Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3672
Revised: June 4, 2024
Accepted: July 5, 2024
Published online: August 15, 2024
Processing time: 123 Days and 19.1 Hours
With the rapid progress of systematic therapy for hepatocellular carcinoma (HCC), therapeutic strategies combining hepatic arterial infusion chemotherapy (HAIC) with systematic therapy arised increasing concentrations. However, there have been no systematic review comparing HAIC and its combination strategies in the first-line treatment for advanced HCC.
To investigate the efficacy and safety of HAIC and its combination therapies for advanced HCC.
A network meta-analysis was performed by including 9 randomized controlled trails and 35 cohort studies to carry out our study. The outcomes of interest comprised overall survival (OS), progression-free survival (PFS), tumor response and adverse events. Hazard ratios (HR) and odds ratios (OR) with a 95% confidence interval (CI) were calculated and agents were ranked based on their ranking probability.
HAIC outperformed Sorafenib (HR = 0.55, 95%CI: 0.42-0.72; HR = 0.51, 95%CI: 0.33-0.78; OR = 2.86, 95%CI: 1.37-5.98; OR = 5.45, 95%CI: 3.57-8.30; OR = 7.15, 95%CI: 4.06-12.58; OR = 2.89, 95%CI: 1.99-4.19; OR = 0.48, 95%CI: 0.25-0.92, respectively) and transarterial chemoembolization (TACE) (HR = 0.50, 95%CI: 0.33-0.75; HR = 0.62, 95%CI: 0.39-0.98; OR = 3.08, 95%CI: 1.36-6.98; OR = 2.07, 95%CI: 1.54-2.80; OR = 3.16, 95%CI: 1.71-5.85; OR = 2.67, 95%CI: 1.59-4.50; OR = 0.16, 95%CI: 0.05-0.54, respectively) in terms of efficacy and safety. HAIC + lenvatinib + ablation, HAIC + ablation, HAIC + anti- programmed cell death 1 (PD-1), and HAIC + radiotherapy had the higher likelihood of providing better OS and PFS outcomes compared to HAIC alone. HAIC + TACE + S-1, HAIC + lenvatinib, HAIC + PD-1, HAIC + TACE, and HAIC + sorafenib had the higher likelihood of providing better partial response and objective response rate outcomes compared to HAIC. HAIC + PD-1, HAIC + TACE + S-1 and HAIC + TACE had the higher likelihood of providing better complete response and disease control rate outcomes compared to HAIC alone.
HAIC proved more effective and safer than sorafenib and TACE. Furthermore, combined with other interventions, HAIC showed improved efficacy over HAIC monotherapy according to the treatment ranking analysis.
Core Tip: Because there are not enough randomized controlled trials to demonstrate the efficacy and safety of hepatic arterial infusion chemotherapy (HAIC) in advanced hepatocellular carcinoma (HCC), HAIC has not yet been recognized in Western countries. Therefore, we conducted a network meta-analysis to compare the safety and efficacy of HAIC and its combination strategies for advanced HCC. Compared to sorafenib and transarterial chemoembolization, HAIC was found to be a better choice in terms of both efficacy and safety. Furthermore, interventions combined with HAIC showed marginally better efficacy compared to HAIC monotherapy.
- Citation: Zhou SA, Zhou QM, Wu L, Chen ZH, Wu F, Chen ZR, Xu LQ, Gan BL, Jin HS, Shi N. Efficacy of hepatic arterial infusion chemotherapy and its combination strategies for advanced hepatocellular carcinoma: A network meta-analysis. World J Gastrointest Oncol 2024; 16(8): 3672-3686
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3672.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3672
Primary liver cancer ranks as the sixth most commonly diagnosed malignant tumor. It is the fourth leading cause of cancer-related mortality worldwide[1]. Its chief cause is liver cirrhosis, including alcoholic cirrhosis, virus-associated cirrhosis, cryptogenic cirrhosis, and other types[2]. Hepatocellular carcinoma (HCC) is the predominant liver cancer subtype[3]. Surgical resection is the leading curative treatment for patients with HCC. However, most patients are diagnosed with HCC at an advanced stage, thus precluding radical surgical resection[4,5]. Patients with advanced HCC demonstrate unsatisfactory outcomes, with a 5-year survival rate of only 5% to 36%[6].
Patients with early-stage HCC may be treated by surgical resection, liver transplantation, or ablation (A). However, locoregional therapies, including transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), and systemic therapy are considered therapeutic options in patients with unresectable HCC. Systemic therapies, such as sorafenib, are primarily used for patients with unresectable HCC with distant metastases. However, many patients either reduce the dosage or discontinue the treatment because of adverse events (AEs) associated with long-term sorafenib use, limiting its therapeutic potential. Conversely, TACE is primarily administered to patients with unresectable HCC without distant metastases. One of its limitations is single-dose administration, which restricts the duration for high-concentration chemotherapy drugs to act on tumors. Japanese guidelines recommend HAIC combined with portal vein thrombosis as the first-line treatment for patients with HCC[7]. Owing to the blood supply characteristics of the liver and HCC cells, HAIC kills tumor cells by the continuous perfusion of cytotoxic drugs with high concentrations through the hepatic artery. Simultaneously, it does not considerably influence healthy liver tissues[8]. However, insufficient phase 3 randomized controlled trials (RCTs) have demonstrated that patients with HCC can benefit from HAIC. Therefore, HAIC has not been recognized in Western countries. Thus, we aimed to conduct a network meta-analysis of RCTs and cohort studies to compare the efficacy and safety of HAIC and its combination therapies with other interventions. We aimed to offer insights into evidence-based medicine for applying HAIC.
To assess and compare the effectiveness of HAIC, both as a standalone treatment and combined with other strategies, in patients diagnosed with advanced HCC.
Specific search terms were applied to the PubMed database to identify relevant RCTs and cohort studies assessing the efficacy of HAIC, either as a standalone treatment or combined with other therapies, published on or before July 10, 2023. Supplementary material describes the search strategy. Additionally, conference papers were manually searched to extract pertinent reference documents and abstracts. The screening process encompassed evaluating the titles, abstracts, and full texts to identify the studies that met the selection criteria.
The inclusion criteria were as follows: (1) RCTs and/or cohort studies; (2) Patients with unresectable HCC (including patients with Barcelona Clinic Liver Cancer stages B and C); (3) One study arm is HAIC or its combination, whereas the other arm is different treatment strategies or best supportive care (BSC); and (4) Overall survival (OS), progression-free survival (PFS), complete response (CR), partial response (PR), objective response rate (ORR), and disease control rate (DCR), AEs, or Kaplan-Meier curves are the outcome indicators. The exclusion criteria were as follows: (1) Articles without a study endpoint (PFS or OS); (2) Therapeutic strategies as a second-line treatment option for patients with unresectable HCC; and (3) Therapeutic strategies that could not be connected to the net graph in the network meta-analysis.
First, the titles and abstracts were read independently by two researchers and screened according to the selection criteria. Second, the full texts of the articles that met the inclusion criteria were read. Third, the articles were screened for network meta-analysis. Information on the pre-specified data, including baseline characteristics, sample size, tumor burden, AEs, and interventions, was extracted independently from each article by the two reviewers using a data form. A third reviewer resolved the disagreements. The outcomes included the OS, PFS, CR, PR, ORR, and DCR, AEs. We selected the data after propensity score matching.
The quality of RCTs was assessed independently by the two reviewers using the Cochrane Risk of Bias assessment tool. This tool considers key criteria, including random sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete outcome data, selective reporting, and other biases. The methodological quality of cohort studies was assessed using the “star” rating system of the Newcastle-Ottawa Scale based on the following three factors: Selecting the research population, comparability of the study group, and evaluating the results.
R software version 4.2.3 (Mathsoft, Cambridge, United States) was used for the statistical analysis. Netmeta package was used to conduct the frequentist Network meta-analysis. The OS and PFS were estimated using hazard ratios (HR) with 95% confidence intervals (CI). The CR, PR, ORR, DCR, AE, and odds ratio (OR) with 95%CI were calculated. I2 was calculated to assess the overall heterogeneity of the data model. For I2 > 50%, the random effects model was selected; conversely, the fixed effects model was selected. Additionally, the P-score was used to rank the treatments[9]. The network graph illustrates the indirect comparative associations among the interventions. Each node represents an intervention, and the node size represents the sample size of the intervention. The funnel plot depicts publication bias; P > 0.05 indicated no publication bias. The study has been registered on PROSPERO (ID: CRD42023463399). The manuscript was prepared and revised according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020[10] and Assessing the Methodological Quality of Systematic Reviews guidelines[11].
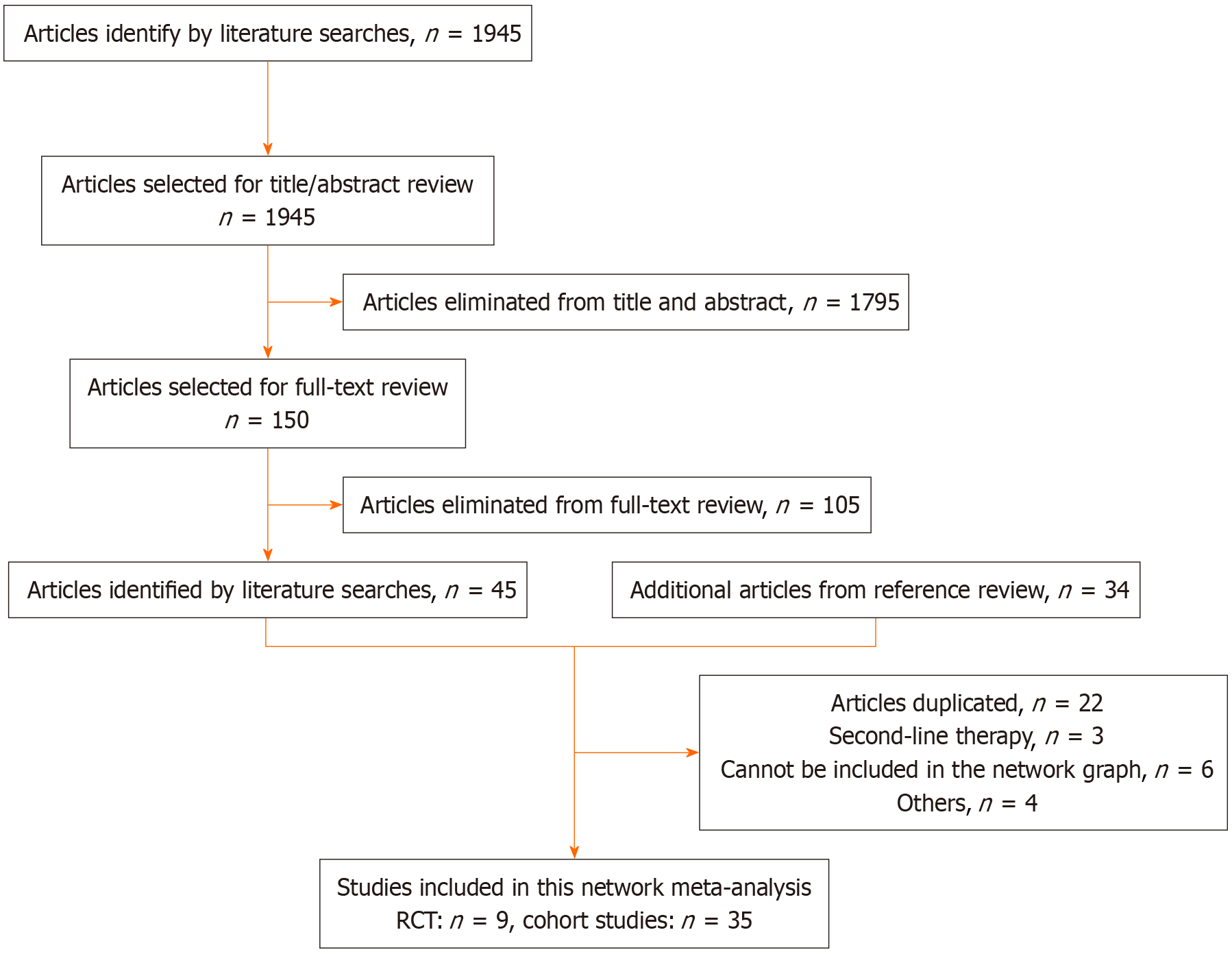
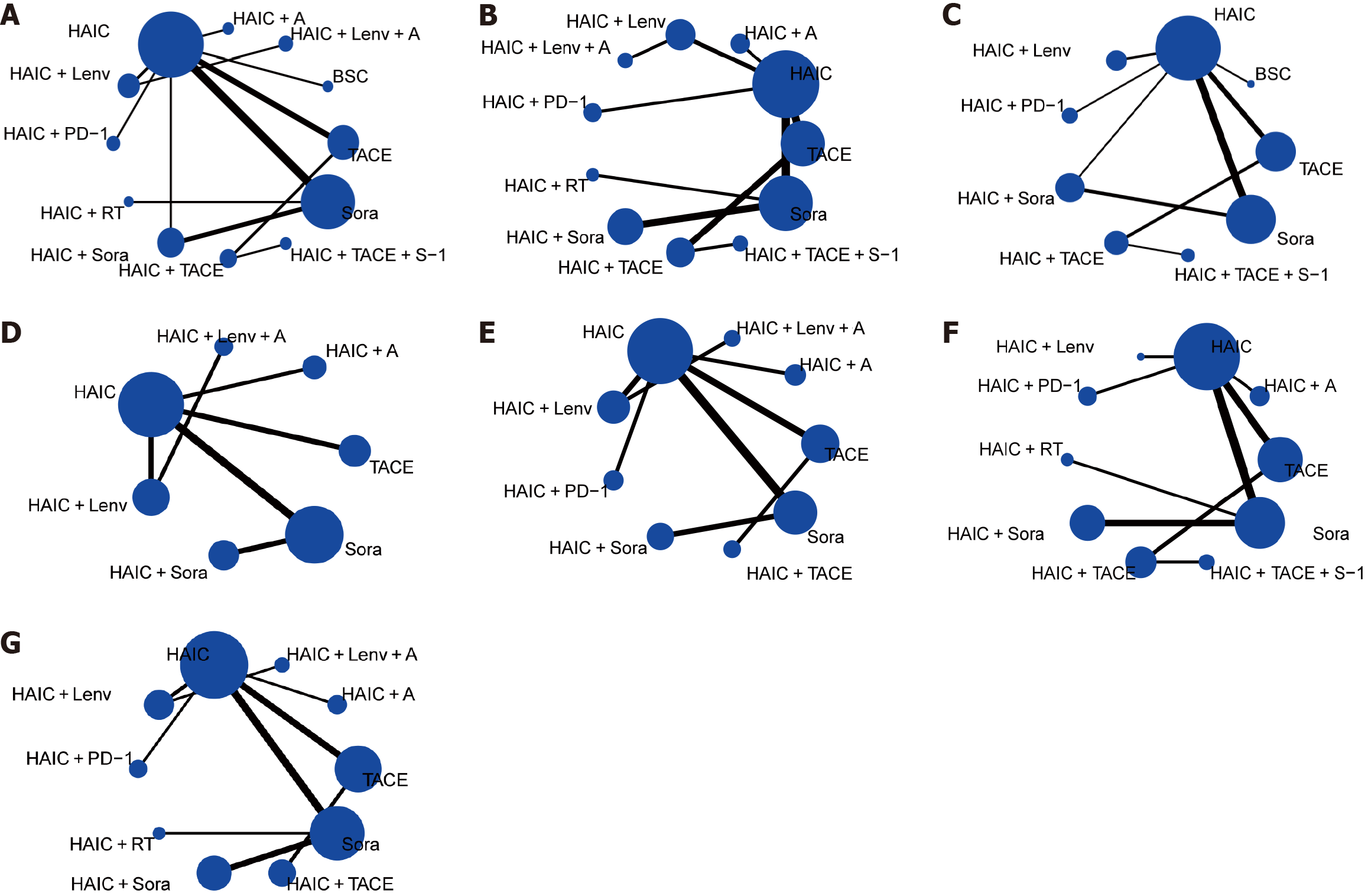
Initially, we retrieved 1945 articles. Of them, 150 met the selection criteria for assessment based on their titles and abstracts. Subsequently, 105 articles were excluded after reading the full text, resulting in 45 articles. Additionally, we included 34 articles from the reference lists of other articles. We excluded 38 articles for various reasons, including duplicates, interventions unsuitable for the network graph, second-line therapies, and others. Finally, we included 44 articles, comprising 9 RCTs and 35 cohort studies[12-55]. Figure 1 presents the flow diagram. The 44 trials included 5789 patients. Supplementary Table 1 summarizes the baseline characteristics of the included studies. Figure 2 illustrates the network graph. Two nodes connected by a line indicate articles directly comparing the effectiveness of the two treatments, with the line thickness representing the article number available for comparison.
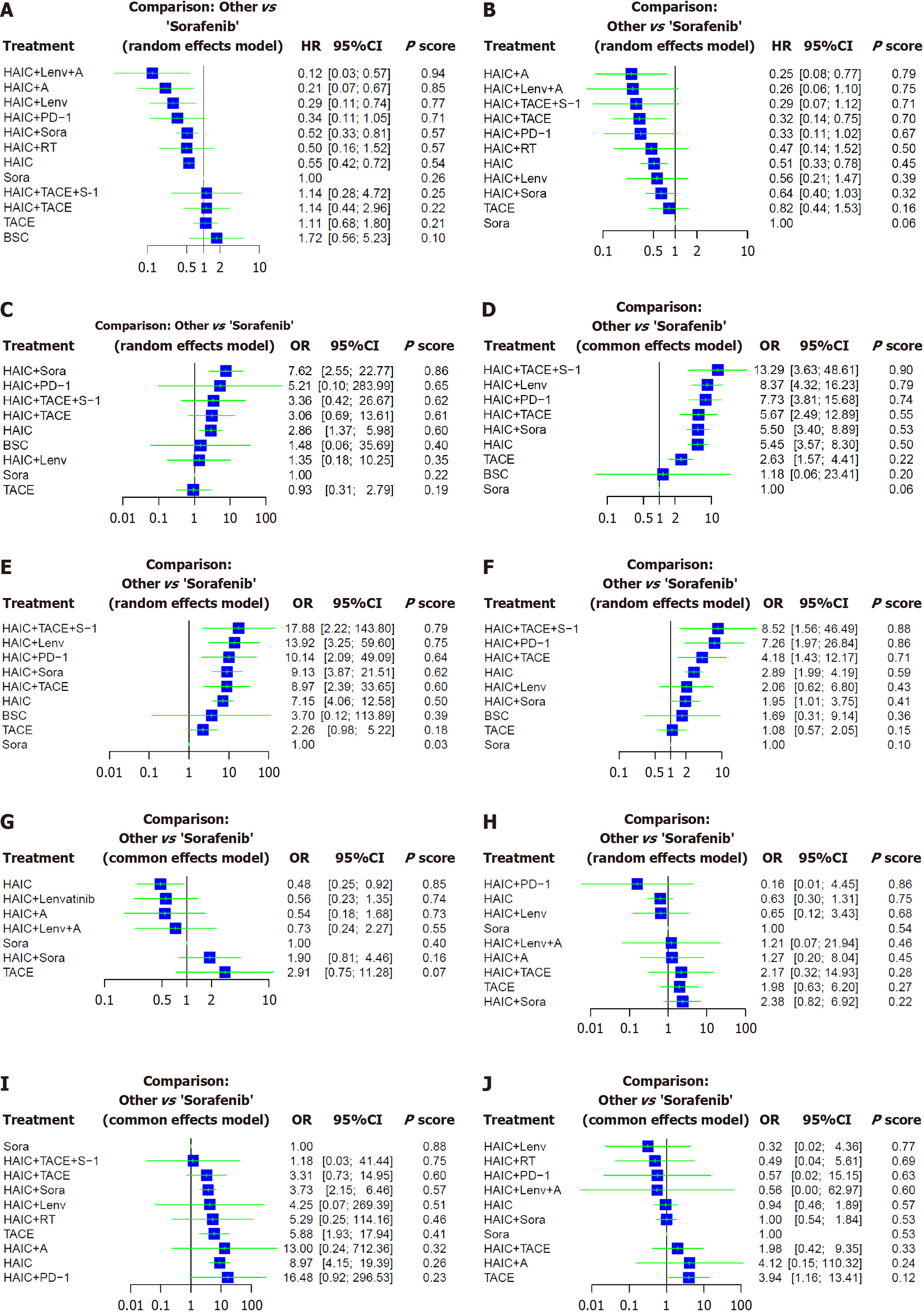
Network meta-analysis of OS: OS data were extracted from 41 articles, including 9 RCTs and 32 cohort studies. They encompassed 5556 patients. Twelve interventions were compared (Figure 2). Patients receiving HAIC + lenvatinib (Lenv) + A (HR = 0.12; 95%CI: 0.03-0.57), HAIC + A (HR = 0.21; 95%CI: 0.07-0.67), HAIC + Lenv (HR = 0.29; 95%CI: 0.11-0.74), HAIC + sorafenib (Sora) (HR = 0.52; 95%CI: 0.33-0.81), and HAIC (HR = 0.55; 95%CI: 0.42-0.72) demonstrated significantly improved OS than patients receiving Sora (Figure 3). Furthermore, the most favorable OS outcomes were associated with HAIC + Lenv + A (P-score: 0.94), followed by HAIC + A (P-score: 0.85) and HAIC + Lenv (P-score: 0.77). Table 1 summarizes direct and indirect comparisons of the interventions for OS.
| League table | |||||||||||
| OS | |||||||||||
| HAIC + Lenv + A | 0.42 (0.12; 1.44) | ||||||||||
| 0.58 (0.09; 3.84) | HAIC + A | 0.38 (0.12; 1.17) | |||||||||
| 0.42 (0.12; 1.44) | 0.73 (0.17; 3.08) | HAIC + Lenv | 0.52 (0.21; 1.28) | ||||||||
| 0.36 (0.05; 2.31) | 0.61 (0.13; 2.93) | 0.84 (0.20; 3.46) | HAIC + PD-1 | 0.62 (0.21; 1.84) | |||||||
| 0.24 (0.05; 1.17) | 0.41 (0.12; 1.39) | 0.56 (0.20; 1.56) | 0.66 (0.20; 2.20) | HAIC + Sora | 0.43 (0.12; 1.47) | 0.58 (0.36; 0.94) | |||||
| 0.24 (0.04; 1.63) | 0.42 (0.08; 2.09) | 0.58 (0.13; 2.47) | 0.68 (0.14; 3.32) | 1.03 (0.31; 3.43) | HAIC + RT | 0.50 (0.16; 1.52) | |||||
| 0.22 (0.05; 1.01) | 0.38 (0.12; 1.17) | 0.52 (0.21; 1.28) | 0.62 (0.21; 1.84) | 0.94 (0.57; 1.55) | 0.91 (0.29; 2.85) | HAIC | 0.53 (0.40; 0.70) | 0.50 (0.33; 0.75) | 0.32 (0.11; 0.95) | ||
| 0.12 (0.03; 0.57) | 0.21 (0.07; 0.67) | 0.29 (0.11; 0.74) | 0.34 (0.11; 1.05) | 0.52 (0.33; 0.81) | 0.50 (0.16; 1.52) | 0.55 (0.42; 0.72) | Sora | ||||
| 0.11 (0.01; 0.84) | 0.18 (0.03; 1.10) | 0.25 (0.05; 1.33) | 0.30 (0.05; 1.76) | 0.45 (0.10; 2.00) | 0.44 (0.07; 2.66) | 0.48 (0.12; 1.95) | 0.88 (0.21; 3.63) | HAIC + TACE + S-1 | 1.00 (0.35; 2.86) | ||
| 0.11 (0.02; 0.63) | 0.18 (0.04; 0.78) | 0.25 (0.07; 0.91) | 0.30 (0.07; 1.24) | 0.45 (0.16; 1.29) | 0.44 (0.10; 1.90) | 0.48 (0.19; 1.21) | 0.88 (0.34; 2.28) | 1.00 (0.35; 2.86) | HAIC + TACE | 1.03 (0.45; 2.34) | |
| 0.11 (0.02; 0.53) | 0.19 (0.06; 0.63) | 0.26 (0.10; 0.70) | 0.31 (0.10; 0.99) | 0.47 (0.24; 0.90) | 0.45 (0.13; 1.52) | 0.50 (0.33; 0.75) | 0.90 (0.55; 1.47) | 1.03 (0.27; 3.91) | 1.03 (0.45; 2.34) | TACE | |
| 0.07 (0.01; 0.46) | 0.12 (0.03; 0.58) | 0.17 (0.04; 0.68) | 0.20 (0.04; 0.92) | 0.30 (0.09; 0.99) | 0.29 (0.06; 1.40) | 0.32 (0.11; 0.95) | 0.58 (0.19; 1.77) | 0.66 (0.11; 3.87) | 0.66 (0.16; 2.73) | 0.64 (0.20; 2.04) | BSC |
| PFS | |||||||||||
| HAIC + A | 0.49 (0.17; 1.39) | ||||||||||
| 0.96 (0.17; 5.46) | HAIC + Lenv + A | 0.46 (0.16; 1.36) | |||||||||
| 0.85 (0.16; 4.50) | 0.89 (0.13; 5.94) | HAIC + TACE + S-1 | 0.90 (0.31; 2.59) | ||||||||
| 0.77 (0.21; 2.77) | 0.80 (0.17; 3.87) | 0.90 (0.31; 2.59) | HAIC + TACE | 0.39 (0.22; 0.70) | |||||||
| 0.75 (0.17; 3.29) | 0.78 (0.14; 4.44) | 0.88 (0.17; 4.61) | 0.97 (0.27; 3.50) | HAIC + PD-1 | 0.65 (0.23; 1.85) | ||||||
| 0.53 (0.10; 2.70) | 0.55 (0.09; 3.58) | 0.62 (0.10; 3.73) | 0.69 (0.16; 2.94) | 0.71 (0.14; 3.61) | HAIC + RT | 0.47 (0.14; 1.52) | |||||
| 0.49 (0.17; 1.39) | 0.51 (0.13; 2.04) | 0.57 (0.16; 2.07) | 0.63 (0.30; 1.32) | 0.65 (0.23; 1.85) | 0.92 (0.26; 3.21) | HAIC | 0.91 (0.38; 2.16) | 0.62 (0.39; 0.98) | 0.51 (0.33; 0.78) | ||
| 0.44 (0.11; 1.72) | 0.46 (0.16; 1.36) | 0.52 (0.11; 2.45) | 0.57 (0.18; 1.80) | 0.59 (0.15; 2.30) | 0.84 (0.18; 3.83) | 0.91 (0.38; 2.16) | HAIC + Lenv | ||||
| 0.39 (0.11; 1.32) | 0.40 (0.09; 1.86) | 0.45 (0.11; 1.91) | 0.50 (0.19; 1.34) | 0.52 (0.15; 1.76) | 0.73 (0.20; 2.61) | 0.79 (0.42; 1.51) | 0.87 (0.30; 2.57) | HAIC + Sora | 0.64 (0.40; 1.03) | ||
| 0.30 (0.10; 0.95) | 0.31 (0.07; 1.36) | 0.35 (0.11; 1.18) | 0.39 (0.22; 0.70) | 0.40 (0.13; 1.26) | 0.57 (0.15; 2.16) | 0.62 (0.39; 0.98) | 0.68 (0.26; 1.82) | 0.78 (0.36; 1.72) | TACE | ||
| 0.25 (0.08; 0.77) | 0.26 (0.06; 1.10) | 0.29 (0.07; 1.12) | 0.32 (0.14; 0.75) | 0.33 (0.11; 1.02) | 0.47 (0.14; 1.52) | 0.51 (0.33; 0.78) | 0.56 (0.21; 1.47) | 0.64 (0.40; 1.03) | 0.82 (0.44; 1.53) | Sora | |
| CR | |||||||||||
| HAIC + Sora | 6.21 (0.28; 138.56) | 6.77 (2.11; 21.68) | |||||||||
| 1.46 (0.02; 90.79) | HAIC + PD-1 | 1.82 (0.04; 92.69) | |||||||||
| 2.27 (0.22; 22.94) | 1.55 (0.02; 123.86) | HAIC + TACE + S-1 | 1.10 (0.26; 4.62) | ||||||||
| 2.49 (0.41; 15.27) | 1.70 (0.03; 106.73) | 1.10 (0.26; 4.62) | HAIC + TACE | 3.30 (1.20; 9.03) | |||||||
| 2.66 (0.75; 9.46) | 1.82 (0.04; 92.69) | 1.17 (0.17; 8.14) | 1.07 (0.29; 3.91) | HAIC | 1.93 (0.09; 42.76) | 2.13 (0.32; 14.10) | 3.01 (1.41; 6.41) | 3.08 (1.36; 6.98) | |||
| 5.15 (0.18; 146.19) | 3.52 (0.02; 524.16) | 2.27 (0.06; 87.52) | 2.07 (0.07; 59.37) | 1.93 (0.09; 42.76) | BSC | ||||||
| 5.67 (0.58; 55.24) | 3.87 (0.05; 303.50) | 2.50 (0.17; 37.42) | 2.27 (0.23; 22.55) | 2.13 (0.32; 14.10) | 1.10 (0.03; 41.42) | HAIC + Lenv | |||||
| 7.62 (2.55; 22.77) | 5.21 (0.10; 283.99) | 3.36 (0.42; 26.67) | 3.06 (0.69; 13.61) | 2.86 (1.37; 5.98) | 1.48 (0.06; 35.69) | 1.35 (0.18; 10.25) | Sora | ||||
| 8.21 (1.82; 37.10) | 5.62 (0.10; 310.71) | 3.62 (0.63; 20.94) | 3.30 (1.20; 9.03) | 3.08 (1.36; 6.98) | 1.59 (0.06; 39.20) | 1.45 (0.18; 11.38) | 1.08 (0.36; 3.24) | TACE | |||
| PR | |||||||||||
| HAIC + TACE + S-1 | 2.34 (0.86; 6.40) | ||||||||||
| 1.59 (0.42; 6.00) | HAIC + Lenv | 1.54 (0.92; 2.56) | |||||||||
| 1.72 (0.44; 6.65) | 1.08 (0.50; 2.33) | HAIC + PD-1 | 1.42 (0.80; 2.51) | ||||||||
| 2.34 (0.86; 6.40) | 1.48 (0.62; 3.53) | 1.36 (0.55; 3.37) | HAIC + TACE | 2.16 (1.14; 4.08) | |||||||
| 2.42 (0.61; 9.51) | 1.52 (0.69; 3.37) | 1.41 (0.61; 3.23) | 1.03 (0.41; 2.62) | HAIC + Sora | 5.00 (1.19; 21.04) | 4.50 (2.71; 7.48) | |||||
| 2.44 (0.72; 8.32) | 1.54 (0.92; 2.56) | 1.42 (0.80; 2.51) | 1.04 (0.51; 2.11) | 1.01 (0.55; 1.85) | HAIC | 2.07 (1.54; 2.80) | 4.62 (0.24; 89.16) | 6.32 (4.08; 9.80) | |||
| 5.05 (1.54; 16.61) | 3.18 (1.76; 5.76) | 2.94 (1.55; 5.59) | 2.16 (1.14; 4.08) | 2.09 (1.06; 4.12) | 2.07 (1.54; 2.80) | TACE | |||||
| 11.28 (0.46; 277.69) | 7.10 (0.35; 143.15) | 6.56 (0.32; 133.57) | 4.81 (0.23; 100.83) | 4.67 (0.23; 95.70) | 4.62 (0.24; 89.16) | 2.23 (0.11; 43.69) | BSC | ||||
| 13.29 (3.63; 48.61) | 8.37 (4.32; 16.23) | 7.73 (3.81; 15.68) | 5.67 (2.49; 12.89) | 5.50 (3.40; 8.89) | 5.45 (3.57; 8.30) | 2.63 (1.57; 4.41) | 1.18 (0.06; 23.41) | Sora | |||
| ORR | |||||||||||
| HAIC + TACE + S-1 | 1.99 (0.40; 9.99) | ||||||||||
| 1.28 (0.12; 14.33) | HAIC + Lenv | 1.95 (0.51; 7.44) | |||||||||
| 1.76 (0.15; 21.24) | 1.37 (0.19; 10.05) | HAIC + PD-1 | 1.42 (0.33; 6.19) | ||||||||
| 1.96 (0.21; 18.19) | 1.53 (0.29; 7.97) | 1.11 (0.19; 6.47) | HAIC + Sora | 8.00 (1.08; 59.18) | 6.08 (2.38; 15.59) | ||||||
| 1.99 (0.40; 9.99) | 1.55 (0.26; 9.34) | 1.13 (0.17; 7.53) | 1.02 (0.22; 4.74) | HAIC + TACE | 3.97 (1.43; 11.04) | ||||||
| 2.50 (0.34; 18.61) | 1.95 (0.51; 7.44) | 1.42 (0.33; 6.19) | 1.28 (0.48; 3.37) | 1.26 (0.38; 4.15) | HAIC | 1.93 (0.07; 56.84) | 3.16 (1.71; 5.85) | 8.36 (4.66 15.01) | |||
| 4.84 (0.09; 246.61) | 3.77 (0.10; 143.00) | 2.74 (0.07; 109.59) | 2.47 (0.07; 83.18) | 2.43 (0.07; 87.58) | 1.93 (0.07; 56.84) | BSC | |||||
| 7.91 (1.17; 53.36) | 6.16 (1.41; 26.89) | 4.48 (0.91; 22.12) | 4.04 (1.28; 12.73) | 3.97 (1.43; 11.04) | 3.16 (1.71; 5.85) | 1.63 (0.05; 50.80) | TACE | ||||
| 17.88 (2.22; 143.80) | 13.92 (3.25; 59.60) | 10.14 (2.09; 49.09) | 9.13 (3.87; 21.51) | 8.97 (2.39; 33.65) | 7.15 (4.06; 12.58) | 3.70 (0.12; 113.89) | 2.26 (0.98; 5.22) | Sora | |||
| DCR | |||||||||||
| HAIC + TACE + S-1 | 2.04 (0.55; 7.61) | ||||||||||
| 1.17 (0.15; 9.35) | HAIC + PD-1 | 2.52 (0.72; 8.81) | |||||||||
| 2.04 (0.55; 7.61) | 1.74 (0.35; 8.65) | HAIC + TACE | 3.87 (1.64; 9.11) | ||||||||
| 2.95 (0.56; 15.45) | 2.52 (0.72; 8.81) | 1.45 (0.53; 3.94) | HAIC | 1.40 (0.45; 4.37) | 0.06 (0.00; 1.40) | 1.71 (0.33; 8.88) | 2.67 (1.59; 4.50) | 3.02 (2.08; 4.40) | |||
| 4.14 (0.56; 30.84) | 3.53 (0.65; 19.15) | 2.03 (0.45; 9.24) | 1.40 (0.45; 4.37) | HAIC + Lenv | |||||||
| 4.38 (0.71; 26.90) | 3.73 (0.87; 16.04) | 2.15 (0.62; 7.49) | 1.48 (0.70; 3.13) | 1.06 (0.27; 4.11) | HAIC + Sora | 1.68 (0.86; 3.29) | |||||
| 5.05 (0.49; 52.13) | 4.30 (0.54; 34.07) | 2.47 (0.36; 17.01) | 1.71 (0.33; 8.88) | 1.22 (0.16; 9.01) | 1.15 (0.19; 7.03) | BSC | |||||
| 7.89 (1.64; 37.97) | 6.73 (1.73; 26.11) | 3.87 (1.64; 9.11) | 2.67 (1.59; 4.50) | 1.90 (0.55; 6.64) | 1.80 (0.73; 4.48) | 1.56 (0.28; 8.79) | TACE | ||||
| 8.52 (1.56; 46.49) | 7.26 (1.97; 26.84) | 4.18 (1.43; 12.17) | 2.89 (1.99; 4.19) | 2.06 (0.62; 6.80) | 1.95 (1.01; 3.75) | 1.69 (0.31; 9.14) | 1.08 (0.57; 2.05) | Sora | |||
Network meta-analysis of PFS: PFS data were extracted from 30 articles, including 9 RCTs and 21 cohort studies. They encompassed 3742 patients. Eleven interventions were compared (Figure 2). Patients receiving HAIC + A (HR = 0.25; 95%CI: 0.08-0.77), HAIC + TACE (HR = 0.32; 95%CI: 0.14-0.75), and HAIC (HR = 0.51; 95%CI: 0.33-0.78) demonstrated significantly improved PFS outcomes than patients receiving Sora (Figure 3). Patients receiving HAIC + Lenv + A (HR = 0.26; 95%CI: 0.06-1.10) and HAIC + anti-programmed death 1 (PD-1) (HR = 0.33; 95%CI: 0.11-1.02) displayed marginally better PFS than patients receiving Sora, though insignificant. According to the treatment ranking analysis, HAIC + A (P-score: 0.79) was associated with the highest likelihood of favorable PFS outcomes, followed by HAIC + Lenv + A (P-score: 0.75) and HAIC + TACE + S-1 (S-1: A composite preparation of a 5-fluorouracil prodrug) (P-score: 0.71). Table 1 summarizes the direct and indirect comparisons of the interventions for PFS.
Network meta-analysis of CR: CR data were extracted from 35 articles, including 8 RCTs and 27 cohort studies. They encompassed 3867 patients. Nine interventions were compared (Figure 2). Patients receiving HAIC + Sora (OR = 7.62; 95%CI: 2.55-22.77) and HAIC (OR = 2.86; 95%CI: 1.37-5.98) demonstrated significantly improved CR outcomes (Figure 3). Patients receiving HAIC + TACE + S-1 (OR = 3.36; 95%CI: 0.42-26.67) and HAIC + TACE (OR = 3.06; 95%CI: 0.69-13.61) displayed marginally better CR outcomes than patients receiving to Sora, though insignificant. The most favorable CR outcomes were associated with HAIC + Sora (P-score: 0.86), followed by HAIC + PD-1 (P-score: 0.65). HAIC + TACE + S-1 (P-score: 0.62) and HAIC (P-score: 0.60) were positioned in fifth and sixth, respectively. The P-scores suggest better CR outcomes with BSC, compared with Sora. The small sample size for BSC may affect this statistically insignificant observation (BSC: OR = 1.48; 95%CI: 0.06-35.69). Table 1 summarizes direct and indirect comparisons of the interventions for CR.
Network meta-analysis of PR: PR data were extracted from 35 articles, including 8 RCTs and 27 cohort studies. They encompassed 3867 patients. Nine interventions were compared (Figure 2). Patients receiving HAIC + TACE + S-1 (OR = 13.29; 95%CI: 3.63-48.61), HAIC + Lenv (OR = 8.37; 95%CI: 4.32-16.23), HAIC + PD-1 (OR = 7.73; 95%CI: 3.81-15.68), HAIC + TACE (OR = 5.67; 95%CI: 2.49-12.89), HAIC + Sora (OR = 5.50; 95%CI: 3.40-8.89), HAIC (OR = 5.45; 95%CI: 3.57-8.30), and TACE (OR = 2.63; 95%CI: 1.57-4.41) demonstrated significantly improved PR outcomes than patients receiving Sora (Figure 3). Furthermore, the most favorable PR outcomes were associated with HAIC + TACE + S-1 (P-score: 0.90), followed by HAIC + Lenv (P-score: 0.79) and HAIC + PD-1 (P-score: 0.74). Additionally, combination therapy for HAIC was highly ranked and generated statistically significant scores. Table 1 summarizes direct and indirect comparisons of the interventions for PR.
Network meta-analysis of ORR: ORR data were extracted from 35 articles, including 8 RCTs and 27 cohort studies. They encompassed 3867 patients. Nine interventions were compared (Figure 2). Patients receiving HAIC + TACE + S-1 (OR = 17.88; 95%CI: 2.22-143.80), HAIC + Lenv (OR = 13.92; 95%CI: 3.25-59.60), HAIC + PD-1 (OR =10.14; 95%CI: 2.09-49.09), HAIC + TACE (OR = 8.97; 95%CI: 2.39-33.65), HAIC + Sora (OR = 9.13; 95%CI: 3.87-21.51), and HAIC (OR = 7.15; 95%CI: 4.06-12.58) demonstrated significantly improved ORR outcomes than patients receiving Sora (Figure 3). Furthermore, the most favorable ORR outcomes were associated with HAIC + TACE + S-1 (P-score: 0.79), followed by HAIC + Lenv (P-score: 0.75) and HAIC + PD-1 (P-score: 0.64). Table 1 summarizes direct and indirect comparisons of the interventions for ORR.
Network meta-analysis of DCR: DCR data were extracted from 35 articles, including 8 RCTs and 27 cohort studies. They encompassed 3867 patients. Nine interventions were compared (Figure 2). Patients receiving HAIC + TACE + S-1 (OR = 8.52; 95%CI: 1.56-46.49), HAIC + PD-1 (OR = 7.26; 95%CI: 1.97-26.84), HAIC + TACE (OR = 4.18; 95%CI: 1.43-12.17), HAIC (OR = 2.89; 95%CI: 1.99-4.19), and HAIC + Sora (OR = 1.95; 95%CI: 1.01-3.75) demonstrated significantly improved DCR outcomes than patients receiving Sora (Figure 3). Furthermore, the most favorable DCR outcomes were associated with HAIC + TACE + S-1 (P-score: 0.88), followed by HAIC + PD-1 (P-score: 0.86) and HAIC + TACE (P-score: 0.71). The League Table summarizes direct and indirect comparisons of the interventions for DCR.
Network meta-analysis of any grade AEs: Any grade AE data were extracted from 12 articles, including 4 RCTs and 12 cohort studies. They encompassed 2095 patients. Seven interventions were compared (Figure 2). Patients receiving HAIC (OR = 0.48; 95%CI: 0.25-0.92) demonstrated a lower trend of any grade AEs than patients receiving Sora (Figure 3). Furthermore, HAIC (P-score: 0.85) generated the lowest incidence of any grade AEs (a higher ranking indicated a lower incidence). Table 2 summarizes direct and indirect comparisons of the interventions for any grade AEs. HAIC + A (OR = 0.19; 95%CI: 0.04-0.84), HAIC (OR = 0.16; 95%CI: 0.05-0.54), and HAIC + Lenv (OR = 0.19; 95%CI: 0.05-0.72) exhibited lower trends of any grade AEs, compared with TACE.
| League table | |||||||||
| Any grade AEs | |||||||||
| HAIC | 0.86 (0.47; 1.56) | 0.88 (0.35; 2.22) | 0.48 (0.25; 0.92) | 0.16 (0.05; 0.54) | |||||
| 0.86 (0.47; 1.56) | HAIC + Lenv | 0.76 (0.38; 1.55) | |||||||
| 0.88 (0.35; 2.22) | 1.03 (0.34; 3.09) | HAIC + A | |||||||
| 0.66 (0.26; 1.66) | 0.76 (0.38; 1.55) | 0.74 (0.20; 2.75) | HAIC + Lenv + A | ||||||
| 0.48 (0.25; 0.92) | 0.56 (0.23; 1.35) | 0.54 (0.18; 1.68) | 0.73 (0.24; 2.27) | Sora | 0.53 (0.22; 1.24) | ||||
| 0.25 (0.09; 0.74) | 0.29 (0.09; 1.00) | 0.29 (0.07; 1.18) | 0.38 (0.09; 1.59) | 0.53 (0.22; 1.24) | HAIC + Sora | ||||
| 0.16 (0.05; 0.54) | 0.19 (0.05; 0.72) | 0.19 (0.04; 0.84) | 0.25 (0.06; 1.13) | 0.34 (0.09; 1.33) | 0.65 (0.13; 3.23) | TACE | |||
| 3-4 grade AEs | |||||||||
| HAIC + PD-1 | 0.26 (0.01; 6.54) | ||||||||
| 0.26 (0.01; 6.54) | HAIC | 0.96 (0.22; 4.26) | 0.63 (0.30; 1.31) | 0.49 (0.09; 2.69) | 0.32 (0.13; 0.75) | ||||
| 0.25 (0.01; 8.71) | 0.96 (0.22; 4.26) | HAIC + Lenv | 0.54 (0.05; 5.76) | ||||||
| 0.16 (0.01; 4.45) | 0.63 (0.30; 1.31) | 0.65 (0.12; 3.43) | Sora | 0.42 (0.14; 1.23) | |||||
| 0.13 (0.00; 9.57) | 0.52 (0.03; 8.50) | 0.54 (0.05; 5.76) | 0.83 (0.05; 14.97) | HAIC + LENV + A | |||||
| 0.13 (0.00; 4.90) | 0.49 (0.09; 2.69) | 0.51 (0.05; 4.89) | 0.79 (0.12; 5.02) | 0.96 (0.04; 25.23) | HAIC + A | ||||
| 0.07 (0.00; 2.97) | 0.29 (0.05; 1.70) | 0.30 (0.03; 3.04) | 0.46 (0.07; 3.16) | 0.56 (0.02; 15.36) | 0.58 (0.05; 6.78) | HAIC + TACE | 1.10 (0.23; 5.19) | ||
| 0.08 (0.00; 2.32) | 0.32 (0.13; 0.75) | 0.33 (0.06; 1.84) | 0.51 (0.16; 1.59) | 0.61 (0.03; 11.50) | 0.64 (0.10; 4.30) | 1.10 (0.23; 5.19) | TACE | ||
| 0.07 (0.00; 2.21) | 0.26 (0.07; 0.97) | 0.27 (0.04; 1.97) | 0.42 (0.14; 1.23) | 0.51 (0.02; 11.17) | 0.53 (0.06; 4.51) | 0.91 (0.10; 8.28) | 0.83 (0.17; 3.97) | HAIC + Sora | |
| 3-4 grade AEs for thrombocytopenia | |||||||||
| Sora | 0.27 (0.15; 0.46) | 0.19 (0.01; 4.08) | 0.11 (0.05; 0.24) | ||||||
| 0.85 (0.02; 29.67) | HAIC + TACE + S-1 | 0.36 (0.01; 8.96) | |||||||
| 0.30 (0.07; 1.37) | 0.36 (0.01; 8.96) | HAIC + TACE | 0.56 (0.20; 1.55) | ||||||
| 0.27 (0.15; 0.46) | 0.32 (0.01; 11.60) | 0.89 (0.18; 4.42) | HAIC + Sora | ||||||
| 0.24 (0.00; 14.92) | 0.28 (0.00; 58.91) | 0.78 (0.01; 56.14) | 0.88 (0.01; 57.66) | HAIC + Lenv | 0.47 (0.01; 27.94) | ||||
| 0.19 (0.01; 4.08) | 0.22 (0.00; 24.56) | 0.63 (0.02; 19.15) | 0.70 (0.03; 15.97) | 0.80 (0.00; 140.28) | HAIC + RT | ||||
| 0.17 (0.06; 0.52) | 0.20 (0.01; 5.89) | 0.56 (0.20; 1.55) | 0.63 (0.18; 2.20) | 0.72 (0.01; 46.14) | 0.90 (0.03; 23.63) | TACE | 0.66 (0.29; 1.47) | ||
| 0.08 (0.00; 4.22) | 0.09 (0.00; 17.21) | 0.25 (0.00; 15.93) | 0.29 (0.01; 16.31) | 0.33 (0.00; 94.05) | 0.41 (0.00; 63.25) | 0.45 (0.01; 24.96) | HAIC + A | 1.45 (0.03; 73.68) | |
| 0.11 (0.05; 0.24) | 0.13 (0.00; 4.24) | 0.37 (0.10; 1.35) | 0.42 (0.16; 1.07) | 0.47 (0.01; 27.94) | 0.59 (0.02; 14.00) | 0.66 (0.29; 1.47) | 1.45 (0.03; 73.68) | HAIC | 0.54 (0.03; 8.82) |
| 0.06 (0.00; 1.09) | 0.07 (0.00; 6.15) | 0.20 (0.01; 4.33) | 0.23 (0.01; 4.28) | 0.26 (0.00; 35.95) | 0.32 (0.00; 21.78) | 0.36 (0.02; 6.48) | 0.79 (0.01; 97.36) | 0.54 (0.03; 8.82) | HAIC + PD-1 |
| 3-4 grade AEs for elevated total bilirubin | |||||||||
| HAIC + Lenv | 0.57 (0.01; 29.09) | 0.34 (0.03; 4.22) | |||||||
| 0.65 (0.02; 23.55) | HAIC + RT | 0.49 (0.04; 5.61) | |||||||
| 0.56 (0.01; 33.37) | 0.86 (0.01; 51.75) | HAIC + PD-1 | 0.60 (0.02; 14.98) | ||||||
| 0.57 (0.01; 29.09) | 0.87 (0.00; 178.10) | 1.01 (0.00; 293.95) | HAIC + LENV + A | ||||||
| 0.34 (0.03; 4.22) | 0.52 (0.04; 6.61) | 0.60 (0.02; 14.98) | 0.60 (0.01; 63.73) | HAIC | 0.94 (0.46; 1.89) | 0.23 (0.01; 5.65) | 0.24 (0.09; 0.65) | ||
| 0.32 (0.02; 4.68) | 0.49 (0.04; 6.06) | 0.57 (0.02; 16.06) | 0.56 (0.00; 65.61) | 0.94 (0.37; 2.38) | HAIC + Sora | 1.00 (0.54; 1.84) | |||
| 0.32 (0.02; 4.36) | 0.49 (0.04; 5.61) | 0.57 (0.02; 15.15) | 0.56 (0.00; 62.97) | 0.94 (0.46; 1.89) | 1.00 (0.54; 1.84) | Sora | |||
| 0.16 (0.01; 2.86) | 0.25 (0.01; 4.46) | 0.29 (0.01; 9.46) | 0.28 (0.00; 36.96) | 0.47 (0.12; 1.90) | 0.51 (0.10; 2.68) | 0.51 (0.11; 2.39) | HAIC + TACE | 0.50 (0.19; 1.30) | |
| 0.08 (0.00; 4.58) | 0.12 (0.00; 7.10) | 0.14 (0.00; 12.89) | 0.14 (0.00; 39.31) | 0.23 (0.01; 5.65) | 0.24 (0.01; 6.87) | 0.24 (0.01; 6.50) | 0.48 (0.01; 15.85) | HAIC + A | |
| 0.08 (0.01; 1.22) | 0.12 (0.01; 1.90) | 0.14 (0.00; 4.16) | 0.14 (0.00; 16.88) | 0.24 (0.09; 0.65) | 0.25 (0.06; 1.00) | 0.25 (0.07; 0.87) | 0.50 (0.19; 1.30) | 1.05 (0.04; 30.26) | TACE |
Network meta-analysis of grade 3 to 4 AEs: Data of grade 3 to 4 AEs were extracted from 16 articles, comprising 5 RCTs and 11 cohort studies. They encompassed 2449 patients. Seven interventions were compared (Figure 2). Patients receiving HAIC + PD-1 (OR = 0.16; 95%CI: 0.01-4.45), HAIC (OR = 0.63; 95%CI: 0.30-1.31), and HAIC + Lenv (OR = 0.65; 95%CI: 0.12-3.43) demonstrated marginally lower trends of grade 3 to 4 AEs than patients receiving Sora, though insignificant (Figure 3). Figure 3 illustrates the P-score for the treatment ranking analysis. Table 2 summarizes direct and indirect comparisons of the interventions for grade 3 to 4 AEs. HAIC demonstrated lower trends of grade 3 to 4 AEs than HAIC + Sora (OR = 0.26; 95%CI: 0.07-0.97) and TACE (OR = 0.32; 95%CI: 0.13-0.75).
Additionally, we examined thrombocytopenia and elevated total bilirubin, the most frequently reported AEs. Sora demonstrated lower trends of grade 3 to 4 AEs for thrombocytopenia than other interventions (Figure 3). TACE demonstrated higher trends of grade 3 to 4 AEs for elevated total bilirubin than Sora (OR = 0.25; 95%CI: 0.07-0.87) and HAIC (OR = 0.24; 95%CI: 0.09-0.65), consistent with the findings of a phase 3 study[48]. Table 2 summarizes direct and indirect comparisons of the interventions for any grade and grade 3 to 4 AEs.
Supplementary material describes the results of quality assessment, publication bias, inconsistency, and heterogeneity analyses.
In this study, we incorporated direct and indirect evidence to compare the efficacy and safety of HAIC and combination therapy in patients with advanced HCC. HAIC was considered a better choice than Sora and TACE regarding efficacy and safety. Moreover, combined interventions displayed marginally better efficacy than HAIC monotherapy. HAIC and its combination are effective for advanced HCC[12,56,57]. The mechanism by which HAIC protects against HCC consists of the blood supply characteristics of the liver and HCC cells. The liver primarily receives its blood supply from the hepatic artery and portal vein, with only approximately 30% of the blood coming from the hepatic artery. In contrast, HCC cells receive approximately 90% of the blood supply from the portal vein[58]. Consequently, chemotherapeutic drugs administered into the hepatic artery predominantly reach the HCC cells, with only a fraction absorbed into the healthy liver tissues. This phenomenon allows HAIC to maintain a high concentration of chemotherapeutic drugs within the HCC cells and a low concentration in other tissues. Additionally, the liver is the primary metabolizing organ; thus, chemotherapeutic drugs reaching the healthy liver tissue can be metabolized to a limited extent, reducing the likelihood of systemic AEs. These findings indicate that HAIC generated a lower incidence of any grade and grade 3 to 4 AEs than TACE. Moreover, HAIC generated a significantly lower incidence of any grade AEs than Sora. In a phase 3 trial, the TACE group exhibited a higher incidence of AEs than the HAIC group (30% vs 19%, P = 0.03)[13].
HAIC + Lenv + A, HAIC + A, HAIC + Lenv, HAIC + Sora, and HAIC outperformed Sora and TACE in improving OS. Additionally, HAIC + A, HAIC + TACE, and HAIC outperformed Sora and TACE in improving PFS. HAIC + Lenv + A and HAIC + A demonstrated better OS and PFS outcomes. This funding may be attributed to ablation that destroys tumors and generates an immune response with anti-tumor effects by activating tumor-specific antigens in the tumor microenvironment. Additionally, these tumor-specific antigens activate anti-tumor responses to vascular endothelial growth factor inhibitors[59]. Furthermore, HAIC combination therapy was superior to monotherapy, though insignificant. A phase 3 study indicated that HAIC + Sora demonstrated higher OS [HR = 0.35; 95%CI: 0.26-0.48, 13.37 (10.27-16.46) vs 7.13 (6.28-7.98) months, P < 0.001] and PFS [HR = 0.33; 95%CI: 0.25-0.43, 7.03 (6.05-8.02) vs 2.6 (2.15-3.05) months, P < 0.001] than HAIC alone[18]. Similarly, a phase 2 study demonstrated better OS and PFS outcomes for HAIC + Sora, compared with Sora for advanced HCC[16]. Furthermore, a phase 3 study indicated that HAIC was superior to TACE, resulting in longer OS [HR = 0.58; 95%CI: 0.45-0.75, 23.1 (18.5-27.7) vs 16.1 (14.3-17.9) months, P < 0.001] and PFS [HR = 0.57; 95%CI: 0.45-0.72, 9.6 (7.4-11.9) vs 5.4 (3.8-7.0) months, P < 0.001][13]. Similar trends can be observed for CR, PR, ORR, and DCR. HAIC resulted in better CR, PR, ORR, and DCR outcomes than Sora and TACE.
Furthermore, combination therapy was more effective than HAIC monotherapy. The statistical insignificance warrants additional clinical trials. You et al[41] reported significantly higher 1-, 2-, and 3-year OS and PFS rates in the HAIC + A group, compared with the HAIC group (OS: 64.3% vs 91.1%, 27.7% vs 74.3%, 16.0% vs 64.1%; PFS: 32.0% vs 61.2%, 16.1% vs 34.4%, 12.1% vs 29.5%; both P < 0.001). Long et al[37] reported significantly higher 1-, 2-, and 3-year cumulative OS rates in the HAIC + Lenv group, compared with the HAIC group (P < 0.001), without significant differences in the PFS, CR, PR, ORR, and DCR. Yuan et al[24] reported significantly increased ORR in the HAIC + Lenv group, compared with the HAIC group, despite no significant differences in the DCR, OS, and PFS between the groups. Mei et al[25] reported that the HAIC + PD-1 group achieved higher OS (HR = 0.62; 95%CI: 0.34-0.91), PFS (HR = 0.65; 95%CI: 0.43-0.87), and DCR (83% vs 66%; P = 0.006), compared with the HAIC group. Nagai et al[51] reported higher OS for HAIC + Sora than for HAIC.
This network meta-analysis has several limitations. First, we did not account for the impact of the HAIC dosing regimen and the dosage of other drugs on the efficacy. For instance, a phase 2 study demonstrated significantly better OS outcomes with Sora + HAIC using low-dose cisplatin than Sora[17]. By contrast, a phase 3 study demonstrated no significant difference in the OS between patients receiving Sora + HAIC using low-dose 5-fluorouracil[19]. This variation may have contributed to significant heterogeneity in some of the comparisons. Second, we included PD-1 without specifying the drug. Some relevant studies were not included because of their small number. For example, a cohort study demonstrated that HAIC + toripalimab was superior to Lenv, resulting in longer OS [17.13 (13.99-20.27) vs 10.1 (8.14-12.06) months; HR = 0.50, 95%CI: 0.31-0.81; P = 0.005], higher DCR (86.8% vs 69.2%, P = 0.002), and higher ORR (47.2% vs 9.2%, P < 0.001)[60]. Third, Japanese guidelines recommend HAIC as a standard treatment for advanced HCC with portal vein tumor thrombus[7]. However, we could not conduct a subgroup network meta-analysis for portal vein tumor thrombus because of the limited number of studies reporting these outcomes.
HAIC is a relatively better choice for advanced HCC than Sora and TACE. It demonstrates a significantly lower trend of any grade AEs than Sora and TACE and of grade 3 to 4 AEs than TACE. Furthermore, combined interventions demonstrated modestly improved OS, PFS, CR, PR, ORR, and DCR, compared with HAIC alone, according to the treatment ranking analysis.
We would like to thank Dr. Zou for his guidance on the statistical methodology of this study.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55686] [Article Influence: 7955.1] [Reference Citation Analysis (132)] |
| 2. | Rinaldi L, Nascimbeni F, Giordano M, Masetti C, Guerrera B, Amelia A, Fascione MC, Ballestri S, Romagnoli D, Zampino R, Nevola R, Baldelli E, Iuliano N, Rosato V, Lonardo A, Adinolfi LE. Clinical features and natural history of cryptogenic cirrhosis compared to hepatitis C virus-related cirrhosis. World J Gastroenterol. 2017;23:1458-1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64104] [Article Influence: 16026.0] [Reference Citation Analysis (174)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6010] [Article Influence: 858.6] [Reference Citation Analysis (3)] |
| 5. | Fitzmaurice C, Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 2006 to 2016: A systematic analysis for the Global Burden of Disease study. J Clin Oncol. 2018;36. [RCA] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, Yim HJ, Yeon JE, Byun KS. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology. 2018;68:977-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 7. | Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T, Yamakado K; Liver Cancer Study Group of Japan. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 488] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 8. | He M, Liu S, Lai Z, Du Z, Li Q, Xu L, Kan A, Shen J, Shi M. Hepatic arterial infusion chemotherapy for patients with hepatocellular carcinoma: Applicability in Western countries. Curr Opin Pharmacol. 2023;70:102362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 9. | Rücker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi-arm studies in network meta-analysis. Stat Med. 2014;33:4353-4369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 10. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 39485] [Article Influence: 9871.3] [Reference Citation Analysis (2)] |
| 11. | Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3100] [Cited by in RCA: 5602] [Article Influence: 700.3] [Reference Citation Analysis (0)] |
| 12. | Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li SL, Deng HJ, He M, Mu LW, Zhao M. Arterial Chemotherapy of Oxaliplatin Plus Fluorouracil Versus Sorafenib in Advanced Hepatocellular Carcinoma: A Biomolecular Exploratory, Randomized, Phase III Trial (FOHAIC-1). J Clin Oncol. 2022;40:468-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 13. | Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, Wei W, Zhang YJ, Guo Y, Guo RP, Chen MS, Shi M. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J Clin Oncol. 2022;40:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 238] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 14. | Kondo M, Morimoto M, Kobayashi S, Ohkawa S, Hidaka H, Nakazawa T, Aikata H, Hatanaka T, Takizawa D, Matsunaga K, Okuse C, Suzuki M, Taguri M, Ishibashi T, Numata K, Maeda S, Tanaka K. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer. 2019;19:954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Choi JH, Chung WJ, Bae SH, Song DS, Song MJ, Kim YS, Yim HJ, Jung YK, Suh SJ, Park JY, Kim DY, Kim SU, Cho SB. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2018;82:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 16. | Zheng K, Zhu X, Fu S, Cao G, Li WQ, Xu L, Chen H, Wu D, Yang R, Wang K, Liu W, Wang H, Bao Q, Liu M, Hao C, Shen L, Xing B, Wang X. Sorafenib Plus Hepatic Arterial Infusion Chemotherapy versus Sorafenib for Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis: A Randomized Trial. Radiology. 2022;303:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 17. | Ikeda M, Shimizu S, Sato T, Morimoto M, Kojima Y, Inaba Y, Hagihara A, Kudo M, Nakamori S, Kaneko S, Sugimoto R, Tahara T, Ohmura T, Yasui K, Sato K, Ishii H, Furuse J, Okusaka T. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol. 2016;27:2090-2096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 18. | He M, Li Q, Zou R, Shen J, Fang W, Tan G, Zhou Y, Wu X, Xu L, Wei W, Le Y, Zhou Z, Zhao M, Guo Y, Guo R, Chen M, Shi M. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019;5:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 377] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 19. | Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, Aikata H, Nagano H, Hatano E, Sasaki Y, Hino K, Kumada T, Yamamoto K, Imai Y, Iwadou S, Ogawa C, Okusaka T, Kanai F, Akazawa K, Yoshimura KI, Johnson P, Arai Y; SILIUS study group. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 225] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 20. | Guo JH, Liu SX, Gao S, Kou FX, Zhang X, Wu D, Li XT, Chen H, Wang XD, Liu P, Zhang PJ, Xu HF, Cao G, Zhu LZ, Yang RJ, Zhu X. Transarterial chemoembolization with hepatic arterial infusion chemotherapy plus S-1 for hepatocellular carcinoma. World J Gastroenterol. 2020;26:3975-3988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Song DS, Song MJ, Bae SH, Chung WJ, Jang JY, Kim YS, Lee SH, Park JY, Yim HJ, Cho SB, Park SY, Yang JM. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol. 2015;50:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Kim HY, Kim JD, Bae SH, Park JY, Han KH, Woo HY, Choi JY, Yoon SK, Jang BK, Hwang JS, Kim SG, Kim YS, Seo YS, Yim HJ, Um SH; Korean Liver Cancer Study Group. A comparative study of high-dose hepatic arterial infusion chemotherapy and transarterial chemoembolization using doxorubicin for intractable, advanced hepatocellular carcinoma. Korean J Hepatol. 2010;16:355-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Yang H, Woo HY, Lee SK, Han JW, Jang B, Nam HC, Lee HL, Lee SW, Song DS, Song MJ, Oh JS, Chun HJ, Jang JW, Lozada A, Bae SH, Choi JY, Yoon SK. A comparative study of sorafenib and metronomic chemotherapy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma with poor liver function. Clin Mol Hepatol. 2017;23:128-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Yuan W, Yue W, Wen H, Wang X, Wang Q. Analysis on Efficacy of Hepatic Artery Infusion Chemotherapy with or without Lenvatinib for Unresectable Hepatocellular Carcinoma. Eur Surg Res. 2023;64:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Mei J, Li SH, Li QJ, Sun XQ, Lu LH, Lin WP, Zheng L, Chen MS, Shi M, Wei W, Guo RP. Anti-PD-1 Immunotherapy Improves the Efficacy of Hepatic Artery Infusion Chemotherapy in Advanced Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:167-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Gao S, Zhang PJ, Guo JH, Chen H, Xu HF, Liu P, Yang RJ, Zhu X. Chemoembolization alone vs combined chemoembolization and hepatic arterial infusion chemotherapy in inoperable hepatocellular carcinoma patients. World J Gastroenterol. 2015;21:10443-10452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 27. | Nakano M, Niizeki T, Nagamatsu H, Tanaka M, Kuromatsu R, Satani M, Okamura S, Iwamoto H, Shimose S, Shirono T, Noda Y, Koga H, Torimura T; Kurume Liver Cancer Study Group of Japan. Clinical effects and safety of intra-arterial infusion therapy of cisplatin suspension in lipiodol combined with 5-fluorouracil versus sorafenib, for advanced hepatocellular carcinoma with macroscopic vascular invasion without extra-hepatic spread: A prospective cohort study. Mol Clin Oncol. 2017;7:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Liu BJ, Gao S, Zhu X, Guo JH, Kou FX, Liu SX, Zhang X, Wang XD, Cao G, Chen H, Liu P, Zhu LZ, Xu HF, Yang RJ. Combination Therapy of Chemoembolization and Hepatic Arterial Infusion Chemotherapy in Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis Compared with Chemoembolization Alone: A Propensity Score-Matched Analysis. Biomed Res Int. 2021;2021:6670367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (2)] |
| 29. | Chen S, Yuan B, Yu W, Wang X, He C, Chen C. Comparison of Arterial Infusion Chemotherapy and Chemoembolization for Locally Advanced Hepatocellular Carcinoma: a Multicenter Retrospective Study. J Gastrointest Surg. 2022;26:2292-2300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Kodama K, Kawaoka T, Aikata H, Uchikawa S, Inagaki Y, Hatooka M, Morio K, Nakahara T, Murakami E, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Masaki K, Honda Y, Mori N, Takaki S, Tsuji K, Kohno H, Kohno H, Moriya T, Nonaka M, Hyogo H, Aisaka Y, Chayama K. Comparison of clinical outcome of hepatic arterial infusion chemotherapy and sorafenib for advanced hepatocellular carcinoma according to macrovascular invasion and transcatheter arterial chemoembolization refractory status. J Gastroenterol Hepatol. 2018;33:1780-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Kang MK, Park JG, Lee HJ. Comparison of clinical outcomes between sorafenib and hepatic artery infusion chemotherapy in advanced hepatocellular carcinoma: A STROBE-compliant article. Medicine (Baltimore). 2018;97:e0611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Nemoto T, Matsuda H, Nosaka T, Saito Y, Ozaki Y, Hayama R, Naito T, Takahashi K, Ofuji K, Ohtani M, Hiramatsu K, Suto H, Nakamoto Y. Comparison of hepatic arterial infusion chemotherapy and sorafenib in elderly patients with advanced hepatocellular carcinoma: A case series. Mol Clin Oncol. 2014;2:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Kawaoka T, Aikata H, Hyogo H, Morio R, Morio K, Hatooka M, Fukuhara T, Kobayashi T, Naeshiro N, Miyaki D, Hiramatsu A, Imamura M, Kawakami Y, Takahashi S, Waki K, Tsuji K, Kohno H, Kohno H, Moriya T, Chayama K. Comparison of hepatic arterial infusion chemotherapy versus sorafenib monotherapy in patients with advanced hepatocellular carcinoma. J Dig Dis. 2015;16:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Kodama K, Kawaoka T, Aikata H, Uchikawa S, Nishida Y, Inagaki Y, Hatooka M, Morio K, Nakahara T, Murakami E, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Masaki K, Honda Y, Mori N, Takaki S, Tsuji K, Kohno H, Kohno H, Moriya T, Nonaka M, Hyogo H, Aisaka Y, Kimura T, Nagata Y, Chayama K. Comparison of Outcome of Hepatic Arterial Infusion Chemotherapy Combined with Radiotherapy and Sorafenib for Advanced Hepatocellular Carcinoma Patients with Major Portal Vein Tumor Thrombosis. Oncology. 2018;94:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Shiozawa K, Watanabe M, Ikehara T, Kogame M, Matsui T, Okano N, Kikuchi Y, Nagai H, Ishii K, Makino H, Igarashi Y, Sumino Y. Comparison of Sorafenib and Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma: A Propensity Score Matching Study. Hepatogastroenterology. 2014;61:885-891. [PubMed] |
| 36. | Ahn YE, Suh SJ, Yim HJ, Seo YS, Yoon EL, Kim TH, Lee YS, Yim SY, Kim HR, Kang SH, Jung YK, Kim JH, Yeon JE, Um SH, Byun KS. Comparison of Sorafenib versus Hepatic Arterial Infusion Chemotherapy-Based Treatment for Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Gut Liver. 2021;15:284-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Long F, Chen S, Li R, Lin Y, Han J, Guo J, Chen Y, Li C, Song P. Efficacy and safety of HAIC alone vs. HAIC combined with lenvatinib for treatment of advanced hepatocellular carcinoma. Med Oncol. 2023;40:147. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Liu Y, Qiao Y, Zhou M, Guo J, Lin Y, Li W, An C, Li C. Efficacy and safety of hepatic arterial infusion chemotherapy combined with lenvatinib and sequential ablation in the treatment of advanced hepatocellular carcinoma. Cancer Med. 2023;12:5436-5449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 39. | Guo W, Gao J, Zhuang W, Wu Z, Li B, Chen S. Efficacy and safety of hepatic arterial infusion chemotherapy combined with transarterial embolization for unresectable hepatocellular carcinoma: A propensity score-matching cohort study. JGH Open. 2020;4:477-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Wu Z, Gao J, Zhuang W, Yang J, Guo W. Efficacy and safety of transcatheter arterial chemoembolization plus hepatic arterial infusion chemotherapy in the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis in the main trunk. J Cancer Res Ther. 2022;18:345-351. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | You H, Liu X, Guo J, Lin Y, Zhang Y, Li C. Hepatic arterial infusion chemotherapy and sequential ablation treatment in large hepatocellular carcinoma. Int J Hyperthermia. 2022;39:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 42. | Tsai WL, Lai KH, Liang HL, Hsu PI, Chan HH, Chen WC, Yu HC, Tsay FW, Wang HM, Tsai HC, Cheng JS. Hepatic arterial infusion chemotherapy for patients with huge unresectable hepatocellular carcinoma. PLoS One. 2014;9:e92784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Hu J, Bao Q, Cao G, Zhu X, Yang R, Ji X, Xu L, Zheng K, Li W, Xing B, Wang X. Hepatic Arterial Infusion Chemotherapy Using Oxaliplatin Plus 5-Fluorouracil Versus Transarterial Chemoembolization/Embolization for the Treatment of Advanced Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis. Cardiovasc Intervent Radiol. 2020;43:996-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 44. | Tsai WL, Sun WC, Chen WC, Chiang CL, Lin HS, Liang HL, Cheng JS. Hepatic arterial infusion chemotherapy vs transcatheter arterial embolization for patients with huge unresectable hepatocellular carcinoma. Medicine (Baltimore). 2020;99:e21489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Zaizen Y, Nakano M, Fukumori K, Yano Y, Takaki K, Niizeki T, Kuwaki K, Fukahori M, Sakaue T, Yoshimura S, Nakazaki M, Kuromatsu R, Okamura S, Iwamoto H, Shimose S, Shirono T, Noda Y, Kamachi N, Koga H, Torimura T. Hepatic Arterial Infusion Chemotherapy with Cisplatin versus Sorafenib for Intrahepatic Advanced Hepatocellular Carcinoma: A Propensity Score-Matched Analysis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Lyu N, Kong Y, Mu L, Lin Y, Li J, Liu Y, Zhang Z, Zheng L, Deng H, Li S, Xie Q, Guo R, Shi M, Xu L, Cai X, Wu P, Zhao M. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 47. | Chen S, Yuan B, Yu W, Wang X, He C, Chen C. Hepatic arterial infusion oxaliplatin plus raltitrexed and chemoembolization in hepatocellular carcinoma with portal vein invasion: A propensity score-matching cohort study. J Surg Oncol. 2022;126:1205-1214. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Li S, Lyu N, Han X, Li J, Lai J, He M, Deng H, Shi M, Wang H, Zhao M. Hepatic Artery Infusion Chemotherapy Using Fluorouracil, Leucovorin, and Oxaliplatin versus Transarterial Chemoembolization as Initial Treatment for Locally Advanced Hepatocellular Carcinoma: A Propensity Score-Matching Analysis. J Vasc Interv Radiol. 2021;32:1267-1276.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 49. | He MK, Le Y, Li QJ, Yu ZS, Li SH, Wei W, Guo RP, Shi M. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. 2017;36:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 50. | Sumie S, Yamashita F, Ando E, Tanaka M, Yano Y, Fukumori K, Sata M. Interventional radiology for advanced hepatocellular carcinoma: comparison of hepatic artery infusion chemotherapy and transcatheter arterial lipiodol chemoembolization. AJR Am J Roentgenol. 2003;181:1327-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Nagai H, Mukozu T, Ogino YU, Matsui D, Matsui T, Wakui N, Momiyama K, Igarashi Y, Sumino Y, Higai K. Sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombus. Anticancer Res. 2015;35:2269-2277. [PubMed] |
| 52. | Hiramine Y, Uto H, Imamura Y, Tabu K, Baba Y, Hiwaki T, Sho Y, Tahara K, Higashi H, Tamai T, Oketani M, Ido A, Tsubouchi H. Sorafenib and hepatic arterial infusion chemotherapy for unresectable advanced hepatocellular carcinoma: A comparative study. Exp Ther Med. 2011;2:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Moriguchi M, Aramaki T, Nishiofuku H, Sato R, Asakura K, Yamaguchi K, Tanaka T, Endo M, Itoh Y. Sorafenib versus Hepatic Arterial Infusion Chemotherapy as Initial Treatment for Hepatocellular Carcinoma with Advanced Portal Vein Tumor Thrombosis. Liver Cancer. 2017;6:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | Iwamoto H, Niizeki T, Nagamatsu H, Ueshima K, Nomura T, Kuzuya T, Kasai K, Kooka Y, Hiraoka A, Sugimoto R, Yonezawa T, Ishihara A, Deguchi A, Arai H, Shimose S, Shirono T, Nakano M, Okamura S, Noda Y, Kamachi N, Sakai M, Suzuki H, Aino H, Matsukuma N, Matsugaki S, Ogata K, Yano Y, Ueno T, Kajiwara M, Itano S, Fukuizumi K, Kawano H, Noguchi K, Tanaka M, Yamaguchi T, Kuromatsu R, Kawaguchi A, Koga H, Torimura T; New Fp Study Group; Kurume Liver Cancer Study Group Of Japan. Survival Benefit of Hepatic Arterial Infusion Chemotherapy over Sorafenib in the Treatment of Locally Progressed Hepatocellular Carcinoma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Jeong SW, Jang JY, Lee JE, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS, Kim KH, Kim YJ. The efficacy of hepatic arterial infusion chemotherapy as an alternative to sorafenib in advanced hepatocellular carcinoma. Asia Pac J Clin Oncol. 2012;8:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Obi S, Yoshida H, Toune R, Unuma T, Kanda M, Sato S, Tateishi R, Teratani T, Shiina S, Omata M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006;106:1990-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 57. | Ueshima K, Kudo M, Takita M, Nagai T, Tatsumi C, Ueda T, Kitai S, Ishikawa E, Yada N, Inoue T, Hagiwara S, Minami Y, Chung H. Hepatic arterial infusion chemotherapy using low-dose 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma. Oncology. 2010;78 Suppl 1:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Obi S, Sato S, Kawai T. Current Status of Hepatic Arterial Infusion Chemotherapy. Liver Cancer. 2015;4:188-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 59. | Qi X, Yang M, Ma L, Sauer M, Avella D, Kaifi JT, Bryan J, Cheng K, Staveley-O'Carroll KF, Kimchi ET, Li G. Synergizing sunitinib and radiofrequency ablation to treat hepatocellular cancer by triggering the antitumor immune response. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 60. | Xu YJ, Lai ZC, He MK, Bu XY, Chen HW, Zhou YM, Xu L, Wei W, Zhang YJ, Chen MS, Guo RP, Shi M, Li QJ. Toripalimab Combined With Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib for Advanced Hepatocellular Carcinoma. Technol Cancer Res Treat. 2021;20:15330338211063848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |