Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3723
Revised: June 3, 2024
Accepted: June 17, 2024
Published online: August 15, 2024
Processing time: 114 Days and 3.3 Hours
Sarcomatoid carcinoma (SCA) of the jejunum is a rare and aggressive neoplasm affecting the smooth muscle cells of the jejunum. This study presents a recent case of jejunal SCA, detailing its diagnosis and treatment, thereby providing a re
A 65-year-old male presented to Yichang Central People's Hospital with a chief complaint of hemorrhoids. A computed tomography (CT) scan incidentally revealed multiple abnormal signals in the liver. Subsequent positron emission tomography/CT at Wuhan Union Hospital indicated malignant tumor pro
This case suggests that chemotherapy, immunotherapy, plus targeted therapy may represent an optimal treatment for intestinal SCA, meriting further investigation.
Core Tip: Sarcomatoid carcinoma (SCA) of the jejunum is a rare and aggressive malignancy. Currently, there are no established treatment guidelines for SCA. This study reports a patient who achieved favorable outcomes with a combination of immunotherapy, targeted therapy, and chemotherapy. A review of the literature was also conducted. We propose that this therapeutic approach may represent a novel treatment option for jejunal SCA.
- Citation: Feng Q, Yu W, Feng JH, Huang Q, Xiao GX. Jejunal sarcomatoid carcinoma: A case report and review of literature. World J Gastrointest Oncol 2024; 16(8): 3723-3731
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3723.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3723
Sarcomatoid carcinoma (SCA) is a rare neoplasm characterized by both malignant epithelial and mesenchymal components[1]. First described by Virchow in 1864[2], the majority of malignant tumors in the small intestine are either adenocarcinomas or neuroendocrine tumors. Primary SCA of the small intestine is particularly uncommon, typically manifesting in the ileum, jejunum, and duodenum. The incidence is approximately 0.5-0.8 cases per 100000 individuals annually[3], with a median age of onset at 57 years, predominantly affecting middle-aged and elderly men[4,5]. Clinical presentation of small intestine SCA is often nonspecific, with common symptoms including abdominal pain, anemia, and bowel obstruction. The tumor usually presents with a polypoid or fungating appearance, occasionally exhibiting ulceration, but frequently accompanied by necrosis and hemorrhage. Computed tomography (CT) scans generally reveal circumferential thickening of the bowel wall or intraluminal rounded soft tissue masses, which may extend to adjacent mesenteric lymph nodes and surrounding tissue. Enhanced CT scans typically show moderate and heterogeneous enhancement. Secondary complications such as bowel obstruction, intussusception, small bowel dilation, and intestinal fistula can also occur. Pathomorphological analysis and immunohistochemical staining remain the gold standards for diagnosing small intestine SCA. Radical surgery is the preferred treatment modality for early-stage disease. Previous studies have indicated that small intestine SCA is largely unresponsive to chemotherapy and radiotherapy, which are reserved for palliative care. Recent research has demonstrated high expression of programmed death-ligand 1 (PD-L1) in small intestine SCA cells[4,5]. Consequently, immunotherapy targeting the PD-L1 pathway may offer potential therapeutic benefits. This article reports on a recent case of small intestine SCA admitted to our hospital. Clinical data are collected and reviewed alongside the relevant literature to enhance clinicians' understanding of this rare disease.
A 65-year-old Chinese man was admitted to our hospital for a hemorrhoidectomy. During a preoperative CT scan, multiple abnormal signals were detected in his liver.
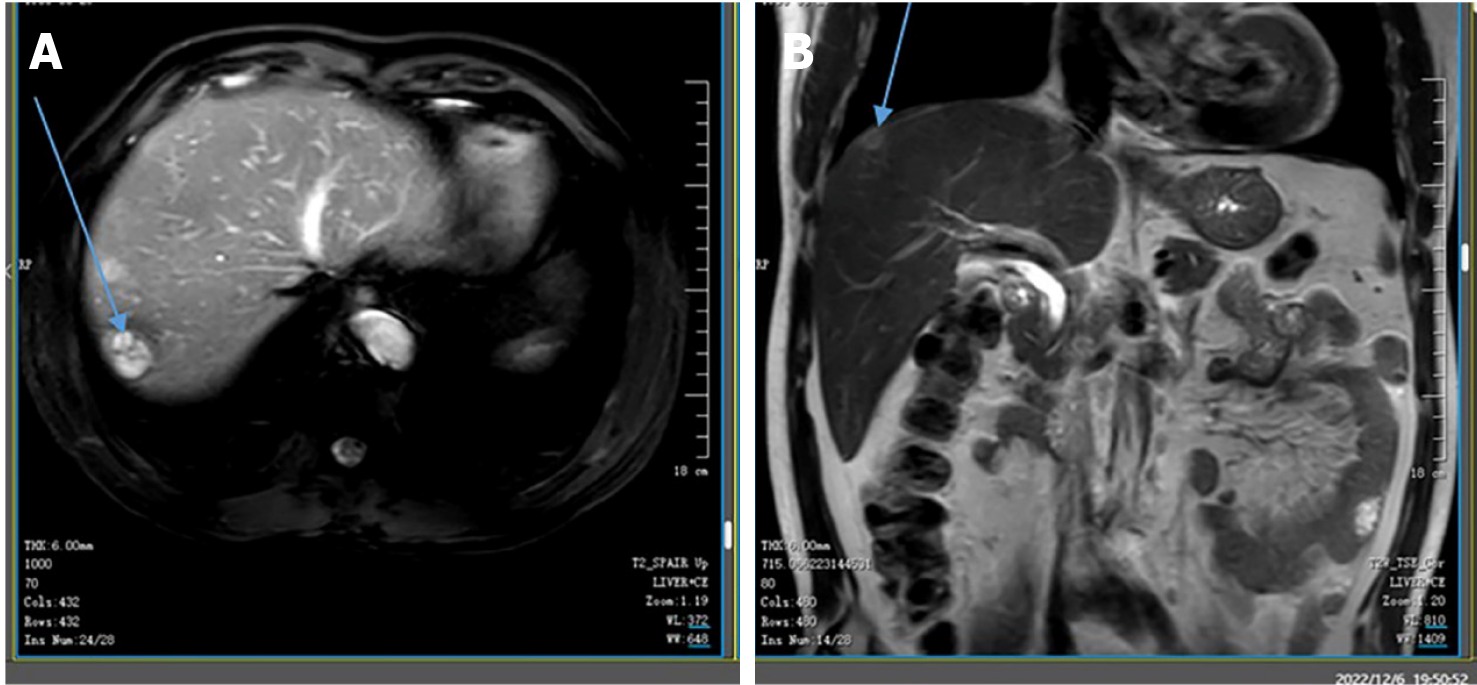
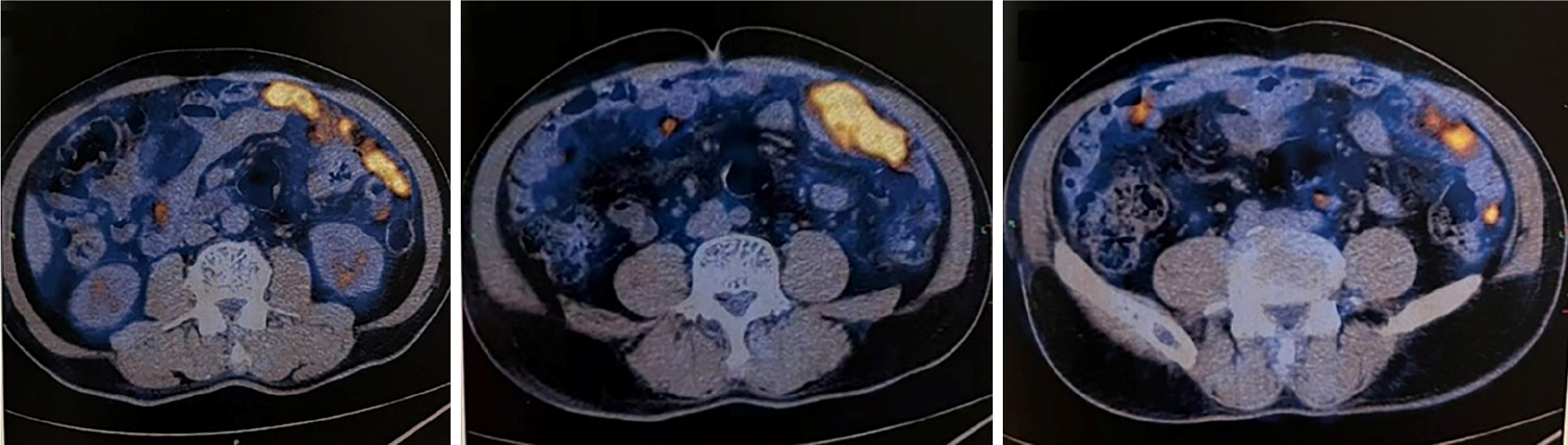
The patient, with no prior medical history, was scheduled for a hemorrhoidectomy at our hospital. A preoperative CT scan revealed multiple abnormal signals in his liver. Subsequent magnetic resonance imaging (MRI) at our hospital demonstrated numerous low-density circular lesions of varying sizes and heterogeneous signals within the liver (Figure 1). The largest lesion, measuring 2.1 cm × 2.0 cm, was located in the right posterior lobe. A positron emission tomography (PET)/CT scan conducted at Wuhan Union Hospital in March 2022 indicated increased metabolic activity in various regions of the intestinal wall, mesentery, omentum, and peritoneum, suggestive of a malignant tumor [maximum standardized uptake value (SUVmax): 16.3] (Figure 2). Exploratory surgery and biopsy revealed a large polypoid tumor in the omentum, measuring 5 cm × 4 cm. This tumor exhibited invasive growth through the muscularis propria into the subserosal soft tissue. Lymphovascular invasion was also observed. Pathological analysis suggested the diagnosis of SCA.
The patient has a documented history of hepatitis B, for which he is currently receiving entecavir therapy. Additionally, he has had hypertension for over a decade and is being treated with metoprolol; however, his blood pressure remains suboptimally controlled.
The patient denied any family history of malignant tumors.
On physical examination, the vital signs were as follows: Body temperature, 36.4 °C; blood pressure, 138/105 mmHg; heart rate, 84 beats per minute; and respiratory rate, 18 breaths per minute.
Serum tumor markers were within normal limits (carcinoembryonic antigen, 1.6 ng/mL; carbohydrate antigen 19-9, 4.7 U/mL; alpha-fetoprotein, 4.5 ng/mL). Routine blood and urine analyses revealed no abnormalities.
A preoperative CT scan revealed multiple abnormal signals in the liver. MRI of the liver performed at our hospital identified numerous low-density circular lesions of varying sizes and heterogeneous signals within the liver parenchyma (Figure 1); the largest lesion, located in the right posterior lobe, measured 2.1 cm × 2.0 cm. A PET/CT scan conducted at Wuhan Union Hospital in March 2022 demonstrated increased metabolic activity in various regions of the intestinal wall, mesentery, omentum, and peritoneum, suggesting a potential malignant tumor (SUV max: 16.3) (Figure 2).
Exploratory surgery and biopsy revealed a large polypoid tumor in the omentum, measuring 5 cm × 4 cm, which had invaded through the muscularis propria into the subserosal soft tissue. Lymphovascular invasion was also observed. Pathological analysis suggested a diagnosis of SCA.
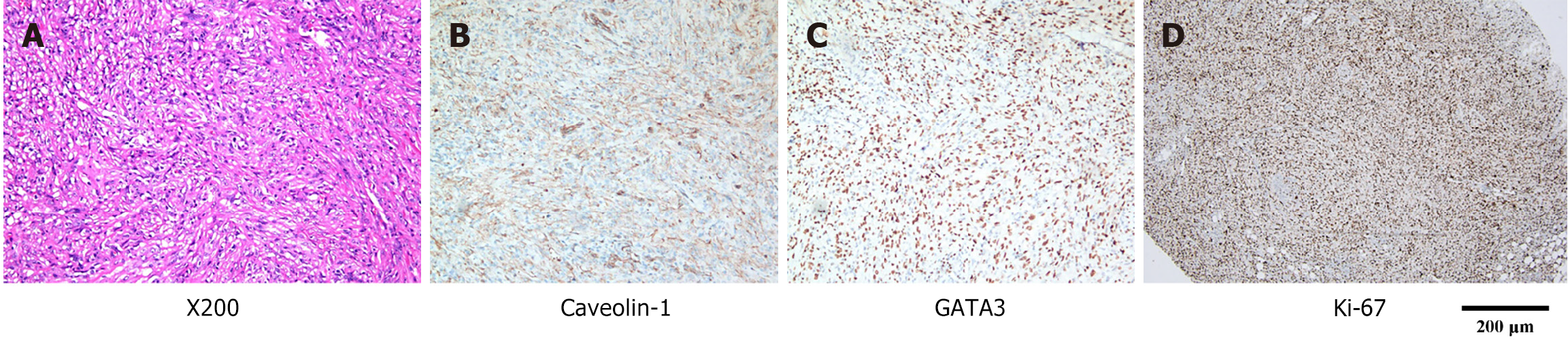
Immunohistochemical staining confirmed the presence of tumor cells in the surgical specimen. The postoperative pathology analysis results consistent with SCA were as follows: PCK (+), CK8/18 (+), CK7 (+), GATA-3 (+), EMA (-), caveolin-1 (weakly positive), calretinin (-), WT1 (-), D2-40 (-), CK5/6 (-), HMHE-1 (-), BAP1 (±), P16 (-), P40 (-), CD34 (-), CD117 (-), DDG-1 (-), SDHB (+), SMA (-), desmin (-), S100 (-), SOX10 (-), HMB45 (-), CD31 (-), ERG (-), E-cadherin (-), ALK (-), CK19 (-), villin (-), CDX2 (-), CEA (-), β-catenin (partially positive), INI-1 (no expression deficiencies detected), and Ki67 (LI > 70%) (Figure 3). Fluorescence in situ hybridization testing indicated a deletion at the cyclin-dependent kinase inhibitor 2A locus on chromosome 9; however, malignant mesothelioma was excluded. The p16 gene deletion test was negative.
Based on the patient's medical history and the diagnostic findings, a diagnosis of jejunal SCA was confirmed.
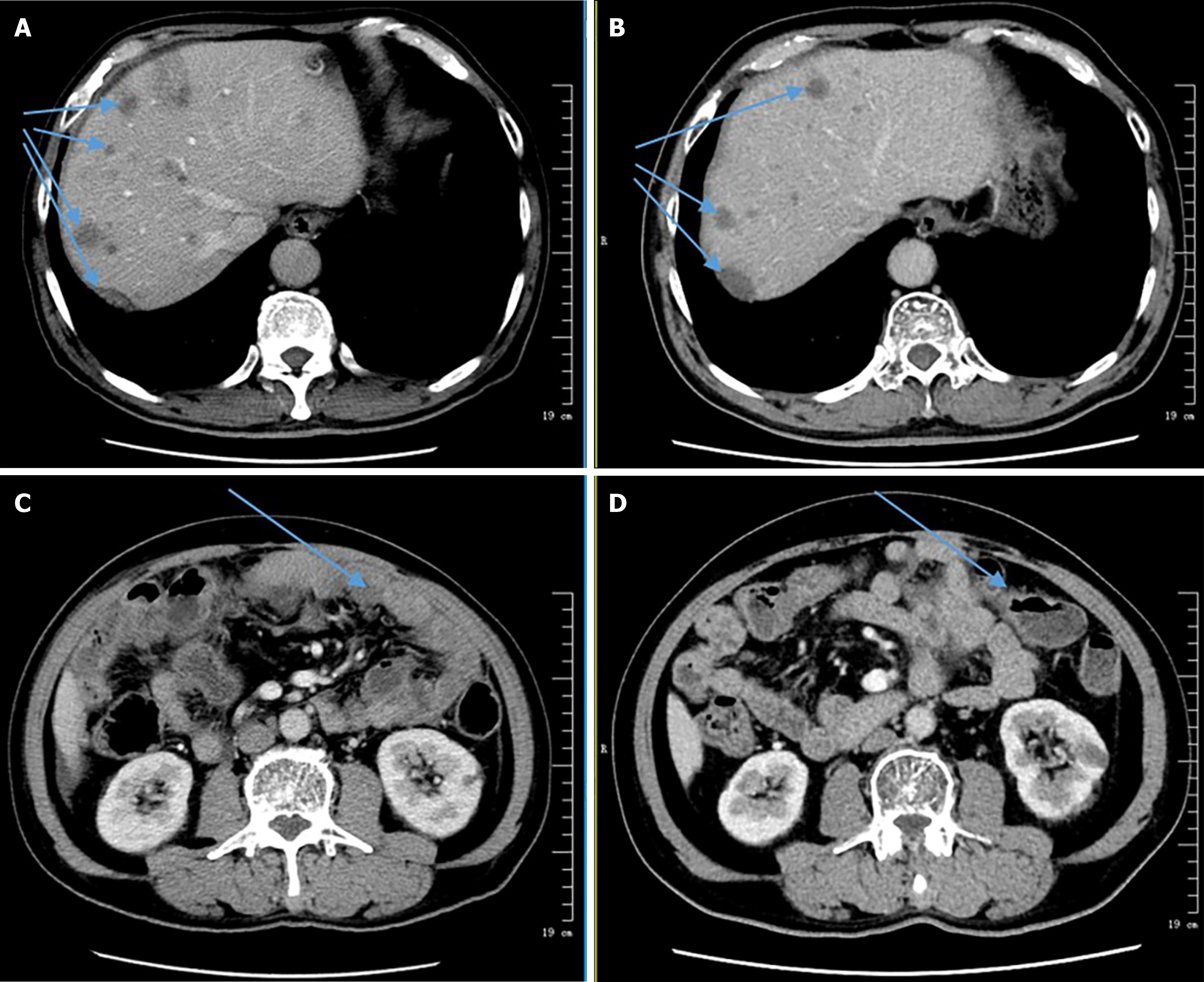
The patient accepted the treatment regimen at our hospital. However, he was unable to complete PD-L1 gene testing due to financial constraints. With informed consent from the patient and his family, he commenced treatment with a combination of albumin-bound paclitaxel [200 mg (125 mg/m²) on day 1] and sintilimab (200 mg on day 1), administered in 21-day cycles. He completed four cycles in total. During treatment, the patient developed significant abdominal ascites, requiring daily drainage of 1000-1500 mL for one week. Cytological analysis of the ascitic fluid showed no malignant cells. The ascites was attributed to abdominal metastases and hypoalbuminemia (serum albumin: 25 g/L). After the first cycle, a CT scan showed no abdominal fluid and a slight enlargement of liver lesions, indicating stable disease. Following the third cycle, CT imaging revealed a reduction in liver nodules and abdominal lesions compared to previous scans. The patient's abdominal fluid and tumor burden was well-controlled, although a complete response was not achieved. Subsequently, the patient underwent three additional cycles of the same treatment regimen, combined with targeted therapy using anlotinib [12 mg orally once daily (days 1–14, 21 days per cycle)]. A CT scan following this treatment indicated a significant reduction in the size of the liver nodule compared to prior imaging (Figure 4), demonstrating a partial response. The patient continued maintenance therapy with anlotinib and sintilimab for an extended period. Unfortunately, due to financial difficulties, the patient discontinued treatment but remained stable for over one-year post-surgery.
The patient remained stable for over one year following surgery.
Primary small intestinal epithelial tumors constitute a mere 2% of all gastrointestinal malignancies, making clinical cases of small intestinal epithelial neoplasms rare[3]. SCA predominantly affects middle-aged and elderly men. Clinical symptoms are often nonspecific and may include abdominal pain, anemia, bowel obstruction, vomiting, and weight loss. The diagnosis of small intestinal SCA cannot rely solely on hematoxylin and eosin staining; a comprehensive assessment incorporating immunohistochemical examination of multiple biomarkers is essential. Due to its nonspecific clinical presentation, small intestinal SCA is frequently misdiagnosed. Differential diagnosis should consider epithelioid angiosarcoma, smooth muscle sarcoma, gastrointestinal stromal tumor, lymphoma, and melanoma. The risk factors for small intestinal SCA remain unclear, though previous studies suggest a potential association with long-term regional intestinal inflammation[6]. The prognosis for this disease is extremely poor, with most patients succumbing to metastasis and recurrence. The 5-year survival rate is essentially 0%, and the median survival time following diagnosis is only 8.25 months[7]. Therefore, small intestinal SCA represents a rare, easily misdiagnosed, and challenging-to-treat malignant tumor.
Currently, no standard treatment guidelines exist for SCA. Surgery is generally recognized as the preferred treatment for early-stage patients. Chemotherapy is utilized for recurrent and metastatic cases, although outcomes are often suboptimal[8]. Earlier studies[8] demonstrated no significant difference in survival rates between patients treated with chemotherapy and those who did not receive it. Radiation therapy is primarily limited to palliative care (Table 1). In 2015, the Marshall University School of Medicine reported a case involving a patient with small bowel SCA and lung metastases who declined surgery. The patient initially received oxaliplatin plus capecitabine for four months, but the disease progressed. Subsequently, the treatment regimen was switched to irinotecan plus capecitabine for four cycles, yet the lung and mediastinal lesions continued to advance[9]. In 2017, the Hofstra School of Medicine reported a case of a 58-year-old woman with a sarcomatoid tumor of the terminal ileum causing small bowel obstruction. A CT scan of the abdomen and pelvis upon admission showed no evidence of metastatic disease. The patient underwent a robotic-assisted right hemicolectomy with resection of the terminal ileum and ileocolic anastomosis. She was followed up for six months and recovered well[10]. The MD Anderson Cancer Center reported a case involving a patient with small bowel SCA who underwent distal pancreatectomy, splenectomy, right hepatectomy, subtotal gastrectomy, and cholecystectomy. Pathological examination revealed a high-grade sarcomatoid malignancy involving the pancreas, liver, adrenal gland, and gastric wall. One month later, a PET-CT scan detected abdominal retroperitoneal nodules, indicating disease progression. The patient was treated with four cycles of a gemcitabine plus docetaxel regimen, achieving stable disease. Subsequently, the patient received the FOLFIRINOX regimen (oxaliplatin 85 mg/m², irinotecan 180 mg/m², leucovorin 400 mg/m², and fluorouracil 2400 mg/m² intravenously every 14 days for six cycles), which resulted in progressive disease. Following this, the patient was treated with a combination of docetaxel, cyclophosphamide, and bevacizumab, but the disease continued to worsen, with new liver metastases developing. The patient succumbed to the disease 12 months after the initial diagnosis[11]. In 2021, Hiroshima University in Japan reported a case of small intestinal SCA with mesenteric lymph node metastasis, abdominal metastasis, and retroperitoneal lymph node metastasis. The patient did not receive additional adjuvant therapy post-surgery and died 15 months after the operation[12]. Previous case reports have indicated that treatments involving moxifloxacin, dacarbazine, cyclophosphamide, gemcitabine, docetaxel, paclitaxel, and platinum compounds did not yield satisfactory outcomes[11,13,14]. In conclusion, the data suggest that surgery is optimal for early-stage SCA patients. However, due to the occult nature of the disease, most patients are diagnosed with lymph node or distant metastases, rendering chemotherapy insufficient. Thus, current chemotherapeutic drugs demonstrate low efficacy for SCA, highlighting the need for exploring alternative treatment options.
| Age/sex | Diagnosis | Tumor Site | No. of lesion (s) | Maximal diameter (cm) | Morphology | Metastasis | Treatment method | OS (months) | Ref. |
| 56/F | SCA | Jejunum | 1 | 6.7 | Ulcerating | Yes | Operation | 6 | [41] |
| 62/M | SCA | Lleum | 1 | 15 | Ulcerating | No | Operation + chemotherapy | 3 | [42] |
| 54/M | SCA | Jejunum | NA | 2.6 | Polypoid | No | Operation | 3 | [18] |
| 56/M | SCA | Jejunum | NA | 14 | Polypoid | Yes | Chemotherapy | 41 | [9] |
| 58/F | SCA | Lleum | 1 | 3 | Polypoid | No | Operation + chemotherapy | 61 | [10] |
| NA | SCA | NA | 1 | 15 | Polypoid | Yes | Operation + chemotherapy | 12 | [11] |
| 68/M | SCA | Lleum | 1 | 9.4 | Polypoid | No | Operation | 0.43 | [3] |
| 60/M | SCA | Jejunum | NA | 5 | Polypoid | Yes | Operation | 0.36 | [7] |
| 67/M | SCA | Jejunum | 2 | 6.5 | Ulcerating | Yes | Operation | 24 | [4] |
| 90/F | SCA | Small intestine | 1 | 4 | Polypoid | Yes | Operation | 15 | [12] |
In recent years, targeted therapies and immunotherapy have been increasingly applied to various solid tumors, yielding sustained survival benefits for patients. In 2019, the MD Anderson Cancer Center reported a case involving a patient who presented with painless lumps in the left axilla. The patient underwent surgical excision of two lymph nodes and was diagnosed with SCA based on biopsy analysis. Following axillary disease progression, the patient received four cycles of gemcitabine plus albumin-bound paclitaxel chemotherapy (administered in a 2-week regimen). Next-generation sequencing identified BRAF V600E and TP53 mutations in the patient. Consequently, the patient was treated with dabrafenib plus trametinib for three months and underwent post-treatment efficacy evaluation. Subsequent axillary clearance was performed, followed by 12 months of adjuvant dabrafenib therapy. At the latest follow-up visit, 45 months post-surgery, the patient's disease remained stable[11]. Additionally, a systematic review and meta-analysis[15] indicated that uterine leiomyosarcoma is almost exclusively driven by the inactivation of tumor suppressor genes, highlighting the necessity of genetic testing. These findings suggest that conventional chemotherapy is less effective for intestinal SCA and may even lead to drug resistance. Therefore, it is recommended to conduct genetic mutation detection and administer appropriate targeted therapies for pathogenic genes, potentially resulting in improved therapeutic outcomes.
Upregulated PD-L1 expression on tumor cells can facilitate immune escape by activating the programmed cell death protein 1 (PD-1)/PD-L1 pathway and inhibiting immune responses[16]. Patients with high levels of PD-1 expression are often treated with PD-L1 inhibitors, which block the interaction between PD-1 and PD-L1, enabling immune cells to maintain their activity and restore their ability to recognize and destroy tumor cells. Consequently, PD-1 inhibitors have demonstrated optimal efficacy in various solid tumors, including lung, cervical, esophageal, and colorectal cancers. Velcheti et al[16] reported that 9 out of 13 (69.2%) patients with lung SCA were PD-L1 positive, with levels higher than those in conventional non-small cell lung cancer (NSCLC)[16]. A case of jejunal SCA with significantly increased PD-L1 protein expression was also reported by Tachibana et al[4]. Although there are currently no published reports on the application of immunotherapy in small intestine SCA, this therapeutic approach has shown excellent anti-tumor efficacy in other cancers, sarcomas, and hematological malignancies. For example, in a study evaluating pembrolizumab for advanced soft tissue sarcoma, 9 out of 40 patients achieved objective remissions, yielding an objective response rate of 23%[17]. Given the overall poor effectiveness of chemotherapy, PD-L1 and driver gene mutation testing, along with immunotherapy and targeted therapy, may be preferred options for patients with SCA.
In 2020, the Department of Oncology at The First Affiliated Hospital of Kunming Medical University identified several key driver gene mutations in small intestinal SCA, including KRAS, TP53, and EGFR[18]. Anlotinib, a novel small-molecule multi-target tyrosine kinase inhibitor, targets vascular endothelial growth factor receptor and platelet-derived growth factor receptor. Anlotinib received its first approval in China on May 10, 2018, as a monotherapy for patients with locally advanced or metastatic NSCLC who have experienced progression or recurrence after at least two lines of systemic chemotherapy. Anlotinib has demonstrated significant antitumor effects in lung cancer and soft tissue sarcoma[19]. Sintilimab, a humanized monoclonal antibody developed in China, binds to PD-L1 expressed on T cells and competes effectively and specifically with PD-1/PD-L1/L2 binding, thereby blocking the immunosuppressive response mediated by the PD-1 pathway. Preclinical data suggest that sintilimab has a potentially greater affinity for PD-1 compared to pembrolizumab or nivolumab due to its distinct structural binding mode to PD-1[20]. By 2020, sintilimab had been approved by the National Medical Products Administration of China to treat relapsed or refractory classical Hodgkin lymphoma[21]. Sintilimab has also shown favorable antitumor effects in lymphoma[22], NSCLC[23], and digestive system cancers[24]. Anti-angiogenesis combined with immunotherapy has a synergistic effect. Mechanistically, anti-angiogenesis can promote antigen presentation and T cell immune responses to tumor antigens by inhibiting dendritic cell maturation disorder[25]. Additionally, anti-angiogenesis can normalize tumor vasculature, enhancing T cell infiltration into the tumor and reducing the number and activity of immunosuppressive cells such as myeloid-derived suppressor cells, tumor-associated macrophages, and regulatory T cells. This combination therapy can transform the tumor microenvironment from immunosuppressive to immunopermissive states[26]. Immunotherapy has demonstrated promising results in the treatment of SCA[17,27,28]. The synergistic effect of combining targeted therapy with immunotherapy in SCA has garnered significant attention[29-31]. Dai et al[32] reported a case where a patient with advanced pulmonary SCA was successfully treated with a combination of sintilimab and anlotinib[32]. Similarly, Piao et al[31] described an advanced pulmonary SCA patient who responded optimally to a regimen of anlotinib, sintilimab, and platinum doublet chemotherapy[31]. Chemotherapy agents such as paclitaxel and docetaxel remain crucial in the treatment of advanced sarcomas[33-38]. Recent clinical trials have demonstrated the promising efficacy of albumin-bound paclitaxel in treating various malignancies, including gastric cancer, melanoma, gynecological malignancies, urothelial carcinoma, head and neck squamous cell carcinoma, biliary carcinoma, and nasopharyngeal carcinoma[38-40]. In the case under discussion, the patient’s condition stabilized following treatment with anlotinib, sintilimab, and albumin-bound paclitaxel. The liver and multiple abdominal nodules showed a significant decrease in size compared to their initial presentation, and the patient experienced no disease progression for over a year post-surgery, indicating substantial therapeutic benefits. The patient reported only mild side effects, such as fatigue and peeling of the hands and feet. However, the patient did not complete genetic testing due to financial constraints and subsequently discontinued treatment. It is crucial to encourage patients who cannot undergo surgery or tolerate chemotherapy to complete genetic testing, as it can significantly inform their treatment responses. Targeted therapy and immunotherapy represent promising treatment options, but further clinical trials are necessary to fully assess their side effects and clinical efficacy.
In conclusion, jejunal SCA is an extremely rare, highly metastatic, and invasive tumor with a very poor prognosis. Surgery is effective for early-stage patients, but for those with late-stage or recurrent disease, chemotherapy remains the mainstay of treatment, often following protocols used for sarcoma and colon cancer. However, the overall efficacy of chemotherapy is limited, and the duration of response is typically short. In the present case report, the patient achieved long-term stability, optimal tolerance, and minimal side effects through a combination of immunotherapy and targeted therapy. This suggests that anti-angiogenic-targeted drugs combined with immunotherapy may offer a novel treatment option for small intestinal SCA. Nevertheless, further clinical research is needed to validate these findings.
| 1. | Oztürk E, Yilmazlar T, Yerci O. A rare tumor located in the anorectal junction: sarcomatoid carcinoma. Turk J Gastroenterol. 2006;17:236-239. [PubMed] |
| 2. | Lam KY, Leung CY, Ho JW. Sarcomatoid carcinoma of the small intestine. Aust N Z J Surg. 1996;66:636-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Rosli N, Shukor NA. Sarcomatoid Carcinoma of Small Intestine: A Case. Chonnam Med J. 2021;57:89-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Tachibana M, Nozawa M, Kamimura K, Tsutsumi Y. Synchronous Jejunal Sarcomatoid Carcinoma and Incidentally Associated Localized Peritoneal Malignant Mesothelioma. Cureus. 2022;14:e26270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Zhang B, Cheng BO, Wang L, Zhao KE, Zhuo GZ, Ding JH. Primary sarcomatoid carcinoma of the jejunum with massive intra-abdominal hemorrhage: A case report and review of the literature. Mol Clin Oncol. 2016;4:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Radi MF, Gray GF Jr, Scott HW Jr. Carcinosarcoma of ileum in regional enteritis. Hum Pathol. 1984;15:385-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Kwok CM. Sarcomatoid carcinoma of the jejunum with gastric metastases: A case report and review of the literature. Int J Surg Case Rep. 2016;28:161-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Ishida H, Ohsawa T, Nakada H, Hashimoto D, Ohkubo T, Adachi A, Itoyama S. Carcinosarcoma of the rectosigmoid colon: report of a case. Surg Today. 2003;33:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Khelfa Y, Alsharedi M, Mehmi I, Raufi A, Arrington A, Lebowicz Y, Pacioles T. Metastatic Sarcomatoid Carcinoma of the Small Intestine: a Case Report of Rare Tumor with Literature Review. J Gastrointest Cancer. 2016;47:478-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Andrawes PA, Shariff M, Chang Q, Grinberg R. Primary sarcomatoid carcinoma of the small intestine: very rare and aggressive tumour. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Huey RW, Makawita S, Xiao L, Matamoros A, Estrella JS, Overman MJ, Varadhachary GR, Raghav K. Sarcomatoid carcinoma presenting as cancers of unknown primary: a clinicopathological portrait. BMC Cancer. 2019;19:965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Karakuchi N, Yanagawa S, Kushitani K, Kodama S, Takeshima Y, Sumimoto K. Primary Small Intestinal Sarcomatoid Carcinoma: Report of a Rare Case and Literature Review. Case Rep Oncol. 2021;14:538-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Lee SE, Park SY. Sarcomatoid carcinoma of the small intestine: a rare and highly aggressive tumor. J Korean Surg Soc. 2012;83:321-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Reid-Nicholson M, Idrees M, Perino G, Hytiroglou P. Sarcomatoid carcinoma of the small intestine: a case report and review of the literature. Arch Pathol Lab Med. 2004;128:918-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Astolfi A, Nannini M, Indio V, Schipani A, Rizzo A, Perrone AM, De Iaco P, Pirini MG, De Leo A, Urbini M, Secchiero P, Pantaleo MA. Genomic Database Analysis of Uterine Leiomyosarcoma Mutational Profile. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 16. | Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol. 2013;8:803-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA, Movva S, Davis LE, Okuno SH, Reed DR, Crowley J, Butterfield LH, Salazar R, Rodriguez-Canales J, Lazar AJ, Wistuba II, Baker LH, Maki RG, Reinke D, Patel S. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18:1493-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 1006] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 18. | Zhu Z, Liu X, Li W, Wen Z, Ji X, Zhou R, Tuo X, Chen Y, Gong X, Liu G, Zhou Y, Chen S, Song L, Huang J. A rare multiple primary sarcomatoid carcinoma (SCA) of small intestine harboring driver gene mutations: a case report and a literature review. Transl Cancer Res. 2021;10:1150-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, Zhao F, Ahmad R, Zhao J. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 439] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 20. | Wang J, Fei K, Jing H, Wu Z, Wu W, Zhou S, Ni H, Chen B, Xiong Y, Liu Y, Peng B, Yu D, Jiang H, Liu J. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. MAbs. 2019;11:1443-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 21. | Hoy SM. Sintilimab: First Global Approval. Drugs. 2019;79:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (2)] |
| 22. | Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W, Zhang W, Gao Y, Jin Z, Zhou J, Jin C, Zou L, Qiu L, Li W, Yang J, Hou M, Zeng S, Zhang Q, Hu J, Zhou H, Xiong Y, Liu P. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019;6:e12-e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 23. | Zhang P, Dai J, Sun F, Xia H, He W, Duan L, Liu M, Zhao D, Zhu Y, Jiang G. Neoadjuvant Sintilimab and Chemotherapy for Resectable Stage IIIA Non-Small Cell Lung Cancer. Ann Thorac Surg. 2022;114:949-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 24. | Zhang L, Mai W, Jiang W, Geng Q. Sintilimab: A Promising Anti-Tumor PD-1 Antibody. Front Oncol. 2020;10:594558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 25. | Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, Wu X, Ma J, Zhou M, Li X, Li Y, Li G, Xiong W, Guo C, Zeng Z. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 1000] [Article Influence: 166.7] [Reference Citation Analysis (0)] |
| 26. | Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 438] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 27. | Brown JT, Bilen MA. Immunotherapy in sarcomatoid renal cell carcinoma: A case for optimism. Cancer Treat Res Commun. 2020;25:100257. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Ayodele O, Razak ARA. Immunotherapy in soft-tissue sarcoma. Curr Oncol. 2020;27:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 29. | Liu Z, Yu M, Zhao F, Zhu C. Anlotinib combined with Sintilimab is win-win cooperation for primary squamous cell carcinoma of the thyroid: A case report and literature review. Front Oncol. 2023;13:976415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Wu S, Wu S, Liao X, Zhou C, Qiu F, Wang C, Zhong W. Pembrolizumab combined with anlotinib improves therapeutic efficacy in pulmonary sarcomatoid carcinoma with TMB-H and PD-L1 expression: a case report and literature review. Front Immunol. 2023;14:1274937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 31. | Piao MN, Ma XT, Tankere P, Liam CK, Li JL, Wang JP. Anlotinib combined with chemotherapy and immunotherapy for advanced pulmonary sarcomatoid cancer: a case report and literature review. Ann Transl Med. 2022;10:1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Dai G, He L, Yan Q, Li Y, Huang Y, Li B, Wang G. Case Report: Advanced pulmonary sarcomatoid carcinoma with adrenal gland metastasis after sintilimab combined with anlotinib treatment. Front Oncol. 2023;13:1167516. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Singhi EK, Moore DC, Muslimani A. Metastatic Soft Tissue Sarcomas: A Review Of Treatment and New Pharmacotherapies. P T. 2018;43:410-429. [PubMed] |
| 34. | Villalobos VM, Byfield SD, Ghate SR, Adejoro O. A retrospective cohort study of treatment patterns among patients with metastatic soft tissue sarcoma in the US. Clin Sarcoma Res. 2017;7:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Metts JL, Alazraki AL, Clark D, Amankwah EK, Wasilewski-Masker KJ, George BA, Olson TA, Cash T. Gemcitabine/nab-paclitaxel for pediatric relapsed/refractory sarcomas. Pediatr Blood Cancer. 2018;65:e27246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Maki RG. Gemcitabine and docetaxel in metastatic sarcoma: past, present, and future. Oncologist. 2007;12:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Nagar SP, Mytelka DS, Candrilli SD, D'yachkova Y, Lorenzo M, Kasper B, Lopez-Martin JA, Kaye JA. Treatment Patterns and Survival among Adult Patients with Advanced Soft Tissue Sarcoma: A Retrospective Medical Record Review in the United Kingdom, Spain, Germany, and France. Sarcoma. 2018;2018:5467057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Tian Z, Yao W. Albumin-Bound Paclitaxel: Worthy of Further Study in Sarcomas. Front Oncol. 2022;12:815900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Sahai V, Catalano PJ, Zalupski MM, Lubner SJ, Menge MR, Nimeiri HS, Munshi HG, Benson AB 3rd, O'Dwyer PJ. Nab-Paclitaxel and Gemcitabine as First-line Treatment of Advanced or Metastatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:1707-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 40. | Hersh EM, Del Vecchio M, Brown MP, Kefford R, Loquai C, Testori A, Bhatia S, Gutzmer R, Conry R, Haydon A, Robert C, Ernst S, Homsi J, Grob JJ, Kendra K, Agarwala SS, Li M, Clawson A, Brachmann C, Karnoub M, Elias I, Renschler MF, Hauschild A. A randomized, controlled phase III trial of nab-Paclitaxel versus dacarbazine in chemotherapy-naïve patients with metastatic melanoma. Ann Oncol. 2015;26:2267-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Han N, Han QH, Liu YZ, Li ZC, Li J. Perforated sarcomatoid carcinoma of the jejunum: Case report. Oncol Lett. 2013;6:562-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Alfonso Puentes N, Jimenez-Alfaro Larrazabal C, García Higuera MI. Sarcomatoid carcinoma of the jejunum presenting as obscure gastrointestinal bleeding in a patient with a history of gliosarcoma. Gastroenterol Rep (Oxf). 2014;2:150-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |