Published online Sep 15, 2024. doi: 10.4251/wjgo.v16.i9.3980
Revised: June 26, 2024
Accepted: August 2, 2024
Published online: September 15, 2024
Processing time: 166 Days and 5.5 Hours
Pancreatic cancer, a formidable gastrointestinal neoplasm, is characterized by its insidious onset, rapid progression, and resistance to treatment, which often lead to a grim prognosis. While the complex pathogenesis of pancreatic cancer is well recognized, recent attention has focused on the oncogenic roles of senescent tumor-associated fibroblasts. However, their precise role in pancreatic cancer remains unknown. Resveratrol is a natural polyphenol known for its multifaceted biological actions, including antioxidative and neuroprotective properties, as well as its potential to inhibit tumor proliferation and migration. Our current investigation builds on prior research and reveals the remarkable ability of resveratrol to inhibit pancreatic cancer proliferation and metastasis.
To explore the potential of resveratrol in inhibiting pancreatic cancer by targeting senescent tumor-associated fibroblasts.
Immunofluorescence staining of pancreatic cancer tissues revealed prominent coexpression of α-SMA and p16. HP-1 expression was determined using immunohistochemistry. Cells were treated with the senescence-inducing factors known as 3CKs. Long-term growth assays confirmed that 3CKs significantly decreased the CAF growth rate. Western blotting was conducted to assess the expression levels of p16 and p21. Immunofluorescence was performed to assess LaminB1 ex
Specifically, we identified the presence of senescent tumor-associated fibroblasts within pancreatic cancer tissues, linking their abundance to cancer progression. Intriguingly, Resveratrol effectively eradicated these fibroblasts and hindered their senescence, which consequently impeded pancreatic cancer progression.
This groundbreaking discovery reinforces Resveratrol's stature as a potential antitumor agent and positions senescent tumor-associated fibroblasts as pivotal contenders in future therapeutic strategies against pancreatic cancer.
Core Tip: This study focused on the effect of resveratrol on pancreatic cancer-associated fibroblasts and confirmed the presence of senescent fibroblasts in pancreatic cancer. Resveratrol has a notable ability to curb pancreatic cancer proliferation and thus has potential as a promising antitumor agent. Therefore, this study identifies a new therapeutic target in pancreatic cancer treatment.
- Citation: Jiang H, Wang GT, Wang Z, Ma QY, Ma ZH. Resveratrol inhibits pancreatic cancer proliferation and metastasis by depleting senescent tumor-associated fibroblasts. World J Gastrointest Oncol 2024; 16(9): 3980-3993
- URL: https://www.wjgnet.com/1948-5204/full/v16/i9/3980.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i9.3980
Pancreatic cancer is a devastating and lethal human malignancy for which there is currently no curative chemotherapy[1]. Despite advances in treatments and improvements in medical technology, pancreatic cancer remains a highly lethal cancer. Although complete tumor resection through surgery is possible for patients with pancreatic cancer, many patients still face the unfortunate reality of local or distant recurrence, which leads to a high mortality rate within 5 years[2]. The elusive nature of pancreatic cancer is evident in its hidden presentation, as the disease often leads to diagnosis at an advanced stage when clinical symptoms become noticeable in patients. Pancreatic cancer frequently metastasizes in early stages, which results in a poor prognosis even in patients who are diagnosed early[3]. A large volume of pancreatic cancer tissue is accompanied by abundant inflammation and a dense stroma. The dense stroma, which comprises approximately 90% of the tumor tissue, is characterized by low oxygen levels[4], poor vascularization, and extensive immune infiltration[5]. This infiltration is associated with variable levels of fibrosis. The fibrotic portion of the tumor is composed of both cellular and acellular elements. The cellular elements mainly consist of cancer-associated fibroblasts (CAFs), endothelial cells, and pancreatic stellate cells (PSCs). In contrast, the acellular portion includes different components of the extracellular matrix, such as collagen, glycosaminoglycans, and growth factors. Importantly, CAFs play crucial roles in the initiation and progression of pancreatic cancer[6-8].
CAFs are recognized as crucial components of the pancreatic cancer tumor microenvironment[9]. Previous research has substantiated the reciprocal promotion of proliferation and differentiation between CAFs and pancreatic cancer cells. This interaction further contributes to the malignant biological characteristics of pancreatic tumor cells[10]. The tumorigenic properties of CAFs are contingent upon their capacity to stimulate tumor cell proliferation, facilitate invasion and metastasis, and exhibit proinflammatory and immunosuppressive properties[11]. Different types of CAFs have different effects on tumors[12]. Senescent CAFs play an oncogenic role in various tumors[13], but few related reports regarding their role in pancreatic cancer have been published. Various stimuli can induce cellular senescence, and senescent cells exhibit several shared characteristics, such as growth arrest, resistance to apoptosis, continuous activation of DNA damage response signaling pathways, and alterations in heterochromatin structure[14].
Senescence is widely recognized as a prominent tumor suppressor mechanism; however, in recent years[15], the focus has shifted toward the tumorigenic effects of paracrine signaling by senescent cells[16]. Although researchers have made significant progress in understanding the role of senescent CAFs in pancreatic cancer, some aspects of their involvement remain unclear. Despite the loss of proliferative capacity during senescence, CAFs maintain their metabolic activity and display a unique characteristic called the senescence-associated secretory phenotype (SASP). This phenotype involves the secretion of a wide range of senescence-related factors by CAFs[17]. The SASP is a pivotal factor that promotes senescence-associated tumorigenesis and thus plays a crucial role in preserving a balanced environment within the pancreatic cancer microenvironment.
Furthermore, the SASP is implicated in the recurrence and metastasis of pancreatic cancer[18,19]. The specific constituents of the secretome associated with the SASP have yet to be fully elucidated. Nonetheless, many studies suggest that most of the fibrotic fraction is composed of soluble signaling molecules, including proinflammatory cytokines, chemokines, growth factors, proteases, MMPs, and extracellular vesicles[20]. Therefore, the objective of this study was to investigate the impact of senescent CAFs on the proliferation and metastasis of pancreatic cancer cells.
Resveratrol has diverse biological effects as well as antioxidant, anti-inflammatory, cardioprotective, neuroprotective, and antidiabetic properties[21-23]. Over the past several years, numerous studies have provided increasing evidence of the direct inhibitory effects of Resveratrol on tumor cell proliferation. These effects are characterized by the induction of growth suppression, cell cycle arrest, and apoptosis, specifically in pancreatic cancer cells[24]. However, the intricate mechanisms through which Resveratrol exerts its therapeutic effects in cancer are not completely understood, and additional investigations for a comprehensive understanding are needed. Our preliminary findings confirmed the inhibitory effects of Resveratrol, a naturally occurring antitumor drug, on the progression and stemness characteristics of pancreatic cancer through the targeting of NAF-1[25]. However, the effect of Resveratrol on the microenvironment of pancreatic cancer, specifically CAFs, including senescent CAFs, is still not fully understood. The objective of this study was to examine the impact of senescent CAFs within the tumor microenvironment of pancreatic cancer. Additionally, we aimed to investigate the potential effects of Resveratrol on senescent CAFs.
The First Affiliated Hospital Ethical Committee, Xi'an Jiaotong University, Xi'an, China, approved all procedures involving human samples. Written informed consent was obtained from all participants before sample collection.
Resveratrol, with a purity of > 99%, was sourced from Sigma-Aldrich (St. Louis, MO, United States) and dissolved in dimethyl sulfoxide (DMSO) to prepare stock solutions of 50 mmol/L and 10 mmol/L, which were stored at -20 °C. DMSO was used as the vehicle control. Primary antibodies against p16 (No. ab93689, 1:1000), α-SMA (No. ab109250, 1:1000), and HP1 (No. ab18976, 1:1000) were purchased from Abcam (Cambridge, MA, United States). Antibodies targeting N-cadherin (No. 13116S, 1:1000), E-cadherin (No. 3195S, 1:1000), and Vimentin (No. 5741, 1:1000) were obtained from Cell Signaling Technology (Danvers, MA, United States). Secondary antibodies, including anti-rabbit IgG (No. ab6721, 1:10000) and anti-mouse IgG (No. ab6728, 1:10000), were also purchased from Abcam. Antibodies specific for p21 (No. 13318-1-AP, 1:1000) and GAPDH (No. 66031-1-Ig, 1:5000) were procured from Protein tech Group (Chicago, IL, United States). All other reagents used in this study were of analytical grade and were purchased from recognized commercial sources.
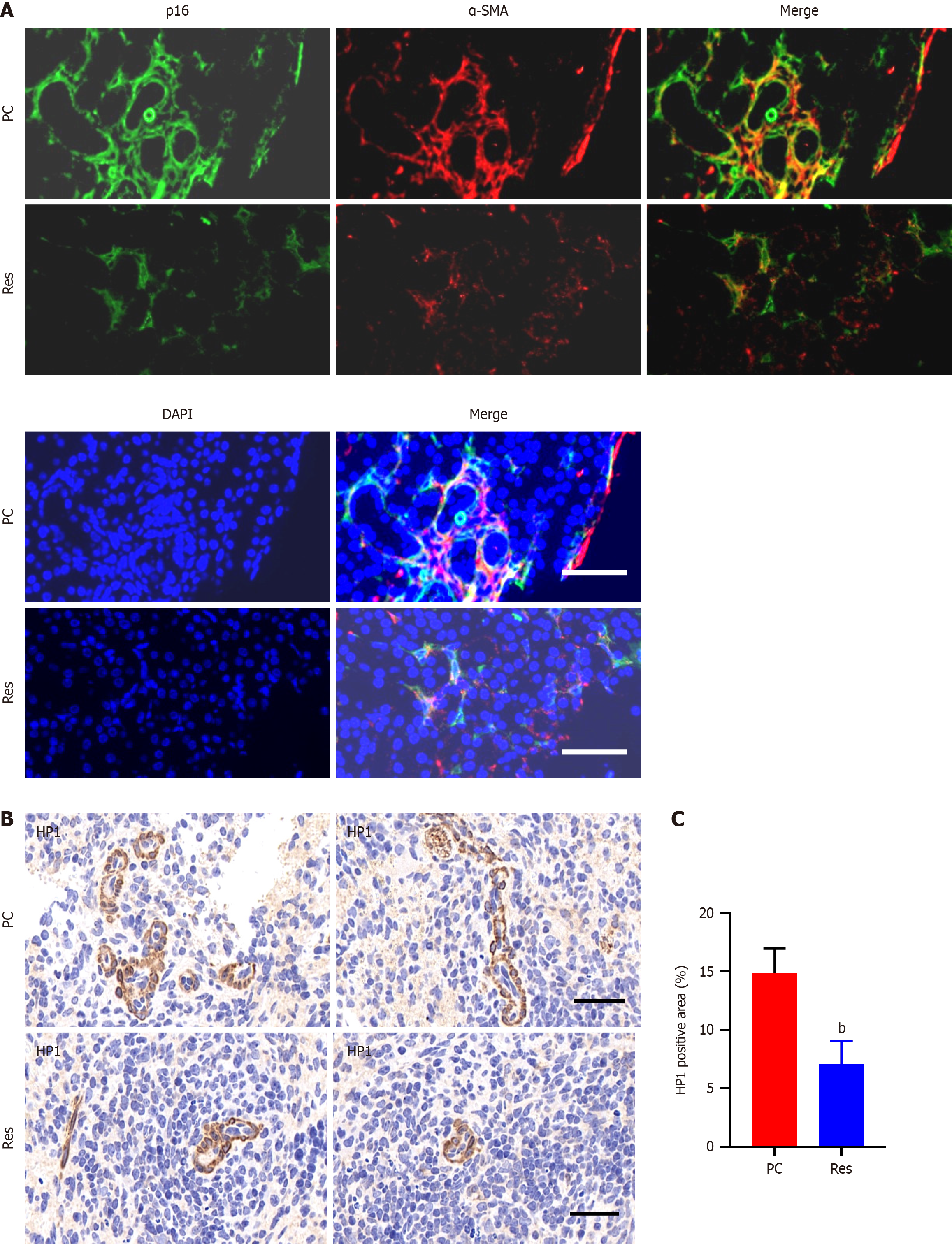
The Department of Hepatobiliary Surgery at the First Affiliated Hospital of Xi'an Jiaotong University provided 91 pancreatic cancer tissue samples for this study. Five standard pancreatic tissue samples were obtained from patients who underwent liver transplantation at the same institution. Immunohistochemistry was conducted according to established protocols. For HP1 expression, evaluation was based on the presence of cytoplasmic staining. NAF-1 staining was assessed by considering both the intensity of staining and the percentage of positively stained cells. The samples were categorized based on a composite score, which was determined by multiplying the percentage score (ranging from 0 to 4, based on the proportion of stained cells) by the intensity score (ranging from 0 to 3, based on the staining intensity). The resulting composite score was used to determine the final categorization: Negative (0) for scores ≤ 3, weak (1 +) for scores > 3 but ≤ 6, moderate (2 +) for scores > 6 but ≤ 9, and vigorous (3 +) for scores > 9.
The PANC-1 and SW1990 human pancreatic cancer cell lines were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). These cell lines were maintained in DMEM (HyClone, Logan, United States) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Cultures were maintained in a humidified incubator at 37 °C with 5% CO2. PSCs were isolated from pancreatic cancer tissue samples and cultured according to established protocols.
PANC-1 and SW1990 cells were rinsed with phosphate-buffered saline (PBS) and then lysed in RIPA buffer (Beyotime, Guangzhou, China). The protein concentration in the lysates was assessed via a bicinchoninic acid protein assay kit (Pierce, Rockford, United States). Equal amounts of proteins were subsequently separated on a 10% SDS-PAGE gel and transferred to PVDF membranes. The membranes were blocked with 10% nonfat milk in TBST for 2 hours at room temperature, after which the membranes were incubated overnight with primary antibodies at 4 °C. After rinsing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 2 hours at room temperature. Detection was accomplished via an enhanced chemiluminescence kit, and the bands were visualized using a Molecular Imager ChemiDoc XRS System (Bio-Rad, Hercules, CA, United States).
The cells were fixed in 4% paraformaldehyde for 15 minutes. Then, 0.3% Triton X-100 was applied to permeabilize the cells. Following a 30-minute block in 5% bovine serum albumin, the cells were incubated with primary antibodies overnight at 4 °C. After a series of washes, the cells were exposed to fluorescently labeled secondary antibodies for 2 hours. Nuclei were counterstained with DAPI for 1 minute, and the cells were subsequently examined under a Zeiss Instruments confocal microscope.
Matrigel-coated Transwell chambers were placed in a 24-well plate and pre hydrated with serum-free DMEM. Then, 700 µl of DMEM containing 10% FBS was added to the lower well. A cell suspension was subsequently introduced into the upper chamber followed by incubation for 36 hours at 37 °C in 5% CO2. After incubation, the fixed cells were stained with 0.3% crystal violet. The invading cells were then observed and imaged using an inverted microscope.
PANC-1 and BxPC-3 cells were cultured in six-well plates until they reached confluency. A sterile pipette tip was then used to create a linear scratch. The cells were subsequently washed to eliminate debris and were then cultured in serum-free medium. The progression of wound healing was monitored using an inverted microscope and was recorded at 0 and 36 hours after the scratch was generated.
Six- to eight-week-old male nude mice were maintained in a specific pathogen-free environment. BxPC-3 cells, which were suspended in a 1:1 mixture of DMEM and Matrigel, were injected subcutaneously into both sides of each mouse. After one week, the mice were divided into two groups: A control group that received daily oral administration of sterile water and a treatment group that received 50 mg/kg/day resveratrol. Each group consisted of 10nude mice. Tumor growth was monitored throughout the study. All experimental protocols were approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University in Xi'an, China.
The experimental data are presented as the means ± SD. Student's t test was used for comparisons between two groups. One-way analysis of variance (ANOVA) followed by Tukey's post hoc test was used for comparisons among multiple groups. Pearson's correlation coefficient was used to assess the correlation between senescent tumor-associated fibroblasts and pancreatic cancer progression. The survival rate of mice used in the in vivo tumor model was estimated using the Kaplan-Meier method and compared using the log-rank test. The presence of senescent tumor-associated fibroblasts in different tissue samples was assessed via a χ2 test for categorical data. Statistical significance was considered at P < 0.05. SPSS software, version 25.0 (IBM Corp., Armonk, NY, United States), was used for all statistical analyses. Graphs and figures were generated with GraphPad Prism software, version 8.0 (GraphPad Software, San Diego, CA, United States).
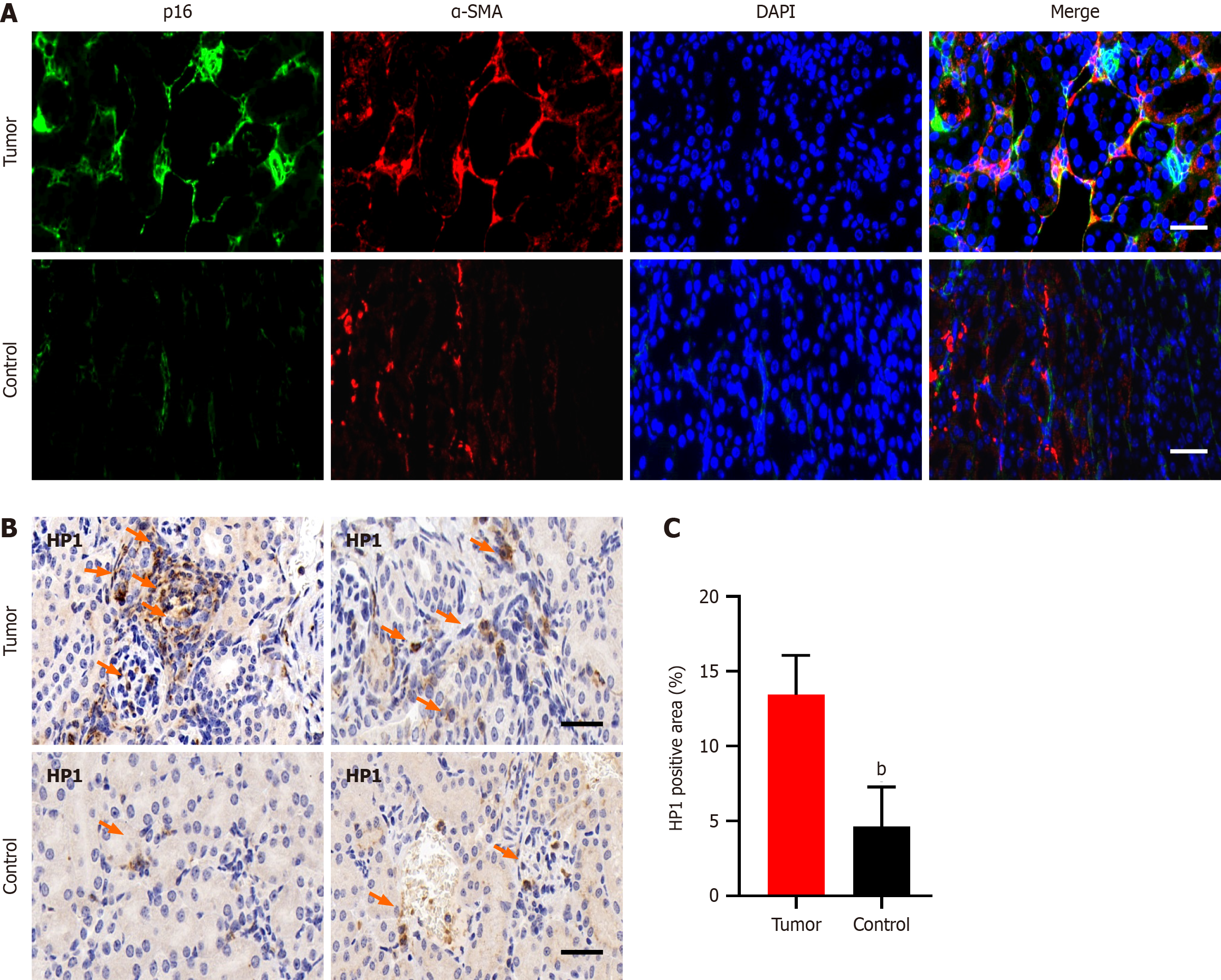
Immunofluorescence staining of pancreatic cancer tissues revealed prominent coexpression of α-SMA and p16, which are markers of CAFs and cellular senescence, respectively. This coexpression was notably greater in tumor tissues than in adjacent or distal normal tissues (Figure 1A). Additionally, HP1, another marker of cellular senescence, was upregulated in tumor tissues compared with normal pancreatic tissues (Figure 1B and C).
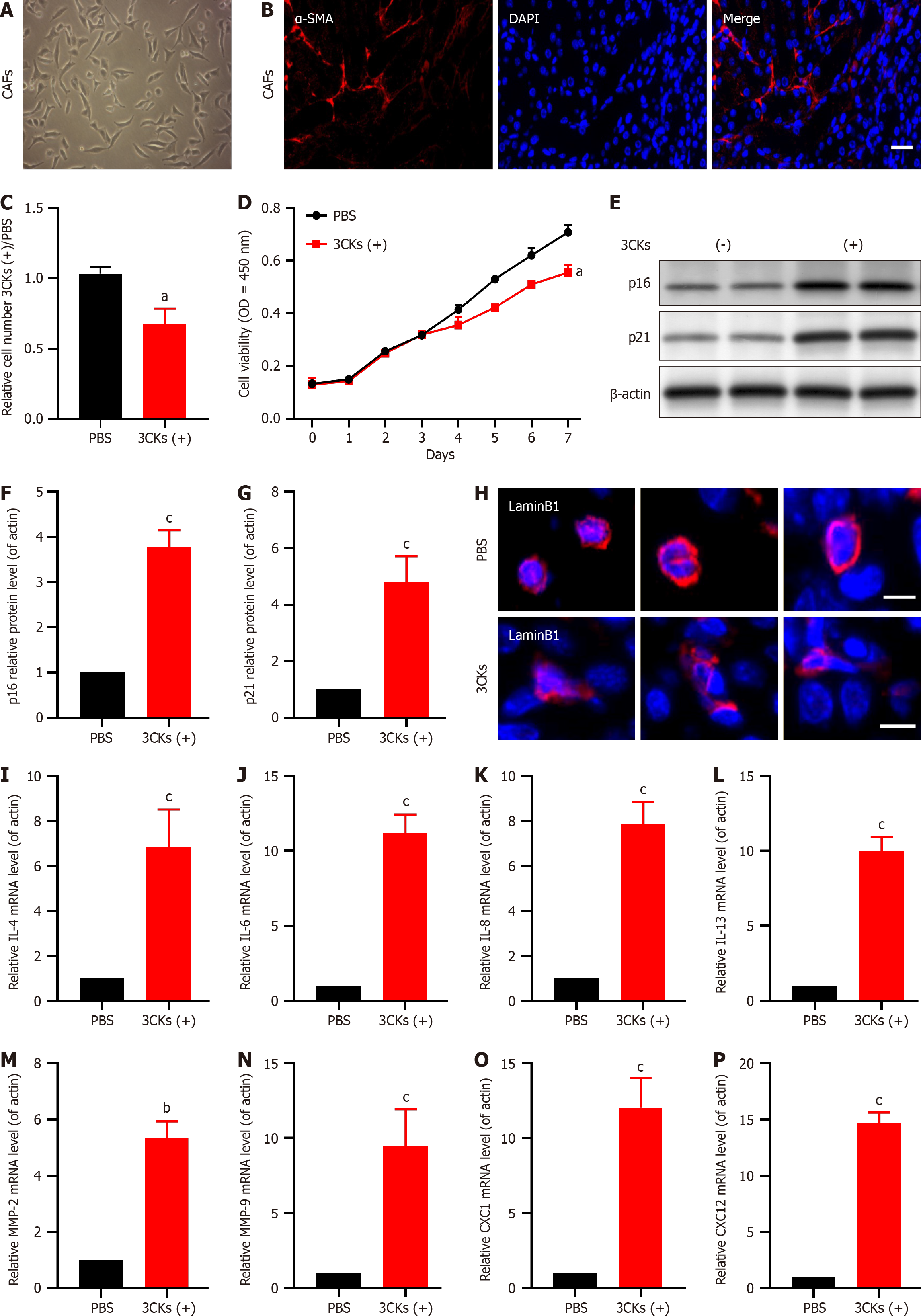
Primary CAF cultures established from pancreatic tumor tissues robustly expressed the CAF marker α-SMA (Figure 2A and B). Treatment of these cells with the senescence-inducing factors 3CKs and a combination of IL-1a, IL-1b, and tumor necrosis factor-α (TNF-α) for 48 hours resulted in significant growth inhibition compared with that in the control group treated with PBS (Figure 2C). Subsequent long-term growth assays confirmed that 3CKs significantly impeded the CAF proliferation rate (Figure 2D). Furthermore, post-3CK stimulation resulted in elevated expression of the senescence markers p21 and p16 (Figure 2E-G). Lamin B1, a protein associated with nuclear integrity, was diminished in 3CKs-treated CAFs, which indicates a loss of nuclear membrane integrity (Figure 2H). Additionally, stimulation with 3CKs increased the levels of several SASP factors, including IL-4, IL-6, IL-8, IL-13, MMP-2, MMP-9, CXCL1, and CXCL12 (Figure 2I-P).
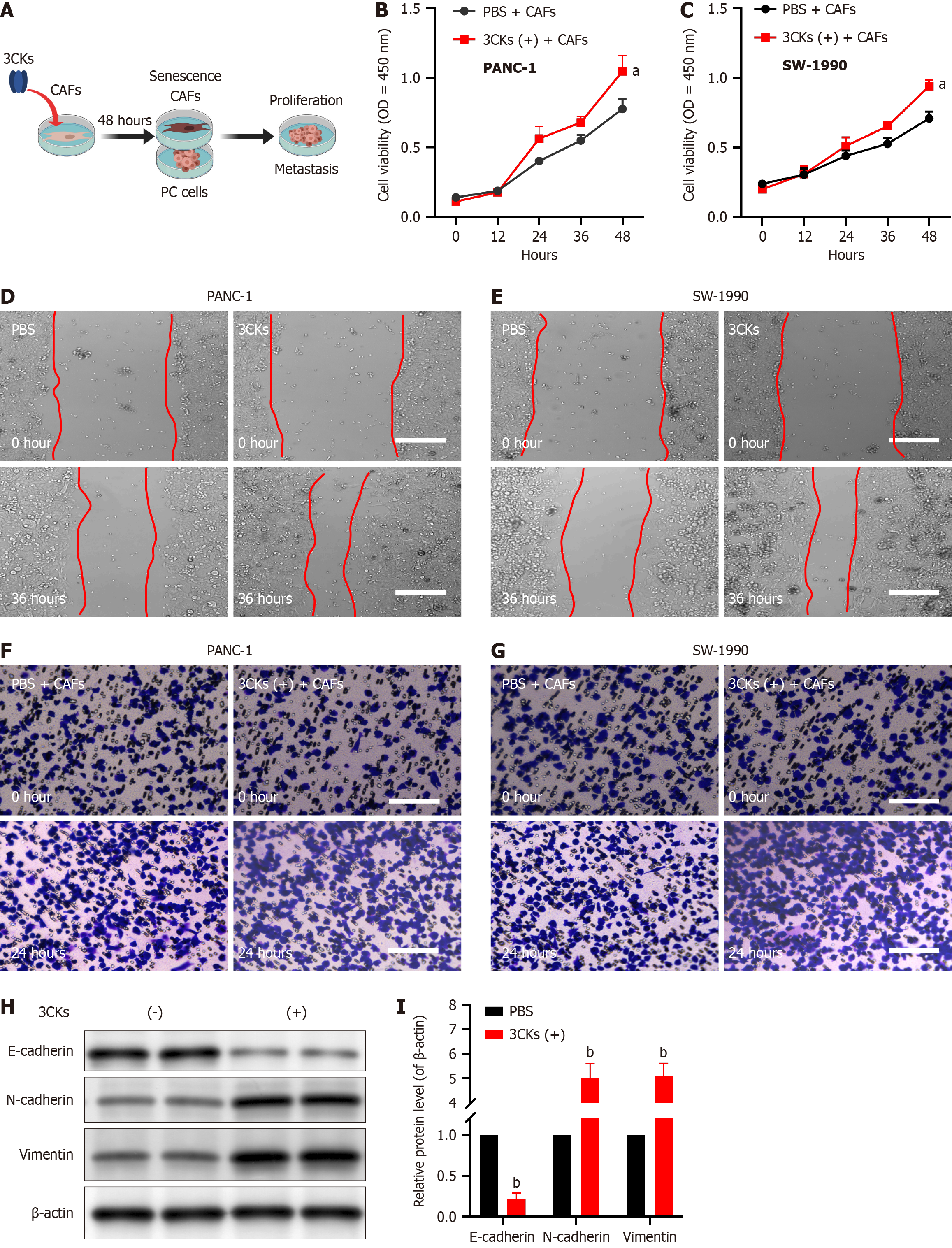
When PANC-1 and SW-1990 pancreatic cancer cells were cocultured with CAFs stimulated by 3CKs, their proliferative capacity was significantly greater than that of cells cocultured with PBS-treated CAFs (Figure 3A-C). Scratch assays demonstrated that when cocultured with CAFs treated with 3CKs, pancreatic cancer cells exhibited a notably greater migratory capacity (Figure 3D and E). Additionally, invasion assays revealed that the invasive potential of pancreatic cancer cells increased when these cells were cocultured with senescent CAFs (Figure 3F and G). At the molecular level, senescent CAFs were found to promote epithelial-mesenchymal transition (EMT) in pancreatic cancer cells, as evidenced by decreased E-cadherin and increased N-cadherin and vimentin expression (Figure 3H and I).
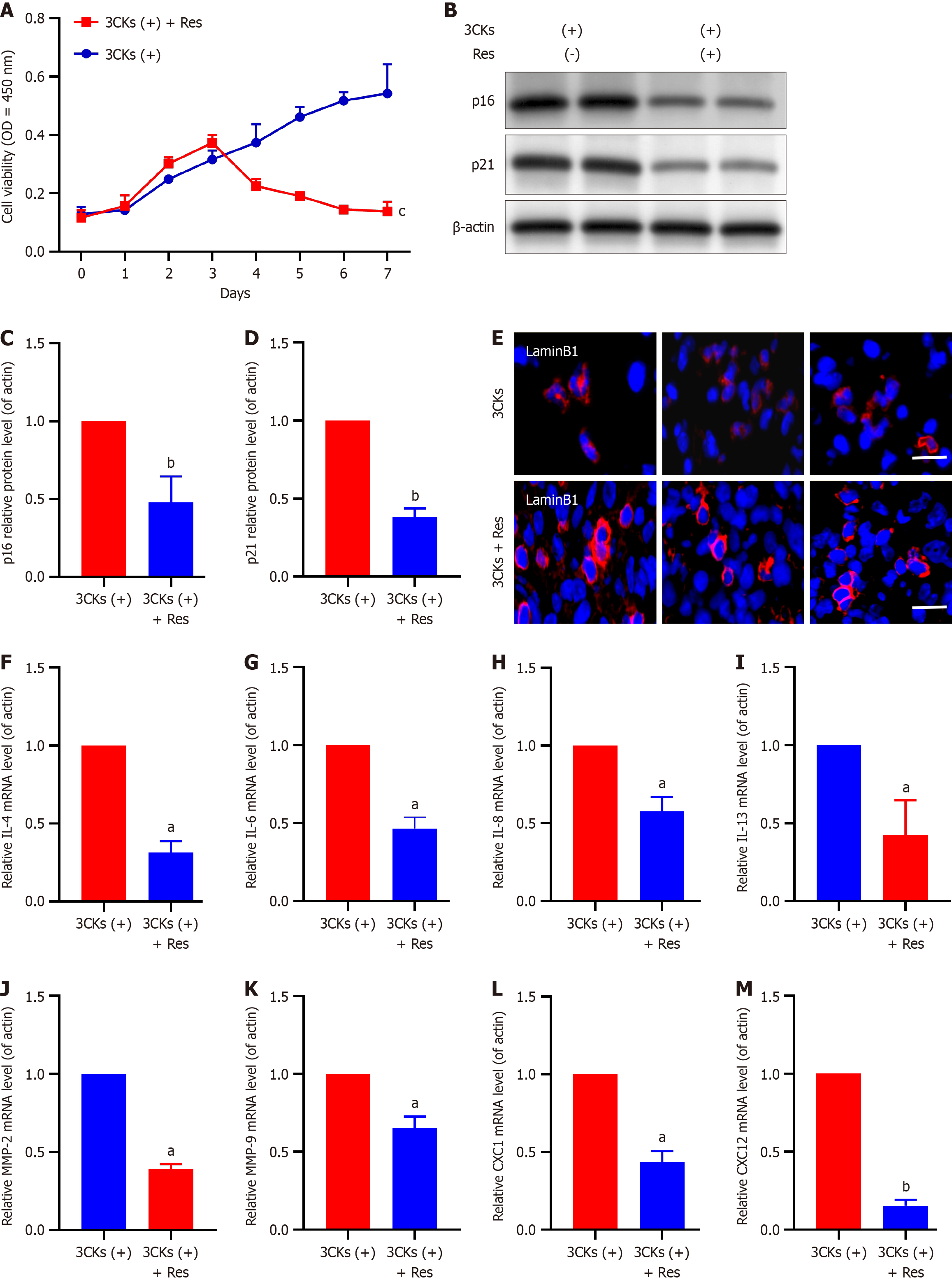
Resveratrol has been the focus of recent research because of its notable effects on inhibiting tumor cell proliferation and inducing growth suppression, cell cycle arrest, and apoptosis in pancreatic cancer cells. In the context of senescent CAFs, our experiments further elucidated the potential of resveratrol. A long-term growth assay demonstrated that resveratrol treatment significantly reduced the number of senescent CAFs, especially compared with CAFs treated with 3CKs alone (Figure 4A). Additionally, the treatment of CAFs stimulated by 3CKs with resveratrol resulted in a significant decrease in the expression levels of the senescence markers p21 and p16 (Figure 4B-D). Additionally, the depleted expression of Lamin B1 in senescent CAFs was restored following resveratrol treatment (Figure 4E), and the expression of various SASP factors was substantially diminished (Figure 4F-M). In vivo experiments further confirmed the ability of resveratrol to reduce senescence in CAFs (Figure 5).
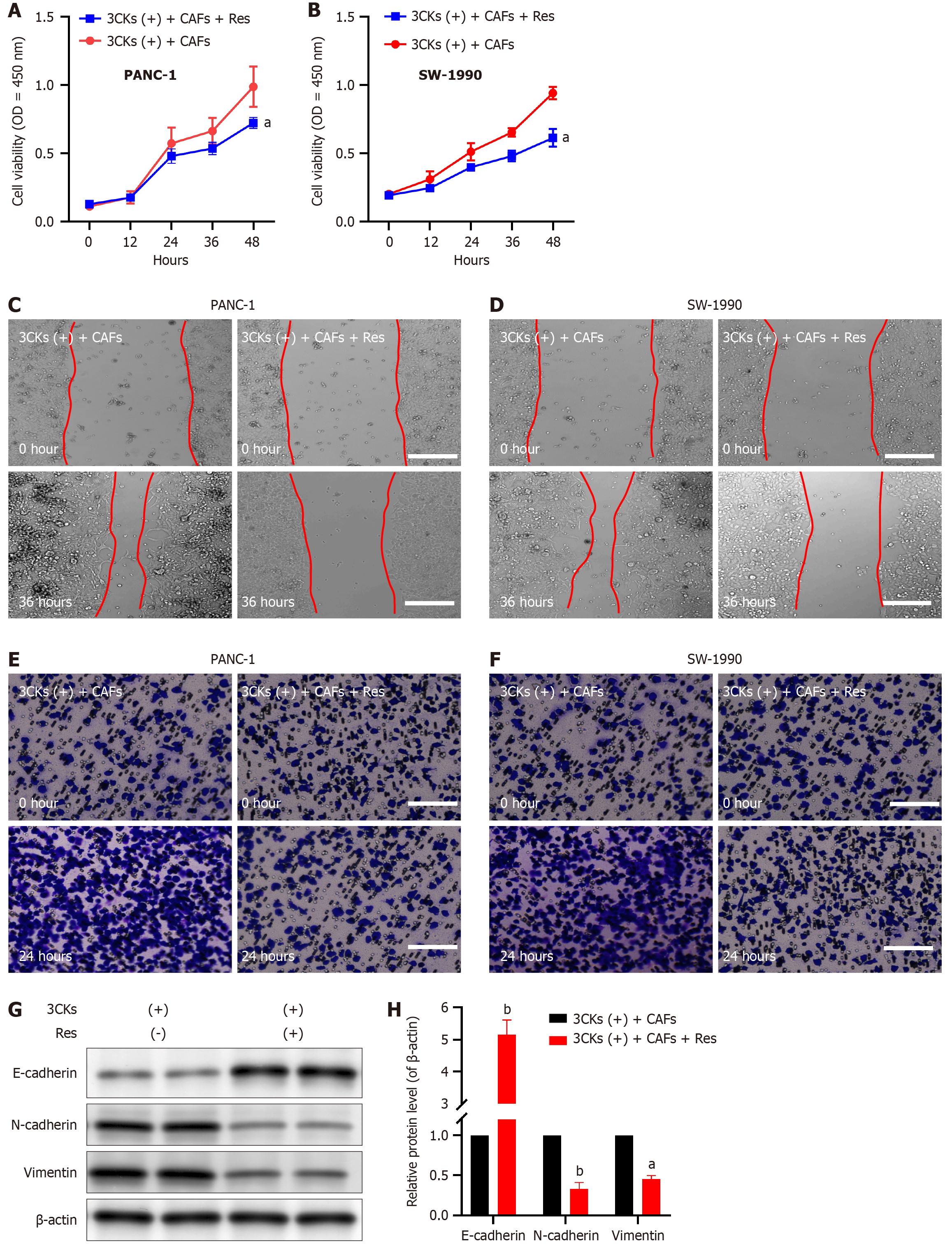
Our subsequent investigations aimed to understand the influence of resveratrol on the growth and metastatic capabilities of pancreatic cancer cells, with particular emphasis on its effects on CAF senescence. Upon treatment with Resveratrol, the CAFs that were pre stimulated with 3CKs were cocultured with PANC-1 and SW-1990 pancreatic cancer cells, which resulted in a significant reduction in the proliferation capacity of these cancer cells (Figure 6A and B). Moreover, resveratrol intervention significantly reduced the migratory capacity of cocultured pancreatic cancer cells, as evidenced by the scratch assay results (Figure 6C and D). Additionally, the invasive potential of cancer cells, which is characterized by their ability to breach the basement membrane, was significantly decreased following resveratrol treatment (Figure 6E and F). Molecular analysis revealed that Resveratrol effectively suppressed the expression of EMT markers in pancreatic cancer cells, which demonstrates its ability to hinder CAF-induced EMT (Figure 6G and H). These results highlight the potent inhibitory effects of resveratrol on pancreatic cancer progression, which are mediated mainly through its impact on CAF senescence.
Pancreatic cancer is recognized as one of the most aggressive malignancies worldwide and poses significant challenges in terms of treatment[1,3]. The formidable prognosis of pancreatic cancer arises from its tendency to have high rates of recurrence and metastasis even after surgical resection. Despite progress in surgical techniques and the use of local and systemic adjuvant therapies, the mortality rate of patients with pancreatic cancer is still alarmingly high. Therefore, a comprehensive understanding of the characteristics of malignant pancreatic cancer has become increasingly important.
In the past few decades, particularly in recent years, significant advances have been made in elucidating the molecular mechanisms underlying pancreatic cancer, and accumulating evidence has demonstrated the vital influence of the tumor microenvironment on pancreatic cancer development. CAFs, which are CAFs, have emerged as an impactful component among the various cell types present in the tumor microenvironments of different cancers, such as breast cancer, prostate cancer, and notably, pancreatic cancer[26-28]. CAFs play pivotal roles in tumorigenesis, disease progression, and metastasis. As activated fibroblasts residing within the cancer stroma, CAFs actively promote cancer progression through intricate interactions with cancer cells[29]. Accumulating evidence suggests that senescence and tumorigenesis participate in crosstalk during tumor progression, and a large body of literature indicates that senescence constitutes a potent tumor suppressor mechanism but that the potential tumor-promoting role of the SASP may lead to cancer recurrence[30-33]. Previous studies have shown that senescent fibroblasts induce tumor cell proliferation through paracrine factors. Yasuda et al[34] reported that the proinflammatory cytokines IL-1a, IL-1b, and TNF-α can activate the NF-ĸB signaling pathway. This activation process leads to the induction of senescence in CAFs by promoting the accumulation of reactive oxygen species. Moreover, senescent CAFs release multiple factors characteristic of the SASP, and of these, IL-6 is particularly notable[34]. IL-6 expression is consistently elevated, which promotes the peritoneal metastasis of gastric cancer via the JAK/STAT3 signaling pathway. However, the extent of interaction and the relationship between senescent CAFs and pancreatic cancer cells are not completely understood. Our study revealed the widespread presence of senescent CAFs within pancreatic cancer tissues. This groundbreaking discovery provides initial evidence of a significant association between senescent CAFs and the progression of pancreatic cancer. We subsequently successfully established primary cultures of pancreatic cancer CAFs and induced senescence in these cells in vitro by stimulating them with the senescence-inducing factors 3CKs. These senescent CAFs exhibited features characteristic of senescent cells, such as growth arrest, substantial increases in the expression of the cellular senescence markers p21 and p16, and loss of Lamin B1 expression. A notable increase was also observed in the expression levels of SASP factors, including IL-4, IL-6, IL-8, IL-13, MMP-2, MMP-9, CXCL1, and CXCL12. The establishment of an in vitro model of senescent CAFs lays the foundation for the next step: Evaluating the malignant biological behaviors of senescent CAFs and their effects on pancreatic cancer cells.
Aberrant cellular proliferation, metastatic potential, and EMT are critical factors that contribute to the refractory nature of pancreatic cancer[35]. To thoroughly assess the influence of senescent CAFs on the aggressive characteristics of pancreatic cancer cells, we conducted experiments involving the coculture of senescent CAFs with PANC-1 or SW-1990 pancreatic cancer cells. We subsequently evaluated the effects on the proliferation, wound healing, and invasiveness of pancreatic cancer cells. Our findings unequivocally demonstrated that coculture with senescent CAFs significantly augmented pancreatic cancer cell proliferation, wound healing, and invasiveness. Notably, EMT plays a crucial role in tumor metastasis by enhancing tumor cell migration and invasion. This process is characterized by decreased expression of E-cadherin and a simultaneous increase in the expression of N-cadherin and vimentin. Furthermore, our investigation revealed the influence of senescent CAFs on the EMT status in pancreatic cancer cells and revealed that coculture with senescent CAFs facilitated the expression of N-cadherin and vimentin but concomitantly suppressed E-cadherin expression, which ultimately culminated in EMT induction in pancreatic cancer cells.
Resveratrol has many biological effects as well as antioxidant, anti-inflammatory, cardio- and neuroprotective, and antidiabetic properties[21,23]. Furthermore, resveratrol has the ability to regulate nontumor cells present in the tumor microenvironment, which hinders their reprogramming and subsequent resistance to anticancer therapy and impedes metastasis. Recent studies have shown that resveratrol directly inhibits the proliferation of tumor cells, which leads to growth suppression, cell cycle arrest, apoptosis, and reduced viability. Resveratrol significantly inhibits tumor cells in different types of cancers, including hepatocellular carcinoma, leukemia, and breast cancer, through diverse mechanisms[22]. In models of pancreatic cancer, resveratrol is acknowledged as a therapeutic or protective agent and has been shown to synergistically enhance the antitumor effects of gemcitabine.
However, the exact role of resveratrol in regulating the senescence of CAFs and its potential therapeutic implications for pancreatic cancer remain uncertain and require further investigation. In this study, we made a compelling discovery that resveratrol effectively inhibits the senescence of CAFs and reduces the expression of SASP factors. The acquisition of the SASP by senescent CAFs is known to play a pivotal role in promoting cancer progression. Remarkably, our subsequent in vivo experiments demonstrated a significant decrease in senescent CAFs within pancreatic cancer tissues following treatment with resveratrol, which highlights its potent inhibitory effect on CAF senescence. Our findings further support the notion that senescent CAFs exert malignant effects on the biological behavior of pancreatic cancer cells and emphasize the inhibitory role of resveratrol in CAF senescence, thus substantiating its function as a tumor suppressing agent in pancreatic cancer. In addition to confirming these conclusions, we found that resveratrol treatment significantly inhibited the proliferation and metastasis of pancreatic cancer cells induced by coculture with senescent CAFs.
Our investigation revealed the significant impact of suppressing senescence in CAFs on the inhibition of pancreatic cancer cell proliferation, invasion, and migration. Furthermore, resveratrol, a natural antitumor agent, was found to hinder the progression of pancreatic cancer by eliminating senescent CAFs and delaying senescence in these cells. These findings highlight the crucial role of senescent CAFs in the modulation of tumor progression through Resveratrol and highlight potential therapeutic opportunities in targeting senescent CAFs for the treatment of pancreatic cancer.
| 1. | Kolbeinsson HM, Chandana S, Wright GP, Chung M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J Invest Surg. 2023;36:2129884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 201] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 2. | Okusaka T. Treatment for postoperative recurrence of pancreatic cancer: a narrative review. Chin Clin Oncol. 2022;11:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Tonini V, Zanni M. Early diagnosis of pancreatic cancer: What strategies to avoid a foretold catastrophe. World J Gastroenterol. 2022;28:4235-4248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 4. | Masugi Y. The Desmoplastic Stroma of Pancreatic Cancer: Multilayered Levels of Heterogeneity, Clinical Significance, and Therapeutic Opportunities. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | LaRue MM, Parker S, Puccini J, Cammer M, Kimmelman AC, Bar-Sagi D. Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer. Proc Natl Acad Sci U S A. 2022;119:e2119168119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 6. | Huang H, Wang Z, Zhang Y, Pradhan RN, Ganguly D, Chandra R, Murimwa G, Wright S, Gu X, Maddipati R, Müller S, Turley SJ, Brekken RA. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell. 2022;40:656-673.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 312] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 7. | McAndrews KM, Chen Y, Darpolor JK, Zheng X, Yang S, Carstens JL, Li B, Wang H, Miyake T, Correa de Sampaio P, Kirtley ML, Natale M, Wu CC, Sugimoto H, LeBleu VS, Kalluri R. Identification of Functional Heterogeneity of Carcinoma-Associated Fibroblasts with Distinct IL6-Mediated Therapy Resistance in Pancreatic Cancer. Cancer Discov. 2022;12:1580-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 177] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 8. | Liu J, Wang Y, Mu C, Li M, Li K, Li S, Wu W, Du L, Zhang X, Li C, Peng W, Shen J, Liu Y, Yang D, Zhang K, Ning Q, Fu X, Zeng Y, Ni Y, Zhou Z, Liu Y, Hu Y, Zheng X, Wen T, Li Z, Liu Y. Pancreatic tumor eradication via selective Pin1 inhibition in cancer-associated fibroblasts and T lymphocytes engagement. Nat Commun. 2022;13:4308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Verginadis II, Avgousti H, Monslow J, Skoufos G, Chinga F, Kim K, Leli NM, Karagounis IV, Bell BI, Velalopoulou A, Salinas CS, Wu VS, Li Y, Ye J, Scott DA, Osterman AL, Sengupta A, Weljie A, Huang M, Zhang D, Fan Y, Radaelli E, Tobias JW, Rambow F, Karras P, Marine JC, Xu X, Hatzigeorgiou AG, Ryeom S, Diehl JA, Fuchs SY, Puré E, Koumenis C. A stromal Integrated Stress Response activates perivascular cancer-associated fibroblasts to drive angiogenesis and tumour progression. Nat Cell Biol. 2022;24:940-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 10. | Hu B, Wu C, Mao H, Gu H, Dong H, Yan J, Qi Z, Yuan L, Dong Q, Long J. Subpopulations of cancer-associated fibroblasts link the prognosis and metabolic features of pancreatic ductal adenocarcinoma. Ann Transl Med. 2022;10:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 11. | Boyd LNC, Andini KD, Peters GJ, Kazemier G, Giovannetti E. Heterogeneity and plasticity of cancer-associated fibroblasts in the pancreatic tumor microenvironment. Semin Cancer Biol. 2022;82:184-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 12. | Shinkawa T, Ohuchida K, Nakamura M. Heterogeneity of Cancer-Associated Fibroblasts and the Tumor Immune Microenvironment in Pancreatic Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Zhang T, Ren Y, Yang P, Wang J, Zhou H. Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Cell Death Dis. 2022;13:897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 106] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 14. | Blagosklonny MV. Cell senescence, rapamycin and hyperfunction theory of aging. Cell Cycle. 2022;21:1456-1467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Schmitt CA, Wang B, Demaria M. Senescence and cancer - role and therapeutic opportunities. Nat Rev Clin Oncol. 2022;19:619-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 423] [Reference Citation Analysis (0)] |
| 16. | Wang X, Ma L, Pei X, Wang H, Tang X, Pei JF, Ding YN, Qu S, Wei ZY, Wang HY, Wang X, Wei GH, Liu DP, Chen HZ. Comprehensive assessment of cellular senescence in the tumor microenvironment. Brief Bioinform. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 17. | Zhang L, Pitcher LE, Yousefzadeh MJ, Niedernhofer LJ, Robbins PD, Zhu Y. Cellular senescence: a key therapeutic target in aging and diseases. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 321] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 18. | Ohtani N. The roles and mechanisms of senescence-associated secretory phenotype (SASP): can it be controlled by senolysis? Inflamm Regen. 2022;42:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 19. | Zhang N, Shang M, Li H, Wu L, Dong M, Huang B, Lu J, Zhang Y. Dual Inhibition of H3K9me2 and H3K27me3 Promotes Tumor Cell Senescence without Triggering the Secretion of SASP. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Takasugi M, Yoshida Y, Ohtani N. Cellular senescence and the tumour microenvironment. Mol Oncol. 2022;16:3333-3351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 21. | Sheng S, Wang X, Liu X, Hu X, Shao Y, Wang G, Mao D, Li C, Chen B, Chen X. The role of resveratrol on rheumatoid arthritis: From bench to bedside. Front Pharmacol. 2022;13:829677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 22. | Li J, Zeng X, Yang F, Wang L, Luo X, Liu R, Zeng F, Lu S, Huang X, Lei Y, Lan Y. Resveratrol: Potential Application in Sepsis. Front Pharmacol. 2022;13:821358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Wang Y, Hong C, Wu Z, Li S, Xia Y, Liang Y, He X, Xiao X, Tang W. Resveratrol in Intestinal Health and Disease: Focusing on Intestinal Barrier. Front Nutr. 2022;9:848400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Qian W, Xiao Q, Wang L, Qin T, Xiao Y, Li J, Yue Y, Zhou C, Duan W, Ma Q, Ma J. Resveratrol slows the tumourigenesis of pancreatic cancer by inhibiting NFκB activation. Biomed Pharmacother. 2020;127:110116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Qin T, Cheng L, Xiao Y, Qian W, Li J, Wu Z, Wang Z, Xu Q, Duan W, Wong L, Wu E, Ma Q, Ma J. NAF-1 Inhibition by Resveratrol Suppresses Cancer Stem Cell-Like Properties and the Invasion of Pancreatic Cancer. Front Oncol. 2020;10:1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Hu D, Li Z, Zheng B, Lin X, Pan Y, Gong P, Zhuo W, Hu Y, Chen C, Chen L, Zhou J, Wang L. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun (Lond). 2022;42:401-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 148] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 27. | Zhang Z, Karthaus WR, Lee YS, Gao VR, Wu C, Russo JW, Liu M, Mota JM, Abida W, Linton E, Lee E, Barnes SD, Chen HA, Mao N, Wongvipat J, Choi D, Chen X, Zhao H, Manova-Todorova K, de Stanchina E, Taplin ME, Balk SP, Rathkopf DE, Gopalan A, Carver BS, Mu P, Jiang X, Watson PA, Sawyers CL. Tumor Microenvironment-Derived NRG1 Promotes Antiandrogen Resistance in Prostate Cancer. Cancer Cell. 2020;38:279-296.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 28. | Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS, Sivajothi S, Armstrong TD, Engle DD, Yu KH, Hao Y, Wolfgang CL, Park Y, Preall J, Jaffee EM, Califano A, Robson P, Tuveson DA. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019;9:1102-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 1376] [Article Influence: 229.3] [Reference Citation Analysis (0)] |
| 29. | Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, Zhang B, Meng Q, Yu X, Shi S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1314] [Cited by in RCA: 1334] [Article Influence: 333.5] [Reference Citation Analysis (0)] |
| 30. | Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A. Cellular Senescence: Aging, Cancer, and Injury. Physiol Rev. 2019;99:1047-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 819] [Article Influence: 136.5] [Reference Citation Analysis (0)] |
| 31. | Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020;34:1565-1576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 709] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 32. | Prieto LI, Baker DJ. Cellular Senescence and the Immune System in Cancer. Gerontology. 2019;65:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 33. | Loo TM, Miyata K, Tanaka Y, Takahashi A. Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer. Cancer Sci. 2020;111:304-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 34. | Yasuda T, Koiwa M, Yonemura A, Miyake K, Kariya R, Kubota S, Yokomizo-Nakano T, Yasuda-Yoshihara N, Uchihara T, Itoyama R, Bu L, Fu L, Arima K, Izumi D, Iwagami S, Eto K, Iwatsuki M, Baba Y, Yoshida N, Ohguchi H, Okada S, Matsusaki K, Sashida G, Takahashi A, Tan P, Baba H, Ishimoto T. Inflammation-driven senescence-associated secretory phenotype in cancer-associated fibroblasts enhances peritoneal dissemination. Cell Rep. 2021;34:108779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 35. | Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29:212-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 1892] [Article Influence: 270.3] [Reference Citation Analysis (0)] |