Published online Sep 15, 2024. doi: 10.4251/wjgo.v16.i9.3898
Revised: August 7, 2024
Accepted: August 9, 2024
Published online: September 15, 2024
Processing time: 46 Days and 21.2 Hours
Gastric cancer, a prevalent malignancy, poses a severe threat to the health of residents in China. Timely intervention in early stages can extend patients’ survival.
To analyze clinical characteristics of patients with early gastric cancer and efficacy and risk of complications associated with endoscopic resection.
This study included 175 patients with early gastric cancer treated at our hospital, with no restrictions on sex or age. General data, pathological information, and endoscopic biopsy results were obtained. The clinical characteristics of early gas
A total of 175 patients with early gastric cancer were included, with 75.43% (n = 132) males and 24.57% (n = 43) females. 38.29% (n = 67) and 35.43% (n = 62) of patients had a history of smoking and alcohol consumption, respectively. Comorbidities included diabetes (8.57%, n = 15), coronary heart disease (10.29%, n = 18), and hypertension (43.43%, n = 76), which was highly prevalent. A history of abdominal surgery and family history of digestive system cancer accounted for 21.14% and 17.14%, respectively. The most common lesion location was the antral part of the stomach (52.00%, n = 91), followed by the gastric angle, body, and fundus. The pathological types were predominantly high-grade intraepithelial neoplasia (28.00%, n = 49) and well-differentiated adenocarcinoma (26.86%, n = 47), followed by moderately differentiated adenocarcinoma, high-moderately differentiated adenocarcinoma, and moderate-lowly differentiated adenocarcinoma. 89.14% of the patients had intestinal metaplasia and 85.14% had atrophy. After endoscopic resection, re-examination revealed that 13 patients had cancer cells at the tissue margin, with a positive margin rate of 7.43%. Postoperative complications included no cases of gastrointestinal obstruction, but incisional infection (2.86%, n = 5), gastric perforation (1.14%, n = 2), and gastric bleeding (4%, n = 7) were present, with an overall incidence of 8.00%.
Analysis of the clinical characteristics indicated that early gastric cancer is more prevalent in males with a history of hypertension, with lesions most commonly occurring in the antral region of the stomach. The pathological types are often high-grade intraepithelial neoplasia and well-differentiated adenocarcinoma, with over 85% of patients having comorbid intestinal metaplasia and atrophy. Despite endoscopic resection, a positive margin rate persisted, indicating a probability of residual cancer at the margins. Postoperative complications, such as gastrointestinal obstruction, incisional infection, gastric perforation, and gastric bleeding can occur and require timely symptomatic treatment.
Core Tip: Understanding the clinical characteristics of patients with early gastric cancer and analyzing the effect of endoscopic resection and the risk of complications can help patients with early gastric cancer prolong their survival.
- Citation: Zhao H, Shi XY, Lv LL, Lai YZ, Bao XX, Hu JW. Clinical characteristics of patients with early gastric prematurity cancer and analysis of complications by endoscopic resection. World J Gastrointest Oncol 2024; 16(9): 3898-3904
- URL: https://www.wjgnet.com/1948-5204/full/v16/i9/3898.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i9.3898
Gastric cancer is a prevalent malignancy posing a severe threat to the health of residents in China[1]. Surveillance data indicate that in 2022, there were 358700 new cases of gastric cancer and 260400 deaths, accounting for 7.43% and 10.11% of all malignant tumors, respectively, with incidence and mortality rates of 25.41 per 100000 and 18.44 per 100000 patients, respectively[2]. Although the 5-year survival rate of patients with gastric cancer in China has improved in recent years, it remains relatively low[3]. The prognosis of gastric cancer varies significantly by stage; patients with advanced disease have a 5-year survival rate of less than 30% even after surgery, whereas the rate for early-stage cancer can be maintained above 90% after surgery[4]. Early detection and treatment can enhance the detection rate of gastric cancer, improve disease prognosis, and extend patient survival. Early gastric cancer often lacks distinct symptoms, is insidious, and can easily be confused with other gastrointestinal diseases because of its low specificity. For instance, many patients mistake common symptoms such as abdominal bloating, nausea, vomiting, and weight loss as being caused by changes in dietary habits or increased life stress, or associate them with common gastrointestinal diseases such as chronic gastritis and indigestion, thus missing the optimal treatment window[5]. In light of this, we believe that for patients with early gastric cancer, analyzing the clinical characteristics and performing endoscopic resection as early as possible while also paying attention to postoperative complication risks and adopting symptomatic intervention measures can greatly improve the prognosis.
This study included 175 patients diagnosed with early gastric cancer at The Second Affiliated Hospital of Anhui Medical University, with no restrictions on sex or age. The inclusion criteria were as follows: (1) Diagnosis was established based on the “Chinese National Clinical Practice Guidelines for the Prevention, Diagnosis, and Treatment of Early Gastric Cancer,”[6] with confirmation through endoscopy and biopsy; (2) No contraindications to endoscopic resection, and all patients underwent endoscopic resection therapy; (3) Complete medical record information; (4) Postoperative follow-up was conducted for all patients; and (5) All participants were informed about the purpose of the study and signed an informed consented voluntarily. The exclusion criteria were as follows: (1) Patients with other malignant tumors; (2) Patients with mental disorders, such as long-term consciousness disturbances or mental abnormalities; (3) Patients who died during the course of the study or abandoned treatment; (4) Presence of cancer cell infiltration or metastasis; (5) Patients who had received other treatment modalities; and (6) Patients with coagulation dysfunction.
Clinical data included gender (male/female), age, smoking history (yes/no), alcohol consumption history (yes/no), presence of hypertension (yes/no), diabetes (yes/no), coronary heart disease (yes/no), history of abdominal surgery (yes/no), family history of digestive system cancer (yes/no), lesion location, pathological type, longest diameter of the lesion, presence of intestinal metaplasia (yes/no), and presence of atrophy (yes/no).
Routine preoperative examinations, including electrocardiography, complete blood count, drug sensitivity tests, and coagulation profiles, were conducted. Patients were instructed to refrain from drinking (for 8 hours) and eating (for 12 hours). Gastrointestinal tract cleansing and endotracheal intubation were performed under general anesthesia with close monitoring of vital signs. Prophylactic ceftriaxone (Shenzhen Lijian Pharmaceutical Co., LTD., National Drug Approval number: H20058023, 1.0 g) was administered to prevent infection. A transparent cap was placed on the front end of the gastroscope, and a disposable endoscopic injection needle (Olympus Trading (Shanghai) Co., LTD.) was inserted through the endoscopic biopsy orifice, multipoint submucosal injections of epinephrine saline (1:10000) around the lesion until the lesion was optimally elevated. Around the lesion, circumferential incision was made from the mucosa to the submucosa with a disposable mucosal incision knife (Jiangsu Wedekang Medical Technology Co., LTD.). The lesion was gradually dissected until the pathological tissue was completely dissociated. Suction was applied using the endoscope at an appropriate force to collect the entire lesion to the transparent cap. The lesion tissue was removed from the stomach with the gastroscope and placed in formalin solution and immediately sent for pathological examination. Routine postoperative care was administered with attention paid to the occurrence of complications.
All the data collected in this study were entered into a spreadsheet. Continuous variables are presented as mean ± SD and categorical variables are expressed as frequency (n) and percentage (%).
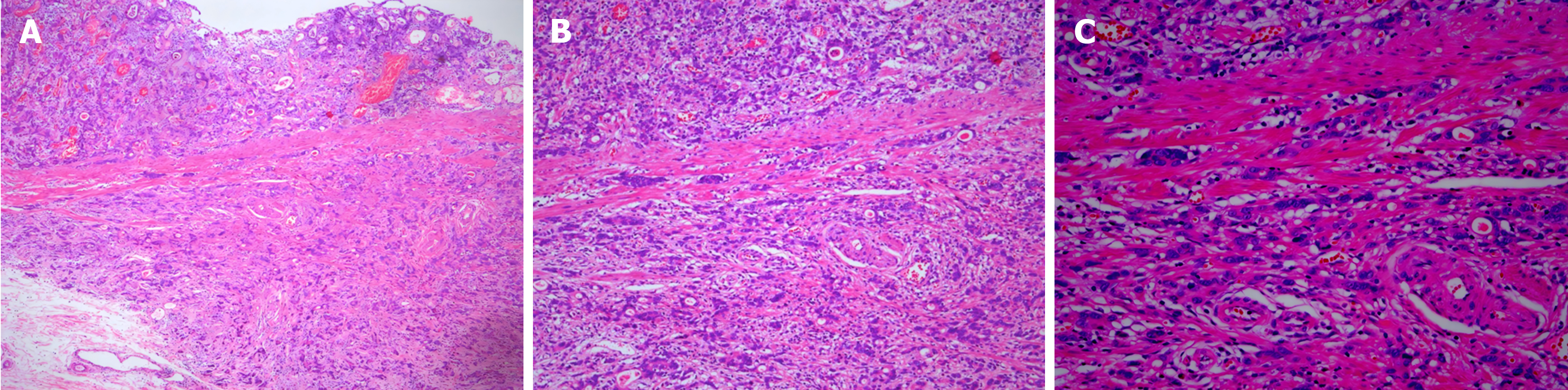
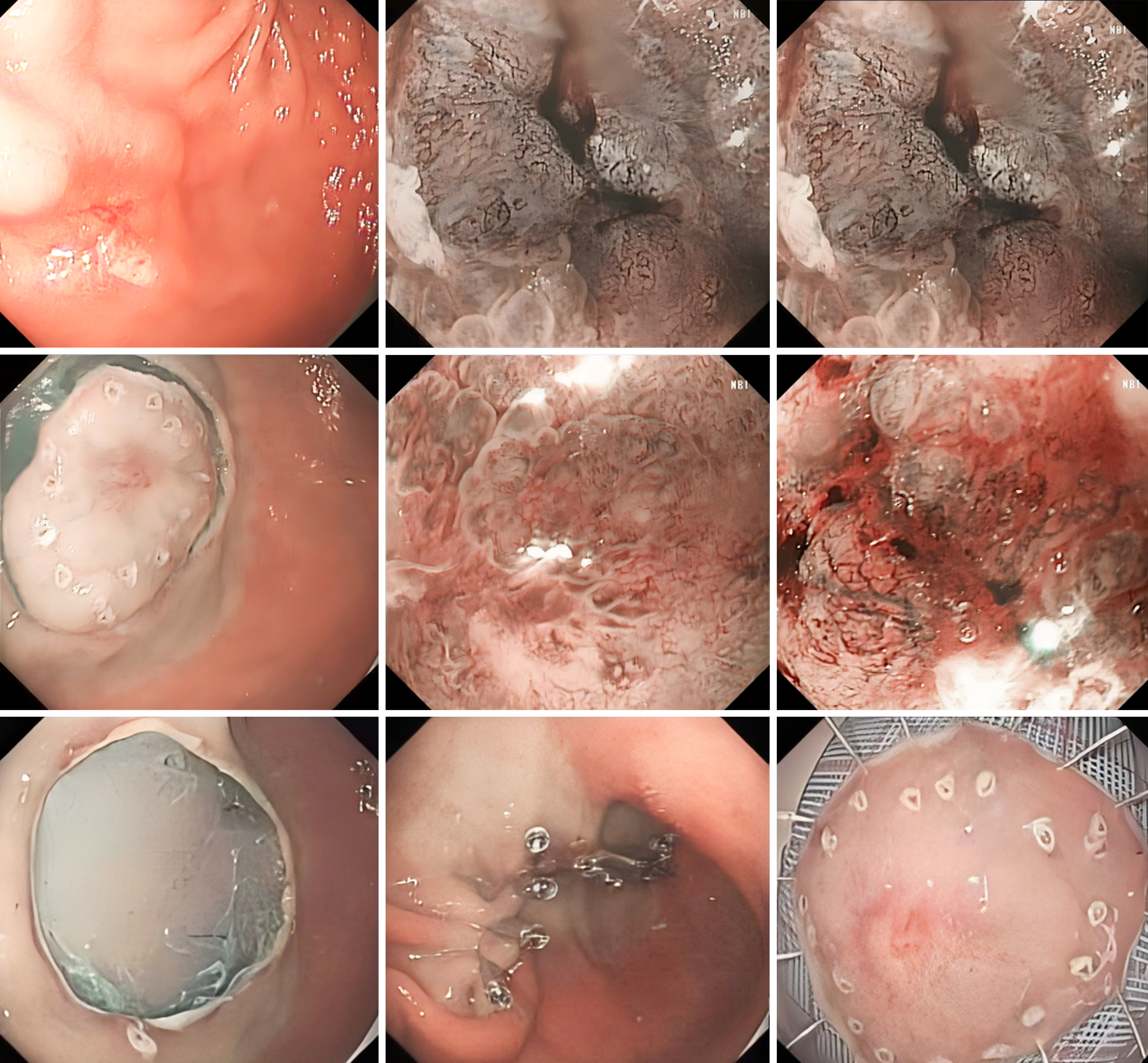
Postoperatively, the patient underwent pathological analysis of the gastrointestinal tissue (Figure 1) and electron microscopy (Figure 2).
Further details are listed in Table 1.
| Classification | Number | Percentage (%) |
| Gender | ||
| Male | 132 | 75.43 |
| Female | 43 | 24.57 |
| Age (years) | 66.54 ± 8.23 | / |
| Smoking history | ||
| Yes | 67 | 38.29 |
| No | 108 | 61.71 |
| Alcohol consumption history | ||
| Yes | 62 | 35.43 |
| No | 113 | 64.57 |
| Hypertension | ||
| Yes | 76 | 43.43 |
| No | 99 | 56.57 |
| Diabetes | ||
| Yes | 15 | 8.57 |
| No | 160 | 91.43 |
| Coronary heart disease | ||
| Yes | 18 | 10.29 |
| No | 157 | 89.71 |
| Abdominal surgery history | ||
| Yes | 37 | 21.14 |
| No | 138 | 78.86 |
| Family history of gastric cancer | ||
| Yes | 30 | 17.14 |
| No | 145 | 82.86 |
| Lesion location | ||
| Antrum of the stomach | 91 | 52.00 |
| Angle of the stomach | 31 | 17.71 |
| Body of the stomach | 18 | 10.29 |
| Fundus of the stomach | 13 | 7.43 |
| Other | 22 | 12.57 |
| Pathological type | ||
| Well-differentiated adenocarcinoma | 47 | 26.86 |
| Moderately- to well-differentiated adenocarcinoma | 16 | 9.14 |
| Moderately differentiated adenocarcinoma | 22 | 12.57 |
| Moderately to poorly differentiated adenocarcinoma | 8 | 4.57 |
| High-grade intraepithelial neoplasia | 49 | 28.00 |
| Other | 33 | 18.86 |
| Longest diameter of lesion (mm) | 2.56 ± 0.43 | / |
| Intestinal metaplasia present | ||
| Yes | 156 | 89.14 |
| No | 19 | 10.86 |
| Atrophy present | ||
| Yes | 149 | 85.14 |
| No | 26 | 14.86 |
All patients underwent endoscopic resection and were followed-up within 3 months postoperatively. It was found that a total of 13 patients had residual cancer cells at the tissue margin, presenting a positive margin rate of 7.43%.
The incidence of complications following endoscopic resection was as follows: None of the patients experienced gastrointestinal obstruction (0%), 5 had incisional infections (2.86%), 2 had gastric perforations (1.14%), and 7 had gastric bleeding (4%), resulting in an overall complication rate of 8.00%.
Gastric cancer is the fifth most common cancer worldwide, and the third leading cause of cancer-related mortality. The indolent nature of early symptoms means that gastric cancer is often diagnosed at an advanced stage, which increases the susceptibility to distant metastasis and postoperative recurrence, leading to a poor prognosis[7]. Research on the mechanisms of gastric cancer development indicates that it is a highly heterogeneous disease characterized by abnormal gene mutations and signaling pathways[8]. With rapid advancements in medical technology, gastric cancer can now be treated with surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy. However, the current 5-year survival rate for gastric cancer is 35.1%, with a median survival of less than one year for advanced stages[9]. Therefore, understanding the disease characteristics, and diagnosing and treating early gastric cancer in a timely and effective manner is crucial for improving the survival rate and quality of life of these patients.
Epidemiological surveys[10] show that East Asia is a high-incidence region for gastric cancer. There are numerous risk factors for gastric cancer, with age being a significant one. The risk of gastric cancer increases with age, with an incidence of less than 1 per 100000 people before the age of 25 years; however, a trend of increasing incidence after the age of 45 years is more common in males. In this study, the clinical characteristics of 175 patients with early gastric cancer were analyzed, revealing that 75.43% (n = 132) were male and 24.57% (n = 43) were female, with an average age of 66.54 ± 8.23 years, consistent with epidemiological findings[11]. We then analyzed the clinical and pathological features of early gastric cancer and found a predilection for the antral region of the stomach, and a majority of high-grade intraepithelial neoplasias and well-differentiated adenocarcinomas. This was corroborated by our study, in which the majority of lesions occurred in the antral region (52.00%, n = 91), with high-grade intraepithelial neoplasia (28.00%, n = 49) and well-differentiated adenocarcinoma (26.86%, n = 47) being the most common pathological types. Additionally, our data indicated that a higher proportion of patients with early gastric cancer had hypertension (43.43%, n = 76), and over 85% of patients had comorbid intestinal metaplasia and atrophy.
In recent years, clinical endoscopic technology has advanced rapidly, making endoscopic resection the preferred treatment for this condition. This allows for swift and effective treatment, enabling clinicians to inspect the lesion with endoscopic assistance, understand the location and nature of the lesion, and more effectively remove the affected tissue[12]. Endoscopic resection has evolved from polypectomy and mucosal injection techniques and is primarily based on the principle of submucosal injection of epinephrine saline to elevate the lesion, separation of the mucosal layer from the muscle layer, and its removal using a high-frequency electrical knife[13,14]. To achieve the goal of curing early gastric cancer, all patients in this study underwent endoscopic resection. Follow-up results showed that 13 patients had residual cancer cells at the tissue margins, with a positive margin rate of 7.43%. In terms of complications, there were no cases of gastrointestinal obstruction (0%), 5 cases of incisional infection (2.86%), 2 cases of gastric perforation (1.14%), and 7 cases of gastric bleeding (4%), with an overall incidence of 8.00%. This procedure is simple and requires a minimally invasive incision to insert the endoscope. However, the depth of resection is difficult to control, and can easily damage the muscle layer, cause an inflammatory response, or lead to an incisional infection, and is not conducive to wound healing or ensuring the completeness of lesion removal. Endoscopic resection does not guarantee a 100% cure rate and carries a risk of complications[15].
This study had certain limitations. Owing to funding and resource constraints, the sample size was relatively small, which may have affected the statistical power of the results. Future research should consider expanding the study to broader geographical and demographic populations to enhance the representativeness and generalizability of the findings.
The analysis of clinical characteristics indicated that early gastric cancer is more prevalent in males with a history of hypertension, with lesions predominantly occurring in the antral region. The most common pathological types are high-grade intraepithelial neoplasia and well-differentiated adenocarcinoma, with more than 85% of patients having comorbid intestinal metaplasia and atrophy. Despite endoscopic resection, a positive margin rate and a certain probability of recurrence persist. Postoperative complications, such as gastrointestinal obstruction, incisional infection, gastric perforation, and gastric bleeding can occur and require timely symptomatic treatment.
| 1. | Kono Y, Kanzaki H, Iwamuro M, Kawano S, Kawahara Y, Okada H. Reality of Gastric Cancer in Young Patients: The Importance and Difficulty of the Early Diagnosis, Prevention and Treatment. Acta Med Okayama. 2020;74:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 2. | Yang K, Lu L, Liu H, Wang X, Gao Y, Yang L, Li Y, Su M, Jin M, Khan S. A comprehensive update on early gastric cancer: defining terms, etiology, and alarming risk factors. Expert Rev Gastroenterol Hepatol. 2021;15:255-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Yuan X, Xiao Y, Luo Y, Wei C, Wang J, Huang J, Liao W, Song S, Jiang Z. Identification and validation of PGLS as a metabolic target for early screening and prognostic monitoring of gastric cancer. J Clin Lab Anal. 2022;36:e24189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Oh YJ, Yang SG, Han WH, Eom BW, Yoon HM, Kim YW, Ryu KW. Effectiveness of Intraoperative Endoscopy for Localization of Early Gastric Cancer during Laparoscopic Distal Gastrectomy. Dig Surg. 2022;39:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Kojima T, Yao K, Ohtsu K, Kuan C, Tanabe H, Imamura K, Kanemitsu T, Miyaoka M, Nagahama T, Ueki T, Iwashita A. A comparative study of demarcation line diagnostic performance between non-magnifying observation with white light and non-magnifying observation with narrow-band light for early gastric cancer. Gastric Cancer. 2022;25:761-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Li P, Li Z, Linghu E, Ji J; Society of Digestive Endoscopy of the Chinese Medical Association, Colorectal Surgery Group of the Chinese Medical Association, Chinese Association of Gastroenterologists & Hepatologists,National Clinical Research Center for Digestive Diseases, Chinese Medical Journal Clinical Practice Guideline Collaborative. Chinese national clinical practice guidelines on the prevention, diagnosis, and treatment of early gastric cancer. Chin Med J (Engl). 2024;137:887-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Zhao B, Huang R, Lu H, Mei D, Bao S, Xu H, Huang B. Risk of lymph node metastasis and prognostic outcome in early gastric cancer patients with mixed histologic type. Curr Probl Cancer. 2020;44:100579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Mazidimoradi A, Momenimovahed Z, Salehiniya H. Barriers and Facilitators Associated with Delays in the Diagnosis and Treatment of Gastric Cancer: a Systematic Review. J Gastrointest Cancer. 2022;53:782-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 9. | Delgado-Guillena P, Morales-Alvarado V, Ramírez Salazar C, Jimeno Ramiro M, Llibre Nieto G, Galvez-Olortegui J, Uchima H. Frequency and clinical characteristics of early gastric cancer in comparison to advanced gastric cancer in a health area of Spain. Gastroenterol Hepatol. 2020;43:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 10. | Hatamian S, Etesam S, Mazidimoradi A, Momenimovahed Z, Salehiniya H. The Barriers and Facilitators of Gastric Cancer Screening: a Systematic Review. J Gastrointest Cancer. 2021;52:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Sato C, Hirasawa K, Tateishi Y, Ozeki Y, Sawada A, Ikeda R, Fukuchi T, Nishio M, Kobayashi R, Makazu M, Kaneko H, Inayama Y, Maeda S. Clinicopathological features of early gastric cancers arising in Helicobacter pylori uninfected patients. World J Gastroenterol. 2020;26:2618-2631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Panarese A. Endoscopic resection for early gastric cancer: Towards a global understanding. World J Gastroenterol. 2022;28:1377-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 13. | Prinz F, Ebigbo A, Probst A, Messmann H. Gastric cancer- endoscopic treatment of early lesions, the West learns from the East. Best Pract Res Clin Gastroenterol. 2021;50-51:101739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Choe Y, Park JM, Kim JS, Cho YK, Kim BW, Choi MG. Factors influencing occurrence of metachronous gastric cancer after endoscopic resection: a systematic review and meta-analysis. Korean J Intern Med. 2023;38:831-843. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Liebenson CS. Thoracic outlet syndrome: diagnosis and conservative management. J Manipulative Physiol Ther. 1988;11:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |