Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3635
Revised: May 24, 2024
Accepted: June 18, 2024
Published online: August 15, 2024
Processing time: 115 Days and 1.3 Hours
Curcumin originates from the natural herb turmeric, and its antitumor effects have been known about for a long time. However, the mechanism by which curcumin affects gastric cancer (GC) has not been elucidated.
To elucidate the potential mechanisms of curcumin in the treatment of GC.
Network pharmacological approaches were used to perform network analysis of Curcumin. We first analyzed Lipinski’s Rule of Five for the use of Curcumin. Curcumin latent targets were predicted using the PharmMapper, SwissTargetPrediction and DrugBank network databases. GC disease targets were mined through the GeneCard, OMIM, DrugBank and TTD network databases. Then, GO enrichment, KEGG enrichment, protein-protein interaction (PPI), and overall survival analyses were performed. The results were further verified through molecular docking, differential expression analysis and cell experiments.
We identified a total of 48 curcumin-related genes with 31 overlapping GC-related targets. The intersection targets between curcumin and GC have been enriched in 81 GO biological processes and 22 significant pathways. Following PPI analysis, 6 hub targets were identified, namely, estrogen receptor 1 (ESR1), epidermal growth factor receptor (EGFR), cytochrome P450 family 3 subfamily A member 4 (CYP3A4), mitogen-activated protein kinase 14 (MAPK
Curcumin can play an anti-GC role through a variety of targets, pathways and biological processes.
Core Tip: This study aimed to elucidate the therapeutic mechanisms of curcumin in gastric cancer (GC). Through network pharmacology, core targets correlating to poor survival in patients with GC were identified, including estrogen receptor 1
- Citation: Yang PH, Wei YN, Xiao BJ, Li SY, Li XL, Yang LJ, Pan HF, Chen GX. Curcumin for gastric cancer: Mechanism prediction via network pharmacology, docking, and in vitro experiments. World J Gastrointest Oncol 2024; 16(8): 3635-3650
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3635.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3635
Gastric cancer (GC) poses a significant threat to human health, with the highest incidence rates observed in East Asia, particularly in China where it ranks third among all cancer incidences, thus constituting a significant public health concern[1,2]. Despite ongoing advancements in treatment modalities for GC, the treatment prospects for patients with advanced GC remain unsatisfactory due to primary or acquired drug resistance and the limited availability of effective treatment methods. However, 5-year survival rate for individuals diagnosed with GC is below 20%[3]. Therefore, the demand for new agents, especially drugs extracted from natural resources, continues to increase.
Natural products are treasures from the natural world, serving not only as treatments for various ailments but also as crucial reservoirs for synthesizing therapeutic drugs[4]. Curcumin, a bioactive phytochemical compound belonging to the Zingiberaceae family, is naturally abundant in the rhizomes of turmeric plants and is notably rich in phenol (diferuloylmethane)[5]. Prior research has indicated that the primary physiological activity of curcumin lies in its anti-inflammatory and antioxidative effects; thus, curcumin exerts an efficient antimutational effect and plays a significant role in anti-inflammatory and antitumor treatment[6,7]. In addition, accumulating evidence has shown that curcumin can not only induce tumor apoptosis by regulating cyclin kinases and their inhibitors through the p53-dependent signaling pathway but also regulate several transcription factors, including the STAT protein, NF-κB protein, and multiple signaling pathways, to inhibit tumor vascular formation[8,9]. However, the mechanism by which curcumin affects GC has not been fully revealed.
Network pharmacology has become a powerful means to understand the potential action of traditional Chinese medicine in cancer treatment. To better explore the therapeutic potential of curcumin in treating GC, we employed a network pharmacology approach to investigate its mechanisms of action.
Lipinski's Rule of Five (RO5) is a set of guidelines used to assess the potential suitability of oral drugs by assessing drug likeness. Including the molecular weight (MW), hydrophobicity (XLogP3), polar surface area, rotatable bonds, H-bond acceptors, and H-bond donors. To explore curcumin’s similarity,
Identifying drug component targets is indispensable in drug discovery, facilitated by unique technologies that pinpoint the genes and proteins associated with the drug. To ensure the comprehensive collection of curcumin, the PharmMapper, Swiss TargetPrediction and DrugBank databases were used for prediction[12,13]. According to the Swiss TargetPrediction database, a correlation value ≥ 0.7 was selected as the molecular target of curcumin. To standardize the gene symbols and facilitate subsequent data sorting, the predicted curcumin targets were sent to UniProt database unified gene symbol after the molecular targets were obtained.
To comprehensively enrich the disease-related targets, we collected GC targets from various public databases, namely, the GeneCard database, the Online Mendelian Inheritance in Man (OMIM) database, the DrugBank database and the TTD database. Notably, targets with a score greater than 10 in the GeneCards database were selected as genes related to GC. We then integrated the disease targets of the four databases and represented each target with the gene symbol uniformly. Eventually, the targets related to the pathogenesis of GC were identified[14].
The target genes of curcumin and GC were introduced into the Weishengxin online mapping tool to identify the putative intersection genes of the drug molecule and the disease target.
The obtained curcumin-related genes and GC-related genes were integrated and then analyzed using the STRING database. Settings were adjusted to "Homo sapiens" and an interaction score of "≥ 0.4". After obtaining the protein interaction TSV format file, the data were imported to Cytoscape (version Cytoscape_v3.7.2) software for analysis via network visual processing, and subsequently, the curcumin-GC target visual network was successfully constructed.
We obtained the connection score between targets through the cythubba tool downloaded from Cytoscape (version Cytoscape_v3.7.2), and then, the hub targets were obtained by Maximal Clique Centrality (MCC) score screening. The network was visualized to obtain the length of the hub target network.
After integrating the hub targets, KEGG and GO analyses were performed on these targets using the DAVID database, proceeded with data mining and visual analysis of the impact of curcumin on GC.
Prognosis holds significant evaluative value in cancer treatment. The prognostic significance of hub targets in GC patients was assessed using the Kaplan-Meier plotter[15]. In the GC dataset, GC patients were divided into high and low expression groups. Hazard ratios, logarithmic rank P values, and their corresponding 95%CI were computed. Comparative analysis was performed using Kaplan-Meier survival curves.
To further ascertain the relationship and mechanism of the interaction of candidate proteins with curcumin, through molecular docking, we can effectively evaluate the binding affinity between curcumin and hub targets. Firstly, the three-dimensional structure of the small molecule was obtained and subjected to energy minimization, followed by saving it in mol2 format. Subsequently, the optimized small molecule was loaded into AutodockTools-1.5.6 for hydrogenation, charge calculation, and charge distribution. After configuring the rotatable bonds, the file was saved in "pdbqt" format. Next, the protein structure corresponding to the provided PDB ID was retrieved from the PDB database. Using PyMOL 2.3.0 software, water molecules and native ligands were removed from the protein crystal structure, which was then loaded into AutoDockTools (v1.5.6) for hydrogenation. Following this, the charge, distribution, and specific atom types of the protein were computed, and the data were saved in "pdbqt" format. Finally, molecular docking simulations were conducted using AutoDock Vina 1.1.2.
The expression levels of estrogen receptor 1 (ESR1), epidermal growth factor receptor (EGFR), cytochrome P450 family 3 subfamily A member 4 (CYP3A4), mitogen-activated protein kinase 14 (MAPK14), cytochrome P450 family 1 subfamily A member 2 (CYP1A2), and cytochrome p450 family 2 subfamily B member 6 (CYP2B6) genes in GC and normal tissues were analyzed using UALCAN (http://ualcan.path.uab.edu/). Utilizing the TCGA database, UALCAN was employed to study the expression levels of these hub targets in clinical tissue samples.
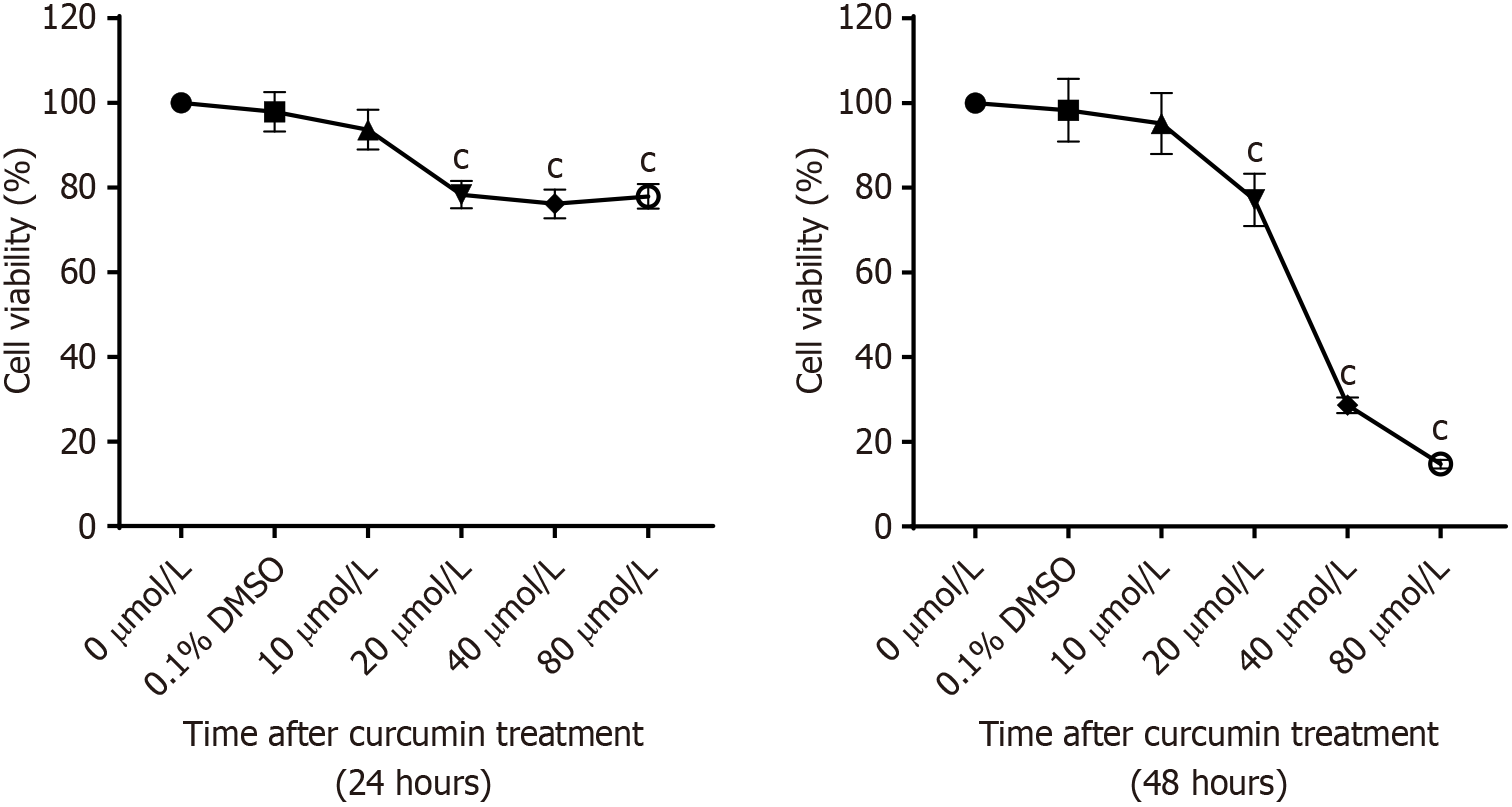
Curcumin was dissolved in 0.1% DMSO (manufactured by Sigma) and subsequently diluted in culture medium to prepare solutions with final concentrations of 0, 10, 20, 40, and 80 µmol/L. Log-phase BGC cells were seeded at a density of 2 × 106 cells per well in 6-well plates. GC BGC cells were subjected to treatment with varying concentrations of each concentration of curcumin as described above in five parallel wells and cultured for 24 hours and 48 hours. Following that, a certain amount of CCK8 was added to each well in a 96-well plate, and the plate was placed in a 37 °C incubator in the dark for 1.5 hours. Absorbance values were measured at a wavelength of 472 nm using a microplate reader, and data were statistically analyzed to calculate the cell survival rate.
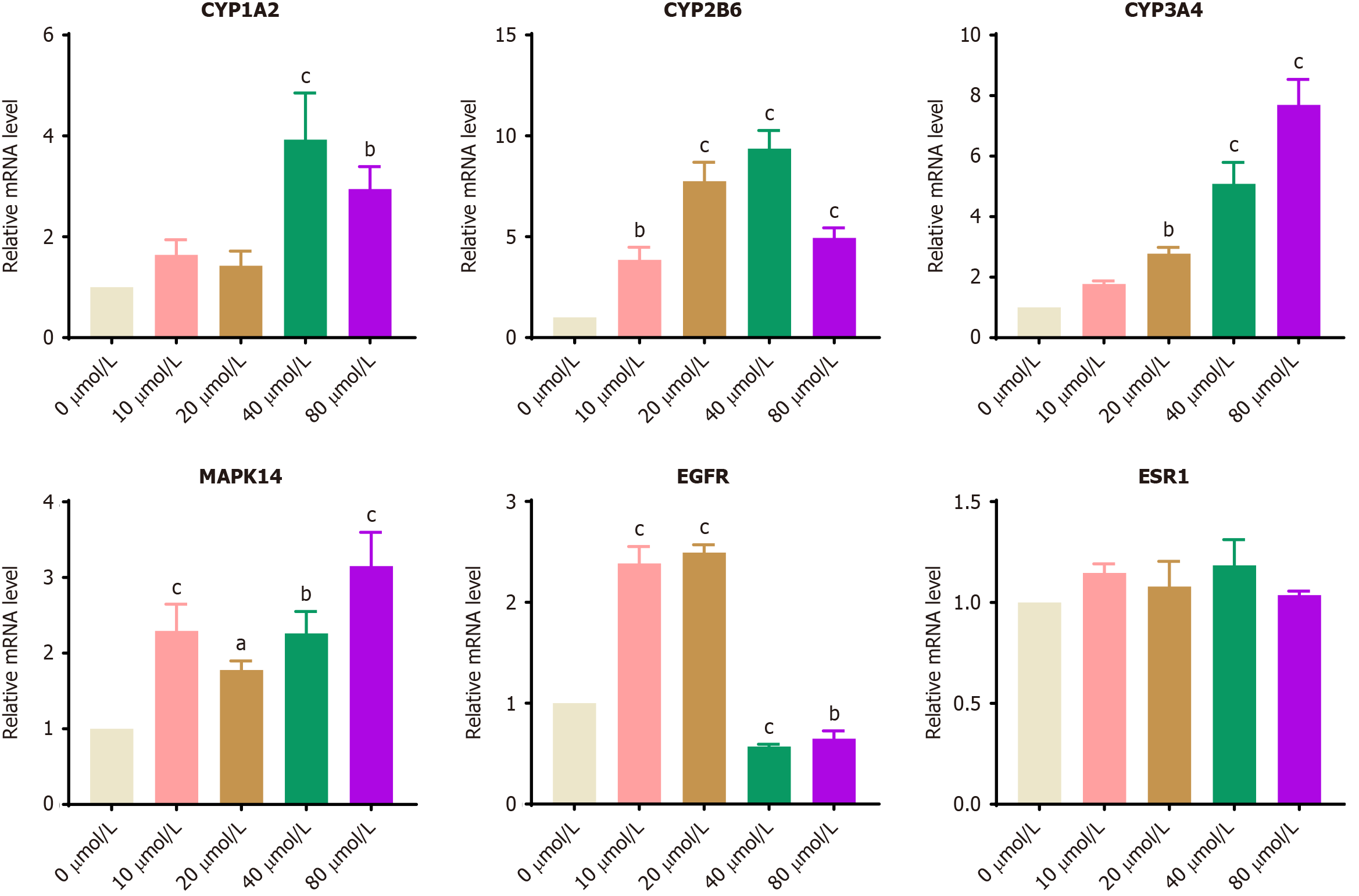
The logarithmic growth phase BGC-823 cells were grouped and treated with curcumin solutions at final concentrations of 0, 10, 20, 40, and 80 µmol/L. After 24 hours of incubation, cells from each group were harvested, and RNA was extracted and quantified. Subsequently, reverse transcription and RT-qPCR were performed. The primer sequences are listed in Table 1. A summary of all databases utilized in this study is provided in Table 2.
| Genes | Primers (5' to 3') |
| ESR1 | F: AGTGCCTTGTTGGATGCT |
| R: TGCCAGGTTGGTCAGTAAG | |
| EGFR | F: GGGTGCAGGAGAGGAGAA |
| R: CTGGTTGTGGCAGCAGTC | |
| CYP3A4 | F: ATGGCACCGTAAGTGGAG |
| R: TGGTGTTCTCAGGCACAG | |
| MAPK14 | F: AATACTGGGGAGGGGACA |
| R: GGCTTCATTCGTTTTCGTT | |
| CYP1A2 | F: AGAATGCCCTCAACACCTT |
| R: CCTTGCTCACATGCTCCT | |
| CYP2B6 | F: GTCTTCCCCAGTCCTCATT |
| R: AGTGCAGAATCCCACAGC | |
| GAPDH | F: TGTGTCCGTCGTGGATCTGA |
| R: TTGCTGTTGAAGTCGCAGGAG |
| Database/tool | Use/version | Database website/version |
| SwissADME | Drug similarity prediction | http://www.swissadme.ch |
| PharmMapper database | Molecular target prediction | http://www.lilab-ecust.cn/pharmmapper/ |
| Swiss TargetPrediction database | http://www.swisstargetprediction.ch/ | |
| Drugbank database | www.drugbank.ca | |
| GeneCard database | Disease target prediction | https://www.genecards.org/ |
| OMIM database | https://omim.org/ | |
| TTD database | http://db.idrblab.net/ttd/ | |
| Drugbank database | www.drugbank.ca | |
| String database | Protein InterNetNetwork | https://string-db.org/ |
| Cytoscape tool | Cytoscape_v3.7.2 | |
| cythubba tool | Hub target filtering | Cytoscape_v3.7.2 |
| DAVID database | KEGG and GO Analysis | https://david.ncifcrf.gov/ |
| Kaplan-Meier tool | Survival analysis | https://kmplot.com/analysis/ |
| AutoDock Vina | Molecular docking | AutoDock Vina1.1.2 |
| Bioinformatics tool | Visualization drawing | http://www.bioinformatics.com.cn/ |
| Ualcan | TCGA | http://ualcan.path.uab.edu/ |
Our results showed that the MW was less than five hundred; the numbers of hydrogen bond donors and receptors were less than 5 and 10, respectively; the number of rotating bonds was not more than ten; and the lipid-water distribution coefficient was less than 5. The results revealed that the nature of curcumin conforms to Lipinski's RO5, which strongly indicates that it has good drug class properties (Table 3).
| Property | Value |
| Molecular weight | 368.38 g/mol |
| PSA | 93.06 A² |
| XLogP3 | 3.20 |
| Rotatable bonds | 8 |
| H-bond donor | 2 |
| H-bond acceptor | 6 |
| Molar refractivity | 102.80 |
| Bioavailability score | 0.55 |
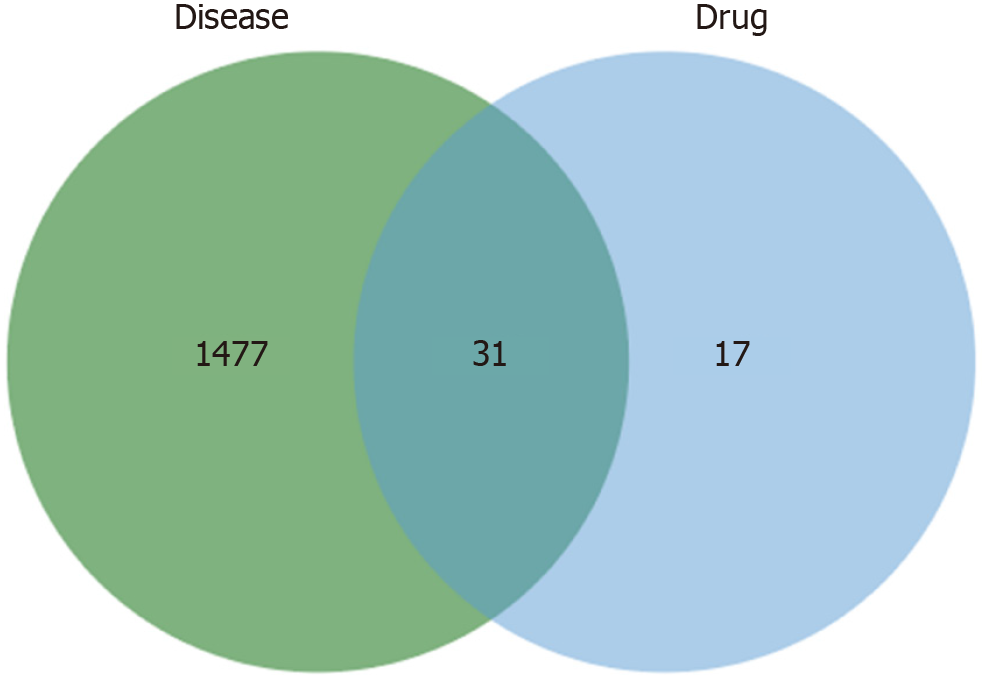
Drugs can usually be combined with multiple targets, namely, multiple pharmacological agents or drugs. Thus, the present study predicted the latent targets of curcumin. After the target data were merged, forty-eight replicates of curcumin were saved. The genes associated with GC were then retrieved from the GeneCard, OMIM, Drugbank, and TTD databases. Subsequently, 1508 GC targets were obtained after eliminating redundant data from the above database, by integrating the targets of curcumin and GC, we identified 31 common targets as potential targets for curcumin in the treatment of GC (Figure 1).
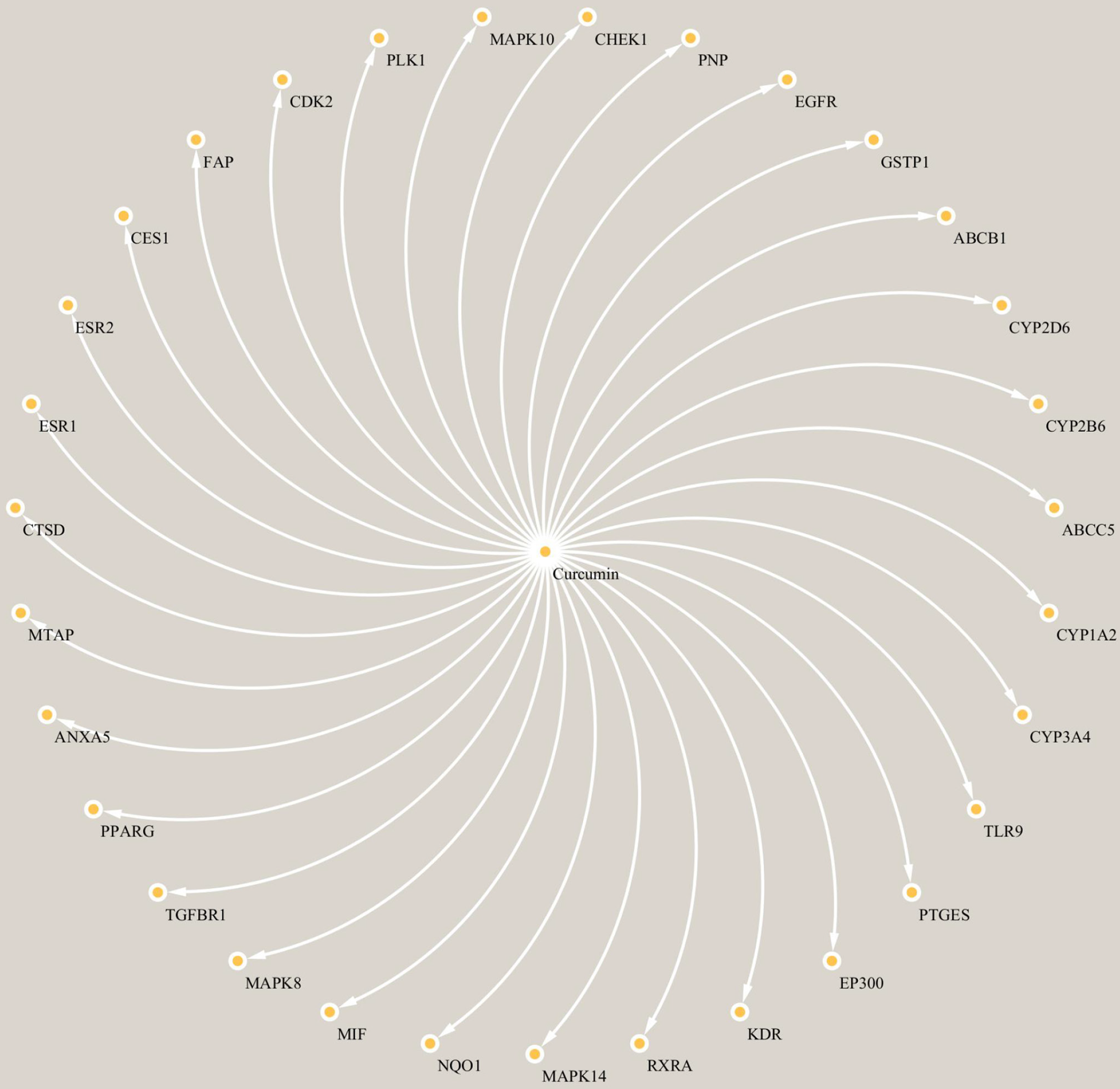
To explore the connections between the potential targets and curcumin, we constructed a composite target network. The network, comprising curcumin and the potential targets, included 32 nodes and 31 edges, as depicted in Figure 2.
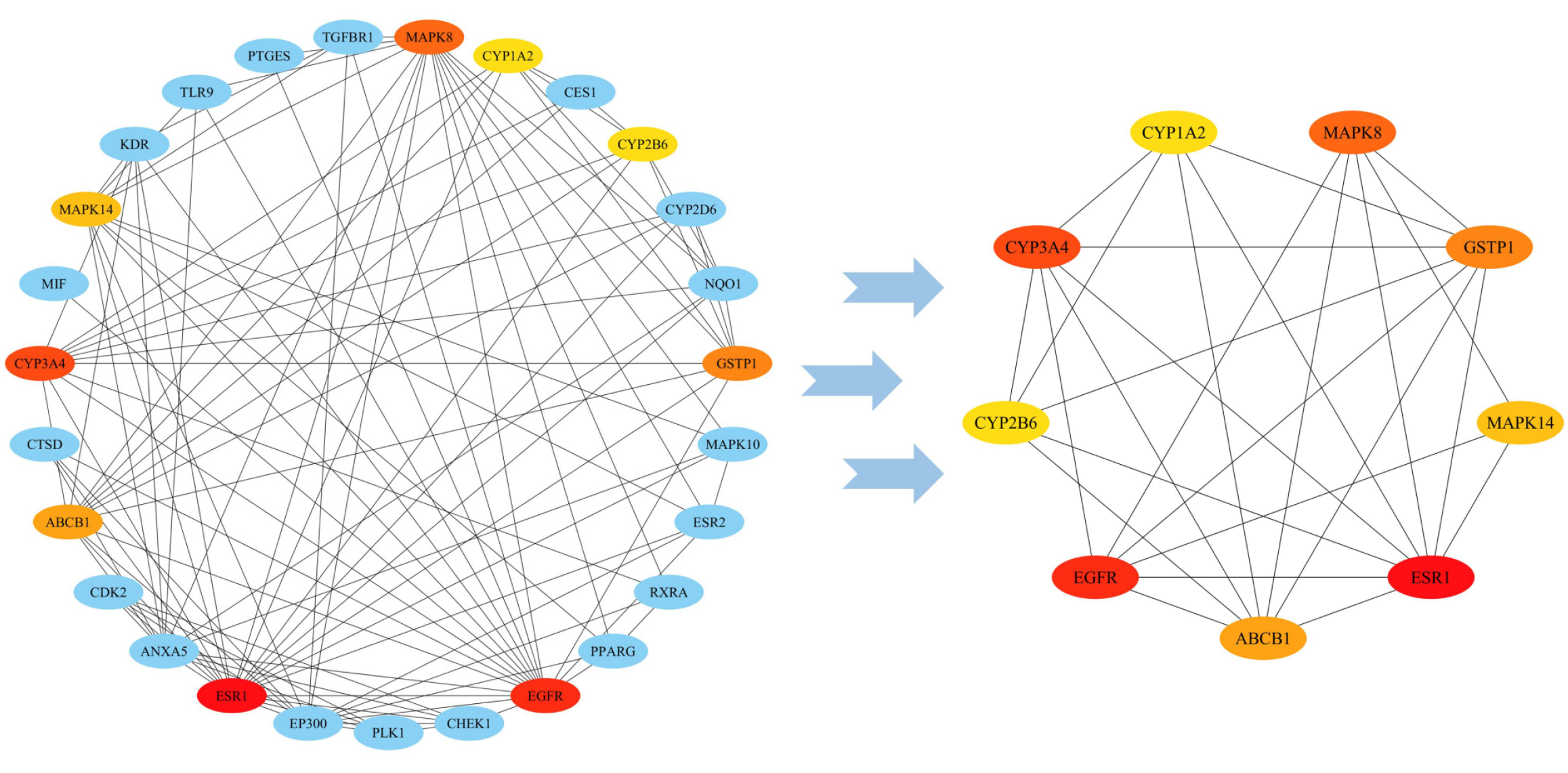
To better understand proteins within cells at a systemic level, through STRING database, we constructed a PPI network consisting of thirty-one targets related to curcumin (Figure 3). Based on the topological analysis of the PPI network, the color varied from yellow to red as the score increased, with ESR1 being the deepest and greatest (MCC score = 732). On the basis of the calculations of the cythubba data, six targets, namely, ESR1, EGFR, CYP3A4, MAPK14, CYP1A2, and CYP2B6, were selected as hub targets based on PPI topological analysis, indicating that they may have significant potential impact on the development of GC, as illustrated in Figure 3.
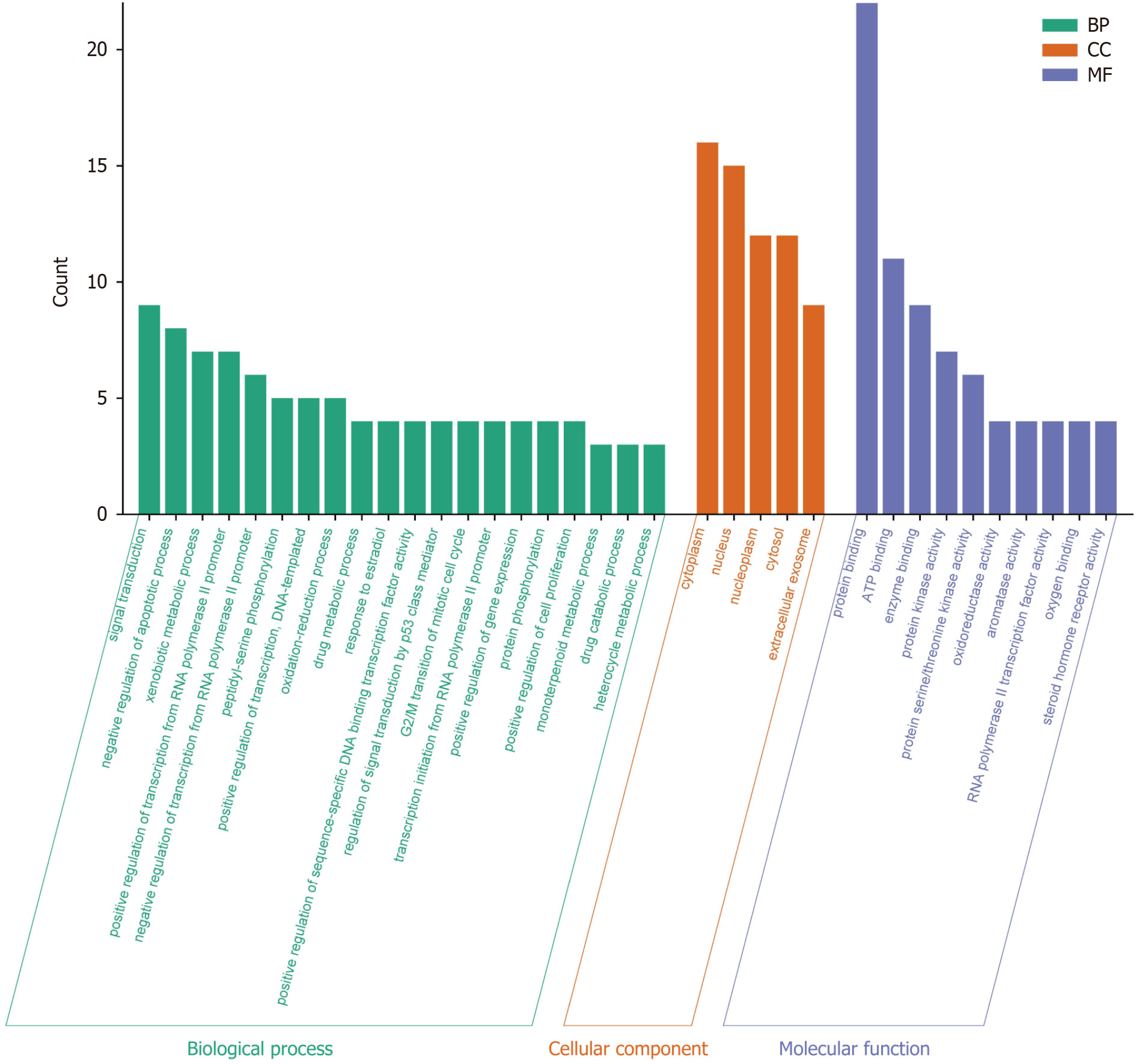
To better analyze the potential biological processes and cellular molecular mechanisms of curcumin in the treatment of GC, we performed GO analysis on 31 potential targets of curcumin for GC treatment. Based on the analysis results, we found that the potential targets were enriched in 81 GO biological processes, including 18 cell component terms, 42 molecular function terms, and the first 20 biological processes, first 5 cellular components, and first 10 molecular functions are presented in Figure 4. As shown in Figure 4, the first 20 biological process terms were significantly associated with "GO:0007165" (GO:0007165), "Negative regulation of apoptotic processes" (GO:0043066), and "Foreign Body Metabolic Process" (GO:0006805). The first 5 cell component terms were significantly associated with the visible sites of action according to GO:0005737 and GO:0005634. The first 10 molecular function terms were significantly associated with "protein binding" (GO:0005515), "ATP binding" (GO:0005524), and "enzyme binding" (GO:0019899). The findings showed that potential targets regulate cell signal transduction, proliferation, apoptosis, phosphorylation, oxidative stress, and metabolism.
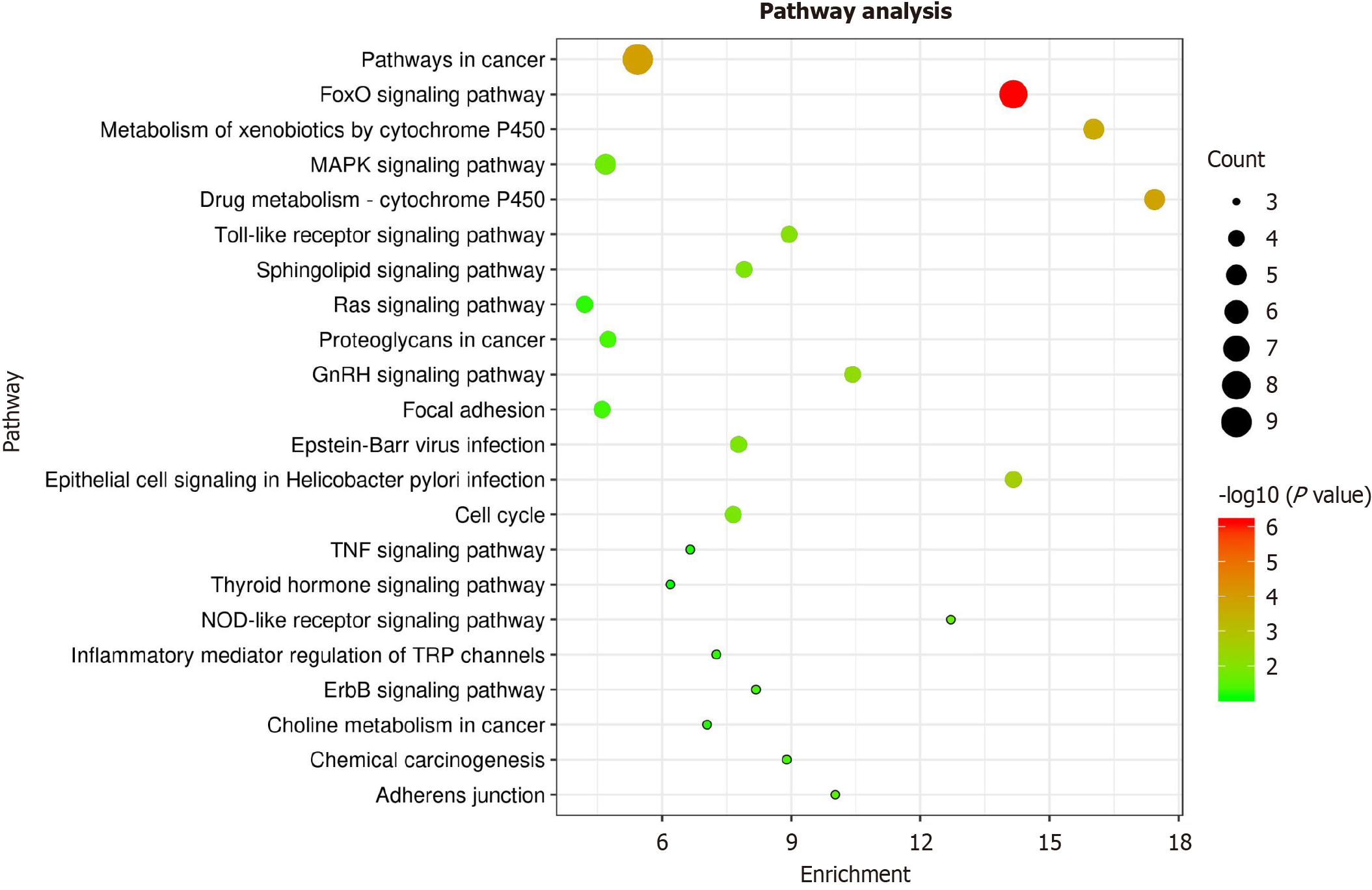
For a deeper comprehending of pharmacological mechanisms underlying efficacy of curcumin in GC, we performed a KEGG pathway analysis of these thirty-one targets via the DAVID database. The results showed that 31 targets were associated with 46 pathways. Combined with the pathogenesis and gene count of GC (gene count ≥ 3), GC-independent pathways such as the prolactin signaling pathway (KEGG: Hsa04917), tuberculosis (KEGG: Hsa05152), and pancreatic cancer (KEGG: Hsa05212) were eliminated. Finally, ten significant enrichment approaches may be the main approaches for GC treatment (as shown in Table 4). A pathway enrichment diagram was then generated by mapping the targets through bioinformatics tools (Figure 5). The aforementioned results indicate that curcumin plays a therapeutic role through FOXO and other signaling pathways that affect cancer.
| Pathway ID | Pathway | Corrected P value | Gene count | Annotated genes |
| KEGG: hsa04068 | FoxO signaling pathway | 7.66E-07 | 8 | MAPK10, MAPK8, PLK1, CDK2, EP300, MAPK14, EGFR, TGFBR1 |
| KEGG: hsa05200 | Pathways in cancer | 1.19E-04 | 9 | MAPK10, MAPK8, RXRA, GSTP1, CDK2, EP300, PPARG, EGFR, TGFBR1 |
| KEGG: hsa00982 | Drug metabolism - cytochrome P450 | 1.50E-04 | 5 | CYP2B6, CYP2D6, GSTP1, CYP1A2, CYP3A4 |
| KEGG: hsa00980 | Metabolism of xenobiotics by cytochrome P450 | 2.08E-04 | 5 | CYP2B6, CYP2D6, GSTP1, CYP1A2, CYP3A4 |
| KEGG: hsa05120 | Epithelial cell signaling in Helicobacter pylori infection | 0.002430862 | 4 | MAPK10, MAPK8, MAPK14, EGFR |
| KEGG: hsa04912 | GnRH signaling pathway | 0.00577562 | 4 | MAPK10, MAPK8, MAPK14, EGFR |
| KEGG: hsa04620 | Toll-like receptor signaling pathway | 0.008806272 | 4 | MAPK10, MAPK8, TLR9, MAPK14 |
| KEGG: hsa04071 | Sphingolipid signaling pathway | 0.012342719 | 4 | MAPK10, MAPK8, MAPK14, CTSD |
| KEGG: hsa05169 | Epstein-Barr virus infection | 0.012905562 | 4 | MAPK10, MAPK8, CDK2, MAPK14 |
| KEGG: hsa04110 | Cell cycle | 0.013483025 | 4 | PLK1, CHEK1, CDK2, EP300 |
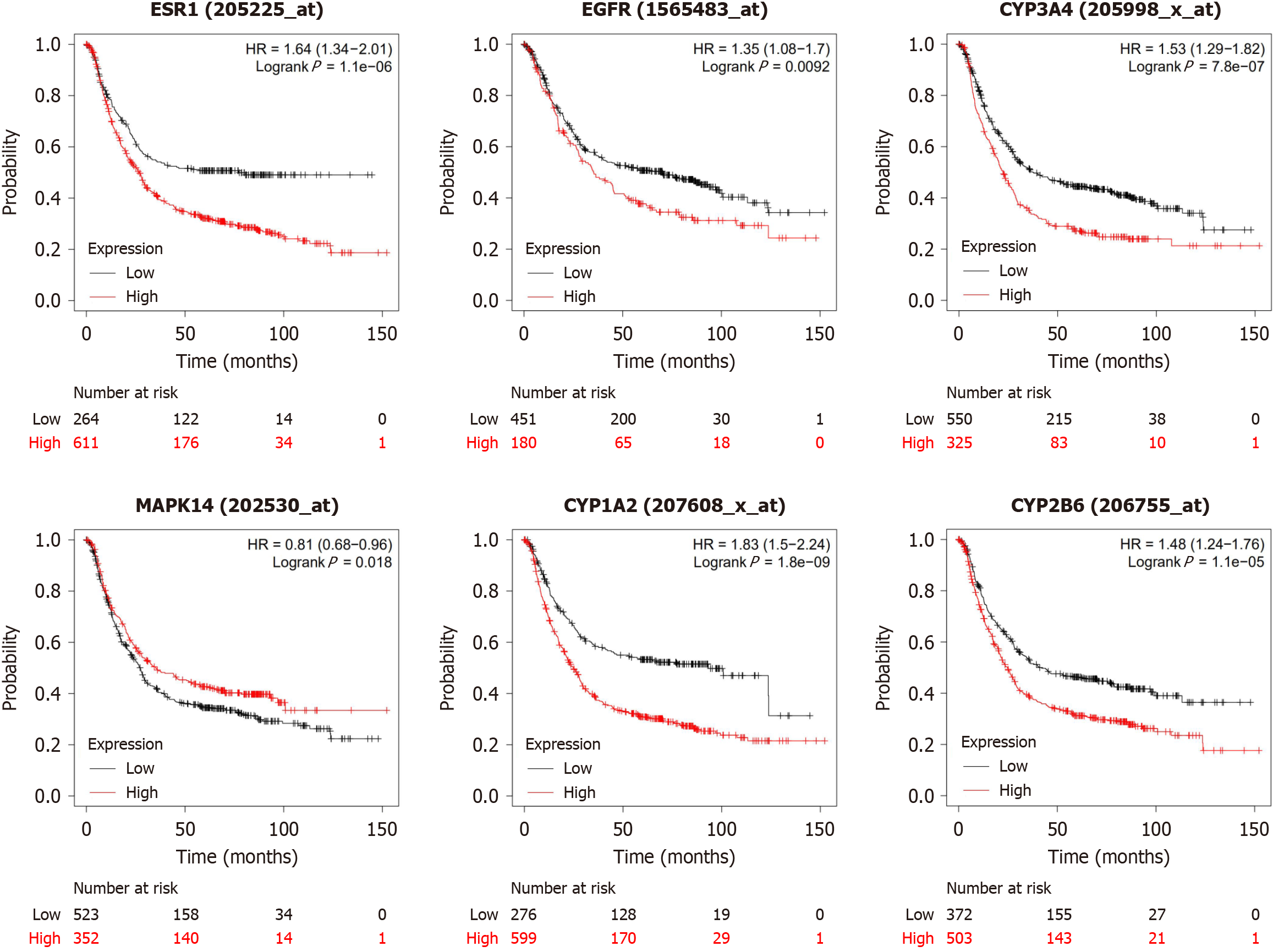
Using the Kaplan-Meier plot database for survival analysis of the hub targets, we have observed high expression of targets (including ESR1, EGFR, CYP3A4, MAPK14, CYP1A2, and CYP2B6) was linked to poor survival in GC patients, as shown in Figure 6.
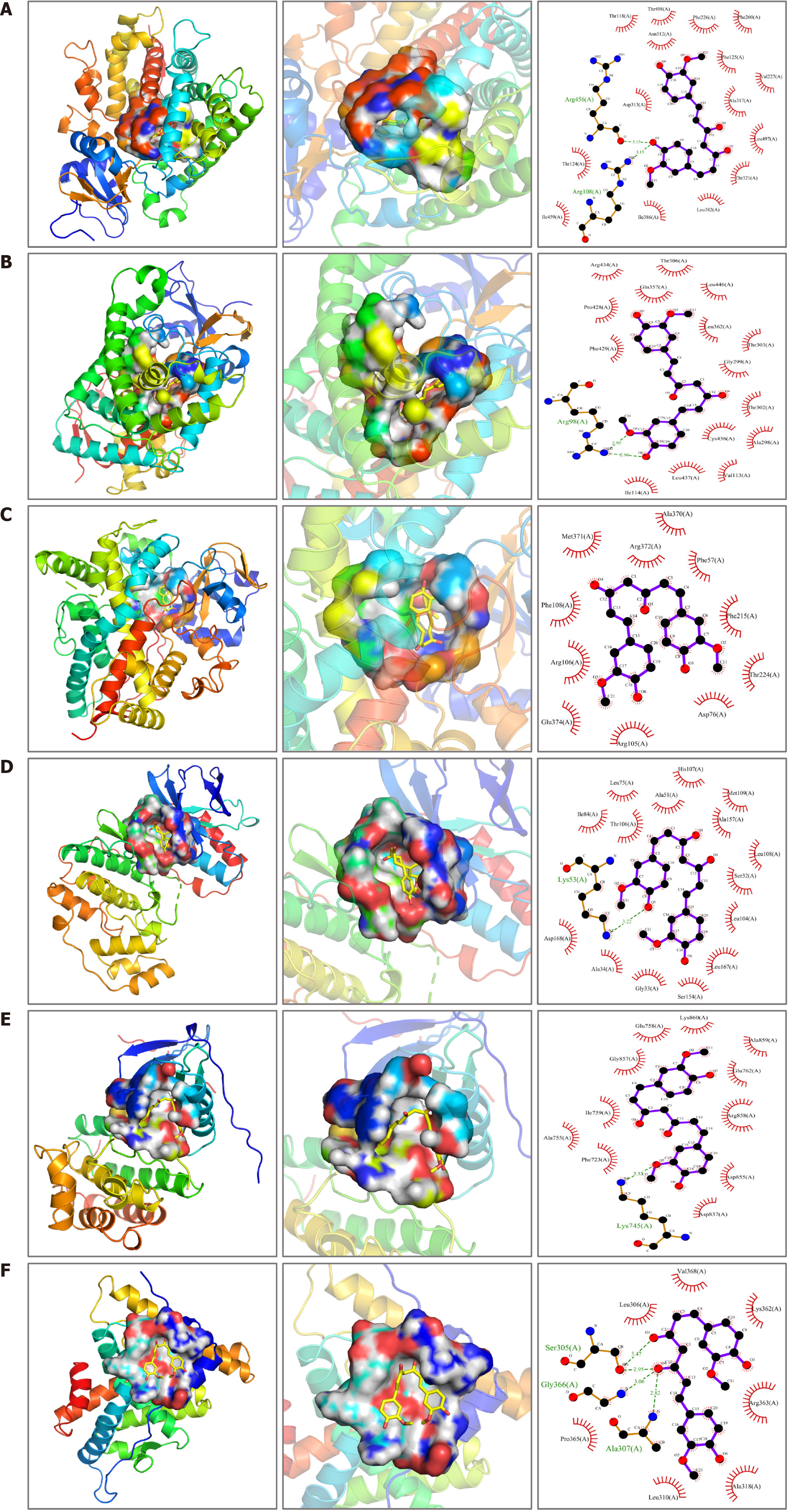
Through molecular docking verification analysis, curcumin was found to be stable in docking with the hub targets ESR1, EGFR, CYP3A4, MAPK14, CYP1A2, and CYP2B6, and the binding energy was low (Table 5). Further examination of results revealed that curcumin exhibited strong binding affinity to CYP1A2, forming hydrogen bonds at Arg108 (A) and Arg456 (A) with lengths of 3.15 Å and 3.15 Å, respectively, and the binding energy was -9.5 kcal/mol. Curcumin has a good binding effect on CYP2B6, forming hydrogen bonds at Arg98 (A) with lengths of 2.95 Å and 2.96 Å, and the binding energy was -9.3 kcal/mol. There was good interaction between curcumin and CYP3A4 only through hydrophobic interactions, and the binding energy was -8.4 kcal/mol. Curcumin has good binding affinity for MAPK14, forming a hydrogen bond at Lys53 (A) with a length of 3.22 Å, and the binding energy was -7.9 kcal/mol. Curcumin has good binding with EGFR, forming a hydrogen bond at Lys745 (A) with a length of 3.31 Å, and the binding energy was -7.8 kcal/mol. Curcumin exhibited strong binding affinity to ESR1, forming hydrogen bonds with Ala307 (A), Gly366 (A) and Ser305 (A), with lengths of 2.82 Å, 3.06 Å, 2.95 Å and 3.47 Å, respectively. The binding energy was -6.3 kcal/mol (Figure 7).
| Hub targets | Binding energy (kcal/mol) | Hydrogen bonds | Hydrogen bonds, lengths | ||||||
| ESR1 | -6.3 | Ala307 (A) | Gly366 (A) | Ser305 (A) | 2.82Å | 3.06Å | 2.95Å | 3.47Å | |
| EGFR | -7.8 | Lys745 (A) | 3.31Å | ||||||
| CYP3A4 | -8.4 | - | - | ||||||
| MAPK14 | -7.9 | Lys53 (A) | 3.22Å | ||||||
| CYP1A2 | -9.5 | Arg108 (A) | Arg456 (A) | 3.15Å | 3.15Å | ||||
| CYP2B6 | -9.3 | Arg98 (A) | 2.95Å | 2.96Å | |||||
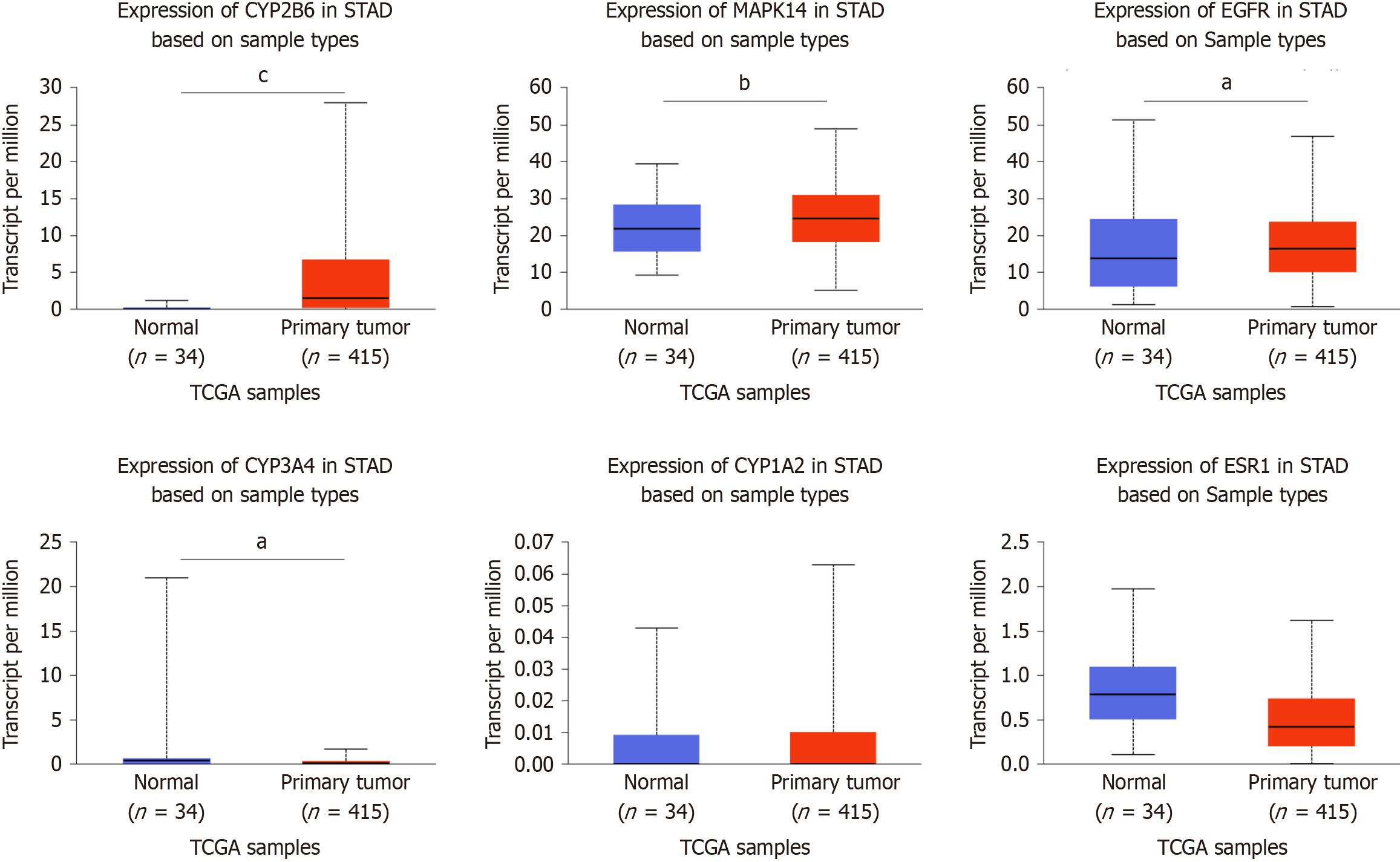
According to the TCGA database, grouping 415 GC tissue samples and 34 normal tissue samples, and analyzing their differential gene expression levels. We found that the levels of expression of EGFR, CYP3A4, MAPK14 and CYP2B6 varied significantly between GC and normal tissues (P < 0.05), while the expression levels of ESR1 and CYP1A2 were not significantly different (Figure 8).
Figure 9 shows that following treatment with curcumin for 24 hours and 48 hours, compared with that after treatment with 0 μmol/L curcumin, cell growth was significantly inhibited after treatment with 10, 20, 40, 80 μmol/L curcumin. At the same time, the higher the concentration of curcumin was, the more obvious was the inhibition of cell growth. Statistical significance was observed when the concentration of curcumin was greater than or equal to 20 μmol/L (P < 0.001).
The experimental results revealed a significant increase in the mRNA expression levels of CYP3A4, MAPK14, CYP1A2, and CYP2B6 in BGC-823 cells treated with curcumin compared to the 0 μmol/L curcumin group (P < 0.05, 0.01, 0.001; Figure 10). Statistical analysis revealed a significant reduction in the mRNA levels of EGFR when the concentration of curcumin reached or exceeded 40 μmol/L (P < 0.01, 0.001). After intervention with a certain concentration of curcumin, significant differences were observed in the mRNA levels of hub target genes across all groups, except for ESR1.
Curcumin as diketone compound derived from rhizome of some plants in the Zingiberaceae and Araceae. It possesses a diverse array of biological functions, including anti-inflammatory, antitumor and antioxidant effects[16]. Its anticancer effects primarily stem from its ability to negatively regulate multiple transcription factors and inhibit cellular proliferation, through eliminating stasis cancer cells at various phases of cell cycle or inducing their apoptosis[17]. Curcumin has been proven to be capable of treating GC by inhibiting cell proliferation, inducing apoptosis, and reducing chemo
Fifteen GC targets were selected, referring to 81 biological processes, 18 cell components and 42 molecular functions. These biological processes mainly involve signal transduction, apoptosis, cell metabolism, proliferation, oxidative stress, and the cell cycle; cell components mainly involve the cytoplasm, nucleus, mitochondria, and cell membrane; molecular functions mainly involve protein binding, protein kinase activity, ATP binding, and enzyme binding. An essential characteristic of cancer cells involves aberrant alterations in both proliferation and apoptosis. Therefore, a promising strategy for treating GC is to regulate the balance between GC cell proliferation and apoptosis. Multiple research findings suggest that manipulating apoptosis holds potential as an effective strategy for cancer treatment. Interruption of orderly apoptosis leads to the overgrowth of malignant cells[19]. Studies have shown that curcumin can significantly activate the activity of Caspase-3 and cleaved PARP to induce apoptosis in GC cells[20]. Furthermore, curcumin can significantly reduce the proliferative capacity of tumors by blocking the cell cycle progression[21]. The rising prominence of metabolomics has led to heightened interest among researchers in understanding the association between metabolic regulation and cancer. Impairment of mitochondrial metabolic reactions leads to the production of reactive species, such as ROS, which are known to instigate oxidative stress and provoke cellular damage, thereby disrupting normal physiological functions. Prolonged elevation of ROS levels can induce the activation of oncogenes, genetic mutations, or chromosomal abnormalities[22,23].
Curcumin exerts its effects through multiple mechanisms of action, thus, we analyzed KEGG pathways. The results indicated that curcumin prevents GC occurrence in a variety of ways. According to pathway analysis, the pathways most highly enriched in GC therapy with curcumin were correlated with the FOXO, P450 metabolic, GnRH, Toll receptor, cell cycle and epithelial cell signaling pathway. FOXO has been found to play a significant role in numerous cell processes, including proliferation, apoptosis, differentiation, stress reactions, and metabolic reactions[24]. Cytochrome P450 (CYP450) is a supergene family that activates carcinogens mainly through epoxidation, lightness, decalkylation, oxidation, and reduction[25]. Toll-like receptors (TLRs) can be used to specifically identify a variety of bacteria, viruses and other pathogenic microorganisms[26]. Recent research suggests a close association between Helicobacter pylori
To elucidate the importance of this curcumin target, we constructed a PPI network. Through this network, we identified the first six hub targets, namely, ESR1, EGFR, CYP3A4, MAPK14, CYP1A2, and CYP2B6. Survival analysis indicates that elevated levels of these genes are associated with poorer prognostic outcomes. To investigate the interaction mechanisms between curcumin and the six central molecules, we employed molecular docking methods. The results showed that curcumin has large binding sites for ESR1, EGFR, CYP3A4, MAPK14, CYP1A2 and CYP2B6, with high binding scores, indicating good affinity for curcumin.
The genetic polymorphism of CYP1A2 not only contributes to a certain extent to the increased risk of GC but particularly enhances the likelihood of developing GC in patients with H. pylori infection[28]. In addition, CYP1A2 is a lipid metabolism-related gene. The biological process of lipid metabolism plays a dual role in regulating proliferation and migration of tumor cells, while also modulating the recruitment and function of tumor-infiltrating immune cells, thereby altering the immune microenvironment[29]. The genetic polymorphisms of various CYP450 enzymes have been thoroughly investigated for their roles in modulating the processes of cancer development[30]. Among these, CYP3A4 is widely expressed in hepatocellular carcinoma, breast cancer, lung cancer, prostate cancer, and GC[31,32]. Additionally, microarray analysis has detected that enhanced expression of CYP3A4 correlates closely with the therapeutic response of metastatic GC to chemotherapy[30]. The expression of ESR1 is associated with fine T staging but has no significant correlation with N staging, suggesting that the estrogen receptor may promote local invasion of GC without affecting its lymph node metastasis mechanism. Estrogen receptor expression may be associated with age, sex, and other factors that promote the development of GC[33]. MAPK14 is one of four p38 MAPKs. Passos et al[34] reported that the activation of the MAPK14/TGF signaling pathway leads to enhanced ROS activation, which participates in aging caused by DNA damage or telomere dysfunction[34,35]. Research has demonstrated that MAPK14 exhibits high expression levels in tumor tissue and in radiotherapy-resistant GC cell lines. High MAPK14 expression mediates radiotherapy resistance in GC by inhibiting apoptosis and affecting the redistribution of the cell cycle. Furthermore, MAPK14 may serve as a predictive marker for radiation sensitivity in patients with advanced GC[36]. CYP is a carcinogen activator enzyme that includes CYP3A4, CYP1A2 and CYP2B6, and the activities of these enzymes have the potential to activate the carcinogen, increasing cancer risk[37]. The pathogenesis of various cancers is closely associated with EGFR. Through the regulation of biological processes such as cell proliferation, survival, and metastasis, EGFR exerts a profound influence on tumor development and progression[38]. Preclinical study data indicate that EGFR is capable of sustaining tumor growth and development[39]. EGFR overexpression or constitutive activation is common in tumor cells and is associated with their proliferation, migration, and invasion, making EGFR an important target for anticancer therapy[40].
In this study, we have used CCK-8 to verify that significant inhibitory effect of curcumin on the proliferation of BGC-823 cells, and a qRT-PCR assay was used to verify that curcumin markedly influenced the hub targets CYP3A4, MAPK14, CYP1A2, and CYP2B6 at the gene level, as determined by network pharmacology. The effect of curcumin on EGFR expression exhibits dose-dependency, and curcumin slightly promoted the expression of ESR1. In summary, ESR1, EGFR, CYP3A4, MAPK14, CYP1A2, and CYP2B6 play key roles in the pathogenesis of GC, indicating that curcumin could have a strong therapeutic effect on GC through these target genes. However, further biological experimental validation is required to determine the exact mechanism of curcumin in GC treatment.
Curcumin, a compound extracted from certain plants, exhibits anti-inflammatory, antitumor, and antioxidant properties, making it a potential treatment for GC. Its anticancer mechanisms involve transcriptional regulation, arrest of the cell cycle, and induction of apoptosis. Despite the efficacy of curcumin in inhibiting cell proliferation and inducing apoptosis, the precise underlying molecular mechanisms remain elusive. Notably, we discuss the cancer risk for polymorphisms of CYP1A2 and the function of CYP450 enzymes. Biological validation demonstrated the inhibitory effect of curcumin on BGC-823 cell proliferation and its impact on hub target gene expression. In summary, curcumin holds promise for GC treatment through the modulation of key molecular targets, warranting further experimental validation. Our current study provides new insight into the mechanism of action of curcumin against GC.
The authors would like to thank all the authors for their contributions to this article.
| 1. | Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 1122] [Article Influence: 187.0] [Reference Citation Analysis (1)] |
| 2. | Chandra R, Balachandar N, Wang S, Reznik S, Zeh H, Porembka M. The changing face of gastric cancer: epidemiologic trends and advances in novel therapies. Cancer Gene Ther. 2021;28:390-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, Liu C, Song W, Wang F, Zhang J, Shen J, Yu J. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Bai Y, Zhao F, Li Y, Wang L, Fang XJ, Wang CY. Ginkgo biloba extract induce cell apoptosis and G0/G1 cycle arrest in gastric cancer cells. Int J Clin Exp Med. 2015;8:20977-20982. [PubMed] |
| 5. | Mohammad Abu-Taweel G, Al-Fifi Z. Protective effects of curcumin towards anxiety and depression-like behaviors induced mercury chloride. Saudi J Biol Sci. 2021;28:125-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Fu H, Wang C, Yang D, Wei Z, Xu J, Hu Z, Zhang Y, Wang W, Yan R, Cai Q. Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. J Cell Physiol. 2018;233:4634-4642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Siriviriyakul P, Chingchit T, Klaikeaw N, Chayanupatkul M, Werawatganon D. Effects of curcumin on oxidative stress, inflammation and apoptosis in L-arginine induced acute pancreatitis in mice. Heliyon. 2019;5:e02222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Marín YE, Wall BA, Wang S, Namkoong J, Martino JJ, Suh J, Lee HJ, Rabson AB, Yang CS, Chen S, Ryu JH. Curcumin downregulates the constitutive activity of NF-kappaB and induces apoptosis in novel mouse melanoma cells. Melanoma Res. 2007;17:274-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1). Cell Cycle. 2007;6:2953-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3717] [Cited by in RCA: 8259] [Article Influence: 1032.4] [Reference Citation Analysis (1)] |
| 11. | Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47:W357-W364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 760] [Cited by in RCA: 2251] [Article Influence: 450.2] [Reference Citation Analysis (0)] |
| 12. | Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074-D1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3266] [Cited by in RCA: 5291] [Article Influence: 881.8] [Reference Citation Analysis (0)] |
| 13. | Wang X, Shen Y, Wang S, Li S, Zhang W, Liu X, Lai L, Pei J, Li H. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45:W356-W360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 951] [Article Influence: 158.5] [Reference Citation Analysis (0)] |
| 14. | Wang Y, Zhang S, Li F, Zhou Y, Zhang Y, Wang Z, Zhang R, Zhu J, Ren Y, Tan Y, Qin C, Li Y, Li X, Chen Y, Zhu F. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020;48:D1031-D1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 407] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 15. | Szász AM, Lánczky A, Nagy Á, Förster S, Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A, Győrffy B. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7:49322-49333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 759] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 16. | Kotha RR, Luthria DL. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 503] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 17. | Shanmugam MK, Rane G, Kanchi MM, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP, Sethi G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules. 2015;20:2728-2769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 320] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 18. | Hassanalilou T, Ghavamzadeh S, Khalili L. Curcumin and Gastric Cancer: a Review on Mechanisms of Action. J Gastrointest Cancer. 2019;50:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Wang YQ, Zhang SJ, Lu H, Yang B, Ye LF, Zhang RS. A C 21 -Steroidal Glycoside Isolated from the Roots of Cynanchum auriculatum Induces Cell Cycle Arrest and Apoptosis in Human Gastric Cancer SGC-7901 Cells. Evid Based Complement Alternat Med. 2013;2013:180839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Gajek A, Denel M, Bukowska B, Rogalska A, Marczak A. Pro-apoptotic activity of new analog of anthracyclines--WP 631 in advanced ovarian cancer cell line. Toxicol In Vitro. 2014;28:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | He YC, He L, Khoshaba R, Lu FG, Cai C, Zhou FL, Liao DF, Cao D. Curcumin Nicotinate Selectively Induces Cancer Cell Apoptosis and Cycle Arrest through a P53-Mediated Mechanism. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1456] [Cited by in RCA: 2270] [Article Influence: 283.8] [Reference Citation Analysis (0)] |
| 23. | Ge W, Zhao K, Wang X, Li H, Yu M, He M, Xue X, Zhu Y, Zhang C, Cheng Y, Jiang S, Hu Y. iASPP Is an Antioxidative Factor and Drives Cancer Growth and Drug Resistance by Competing with Nrf2 for Keap1 Binding. Cancer Cell. 2017;32:561-573.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 24. | van der Vos KE, Coffer PJ. FOXO-binding partners: it takes two to tango. Oncogene. 2008;27:2289-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Cai L, Yu SZ, Zhan ZF. Cytochrome P450 2E1 genetic polymorphism and gastric cancer in Changle, Fujian Province. World J Gastroenterol. 2001;7:792-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Castaño-Rodríguez N, Kaakoush NO, Mitchell HM. Pattern-recognition receptors and gastric cancer. Front Immunol. 2014;5:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Tongtawee T, Simawaranon T, Wattanawongdon W, Dechsukhum C, Leeanansaksiri W. Toll-like receptor 2 and 4 polymorphisms associated with Helicobacter pylori susceptibility and gastric cancer. Turk J Gastroenterol. 2019;30:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Wei XL, Luo TQ, Li JN, Xue ZC, Wang Y, Zhang Y, Chen YB, Peng C. Development and Validation of a Prognostic Classifier Based on Lipid Metabolism-Related Genes in Gastric Cancer. Front Mol Biosci. 2021;8:691143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | He S, Cai T, Yuan J, Zheng X, Yang W. Lipid Metabolism in Tumor-Infiltrating T Cells. Adv Exp Med Biol. 2021;1316:149-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Zhang F, Wang F, Chen C, Wang T, Hu J, Su R, Li X, Gu B, Tang S, Chen H, Li Y. Prediction of progression of chronic atrophic gastritis with Helicobacter pylori and poor prognosis of gastric cancer by CYP3A4. J Gastroenterol Hepatol. 2020;35:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Fanni D, Manchia M, Lai F, Gerosa C, Ambu R, Faa G. Immunohistochemical markers of CYP3A4 and CYP3A7: a new tool towards personalized pharmacotherapy of hepatocellular carcinoma. Eur J Histochem. 2016;60:2614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Angelini S, Botticelli A, Onesti CE, Giusti R, Sini V, Durante V, Strigari L, Gentile G, Cerbelli B, Pellegrini P, Sgroi V, Occhipinti M, DI Pietro FR, Rossi A, Simmaco M, Mazzuca F, Marchetti P. Pharmacogenetic Approach to Toxicity in Breast Cancer Patients Treated with Taxanes. Anticancer Res. 2017;37:2633-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Luo Y, Zheng S, Wu Q, Wu J, Zhou R, Wang C, Wu Z, Rong X, Huang N, Sun L, Bin J, Liao Y, Shi M, Liao W. Long noncoding RNA (lncRNA) EIF3J-DT induces chemoresistance of gastric cancer via autophagy activation. Autophagy. 2021;17:4083-4101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 34. | Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, Saretzki G, Rudolph KL, Kirkwood TB, von Zglinicki T. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6:347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 816] [Cited by in RCA: 734] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 35. | Kim CG, Castro-Aceituno V, Abbai R, Lee HA, Simu SY, Han Y, Hurh J, Kim YJ, Yang DC. Caspase-3/MAPK pathways as main regulators of the apoptotic effect of the phyto-mediated synthesized silver nanoparticle from dried stem of Eleutherococcus senticosus in human cancer cells. Biomed Pharmacother. 2018;99:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Mesquita FP, Moreira-Nunes CA, da Silva EL, Lima LB, Daniel JP, Zuerker WJ, Brayner M, de Moraes MEA, Montenegro RC. MAPK14 (p38α) inhibition effects against metastatic gastric cancer cells: A potential biomarker and pharmacological target. Toxicol In Vitro. 2020;66:104839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Ghoshal U, Tripathi S, Kumar S, Mittal B, Chourasia D, Kumari N, Krishnani N, Ghoshal UC. Genetic polymorphism of cytochrome P450 (CYP) 1A1, CYP1A2, and CYP2E1 genes modulate susceptibility to gastric cancer in patients with Helicobacter pylori infection. Gastric Cancer. 2014;17:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 38. | Fu J, Guo Q, Feng Y, Cheng P, Wu A. Dual role of fucosidase in cancers and its clinical potential. J Cancer. 2022;13:3121-3132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 39. | Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 40. | Corso S, Pietrantonio F, Apicella M, Migliore C, Conticelli D, Petrelli A, D'Errico L, Durando S, Moya-Rull D, Bellomo SE, Ughetto S, Degiuli M, Reddavid R, Fumagalli U, De Pascale S, Sgroi G, Rausa E, Baiocchi GL, Molfino S, De Manzoni G, Bencivenga M, Siena S, Sartore-Bianchi A, Morano F, Corallo S, Prisciandaro M, Di Bartolomeo M, Gloghini A, Marsoni S, Sottile A, Sapino A, Marchiò C, Dahle-Smith A, Miedzybrodzka Z, Lee J, Ali SM, Ross JS, Alexander BM, Miller VA, Petty R, Schrock AB, Giordano S. Optimized EGFR Blockade Strategies in EGFR Addicted Gastroesophageal Adenocarcinomas. Clin Cancer Res. 2021;27:3126-3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |