Published online Sep 15, 2024. doi: 10.4251/wjgo.v16.i9.3781
Revised: June 30, 2024
Accepted: July 24, 2024
Published online: September 15, 2024
Processing time: 137 Days and 16.7 Hours
Erythropoietin-induced hepatocyte receptor A2 (EphA2) is a receptor tyrosine kinase that plays a key role in the development and progression of a variety of tumors. This article reviews the expression of EphA2 in gastrointestinal (GI) colorectal cancer (CRC) and its regulation of pyroptosis. Pyroptosis is a form of programmed cell death that plays an important role in tumor suppression. Studies have shown that EphA2 regulates pyrodeath through various signaling pathways, affecting the occurrence, development and metastasis of GI CRC. The overexpression of EphA2 is closely related to the aggressiveness and metastasis of GI CRC, and the inhibition of EphA2 can induce pyrodeath and improve the sensitivity of cancer cells to treatment. In addition, EphA2 regulates intercellular communication and the microenvironment through interactions with other cytokines and receptors, further influencing cancer progression. The role of EphA2 in GI CRC and its underlying mechanisms provide us with new per
Core Tip: This study investigated the expression of erythropoietin-induced hepatocyte receptor A2 (EphA2) in gastrointestinal (GI) colorectal cancer (CRC) and the mechanism by which EphA2 regulates pyroptosis. By reviewing the relevant literature, we found that EphA2 regulates pyroptosis through a variety of signaling pathways (such as the phosphatidylinositol 3 kinase/protein kinase B and Ras/mitogen-activated protein kinase pathways), thereby affecting the survival, proliferation and metastasis of cancer cells. The abnormal expression of EphA2 is closely related to the malignant behavior of GI CRC, and EphA2 further regulates the tumor microenvironment and immune response through interactions with inflammatory factors (such as tumor necrosis factor-α and interferon-γ). Studies have shown that targeting EphA2 can induce pyrodeath in cells, improve the sensitivity of cancer cells to treatment, and subsequently inhibit the occurrence and metastasis of tumors. Therefore, an in-depth understanding of the molecular mechanism by which EphA2 regulates pyrodeath provides new ideas and potential targets for the treatment of GI CRC.
- Citation: Zhang YK, Shi R, Meng RY, Lin SL, Zheng M. Erythropoietin-induced hepatocyte receptor A2 regulates effect of pyroptosis on gastrointestinal colorectal cancer occurrence and metastasis resistance. World J Gastrointest Oncol 2024; 16(9): 3781-3797
- URL: https://www.wjgnet.com/1948-5204/full/v16/i9/3781.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i9.3781
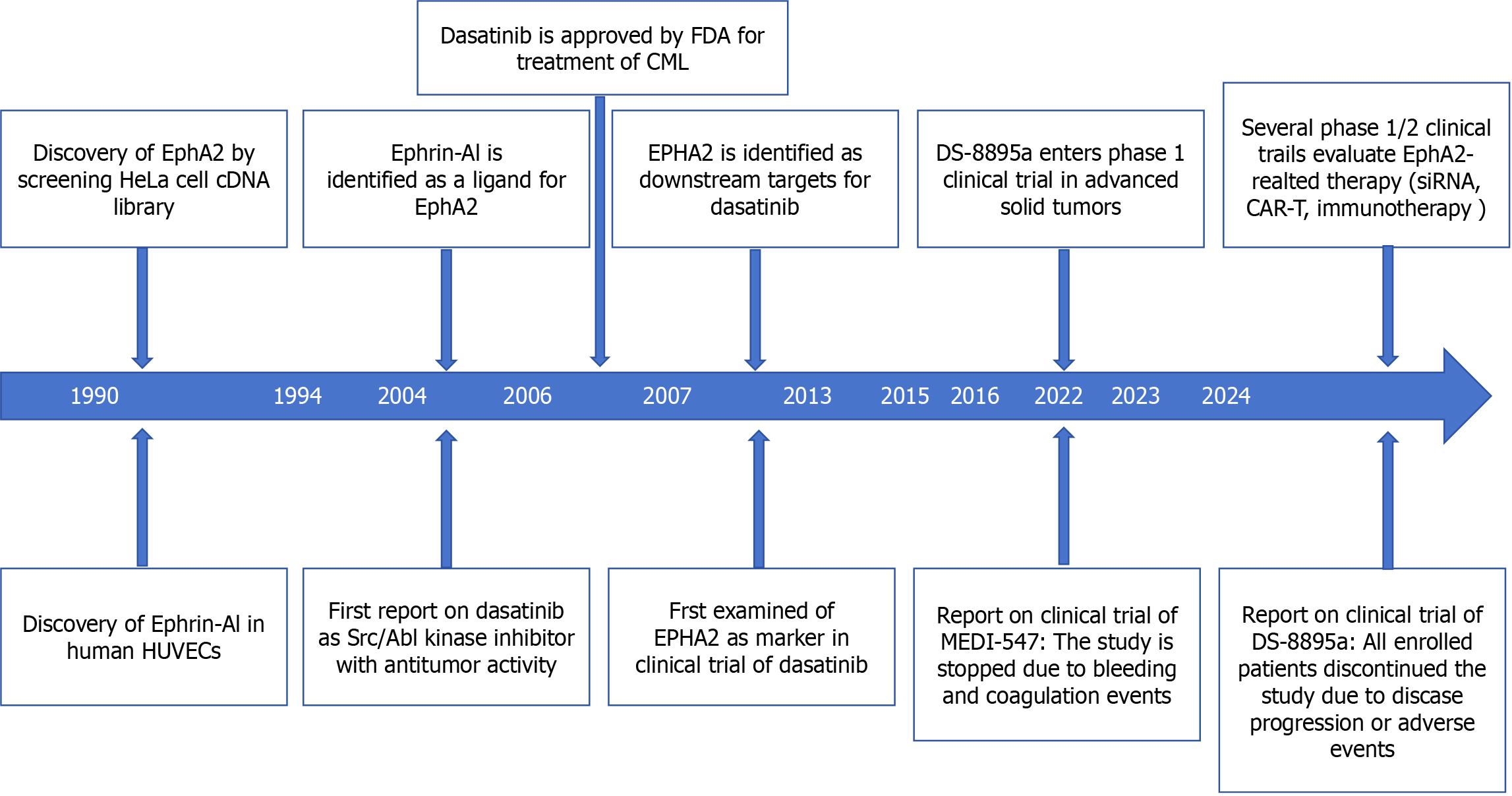
Erythropoietin-induced hepatocyte receptor (Eph) is the largest receptor tyrosine kinase (RTK) family in the spinal cord animal genome[1-4]. According to their extracellular structure, Eph ligands can be divided into two categories: A (EPHA1-A8 and EphA10) and B (EphB1-B4 and EphB6), and EPH ligands can also be divided into two categories: Ephrin A1-5 and Ephrin B1-3. EphA2 has received increasing attention due to its role in regulating the progression and prognosis of malignant tumors[5]. The EphA2 gene is located in band 6 of the short arm 3 region of human chromosome 1 and has been shown to be a region that is often altered in cancer. EphA2 was discovered in 1990 during the screening of HeLa cell cDNA libraries and was originally called epithelial cell kinase because EphA2 is expressed in most epithelial cells[6-8]. The EphA2 receptor is a type I transmembrane protein composed of 976 amino acid polypeptides, and its structure is usually conserved[9]. It includes an N-terminal extracellular region, a transmembrane region and a C-terminal intracellular region[10-12]. The extracellular region consists of a ligand-binding domain at the N-terminus, a Sushi domain, a cysteine-rich domain, and two fibronectin III repeats (FN III)[13-15]. The intracellular portion consists of a near-membrane tyrosine kinase domain, followed by a tyrosine kinase domain, an S-adenosylmethionine (SAM) domain, and a PDZ-binding motif at the C-terminus, and the following is a timeline summary of EphA2-related research and findings (Figure 1).
Gastrointestinal (GI) colorectal cancer (CRC) is one of the most common and lethal malignancies in the world. The molecular mechanisms of its occurrence and metastasis are complex and diverse. EphA2, as a RTK, has been found to be closely related to the progression of CRC in recent years. Specifically, EphA2 regulates cell pyrodeath by activating signaling pathways such as phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt) and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), thereby affecting the proliferation, migration and invasion of tumor cells. In addition, experimental studies have shown that high expression of EphA2 is associated with poor prognosis of CRC, and inhibition of EphA2 can significantly reduce tumor growth and metastasis. These studies reveal the key role of EphA2 in CRC and provide an important theoretical basis for developing new treatment strategies.
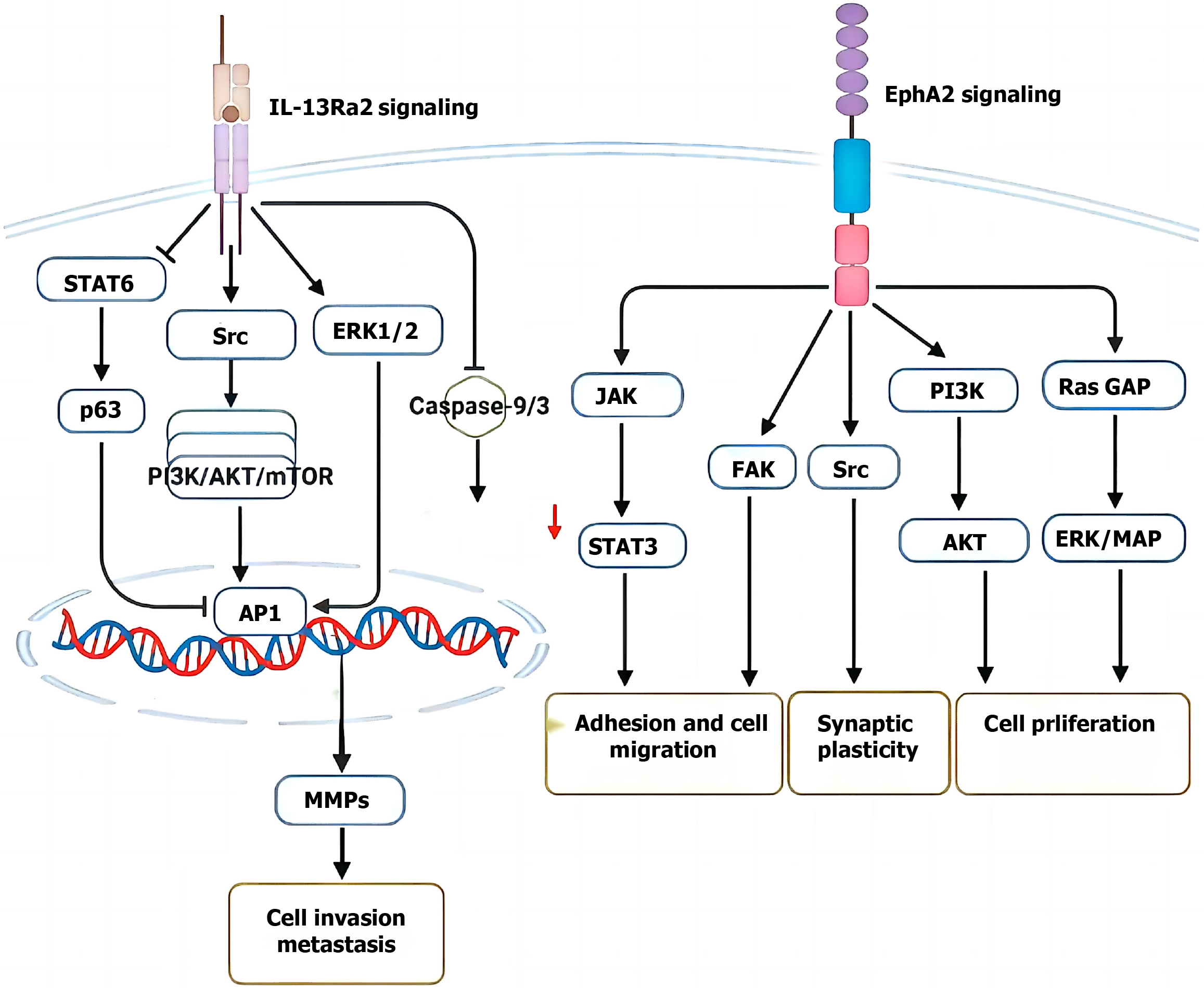
EphA2 can interact with any of the eight ligands of Eprin class A but has a particular preference for Ephrin A1[16-18]. Unlike classical RTKs, which usually only mediate one-way transmission, cells expressing EphA2 receptors can transmit forward signals from Ephrin A1 to Ephrin A1 during intercellular contact, while cells expressing Ephrin A1 receive reverse signals from EphA2 to Ephrin A1[19]. This bidirectional signaling pathway, also known as the ephrin A1-EphA2 pathway, can cause signal transduction on the cell surface, regulating cell activity and cell-cell interactions[20-24]. This signaling pathway plays an important role in regulating the growth and metastasis of malignant tumors and the proliferation and invasion of cancer cells[25]. Many downstream signaling pathways are related to Ephrin-Eph complexes[26-30]. For example, EphA2 can activate the PI3K/Akt signaling pathway and subsequently inhibit cell apoptosis[31]. EphA2 can also activate the MAPK signaling pathway, which is closely related to cell proliferation, metastasis, invasion and other processes[32-36]. The interaction of EphA2 with epidermal growth factor receptor (EGFR) can activate signaling pathways such as the EGFR/Akt and EGFR/MAPK pathways, thus promoting cell proliferation and migration[37-40]. In conclusion, the signal transduction pathway of EphA2 involves multiple molecules and pathways, and this complex regulatory network plays an important role in the growth, metastasis, invasion and other processes of CRC[41-45].
Eph receptors are the largest RTK family in vertebrates and have a single transmembrane structure[46]. Fourteen Eph receptors and 8 Eph ligands have been identified. In recent years, as research has progressed, Eph receptors such as EphA2 have been found to be closely related to the occurrence, development and drug resistance of tumors[47-50]. EphA2 is a 130 kDa transmembrane glycoprotein composed of 976 amino acid residues[51]. Due to its multiple phosphorylation sites, EPHA2 exerts different functions, so its biological effects are very complex, and EPHA2 plays a bidirectional role in the occurrence and development of tumors[52-55]. Structurally, the extracellular segment of EphA2 has ligand-binding domains, EGF-like domains and FN-III repeats[56]. The intracellular near-membrane regions Y588 and Y594 are phosphorylated when ligands are bound to extracellular regions, activating classical ligand-dependent pathways[57]. The tyrosine kinase domain contains two phosphorylation sites, Y735 and Y772, which can interact with the p85 regulatory subunit of PI3K. The intracellular terminal SAM structure contains the phosphorylable site S897[58-60]. It is regulated by intracellular kinases and performs nonclassical functions that are not dependent on ligands (Table 1).
| Cancer type | Eph receptor | Aberrant function | Role of Eph receptor |
| Colorectal cancer | EphA1 | Overexpressed in early stages; low expression in later stages | Tumour suppressive |
| EphA2 | Overexpressed in early stages; low expression in later stages | Tumour suppressive | |
| EphA3 | Low expression | Tumour suppressive | |
| EphA4 | Overexpressed | Tumour promoting | |
| EphA7 | Low expression | Tumour suppressive | |
| EphB2 | Overexpressed in early stages; low expression in later stages | Tumour suppressive | |
| EphB3 | Overexpressed in early stages; low expression in later stages | Tumour suppressive | |
| EphB4 | Overexpressed | Tumour promoting | |
| EphB6 | Low expression | Tumour suppressive |
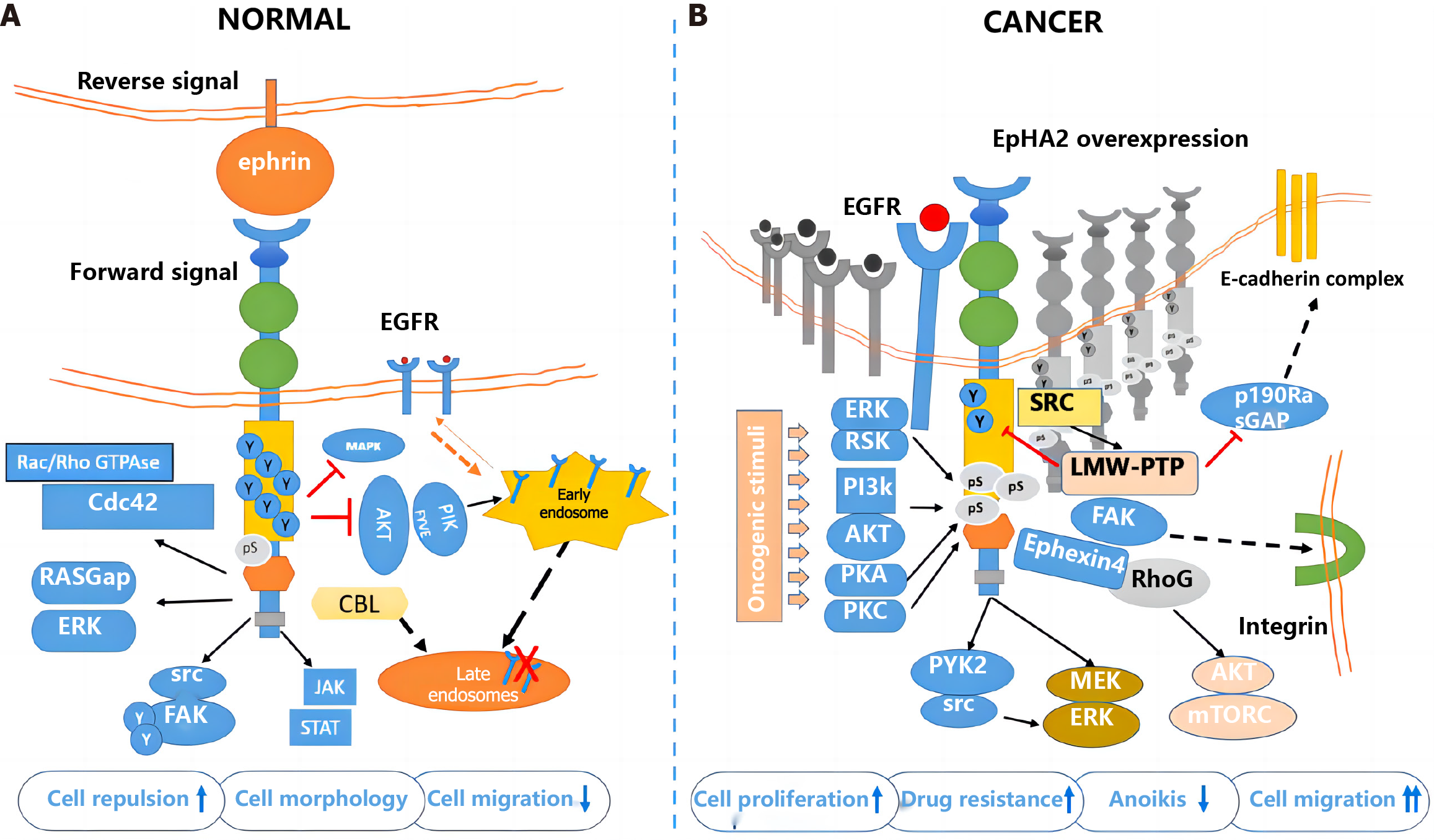
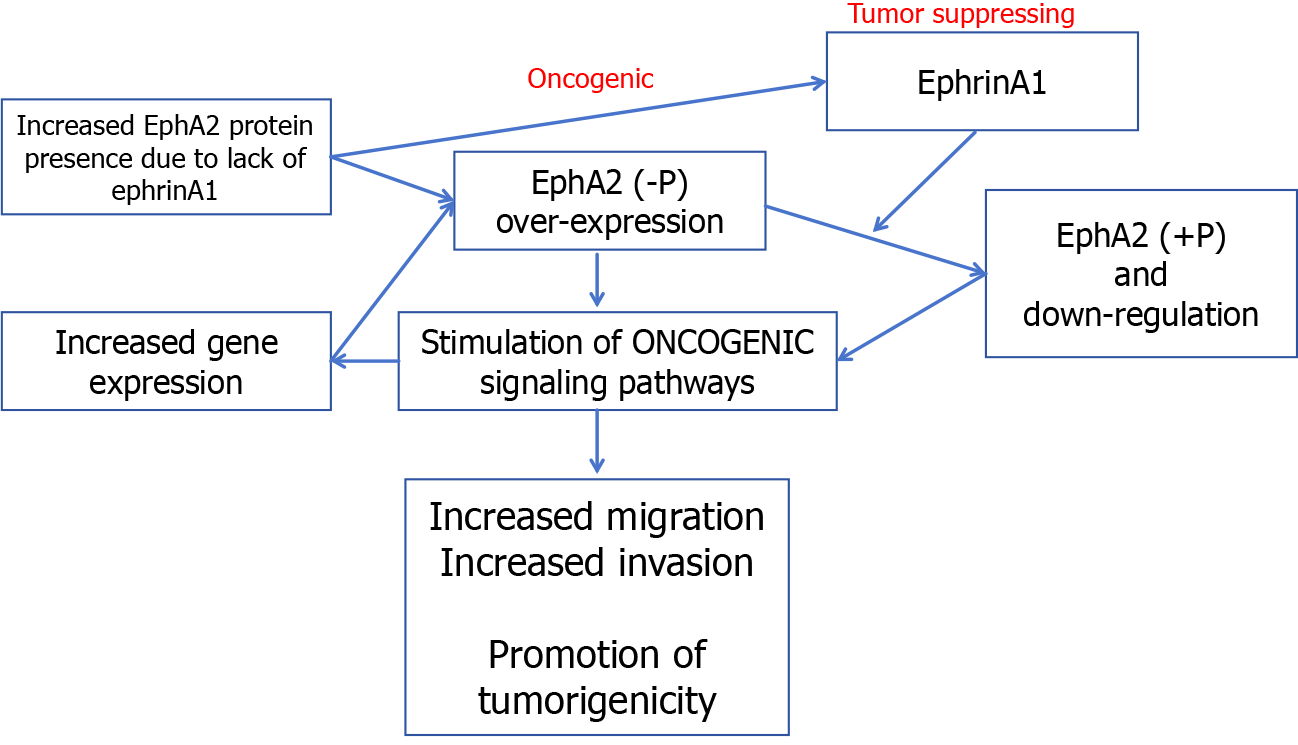
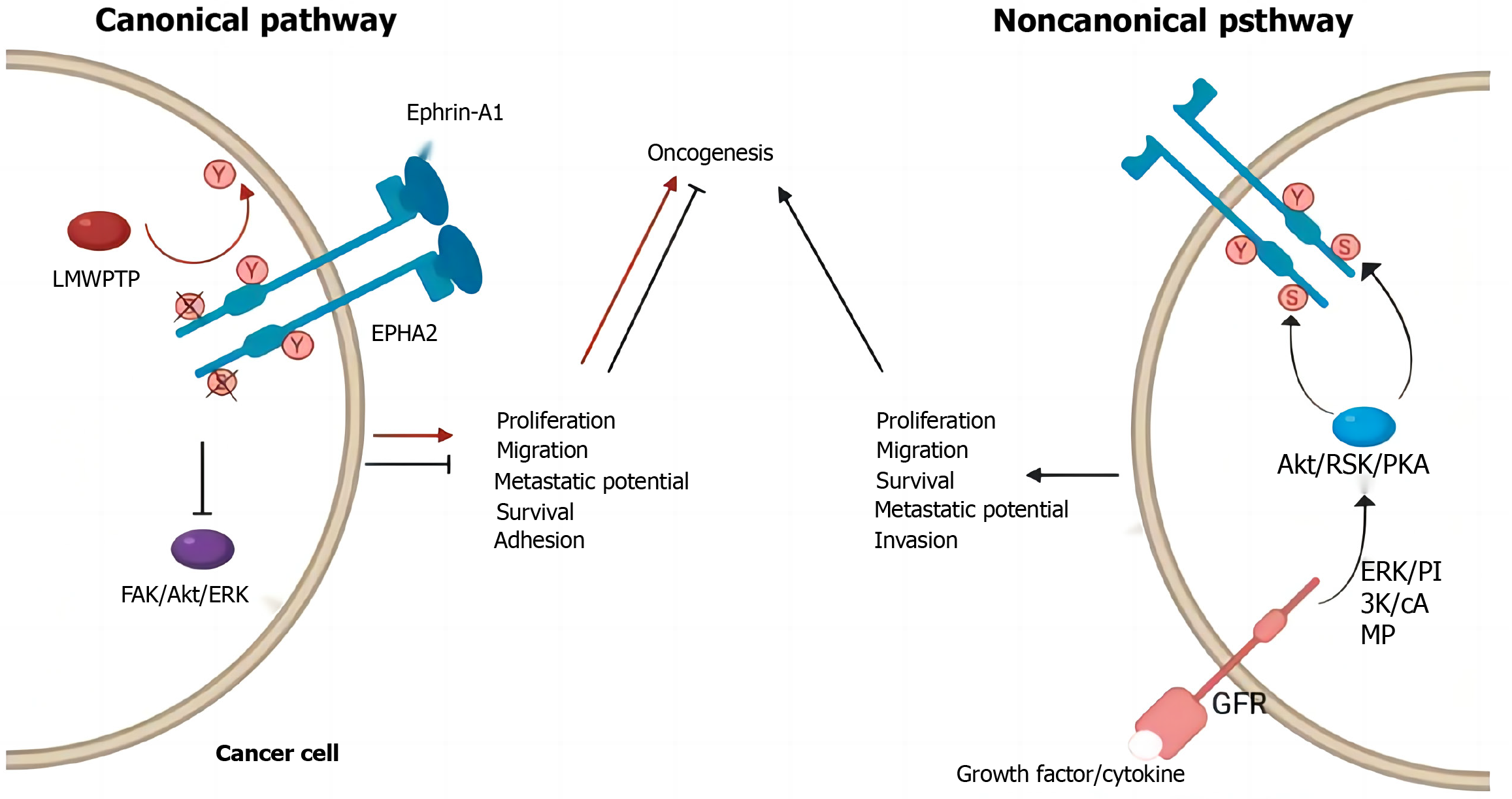
The classical signaling pathway of EphA2 begins with the binding of EphA2 to ephrin, which is expressed on the cell surface but not in the free form[61-64]. Therefore, receptor ligand binding not only activated the downstream signal of the receptor but also negatively affected the expression of the ligand in the intracellular signaling pathway. After binding with ligands, self-phosphorylation of the EphA2 intracellular near-membrane regions Y588 and Y594 occurs, initiating the phosphorylation cascade and recruiting intracellular effector molecules[65]. GTPase activating protein, guanylic acid exchange factor, FAK, Src, p85, etc., can interact with this activated form of EphA2 to regulate cell proliferation, apoptosis, adhesion, migration, and morphological development[66-70]. According to previous reports, the classical signaling pathway of EphA2 mainly plays a role in cancer inhibition (Figure 2).
EphA2 regulates cell proliferation in normal epithelial tissues but is highly expressed in breast cancer, lung cancer and CRC[71]. Several glioma-related studies have reported the role of RSK-EphA2 pathway activation in regulating cell proliferation and its association with poor prognosis[72-75]. Knockdown of EphA2 or interference with EphA2 through microRNA (miRNA) can inhibit EGF-dependent cell proliferation, while overexpression of the EphA2S897A mutant does not cause abnormal cell proliferation, suggesting that the regulation of proliferation by EphA2 is ligand independent[76]. Some studies reported that EphrinA1 combined with EphA2 inhibited the proliferation and metastasis of breast cancer cells and that Y594 and Y588 phosphorylation and S897 dephosphorylation occurred[77-80]. EphrinA1 expression decreased significantly in breast cancer tissues[81]. These results suggest that the ligand-dependent classical EphA2 pathway plays a major role in cancer inhibition and antagonizes nonclassical functions. Previously reported EphA2-mediated regulation of cell proliferation may affect the cell cycle by decreasing p27KIP1 and inhibiting the Cdk2/CycinE1/2 complex[82]. EphA2 can also interact with FAK, HER2 and a variety of cytokines to promote proliferation and metastasis.
The most widely studied nonclassical pathway of EphA2 is its involvement in cell motility and morphological development[83-85]. A variety of cytokines can phosphorylate EphA2 expressed in cellular pseudopods at S897 through RSK1/2 to regulate cell migration and allow cells to move to the side of high-concentration chemokines[86-90]. For example, RSK1/2 phosphorylates EphA2 (S897) to promote invasion and metastasis in the MDA-MB-231 cell line. It has also been reported that tropomyosin-associated kinase A activates SRC-mediated cell migration via the AKT-EphA2 (S897) pathway[91]. Treatment of cells with RSK inhibitors or EphA2-targeting miRNA200a reduced cell motility, while overexpression of the EphA25897A mutant did not restore cell migration after downregulation, suggesting that RSK activates the EphA2S897 site to regulate cell motility[92-95]. Immunohistochemistry showed that RSK inhibition not only decreased pEphA2 S897 staining intensity but also disrupted EphA2 distribution in cells, indicating that RSK also regulated the phosphorylation of EphA2 and its intracellular localization. In addition, EphB6 inhibited the migration-promoting effect of EphA2, and EphA2 and EphB6 expression was often negatively correlated in tissues, suggesting the existence of a common upstream regulatory mechanism[96]. Chemokines such as nerve growth factor precursors phosphorylate EphA2 S897 via AKT, thereby activating SRC-mediated cell migration[97].
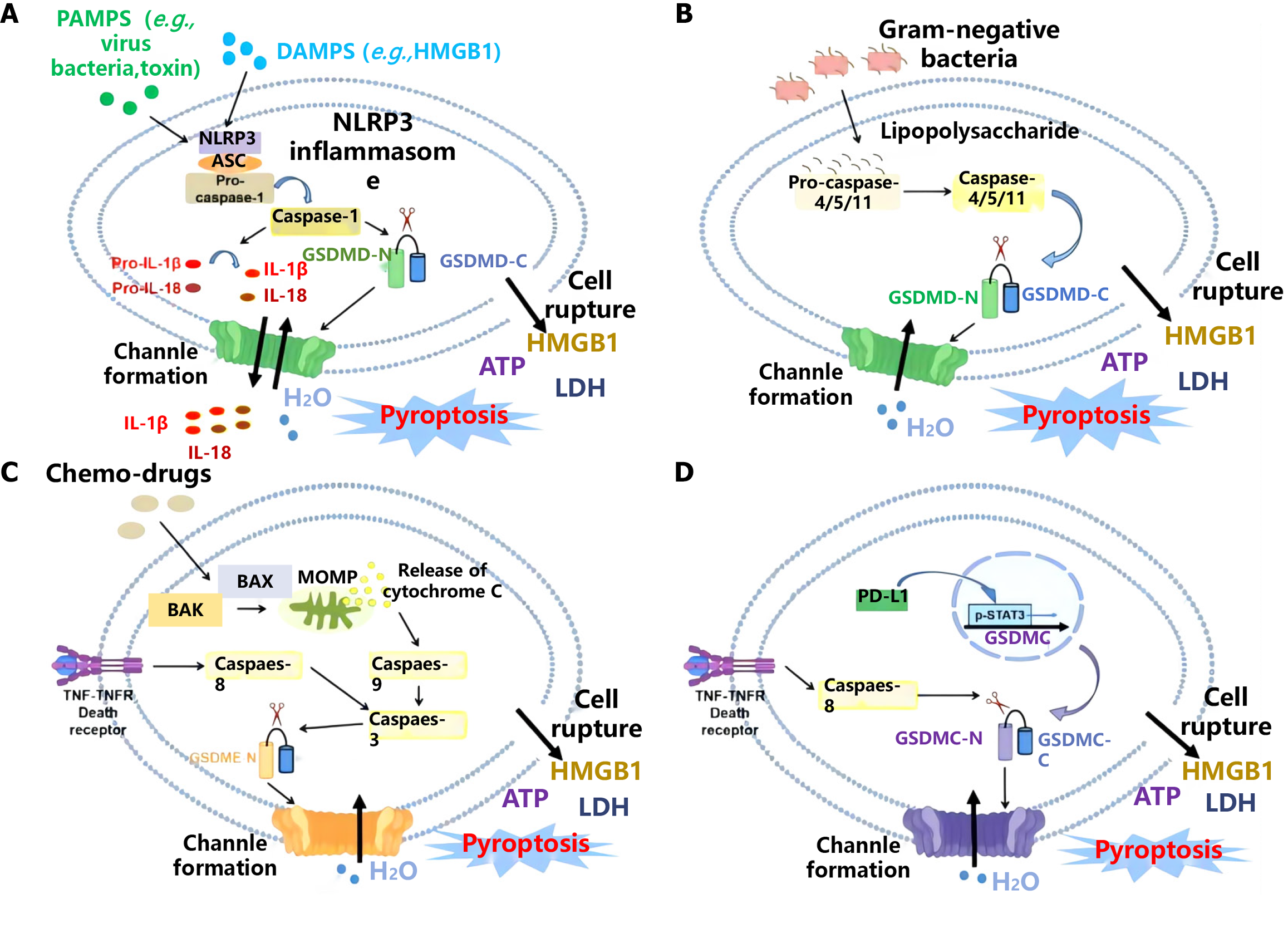
Pyroptosis is a form of inflammatory programmed cell death that, unlike cell necrosis, plays a key role in tumor suppression and the immune response. Studies have shown that EphA2 regulates pyrodeath through a variety of signaling pathways. For example, EphA2 can activate the PI3K/AKT and Ras/MAPK signaling pathways, which are commonly associated with cell survival and antiapoptotic mechanisms. However, under specific physiological or pathological conditions, the overexpression or abnormal activation of EphA2 can lead to pyroptosis, thereby inhibiting the growth and spread of cancer cells. Binding of EphA2 to its ligand triggers a series of downstream signal transduction events that promote the release of intracellular inflammatory response factors, such as interleukin (IL)-1β and IL-18, thus initiating the pyroptosis process.
In addition, the interaction of EphA2 with other cytokines and receptors is also an important mechanism regulating pyrodeath. EphA2 can synergize with inflammatory factors such as tumor necrosis factor-α and interferon-γ to enhance pyroptotic signal transduction. These interactions not only affect the survival of the cancer cells themselves but also alter the tumor microenvironment and enhance the ability of immune cells to recognize and clear cancer cells.
In GI CRC, EPHA2-regulated pyroptosis has dual effects on cancer initiation and metastasis. On the one hand, EPHA2-mediated pyroptosis can limit the growth and metastasis of cancer cells and improve therapeutic efficacy. On the other hand, abnormal EphA2 expression and signaling may cause cancer cells to evade pyrodeath and increase drug resistance and metastasis. Therefore, a thorough understanding of the regulatory mechanism of EphA2 in pyroptosis is highly important for the development of new therapeutic strategies. In summary, EphA2 regulates pyroptosis through a variety of complex signaling pathways and interactions and plays a key role in the occurrence, development and metastasis resistance of GI CRC.
CRC is a highly complex disease whose pathological features involve abnormal changes in multiple biological processes, including cell proliferation, differentiation, apoptosis, cycle regulation, migration and invasion, and angiogenesis[98-100]. These biological processes interact and together drive the onset and development of cancer[101-103]. Serum EphA2 levels are greater in patients with advanced TNM, deeply invasive tumors, multiple lymph node metastases, and distant metastases[104]. The abundance of 21 RTKSs and reported that the expression levels of some RTKSs, including EphA2, were closely related to liver metastasis and the prognosis of CRC patients[105-110]. In conclusion, EphA2 plays a crucial role in the development and metastasis of CRC, and its mechanism of action involves several biological processes, such as angiogenesis, epithelial-to-mesenchymal transition (EMT) and intracellular signal transduction (Figure 3).
Neovascularization plays an important role in the development and metastasis of CRC[111-114]. Tumor cells release large amounts of vascular endothelial growth factor (VEGF) in harsh environments, stimulating angiogenesis to meet the oxygen and nutrient requirements for tumor growth[115]. The formation of blood vessels and lymphatic vessels can also provide pathways for metastasis, which can promote hematologic or lymphatic metastasis of tumors[116-120]. EphA2 is an important factor that promotes vascular mimicry, and its mechanism of action mainly involves the regulation of the VE-cadherin/EphA2 pathway in vascular endothelial cells. Serum EphA2, VEGF-A and carcinoembryonic antigen levels in CRC patients were significantly greater than those in controls, and serum EphA2 levels were positively correlated with VEGF-A content. One study revealed a close relationship between EphA2 and VEGF[121]. The level of N6-methyladenosine methylation of EphA2 and VEGF-A in CRC was significantly greater than that in normal tissues. This modification allows its messenger RNA to be stabilized and translated[122-125]. Thus, the protein expression of EphA2 and VEGF-A is synergistically upregulated, and this process is caused by insulin-like growth factor 2 (IGF2) mRNA binding protein 2/3. With the activation of IGF2BP2/3, EphA2 and VFGRA promote the formation of intravascular structures in tumor blast cells through the PI3K/Akt and ERK1/2 pathways to promote tumor growth and development[126-130]. EphA2 is a functional receptor for growth factor progranulin (PGRN). In EPHA2-deficient endothelial cells, the role of PGRN in promoting endothelial cell angiogenesis is significantly weakened. EphA2 plays a promoting role in angiogenesis and promotes the development and metastasis of CRC[131-134].
EMT is a complex process involving cytoskeletal and phenotypic changes. During EMT, epithelial cells gradually lose their characteristic cell-cell connections and epithelial cell morphology and gradually acquire the morphological and biological characteristics of mesenchymal cells[135]. EMT can increase the migration and invasion ability of CRC cells so that they can more easily metastasize and spread to other tissues and organs[136]. EphA2 regulates EMT in CRC by downregulating the expression of epithelial E-cadherin, thereby reducing intercellular adhesion and enhancing cell metastasis[137]. SW480 cell line, the phosphorylation of EphA2 was reduced through the Akt-EphA2 pathway, resulting in a significant increase in E-cadherin. The inhibition of EphA2 phosphorylation was also significantly associated with low vimentin expression[138-140]. Further studies revealed that EphA2 promotes EMT through the Notch and Snail signaling pathways, thereby enhancing the invasion and migration ability of the CRC cell line LoVo. Further studies revealed that EphA2 promotes EMT through the Notch and Snail signaling pathways, thereby enhancing the invasion and migration ability of the CRC cell line LoVo[141-145].
EphA2 not only promotes the development and metastasis of CRC by directly affecting cell biological processes such as cell adhesion, migration and invasion but also regulates the growth, apoptosis and metastasis of CRC cells by affecting a variety of intracellular signaling pathways and gene regulation[146-148]. PGRN can form a complex with EphA2 on the surface of the tumor cell membrane, thereby activating the EphA2 signaling pathway[149]. This signaling pathway promotes the proliferation and development of tumor cells through the EphA2-mediated activation of Akt and MAPK. Cui et al[126] showed that EPHA2-superenhancer promotes tumor progression by recruiting FOSL2 and TCF7 L2 to activate the expression of the target gene EphA2. The deletion of EPHA2-superenhancer promoted apoptosis, inhibited cell growth and enhanced cell invasion by blocking the PI3K/Akt and Wnt/β-catenin pathways in HCT-116 cells. Liu et al[128] showed that the upregulation of Smad4 inhibits EphA2 phosphorylation by blocking the PI3K/Akt/EphA2 axis, thus weakening the migration and invasion ability of CRC cells. In conclusion, EphA2 is an important regulatory factor that is closely related to various biological processes related to CRC development and metastasis.
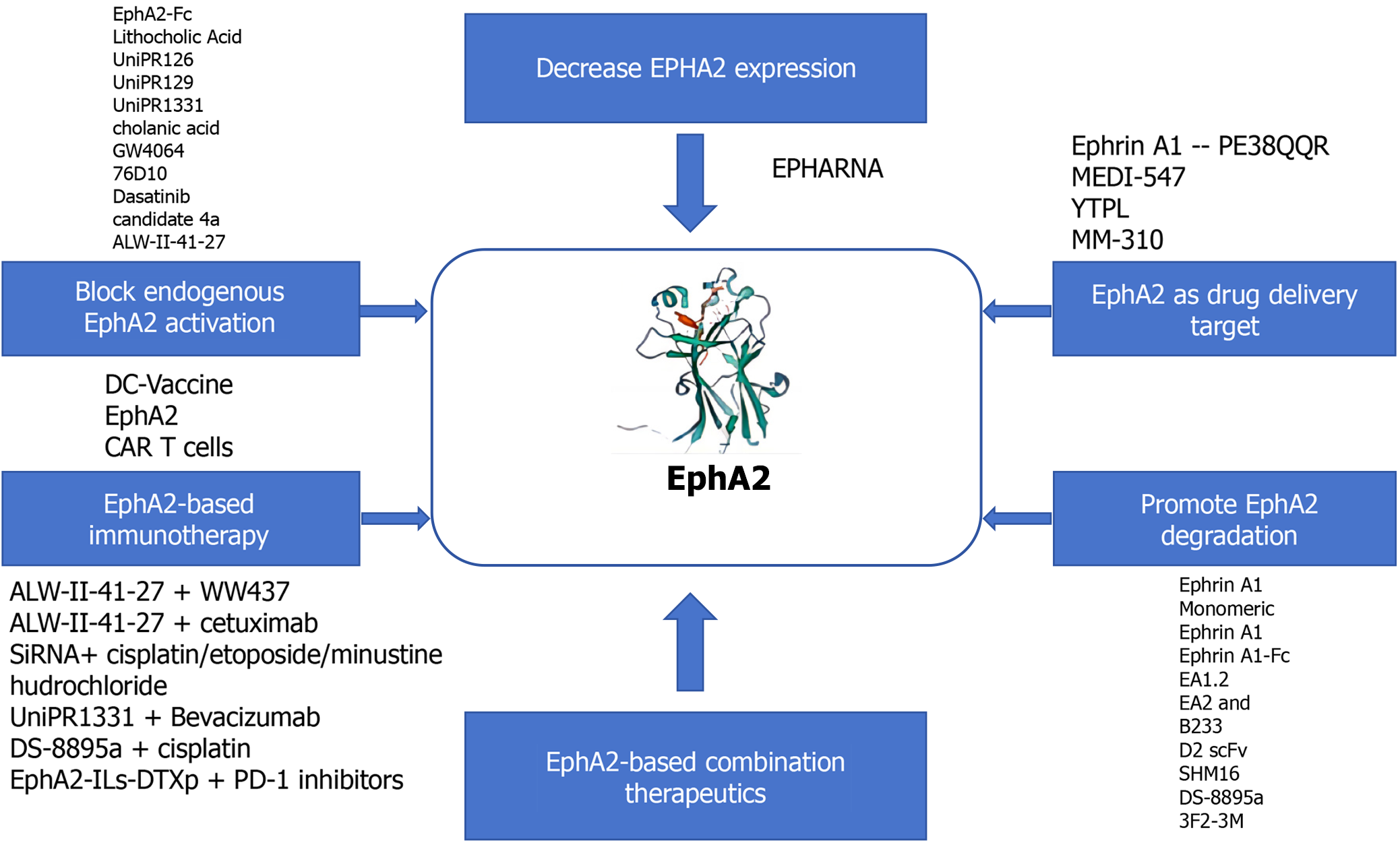
In addition to promoting the proliferation, invasion and angiogenesis of CRC cells, a series of studies in recent years have shown that EphA2 is closely related to chemotherapy resistance and targeted therapy resistance in CRC[150-154]. Drug resistance occurs in tumor cells through a series of ways to reduce their sensitivity to drugs, resulting in reduced or ineffective therapeutic effects, which is still the primary problem of treatment (Figure 4).
The protein and mRNA expression levels of EphA2 in drug-resistant CRC cells were significantly greater than those in their parental cells and gradually increased with increasing chemotherapeutic drug concentration[155-160]. When EphA2 expression is disrupted in drug-resistant cells, the sensitivity of these cells to chemotherapy drugs is significantly increased[161]. Yao et al[11] showed that EphA2, which is equivalent to that in the CRC parent strain HCT8, is overexpressed in 5-fluorouracil/cisplatin (5-Fu/DDP)-resistant cell lines and mediates the resistance of CRC cells to chemotherapy, which is closely related to long noncoding RNAs. LINC02418 upregulates EphA2 expression through competitive binding of miR-372-3p, thereby promoting CRC chemotherapy resistance to 5-Fu/DDP. This study revealed the potential mechanism of 5-FU/DDP resistance in CRC through the LINC02418/miR-372-3p/EphA2 axis. At the same time, treatment with ALW-II-41-27 can significantly improve the chemotherapy sensitivity of CRC-resistant cells, reduce cell proliferation, promote cell apoptosis, and block the cell cycle in the G2/M phase, indicating that inhibition of EphA2 kinase activity can have a series of effects on the function of drug-resistant cells, increasing the sensitivity of drug-resistant cells to chemotherapy drugs[162-166]. EphA2 also promotes the differentiation of cancer stem cells in CRC. Cancer stem cells are a subgroup of cancer cells with strong adaptability that enable them to survive chemotherapy drugs and promote tumor recurrence and metastasis, which is closely related to chemotherapy resistance[167-170]. When EphA2 binds to PGRN or is activated by IGF2BP2/3, the mammalian target of rapamycin (mTOR) pathway of PI3K-Akt-rapamycin is activated, and the activation of this pathway promotes the survival and anti-apoptosis of cells[171-173]. Therefore, in the treatment of cancer, the mTOR pathway is activated. The activation of this pathway often leads to the occurrence of chemotherapy resistance, so inhibiting the interaction between EphA2 and its upstream molecules or blocking the PI3K-Akt-mTOR pathway may be one of the strategies for preventing chemotherapy resistance (Figure 5).
Using transcriptomic sequencing technology (RNA-seq), high basal EphA2 expression was associated with resistance to regorafenib in metastatic CRC patients. Martini et al[9] showed that high EphA2 expression in CRC tissues leads to increased cetuximab (CET) resistance in cancer cells, and high EphA2 levels are significantly correlated with poor progression-free survival. Moreover, by combining CET with ALW-II-41-27 (an EphA2 kinase inhibitor), the sensitivity of tumor cells to chemotherapy drugs can be significantly enhanced, reversing primary and acquired resistance to CET[174-176]. CET is a monoclonal antibody that acts on CRC cells and binds to EGFR, thereby inhibiting the EGFR signaling pathway and preventing cancer cell growth and spread. According to the results of differential proteomic analysis, the EphA2 protein was significantly upregulated in drug-resistant cells, highlighting the role of EphA2 in KRAS mutation-acquired CET resistance in metastatic CRC. Studies have shown that the expression status of EphA2/Efna1/EGFR genes is closely related to the response of CRC patients to CET treatment, and the expression of these genes is not related to the genetic status of KRAS, which contradicts the findings of previous studies and needs further research[177]. Specifically, in CRC patients, progression-free survival in patients with high EphA2 expression under CET treatment is significantly lower than that in patients with low EphA2 expression, while in patients with high EGFR and EphA2 expression, the shortening of progression-free survival duration with CET suggests that EphA2 may play a role in circumventing CET’s inhibition of the EGFR pathway and that patients with abnormal EphA2 gene expression are more likely to show resistance to CET and have less effective treatment. Further studies have shown that the Akt signaling pathway can promote the interaction of EphA2 with EGFR and Ephexin1, thereby activating the Ephexin1 signaling pathway[178-180]. Akt promotes the interaction between EGFR and Ephexin1 by inducing EphA2 phosphorylation at Ser897, thereby promoting the development of CRC and resistance to CET. In general, EphA2 can enhance drug resistance in CRC cells by interacting with a variety of signaling pathways. These studies suggest that EphA2 is an important regulator of CRC resistance (Figure 6).
RTKs are responsible for the transmission of external stimulus signals to the nucleus[181]. The EPH gene family, which is a key component of the signal transduction pathway that is involved in cell effects, is the largest member of the newly discovered RTK family and is widely expressed in cells of epithelial origin. Its structure includes an amino terminal extracellular ligand binding region, a transmembrane domain and an intracellular enzyme domain[182]. EphA2 was the first gene found to have tyrosine kinase activity in the family. EphrinA1 can bind to the EphrinA1 ligand through the extracellular ligand binding region to form a receptor-ligand complex, which activates the cytoplasmic tyrosine phosphatase and leads to self-phosphorylation and tyrosine phosphorylation of a large number of downstream intracellular substrate protein molecules[183]. These pathways participate in cell growth, migration and differentiation activities and play important roles in embryonic development, blood vessel formation, tumor formation and so on. Studies have shown that EphA2 is highly expressed in many tumor tissues, including breast cancer, colon cancer, esophageal cancer, prostate cancer, etc., especially in highly invasive tumor cells (Figure 7).
Since the hypothesis that tumor growth can be slowed by inhibiting tumor nutrient vessels was proposed by Folkman in the early 1970s, a large number of studies[184-186] have shown that angiogenesis is a prerequisite for tumor growth and metastasis. The microvascular density (MVD) of tumors is an important indicator of the biological behavior of malignant tumors. The so-called MVD refers to the small blood vessel count performed on the most densely populated part of the tumor blood vessels. As the gold standard for evaluating tumor angiogenesis, the MVD can reflect the tumor’s ability to induce angiogenesis and is closely related to malignant behavior and tumor recurrence and metastasis. The MVD reflects the inevitable relationship between the intensity of tumor angiogenesis and tumor aggressiveness and can be used as an indicator to judge the prognosis of patients with CRC for clinical reference[187]. A study showed that the MVD was greater in patients with CRC and could predict that the tumor is more aggressive and has a poor prognosis[188]. Tumor angiogenesis is regulated by many factors, among which Eph RTK family members are central regulators of angiogenesis. Our results showed that in addition to EphA2 expression in CRC cells, EphA2 was also expressed in microvascular endothelial cells in tumors, and tumors with high EphA2 expression had a greater MVD, suggesting that EphA2 may affect the invasion and metastasis of CRC cells by regulating tumor angiogenesis.
As a key RTK, EphA2 plays a crucial role in the occurrence, development and metastasis of GI CRC by regulating cell pyrodeath. It regulates the initiation and execution of pyrodeath through various signaling pathways and interactions with other cytokines, thus affecting the survival and spread of cancer cells. Abnormal expression of EphA2 not only promotes cancer invasion and metastasis but also may lead to treatment resistance. However, by targeting EphA2 and its related signaling pathways, it is expected to induce pyrodeath in cells, inhibit tumor growth, and enhance therapeutic efficacy. Therefore, further study of the mechanism by which EphA2 regulates pyroptosis will provide new strategies and potential targets for the treatment of GI CRC.
| 1. | Liu X, He H, Zhang F, Hu X, Bi F, Li K, Yu H, Zhao Y, Teng X, Li J, Wang L, Zhang Y, Wu Q. m6A methylated EphA2 and VEGFA through IGF2BP2/3 regulation promotes vasculogenic mimicry in colorectal cancer via PI3K/AKT and ERK1/2 signaling. Cell Death Dis. 2022;13:483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 2. | Primeaux M, Liu X, Gowrikumar S, Fatima I, Fisher KW, Bastola D, Vecchio AJ, Singh AB, Dhawan P. Claudin-1 interacts with EPHA2 to promote cancer stemness and chemoresistance in colorectal cancer. Cancer Lett. 2023;579:216479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Tröster A, Jores N, Mineev KS, Sreeramulu S, DiPrima M, Tosato G, Schwalbe H. Targeting EPHA2 with Kinase Inhibitors in Colorectal Cancer. ChemMedChem. 2023;18:e202300420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Li Y, Peng Q, Wang L. EphA2 as a phase separation protein associated with ferroptosis and immune cell infiltration in colorectal cancer. Aging (Albany NY). 2023;15:12952-12965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 5. | Stammes MA, Prevoo HA, Ter Horst MC, Groot SA, Van de Velde CJ, Chan AB, de Geus-Oei LF, Kuppen PJ, Vahrmeijer AL, Pasquale EB, Sier CF. Evaluation of EphA2 and EphB4 as Targets for Image-Guided Colorectal Cancer Surgery. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Gunji D, Narumi R, Muraoka S, Isoyama J, Ikemoto N, Ishida M, Tomonaga T, Sakai Y, Obama K, Adachi J. Integrative analysis of cancer dependency data and comprehensive phosphoproteomics data revealed the EPHA2-PARD3 axis as a cancer vulnerability in KRAS-mutant colorectal cancer. Mol Omics. 2023;19:624-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Wang G, Wang Y, Yang X, Zhang Y, Lu Y, Li Y. The expression and diagnostic value of serum levels of EphA2 and VEGF-A in patients with colorectal cancer. Cancer Biomark. 2021;31:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Mao L, Yuan W, Cai K, Lai C, Huang C, Xu Y, Zhong S, Yang C, Wang R, Zeng P, Huang H, Chen Z, Chen Z. EphA2-YES1-ANXA2 pathway promotes gastric cancer progression and metastasis. Oncogene. 2021;40:3610-3623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Martini G, Cardone C, Vitiello PP, Belli V, Napolitano S, Troiani T, Ciardiello D, Della Corte CM, Morgillo F, Matrone N, Sforza V, Papaccio G, Desiderio V, Paul MC, Moreno-Viedma V, Normanno N, Rachiglio AM, Tirino V, Maiello E, Latiano TP, Rizzi D, Signoriello G, Sibilia M, Ciardiello F, Martinelli E. EPHA2 Is a Predictive Biomarker of Resistance and a Potential Therapeutic Target for Improving Antiepidermal Growth Factor Receptor Therapy in Colorectal Cancer. Mol Cancer Ther. 2019;18:845-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Dunne PD, Dasgupta S, Blayney JK, McArt DG, Redmond KL, Weir JA, Bradley CA, Sasazuki T, Shirasawa S, Wang T, Srivastava S, Ong CW, Arthur K, Salto-Tellez M, Wilson RH, Johnston PG, Van Schaeybroeck S. EphA2 Expression Is a Key Driver of Migration and Invasion and a Poor Prognostic Marker in Colorectal Cancer. Clin Cancer Res. 2016;22:230-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Yao F, Huang X, Xie Z, Chen J, Zhang L, Wang Q, Long H, Jiang J, Wu Q. LINC02418 upregulates EPHA2 by competitively sponging miR-372-3p to promote 5-Fu/DDP chemoresistance in colorectal cancer. Carcinogenesis. 2022;43:895-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 12. | Feng J, Xiao T, Lu SS, Hung XP, Yi H, He QY, Huang W, Tang YY, Xiao ZQ. ANXA1derived peptides suppress gastric and colon cancer cell growth by targeting EphA2 degradation. Int J Oncol. 2020;57:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Saito T, Masuda N, Miyazaki T, Kanoh K, Suzuki H, Shimura T, Asao T, Kuwano H. Expression of EphA2 and E-cadherin in colorectal cancer: correlation with cancer metastasis. Oncol Rep. 2004;11:605-611. [PubMed] |
| 14. | Torlot L, Jarzab A, Albert J, Pók-Udvari Á, Stahler A, Holch JW, Gerlinger M, Heinemann V, Klauschen F, Kirchner T, Kumbrink J, Küster B, Jung A. Proteomics uncover EPHA2 as a potential novel therapeutic target in colorectal cancer cell lines with acquired cetuximab resistance. J Cancer Res Clin Oncol. 2023;149:669-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Cuyàs E, Queralt B, Martin-Castillo B, Bosch-Barrera J, Menendez JA. EphA2 receptor activation with ephrin-A1 ligand restores cetuximab efficacy in NRAS-mutant colorectal cancer cells. Oncol Rep. 2017;38:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | De Robertis M, Loiacono L, Fusilli C, Poeta ML, Mazza T, Sanchez M, Marchionni L, Signori E, Lamorte G, Vescovi AL, Garcia-Foncillas J, Fazio VM. Dysregulation of EGFR Pathway in EphA2 Cell Subpopulation Significantly Associates with Poor Prognosis in Colorectal Cancer. Clin Cancer Res. 2017;23:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Eriksson O, Thulin Å, Asplund A, Hegde G, Navani S, Siegbahn A. Cross-talk between the Tissue Factor/coagulation factor VIIa complex and the tyrosine kinase receptor EphA2 in cancer. BMC Cancer. 2016;16:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Naudin C, Sirvent A, Leroy C, Larive R, Simon V, Pannequin J, Bourgaux JF, Pierre J, Robert B, Hollande F, Roche S. SLAP displays tumour suppressor functions in colorectal cancer via destabilization of the SRC substrate EPHA2. Nat Commun. 2014;5:3159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Kiewlich D, Zhang J, Gross C, Xia W, Larsen B, Cobb RR, Biroc S, Gu JM, Sato T, Light DR, Heitner T, Willuda J, Vogel D, Monteclaro F, Citkowicz A, Roffler SR, Zajchowski DA. Anti-EphA2 antibodies decrease EphA2 protein levels in murine CT26 colorectal and human MDA-231 breast tumors but do not inhibit tumor growth. Neoplasia. 2006;8:18-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Kataoka H, Igarashi H, Kanamori M, Ihara M, Wang JD, Wang YJ, Li ZY, Shimamura T, Kobayashi T, Maruyama K, Nakamura T, Arai H, Kajimura M, Hanai H, Tanaka M, Sugimura H. Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer. Cancer Sci. 2004;95:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Arabzadeh A, McGregor K, Breton V, Van Der Kraak L, Akavia UD, Greenwood CMT, Beauchemin N. EphA2 signaling is impacted by carcinoembryonic antigen cell adhesion molecule 1-L expression in colorectal cancer liver metastasis in a cell context-dependent manner. Oncotarget. 2017;8:104330-104346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Lam D, Jones O. Changes to gastrointestinal function after surgery for colorectal cancer. Best Pract Res Clin Gastroenterol. 2020;48-49:101705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Keku TO, Dulal S, Deveaux A, Jovov B, Han X. The gastrointestinal microbiota and colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2015;308:G351-G363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 24. | Oshima K, Yamazaki K. Immune checkpoint inhibitor therapy in neoadjuvant and adjuvant treatment for cancer: A paradigm shift in the treatment of resectable gastrointestinal cancer 3)A paradigm shift in the treatment of colorectal cancer. Int J Clin Oncol. 2023;28:1442-1450. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Rowland IR. The role of the gastrointestinal microbiota in colorectal cancer. Curr Pharm Des. 2009;15:1524-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Duffy MJ, Lamerz R, Haglund C, Nicolini A, Kalousová M, Holubec L, Sturgeon C. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. 2014;134:2513-2522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 27. | Hoffmeister M, Jansen L, Stock C, Chang-Claude J, Brenner H. Smoking, lower gastrointestinal endoscopy, and risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Azcárate-Peril MA, Sikes M, Bruno-Bárcena JM. The intestinal microbiota, gastrointestinal environment and colorectal cancer: a putative role for probiotics in prevention of colorectal cancer? Am J Physiol Gastrointest Liver Physiol. 2011;301:G401-G424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | O'Dwyer PJ, Eckhardt SG, Haller DG, Tepper J, Ahnen D, Hamilton S, Benson AB 3rd, Rothenberg M, Petrelli N, Lenz HJ, Diasio R, DuBois R, Sargent D, Sloan J, Johnson CD, Comis RL, O'Connell MJ; Gastrointestinal Scientific Leadership Council of the Coalition of Cancer Cooperative Groups. Priorities in colorectal cancer research: recommendations from the Gastrointestinal Scientific Leadership Council of the Coalition of Cancer Cooperative Groups. J Clin Oncol. 2007;25:2313-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Schoormans D, van Es B, Mols F, Wasowicz D, Beijer S, Ezendam NPM. The relation between sleep quality, sleep quantity, and gastrointestinal problems among colorectal cancer survivors: result from the PROFILES registry. Support Care Cancer. 2022;30:1391-1398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Fang L, Yao Y, Guan X, Liao Y, Wang B, Cui L, Han S, Zou H, Su D, Ma Y, Liu B, Wang Y, Huang R, Ruan Y, Yu X, Yao Y, Liu C, Zhang Y. China special issue on gastrointestinal tumors-Regulatory-immunoscore-A novel indicator to guide precision adjuvant chemotherapy in colorectal cancer. Int J Cancer. 2023;153:1904-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Fleet JC. Animal models of gastrointestinal and liver diseases. New mouse models for studying dietary prevention of colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2014;307:G249-G259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Loaiza-Bonilla A, Benson AB 3rd, Grothey A, Karimi M, Klempner SJ, Lin D, Mahtani R, Soares HP. Use of Molecular Assays and Circulating Tumor DNA in Early-Stage Colorectal Cancer: A Roundtable Discussion of the Gastrointestinal Cancer Therapy Expert Group. Oncologist. 2021;26:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Winawer SJ. The multidisciplinary management of gastrointestinal cancer. Colorectal cancer screening. Best Pract Res Clin Gastroenterol. 2007;21:1031-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Niu C, Zhang J, Lian J, Utsav J, Iyer C, Low S, Saeed H, Zahid S, Okolo PI 3rd. Anatomical location, risk factors, and outcomes of lower gastrointestinal bleeding in colorectal cancer patients: a national inpatient sample analysis (2009-2019). Int J Colorectal Dis. 2023;38:205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Tejpar S. The multidisciplinary management of gastrointestinal cancer. The use of molecular markers in the diagnosis and treatment of colorectal cancer. Best Pract Res Clin Gastroenterol. 2007;21:1071-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Kong F, Cai Y. Study Insights into Gastrointestinal Cancer through the Gut Microbiota. Biomed Res Int. 2019;2019:8721503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Calip GS, Meropol NJ, Weinberg DS. Colorectal Cancer Incidence Among Adults Younger Than 50 Years-Understanding Findings From Observational Studies of Lower Gastrointestinal Endoscopy. JAMA Oncol. 2022;8:981-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Grady WM. Epigenetic alterations in the gastrointestinal tract: Current and emerging use for biomarkers of cancer. Adv Cancer Res. 2021;151:425-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 40. | Moazzen S, Cortes-Ibañez FO, van der Vegt B, Alizadeh BZ, de Bock GH. Diet quality indices and gastrointestinal cancer risk: results from the Lifelines study. Eur J Nutr. 2022;61:317-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Tveit KM, Dahl O, Gerner T. [Chemotherapy in colorectal cancer. Recommendations of the Norwegian Gastrointestinal Cancer Group]. Tidsskr Nor Laegeforen. 1996;116:357-360. [PubMed] |
| 42. | Ciombor KK, Goldberg RM. Highlights in Gastrointestinal (Colorectal) Cancer Treatment: The Primary Tumor Sidedness Debate and Advances in Immunotherapy. JAMA Oncol. 2016;2:1537-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Sial SH, Catalano MF. Gastrointestinal tract cancer in the elderly. Gastroenterol Clin North Am. 2001;30:565-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Yozu M, Kumarasinghe MP, Brown IS, Gill AJ, Rosty C. Australasian Gastrointestinal Pathology Society (AGPS) consensus guidelines for universal defective mismatch repair testing in colorectal carcinoma. Pathology. 2019;51:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Rasmussen M, Kronborg O. Upper gastrointestinal cancer in a population-based screening program with fecal occult blood test for colorectal cancer. Scand J Gastroenterol. 2002;37:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Brazowski E, Rozen P, Pel S, Samuel Z, Solar I, Rosner G. Can a gastrointestinal pathologist identify microsatellite instability in colorectal cancer with reproducibility and a high degree of specificity? Fam Cancer. 2012;11:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Hafström L, Domellöf L, Rudenstam CM, Norryd C, Bergman L, Nilsson T, Hansson K, Wählby L, Asklöf G, Kugelberg C. Adjuvant chemotherapy with 5-fluorouracil, vincristine and CCNU for patients with Dukes' C colorectal cancer. The Swedish Gastrointestinal Tumour Adjuvant Therapy Group. Br J Surg. 1990;77:1345-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Wu D, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Zhang SY, Du YQ. [Effects of warming needle moxibustion on improvement of gastrointestinal and immune function in patients with postoperation of colorectal cancer]. Zhongguo Zhen Jiu. 2011;31:513-517. [PubMed] |
| 50. | Wils J, Sahmoud T, Sobrero A, Bleiberg H, Ahmedzai S, Blazeby J, Blijham G, Conroy T, Cunningham D, Curran D, Díaz-Rubio E, Ducreux M, Evans J, Glimelius B, Hutchinson G, Kerr D, Kiebert G, Köhne H, Labianca R, Langendijk R, Nitti D, Nordlinger B, Rougier P, Scheithauer W, Therasse P. Evaluation of clinical efficacy of new medical treatments in advanced colorectal cancer. Results of a workshop organized by the EORTC GITCCG. European Organization for Research and Treatment of Cancer. Gastrointestinal Tract Cancer Cooperative Group. Tumori. 1998;84:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Nehal M, Khatoon J, Akhtar S, Khan MKA. Exploring the potential of EphA2 receptor signaling pathway: a comprehensive review in cancer treatment. Mol Biol Rep. 2024;51:337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 52. | Morrow L, Greenwald B. The American Society for Gastrointestinal Endoscopy Quality Assurance in Endoscopy Committee's Three Priority Quality Indicators for Screening Colonoscopy Services. Gastroenterol Nurs. 2022;45:407-409. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 53. | . Colonoscopy in the screening and surveillance of individuals at increased risk for colorectal cancer. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 1998;48:676-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Ida A, Miyaki A, Miyauchi T, Yamaguchi K, Naritaka Y. [Three Cases of Unresectable, Advanced, and Recurrent Colorectal Cancer Associated with Gastrointestinal Obstruction That Were Treated with Small Intestine-Transverse Colon Bypass Surgery]. Gan To Kagaku Ryoho. 2016;43:1632-1634. [PubMed] |
| 55. | Ho JW, Chu KM, Tse CW, Yuen ST. Phenotype and management of patients with familial adenomatous polyposis in Hong Kong: perspective of the Hereditary Gastrointestinal Cancer Registry. Hong Kong Med J. 2002;8:342-347. [PubMed] |
| 56. | Wilson K, Shiuan E, Brantley-Sieders DM. Oncogenic functions and therapeutic targeting of EphA2 in cancer. Oncogene. 2021;40:2483-2495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 57. | London M, Gallo E. The EphA2 and cancer connection: potential for immune-based interventions. Mol Biol Rep. 2020;47:8037-8048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Zhou Y, Sakurai H. Emerging and Diverse Functions of the EphA2 Noncanonical Pathway in Cancer Progression. Biol Pharm Bull. 2017;40:1616-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 59. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 60. | Zhao P, Sun J, Huang X, Zhang X, Liu X, Liu R, Du G, Gan W, Yang C, Tang Y, Chen C, Jiang D. Targeting the KLF5-EphA2 axis can restrain cancer stemness and overcome chemoresistance in basal-like breast cancer. Int J Biol Sci. 2023;19:1861-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 61. | Dasari SK, Joseph R, Umamaheswaran S, Mangala LS, Bayraktar E, Rodriguez-Aguayo C, Wu Y, Nguyen N, Powell RT, Sobieski M, Liu Y, Chowdhury MA, Amero P, Stephan C, Lopez-Berestein G, Westin SN, Sood AK. Combination of EphA2- and Wee1-Targeted Therapies in Endometrial Cancer. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Huang C, Yuan W, Lai C, Zhong S, Yang C, Wang R, Mao L, Chen Z, Chen Z. EphA2-to-YAP pathway drives gastric cancer growth and therapy resistance. Int J Cancer. 2020;146:1937-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 63. | Husain A, Chiu YT, Sze KM, Ho DW, Tsui YM, Suarez EMS, Zhang VX, Chan LK, Lee E, Lee JM, Cheung TT, Wong CC, Chung CY, Ng IO. Ephrin-A3/EphA2 axis regulates cellular metabolic plasticity to enhance cancer stemness in hypoxic hepatocellular carcinoma. J Hepatol. 2022;77:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 64. | Joseph R, Dasari SK, Umamaheswaran S, Mangala LS, Bayraktar E, Rodriguez-Aguayo C, Wu Y, Nguyen N, Powell RT, Sobieski M, Liu Y, Kim MS, Corvigno S, Foster K, Hanjra P, Vu TC, Chowdhury MA, Amero P, Stephan C, Lopez-Berestein G, Westin SN, Sood AK. EphA2- and HDAC-Targeted Combination Therapy in Endometrial Cancer. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 65. | Tomiyama E, Fujita K, Matsuzaki K, Narumi R, Yamamoto A, Uemura T, Yamamichi G, Koh Y, Matsushita M, Hayashi Y, Hashimoto M, Banno E, Kato T, Hatano K, Kawashima A, Uemura M, Ukekawa R, Takao T, Takada S, Uemura H, Adachi J, Tomonaga T, Nonomura N. EphA2 on urinary extracellular vesicles as a novel biomarker for bladder cancer diagnosis and its effect on the invasiveness of bladder cancer. Br J Cancer. 2022;127:1312-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 66. | Kinch MS, Carles-Kinch K. Overexpression and functional alterations of the EphA2 tyrosine kinase in cancer. Clin Exp Metastasis. 2003;20:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Veiga RN, de Azevedo ALK, de Oliveira JC, Gradia DF. Targeting EphA2: a promising strategy to overcome chemoresistance and drug resistance in cancer. J Mol Med (Berl). 2024;102:479-493. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 68. | Wu L, Li X, Yan J. Commentary: Machine learning developed an intratumor heterogeneity signature for predicting prognosis and immunotherapy benefits in cholangiocarcinoma. Transl Oncol. 2024;45:101995. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 69. | Sachdeva A, Hart CA, Kim K, Tawadros T, Oliveira P, Shanks J, Brown M, Clarke N. Non-canonical EphA2 activation underpins PTEN-mediated metastatic migration and poor clinical outcome in prostate cancer. Br J Cancer. 2022;127:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 70. | Chang FL, Lee CC, Tsai KC, Lin TY, Chiang CW, Pan SL, Lee YC. An auristatin-based antibody-drug conjugate targeting EphA2 in pancreatic cancer treatment. Biochem Biophys Res Commun. 2023;688:149214. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 71. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 72. | Merritt WM, Kamat AA, Hwang JY, Bottsford-Miller J, Lu C, Lin YG, Coffey D, Spannuth WA, Nugent E, Han LY, Landen CN, Nick AM, Stone RL, Coffman K, Bruckheimer E, Broaddus RR, Gershenson DM, Coleman RL, Sood AK. Clinical and biological impact of EphA2 overexpression and angiogenesis in endometrial cancer. Cancer Biol Ther. 2010;10:1306-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 73. | Larsen AB, Stockhausen MT, Poulsen HS. Cell adhesion and EGFR activation regulate EphA2 expression in cancer. Cell Signal. 2010;22:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | El Zawily A, Vizeacoumar FS, Dahiya R, Banerjee SL, Bhanumathy KK, Elhasasna H, Hanover G, Sharpe JC, Sanchez MG, Greidanus P, Stacey RG, Moon KM, Alexandrov I, Himanen JP, Nikolov DB, Fonge H, White AP, Foster LJ, Wang B, Toosi BM, Bisson N, Mirzabekov TA, Vizeacoumar FJ, Freywald A. A Multipronged Unbiased Strategy Guides the Development of an Anti-EGFR/EPHA2-Bispecific Antibody for Combination Cancer Therapy. Clin Cancer Res. 2023;29:2686-2701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Wu L, Zhou J, Zhou W, Huang XF, Chen Q, Wang W, Zhai L, Li S, Tang Z. Sorafenib blocks the activation of the HIF-2α/VEGFA/EphA2 pathway, and inhibits the rapid growth of residual liver cancer following high-intensity focused ultrasound therapy in vivo. Pathol Res Pract. 2021;220:153270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Brannan JM, Sen B, Saigal B, Prudkin L, Behrens C, Solis L, Dong W, Bekele BN, Wistuba I, Johnson FM. EphA2 in the early pathogenesis and progression of non-small cell lung cancer. Cancer Prev Res (Phila). 2009;2:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 77. | Wei Q, Wei L, Zhang J, Li Z, Feng H, Ren L. EphA2enriched exosomes promote cell migration and are a potential diagnostic serum marker in pancreatic cancer. Mol Med Rep. 2020;22:2941-2947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Xia L, Liu X, Mao W, Guo Y, Huang J, Hu Y, Jin L, Liu X, Fu H, Du Y, Shou Q. Panax notoginseng saponins normalises tumour blood vessels by inhibiting EphA2 gene expression to modulate the tumour microenvironment of breast cancer. Phytomedicine. 2023;114:154787. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 79. | Lee PC, Chen ST, Kuo TC, Lin TC, Lin MC, Huang J, Hung JS, Hsu CL, Juan HF, Lee PH, Huang MC. C1GALT1 is associated with poor survival and promotes soluble Ephrin A1-mediated cell migration through activation of EPHA2 in gastric cancer. Oncogene. 2020;39:2724-2740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 80. | Asakura N, Nakamura N, Muroi A, Nojima Y, Yamashita T, Kaneko S, Ikeda K, Koshikawa N, Suzuki T. Expression of Cancer Stem Cell Markers EpCAM and CD90 Is Correlated with Anti- and Pro-Oncogenic EphA2 Signaling in Hepatocellular Carcinoma. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Vincenzi M, Mercurio FA, Autiero I, Leone M. Cancer-Related Mutations in the Sam Domains of EphA2 Receptor and Ship2 Lipid Phosphatase: A Computational Study. Molecules. 2024;29. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 82. | Okuyama T, Sakamoto R, Kumagai K, Nishizawa M, Kimura T, Sugie T, Kimura T. EPHA2 antisense RNA modulates EPHA2 mRNA levels in basal-like/triple-negative breast cancer cells. Biochimie. 2020;179:169-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Guo Z, He B, Yuan L, Dai W, Zhang H, Wang X, Wang J, Zhang X, Zhang Q. Dual targeting for metastatic breast cancer and tumor neovasculature by EphA2-mediated nanocarriers. Int J Pharm. 2015;493:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Liang S, Wang Q, Wen Y, Wang Y, Li M, Wang Q, Peng J, Guo L. Ligand-independent EphA2 contributes to chemoresistance in small-cell lung cancer by enhancing PRMT1-mediated SOX2 methylation. Cancer Sci. 2023;114:921-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 85. | Behr M, Kaufmann JK, Ketzer P, Engelhardt S, Mück-Häusl M, Okun PM, Petersen G, Neipel F, Hassel JC, Ehrhardt A, Enk AH, Nettelbeck DM. Adenoviruses using the cancer marker EphA2 as a receptor in vitro and in vivo by genetic ligand insertion into different capsid scaffolds. PLoS One. 2014;9:e95723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Yuan W, Chen Z, Wu S, Ge J, Chang S, Wang X, Chen J, Chen Z. Expression of EphA2 and E-cadherin in gastric cancer: correlated with tumor progression and lymphogenous metastasis. Pathol Oncol Res. 2009;15:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Moyano-Galceran L, Pietilä EA, Turunen SP, Corvigno S, Hjerpe E, Bulanova D, Joneborg U, Alkasalias T, Miki Y, Yashiro M, Chernenko A, Jukonen J, Singh M, Dahlstrand H, Carlson JW, Lehti K. Adaptive RSK-EphA2-GPRC5A signaling switch triggers chemotherapy resistance in ovarian cancer. EMBO Mol Med. 2020;12:e11177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 88. | Ezenwafor TC, Uzonwanne VO, Madukwe JUA, Amin SM, Anye VC, Obayemi JD, Odusanya OS, Soboyejo WO. Adhesion of LHRH/EphA2 to human Triple Negative Breast Cancer tissues. J Mech Behav Biomed Mater. 2022;136:105461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 89. | Salem AF, Wang S, Billet S, Chen JF, Udompholkul P, Gambini L, Baggio C, Tseng HR, Posadas EM, Bhowmick NA, Pellecchia M. Reduction of Circulating Cancer Cells and Metastases in Breast-Cancer Models by a Potent EphA2-Agonistic Peptide-Drug Conjugate. J Med Chem. 2018;61:2052-2061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 90. | Taddei ML, Parri M, Angelucci A, Onnis B, Bianchini F, Giannoni E, Raugei G, Calorini L, Rucci N, Teti A, Bologna M, Chiarugi P. Kinase-dependent and -independent roles of EphA2 in the regulation of prostate cancer invasion and metastasis. Am J Pathol. 2009;174:1492-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 91. | Liu X, Li Y, Chen C, Dong J, Zhou J, Tong D, Wang L, Gao X, Kang X. Exosomal EphA2 promotes tumor metastasis of triple-negative breast cancer by damaging endothelial barrier. Clin Exp Metastasis. 2023;40:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 92. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Tandon M, Vemula SV, Mittal SK. Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opin Ther Targets. 2011;15:31-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 94. | Gan Q, Cui K, Cao Q, Zhang N, Yang MF, Yang X. Development of a (18)F-Labeled Bicyclic Peptide Targeting EphA2 for Molecular Imaging of PSMA-Negative Prostate Cancer. J Med Chem. 2023;66:14623-14632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 95. | Buraschi S, Neill T, Xu SQ, Palladino C, Belfiore A, Iozzo RV, Morrione A. Progranulin/EphA2 axis: A novel oncogenic mechanism in bladder cancer. Matrix Biol. 2020;93:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 96. | Wu B, Wang S, De SK, Barile E, Quinn BA, Zharkikh I, Purves A, Stebbins JL, Oshima RG, Fisher PB, Pellecchia M. Design and Characterization of Novel EphA2 Agonists for Targeted Delivery of Chemotherapy to Cancer Cells. Chem Biol. 2015;22:876-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 97. | Hasegawa J, Sue M, Yamato M, Ichikawa J, Ishida S, Shibutani T, Kitamura M, Wada T, Agatsuma T. Novel anti-EPHA2 antibody, DS-8895a for cancer treatment. Cancer Biol Ther. 2016;17:1158-1167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 98. | Landen CN Jr, Lu C, Han LY, Coffman KT, Bruckheimer E, Halder J, Mangala LS, Merritt WM, Lin YG, Gao C, Schmandt R, Kamat AA, Li Y, Thaker P, Gershenson DM, Parikh NU, Gallick GE, Kinch MS, Sood AK. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 99. | Buckens OJ, El Hassouni B, Giovannetti E, Peters GJ. The role of Eph receptors in cancer and how to target them: novel approaches in cancer treatment. Expert Opin Investig Drugs. 2020;29:567-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 100. | Li R, Yuan W, Mei W, Yang K, Chen Z. MicroRNA 520d-3p inhibits gastric cancer cell proliferation, migration, and invasion by downregulating EphA2 expression. Mol Cell Biochem. 2014;396:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 101. | Gao Z, Han X, Zhu Y, Zhang H, Tian R, Wang Z, Cui Y, Wang Z, Niu R, Zhang F. Drug-resistant cancer cell-derived exosomal EphA2 promotes breast cancer metastasis via the EphA2-Ephrin A1 reverse signaling. Cell Death Dis. 2021;12:414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 102. | Hong HN, Won YJ, Shim JH, Kim HJ, Han SH, Kim BS, Kim HS. Cancer-associated fibroblasts promote gastric tumorigenesis through EphA2 activation in a ligand-independent manner. J Cancer Res Clin Oncol. 2018;144:1649-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 103. | Song W, Ma Y, Wang J, Brantley-Sieders D, Chen J. JNK signaling mediates EPHA2-dependent tumor cell proliferation, motility, and cancer stem cell-like properties in non-small cell lung cancer. Cancer Res. 2014;74:2444-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 104. | Liu K, Wu L, Jie Y, Na L, Zhang Q, Tian R, Li G, Lu G, Ma S. [Overexpression of ephrin-A receptor 2 (EphA2) in invasive breast cancer tissues and its negative correlation with pyroptosis]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2021;37:981-986. [PubMed] |
| 105. | Hou F, Yuan W, Huang J, Qian L, Chen Z, Ge J, Wu S, Chen J, Wang J, Chen Z. Overexpression of EphA2 correlates with epithelial-mesenchymal transition-related proteins in gastric cancer and their prognostic importance for postoperative patients. Med Oncol. 2012;29:2691-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 106. | Wei Q, Zhang J, Li Z, Wei L, Ren L. Serum Exo-EphA2 as a Potential Diagnostic Biomarker for Pancreatic Cancer. Pancreas. 2020;49:1213-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 107. | Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 108. | Yuan W, Chen Z, Chen Z, Wu S, Guo J, Ge J, Yang P, Huang J. Silencing of EphA2 inhibits invasion of human gastric cancer SGC-7901 cells in vitro and in vivo. Neoplasma. 2012;59:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 109. | Nowakowski J, Cronin CN, McRee DE, Knuth MW, Nelson CG, Pavletich NP, Rogers J, Sang BC, Scheibe DN, Swanson RV, Thompson DA. Structures of the cancer-related Aurora-A, FAK, and EphA2 protein kinases from nanovolume crystallography. Structure. 2002;10:1659-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 110. | Kamoun WS, Dugast AS, Suchy JJ, Grabow S, Fulton RB, Sampson JF, Luus L, Santiago M, Koshkaryev A, Sun G, Askoxylakis V, Tam E, Huang ZR, Drummond DC, Sawyer AJ. Synergy between EphA2-ILs-DTXp, a Novel EphA2-Targeted Nanoliposomal Taxane, and PD-1 Inhibitors in Preclinical Tumor Models. Mol Cancer Ther. 2020;19:270-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 111. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 112. | Annamalai B, Liu X, Gopal U, Isaacs JS. Hsp90 is an essential regulator of EphA2 receptor stability and signaling: implications for cancer cell migration and metastasis. Mol Cancer Res. 2009;7:1021-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 113. | Chang Q, Jorgensen C, Pawson T, Hedley DW. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer. 2008;99:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 114. | Kamat AA, Coffey D, Merritt WM, Nugent E, Urbauer D, Lin YG, Edwards C, Broaddus R, Coleman RL, Sood AK. EphA2 overexpression is associated with lack of hormone receptor expression and poor outcome in endometrial cancer. Cancer. 2009;115:2684-2692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 115. | O'Malley Y, Lal G, Howe JR, Weigel RJ, Komorowski RA, Shilyansky J, Sugg SL. Invasion in follicular thyroid cancer cell lines is mediated by EphA2 and pAkt. Surgery. 2012;152:1218-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Tandon M, Vemula SV, Sharma A, Ahi YS, Mittal S, Bangari DS, Mittal SK. EphrinA1-EphA2 interaction-mediated apoptosis and FMS-like tyrosine kinase 3 receptor ligand-induced immunotherapy inhibit tumor growth in a breast cancer mouse model. J Gene Med. 2012;14:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 117. | Song W, Hwang Y, Youngblood VM, Cook RS, Balko JM, Chen J, Brantley-Sieders DM. Targeting EphA2 impairs cell cycle progression and growth of basal-like/triple-negative breast cancers. Oncogene. 2017;36:5620-5630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 118. | Petty A, Myshkin E, Qin H, Guo H, Miao H, Tochtrop GP, Hsieh JT, Page P, Liu L, Lindner DJ, Acharya C, MacKerell AD Jr, Ficker E, Song J, Wang B. A small molecule agonist of EphA2 receptor tyrosine kinase inhibits tumor cell migration in vitro and prostate cancer metastasis in vivo. PLoS One. 2012;7:e42120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 119. | Brannan JM, Dong W, Prudkin L, Behrens C, Lotan R, Bekele BN, Wistuba I, Johnson FM. Expression of the receptor tyrosine kinase EphA2 is increased in smokers and predicts poor survival in non-small cell lung cancer. Clin Cancer Res. 2009;15:4423-4430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 120. | Li X, Wang F, Huang L, Yang M, Kuang E. Downregulation of EphA2 stability by RNF5 limits its tumor-suppressive function in HER2-negative breast cancers. Cell Death Dis. 2023;14:662. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 121. | Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. IJB. 2024;10:1256. [DOI] [Full Text] |
| 122. | Li W, Yang X, Bai T, Xu J, Qian Z, Li Y, Guo Z, Zhu Y. Detection of serum EphA2-EVs for pancreatic cancer diagnosis by light initiated chemiluminescent assay. Anal Methods. 2022;14:1335-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 123. | Du J, He Y, Wu W, Li P, Chen Y, Hu Z, Han Y. Targeting EphA2 with miR-124 mediates Erlotinib resistance in K-RAS mutated pancreatic cancer. J Pharm Pharmacol. 2019;71:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 124. | Fan J, Wei Q, Koay EJ, Liu Y, Ning B, Bernard PW, Zhang N, Han H, Katz MH, Zhao Z, Hu Y. Chemoresistance Transmission via Exosome-Mediated EphA2 Transfer in Pancreatic Cancer. Theranostics. 2018;8:5986-5994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 125. | Chen YH, Lv H, Shen N, Wang XM, Tang S, Xiong B, Ding J, Geng MY, Huang M. EPHA2 feedback activation limits the response to PDEδ inhibition in KRAS-dependent cancer cells. Acta Pharmacol Sin. 2020;41:270-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 126. | Cui S, Wu Q, Liu M, Su M, Liu S, Shao L, Han X, He H. EphA2 super-enhancer promotes tumor progression by recruiting FOSL2 and TCF7L2 to activate the target gene EphA2. Cell Death Dis. 2021;12:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 127. | Koizumi M, Sato S, Yoshihara M, Nakamura Y, Terao H, Okubo Y, Washimi K, Yoshioka E, Yokose T, Kishida T, Koshikawa N, Miyagi Y. Chronological Change in EPHA2 Protein Expression Is Associated With Recurrence of Bladder Cancer. Anticancer Res. 2022;42:5783-5794. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 128. | Liu Z, Tao Z, Zhang Q, Wan S, Zhang F, Zhang Y, Wu G, Wang J. YSA-conjugated mesoporous silica nanoparticles effectively target EphA2-overexpressing breast cancer cells. Cancer Chemother Pharmacol. 2018;81:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 129. | Gong S, Li Y, Lv L, Men W. Restored microRNA-519a enhances the radiosensitivity of non-small cell lung cancer via suppressing EphA2. Gene Ther. 2022;29:588-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 130. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 131. | Gan HK, Parakh S, Lee FT, Tebbutt NC, Ameratunga M, Lee ST, O'Keefe GJ, Gong SJ, Vanrenen C, Caine J, Giovannetti M, Murone C, Scott FE, Guo N, Burvenich IJG, Paine C, Macri MJ, Kotsuma M, Senaldi G, Venhaus R, Scott AM. A phase 1 safety and bioimaging trial of antibody DS-8895a against EphA2 in patients with advanced or metastatic EphA2 positive cancers. Invest New Drugs. 2022;40:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 132. | Sugiyama N, Gucciardo E, Tatti O, Varjosalo M, Hyytiäinen M, Gstaiger M, Lehti K. EphA2 cleavage by MT1-MMP triggers single cancer cell invasion via homotypic cell repulsion. J Cell Biol. 2013;201:467-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 133. | Amato KR, Wang S, Tan L, Hastings AK, Song W, Lovly CM, Meador CB, Ye F, Lu P, Balko JM, Colvin DC, Cates JM, Pao W, Gray NS, Chen J. EPHA2 Blockade Overcomes Acquired Resistance to EGFR Kinase Inhibitors in Lung Cancer. Cancer Res. 2016;76:305-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 134. | Ishigaki H, Minami T, Morimura O, Kitai H, Horio D, Koda Y, Fujimoto E, Negi Y, Nakajima Y, Niki M, Kanemura S, Shibata E, Mikami K, Takahashi R, Yokoi T, Kuribayashi K, Kijima T. EphA2 inhibition suppresses proliferation of small-cell lung cancer cells through inducing cell cycle arrest. Biochem Biophys Res Commun. 2019;519:846-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 135. | Rezaie E, Amani J, Bidmeshki Pour A, Mahmoodzadeh Hosseini H. A new scfv-based recombinant immunotoxin against EPHA2-overexpressing breast cancer cells; High in vitro anti-cancer potency. Eur J Pharmacol. 2020;870:172912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 136. | Wen Q, Chen Z, Chen Z, Chen J, Wang R, Huang C, Yuan W. EphA2 affects the sensitivity of oxaliplatin by inducing EMT in oxaliplatin-resistant gastric cancer cells. Oncotarget. 2017;8:47998-48011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 137. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 138. | Liu F, Park PJ, Lai W, Maher E, Chakravarti A, Durso L, Jiang X, Yu Y, Brosius A, Thomas M, Chin L, Brennan C, DePinho RA, Kohane I, Carroll RS, Black PM, Johnson MD. A genome-wide screen reveals functional gene clusters in the cancer genome and identifies EphA2 as a mitogen in glioblastoma. Cancer Res. 2006;66:10815-10823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 139. | Quinn BA, Wang S, Barile E, Das SK, Emdad L, Sarkar D, De SK, Morvaridi SK, Stebbins JL, Pandol SJ, Fisher PB, Pellecchia M. Therapy of pancreatic cancer via an EphA2 receptor-targeted delivery of gemcitabine. Oncotarget. 2016;7:17103-17110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 140. | Liu B, Sun W, Gao W, Li L, Cao Z, Yang X, Liu J, Guo Y. microRNA-451a promoter methylation regulated by DNMT3B expedites bladder cancer development via the EPHA2/PI3K/AKT axis. BMC Cancer. 2020;20:1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 141. | Huang J, Xiao D, Li G, Ma J, Chen P, Yuan W, Hou F, Ge J, Zhong M, Tang Y, Xia X, Chen Z. EphA2 promotes epithelial-mesenchymal transition through the Wnt/β-catenin pathway in gastric cancer cells. Oncogene. 2014;33:2737-2747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 142. | Wang S, Noberini R, Stebbins JL, Das S, Zhang Z, Wu B, Mitra S, Billet S, Fernandez A, Bhowmick NA, Kitada S, Pasquale EB, Fisher PB, Pellecchia M. Targeted delivery of paclitaxel to EphA2-expressing cancer cells. Clin Cancer Res. 2013;19:128-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 143. | Patel AR, Chougule M, Singh M. EphA2 targeting pegylated nanocarrier drug delivery system for treatment of lung cancer. Pharm Res. 2014;31:2796-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 144. | Song W, Kim LC, Han W, Hou Y, Edwards DN, Wang S, Blackwell TS, Cheng F, Brantley-Sieders DM, Chen J. Phosphorylation of PLCγ1 by EphA2 Receptor Tyrosine Kinase Promotes Tumor Growth in Lung Cancer. Mol Cancer Res. 2020;18:1735-1743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 145. | Jukonen J, Moyano-Galceran L, Höpfner K, Pietilä EA, Lehtinen L, Huhtinen K, Gucciardo E, Hynninen J, Hietanen S, Grénman S, Ojala PM, Carpén O, Lehti K. Aggressive and recurrent ovarian cancers upregulate ephrinA5, a non-canonical effector of EphA2 signaling duality. Sci Rep. 2021;11:8856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 146. | Nakamura R, Kataoka H, Sato N, Kanamori M, Ihara M, Igarashi H, Ravshanov S, Wang YJ, Li ZY, Shimamura T, Kobayashi T, Konno H, Shinmura K, Tanaka M, Sugimura H. EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci. 2005;96:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 147. | Kurose H, Ueda K, Kondo R, Ogasawara S, Kusano H, Sanada S, Naito Y, Nakiri M, Nishihara K, Kakuma T, Akiba J, Igawa T, Yano H. Elevated Expression of EPHA2 Is Associated With Poor Prognosis After Radical Prostatectomy in Prostate Cancer. Anticancer Res. 2019;39:6249-6257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 148. | Xu Y, Fu L, Pan D, Wei J, Xia H, Wang S, Sun G. Folic Acid Inhibited Vasculogenic Mimicry in Esophageal Cancer Cell Line Eca-109, the One Target Was EphA2. Nutr Cancer. 2022;74:2235-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 149. | Zhang T, Li J, Ma X, Yang Y, Sun W, Jin W, Wang L, He Y, Yang F, Yi Z, Hua Y, Liu M, Chen Y, Cai Z. Inhibition of HDACs-EphA2 Signaling Axis with WW437 Demonstrates Promising Preclinical Antitumor Activity in Breast Cancer. EBioMedicine. 2018;31:276-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 150. | Conejo-Garcia JR. Breaking barriers for T cells by targeting the EPHA2/TGF-β/COX-2 axis in pancreatic cancer. J Clin Invest. 2019;129:3521-3523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 151. | Lohmüller T, Xu Q, Groves JT. Nanoscale obstacle arrays frustrate transport of EphA2-Ephrin-A1 clusters in cancer cell lines. Nano Lett. 2013;13:3059-3064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 152. | Walker-Daniels J, Coffman K, Azimi M, Rhim JS, Bostwick DG, Snyder P, Kerns BJ, Waters DJ, Kinch MS. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275-280. [PubMed] [DOI] [Full Text] |
| 153. | Lin YG, Han LY, Kamat AA, Merritt WM, Landen CN, Deavers MT, Fletcher MS, Urbauer DL, Kinch MS, Sood AK. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer. 2007;109:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 154. | Huang J, He Y, Mcleod HL, Xie Y, Xiao D, Hu H, Chen P, Shen L, Zeng S, Yin X, Ge J, Li L, Tang L, Ma J, Chen Z. miR-302b inhibits tumorigenesis by targeting EphA2 via Wnt/ β-catenin/EMT signaling cascade in gastric cancer. BMC Cancer. 2017;17:886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 155. | Koshikawa N, Minegishi T, Kiyokawa H, Seiki M. Specific detection of soluble EphA2 fragments in blood as a new biomarker for pancreatic cancer. Cell Death Dis. 2017;8:e3134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 156. | Yu L, Chen D, Song J. LncRNA SNHG16 promotes non-small cell lung cancer development through regulating EphA2 expression by sponging miR-520a-3p. Thorac Cancer. 2020;11:603-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 157. | Kinch MS, Moore MB, Harpole DH Jr. Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9:613-618. [PubMed] |
| 158. | Allocca C, Cirafici AM, Laukkanen MO, Castellone MD. Serine 897 Phosphorylation of EPHA2 Is Involved in Signaling of Oncogenic ERK1/2 Drivers in Thyroid Cancer Cells. Thyroid. 2021;31:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 159. | Noblitt LW, Bangari DS, Shukla S, Mohammed S, Mittal SK. Immunocompetent mouse model of breast cancer for preclinical testing of EphA2-targeted therapy. Cancer Gene Ther. 2005;12:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 160. | Guo JQ, Zheng QH, Chen H, Chen L, Xu JB, Chen MY, Lu D, Wang ZH, Tong HF, Lin S. Ginsenoside Rg3 inhibition of vasculogenic mimicry in pancreatic cancer through downregulation of VEcadherin/EphA2/MMP9/MMP2 expression. Int J Oncol. 2014;45:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 161. | Lu C, Shahzad MM, Wang H, Landen CN, Kim SW, Allen J, Nick AM, Jennings N, Kinch MS, Bar-Eli M, Sood AK. EphA2 overexpression promotes ovarian cancer growth. Cancer Biol Ther. 2008;7:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 162. | Larsen AB, Pedersen MW, Stockhausen MT, Grandal MV, van Deurs B, Poulsen HS. Activation of the EGFR gene target EphA2 inhibits epidermal growth factor-induced cancer cell motility. Mol Cancer Res. 2007;5:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 163. | Giorgio C, Mena P, Del Rio D, Brighenti F, Barocelli E, Hassan-Mohamed I, Callegari D, Lodola A, Tognolini M. The ellagitannin colonic metabolite urolithin D selectively inhibits EphA2 phosphorylation in prostate cancer cells. Mol Nutr Food Res. 2015;59:2155-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 164. | Torres-Adorno AM, Vitrac H, Qi Y, Tan L, Levental KR, Fan YY, Yang P, Chapkin RS, Eckhardt BL, Ueno NT. Eicosapentaenoic acid in combination with EPHA2 inhibition shows efficacy in preclinical models of triple-negative breast cancer by disrupting cellular cholesterol efflux. Oncogene. 2019;38:2135-2150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 165. | Giannoni E, Taddei ML, Parri M, Bianchini F, Santosuosso M, Grifantini R, Fibbi G, Mazzanti B, Calorini L, Chiarugi P. EphA2-mediated mesenchymal-amoeboid transition induced by endothelial progenitor cells enhances metastatic spread due to cancer-associated fibroblasts. J Mol Med (Berl). 2013;91:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 166. | Jannu AK, Puppala ER, Gawali B, Syamprasad NP, Alexander A, Marepally S, Chella N, Gangasani JK, Naidu VGM. Lithocholic acid-tryptophan conjugate (UniPR126) based mixed micelle as a nano carrier for specific delivery of niclosamide to prostate cancer via EphA2 receptor. Int J Pharm. 2021;605:120819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 167. | Xiang YP, Xiao T, Li QG, Lu SS, Zhu W, Liu YY, Qiu JY, Song ZH, Huang W, Yi H, Tang YY, Xiao ZQ. Y772 phosphorylation of EphA2 is responsible for EphA2-dependent NPC nasopharyngeal carcinoma growth by Shp2/Erk-1/2 signaling pathway. Cell Death Dis. 2020;11:709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 168. | Meade-Tollin L, Martinez JD. Loss of p53 and overexpression of EphA2 predict poor prognosis for ovarian cancer patients. Cancer Biol Ther. 2007;6:288-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 169. | Tognolini M, Giorgio C, Hassan Mohamed I, Barocelli E, Calani L, Reynaud E, Dangles O, Borges G, Crozier A, Brighenti F, Del Rio D. Perturbation of the EphA2-EphrinA1 system in human prostate cancer cells by colonic (poly)phenol catabolites. J Agric Food Chem. 2012;60:8877-8884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 170. | Foveau B, Boulay G, Pinte S, Van Rechem C, Rood BR, Leprince D. The receptor tyrosine kinase EphA2 is a direct target gene of hypermethylated in cancer 1 (HIC1). J Biol Chem. 2012;287:5366-5378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 171. | Noblitt LW, Bangari DS, Shukla S, Knapp DW, Mohammed S, Kinch MS, Mittal SK. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther. 2004;11:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 172. | Tawadros T, Brown MD, Hart CA, Clarke NW. Ligand-independent activation of EphA2 by arachidonic acid induces metastasis-like behaviour in prostate cancer cells. Br J Cancer. 2012;107:1737-1744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 173. | Yamaguchi S, Tatsumi T, Takehara T, Sakamori R, Uemura A, Mizushima T, Ohkawa K, Storkus WJ, Hayashi N. Immunotherapy of murine colon cancer using receptor tyrosine kinase EphA2-derived peptide-pulsed dendritic cell vaccines. Cancer. 2007;110:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 174. | Bekaii-Saab TS, Raghav K, Wainberg ZA. How quality of life can inform management decisions in later-line metastatic colorectal cancer: Q&A. Clin Adv Hematol Oncol. 2021;19 Suppl 22:13-14. [PubMed] |
| 175. | Katayama S, Shiota G, Oshimura M, Kawasaki H. Clinical usefulness of telomerase activity and telomere length in the preoperative diagnosis of gastric and colorectal cancer. J Cancer Res Clin Oncol. 1999;125:405-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 176. | Saidi HS, Karuri D, Nyaim EO. Correlation of clinical data, anatomical site and disease stage in colorectal cancer. East Afr Med J. 2008;85:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 177. | Chen P, Wang HJ, Zhu L, Jia R, Jian HY. [Factors affecting quality of life of patients with gastrointestinal cancer]. Ai Zheng. 2007;26:1116-1121. [PubMed] |
| 178. | Gastric Cancer Group; Oncology Branch; Chinese Medical Association; Colorectal Cancer Professional Committee, Chinese Medical Doctor Association; Colorectal Cancer Professional Committee, Chinese Anti-Cancer Association; Gastric Cancer Professional Committee, Chinese Anti-Cancer Association; Digestive Tract Polyp and Precancerous Lesion Professional Committee, Chinese Anti-Cancer Association. [Chinese expert consensus on the application of circulating tumor cell detection in the diagnosis and treatment of gastrointestinal neoplasms (2023 edition)]. Zhonghua Wei Chang Wai Ke Za Zhi. 2023;26:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 179. | Chen XZ, Hu JK, Zhou ZG. Importance of organized screening and surveillance for colorectal cancer in China: epidemiological differences from Europe. Eur J Cancer Prev. 2015;24:459-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 180. | Boland CR. Molecular basis for stool-based DNA tests for colorectal cancer: a primer for clinicians. Rev Gastroenterol Disord. 2002;2 Suppl 1:S12-S19. [PubMed] |
| 181. | Lévy J, Romagnolo B. [Antitumoral functions of autophagy inhibition in colorectal cancer: the intestinal microbiota and the immune system come to the rescue…]. Med Sci (Paris). 2016;32:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 182. | Briggman J, Genduso R, Camara C, Healy B, Shapiro K, Roos R, Merrifield S, Lifter J, Wu YJ, Elder E, Talamonti M. NuMA: evaluation of a new biomarker for the detection of low stage colorectal cancer. Anticancer Res. 1999;19:2411-2414. [PubMed] |
| 183. | McGill SK, Kaltenbach T, Friedland S, Soetikno R. The learning curve for detection of non-polypoid (flat and depressed) colorectal neoplasms. Gut. 2015;64:184-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 184. | Bini EJ, Rajapaksa RC, Weinshel EH. The findings and impact of nonrehydrated guaiac examination of the rectum (FINGER) study: a comparison of 2 methods of screening for colorectal cancer in asymptomatic average-risk patients. Arch Intern Med. 1999;159:2022-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 185. | Durrant LG, Jones H, Armitage NC, Baldwin RW. Development of an ELISA to detect early local relapse of colorectal cancer. Br J Cancer. 1989;60:533-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 186. | Wauters H, Van Casteren V, Buntinx F. Rectal bleeding and colorectal cancer in general practice: diagnostic study. BMJ. 2000;321:998-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 187. | Mai M, Omote K, Minamoto T, Fujioka N, Yasumoto K, Dong HC, Takahashi Y. [Controversy between endoscopic and surgical treatment against early gastric and colorectal cancer]. Nihon Geka Gakkai Zasshi. 1992;93:1075-1078. [PubMed] |
| 188. | Aste H, Picasso M, Allegretti A, Conio M. Correlation between family history of colorectal cancer and pathological and clinical features of the disease. Cancer Detect Prev. 1994;18:343-348. [PubMed] |