Published online Sep 15, 2024. doi: 10.4251/wjgo.v16.i9.3887
Revised: July 31, 2024
Accepted: August 12, 2024
Published online: September 15, 2024
Processing time: 97 Days and 19 Hours
Immunochemotherapy involving the combination of programmed cell death 1/programmed cell death ligand 1 inhibitors with chemotherapy has advanced the treatment of locally advanced esophageal squamous cell carcinoma (ESCC). The use of corticosteroids as pretreatment might reduce immunotherapy efficacy.
To investigate the impact of baseline corticosteroid use on neoadjuvant immunochemotherapy (nIC) outcomes in locally advanced ESCC patients.
Patients with locally advanced ESCC who received nIC at Sun Yat-sen University Cancer Center and the Third Affiliated Hospital of Sun Yat-sen University were included. Patients were divided into dexamethasone and antihistamine groups on the basis of the administered pretreatment. Antiallergic efficacy and safety were evaluated, as well as its impact on short-term efficacy [complete pathological response (pCR), major pathological response (MPR)] and long-term efficacy [overall survival (OS), progression-free survival (PFS)] of nIC.
From September 2019 to September 2023, 142 patients were analyzed. No severe treatment-related adverse events or deaths were observed. Allergy occurrence was greater in the antihistamine group (P = 0.014). Short-term efficacy was not significantly different: The pCR rates were 29.9% and 40.0%, and the MPR rates were 57.9% and 65.7% in the dexamethasone and antihistamine groups, respectively. The long-term efficacy was not significantly different: The 2 years OS rates were 95.2% and 93.5%, and the 2 years PFS rates were 90.3% and 87.8%. Subgroup analysis revealed no difference in OS between the 20 mg dexamethasone group and the < 20 mg dexamethasone group, but PFS was significantly greater in the 20 mg dexamethasone group (93.9% vs 56.4%, P = 0.001).
Dexamethasone or antihistamines can be used before nIC in locally advanced ESCC without affecting short- or long-term efficacy. Administering 20 mg dexamethasone before nIC may improve PFS in ESCC.
Core Tip: This study evaluated the impact of baseline corticosteroid use on the outcomes of neoadjuvant immunochemotherapy (nIC) in patients with locally advanced esophageal squamous cell carcinoma (ESCC). These findings indicate that low-dose dexamethasone or antihistamines can be used for pretreatment without compromising short-term or long-term efficacy. Notably, administering 20 mg of dexamethasone before nIC significantly improved progression-free survival in ESCC patients, highlighting the potential benefit of optimizing the corticosteroid dose in clinical practice. These results support further investigations in larger, prospective trials.
- Citation: Huang YH, Yang GZ, Chen HG, Li XJ, Wu YH, Zhang K, Xu JN, Zhang J. Impact of baseline steroids on the efficacy of neoadjuvant immunochemotherapy in locally advanced esophageal squamous cell carcinoma. World J Gastrointest Oncol 2024; 16(9): 3887-3897
- URL: https://www.wjgnet.com/1948-5204/full/v16/i9/3887.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i9.3887
Currently, the use of programmed cell death 1 (PD-1)/programmed cell death ligand 1 inhibitors has facilitated significant progress in the treatment of advanced esophageal squamous cell carcinoma (ESCC). Studies such as ESCORT, ATTRACTION-3, KEYNOTE-181, and KEYNOTE-590[1-4] have established the role of PD-1 antibody therapy in advanced ESCC. In 2021, the ESCORT-1st trial[5] also established immunochemotherapy as the first-line treatment for advanced ESCC. Increasingly, phase II and III clinical trials have demonstrated the efficacy and safety of immunochemotherapy in locally advanced ESCC, making it a promising standard treatment for neoadjuvant therapy in this context[5,6].
In neoadjuvant therapy, paclitaxel is a commonly used anti-tubulin chemotherapeutic drug widely applied in the treatment of various solid tumors, especially for the neoadjuvant treatment of locally advanced esophageal cancer[7]. Owing to the potential for allergic reactions during paclitaxel infusion, which can be fatal, adequate pretreatment is needed before its administration[8]. The standard pretreatment regimen involves the oral or intravenous administration of corticosteroids[9], such as dexamethasone, to prevent allergic reactions. Corticosteroids are routinely used in neoadjuvant chemotherapy to prevent allergies and reduce the toxic side effects of chemotherapeutic drugs. However, recent studies[10,11] have suggested that the use of corticosteroids before treatment may reduce the effectiveness of immunotherapy.
Nevertheless, the potential impact of baseline corticosteroid use on paclitaxel-based neoadjuvant immunochemotherapy for locally advanced ESCC remains unclear. This study real-world data were utilized to assess the effects of corticosteroid use before immunochemotherapy on the treatment outcomes of patients with locally advanced ESCC.
Patients with locally advanced ESCC who received neoadjuvant immunochemotherapy at two centers, the Sun Yat-sen University Cancer Center (SYSUCC), and the Third Affiliated Hospital of Sun Yat-sen University (3rd HSYSU), from September 2019 to September 2023 were retrospectively included. The current study was approved by the ethics committees of SYSUCC and 3rd HSYSU.
Inclusion criteria: The main inclusion criteria were as follows: (1) Locally advanced thoracic ESCC (cT2-4aNany), Treatment with any oral or intravenous corticosteroids or antihistamine drugs before the initiation of neoadjuvant immunochemotherapy; (2) Completion of all courses of neoadjuvant chemotherapy combined with anti-PD-1 therapy; and (3) Radical surgical resection following neoadjuvant therapy.
Exclusion criteria: (1) Diagnosis of non-ESCC histologies; (2) Continuous use of corticosteroids or antihistamines before the initiation of neoadjuvant immunochemotherapy; or (3) Presence of another malignant tumor.
By reviewing patients' medical records, including prescription records, we determined whether patients had received oral or intravenous corticosteroids or other antiallergic medications on the day they began immunochemotherapy. We collected the clinical and pathological characteristics of all patients, including age, sex, histological type, and Eastern Cooperative Oncology Group performance status.
We primarily refer to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0[12], in combination with the World Allergy Organization grading criteria for common side effects of anticancer drugs[13]. We categorized allergic reactions into five grades:
Grade 1 - mild: Asymptomatic or mild symptoms; clinical or diagnostic observations only; no intervention needed (e.g., transient facial flushing or rash, drug fever < 38 °C).
Grade 2 - moderate: Minimal, local, or noninvasive intervention indicated; limiting age-appropriate instrumental activities of daily living (ADL) (e.g., rash, facial flushing, urticaria, dyspnea). Instrumental ADL refer to tasks such as preparing meals, shopping for groceries, using the telephone, managing finances, etc.
Grade 3 - severe or medically significant but not immediately life-threatening: Hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL (e.g., symptomatic bronchospasm with/without urticaria; parenteral medication indicated). Self-care ADL refer to tasks such as bathing, dressing, eating, using the toilet, and taking medication but not bedridden activities.
Grade 4 - life-threatening: Urgent intervention indicated (e.g., anaphylaxis-related edema/angioedema, hypotension).
Grade 5 - death.
The pathological efficacy of immunochemotherapy after surgery was evaluated by experienced pathologists. A pathological complete response (pCR)[14] was defined as no residual tumor cells in the primary lesion or lymph nodes (ypT0N0; y indicates that the staging was performed after neoadjuvant therapy; p indicates that the staging was based on pathological examination) after hematoxylin and eosin (H&E) staining of surgically resected samples. A major pathological response (MPR)[15] was defined as less than 10% residual tumor cells in the primary lesion and lymph nodes according to H&E staining.
All patients were followed until death or the end of the follow-up period (December 31, 2023). Progression-free survival (PFS) was defined as the time from the initiation of immune checkpoint blockade (ICB) to the first event (tumor progression or death from any cause). Overall survival (OS) was defined as the time from the initiation of ICB to death from any cause.
The baseline characteristics of the patients were described using totals and frequencies for categorical variables and medians for continuous variables. Student's t test was used to compare approximately normally distributed continuous variables, whereas the Mann-Whitney U test was used for nonnormally distributed continuous variables. Fisher's exact test or the χ² test was used to compare categorical variables. PFS and OS were evaluated via the Kaplan-Meier method to generate survival curves, with comparisons made via the log-rank test (univariate analysis). All the statistical tests were two-sided, and a P value of less than 0.05 was considered to indicate statistical significance. Statistical analyses and plotting were performed via Python 3.11.5, R 3.4.3, and GraphPad Prism 8 software.
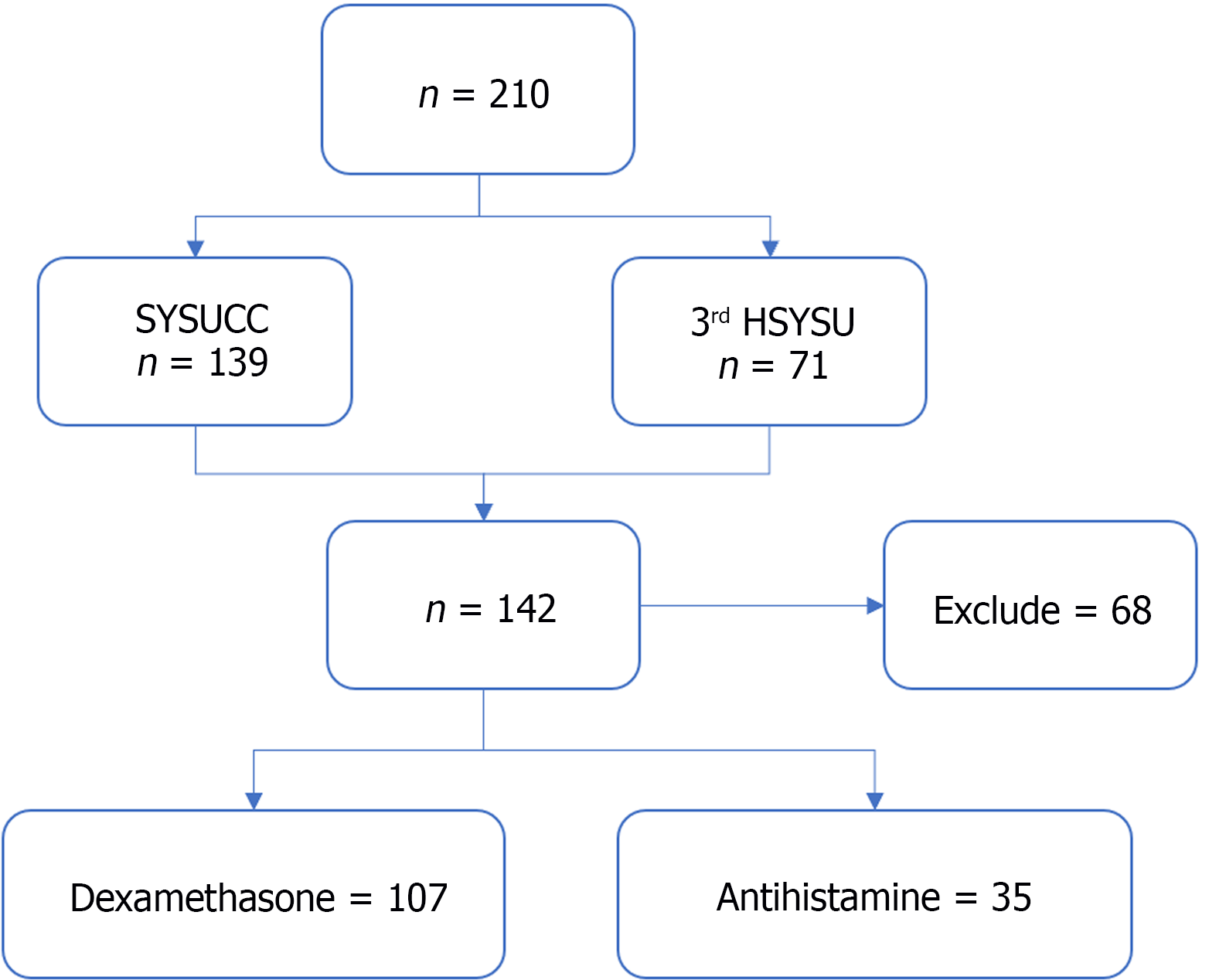
We included a total of 210 patients with locally advanced ESCC who received nIC at SYSUCC and the 3rd HSYSU. Among them, 139 patients were from SYSUCC, and 71 patients were from the 3rd HSYSU. After excluding 68 patients who were lost to follow-up or did not meet the inclusion criteria, a total of 142 patients were included in the final analysis (Figure 1).
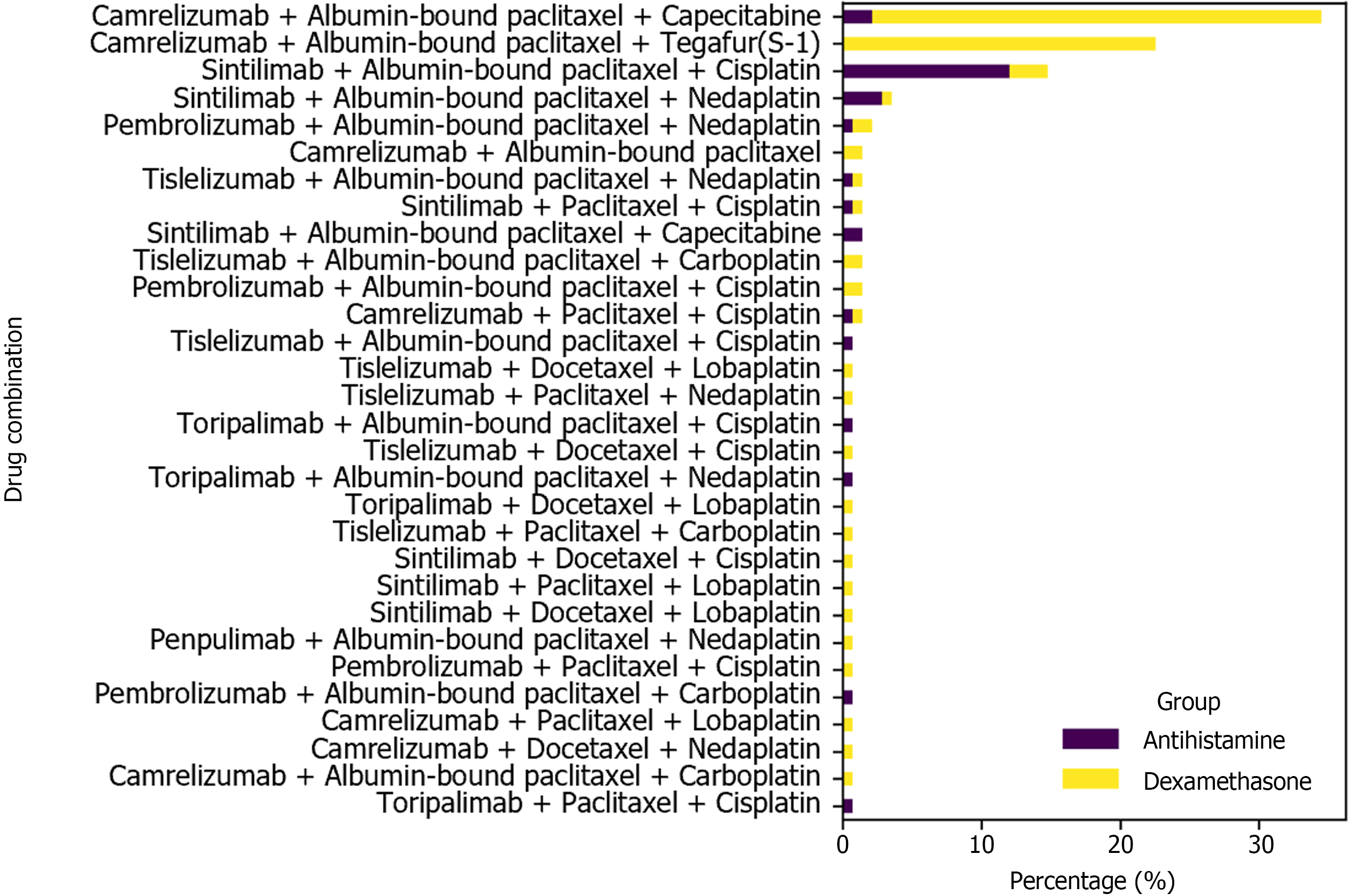
Among the 142 patients, 114 were male (80.3%), and 28 were female (19.7%), with a median age of 62 years (range: 35-80 years). The clinical stages before treatment were as follows: Stage I, 1 patient (0.7%); Stage II, 33 patients (23.2%); Stage III, 86 patients (60.6%); and Stage IV, 22 patients (15.5%) (Table 1). The most frequently used nIC regimen was camrelizumab combined with albumin-bound paclitaxel and capecitabine (34.5%), followed by camrelizumab combined with albumin-bound paclitaxel and tegafur-gimeracil-oteracil potassium (S-1) (22.5%), and sintilimab combined with albumin-bound paclitaxel and cisplatin (14.8%) (Figure 2).
| Characteristics | N = 142 |
| Age, median (IQR) (years) | 62 (35-80) |
| Sex | |
| Male | 114 (80.3) |
| Female | 28 (19.7) |
| ECOG | |
| 0 | 92 (64.8) |
| 1 | 50 (35.2) |
| ≥2 | 0 (0) |
| Premedication | |
| Dexamethasone | 107 (75.4) |
| Antihistamine | 35 (24.6) |
| cTNM stage | |
| I | 1 (0.7) |
| II | 33 (23.2) |
| III | 86 (60.6) |
| IV | 22 (15.5) |
| cT stage | |
| T1 | 0 (0) |
| T2 | 36 (25.4) |
| T3 | 96 (67.6) |
| T4 | 10 (7.0) |
| cN stage | |
| N0 | 20 (14.1) |
| N1 | 54 (38.0) |
| N2 | 51 (35.9) |
| N3 | 17 (12.0) |
At the start of immunochemotherapy, 107 patients (75.4%) received corticosteroid pretreatment with dexamethasone. Thirty-five patients (24.6%) received antihistamine pretreatment, and the distribution was as follows: Diphenhydramine in 33 patients (94.3%), chlorpromazine in 1 patient (3%), and loratadine in 1 patient (3%).
Among the 107 patients who received dexamethasone pretreatment, the median dose was 20 mg (range: 5-20 mg). Therefore, the patients were divided into two groups on the basis of a cutoff of 20 mg: The 20 mg group and the < 20 mg group. Among the patients who received dexamethasone, 82 (76.6%) received 20 mg of dexamethasone, whereas 25 patients (23.4%) received less than 20 mg of dexamethasone at the start of immunochemotherapy.
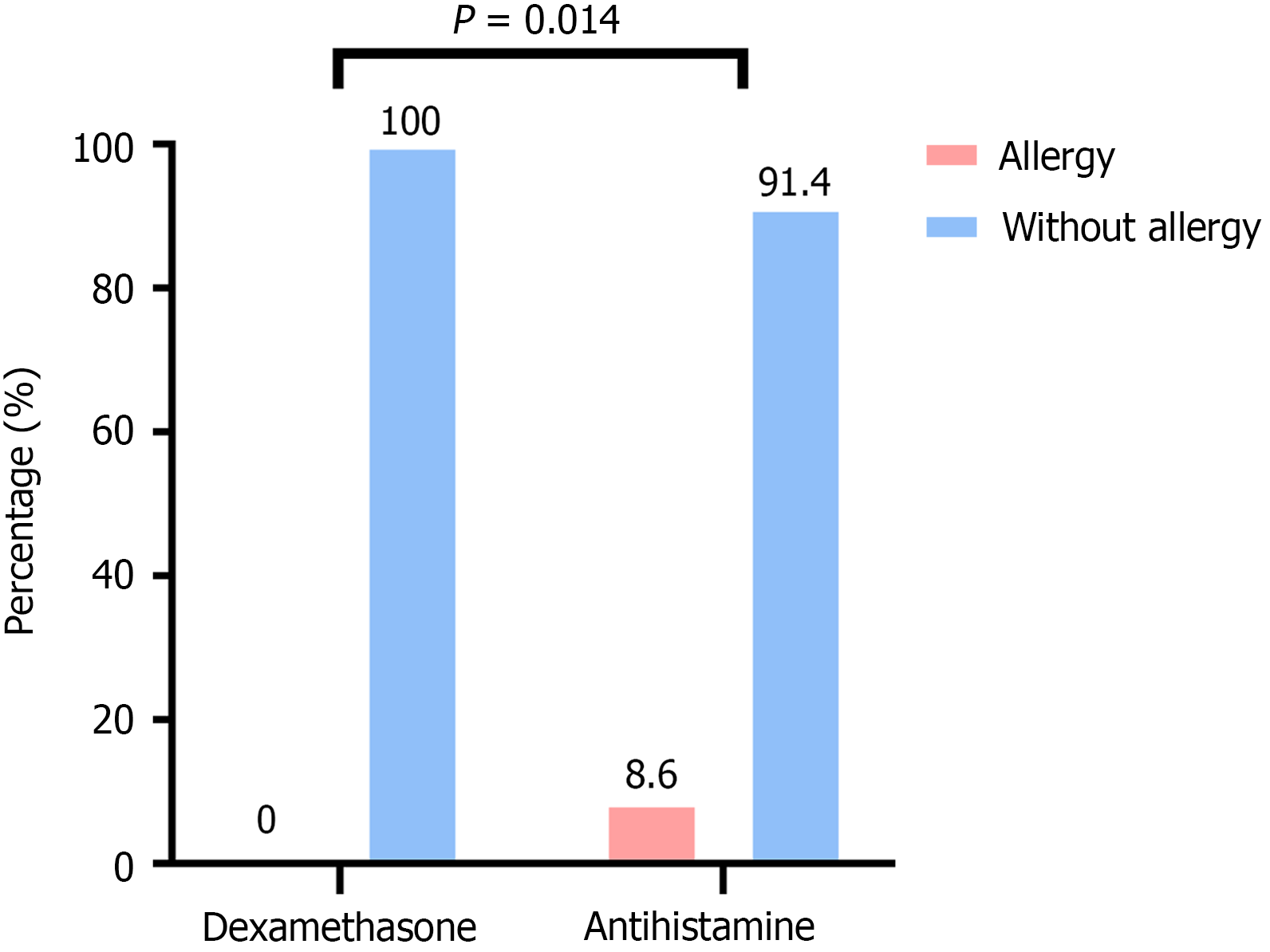
No severe treatment-related adverse events (TRAEs) or deaths were observed with the use of dexamethasone or antihistamines before nIC. In the dexamethasone group, no allergic reactions were reported, resulting in an allergy rate of 0%. In contrast, the antihistamine group had a higher incidence of allergic reactions (8.6%), all of which were Grade 1-2 allergic reactions. These reactions were alleviated after treatment with corticosteroids such as prednisone. The difference between the two groups was statistically significant (P = 0.014; Figure 3).
Postoperative pathological results revealed the following stage distribution among the 142 patients: Stage I, 46 patients (32.4%); Stage II, 19 patients (13.4%); Stage III, 40 patients (28.2%); and Stage IV, 1 patient (0.7%). The overall pCR (ypT0N0) rate was 32.4%, and the MPR rate was 59.9% (Table 2).
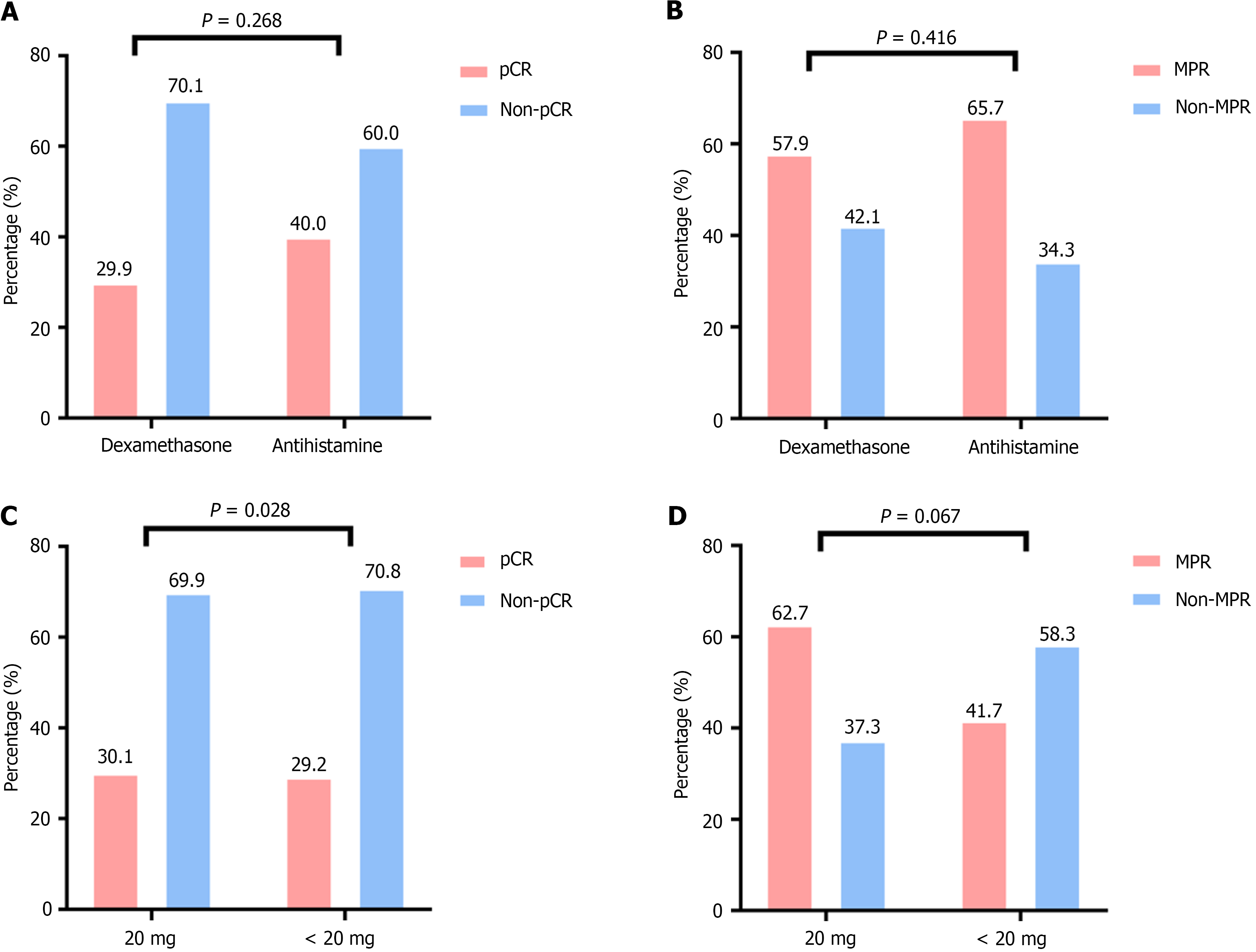
There was no statistically significant difference in pCR rates between the dexamethasone and antihistamine groups. The pCR rate in the dexamethasone group was 29.9%, whereas it was 40.0% in the antihistamine group (P = 0.268). The MPR rate was 57.9% in the dexamethasone group and 65.7% in the antihistamine group (P = 0.416); no significant difference was observed (Figure 4A and B).
For further analysis, the dexamethasone group was divided into < 20 mg and 20 mg subgroups on the basis of a 20 mg cutoff. Subgroup analysis revealed that the pCR rates for the < 20 mg and 20 mg subgroups were 30.1% and 29.2%, respectively, with no significant difference (P = 0.928). The MPR rates were 62.7% and 41.7%, respectively (P = 0.067), and no statistically significant difference was found (Figure 4C and D).
For this study cohort, the median follow-up time was 32.8 months (range: 12.1-51.3 months), and the database cutoff date was December 31, 2023. Among the patients who underwent surgery after neoadjuvant therapy, 21 patients experienced local recurrence or distant metastasis, and 12 patients died. The 2-year PFS and OS rates were 90.6% and 93.7%, respectively.
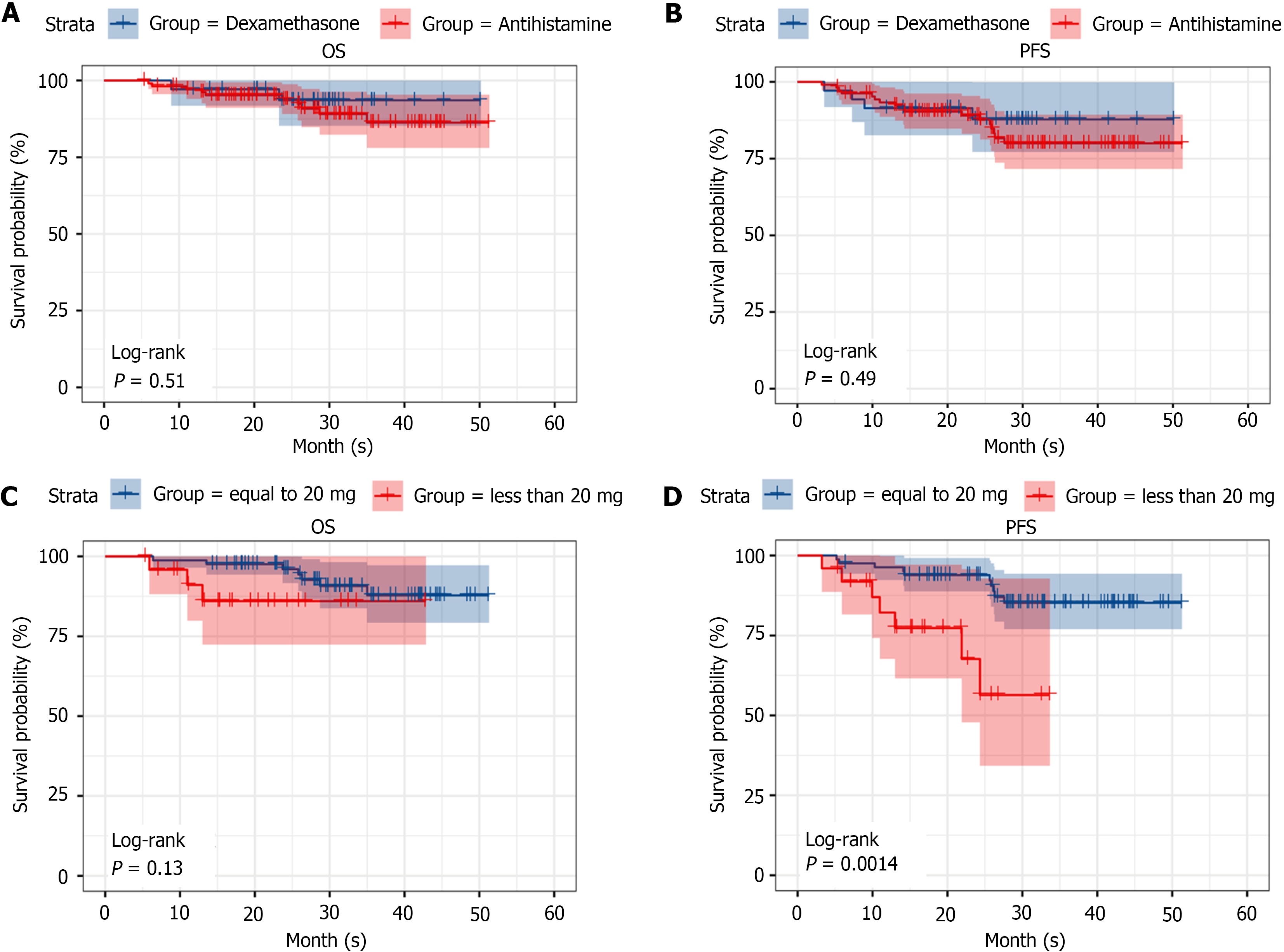
In the dexamethasone and antihistamine groups, the OS rates were 95.2% and 93.5%, respectively, and the PFS rates were 90.3% and 87.8%, respectively; no statistically significant differences were observed between the two groups in terms of PFS and OS (P = 0.51 and P = 0.49, respectively; Figure 5A and B).
For the dexamethasone group, a subgroup analysis was performed on the basis of a 20 mg cutoff (< 20 and 20 mg). The PFS rates were 93.9% for the 20 mg group and 56.4% for the < 20 mg group, whereas the OS rates were 96.4% for the 20 mg group and 86.0% for the < 20 mg group. Although no statistically significant difference was observed in OS between the two subgroups, the PFS in the 20 mg group was significantly greater than that in the < 20 mg group (P = 0.13 and P = 0.0014, respectively; Figure 5C and D).
Paclitaxel-based chemotherapy regimens are among the standard chemotherapy options for locally advanced ESCC. Previous studies reported that allergic reactions to paclitaxel formulations are mostly Type I hypersensitivity reactions, with an incidence rate of approximately 30%[16,17]. Mild reactions often include skin flushing and urticaria, whereas severe allergic reactions can lead to dyspnea, hypotension, shock, and even death. Therefore, pretreatment before the administration of paclitaxel is essential. Corticosteroids and antihistamines are routinely used as pretreatments before paclitaxel-based chemotherapy[18]. Neoadjuvant treatment for ESCC is gradually transitioning towards immunochemotherapy. Some studies[19,20] suggest that the use of corticosteroids may inhibit immune cells and induce resistance in malignant solid tumors, potentially affecting the efficacy of immunotherapy. Therefore, it is crucial to further explore whether corticosteroid pretreatment is necessary before paclitaxel-based immunochemotherapy.
This study utilized real-world data from two centers for a retrospective analysis and revealed that pretreatment with corticosteroids and noncorticosteroid drugs before chemotherapy is safe and feasible. In the dexamethasone and antihistamine groups, we detected no severe TRAEs or death events. Interestingly, 8.6% (3 patients) of the patients premedicated with noncorticosteroid drugs experienced allergic reactions, which were alleviated by corticosteroid treatment. In contrast, no allergic reactions were found in the corticosteroid pretreatment group, and the difference between the two groups was statistically significant. A previous study[21] suggested that noncorticosteroid drugs, such as diphenhydramine, work primarily by blocking H-1 receptors and inhibiting mast cell degranulation, thereby preventing allergic reactions. On the other hand, dexamethasone inhibits the aggregation of inflammatory cells, including macrophages and lymphocytes, at the site of inflammation, suppresses phagocyte function, stabilizes lysosomal membranes, prevents complement involvement in inflammatory responses, and inhibits the synthesis and release of inflammatory chemical mediators (such as prostaglandins, thromboxanes, and leukotrienes)[22]. This comprehensive anti-inflammatory and anti-allergic effect likely increases the effectiveness of corticosteroids in preventing paclitaxel-induced allergic reactions. This could be one of the reasons why the corticosteroid group had a lower incidence of allergic reactions than did the noncorticosteroid group.
Premedication with dexamethasone and antihistamines before paclitaxel-based chemotherapy does not affect the short-term or long-term efficacy of nIC in locally advanced ESCC patients. In this study, no significant difference was observed between the two groups. Similarly, several phase III studies in lung cancer, KEYNOTE-189 and KEYNOTE-407[23,24], have also demonstrated that the standard pretreatment dose of steroids before PD-1 antibody treatment does not affect the survival advantage of patients in the PD-1 antibody combination group. Interestingly, a study on melanoma[25] also suggested that the use of low-dose corticosteroids does not affect the efficacy of immunotherapy, whereas high-dose corticosteroids may impact the efficacy of immunotherapy. Additionally, some studies[26] have shown that histamine and histamine receptor H1 are often increased in the tumor microenvironment and induce T-cell dysfunction; thus, blocking histamine receptors can increase the effectiveness of immunotherapy in patients. Therefore, it is feasible to use both corticosteroids and antihistamines as pretreatments before neoadjuvant immunochemotherapy.
Studies have suggested that the use of corticosteroids may inhibit immune cells[19], thereby contributing to tumor progression. However, in the subgroup analysis of this study, although there was no difference in short-term efficacy between the < 20 mg dexamethasone subgroup and the 20 mg dexamethasone subgroup, interestingly, the long-term efficacy evaluation revealed that the PFS of the 20 mg subgroup was significantly greater than that of the < 20 mg subgroup, which is contrary to the conclusions of previous studies. Several research[27,28] has indicated that corticosteroids, such as glucocorticoids, can alleviate peritumoral edema. The possible mechanism is that glucocorticoids inhibit phospholipase A2 activity, leading to a reduction in lipoxygenase products, thereby decreasing the capillary permeability of tumor cells and further reducing stromal edema around the tumor. Therefore, the use of 20 mg dexamethasone before neoadjuvant chemotherapy may help alleviate peritumoral edema, facilitating better penetration of the drug into the tumor and enhancing its cytotoxic effects, thereby improving PFS. However, this mechanism requires further investigation and exploration.
This study aimed to preliminarily explore the impact of corticosteroids or antihistamines administered before neoadjuvant immunochemotherapy on short-term and long-term efficacy in patients with locally advanced ESCC through a retrospective analysis of data from two centers. Owing to the relatively small sample size, further studies with larger sample sizes are needed to validate these findings.
Dexamethasone or antihistamines can be administered as pretreatment before nIC in locally advanced ESCC without compromising short-term or long-term efficacy. Administration of 20 mg of dexamethasone before neoadjuvant treatment may help improve PFS in locally advanced ESCC patients.
The authors would like to acknowledge all patients participating in the study.
| 1. | Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, Chen J, Zhang H, Niu Z, Fan Q, Lin L, Gu K, Liu Y, Ba Y, Miao Z, Jiang X, Zeng M, Chen J, Fu Z, Gan L, Wang J, Zhan X, Liu T, Li Z, Shen L, Shu Y, Zhang T, Yang Q, Zou J; ESCORT Study Group. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21:832-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 414] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 2. | Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 791] [Article Influence: 131.8] [Reference Citation Analysis (0)] |
| 3. | Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, Bennouna J, Kato K, Shen L, Enzinger P, Qin SK, Ferreira P, Chen J, Girotto G, de la Fouchardiere C, Senellart H, Al-Rajabi R, Lordick F, Wang R, Suryawanshi S, Bhagia P, Kang SP, Metges JP; KEYNOTE-181 Investigators. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol. 2020;38:4138-4148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 685] [Article Influence: 137.0] [Reference Citation Analysis (0)] |
| 4. | Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K; KEYNOTE-590 Investigators. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 888] [Article Influence: 222.0] [Reference Citation Analysis (0)] |
| 5. | Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, Zhang Y, Zhao K, Chen Z, Gao S, Li J, Fu Z, Gu K, Liu Z, Wu L, Zhang X, Feng J, Niu Z, Ba Y, Zhang H, Liu Y, Zhang L, Min X, Huang J, Cheng Y, Wang D, Shen Y, Yang Q, Zou J, Xu RH; ESCORT-1st Investigators. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA. 2021;326:916-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 457] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 6. | Yang G, Su X, Huang Y, Luo G, Wang Z, Cai P, Zheng Y, Bei T, Huang M, Bai Y, He H, Xiang J, Cai M, Zhong J, Guo Q, Zhang X. Intensive cycles of neoadjuvant camrelizumab combined with chemotherapy in locally advanced esophageal squamous cell carcinoma: a single-arm, phase II trial. J Transl Med. 2023;21:411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 7. | Shen L, Kato K, Kim SB, Ajani JA, Zhao K, He Z, Yu X, Shu Y, Luo Q, Wang J, Chen Z, Niu Z, Zhang L, Yi T, Sun JM, Chen J, Yu G, Lin CY, Hara H, Bi Q, Satoh T, Pazo-Cid R, Arkenau HT, Borg C, Lordick F, Li L, Ding N, Tao A, Shi J, Van Cutsem E; RATIONALE-302 Investigators. Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study. J Clin Oncol. 2022;40:3065-3076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 174] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 8. | Joerger M. Prevention and handling of acute allergic and infusion reactions in oncology. Ann Oncol. 2012;23 Suppl 10:x313-x319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Lansinger OM, Biedermann S, He Z, Colevas AD. Do Steroids Matter? A Retrospective Review of Premedication for Taxane Chemotherapy and Hypersensitivity Reactions. J Clin Oncol. 2021;39:3583-3590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, Martínez-Bernal G, Ferrara R, Lai WV, Hendriks LEL, Sabari JK, Caramella C, Plodkowski AJ, Halpenny D, Chaft JE, Planchard D, Riely GJ, Besse B, Hellmann MD. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36:2872-2878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 746] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 11. | Libert C, Dejager L. How steroids steer T cells. Cell Rep. 2014;7:938-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Arrieta O, Barrón F, Ramírez-Tirado LA, Zatarain-Barrón ZL, Cardona AF, Díaz-García D, Yamamoto Ramos M, Mota-Vega B, Carmona A, Peralta Álvarez MP, Bautista Y, Aldaco F, Gerson R, Rolfo C, Rosell R. Efficacy and Safety of Pembrolizumab Plus Docetaxel vs Docetaxel Alone in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer: The PROLUNG Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 13. | Cox LS, Sanchez-Borges M, Lockey RF. World Allergy Organization Systemic Allergic Reaction Grading System: Is a Modification Needed? J Allergy Clin Immunol Pract. 2017;5:58-62.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Donohoe CL, O'Farrell NJ, Grant T, King S, Clarke L, Muldoon C, Reynolds JV. Classification of pathologic response to neoadjuvant therapy in esophageal and junctional cancer: assessment of existing measures and proposal of a novel 3-point standard. Ann Surg. 2013;258:784-92; discussion 792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Wang H, Jiang Z, Wang Q, Wu T, Guo F, Xu Z, Yang W, Yang S, Feng S, Wang X, Chen S, Cheng C, Chen W. Pathological response and prognostic factors of neoadjuvant PD-1 blockade combined with chemotherapy in resectable oesophageal squamous cell carcinoma. Eur J Cancer. 2023;186:196-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Micha JP, Rettenmaier MA, Dillman R, Fraser P, Birk C, Brown JV. Single-dose dexamethasone paclitaxel premedication. Gynecol Oncol. 1998;69:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Parikh B, Khanolkar S, Advani SH, Dhabhar B, Chandra M. Safety profile of single-dose dexamethasone premedication for paclitaxel. J Clin Oncol. 1996;14:2189-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | ALMuhizi F, De Las Vecillas Sanchez L, Gilbert L, Copaescu AM, Isabwe GAC. Premedication Protocols to Prevent Hypersensitivity Reactions to Chemotherapy: a Literature Review. Clin Rev Allergy Immunol. 2022;62:534-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Oppong E, Cato AC. Effects of Glucocorticoids in the Immune System. Adv Exp Med Biol. 2015;872:217-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Matasić R, Dietz AB, Vuk-Pavlović S. Dexamethasone inhibits dendritic cell maturation by redirecting differentiation of a subset of cells. J Leukoc Biol. 1999;66:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Wolfson AR, Wong D, Abrams EM, Waserman S, Sussman GL. Diphenhydramine: Time to Move on? J Allergy Clin Immunol Pract. 2022;10:3124-3130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, Brown JP, Cohen A, Kim H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 777] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 23. | Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, Hochmair MJ, Powell SF, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Kurata T, Gray JE, Schwarzenberger P, Jensen E, Pietanza MC, Rodríguez-Abreu D. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol. 2023;41:1992-1998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 311] [Article Influence: 155.5] [Reference Citation Analysis (0)] |
| 24. | Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, Hermes B, Cicin I, Medgyasszay B, Rodríguez-Cid J, Okamoto I, Lee S, Ramlau R, Vladimirov V, Cheng Y, Deng X, Zhang Y, Bas T, Piperdi B, Halmos B. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J Thorac Oncol. 2020;15:1657-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 415] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 25. | Bai X, Hu J, Betof Warner A, Quach HT, Cann CG, Zhang MZ, Si L, Tang B, Cui C, Yang X, Wei X, Pallan L, Harvey C, Manos MP, Ouyang O, Kim MS, Kasumova G, Cohen JV, Lawrence DP, Freedman C, Fadden RM, Rubin KM, Sharova T, Frederick DT, Flaherty KT, Rahma OE, Long GV, Menzies AM, Guo J, Shoushtari AN, Johnson DB, Sullivan RJ, Boland GM. Early Use of High-Dose Glucocorticoid for the Management of irAE Is Associated with Poorer Survival in Patients with Advanced Melanoma Treated with Anti-PD-1 Monotherapy. Clin Cancer Res. 2021;27:5993-6000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 26. | Li H, Xiao Y, Li Q, Yao J, Yuan X, Zhang Y, Yin X, Saito Y, Fan H, Li P, Kuo WL, Halpin A, Gibbons DL, Yagita H, Zhao Z, Pang D, Ren G, Yee C, Lee JJ, Yu D. The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer Cell. 2022;40:36-52.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 158] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 27. | Ohnishi T, Sher PB, Posner JB, Shapiro WR. Capillary permeability factor secreted by malignant brain tumor. Role in peritumoral brain edema and possible mechanism for anti-edema effect of glucocorticoids. J Neurosurg. 1990;72:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Andersen C, Astrup J, Gyldensted C. Quantitative MR analysis of glucocorticoid effects on peritumoral edema associated with intracranial meningiomas and metastases. J Comput Assist Tomogr. 1994;18:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |