Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3521
Revised: May 2, 2024
Accepted: June 11, 2024
Published online: August 15, 2024
Processing time: 168 Days and 23 Hours
Bevacizumab, an anti-vascular endothelial growth factor (VEGF) monoclonal antibody, inhibits angiogenesis and reduces tumor growth. Serum VEGF-C, lactate dehydrogenase, and inflammatory markers have been reported as predictive markers related to bevacizumab treatment. Programmed cell death ligand 1 (PD-L1) could act upon VEGF receptor 2 to induce cancer cell angioge
To investigate the efficacy of bevacizumab-containing chemotherapy in patients with metastatic colorectal cancer (CRC) according to the expression of PD-L1.
This analysis included CRC patients who received bevacizumab plus FOLFOX or FOLFIRI as first-line therapy between June 24, 2014 and February 28, 2022, at Samsung Medical Center (Seoul, South Korea). Analysis of patient data included evaluation of PD-L1 expression by the combined positive score (CPS). We analyzed the efficacy of bevacizumab according to PD-L1 expression status in patients with CRC.
A total of 124 patients was included in this analysis. Almost all patients were treated with bevacizumab plus FOLFIRI or FOLFOX as the first-line chemotherapy. While 77% of patients received FOLFOX, 23% received FOLFIRI as backbone first-line chemotherapy. The numbers of patients with a PD-L1 CPS of 1 or more, 5 or more, or 10 or more were 105 (85%), 64 (52%), and 32 (26%), respectively. The results showed no significant difference in progression-free survival (PFS) and overall survival (OS) with bevacizumab treatment between patients with PD-L1 CPS less than 1 and those with PD-L1 CPS of 1 or more (PD-L1 < 1% vs PD-L1 ≥ 1%; PFS: P = 0.93, OS: P = 0.33), between patients with PD-L1 CPS less than 5 and of 5 or more (PD-L1 < 5% vs PD-L1 ≥ 5%; PFS: P = 0.409, OS: P = 0.746), and between patients with PD-L1 CPS less than 10 and of 10 or more (PD-L1 < 10% vs PD-L1 ≥ 10%; PFS: P = 0.529, OS: P = 0.568).
Chemotherapy containing bevacizumab can be considered as first-line therapy in metastatic CRC irrespective of PD-L1 expression.
Core Tip: The efficacy of first line chemotherapy (FOLFOX or FOLFIRI) plus bevacizumab in metastatic colorectal cancer was not significantly different according to the status of tumor tissue programmed cell death ligand 1 (PD-L1) combined positive score. Further research regarding dual inhibition of the vascular endothelial growth factor and PD-L1/programmed death 1 axes in various tumor types including colorectal cancer is required.
- Citation: Kang SW, Lim SH, Kim MJ, Lee J, Park YS, Lim HY, Kang WK, Kim ST. Efficacy of chemotherapy containing bevacizumab in patients with metastatic colorectal cancer according to programmed cell death ligand 1. World J Gastrointest Oncol 2024; 16(8): 3521-3528
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3521.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3521
Globally, colorectal cancer (CRC) is ranked the second most frequent cancer diagnosed in women and the third most frequent in men[1]. CRC represents a significant public health challenge, contributing to 8.5% of all cancer-related deaths and ranking as the fourth most common cause of cancer mortality worldwide[1]. Angiogenesis has been identified as a crucial hallmark of cancer development and has become a major target in the treatment of solid tumors, including CRC[2,3]. Tumor angiogenesis is a critical process for tumor growth and survival and is heavily reliant on the activity of vascular endothelial growth factor (VEGF)[4]. Treatment with bevacizumab, which is a monoclonal antibody (mAb) directed against VEGF, could result in reduction of tumor vasculature and prevention of new blood vessel growth, impeding tumor progress. Bevacizumab-based therapy has improved the survival of patients with metastatic CRC (mCRC)[5-7]. The Food and Drug Administration approved bevacizumab as a first- or second-line treatment for mCRC when combined with chemotherapy based on survival benefit, as shown in a landmark trial.
Immunotherapy, emerging as a novel anticancer treatment has become a key strategy for combating various solid tumors. This approach primarily targets programmed cell death ligand 1 (PD-L1) or programmed death 1 (PD-1), which are crucial in regulating the immune response against tumors. PD-L1 expression is controlled by various pathways, and tumors with low PD-L1 levels typically exhibit less T cell infiltration compared to those with high PD-L1 expression[8,9]. Anti-VEGF mAb inhibits angiogenesis and reduces tumor activity while also inducing hypoxia to enhance effector T cells[10-12]. PD-L1 may interact with VEGF receptor 2 (VEGFR2), promoting cancer cell angiogenesis and metastasis[13].
PD-L1 and VEGF signaling are cross-linked, and the synergistic effect of anti-PD-L1/PD-1 inhibition and anti-angiogenic inhibition has been reported in various tumor types. However, in mCRC, there is little information regarding relationships between PD-L1 expression and clinical outcomes. Therefore, we investigated the clinical outcomes of bevacizumab plus chemotherapy in patients with mCRC according to PD-L1 expression.
We analyzed all 124 metastatic CRC patients who received first-line bevacizumab and were tested for PD-L1 expression at Samsung Medical Center, Korea, between June 24, 2014 and February 28, 2022. The following clinicopathologic characteristics were collected for all 124 patients: Age, sex, tumor site, initial disease status, pathology, chemotherapy, and survival. This study was approved by the Institutional Review Board (IRB No. 2021-09-052-004) at Samsung Medical Center, and individual consent for this analysis was waived. Patients in the database were identified by patient number only, and patient information was kept confidential according to the IRB protocol. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and the Korea Good Clinical Practice guidelines.
Samples for analysis were collected from 501 solid tumors and were processed into formalin-fixed paraffin-embedded material. Tumor samples were collected as biopsies at diagnosis, surgical specimens, or repeat biopsies at the time of disease progression. Although the collection times of the tumor samples were not uniform, they were all collected prior to the start of first-line bevacizumab.
Tissue samples were freshly cut into 4 μm sections, mounted on Fisherbrand Superfrost Plus Microscope Slides (ThermoFisher), and then dried at 60°C for one hour. Immunohistochemistry (IHC) staining was carried out on a Dako Autostainer Link 48 system (Agilent Technologies) using a Dako PD-L1 IHC 22C3 pharmDx kit (Agilent Technologies) with an EnVision FLEX visualization system. The samples were then counterstained with hematoxylin according to the manufacturer’s instructions. The expression of PD-L1 protein was quantitated using a combined positive score (CPS) calculated as the number of PD-L1-stained cells (tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells, multiplied by 100.
Descriptive statistics were reported as proportions and medians. Data are presented as the n (%) for categorical variables. Response categories were assessed according to RECIST 1.1. Progression-free survival (PFS) was defined as the time from the start of bevacizumab until the date of disease progression or death from any cause. Overall survival (OS) was defined as the time from the start of bevacizumab until death from any cause. Survival analysis between the two subgroups was performed using the Kaplan-Meier method, and the hazard ratio between the two subgroups was analyzed using Cox-proportional hazard models. All P values were two-sided, and statistical significance was set at P <0.05. Statistical analysis was performed using IBM SPSS Statistics 25 (Armonk, NY, United States).
Table 1 presents the clinical characteristics including expression of PD-L1. A total of 124 patients was analyzed, with a median age was 56 years. Among the patients, 69 (56%) were male and 55 (44%) were female. Almost all patients were treated with bevacizumab plus FOLFIRI or FOLFOX as the first-line chemotherapy. Of these, 77% of patients received FOLFOX, while 23% received FOLFIRI as backbone first-line chemotherapy. When dividing patients according to tumor mutational burden (TMB) status, 99 (80%) were TMB-low and 25 (20%) were TMB-high. In this study, TMB high was defined as ≥ 10 mutations/Mb and TMB low was defined as < 10 mutations/Mb. When sorting patients according to microsatellite instability (MSI) status, 120 (97%) were MSS and 4 (3%) were MSI-high. A total of 105 patients (85%) had a PD-L1 CPS of 1 or more, 64 patients (52%) had a PD-L1 CPS of 5 or more, and 32 patients (26%) had a PD-L1 CPS of 10 or more (Table 1).
| Characteristics | |
| Number of patients | 124 |
| Age (yr) | 56 (29-83) |
| Sex, n (%) | |
| Male | 69 (56) |
| Female | 55 (44) |
| Tumor sidedness, n (%) | |
| Right | 32 (26) |
| Left | 92 (74) |
| RAS mutational status, n (%) | |
| Wild type | 64 (52) |
| Mutation | 60 (48) |
| TMB status, n (%) | |
| TMB-low | 99 (80) |
| TMB-high | 25 (20) |
| Status of MSI (by IHC), n (%) | |
| MSS | 120 (97) |
| MSI-high | 4 (3) |
| PD-L1 status, n (%) | |
| PD-L1 CPS ≥ 1 | 105 (85) |
| PD-L1 CPS ≥ 5 | 64 (52) |
| PD-L1 CPS ≥ 10 | 32 (26) |
| Chemotherapy backbone with bevacizumab as first-line treatment, n (%) | |
| FOLFOX | 96 (77) |
| FOLFIRI | 28 (23) |
The tumor responses to bevacizumab were partial response (PR) in 51 patients (41%), stable disease (SD) in 56 patients (45%), and disease progression (PD) in 9 patients (7%) (Table 2). We re-analyzed the tumor response to bevacizumab at cutoff values of PD-L1 CPS of 1, 5, and 10. When the PD-L1 CPS cutoff value was 1, patients with a PD-L1 CPS value of 1 or more numbered 105, and a PR was noted in 44 patients (42%), SD in 47 patients (45%), and PD in 8 patients (8%). When the PD-L1 CPS cutoff value was 5, patients with a PD-L1 CPS of 5 or more numbered 64, and a PR was noted in 29 patients (45%), SD in 16 patients (25%), and PD in 4 patients (6%). At a PD-L1 CPS cutoff of 10, patients with a PD-L1 CPS of 10 or more numbered 32, and a PR was noted in six patients (53%), SD in 10 patients (31%), and PD in 2 patients (5%).
| Response | |
| CR | 3 (3) |
| PR | 51 (41) |
| SD | 56 (45) |
| PD | 9 (7) |
| Unknown | 5 (4) |
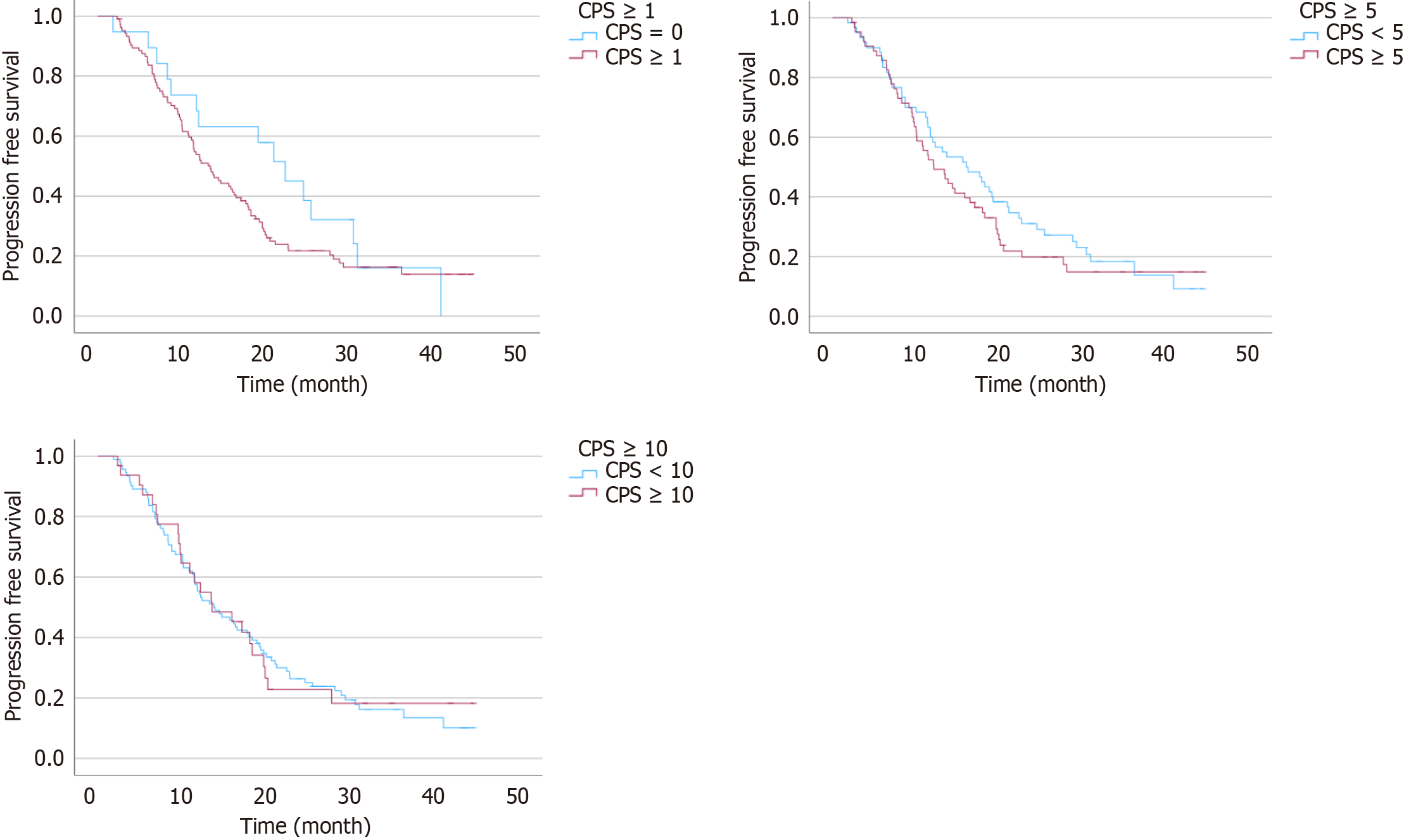
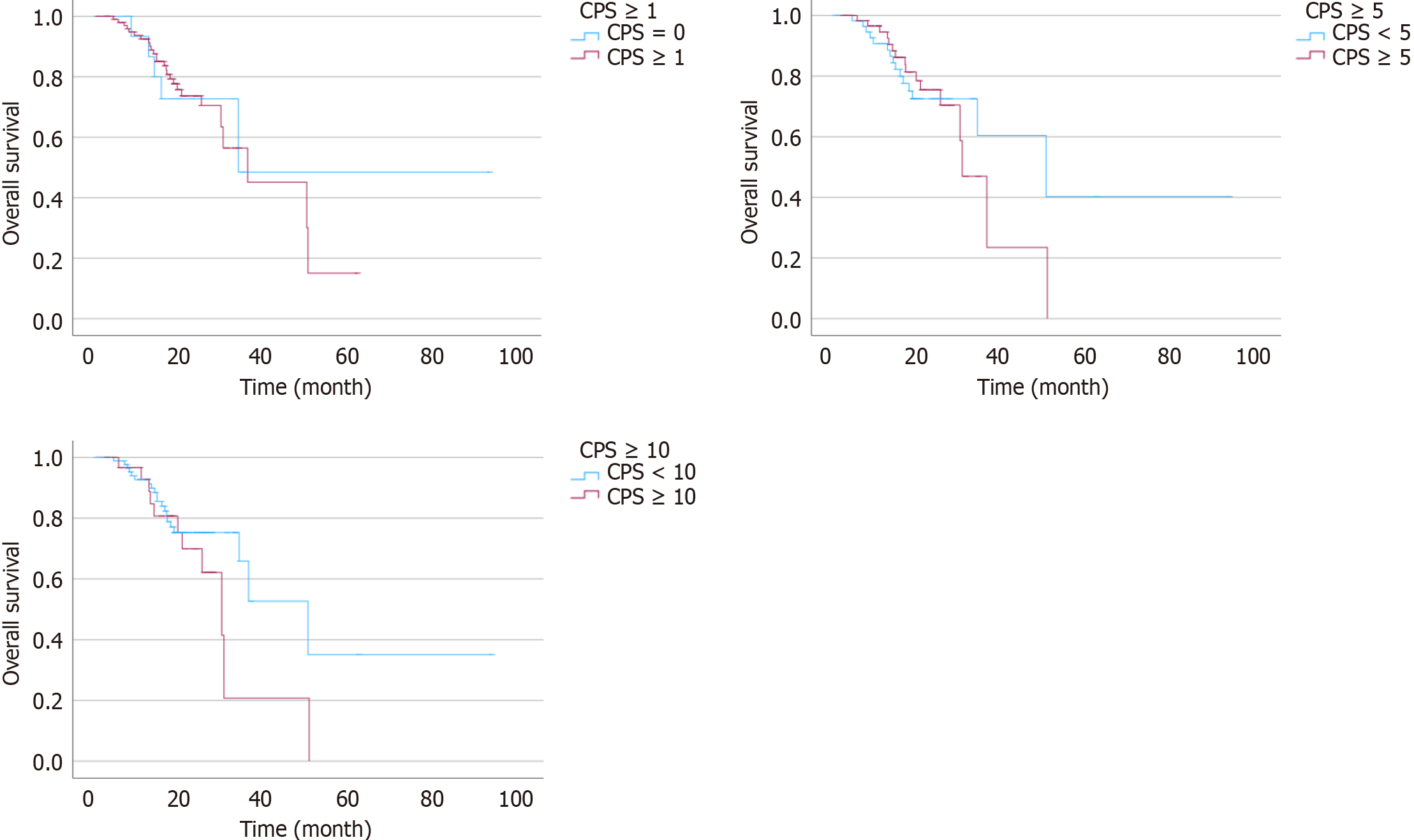
We analyzed patient survival with bevacizumab treatment based on the PD-L1 CPS (Table 3). At the cutoff value of one, there was no significant difference in OS and PFS with bevacizumab between patients with a PD-L1 CPS less than 1 and 1 or more (P = 0.863, and P = 0.152, respectively) (Figure 1). At a cutoff value of 5, there was no significant difference in OS and PFS with bevacizumab between patients with a PD-L1 CPS less than 5 and 5 or more (P = 0.746, and P = 0.409, respectively) (Figure 2).
| Patients, n (%) | Progression-free survival (months) | P value2 | Overall survival (months) | P value2 | |
| PD-L1 CPS < 11 | 19 (15) | 22.4 (15.63-27.30) | P = 0.152 | 33.7 (NA) | P = 0.863 |
| PD-L1 CPS ≥ 1 | 105 (85) | 13.2 (14.91-20.10) | 35.9 (16.84-55.02) | ||
| PD-L1 CPS < 5 | 60 (48) | 16.7 (15.80-22.60) | P = 0.409 | 49.6 (20.89-78.97) | P = 0.746 |
| PD-L1 CPS ≥ 5 | 64 (52) | 12.1 (13.81-20.47) | 30.1 (24.96-34.24) | ||
| PD-L1 CPS < 10 | 92 (74) | 13.7 (15.35-20.77) | P = 0.529 | 49.9 (32.10-67.77) | P = 0.529 |
| PD-L1 CPS ≥ 10 | 32 (26) | 13.5 (13.52-23.45) | 29.6 (21.65-37.49) |
Also, at the cutoff value of 10, there was no significant difference in OS and PFS with bevacizumab between patients with PD-L1 CPS less than 10 and 10 or more (P = 0.568, and P = 0.529, respectively) (Figures 1 and 2). Supplementary Figure 1 shows PFS and OS in the patients divided into 4 subgroups according to PD-L1 CPS, and there was no statistically significant difference between each subgroup.
This study intended to investigate the clinical outcomes of bevacizumab plus chemotherapy in patients with mCRC according to PD-L1 expression. In the present analysis, PFS and OS with chemotherapy containing bevacizumab were not different in patients with a PD-L1 CPS less than one or one or more (PD-L1 < 1% vs PD-L1 ≥ 1%; PFS: P = 0.93, OS: P = 0.33), between those with a PD-L1 CPS less than five or five or more (PD-L1 < 5% vs PD-L1 ≥ 5%; PFS: P = 0.409, OS: P = 0.746), and between those with a PD-L1 CPS less than 10 or 10 or more (PD-L1 < 10% vs PD-L1 ≥ 10%; PFS: P = 0.529, OS: P = 0.568). These findings suggest that chemotherapy containing bevacizumab can still be used as a first-line therapy in mCRC irrespective of PD-L1 expression.
In tumors, VEGF released by hypoxic cancer cells and vascular endothelial cells promotes tumor growth, invasion, and metastasis by increasing neovascularization[14]. Simultaneously, VGEF promotes immune escape at almost every step of the cancer immunity cycle. Administration of a molecular targeted drug that inhibits VEGF, such as bevacizumab, leads to an increase in antigen presentation by dendritic cells, promotes T-cell activation, and negatively regulates the ex
In this study, we analyzed the response and survival to chemotherapy containing bevacizumab according to the status of PD-L1 CPS in patients with mCRC. Our hypothesis was that the antiangiogenic effect of bevacizumab would differ according to PD-L1 expression, indicating PD-L1 expression as a novel biomarker of bevacizumab in mCRC. However, this study showed that there was no correlation between the efficacy of bevacizumab and PD-L1 expression. The potential mechanism for this result is not yet known. Even in previous studies among patients with MSI-H mCRC, the predictive value of baseline PD-L1 level for immune checkpoint inhibitors remains unclear[23,24]. Similarly, in the recent published results of first-line FOLFOX plus bevacizumab with or without nivolumab trial showed that baseline PD-L1 CPS does not impact PFS[25]. In mCRC, further research is needed to examine the changes in PD-L1 expression during antiangiogenic treatment rather than baseline PD-L1 status.
Our study has several limitations. It had a small sample size, was retrospective in nature, and utilized a heterogeneous population, leading to bias. Second, only Asian patients with CRC were analyzed, limiting the generalizability due to differences in molecular profiles and clinical features between Western and Eastern patients with CRC. Third, the patients analyzed in this study received the anti-angiogenetic agent bevacizumab as well as cytotoxic chemotherapy, limiting conclusions on bevacizumab effects. The finding of the effect of bevacizumab based on PD-L1 expression should be interpreted with caution. In conclusion, this study showed that chemotherapy containing bevacizumab can be considered as a first-line therapy in mCRC irrespective of PD-L1 expression. Further research on dual inhibition of the VEGF and PD-L1/PD-1 axes in various tumor types including CRC is required.
This study investigated the clinical outcomes of bevacizumab plus chemotherapy in patients with mCRC according to PD-L1 expression. The findings suggest that chemotherapy containing bevacizumab could be considered as a first-line therapy in mCRC irrespective of PD-L1 expression.
| 1. | International Agency for Research R on Cancer; World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. [cited 1 December 2023]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. |
| 2. | Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1925] [Cited by in RCA: 1997] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 3. | Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1666] [Cited by in RCA: 1757] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 4. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6952] [Article Influence: 316.0] [Reference Citation Analysis (0)] |
| 5. | Riechelmann R, Grothey A. Antiangiogenic therapy for refractory colorectal cancer: current options and future strategies. Ther Adv Med Oncol. 2017;9:106-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2432] [Article Influence: 270.2] [Reference Citation Analysis (31)] |
| 7. | Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd. Bevacizumab in Combination With Oxaliplatin, Fluorouracil, and Leucovorin (FOLFOX4) for Previously Treated Metastatic Colorectal Cancer: Results From the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2023;41:3670-3675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 8. | Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, Lee S, Park SH, Park JO, Park YS, Lim HY, Lee H, Choi M, Talasaz A, Kang PS, Cheng J, Loboda A, Lee J, Kang WK. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 1166] [Article Influence: 166.6] [Reference Citation Analysis (0)] |
| 9. | Schoemig-Markiefka B, Eschbach J, Scheel AH, Pamuk A, Rueschoff J, Zander T, Buettner R, Schroeder W, Bruns CJ, Loeser H, Alakus H, Quaas A. Optimized PD-L1 scoring of gastric cancer. Gastric Cancer. 2021;24:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Hack SP, Zhu AX, Wang Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front Immunol. 2020;11:598877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 11. | de Almeida PE, Mak J, Hernandez G, Jesudason R, Herault A, Javinal V, Borneo J, Kim JM, Walsh KB. Anti-VEGF Treatment Enhances CD8(+) T-cell Antitumor Activity by Amplifying Hypoxia. Cancer Immunol Res. 2020;8:806-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 418] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 13. | Yang Y, Xia L, Wu Y, Zhou H, Chen X, Li H, Xu M, Qi Z, Wang Z, Sun H, Cheng X. Programmed death ligand-1 regulates angiogenesis and metastasis by participating in the c-JUN/VEGFR2 signaling axis in ovarian cancer. Cancer Commun (Lond). 2021;41:511-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 1396] [Article Influence: 199.4] [Reference Citation Analysis (0)] |
| 15. | Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 16. | Wang S, Li J, Xie J, Liu F, Duan Y, Wu Y, Huang S, He X, Wang Z, Wu X. Programmed death ligand 1 promotes lymph node metastasis and glucose metabolism in cervical cancer by activating integrin β4/SNAI1/SIRT3 signaling pathway. Oncogene. 2018;37:4164-4180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 17. | Cao D, Qi Z, Pang Y, Li H, Xie H, Wu J, Huang Y, Zhu Y, Shen Y, Zhu Y, Dai B, Hu X, Ye D, Wang Z. Retinoic Acid-Related Orphan Receptor C Regulates Proliferation, Glycolysis, and Chemoresistance via the PD-L1/ITGB6/STAT3 Signaling Axis in Bladder Cancer. Cancer Res. 2019;79:2604-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4696] [Article Influence: 939.2] [Reference Citation Analysis (2)] |
| 19. | Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M; IMpower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2770] [Article Influence: 395.7] [Reference Citation Analysis (0)] |
| 20. | Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, Bracarda S, Stadler WM, Donskov F, Lee JL, Hawkins R, Ravaud A, Alekseev B, Staehler M, Uemura M, De Giorgi U, Mellado B, Porta C, Melichar B, Gurney H, Bedke J, Choueiri TK, Parnis F, Khaznadar T, Thobhani A, Li S, Piault-Louis E, Frantz G, Huseni M, Schiff C, Green MC, Motzer RJ; IMmotion151 Study Group. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 762] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 21. | Reckamp KL, Redman MW, Dragnev KH, Minichiello K, Villaruz LC, Faller B, Al Baghdadi T, Hines S, Everhart L, Highleyman L, Papadimitrakopoulou V, Neal JW, Waqar SN, Patel JD, Gray JE, Gandara DR, Kelly K, Herbst RS. Phase II Randomized Study of Ramucirumab and Pembrolizumab Versus Standard of Care in Advanced Non-Small-Cell Lung Cancer Previously Treated With Immunotherapy-Lung-MAP S1800A. J Clin Oncol. 2022;40:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 139] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 22. | Kim HR, Sugawara S, Lee JS, Kang JH, Inui N, Hida T, Lee KH, Yoshida T, Tanaka H, Yang CT, Nishio M, Ohe Y, Tamura T, Yamamoto N, Yu CJ, Akamatsu H, Takahashi S, Nakagawa K. First-line nivolumab, paclitaxel, carboplatin, and bevacizumab for advanced non-squamous non-small cell lung cancer: Updated survival analysis of the ONO-4538-52/TASUKI-52 randomized controlled trial. Cancer Med. 2023;12:17061-17067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 23. | Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, García-Alfonso P, Neyns B, Luppi G, Cardin DB, Dragovich T, Shah U, Abdullaev S, Gricar J, Ledeine JM, Overman MJ, Lonardi S. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol. 2022;40:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 417] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 24. | Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1775] [Cited by in RCA: 2091] [Article Influence: 261.4] [Reference Citation Analysis (0)] |
| 25. | Lenz HJ, Parikh A, Spigel DR, Cohn AL, Yoshino T, Kochenderfer M, Elez E, Shao SH, Deming D, Holdridge R, Larson T, Chen E, Mahipal A, Ucar A, Cullen D, Baskin-Bey E, Kang T, Hammell AB, Yao J, Tabernero J. Modified FOLFOX6 plus bevacizumab with and without nivolumab for first-line treatment of metastatic colorectal cancer: phase 2 results from the CheckMate 9X8 randomized clinical trial. J Immunother Cancer. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |