INTRODUCTION
Gastric cancer (GC) ranks as the fifth most common malignancy worldwide and holds the third highest position in cancer-associated mortality[1]. Its development is intricately linked to genetics, dietary habits, lifestyle choices, alcohol consumption, and smoking. Notably, infection with Helicobacter pylori (H. pylori), a microaerophilic gram-negative bacillus that colonizes the gastric mucosa of over half the global population, is recognized as a major risk factor for the development of malignant gastric tumors[2]. First isolated in 1983 from the biopsy tissues of patients with chronic active gastritis by Australian researchers, H. pylori was designated as a Group 1 carcinogen in 1994. It produces an array of toxins and effector proteins that damage cellular and gastric mucosal tissues, leading to neutrophil infiltration and edema of the gastric mucosal layer. This sequence of events initiates chronic gastritis, advances to atrophic gastritis, proceeds to intestinal metaplasia, results in dysplasia, and ultimately culminates in GC[3].
During infection of the gastric mucosa, H. pylori triggers inflammatory responses in host gastric epithelial cells through various mechanisms. Inflammatory diseases are key risk factors for the development of various cancers, and H. pylori-induced chronic inflammation of the gastric mucosa plays a significant role in the genesis and progression of GC[4]. Numerous studies have revealed that the apoptosis of gastric epithelial cells induced by H. pylori infection constitutes one of its pathogenic mechanisms[5,6]. Dysregulation of apoptosis coupled with uncontrolled cell proliferation may lead to tumorigenesis[7,8]. Therefore, rectifying the imbalance in apoptosis regulation is critical for preventing cancer development. In this context, measures to eradicate H. pylori infections are particularly crucial for improving global public health and preventing bacterium-induced GC.
According to both international and domestic consensus, the current preferred treatment strategy for eradicating H. pylori infection is standard quadruple therapy, which combines a proton pump inhibitor, a bismuth compound, and two antibiotics. The use of antibiotics in the eradication of H. pylori may lead to bacterial resistance, disruption of gastrointestinal microbiota, and adverse gastrointestinal reactions such as dyspepsia[9,10]. Therefore, there is an urgent need to develop safe and effective therapeutic drugs associated with fewer side effects. Traditional Chinese medicine, a medical system originating from China with at least 2200 years of history of studying human physiological processes, pathological states, clinical diagnoses, and therapeutic strategies, has played an undeniable role in enhancing national health[11]. In recent years, a plethora of research has demonstrated the efficacy of traditional Chinese medicine in the treatment of various diseases[12,13]. In particular, Chinese herbal medicines (CHM), owing to their multitarget mechanisms of action and fewer side effects, have been widely applied in anti-infective treatments[14,15]. Specific CHMs have shown significant potential in the treatment of H. pylori-induced gastritis[16-18].
Acacetin (5,7-dihydroxy-4’-methoxyflavone) is a natural flavonoid compound ubiquitously present in various plants, existing either in its free form or as a glycoside, and is particularly common in plants such as black locust, wild chrysanthemum, and patchouli. This compound exhibits a wide range of biological effects such as anti-inflammatory, antioxidative, antitumor, neuroprotective, and cardioprotective properties[19-21]. Recent studies have revealed that acacetin attenuates tumor invasion, metastasis, and epithelial-mesenchymal transition in gastrointestinal tumors, especially in GC, by inhibiting the PI3K/Akt/Snail signaling pathway and exerting antitumor effects by targeting the EGFR receptor[22,23]. Previous studies have shown that acacetin can inhibit the growth of various malignant tumor cell lines through multiple signaling pathways[24-26]. However, the role of acacetin in the H. pylori-induced apoptosis of gastric epithelial cells has not yet been thoroughly investigated. This study aimed to explore the effects and potential mechanisms of action of acacetin on H. pylori-infected gastric mucosal epithelial cells. To the best of our knowledge, this is the first study to confirm that acacetin protects against apoptosis in gastric epithelial GES-1 cells induced by H. pylori infection, with mechanisms identified through the expression of cleaved caspase-3, Bcl-2, and Bax proteins. This study offers a new perspective for early intervention in H. pylori-induced chronic gastritis and may provide synergistic effects and novel therapeutic directions for the clinical eradication of H. pylori using standard quadruple therapy.
MATERIALS AND METHODS
Reagents
Acacetin was acquired from MCE (Shanghai, China) and dissolved in dimethyl sulfoxide to achieve a concentration of 20 mmol/L. The solution was subsequently preserved at −80 °C. Before use, the acacetin stock solution was thawed and diluted to the desired concentration in a complete culture medium containing fetal bovine serum (FBS).
Cell culture methods and conditions
The normal human gastric epithelial cell line (GES-1; Cell Bank of the Chinese Academy of Sciences, Shanghai, China) was preserved in liquid nitrogen. Cells were cultured in complete medium, consisting of Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Grand Island, NY, United States) supplemented with 10% FBS (Gibco, Zhejiang Province, China), and incubated in a humidified environment of 95% air and 5% CO2 at 37 °C.
H. pylori strain culture and infection process
The H. pylori Sydney strain HPSS1 (cagA positive, vacA positive) was provided by the Army Medical University and stored at −80 °C. The bacteria were cultured on Columbia agar plates containing sterile defibrinated sheep blood and H. pylori antibiotic selection supplement (OXOID, Hampshire, United Kingdom) in a microaerophilic environment of 5% O2, 10% CO2, and 85% N2 at 37 °C for 48-72 h. GES-1 cells were cultured overnight in an antibiotic-free medium to reach 70%-80% confluency. Bacterial colonies were scraped from the Columbia agar plates and suspended in antibiotic-free RPMI 1640 medium supplemented with 10% FBS. The optical density at 600 nm was measured using a spectrophotometer to calculate the concentration of the bacterial suspension. The suspension was then used to infect GES-1 cells. The cells were co-cultured with H. pylori for 24 or 48 h, with a cell-to-bacteria ratio of 1:100 (multiplicity of infection [MOI] of 100).
Experimental protocol
To investigate the effect of acacetin, GES-1 cells were pretreated with acacetin (20 µmol/L) for 2 h, followed by stimulation with H. pylori for 24 h and 48 h to analyze cell viability, lactate dehydrogenase (LDH) release, and cell migration distance. The pretreatment duration of acacetin was adjusted based on previous studies[22,23]. After determining the infection multiplicity, the cells were cultured with H. pylori at a ratio of 1:100 for 24 h. Apoptosis levels were assessed using an apoptosis assay kit and TUNEL assay kit, while the protein expression of Bcl-2, Bax, and cleaved caspase-3 was quantified using western blot analysis.
Cell viability assay
The viability of the human gastric epithelial cell line GES-1 was evaluated through the CCK-8 assay. The cells were seeded at a density of 8 × 103 cells/well in 96-well plates and cultured overnight. Subsequently, the cells were infected with H. pylori at specified ratios (1:10, 1:30, 1:50, 1:80, and 1:100) for 24 h and 48 h, with the 1:100 infection ratio selected for further experiments. Cells were pretreated with varying concentrations of acacetin (1 µmol/L, 3 µM, 10 µM, 20 µM, and 40 µM) before H. pylori infection, and changes in cell viability after acacetin pretreatment were assessed at 24 h and 48 h. CCK-8 solution (10 µL/well) was added to each well, and the plates were incubated for 1 h, after which the optical density at 450 nm was measured using a microplate reader (Synergy H1; Bio-Tek, Winooski, VT, United States).
LDH release assay
The cell culture supernatants were collected and centrifuged at 400 × g for 10 min. Following the manufacturer’s instructions, 20 µL of the cell supernatant was used to assess LDH release via an LDH assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China). The absorbance was measured at 450 nm.
Wound healing assay
The cells were plated at a density of 2 × 105 cells per well into 6-well plates and incubated overnight to ensure full attachment. On reaching 90% confluency, scratches were made using the tip of a 200-µL sterile pipette. The cells were then washed with PBS and cultured in RPMI 1640 medium containing 5% FBS at 37 °C. Wound healing stages were imaged at 0, 12, 24, and 48 h using an optical microscope. The wound area was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, United States), and the migration rate was calculated as follows: Migration rate (%) = (Scratch area at 0 h − Scratch area at a specific time point)/Scratch area at 0 h × 100%. Data are reported as the average from three independent experiments.
Flow cytometry analysis of apoptosis
Cells were plated at a density of 2 × 105 cells per well into 6-well plates and treated with acacetin (20 µmol/L) and H. pylori for 24 h. The cells were then digested with trypsin without EDTA, collected, resuspended in PBS, and counted. A total of 1 × 104 resuspended cells were stained using an Annexin V-FITC apoptosis detection kit (Beyotime Biotechnology, Jiangsu, China) according to the manufacturer’s instructions and incubated in the dark at room temperature for 15 min. Apoptosis was analyzed using a flow cytometer (FACSMelody; BD Biosciences, Franklin Lakes, NJ, United States).
TUNEL staining
The TUNEL assay was conducted following the protocol provided with the one-step TUNEL apoptosis assay kit (Beyotime Biotechnology). GES-1 cells were seeded on sterile coverslips in 6-well plates and treated with acacetin (20 µM) and H. pylori for 24 h. The cells were then fixed with 4% paraformaldehyde for 30 min, washed twice with PBS, permeabilized with 0.3% Triton X-100 in PBS, washed again, and incubated with the TUNEL detection solution for 1 h, followed by DAPI staining for 10 min. Apoptotic cells were imaged using an inverted fluorescence microscope (Olympus, Tokyo, Japan) at a × 40 magnification. Three independent experiments were conducted, and each experiment was repeated three times.
Western blot analysis
Cells were harvested, and the total protein was extracted. The lysate mixture was centrifuged at 4 °C at 12,000 rpm for 15 min, and protein concentration was determined using a BCA protein assay kit (Beyotime Biotechnology). Whole-cell extracts were loaded onto lanes and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by transfer to PVDF membranes (Millipore, Billerica, MA, United States). The membranes were blocked with 3% skimmed milk in tris-buffered saline (TBST and 0.2% Tween 20) for 1 h and incubated overnight at 4 °C with the following primary antibodies: mouse anti-cleaved-caspase 3 (diluted 1:2000; Proteintech, Rosemont, IL, United States), rabbit anti-Bcl-2 (diluted 1:2000; Proteintech), rabbit anti-Bax (diluted 1:2000; Proteintech), and mouse anti-GAPDH (diluted 1:1000; Proteintech). After washing the membranes three times with TBST, they were incubated with secondary antibodies (BBI Life Sciences Corp., Shanghai, China) at room temperature for 1 h. Protein bands were detected using an ECL chemiluminescence system. Blots were visualized using an ECL luminescent solution (Oriscience Biotechnology Co., Ltd., Chengdu, China). GAPDH was used as an internal control. Densitometric quantification of each protein band was performed using ImageJ software (NIH), and the density ratios of cleaved-caspase3/GAPDH, Bcl-2/GAPDH, and Bax/GAPDH were determined. Data are presented as mean ± SD of three independent experiments, with four samples per experimental group. The ratio of the blank control group (cells unstimulated with H. pylori and untreated with acacetin) was set at 100%.
Statistical analysis
Data were analyzed using GraphPad Prism software (La Jolla, CA, United States), using an unpaired Student’s t-test or ANOVA for statistical analysis. All data are represented as mean ± SE. P < 0.05 was considered statistically significant.
RESULTS
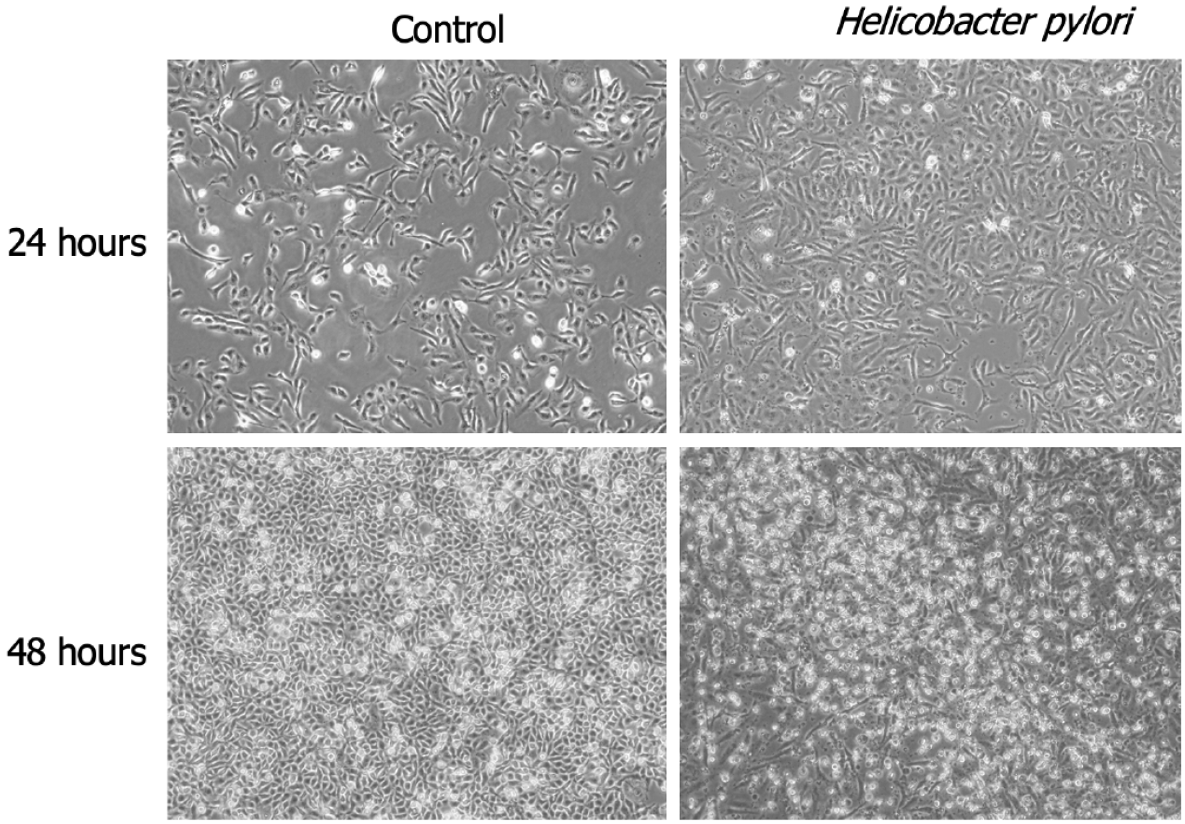
H. pylori infection influences cellular morphology
Using optical microscopy, we examined the morphology of GES-1 cells infected with H. pylori SS1 (CagA+ and VacA+) for 24 h and 48 h. Compared to the untreated normal control group cells (NC), the infected cells showed significant morphological changes. H. pylori infection resulted in the loss of cell polarity, with cell morphology transitioning from blunt to elongated, spindle, and irregular forms, such as fusiform and hummingbird-like shapes (Figure 1).
Figure 1 Significant morphological alterations in GES-1 cells following exposure to Helicobacter pylori for 24 h and 48 h.
A: 24 h; B: 48 h. × 40 magnification.
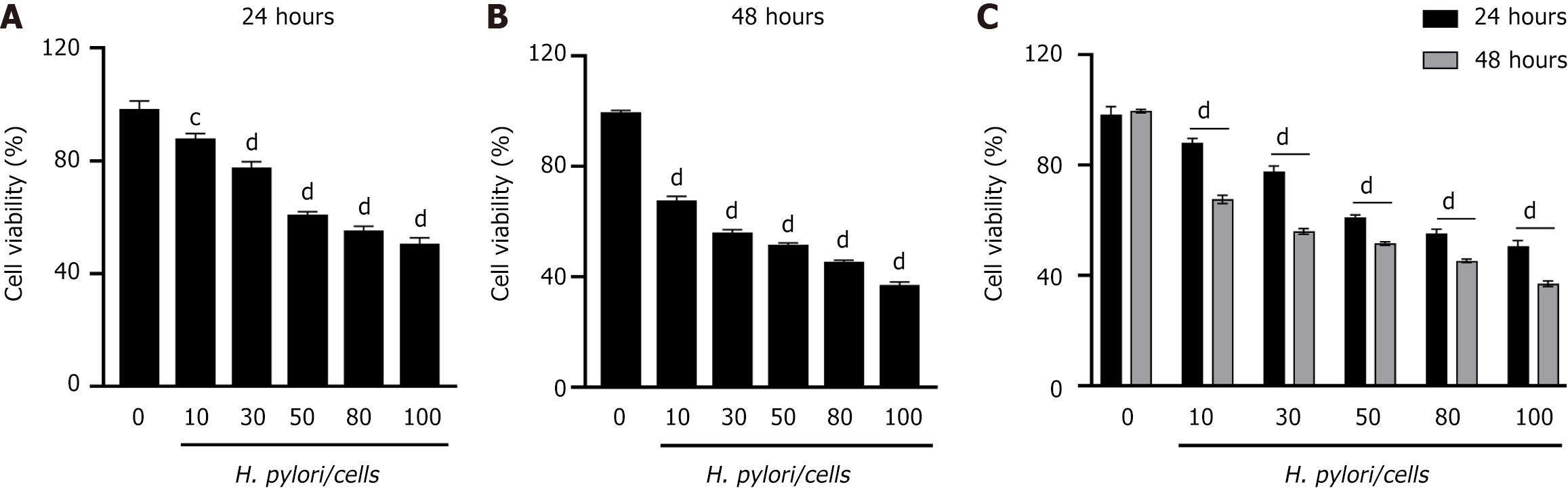
H. pylori induces a decrease in GES-1 cell viability
To investigate whether H. pylori infection promotes a decrease in cellular viability, we measured the viability of GES-1 cells at 24 h (Figure 2A) and 48 h (Figure 2B) after infection, using the CCK-8 assay at specified ratios of cells to H. pylori (MOI = 1:10, 1:30, 1:50, 1:80, and 1:100). We observed a concentration-dependent decrease in GES-1 cell viability with increasing H. pylori infection concentrations. Furthermore, cell viability was significantly reduced at 48 h compared to 24 h (Figure 2C). At a cell-to-H. pylori ratio of 1:100, GES-1 cell viability was suppressed to 50% of that in the normal group; hence, a 1:100 infection ratio was used for subsequent experiments.
Figure 2 Helicobacter pylori infection reduces the viability of GES-1 gastric epithelial cells.
A and B: Stimulation of cells for 24 h or 48 h at specified ratios of cells to Helicobacter pylori (H. pylori); C: Viability of GES-1 cells treated with different ratios of H. pylori over various time periods. The viability of the control group (GES-1 cells not stimulated with H. pylori) was set at 100%. Significant differences were observed: aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001 vs control group.
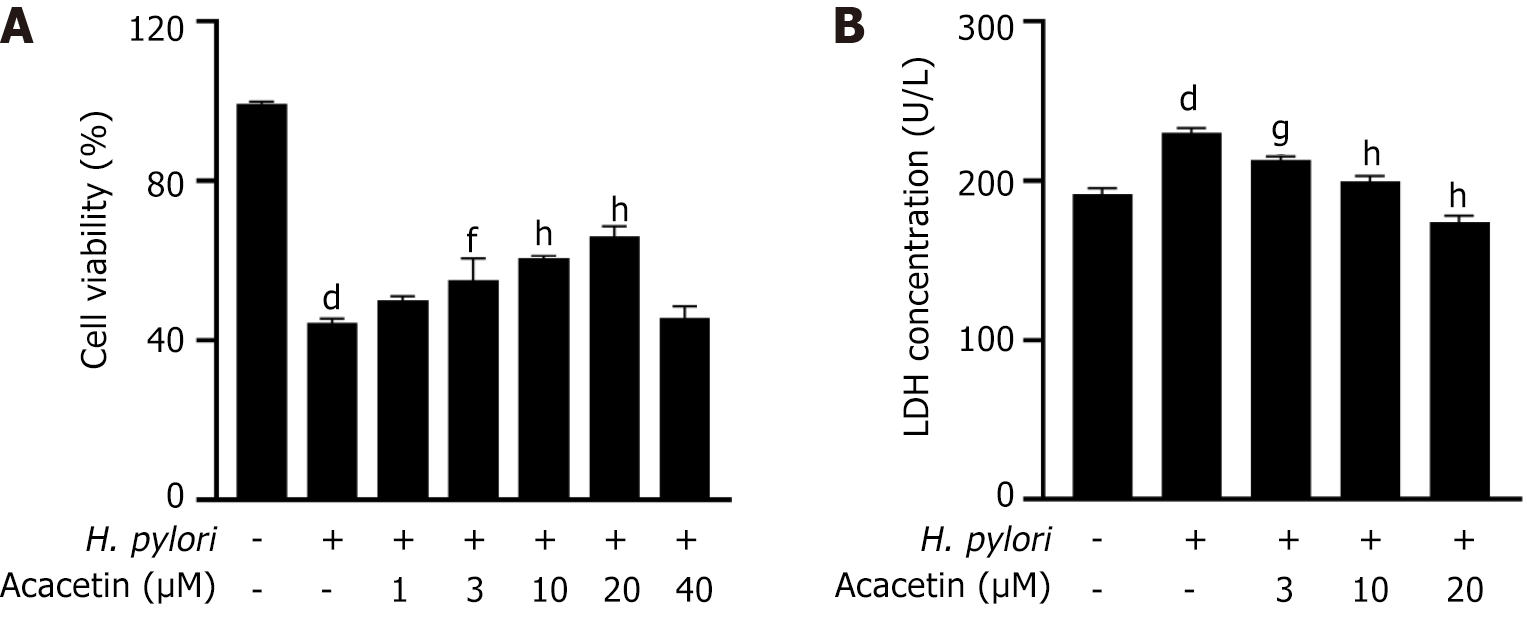
Acacetin mitigates the cytotoxic effects induced by H. pylori in GES-1 cells
GES-1 cells were pretreated with various concentrations of acacetin (1 µM, 3 µM, 10 µmol/L, 20 µmol/L, 40 µmol/L). The CCK-8 assay revealed that acacetin inhibited the H. pylori-induced decrease in cell viability in a dose-dependent manner (Figure 3A). Compared to the model group (cells infected with H. pylori), pretreatment with acacetin at concentrations of 10 µmol/L and 20 µmol/L significantly reduced the inhibitory effect of H. pylori on GES-1 cell viability. The most notable increase in cell viability was observed at an acacetin concentration of 20 µmol/L. Therefore, this concentration was used in subsequent experiments. To investigate the protective effect of acacetin against H. pylori-induced cytotoxicity in GES-1 cells, cell viability and mortality were measured using the CCK-8 assay and LDH release experiments with and without the addition of acacetin. These results suggest that acacetin mitigated the H. pylori-induced decrease in cell viability (Figure 3A). The LDH release results (Figure 3B) were consistent with those of the CCK-8 assay, showing that H. pylori infection led to increased LDH release from GES-1 cells, which was mitigated by acacetin.
Figure 3 Acacetin inhibits the decline in cell viability and lactate dehydrogenase release induced by Helicobacter pylori infection.
A: Cells were pre-treated with specified concentrations of acacetin extract for 2 h, followed by stimulation with Helicobacter pylori (H. pylori) [multiplicity of infection (MOI) = 1:100] for 24 h; B: After pretreatment with specified concentrations of acacetin extract for 2 h and subsequent stimulation with H. pylori (MOI = 1:100) for 24 h, the amount of lactate dehydrogenase released into the cell culture supernatant was measured. aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001 vs control group; eP < 0.05, fP < 0.01, gP < 0.001, hP < 0.0001 vs H. pylori group.
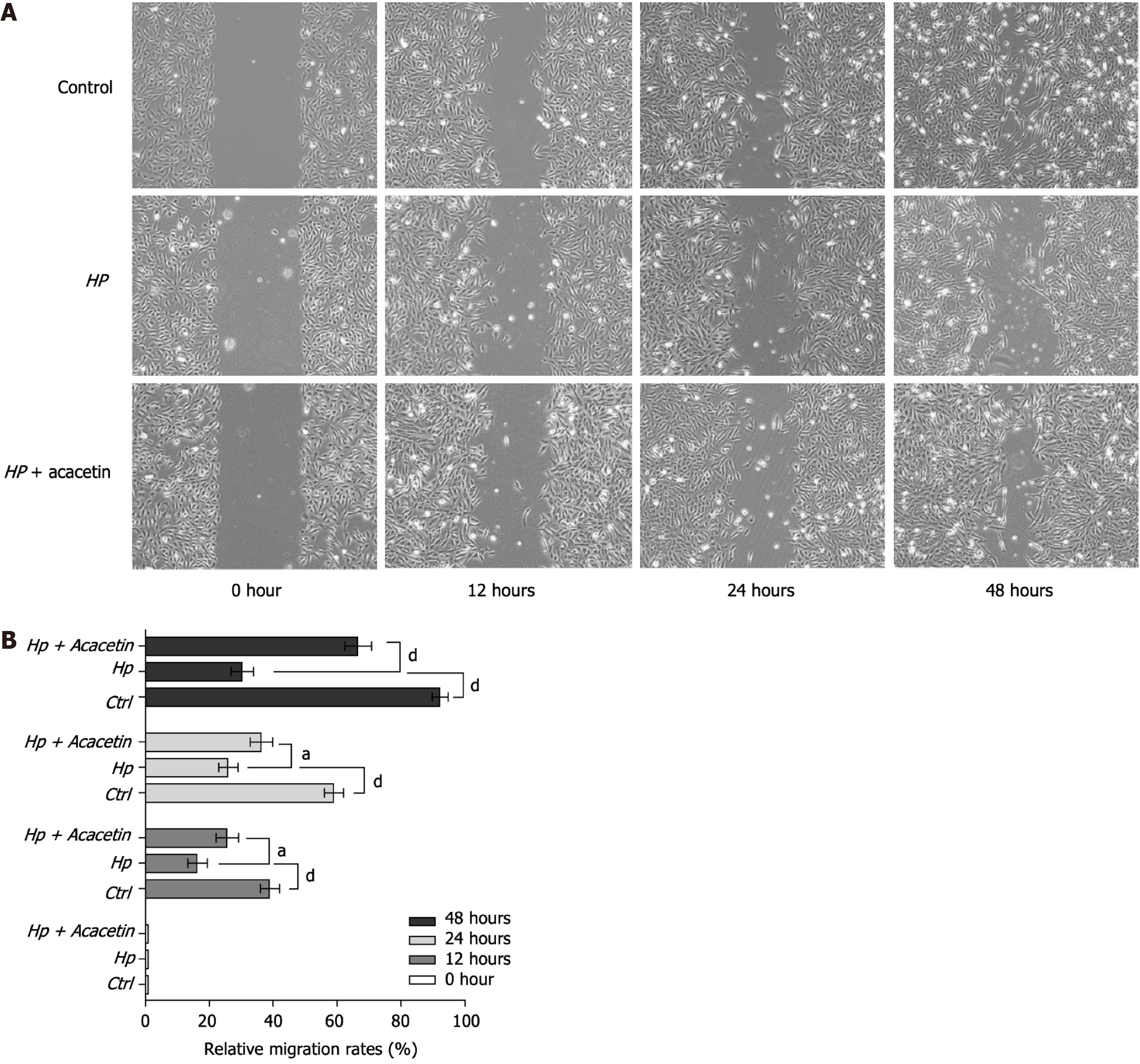
Acacetin reverses H. pylori-induced migration inhibition in GES-1 cells
We assessed the effect of H. pylori infection on the migration of GES-1 cells using a wound healing assay. As depicted in Figure 4A, compared to the blank control group (untreated GES-1 cells), GES-1 cells showed significantly reduced migration and repair capabilities at 12, 24, and 48 h after infection with H. pylori (MOI = 1:100). In contrast, GES-1 cells pretreated with acacetin (20 µM) exhibited enhanced migration and repair compared to the H. pylori-infected group. In summary, these results indicate that acacetin reversed the migration inhibition induced by H. pylori in GES-1 cells (Figure 4B).
Figure 4 Migration analysis was performed through a wound healing assay.
A: Images of the wound region captured at four distinct time intervals (0, 12, 24, and 48 h). × 40 magnification; B: The area of wound closure was analyzed and converted into migration rates using ImageJ software. HP: Helicobacter pylori.
Acacetin inhibits H. pylori-induced apoptosis in GES-1 cells
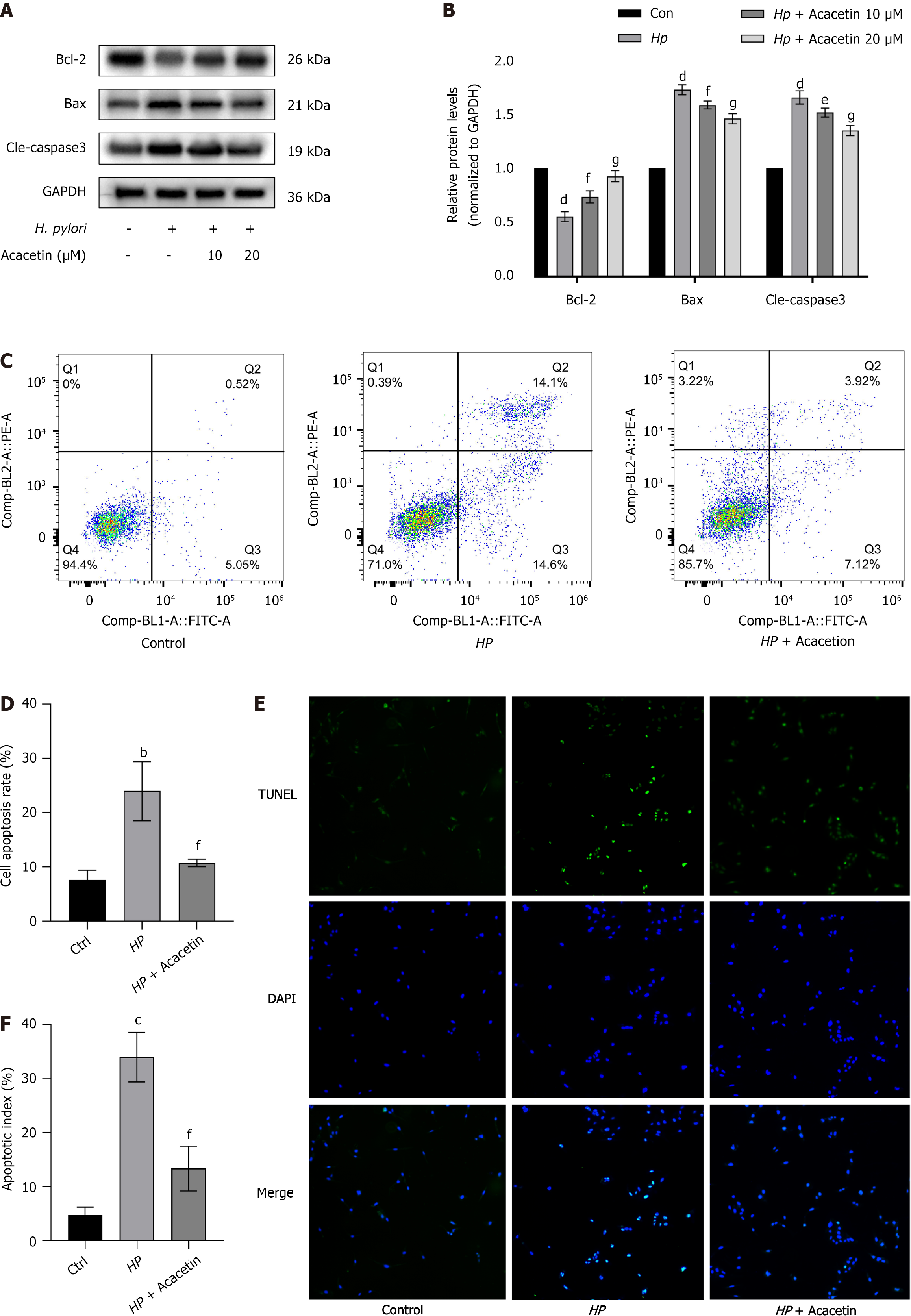
To examine the influence of acacetin on H. pylori-induced apoptosis in GES-1 cells, the expression levels of apoptosis-associated proteins were quantified by western blot analysis under various concentrations of acacetin (10 µmol/L, 20 µmol/L) or without it. Our immunoblotting results revealed that compared to the blank control group, H. pylori infection significantly reduced the expression of Bcl-2 protein and significantly upregulated the expression of Bax and cleaved caspase-3 (Figure 5A); these effects were inhibited by acacetin in a concentration-dependent manner (Figure 5B). Additionally, flow cytometry analysis (Figure 5C and D) and TUNEL staining of cell nuclei (Figure 5E and F) were performed on GES-1 cells. Our findings indicated that compared to the blank control group, the model group (H. pylori-infected group) showed a significantly increased apoptosis rate (Figures 5D and 5F), further suggesting that H. pylori infection significantly induced apoptosis. Compared to no treatment in the model group (H. pylori-infected group), acacetin treatment significantly reduced H. pylori-induced apoptosis in GES-1 cells (Figure 5D and F), further supporting the anti-apoptotic effect of acacetin on H. pylori-infected GES-1 cells.
Figure 5 Acacetin extract inhibits Helicobacter pylori-induced apoptosis in GES-1 cells.
A and B: Protein levels of cleaved caspase-3, Bcl-2, and Bax were analyzed by immunoblotting following pre-treatment with acacia extract (10 µmol/L, 20 µmol/L) and subsequent stimulation with Helicobacter pylori(H. pylori) (cell to H. pylori ratio of 1:100) for 24 h. GAPDH served as an internal control, with the ratio in the blank control group set to 1; C and D: Apoptosis levels in the blank group, H. pylori-infected group, and H. pylori plus acacetin extract group were measured by flow cytometry; E: Representative images of cells stained with TUNEL and DAPI. × 40 magnification; F: Percentage of apoptotic cells quantified as the apoptosis index.
DISCUSSION
Previous studies have indicated that H. pylori infection can disrupt the gastric mucosal barrier by inducing apoptosis in gastric epithelial cells and causing oxidative DNA damage, leading to chronic gastritis and peptic ulcers that may further progress to GC[27,28]. The pathogenic mechanisms involved are complex, encompassing disturbances in the dynamics of gastric mucosal epithelial cells; an imbalance between cell division, proliferation, and apoptosis; and abnormalities in regulatory genes. H. pylori, a human pathogen, flourishes in the acidic environment of the stomach by secreting urease to neutralize the acidity[29]. It colonizes the gastric mucosa and secretes cytotoxic proteins that increase the rate of apoptosis both intracellularly and extracellularly, thereby playing a key role in the transformation and development of precancerous conditions[30]. Therefore, early eradication of H. pylori is widely considered an effective means of reducing the risk of GC. With the widespread adoption of quadruple therapy for the eradication of H. pylori, adverse drug reactions such as allergic reactions, strain resistance, and dysbiosis of the original intestinal flora due to the use of multiple antibiotics have further increased the difficulty of clinical treatment, reducing patient compliance and leading to failure of H. pylori eradication[31,32]. New treatment methods are urgently required to overcome this bottleneck.
Apoptosis can be initiated or inhibited through multiple pathways, with each cell possessing an intrinsic signal capable of activating apoptosis[33]. It is primarily triggered by death receptor (extrinsic pathway) and mitochondrial (intrinsic pathway) pathways. The extrinsic pathway is triggered by the binding of ligands to receptors, which activates caspase-8 and promotes the activation of caspase-3. Caspase-3, the executor of apoptosis, converges to both the intrinsic and extrinsic apoptotic pathways at this node, leading to cell death[34,35]. Our research found that caspase-3 activation and cleavage occurred 24 h after H. pylori infection, and acacetin inhibited this activation, suggesting that the anti-apoptotic effect of acacetin on H. pylori-induced cell apoptosis may involve caspase-3-related pathways. Internal apoptosis is triggered by increased permeability of the mitochondrial outer membrane, governed by the Bcl-2 family of proteins[36]. We speculate that Bcl-2 family proteins may be involved in acacetin-mediated apoptosis in H. pylori-infected GES-1 cells. In this study, H. pylori infection reduced the expression of Bcl-2 in GES-1 cells and enhanced the expression of Bax, while acacetin treatment upregulated Bcl-2 and downregulated Bax expression. In summary, we demonstrated that acacetin inhibits the expression of pro-apoptotic proteins such as cleaved caspase-3 and Bax and promotes the expression of the anti-apoptotic protein Bcl-2, thereby causing an imbalance in Bax/Bcl-2 and inhibiting apoptosis induced by H. pylori infection.
In this study, we demonstrate the protective and anti-apoptotic effects of acacetin on H. pylori-infected gastric mucosal epithelial cells. The results showed that acacetin increased cell viability and reduced LDH release in H. pylori-infected GES-1 cells. Additionally, acacetin reversed the reduced migration and repair capabilities of H. pylori-infected GES-1 cells. Most importantly, acacetin inhibited H. pylori-induced apoptosis in GES-1 cells by regulating the expression of Bcl-2 and Bax family members and inhibiting caspase-3 activity in a concentration-dependent manner. Our findings suggest that acacetin protects against H. pylori-induced apoptosis of gastric mucosal epithelial cells, indicating its potential for treating H. pylori-related diseases and aiding in the eradication of H. pylori in conjunction with the current standard quadruple therapy.
In clinical practice, the two antibiotics included in standard quadruple therapy remain the primary means of eradicating H. pylori; however, the use of antibiotic drugs may lead to bacterial resistance and the risk of reinfection[37]. The continuous increase in antibiotic resistance has become a major factor leading to a gradual decline in the success rate of current treatments for H. pylori infections. Globally, the main patterns of H. pylori infection resistance are increasing, making prevention and treatment of H. pylori infections an urgent public health concern. Therefore, the development of non-antibiotic therapies based on phytochemicals and antioxidants has become an extremely attractive new trend in the development of all-natural treatments for H. pylori infections. Traditional Chinese medicine can enhance the inhibitory effects on resistant bacterial strains, alleviate gastric mucosal inflammation, and promote regeneration and repair, thus offering advantages over monotherapy with Western medicines for eliminating H. pylori. To date, no studies have reported the protective effects of acacetin against apoptosis in H. pylori-infected gastric epithelial cells. In this study, we found that acacetin acts as a cellular protector, enhancing cell viability and reducing LDH release, thereby combating apoptosis in H. pylori-infected GES-1 cells, demonstrating the antibacterial effect of acacetin on normal gastric epithelial cells.
Acacetin is an orally effective flavonoid herbal compound with almost no side effects and possesses effective anti-cancer and anti-inflammatory activities, causing cancer cell cycle arrest and inducing apoptosis. Previous in vitro studies have shown that acacetin exhibits broad anti-cancer activities by regulating various signaling pathways and inducing apoptosis in human breast cancer MCF-7 cells, oral squamous carcinoma cells, human non-small cell lung cancer, glioblastoma, human osteosarcoma cells, human liver cancer cells, etc[38-43]. In gastrointestinal disease research, acacetin has been shown to inhibit the persistent proliferation of GC cells by inhibiting the PI3K/Akt/Snail signaling pathway, exert anti-tumor effects on gastric cancer by targeting EGFR, and induce apoptosis in colorectal cancer cells by activating apoptosis-inducing factors[44]. However, the effects and mechanisms of action of acacetin on normal H. pylori-infected human gastric mucosal epithelial cells have not been reported. Therefore, through western blotting and cell function-related experiments, this study validated that acacetin protects the gastric mucosa from H. pylori toxins by inhibiting apoptosis.
Previous animal studies have shown that acacetin alleviates ischemia-reperfusion kidney injury in mice[45], inhibits oxidative stress through the Nrf-2/HO-1 pathway, reduces myocardial ischemia/reperfusion injury[46], improves the oxidative-antioxidative system, accelerates testicular cell regeneration, and inhibits cell apoptosis[47]. These studies demonstrate that acacetin can prevent mitochondrial dysfunction and organ damage caused by oxidative stress. Thus, acacetin might suppress apoptosis by mitigating mitochondrial dysfunction in H. pylori-infected mice. Prior research has introduced animal models of H. pylori infection and shown that Korean red ginseng extract and N-acetylcysteine reduce gastric inflammation in mice and gerbils exposed to H. pylori[48,49]. Consequently, acknowledging the limitations of this study, further research is needed to determine whether acacetin inhibits apoptosis in the gastric mucosal tissues of animals infected with H. pylori.