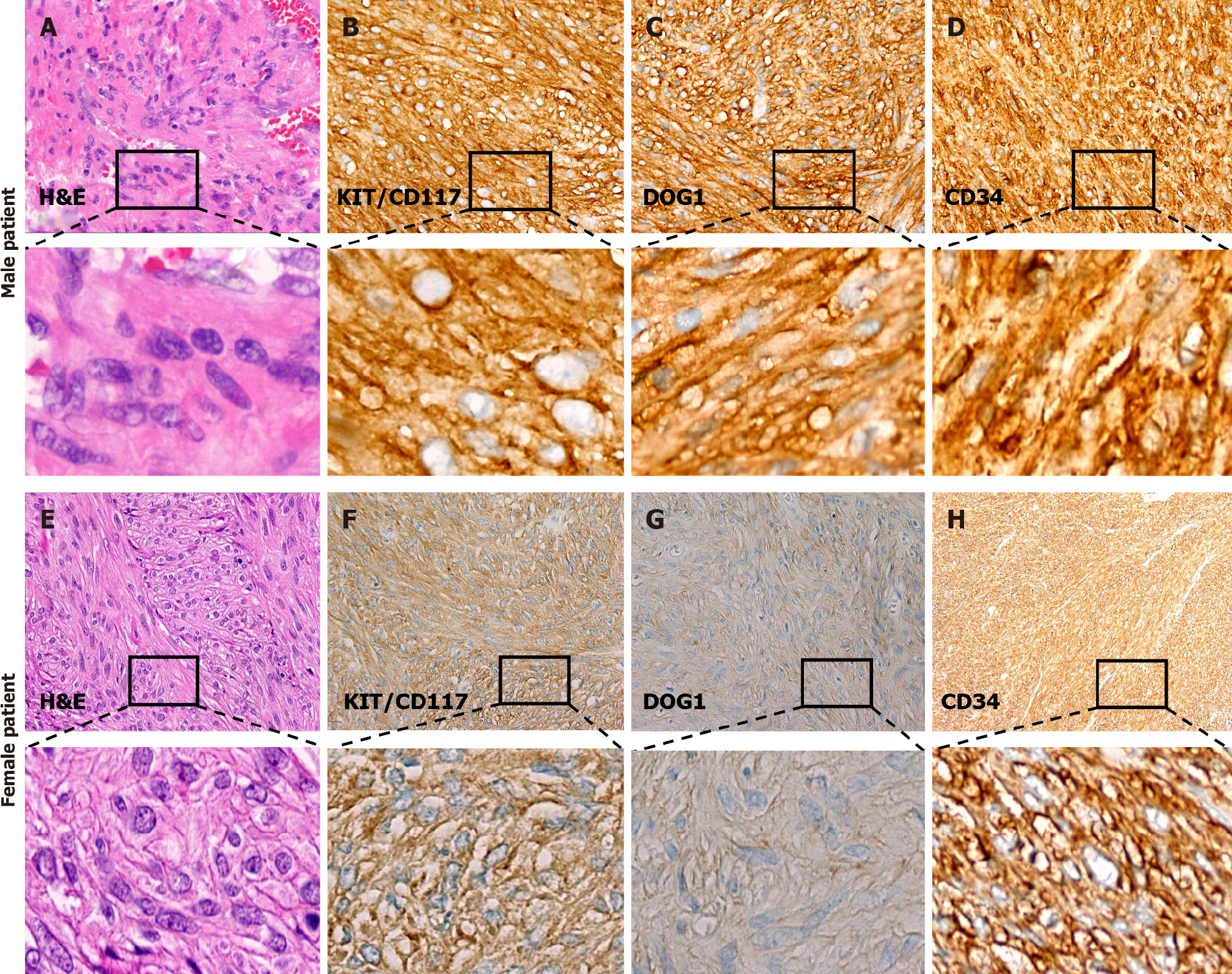
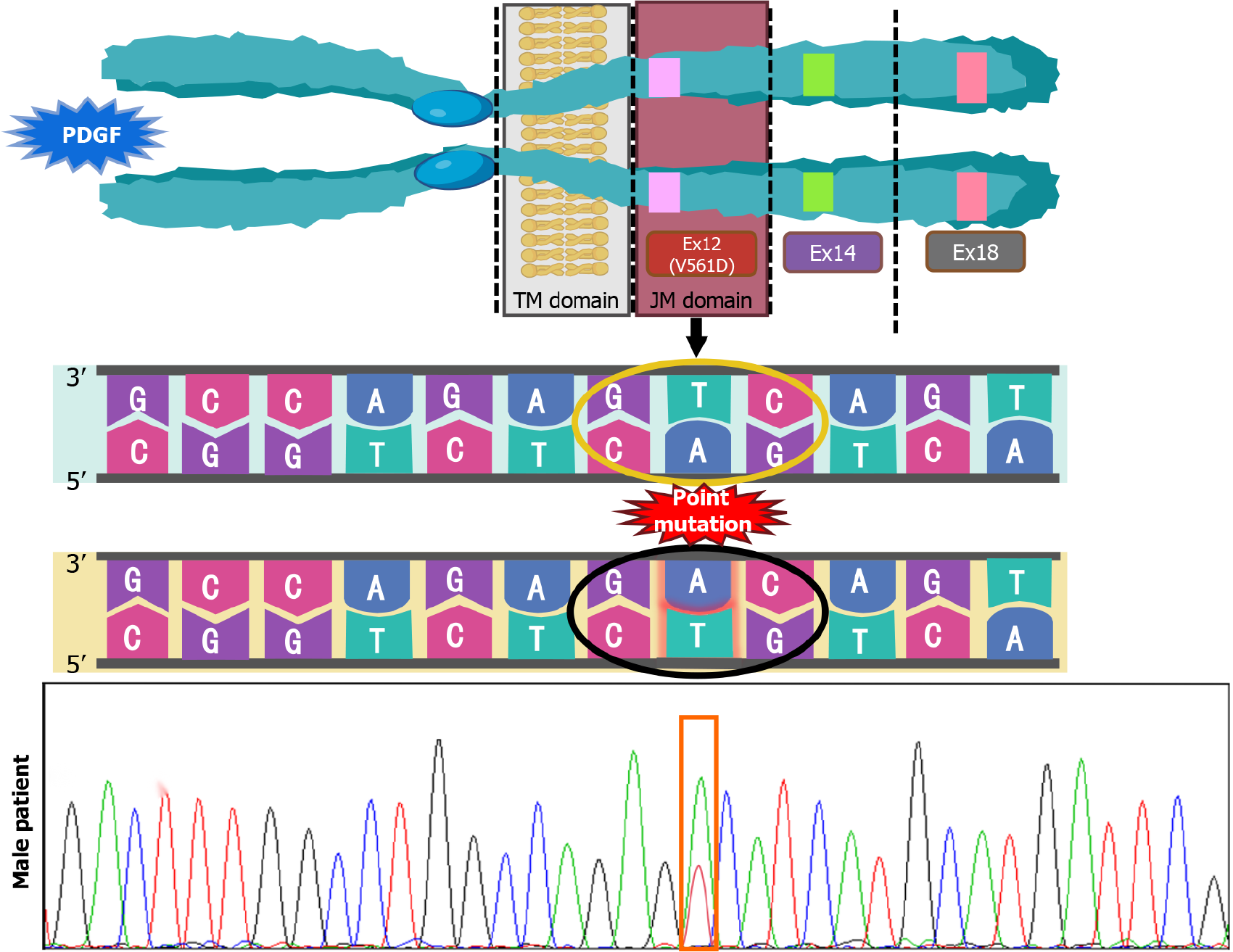
Postoperative histopathological examination of tumor tissue from the male patient showed that the tumor was composed of fusiform cells with a fasciculate arrangement (Figure 4A). The tumor locally invaded the submucosa and lamina propria, and no abnormalities were found in the mucosal layer. Immunohistochemical staining showed that KIT/CD117 (Figure 4B), DOG-1 (Figure 4C) and CD34 were positive (Figure 4D), and the Ki-67 index was 8%-10%. GIST was diagnosed by pathological examination, and the risk grade was high (nuclear fission image: 18/50 HPF). The results of gene detection showed that there was a V561D mutation in exon 12 of the PDGFRA gene (Figure 5).
Postoperative histopathological examination of tissue from the female patient showed that there were two lesions, tumor 1: Size 0.8 cm × 0.8 cm × 0.8 cm, nuclear fission picture: < 5/50 HPF, very low risk; tumor 2: Size 1.5 cm × 1.5 cm × 1.5 cm, nuclear fission image: 6/50 HPF, moderate risk. The tumor tissues were composed of spindle cells with uneven density, arranged in bundles or weaves, and the nuclei were oval or rod-shaped (Figure 4E). Immunohistochemical staining showed that KIT/CD117 (Figure 4F), DOG-1 (Figure 4G) and CD34 were diffusely positive (Figure 4H) and the Ki-67 index was 5%. The results of gene detection showed no KIT/PDGFRA mutation, and that the tumors were wtGISTs.
According to the clinical manifestations and imaging findings, both patients were eventually diagnosed with GISTs.
According to the genetic test results of both patients, the male patient received treatment with oral imatinib 400 mg/day and was followed up every 3 months until now. The female patient received treatment with oral imatinib 400 mg/day and was followed up every 3 months for three years.
According to our recent follow-up results, both patients had a good prognosis and there was no recurrence or metastasis observed (Figure 6).
In the study of familial GISTs, not only activation of KIT or PDGFRA mutations, but possibly other genetic changes are required during the development of GISTs from polyclonal proliferation of diffuse ICC to monoclonal ones[14]. At the structural level, PDGFRA and KIT belong to the same receptor tyrosine kinase (RTK) subfamily, and at the functional level, PDGFRA and KIT mutations lead to the same biological consequences during tumor progression, e.g., leading to the activation of common pathways such as the PI3K/AKT anti-apoptotic pathway, the JAK/STAT3 transcriptional pathway, and the Ras/MAPK mitotic pathway. Response to analogs such as imatinib, demonstrates that PDGFRA is likely to be the second familial GIST susceptibility gene after KIT[8,15,16]. Mutations in exon 18, followed by exon 12, are the most common mutations in the PDGFRA gene, and only one type of mutation, the V561D mutation in exon 12, is known[17].
A total of 54 cases of familial GISTs have been reported to date, of which 49 cases described hereditary GISTs due to germline KIT mutations, 6 cases described familial GISTs associated with germline PDGFRA mutations, and only 1 case of V561D mutation in exon 12 of the PDGFRA gene has been reported in relation to the present case[8]. KIT and PDGFRA are both homologous type III RTKs consisting of five immunoglobulin (Ig)-like structural domains, i.e., ligand-binding extracellular, transmembrane, intracellular juxtamembrane (JM), and two TK structural domains[18], of which the V561D missense mutation is the most common mutation in the PDGFRA JM structural domain[19]. It has been shown that deletions in chromosomal regions 1p33-36, 9q12-24, 11q13, 16q, and 14q may be involved in PDGFRA (V561D) tumorigenesis, and that the V561D mutation can occur in the germline state and results in a syndrome that is distinct from neuroendocrine tumors[16]. As the sensitivity of familial GISTs to TKI is similar to that of sporadic GISTs with the same mutation[20], surgical resection and imatinib supplemented with chemotherapy is the best strategy to potentially improve the patient’s prognosis; therefore, we treated our male patient with imatinib 400 mg/day continuously postoperatively until now, and the patient is doing well during follow-up. Due to the small number of case reports and the large number of patients who were lost due to various factors during late follow-up, there is a lack of more solid evidence to support the duration of treatment, and therefore it is controversial whether to extend the duration of imatinib treatment in familial GISTs. However, the fact that no tumor recurrence was seen in our patient in up to 7 years of imatinib treatment could indicate that long-term continuous treatment is at least prognostically beneficial for patients with this mutation type.
STI571 (imatinib) was first used in the clinic in 2002 and achieved significant efficacy in the treatment of a patient with metastatic GI mesenchymal stromal tumor[21], and since then, a new era of imatinib treatment for GISTs has developed. Imatinib has been used as a first-line treatment for KIT/PDGFRA mutant GISTs, but wtGISTs, a mutant subtype, are often resistant to imatinib. Compared with mutant GISTs, wtGISTs show higher chromosomal stability and little genomic imbalance[22], which leads to wtGISTs being less aggressive than the KIT/PDGFRA mutant type, so the proportion of high-risk wtGISTs is low which are usually inert, and patients with wtGISTs generally have better survival, and some studies have found that the median recurrence-free survival (RFS) time in patients with wtGISTs was 7.5 years, and the median OS was 10.1 years[11]. With these in mind, we terminated imatinib treatment in our female patient after 3 years of adjuvant therapy. The good performance of our female patient during long-term follow-up also reinforces the relative inertia of wtGISTs. However, a recent phase III clinical trial found that during a 10-year follow-up period, patients in the 3-year imatinib group had a longer RFS and OS compared to the 1-year group[23]. Moreover, some studies found that regorafenib had therapeutic superiority for wtGISTs, especially in patients with SDHBIHC- and should be considered as a pre-treatment for advanced wtGISTs[22]. However, due to the limited conditions at the time, we only performed primary genetic testing on the female patient and were unable to perform next-generation sequencing (NGS) to determine more precise typing of wtGISTs as a means of more accurately evaluating the treatment and prognosis of this patient.
As solid tumors require targeted therapy, the process from preoperative computed tomography and gastroscopy to postoperative pathology to confirm the diagnosis, and genetic testing in order to specify the targeted drug has become more mature, but there is still much to learn about familial GISTs. For example, emphasis should be placed on screening for familial GISTs: Patients with GISTs in first-degree relatives; age less than 50 years at diagnosis; localized multifocal GISTs; and those with skin pigmentation changes, mastocytosis, large hands, and other specific symptoms should be highly suspected to have familial GISTs, and germline testing for the KIT and PDGFRA genes should be performed promptly[8]. For familial wtGISTs, NGS should also be performed, to provide patients with matching therapeutic programs and improve their prognosis. For patients with confirmed familial GIST, appropriate targeted drugs should be given according to the results of genetic testing, and regular observation and follow-up should be performed, in order to understand the efficacy of the patient’s treatment and prognosis in a timely manner (Figure 7).
Familial GISTs are extremely rare, and the present report provides good strategies and recommendations for the treatment, prognosis, and screening of familial GISTs, with a view to recognizing GISTs as a disease from a different perspective.
We appreciate the cooperation of the patients and their families during the treatment period.
| 1. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (33)] |
| 2. | Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 520] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 3. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1177] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 4. | Ge Q, Liu Y, Yang F, Sun G, Guo J, Sun S. Chinese Pedigree with Hereditary Gastrointestinal Stromal Tumors: A Case Report and Literature Review. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 5. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1721] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 6. | von Mehren M, Joensuu H. Gastrointestinal Stromal Tumors. J Clin Oncol. 2018;36:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 7. | Gopie P, Mei L, Faber AC, Grossman SR, Smith SC, Boikos SA. Classification of gastrointestinal stromal tumor syndromes. Endocr Relat Cancer. 2018;25:R49-R58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Yan M, Lin J, Shu M, Luo Y, Sun K, Yang S, Zhang X. Diagnosis, Treatment, and Prognosis of Patients with Primary Familial Gastrointestinal Stromal Tumor: A Case Report and Literature Review. Oncologist. 2023;28:e1134-e1141. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Zhao C, Jin L, Tan Y, Chen Y, Su Z, Li W, Yang Q. Case Report: Multiple gastrointestinal stromal tumors along with numerous cutaneous neurofibromas: a case description and literature analysis. Front Oncol. 2023;13:1206991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Kelly CM, Gutierrez Sainz L, Chi P. The management of metastatic GIST: current standard and investigational therapeutics. J Hematol Oncol. 2021;14:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 165] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 11. | Nishida T, Naito Y, Takahashi T, Saito T, Hisamori S, Manaka D, Ogawa K, Hirota S, Ichikawa H. Molecular and clinicopathological features of KIT/PDGFRA wild-type gastrointestinal stromal tumors. Cancer Sci. 2024;115:894-904. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Gaal J, Stratakis CA, Carney JA, Ball ER, Korpershoek E, Lodish MB, Levy I, Xekouki P, van Nederveen FH, den Bakker MA, O'Sullivan M, Dinjens WN, de Krijger RR. SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol. 2011;24:147-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Gheorghe G, Bacalbasa N, Ceobanu G, Ilie M, Enache V, Constantinescu G, Bungau S, Diaconu CC. Gastrointestinal Stromal Tumors-A Mini Review. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Chen H, Hirota S, Isozaki K, Sun H, Ohashi A, Kinoshita K, O'Brien P, Kapusta L, Dardick I, Obayashi T, Okazaki T, Shinomura Y, Matsuzawa Y, Kitamura Y. Polyclonal nature of diffuse proliferation of interstitial cells of Cajal in patients with familial and multiple gastrointestinal stromal tumours. Gut. 2002;51:793-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Chompret A, Kannengiesser C, Barrois M, Terrier P, Dahan P, Tursz T, Lenoir GM, Bressac-De Paillerets B. PDGFRA germline mutation in a family with multiple cases of gastrointestinal stromal tumor. Gastroenterology. 2004;126:318-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Pasini B, Matyakhina L, Bei T, Muchow M, Boikos S, Ferrando B, Carney JA, Stratakis CA. Multiple gastrointestinal stromal and other tumors caused by platelet-derived growth factor receptor alpha gene mutations: a case associated with a germline V561D defect. J Clin Endocrinol Metab. 2007;92:3728-3732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Li B, Chen H, Yang S, Chen F, Xu L, Li Y, Li M, Zhu C, Shao F, Zhang X, Deng C, Zeng L, He Y, Zhang C. Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers. Mol Cancer. 2023;22:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 18. | Dermawan JK, Rubin BP. Molecular Pathogenesis of Gastrointestinal Stromal Tumor: A Paradigm for Personalized Medicine. Annu Rev Pathol. 2022;17:323-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Lasota J, Dansonka-Mieszkowska A, Sobin LH, Miettinen M. A great majority of GISTs with PDGFRA mutations represent gastric tumors of low or no malignant potential. Lab Invest. 2004;84:874-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 227] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Blay JY, Kang YK, Nishida T, von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Primers. 2021;7:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 21. | Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, Demetri GD. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1324] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 22. | Martin-Broto J, Valverde C, Hindi N, Vincenzi B, Martinez-Trufero J, Grignani G, Italiano A, Lavernia J, Vallejo A, Tos PD, Le Loarer F, Gonzalez-Campora R, Ramos R, Hernández-Jover D, Gutierrez A, Serrano C, Monteagudo M, Letón R, Robledo M, Moura DS, Martin-Ruiz M, López-Guerrero JA, Cruz J, Fernandez-Serra A, Blay JY, Fumagalli E, Martinez-Marin V. REGISTRI: Regorafenib in first-line of KIT/PDGFRA wild type metastatic GIST: a collaborative Spanish (GEIS), Italian (ISG) and French Sarcoma Group (FSG) phase II trial. Mol Cancer. 2023;22:127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Joensuu H, Wardelmann E, Eriksson M, Reichardt A, Hall KS, Schütte J, Cameron S, Hohenberger P, Sihto H, Jost PJ, Lindner LH, Bauer S, Nilsson B, Kallio R, Pesonen T, Reichardt P. KIT and PDGFRA Mutations and Survival of Gastrointestinal Stromal Tumor Patients Treated with Adjuvant Imatinib in a Randomized Trial. Clin Cancer Res. 2023;29:3313-3319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |