Published online Sep 15, 2024. doi: 10.4251/wjgo.v16.i9.3932
Revised: June 24, 2024
Accepted: July 15, 2024
Published online: September 15, 2024
Processing time: 155 Days and 13.9 Hours
Cancer is one of the most serious threats to human health worldwide. Conventional treatments such as surgery and chemotherapy are associated with some drawbacks. In recent years, traditional Chinese medicine treatment has been in
To investigate the mechanism of Xiaojianzhong decoction (XJZ) in the treatment of gastric cancer (GC) by utilizing network pharmacology and experimental vali
We analyzed the mechanism and targets of XJZ in the treatment of GC through network pharmacology and bioinformatics. Subsequently, we verified the impact of XJZ treatment on the proliferative ability of GC cells through CCK-8, apoptosis, cell cycle, and clone formation assays. Additionally, we performed Western blot analysis and real-time quantitative PCR to assess the protein and mRNA expression of the core proteins.
XJZ mainly regulates IL6, PTGS2, CCL2, MMP9, MMP2, HMOX1, and other target genes and pathways in cancer to treat GC. The inhibition of cell viability, the increase of apoptosis, the blockage of the cell cycle at the G0/G1 phase, and the inhibition of the ability of cell clone formation were observed in AGS and HGC-27 cells after XJZ treatment. In addition, XJZ induced a decrease in the mRNA expression of IL6, PTGS2, MMP9, MMP2, and CCL2, and an increase in the mRNA expression of HOMX1. XJZ significantly inhibited the expression of IL6, PTGS2, MMP9, MMP2, and CCL2 proteins and promoted the expression of the heme oxygenase-1 protein.
XJZ exerts therapeutic effects against GC through multiple components, multiple targets, and multiple pathways. Our findings provide a new idea and scientific basis for further research on the molecular mechanisms underlying the therapeutic effects of XJZ in the treatment of GC.
Core Tip: We analyzed the mechanism and targets of Xiaojianzhong decoction (XJZ) in the treatment of gastric cancer (GC) through network pharmacology and bioinformatics. Subsequently, we verified the impact of XJZ treatment on the proliferative ability of GC cells through CCK-8, apoptosis, cell cycle, and clone formation assays. Additionally, we performed Western blot analysis and real-time quantitative PCR to assess the protein and mRNA expression of the hub genes, respectively. Our findings provide a new idea and scientific basis for further research on the molecular mechanisms underlying the therapeutic effects of XJZ in the treatment of GC.
- Citation: Chen GQ, Nan Y, Ning N, Huang SC, Bai YT, Zhou ZY, Qian G, Li WQ, Yuan L. Network pharmacology study and in vitro experimental validation of Xiaojianzhong decoction against gastric cancer. World J Gastrointest Oncol 2024; 16(9): 3932-3954
- URL: https://www.wjgnet.com/1948-5204/full/v16/i9/3932.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i9.3932
Gastric cancer (GC) stands as the third most prevalent form of malignant tumor in terms of both morbidity and mortality. It accounts for over 20.18 million newly diagnosed cases globally every year[1]. Among cancers, it ranks fourth in prevalence among males and seventh among females[2]. In China, a developing country, GC has the second highest incidence and mortality rates only to lung cancer and liver cancer. In the early stage of GC, clinical symptoms are not significant, specific indicators are poor, and the condition is hidden, so it is easy to be overlooked and clinical diagnosis is difficult. Once detected, the cancer has already reached the middle or late stage, which is difficult to treat and has a high mortality rate[3]. At present, surgery, radiotherapy, chemotherapy, and molecular target therapy are the main treatment methods[4,5]. The use of chemotherapeutic drugs in the treatment process will lead to adverse reactions and drug resistance[6]. A number of molecularly targeted drugs such as ramucirumab[7] and apatinib[8] have been approved for use as second- and third-line cancer clinical therapies. Molecularly targeted drugs have manageable adverse events and longer lasting responses than conventional chemotherapy. However, molecularly targeted drugs are currently only effective in specific patients with known target overexpression or activation and therefore have limited clinical use. On the contrary, traditional Chinese medicine (TCM), under the guidance of the holistic view, recognizes the evidence and treats the disease. This has improved the clinical symptoms and quality of life and prolonged the survival period of GC patients, as well as reduced the toxic side effects brought by radiotherapy and chemotherapy[9].
Xiaojianzhong decoction (XJZ) is derived from the Treatise on Febrile Diseases and Miscellaneous Diseases, which contains cassia twigs, paeonia, roasted licorice, ginger, jujube, and maltose sugar. More than 2000 years have passed since it began to be used for preventing and treating gastrointestinal diseases with remarkable clinical efficacy. Chen et al[10] found that XJZ could effectively reduce damage caused by oxidative stress and ferroptosis to the gastric mucosa. Yu et al[11] found that XJZ can quickly resolve inflammation and biological disorders, has a good therapeutic effect on colitis, and prevents carcinogenesis by re-establishing the microbiome structure that is conducive to re-epithelialization. XJZ appears to be able to restore bile acid metabolism in patients with atrophic gastritis, according to Liu et al[12]. Other studies have reported that XJZ can prevent gastric precancerous lesions in rats by inhibiting autophagy and glycolysis in gastric mucosa cells[13]. Therefore, it is feasible to treat gastrointestinal diseases with XJZ.
In recent years, there has been much research on how TCM compounds may be used to prevent and treat GC. Song et al[14] found that Yiqi Huayu decoction can prevent GC recurrence and metastasis by inducing ferroptosis. According to Shi et al[15], Xiaotan Sanjie decoction inhibits angiogenesis in GC via IL-8 to regulate the VEGF pathway. Ding et al[16] found that Sijunzi decoction exerts its anti-GC effects by inhibiting angiogenesis and inducing apoptosis. Li et al[17] found that compound matrine injections inhibit the epithelial-mesenchymal transition (EMT) of GC cells via the PI3K/AKT signaling. Thus, TCM compounds can be considered very effective in treating GC. Therefore, we propose using XJZ to treat GC.
Chinese medicines have unique advantages in the treatment of tumor diseases. In addition to inducing apoptosis[18], inhibiting cell proliferation[19], and suppressing tumor metastasis[20], they can also reduce the toxic side effects of radiotherapy[21]. Chinese medicines and their compound formulas are characterized by multiple components, multiple targets, and multi-level effects[22]. Similarly, XJZ also has many monomers and active ingredients. Network pharmacology is an innovative method that combines various histological techniques and network analysis methods. Its main goal is to establish and uncover the intricate connections existing between drugs, their components, target molecules, and diseases[23]. By doing so, it aims to elucidate the mechanisms underlying drug action and investigate the potential relationships between drugs and specific diseases[24]. Cyberpharmacology focuses on the correlative relationships between drugs and diseases based on systems biology and biological networks. This coincides with the mode of action of TCM. The current study aimed to utilize network pharmacology to systematically forecast the potential targets of action and signaling pathways of XJZ for treating GC. In addition, we conducted in vitro cellular experiments to explore the molecular mechanism of XJZ in GC treatment. These findings will serve as a theoretical foundation for future clinical interventions. The flow chart of this study is shown in Figure 1.
Identification of active ingredients and target prediction of XJZ: The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (https://tcmsp-e.com/) was utilized to search for the active com
GC transcriptome data downloading and identification of differentially expressed genes: The file containing transcriptomic GC dataset GSE118916 was acquired from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). Using the GEO2R online analysis tool (https://www.ncbi.nlm.nih.gov/geo/geo2r/), the dataset was grouped and evaluated for differentially expressed genes (DEGs). Genes with a LogFC ≥ 1 were classified as up-regulated, while those with a LogFC ≤ -1 were categorized as down-regulated. To provide a visual representation, the number of up- and down-regulated genes was depicted as a Venn diagram, with XJZ targets being the focus. Additionally, the overlapping DEGs were illustrated as heatmaps and histograms utilizing the online platform of Microbiotics (http://www.bioinformatics.com.cn/). Volcano plot depicting differential gene expression was made using GraphPad Prism 8.
Acquisition of targets for treatment of GC with XJZ: We utilized the Venny 2.1.0 platform (https://bioinfogp.cnb.csic.es/tools/venny/) to determine the overlap between the genes that were differentially expressed in GC and the target genes of XJZ. A Venn diagram was generated to display the target genes of XJZ associated with GC.
Constructing protein-protein interaction network and acquiring core targets: To construct a protein-protein interaction (PPI) network for GC, the identified gene targets of XJZ were imported into the String database. The resulting network was then visualized. To proceed, the tsv. file was downloaded and imported into the CytoScape 3.9.1 software. The CytoNCA plug-in was used to filter the core targets based on the node's degree value. Finally, a PPI network graph was constructed.
Constructing component-target network: The component-target network was constructed by combining the predicted targets of XJZ for GC with the active ingredients of XJZ using Cytoscape 3.9.1 software. The main components and targets of XJZ for GC were analyzed according to the degree value of the nodes using CytoNCA plug-in, and the results were visualized.
Gene ontology and Kyoto encyclopedia of genes and genomes analysis: The identified core targets were analyzed using the Metascape (http://metascape.org) online platform for gene ontology (GO) biological function and Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analyses identify the specific biological processes (BP), molecular functions (MF), cellular components (CC), and related biological pathways associated with the core targets. The results of this analysis were then visualized using the Microbiome (http://www.bioinformatics.com.cn/) and Sangerbox (http://sangerbox.com/) online platforms.
Molecular docking: The crucial chemical components of XJZ were obtained from the TCMSP database, where the three-dimensional (3D) structures were downloaded. To analyze the core target proteins, the 3D structures were acquired from the PDB database (https://www.rcsb.org/). The Pymol software was utilized to eliminate the water molecules and small molecule ligands from the proteins. Subsequently, the AutoDock 4.2.6 software was employed for hydrogenation, followed by molecular docking of the receptor and ligand. The outcomes of the docking process were visualized using the Pymol software and detailed in Supplementary Table 1.
Expression difference analysis of the hub genes and assessment of their correlation with clinical stage were conducted using the Sangerbox tool. The analysis aimed to compare the expression levels of the hub genes in tumor tissues vs normal tissues and assess their copy number variation. Additionally, the GEPIA database (http://gepia2.cancer-pku.cn) was employed to investigate the relationship between hub gene expression and survival outcomes in patients with GC.
Immune cells from GEO probe files were analyzed using CIBERCORTx (https://cibersortx.stanford.edu/), and the results were plotted in box plots using the Microbiotics platform. The expression of the hub genes in various immune subtypes of GC was analyzed on the TISIDB platform (http://cis.hku.hk/TISIDB/). Pearson's correlation coefficients of the hub genes with immune infiltration scores in GC were analyzed using the Sangerbox tool. In order to determine the presence of immune infiltration in each tumor, stromal, immune, and ESTIMATE scores were calculated for each patient. These scores were derived from gene expression data, allowing for a comprehensive assessment of the immune response within the tumor microenvironment. Expression data of the hub genes and immune checkpoint pathway genes in GC were analyzed using the Sangerbox tool, and Pearson's correlation was calculated between the hub genes and marker genes of the five immune pathways.
Experimental materials: XJZ is composed of cassia twigs, paeonia, roasted licorice, ginger, jujube, and maltose sugar. The herbs were purchased from Ningxia Mingde Traditional Chinese Medicine Drinking Tablets Co. The human GC cell lines AGS (Cat. No. CL-0022) and HGC-27 (CAT: CL-0107), and the human gastric mucosal epithelial cell line GES-1 (CAT: CL-0563) were purchased from Wuhan Prosperity Life Technology Co. The cells were confirmed to be free of cross-contamination by short tandom repeat profiling. Fetal bovine serum (CAT: 900-108) was provided by Gemini. RPMI-1640 medium (Cat. No. 11875-119), DMEM medium (Cat. No. 11960-069), and DMEM/F12 medium (Cat. No. SH30272.1) were purchased from Gemini. Basal Medium Eagle (Cat. No. SH30272.01) was purchased from Hyclone. Annexin-V FITC/PI Double Staining Apoptosis Detection Kit (CAT: KGA1023) and Cell Cycle Detection Kit (CAT: KGA511, China) were provided by Jiangsu Kagan Biotechnology Co.
Cell viability assessment by CCK-8 assay: GES-1, AGS, and HGC-27 cells were cultured in 96-well plates and exposed to various concentrations of XJZ for 12, 24, and 36 h, respectively. Each group was set up with five replicate wells. After that, 10 μL of CCK-8 was added to each well containing 100 μL of medium and incubated for 2 h. The optical density (OD) values were measured at 450 nm to calculate cell viability.
Detection of apoptosis by flow cytometry: AGS and HGC-27 cells were cultured in 6-well plates until reaching approximately 70%-80% confluence. The supernatant was then removed and replaced with a drug-containing medium for further culture. After 12 h of XJZ intervention, the cells were digested. Subsequently, the cells were treated according to the apoptosis kit instructions, and the samples were tested using a flow cytometer within 1 h. The experiment was repeated three times.
Detection of cell cycle progression by flow cytometry: AGS and HGC-27 cells were synchronized for 12 h after inoculation in 6-well plates. After adding drug-containing culture medium for 12 h, the cells were digested and collected. The cells were then processed following the instructions provided in the cell cycle detection kit. Subsequently, the cells were tested using a flow cytometer to analyze the percentage of cells in each cycle. The experiment was repeated three times.
Clone formation assay: AGS and HGC-27 cells were inoculated into 6-well plates with 500 cells per well. After treatment with XJZ, the cells were further cultivated for 10-14 d. When cell clones were obviously formed, the culture was terminated. The cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The number of clones in each well was then counted and analyzed. The experiment was repeated three times.
Real-time quantitative PCR: Total RNA was extracted from the cells of the control group and XJZ group with Trizol reagent, and then cDNA was synthesized. The relative gene expression was determined by the 2-ΔΔCT method. The primer sequences are shown in Table 1. The experiment was repeated three times.
| Gene | Direction | Sequence |
| IL-6 | FORWARD | TGGTGTTGCCTGCTGCCTTC |
| REVERSE | GCTGAGATGCCGTCGAGGATG | |
| CCL2 | FORWARD | ATGAAAGTCTCTGCCGCCCTTC |
| REVERSE | GACACTTGCTGCTGGTGATTCTTC | |
| PTGS2 | FORWARD | AAGTCCCTGAGCATCTACGGTTTG |
| REVERSE | TGTTCCCGCAGCCAGATTGTG | |
| HMOX1 | FORWARD | CTACCGCTCCCGCATGAACTC |
| REVERSE | AGCAGGAACGCAGTCTTGGC | |
| MMP9 | FORWARD | TGGTCCTGGTGCTCCTGGTG |
| REVERSE | TGCCTGTCGGTGAGATTGGTTC | |
| MMP2 | FORWARD | CCTACACCAAGAACTTCCGTCTG |
| REVERSE | GTGCCAAGGTCAATGTCAGGAG |
Western blot analysis: Total protein was extracted from normal HGC-27 cells and XJZ-treated cells, and the protein concentration was measured by the Bicinchoninic acid assay. After the total protein was isolated by electrophoresis, the protein was transferred to a membrane. After the membrane were blocked with skimmed milk for 2 h, the membrane was incubated with primary antibodies for 12-24 h, followed by incubation with the corresponding secondary antibody. Finally, an ultrasensitive multifunctional imager and luminescent solution were used for detection. The resulting bands were analyzed using Image J software to calculate protein expression.
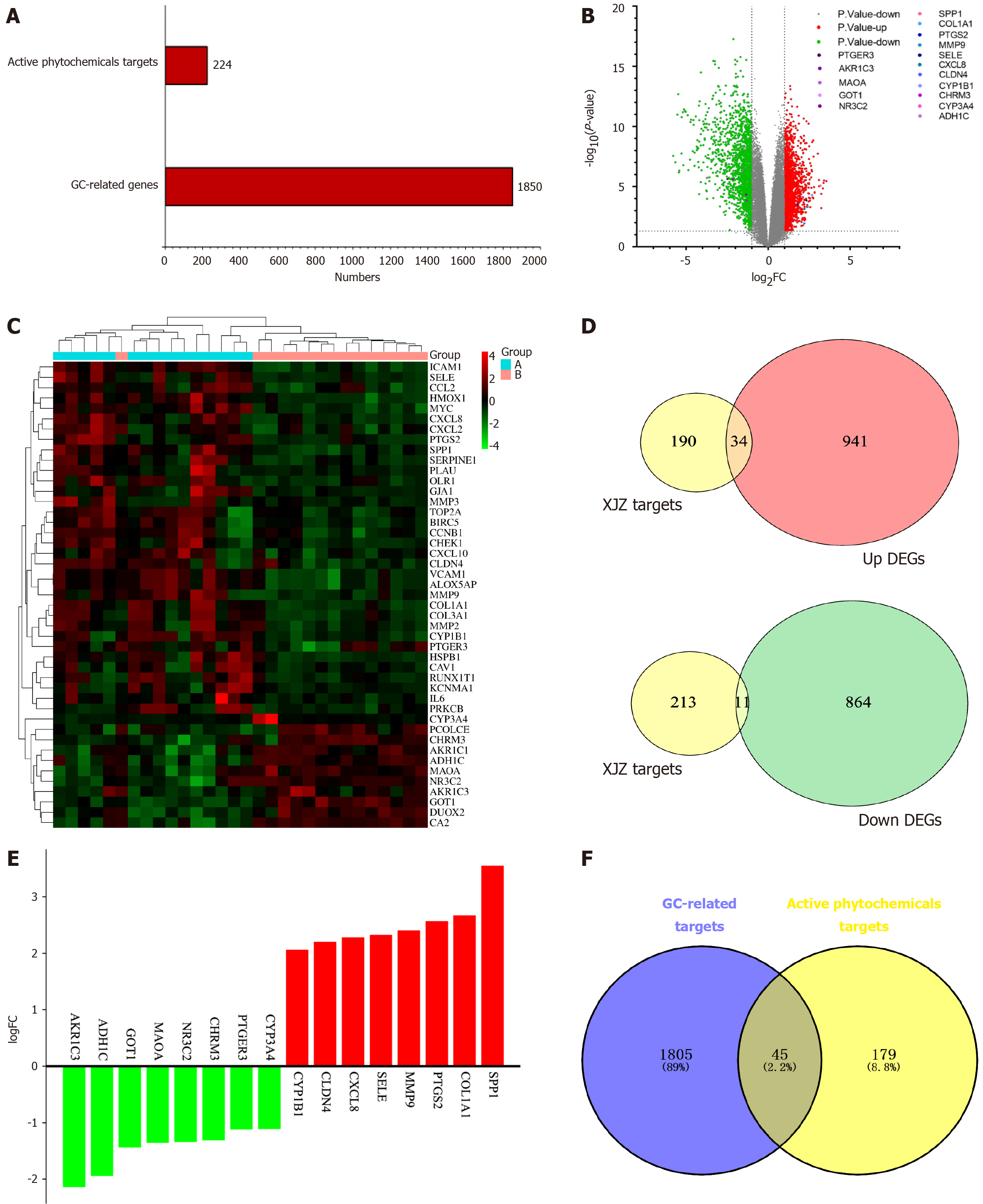
Identification of active ingredients and target prediction of XJZ: All the effective ingredients of each herb in XJZ were searched using TCMSP, among which six active ingredients of cassia twigs were obtained, corresponding to 74 targets. There were 19 active ingredients in jujube, corresponding to 213 targets. There were 88 active ingredients in roasted licorice, corresponding to 238 targets. There were 6 active ingredients in paeonia, corresponding to 82 targets. There were 4 active ingredients in ginger, corresponding to 56 targets. After removing duplicates, 112 active ingredients were obtained. Finally, 224 identical targets were obtained.
Differential analysis of gastric cancer transcriptome data: The dataset GSE118916 was downloaded from the GEO database. This dataset has 30 samples, including 15 normal gastric tissue samples and 15 GC samples. The histogram of the number of GC-related targets and XJZ's core component targets is shown in Figure 2A. The matrix file and platform file were downloaded, and the data were filtered and normalized with the help of the GEO2R online tool. With |logFC| < 1 and adjusted P < 0.05 as the criteria, 1850 differential genes were identified between the normal group and GC tissues, among which 975 were up-regulated and 875 down-regulated. A volcano plot of GC genes was generated, showing the distribution of up-regulated (red), down-regulated (green), and non-differentially expressed genes (gray) in the dataset (Figure 2B). The up- and down-regulated differential genes were plotted in a clustered heatmap, with red showing that the genes were highly expressed in this sample and green showing low expression (Figure 2C). The up- and down-regulated genes were plotted against the predicted targets of XJZ to obtain 34 up-regulated genes and 11 down-regulated genes (Figure 2D). The up- and down-regulated genes were then visualized in a histogram (Figure 2E).
Common targets of GC and XJZ: The 1850 GC-related differential genes and 224 target genes of XJZ were used to generate a Venn diagram. After taking the intersection of the two data sets, 45 common target genes for XJZ and GC were obtained (Figure 2F).
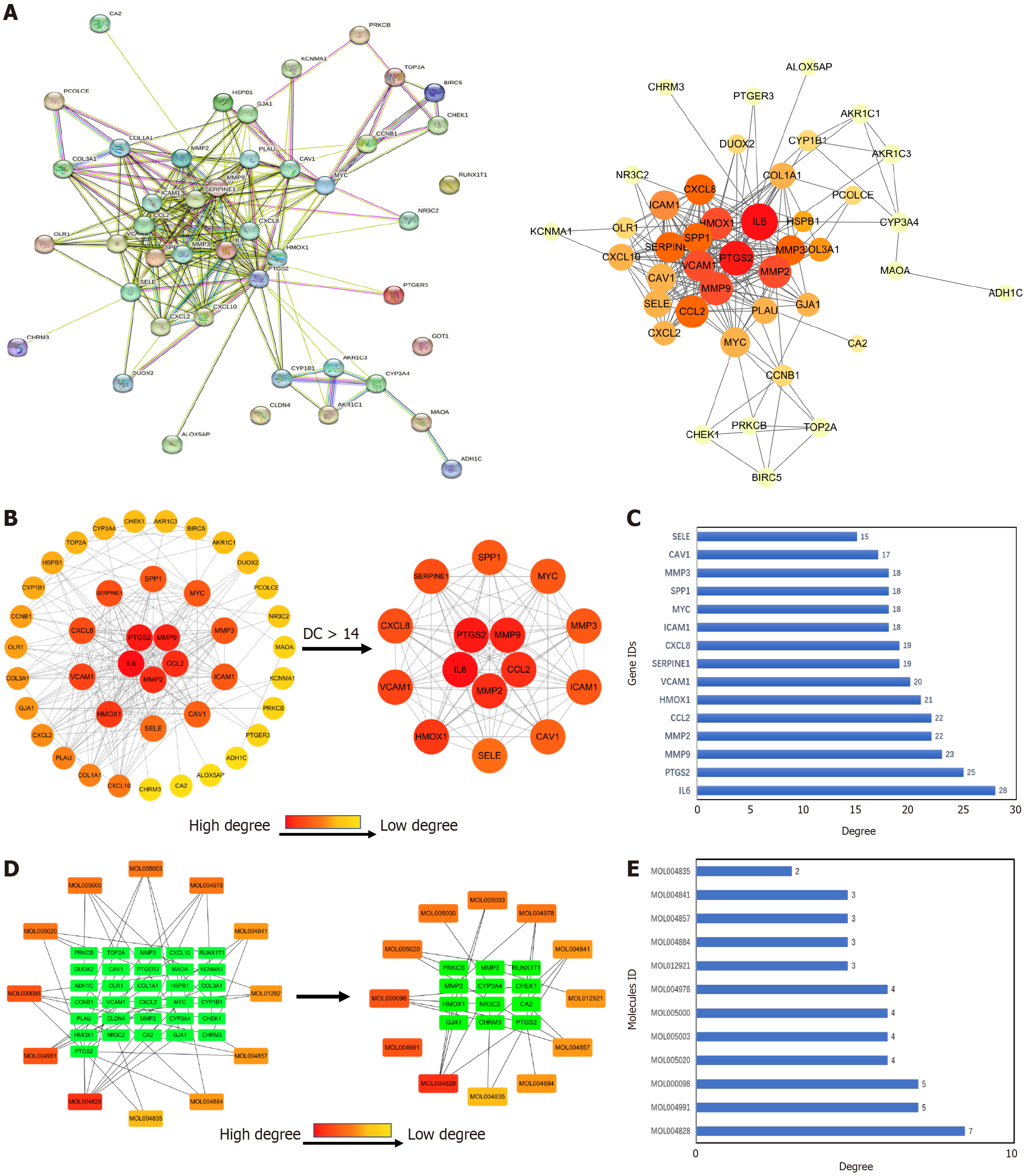
Construction of PPI network and identification of core targets: The predicted gene targets of XJZ for GC were imported into the STRING database to obtain the PPI network graph (Figure 3A). There were 42 nodes and 224 edges in the graph, with the nodes representing proteins. The TSV file of the PPI network graph was downloaded, and the network features were analyzed with the CytoNCA plug-in in Cytoscape 3.9.1 software. According to the results of the topological analysis, the nodes with a degree greater than or equal to 14 were screened out, and the core target PPI network of XJZ for GC was initially obtained. The core targets were IL6, PTGS2, MMP9, MMP2, CCL2, HMOX1, VCAM1, SERPINE1, CXCL8, ICAM1, MYC, SPP1, MMP3, CAV1, and SELE (Figure 3B). A darker color in the graph represents a larger degree value of the 15 core targets represented visually (Figure 3C).
Construction of component-target network: A network was reconstructed by combining the targets of XJZ for GC with the active ingredients of XJZ using Cytoscape 3.9.1 software. The CytoNCA plug-in was applied for analysis, and 12 key active ingredients were obtained (Figure 3D). A darker color in the figure represents a larger value of the degree. And the degree values of the 12 key core components were visually represented (Figure 3E).
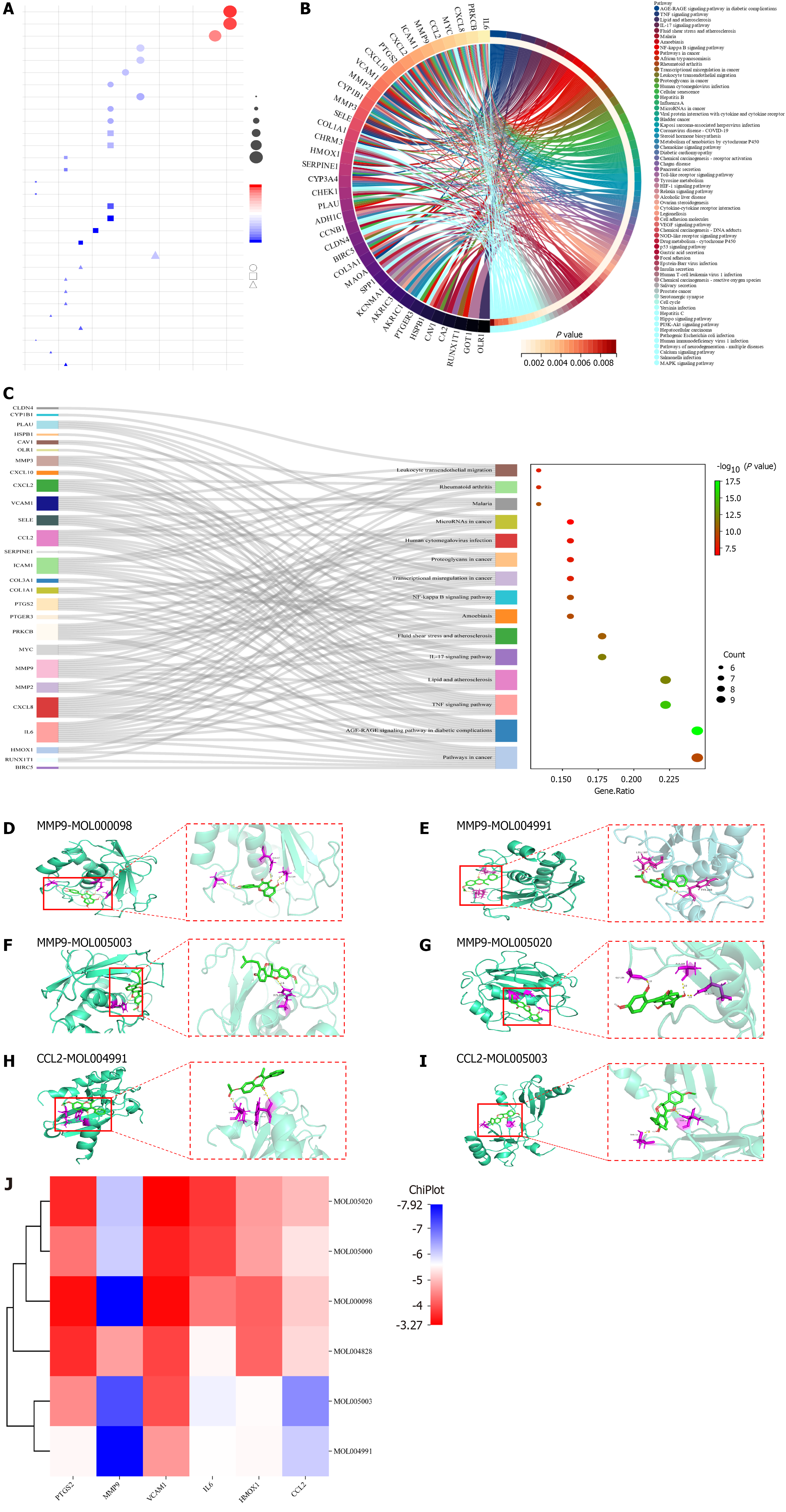
GO biological function and KEGG pathway enrichment analysis: GO analysis showed that XJZ for GC was associated with BPs such as positive regulation of locomotion, positive regulation of cell migration, etc. CCs included the membrane raft, membrane microdomain, etc. MFs included oxidoreductase activity, acting on paired donors, incorporation or reduction of molecular oxygen, etc. The top ten entries of each of the BP, CC, and MF enrichment results were selected for visualization (Figure 4A). The KEGG analysis is shown in Figure 4B. The Sankey diagrams of the top 15 pathways in terms of enrichment results are shown in Figure 4C. The top 15 enrichment results involved pathways in cancer and so on, suggesting that these pathways may be important in the treatment of GC by XJZ.
Molecular docking: To further analyze the feasibility of XJZ for the treatment of GC, quercetin, glepidotin A, 7-acetoxy-2-methylisoflavone, gancaonin G, and licoagrocarpin, which are the active ingredients with the top 6 rankings of DEGREE, were selected. Dehydroglyasperins C was molecularly docked with the core targets PTGS2, MMP9, MMP2, IL6, HMOX1, and CCL2. Binding activity was evaluated based on binding energy after docking using AutoDock: Different levels of binding activity can be determined based on the binding energy. The binding energy results of molecular docking are shown in Table 2. A binding energy of -4.25 kcal/mol suggests some binding activity, while a binding energy of -5.0 kcal/mol indicates good binding activity. For strong binding activity, a binding energy of -7.0 kcal/mol is needed. The six docking pairs with the lowest binding energies were visualized using PyMol (Figure 4D-I), and the molecular binding activity results were heatmapped using the ChiPlot online platform (Figure 4J). The docking results showed that MMP9-quercetin had the lowest binding energy of -7.92 kcal/mol.
| Mol ID | Molecule name | Binding affinity (kcal/mol) | |||||
| PTGS2 | MMP9 | MMP2 | IL6 | HMOX1 | CCL2 | ||
| MOL000098 | Quercetin | -3.38 | -7.92 | -3.33 | -4.36 | -4.15 | -5.12 |
| MOL004828 | Glepidotin A | -3.67 | -4.72 | -3.88 | -5.52 | -4.18 | -5.24 |
| MOL004991 | 7-Acetoxy-2-methylisoflavone | -5.52 | -7.89 | -4.66 | -5.59 | -5.54 | -6.06 |
| MOL005000 | Gancaonin G | -4.33 | -6.05 | -3.56 | -3.88 | -4.73 | -5.34 |
| MOL005003 | Licoagrocarpin | -4.54 | -7.22 | -3.98 | -5.72 | -5.56 | -6.65 |
| MOL005020 | Dehydroglyasperins C | -3.62 | -6.12 | -3.27 | -3.76 | -4.69 | -4.97 |
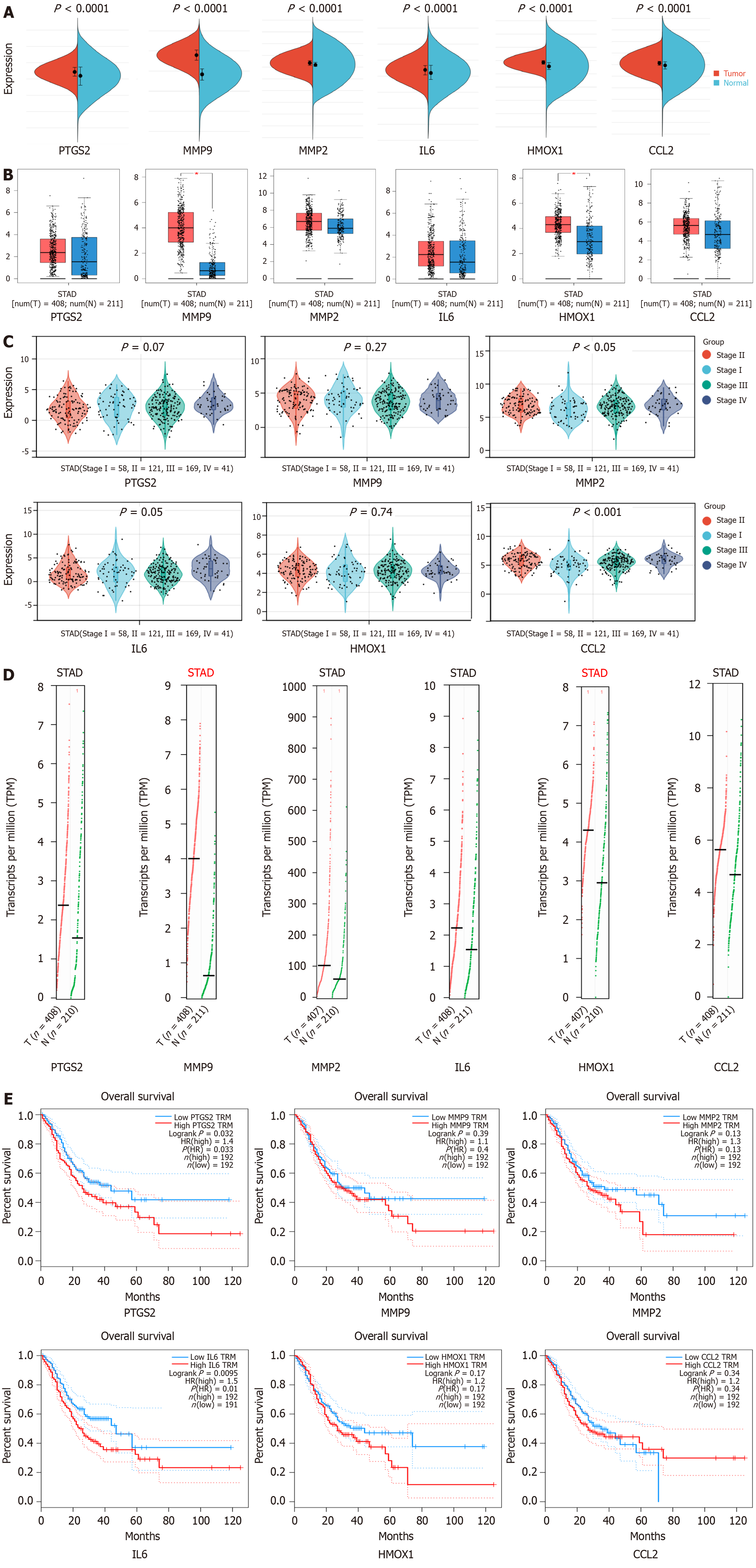
GC samples from the TCGA and GTEx databases were analyzed on the Sangerbox website. As shown in Figure 5A, the mRNA expression of PTGS2 (P < 0.0001), MMP9 (P < 0.0001), MMP2 (P < 0.0001), IL-6 (P < 0.0001), HMOX1 (P < 0.0001), and CCL2 (P < 0.0001) was significantly up-regulated in the GC samples relative to normal samples. In the GEPIA database, the results of comparing GC tissues with normal tissues showed that MMP9 (P < 0.05) and HMOX (P < 0.05) were significantly up-regulated (Figure 5B). As shown in Figure 5C, MMP2 (P < 0.05) and CCL2 (P < 0.001) expression had significance in the grading and staging of GC. In addition, the copy number of the genes MMP9 and HMOX1 was up-regulated (Figure 5D), and the genes PTGS2 (P = 0.033) and IL6 (P = 0.01) had a statistically significant effect on the prognosis of GC (Figure 5E).
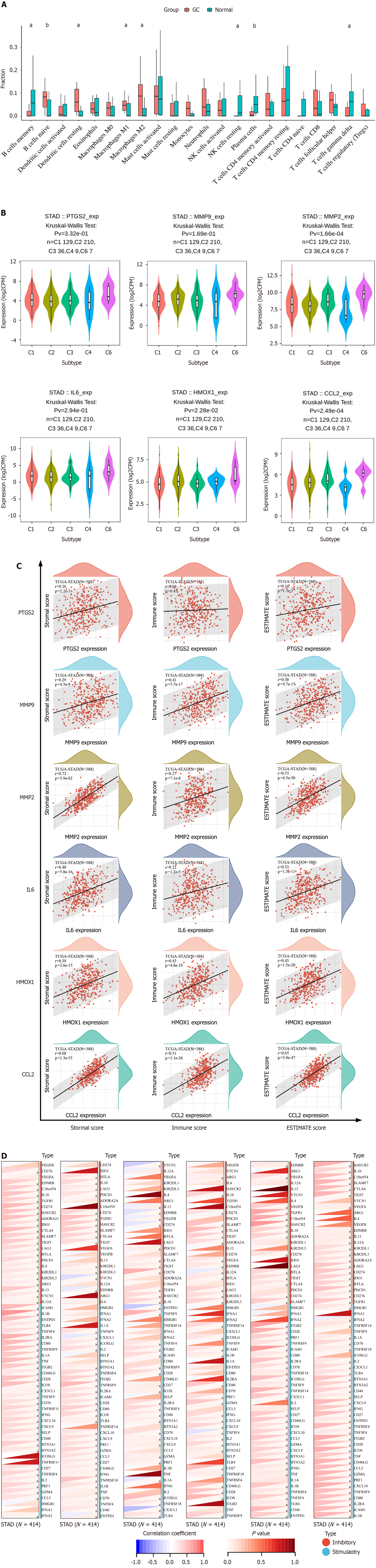
The results of immune infiltration analysis showed that eight types of immune cells were significantly changed in GC. Four of them were up-regulated, and the other four were down-regulated (Figure 6A). This indicates that there are different immune cell infiltration patterns in GC. The expression of hub genes in different immune subtypes in GC was analyzed using TISCH, and the results are shown in Figure 6B. Based on the immune infiltration scores, we finally observed that hub gene expression was significantly and positively correlated with immune infiltration in GC (Figure 6C). A heatmap of the correlation between hub genes and immune checkpoints shows that hub genes were positively correlated with the majority of immune checkpoints in GC (Figure 6D).
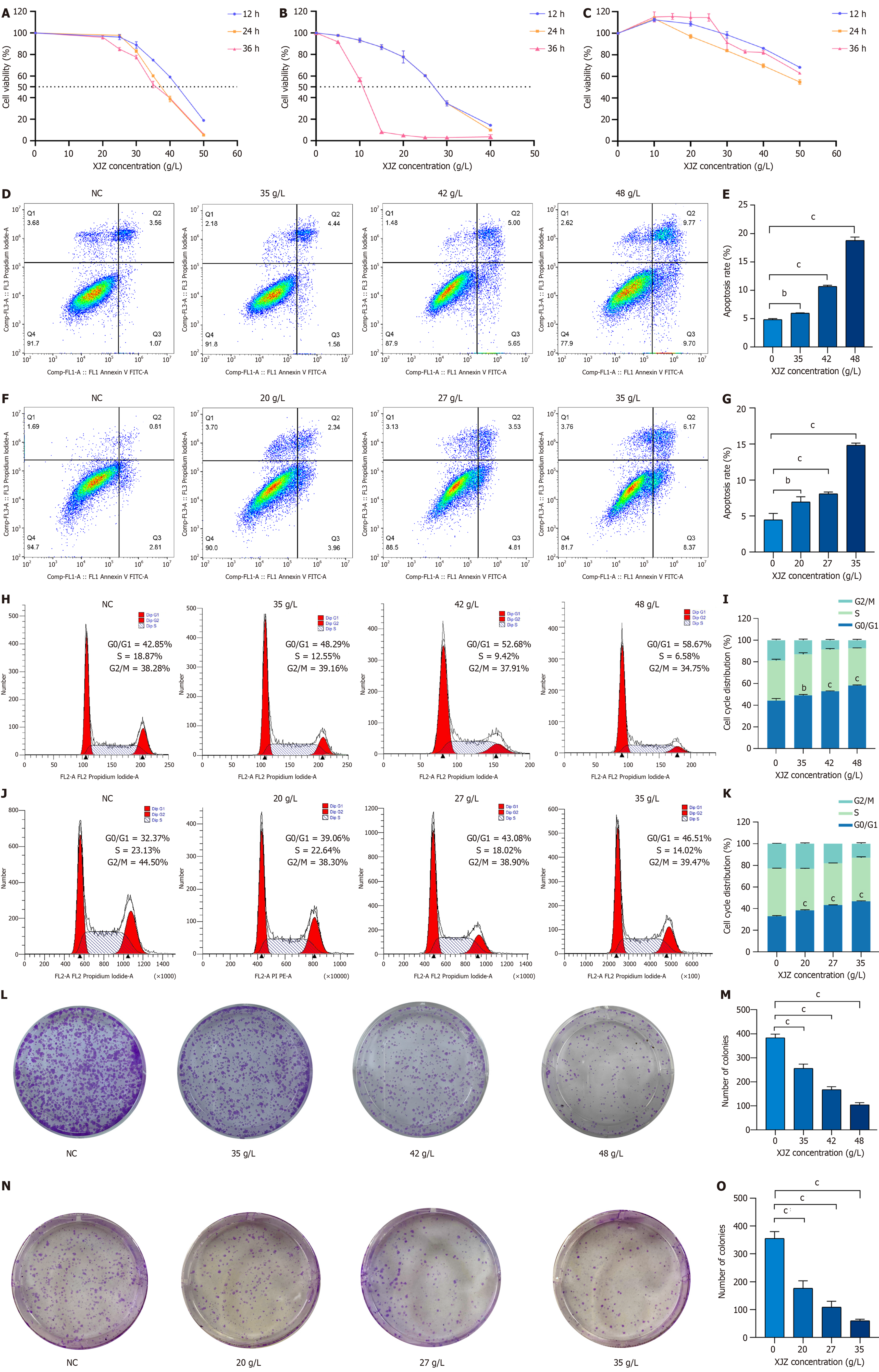
Inhibition of viability of GC cells by XJZ: To examine the impact of XJZ on the viability of GC cells, AGS and HGC-27 cells were administered various doses of XJZ for different durations (12, 24, and 36 h). The viability of AGS cells was significantly suppressed by XJZ at concentrations ranging from 20 to 50 g/L. Moreover, the inhibitory effect on cell viability displayed a concentration- and time-dependent manner (Figure 7A). The half maximal inhibitory concentration (IC50) of XJZ intervention on AGS cells at 12, 24, and 36 h was 41.82 g/L, 37.45 g/L, and 36.46 g/L, respectively. Drug intervention for 12 h significantly inhibited the proliferation of AGS cells, so 12 h was chosen as the duration of drug intervention. Finally, the low, medium, and high concentrations of the drug (38, 42, and 48 g/L) were determined as the concentrations for the subsequent experiments based on the IC50 at 12 h. XJZ at 5-40 g/L also significantly inhibited the viability of HGC-27 cells in a time- and concentration-dependent manner (Figure 7B). The IC50 of XJZ intervention on HGC-27 cells was 27.82 g/L, 37.45 g/L, and 36.46 g/L for the 12, 24 and 36 hours interventions, respectively. The IC50s at 12, 24, and 36 hours were 27.13 g/L, 26.89 g/L, and 10.47 g/L, respectively. We chose 12 h as the duration of subsequent drug intervention and finalized the low, medium, and high doses of the drug (20 g/L, 27 g/L, and 35 g/L) as the concentrations of subsequent drug administration based on the IC50 at 12 h. When AGS and HGC-27 cells were treated with XJZ at the half inhibitory concentration, XJZ showed very little damage to normal gastric mucosal cells (GES-1) (Figure 7C). Taken together, these findings suggest that XJZ has potential for use in GC therapy.
XJZ induces apoptosis of GC cells: XJZ effectively triggered apoptosis in AGS cells, exhibiting a dose-dependent pattern when compared to the control group (Figure 7D). The apoptosis rates observed in the low, medium, and high dose groups were 5.97% ± 0.09%, 10.69% ± 0.21%, and 18.80% ± 0.58%, respectively (Figure 7E). In HGC-27 cells, XJZ also induced apoptosis in a dose-dependent manner when compared to the control group (Figure 7F). The apoptosis rates were 6.95% ± 0.73%, 8.08% ± 0.25%, and 14.86% ± 0.28% in the low, medium, and high dose groups, respectively (Figure 7G).
XJZ blocks cell cycle progression of GC cells: There was a concentration-dependent increase in the percentage of cells in the G0/G1 phase when AGS cells were exposed to varying concentrations of XJZ. The respective percentages for cells treated with low, medium, and high concentrations of XJZ were 49.17% ± 0.78%, 52.82% ± 0.44%, and 58.26% ± 0.50% (Figure 7H and I). The proportion of cells in the G0/G1 phase increased in HGC cells as the concentration of XJZ increased. The cells treated with low, medium, and high concentrations of XJZ had a proportion of 38.44% ± 0.54%, 43.34% ± 0.23%, and 46.73% ± 0.50% in the G0/G1 phase, respectively (Figure 7J and K).
XJZ inhibits clone formation of GC cells: In order to assess the impact of XJZ on the clone formation capability of AGS and HGC-27 cells, we subjected AGS and HGC cells to varying concentrations of XJZ (20, 27, 35, 38, 42, and 48 g/L) for a duration of 12 h. XJZ at low, medium, and high doses resulted in a noticeable decrease in clone formation ability in a dose-dependent manner when compared to the control group (Figure 7L-O).
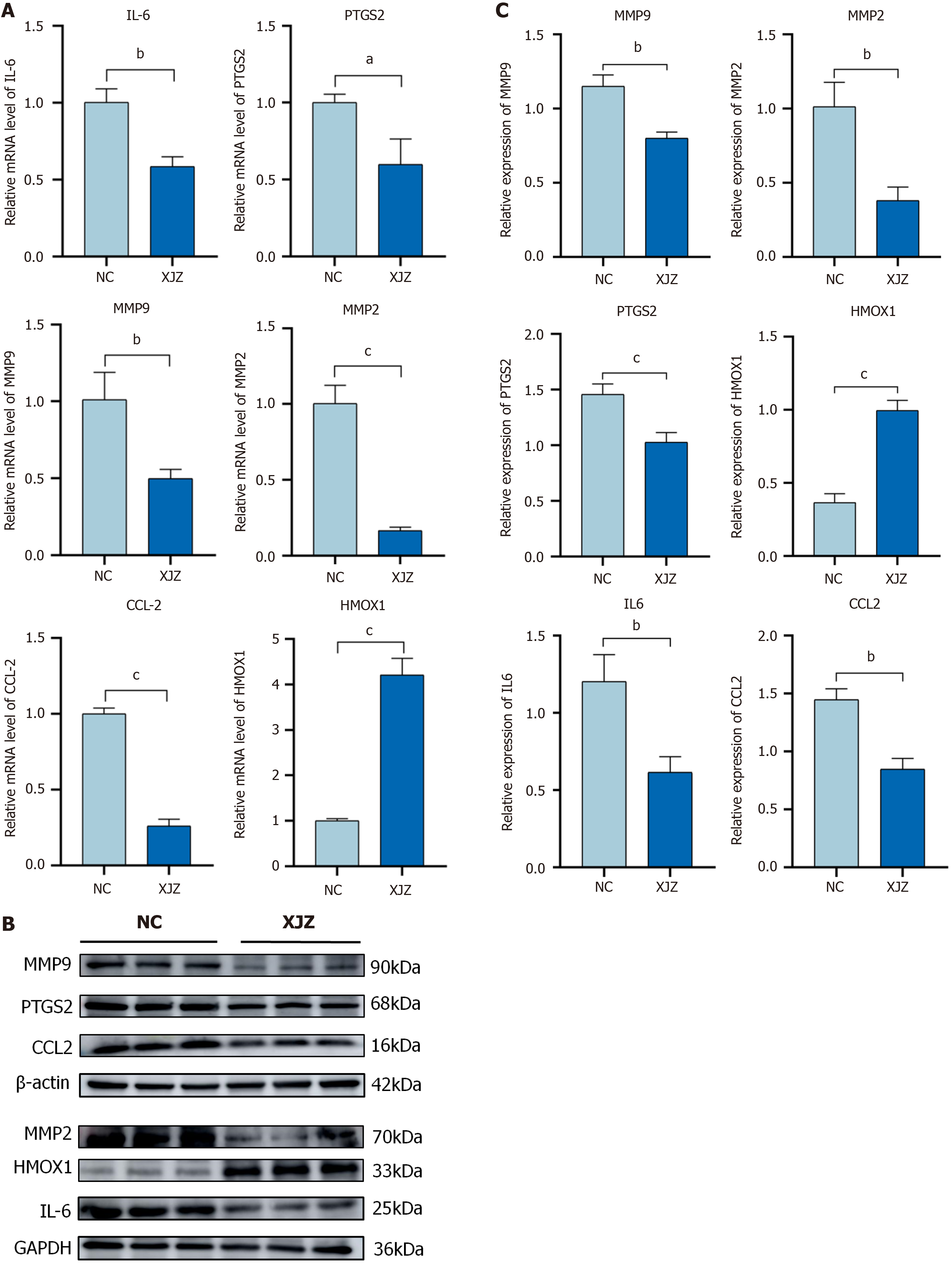
Real-time quantitative PCR: As shown in Figure 8A, there was a significant decrease in the mRNA expression levels of IL-6 (P < 0.01), MMP2 (P < 0.01), MMP9 (P < 0.01), CCL2 (P < 0.001), and PTGS2 (P < 0.05) in the XJZ-treated group compared to the control group. Moreover, we observed a significant elevation in the mRNA expression of HMOX1 (P < 0.001) in the XJZ group relative to the control group.
Western blot analysis: Western blot analysis showed that the protein expression of IL-6 (P < 0.01), MMP2 (P < 0.01), MMP9 (P < 0.01), CCL2 (P < 0.01), and PTGS2 (P < 0.001) decreased significantly and that of HMOX1 increased significantly in the XJZ group compared with the control group (Figure 8B and C). This is consistent with the results of real-time quantitative PCR.
Based on network pharmacology, a total of 112 active ingredients and 45 anti-GC targets of XJZ were screened. PPI network analysis indicated that XJZ might exert anti-GC effects by acting on the targets of IL6, PTGS2, MMP9, MMP2, CCL2, and HMOX1. Among them, the docking of MMP9 with quercetin was the best combination of target protein and active compound, indicating that quercetin could regulate the main target. GO function and KEGG pathway enrichment analyses uncovered the pathways, biological functions, and potential mechanisms engaged in the management of GC by XJZ. This includes the cancer signaling pathway as a pivotal pathway in the treatment of GC.
Using in vitro cellular experiments, the efficacy of XJZ in suppressing the viability of GC cells, inducing apoptosis, arresting these cells in the G0/G1 phase, and inhibiting cell clone formation was discovered. By means of qRT-PCR analysis, it was observed that XJZ treatment resulted in decreased expression of IL6, PTGS2, MMP9, MMP2, and CCL2 hub genes, while HOMX1 expression levels increased. Furthermore, Western blot assays demonstrated that XJZ effectively suppressed the expression of IL6, PTGS2, MMP9, MMP2, and CCL2 proteins and triggered the upregulation of the heme oxygenase-1 (HO-1) protein.
IL-6 is a cytokine that is known to play a significant role in inflammatory conditions and inflammation-related cancers[25]. It has the ability to activate the Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) signaling pathway by binding with IL-6R[26]. This pathway is considered to be a major contributor to inflammation in cancer, as it is frequently activated in malignant cells and controls the expression of multiple genes associated with inflammation[27]. Various studies have demonstrated the involvement of IL-6 in the development and spread of different types of tumors, such as gastric[28], cervical[29], colorectal[30], and breast cancers[31]. Additionally, IL-6 has been found to stimulate the process of EMT, which is critical for cancer progression, metastasis, and resistance to treatment. This suggests that IL-6 plays a crucial role in promoting tumor development and enhancing cancer aggressiveness through its effects on EMT and inflammation-related gene expression[32].
PTGS2, also known as COX-2, is an enzyme that gets released at the location of tissue damage in order to generate a substance resembling a hormone known as prostaglandin E2 (PGE2)[33]. PGE2, in turn, triggers both pain and inflammation. Investigations conducted have revealed the significance of PTGS2 expression in the development of gas
HO-1 is an intracellular enzyme that can be induced by various stimuli to break down heme and produce important catabolic products such as carbon monoxide (CO), biliverdin, and ferrous iron (Fe2+)[37]. The role of HO-1 in tumorigenesis is complex. On the one hand, HO-1 is found in many types of cancers and has been shown to promote tumor progression through various mechanisms[38]. In advanced stages of tumorigenesis, the overexpression of HO-1 contributes to increased proliferation and invasiveness of cancer cells[39]. However, excessive activation of HO-1 leads to the excessive accumulation of unstable iron pools and the generation of reactive oxygen species through accelerated heme metabolism[40]. This, in turn, results in severe cytotoxic effects, which are known as iron death. Iron death is a newly discovered form of regulated cell death that is distinct from traditional types of cell death like apoptosis, necrosis, pyroptosis, and autophagy. It is characterized by disturbances in iron metabolism and the accumulation of lipid peroxides[41]. Considering its potential for inducing iron death, HO-1 has emerged as a promising candidate for cancer treatment. Han et al[42] found that lignans induced iron death in renal cell carcinoma by up-regulating HO-1 expression, activating unstable iron pools, and promoting lipid peroxidation, thus exerting anticancer effects. Abietic acid induces iron death by activating the HO-1 pathway in bladder cancer cells[43]. We speculate that the increase in HO-1 protein expression and HOMX1 mRNA expression after XJZ intervention in HGC-27 GC cells might be due to the toxic effects of XJZ-regulated HO-1-induced iron death on GC cells. The mechanism of iron death is extremely complex and not fully understood, and the dual role of HO-1 in tumor cells and its mechanism need to be further investigated. We still need to verify our current findings with other GC cell lines.
Chemokines and cytokines function as vital regulators within the cancer microenvironment, guiding a diverse array of immune/inflammatory cells towards the tumor site during tumorigenesis. They have been recognized as pivotal drivers of cancer development[44]. Chemokines have been established to promote tumorigenesis and tumor progression through various means, encompassing augmented proliferation, heightened invasiveness, increased angiogenesis, and the recruitment of immune cells[45]. Several investigations have demonstrated a close correlation between CCL2 and HIF-1α expression in GC, suggesting CCL2 as a potentially independent prognostic marker for GC[46]. In the context of colorectal cancer, escalated activation of AKT-mTOR heightens the phosphorylation level of STAT3, thus eliciting CCL2 expression while repressing the activation of the cancer immune response[47]. Extensive research has highlighted the probable oncogenic nature of CCL2 in GC, with experiments showcasing that suppressing CCL2 yields beneficial outcomes in reducing the incidence and progression of GC[48]. A comprehensive comprehension of the pharmacological impacts of CCL2 can substantially augment our understanding of the molecular mechanisms driving GC progression and engender novel potential strategies for GC treatment.
Matrix metalloproteinases (MMPs) belong to a class of recognizable protein hydrolases responsible for degrading a wide range of proteins in the extracellular matrix[49]. In normal physiological conditions, MMPs are tightly regulated and expressed at low levels. However, dysregulation and overexpression of these enzymes have been associated with malignant tumor growth, invasion, metastasis, and angiogenesis[50]. It has been demonstrated that upregulation of MMP9 can enhance the migration and invasion of GC cells[51]. Furthermore, Helicobacter pylori has been found to promote the progression of GC through the ERK/MMP9 signaling pathway[52]. The activity of the MMP9 promoter may serve as a predictor of high risk of GC and the subsequent metastasis[53]. Multiple studies have indicated that USP19 induces the expression and enzymatic activities of MMP2 and MMP9, thereby promoting tumor progression[54]. In summary, there is ample evidence supporting the crucial role of MMPs in the invasion and growth of various malignant tumors. The expression levels of specific MMPs can be utilized as indicators for estimating the metastatic potential, recurrence risk, and prognosis of patients with malignant tumors. However, the current major challenge facing the field of MMPs in cancer therapy lies in the lack of clinical trials and insufficient research on MMP inhibitors.
For this investigation, we solely applied network pharmacology to identify the targets and signaling pathways involved in the therapeutic effects of XJZ on GC. Additionally, we conducted in vitro experiments to confirm the molecular mechanism by which XJZ operates for GC treatment. Next, we can analyze the components contained in XJZ by mass spectrometry, conduct in vivo experiments, and perform metabolomics analysis on rat serum containing the drug, which can provide a more reliable basis for XJZ to treat GC. Then, the main gene targets are silenced by lentiviral transfection. Next, high-throughput screening methods can be used for proteomics, genomics, and metabolomics analysis. Finally, a dual luciferase reporter system combined with the chromatin immunoprecipitation method is used to detect the interaction between the transcription factors and the promoter regions of the target genes. These experiments will provide new ideas and insights for the mechanisms of XJZ in the treatment of GC.
XJZ exerts therapeutic effects against GC through multiple components, multiple targets, and multiple pathways. We preliminary revealed the active components and molecular mechanisms of XJZ in the treatment of GC and carried out preliminary validation using molecular docking and in vitro experiments, which provided a new idea and scientific basis for further research on the molecular mechanisms underlying the therapeutic effects of XJZ in the treatment of GC.
The authors would like to acknowledge Joanna Tibenda for revising the article. Thanks to the Key Laboratory of Hui Ethnic Medicine Modernization of Ministry of Education for providing experimental equipment and space.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39:1179-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 458] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 3. | Tan Z. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med Sci Monit. 2019;25:3537-3541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 309] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 4. | Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Li H, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Chen J, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555-e567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 973] [Article Influence: 139.0] [Reference Citation Analysis (2)] |
| 5. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13210] [Article Influence: 1467.8] [Reference Citation Analysis (3)] |
| 6. | Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 1082] [Article Influence: 270.5] [Reference Citation Analysis (0)] |
| 7. | Casak SJ, Fashoyin-Aje I, Lemery SJ, Zhang L, Jin R, Li H, Zhao L, Zhao H, Zhang H, Chen H, He K, Dougherty M, Novak R, Kennett S, Khasar S, Helms W, Keegan P, Pazdur R. FDA Approval Summary: Ramucirumab for Gastric Cancer. Clin Cancer Res. 2015;21:3372-3376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Roviello G, Ravelli A, Fiaschi AI, Cappelletti MR, Gobbi A, Senti C, Zanotti L, Polom K, Reynolds AR, Fox SB, Generali D. Apatinib for the treatment of gastric cancer. Expert Rev Gastroenterol Hepatol. 2016;10:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Xu W, Li B, Xu M, Yang T, Hao X. Traditional Chinese medicine for precancerous lesions of gastric cancer: A review. Biomed Pharmacother. 2022;146:112542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 10. | Chen J, Zhang J, Chen T, Bao S, Li J, Wei H, Hu X, Liang Y, Liu F, Yan S. Xiaojianzhong decoction attenuates gastric mucosal injury by activating the p62/Keap1/Nrf2 signaling pathway to inhibit ferroptosis. Biomed Pharmacother. 2022;155:113631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Yu W, Liang Z, Li Q, Liu Y, Liu X, Jiang L, Liu C, Zhang Y, Kang C, Yan J. The pharmacological validation of the Xiao-Jian-Zhong formula against ulcerative colitis by network pharmacology integrated with metabolomics. J Ethnopharmacol. 2022;298:115647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Liu Y, Lian X, Qin X. Bile acid metabolism involved into the therapeutic action of Xiaojianzhong Tang via gut microbiota to treat chronic atrophic gastritis in rats. Phytomedicine. 2023;109:154557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 13. | Zhang JX, Bao SC, Chen J, Chen T, Wei HL, Zhou XY, Li JT, Yan SG. Xiaojianzhong decoction prevents gastric precancerous lesions in rats by inhibiting autophagy and glycolysis in gastric mucosal cells. World J Gastrointest Oncol. 2023;15:464-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Song S, Wen F, Gu S, Gu P, Huang W, Ruan S, Chen X, Zhou J, Li Y, Liu J, Shu P. Network Pharmacology Study and Experimental Validation of Yiqi Huayu Decoction Inducing Ferroptosis in Gastric Cancer. Front Oncol. 2022;12:820059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Shi J, Lu Y, Wei P. Xiaotan Sanjie decoction inhibits angiogenesis in gastric cancer through Interleukin-8-linked regulation of the vascular endothelial growth factor pathway. J Ethnopharmacol. 2016;189:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Ding P, Guo Y, Wang C, Chen J, Guo C, Liu H, Shi Q. A Network Pharmacology Approach for Uncovering the Antitumor Effects and Potential Mechanisms of the Sijunzi Decoction for the Treatment of Gastric Cancer. Evid Based Complement Alternat Med. 2022;2022:9364313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Li L, Wang K, Liu Z, Lü Y, Wang C, Yi X, Guo J. Compound Kushen injection inhibits EMT of gastric cancer cells via the PI3K/AKT pathway. World J Surg Oncol. 2022;20:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 18. | Deng YQ, Gao M, Lu D, Liu QP, Zhang RJ, Ye J, Zhao J, Feng ZH, Li QZ, Zhang H. Compound-composed Chinese medicine of Huachansu triggers apoptosis of gastric cancer cells through increase of reactive oxygen species levels and suppression of proteasome activities. Phytomedicine. 2024;123:155169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 19. | Zhang L, Liang B, Xu H, Gong Y, Hu W, Jin Z, Wu X, Chen X, Li M, Shi L, Shi Y, Wang Y, Yang L. Cinobufagin induces FOXO1-regulated apoptosis, proliferation, migration, and invasion by inhibiting G9a in non-small-cell lung cancer A549 cells. J Ethnopharmacol. 2022;291:115095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Wang J, Cai H, Liu Q, Xia Y, Xing L, Zuo Q, Zhang Y, Chen C, Xu K, Yin P, Chen T. Cinobufacini Inhibits Colon Cancer Invasion and Metastasis via Suppressing Wnt/β-Catenin Signaling Pathway and EMT. Am J Chin Med. 2020;48:703-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 21. | Chen GQ, Nan Y, Huang SC, Ning N, Du YH, Lu DD, Yang YT, Meng FD, Yuan L. Research progress of ginger in the treatment of gastrointestinal tumors. World J Gastrointest Oncol. 2023;15:1835-1851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
| 22. | Ren X, Yan CX, Zhai RX, Xu K, Li H, Fu XJ. Comprehensive survey of target prediction web servers for Traditional Chinese Medicine. Heliyon. 2023;9:e19151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 23. | Zhao L, Zhang H, Li N, Chen J, Xu H, Wang Y, Liang Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 372] [Article Influence: 186.0] [Reference Citation Analysis (0)] |
| 24. | Zhou Z, Chen B, Chen S, Lin M, Chen Y, Jin S, Chen W, Zhang Y. Applications of Network Pharmacology in Traditional Chinese Medicine Research. Evid Based Complement Alternat Med. 2020;2020:1646905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 25. | Rose-John S. Blocking only the bad side of IL-6 in inflammation and cancer. Cytokine. 2021;148:155690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1929] [Cited by in RCA: 2093] [Article Influence: 299.0] [Reference Citation Analysis (0)] |
| 27. | Abaurrea A, Araujo AM, Caffarel MM. The Role of the IL-6 Cytokine Family in Epithelial-Mesenchymal Plasticity in Cancer Progression. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 28. | Liu M, Li H, Zhang H, Zhou H, Jiao T, Feng M, Na F, Sun M, Zhao M, Xue L, Xu L. RBMS1 promotes gastric cancer metastasis through autocrine IL-6/JAK2/STAT3 signaling. Cell Death Dis. 2022;13:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 29. | Su K, Zhao Q, Bian A, Wang C, Cai Y, Zhang Y. A novel positive feedback regulation between long noncoding RNA UICC and IL-6/STAT3 signaling promotes cervical cancer progression. Am J Cancer Res. 2018;8:1176-1189. [PubMed] |
| 30. | Heichler C, Scheibe K, Schmied A, Geppert CI, Schmid B, Wirtz S, Thoma OM, Kramer V, Waldner MJ, Büttner C, Farin HF, Pešić M, Knieling F, Merkel S, Grüneboom A, Gunzer M, Grützmann R, Rose-John S, Koralov SB, Kollias G, Vieth M, Hartmann A, Greten FR, Neurath MF, Neufert C. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut. 2020;69:1269-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 31. | Abana CO, Bingham BS, Cho JH, Graves AJ, Koyama T, Pilarski RT, Chakravarthy AB, Xia F. IL-6 variant is associated with metastasis in breast cancer patients. PLoS One. 2017;12:e0181725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Soler MF, Abaurrea A, Azcoaga P, Araujo AM, Caffarel MM. New perspectives in cancer immunotherapy: targeting IL-6 cytokine family. J Immunother Cancer. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 33. | Desai SJ, Prickril B, Rasooly A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr Cancer. 2018;70:350-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 34. | Nagaraju GP, El-Rayes BF. Cyclooxygenase-2 in gastrointestinal malignancies. Cancer. 2019;125:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Cheng J, Fan XM. Role of cyclooxygenase-2 in gastric cancer development and progression. World J Gastroenterol. 2013;19:7361-7368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 36. | Thiel A, Mrena J, Ristimäki A. Cyclooxygenase-2 and gastric cancer. Cancer Metastasis Rev. 2011;30:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (2)] |
| 37. | Luu Hoang KN, Anstee JE, Arnold JN. The Diverse Roles of Heme Oxygenase-1 in Tumor Progression. Front Immunol. 2021;12:658315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 38. | Nitti M, Piras S, Marinari UM, Moretta L, Pronzato MA, Furfaro AL. HO-1 Induction in Cancer Progression: A Matter of Cell Adaptation. Antioxidants (Basel). 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 39. | Was H, Cichon T, Smolarczyk R, Rudnicka D, Stopa M, Chevalier C, Leger JJ, Lackowska B, Grochot A, Bojkowska K, Ratajska A, Kieda C, Szala S, Dulak J, Jozkowicz A. Overexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of mice. Am J Pathol. 2006;169:2181-2198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 40. | Chiang SK, Chen SE, Chang LC. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int J Mol Sci. 2018;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 336] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 41. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11690] [Article Influence: 899.2] [Reference Citation Analysis (1)] |
| 42. | Han S, Lin F, Qi Y, Liu C, Zhou L, Xia Y, Chen K, Xing J, Liu Z, Yu W, Zhang Y, Zhou X, Rao T, Cheng F. HO-1 Contributes to Luteolin-Triggered Ferroptosis in Clear Cell Renal Cell Carcinoma via Increasing the Labile Iron Pool and Promoting Lipid Peroxidation. Oxid Med Cell Longev. 2022;2022:3846217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 43. | Xu Y, Tong Y, Lei Z, Zhu J, Wan L. Abietic acid induces ferroptosis via the activation of the HO-1 pathway in bladder cancer cells. Biomed Pharmacother. 2023;158:114154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 44. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47087] [Article Influence: 3363.4] [Reference Citation Analysis (5)] |
| 45. | Hao Q, Vadgama JV, Wang P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun Signal. 2020;18:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 46. | Tao LL, Shi SJ, Chen LB, Huang GC. Expression of monocyte chemotactic protein-1/CCL2 in gastric cancer and its relationship with tumor hypoxia. World J Gastroenterol. 2014;20:4421-4427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Xue J, Ge X, Zhao W, Xue L, Dai C, Lin F, Peng W. PIPKIγ Regulates CCL2 Expression in Colorectal Cancer by Activating AKT-STAT3 Signaling. J Immunol Res. 2019;2019:3690561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Ma L, Jiang Y, Wu N. Long non-coding RNA CCL2 promoted gastric cancer function via miR-128/ PARP2 signal pathway. Bioengineered. 2022;13:1602-1611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Almutairi S, Kalloush HM, Manoon NA, Bardaweel SK. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013-2023). Molecules. 2023;28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 50. | Vihinen P, Kähäri VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 476] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 51. | Chen SW, Zhang Q, Xu ZF, Wang HP, Shi Y, Xu F, Zhang WJ, Wang P, Li Y. HOXC6 promotes gastric cancer cell invasion by upregulating the expression of MMP9. Mol Med Rep. 2016;14:3261-3268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Pan G, Wang X, Wang Y, Li R, Li G, He Y, Liu S, Luo Y, Wang L, Lei Z. Helicobacter pylori promotes gastric cancer progression by upregulating semaphorin 5A expression via ERK/MMP9 signaling. Mol Ther Oncolytics. 2021;22:256-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 53. | Fu CK, Chang WS, Tsai CW, Wang YC, Yang MD, Hsu HS, Chao CY, Yu CC, Chen JC, Pei JS, Bau DT. The Association of MMP9 Promoter Rs3918242 Genotype With Gastric Cancer. Anticancer Res. 2021;41:3309-3315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Dong Z, Guo S, Wang Y, Zhang J, Luo H, Zheng G, Yang D, Zhang T, Yan L, Song L, Liu K, Sun Z, Meng X, Zheng Z, Zhang J, Zhao Y. USP19 Enhances MMP2/MMP9-Mediated Tumorigenesis in Gastric Cancer. Onco Targets Ther. 2020;13:8495-8510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |