Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3496
Revised: May 14, 2024
Accepted: June 11, 2024
Published online: August 15, 2024
Processing time: 140 Days and 21.7 Hours
The incidence of early-onset colorectal cancer (EO-CRC) is rising in the United States, and is often diagnosed at advanced stages. Low serum ferritin is often incidentally discovered in young adults, however, the indication for endoscopy in EO-CRC is unclear.
To compare serum ferritin between patients with EO-CRC and healthy controls (HCs), and examine the association of serum ferritin in EO-CRC with patient- and disease-specific characteristics.
A retrospective study of patients < 50 years with newly-diagnosed EO-CRC was conducted from 1/2013-12/2023. Patients were included if serum ferritin was measured within 2 years prior to 1 year following CRC histologic diagnosis. To supplement the analysis, a cohort of HCs meeting similar inclusion and exclusion criteria were identified for comparison. A sensitivity analysis including only patients with serum ferritin obtained at or before diagnosis was separately performed to minimize risk of confounding.
Among 85 patients identified with EO-CRC (48 females), the median serum ferritin level was 26 ng/mL (range < 1-2759 ng/mL). Compared to HCs (n = 80211), there were a higher proportion of individuals with EO-CRC with serum ferritin < 20 ng/mL (female 65%, male 40%) versus HCs (female 32.1%, male 7.2%) age 29-39 years (P = 0.002 and P < 0.00001, respectively). Stage IV disease was associated with significantly higher serum ferritin compared to less advanced stages (P < 0.001). Serum ferritin obtained before or at the time of diagnosis was lower than levels obtained after diagnosis. Similar findings were confirmed in the sensitivity analysis.
Severe iron deficiency may indicate an increased risk of EO-CRC, particularly at earlier stages. Further studies defining the optimal serum ferritin threshold and routine incorporation of serum ferritin in screening algorithms is essential to develop more effective screening strategies for EO-CRC.
Core Tip: This is a retrospective study that compares serum ferritin between patients with early-onset colorectal cancer (EO-CRC) and healthy controls (HCs), and examines the association of serum ferritin in EO-CRC with patient- and disease-specific characteristics. We found a higher proportion of individuals with EO-CRC with serum ferritin < 20 ng/mL compared to HCs. Lower serum ferritin in patients with EO-CRC was associated with an earlier stage of disease and a younger age among females. These findings suggest that serum ferritin may be a useful marker in patients with localized disease, emphasizing the importance of early and appropriate gastrointestinal screening in patients found to have iron deficiency.
- Citation: Urback AL, Martens K, McMurry HS, Chen EY, Citti C, Sharma A, Kardosh A, Shatzel JJ. Serum ferritin and the risk of early-onset colorectal cancer. World J Gastrointest Oncol 2024; 16(8): 3496-3506
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3496.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3496
The incidence of colorectal cancer (CRC) in the United States is increasing in younger adults, while simultaneously decreasing in older adults[1]. Early-onset CRC (EO-CRC) defined as those < 50 years of age at the time of diagnosis, tends to manifest with advanced disease, an observation that is only partly explained by inconsistent screening practices[2,3]. Furthermore, there is a higher incidence of EO-CRC in women, while being the second leading cause of death in men younger than 50 years[1,2]. Acknowledging these trends, the United States Preventative Services Task Force and the American Cancer Society have recommended lowering the age of CRC screening from 50 to 45 years[4,5]. The increased incidence of EO-CRC is most likely linked to modifiable risk factors, including obesity[6,7], diabetes[8], alcohol use[9], tobacco use[10], and Western diet[11], as well as other non-modifiable risk factors including genetics[12] and the gut microbiome[13]. Additionally, EO-CRC more commonly presents with left-sided and rectal involvement[14].
Iron deficiency is the most common extraintestinal sign of CRC[15] and is defined by the American Gastroenterological Association (AGA) as a serum ferritin level < 45 ng/mL[16]. Both iron deficiency with and without anemia have been shown to increase risk of gastrointestinal (GI) malignancy[17]. The AGA guidelines recommend bidirectional endoscopy in males and postmenopausal females with iron deficiency anemia (IDA) and issue a conditional recommendation for premenopausal females without other obvious causes, such as heavy menstrual bleeding, malabsorption syndromes or pregnancy. Available studies evaluating the association between serum ferritin and CRC have demonstrated that those at highest-risk exhibited serum ferritin levels < 100 ng/mL, with an escalated risk associated with levels < 50 ng/mL[18-20]. Interestingly, evidence of increased risk of CRC in those with serum ferritin > 300 ng/mL has also been reported[21,22], particularly in older individuals and with a higher stage at diagnosis, potentially attributable to chronic inflammation, given serum ferritin can act as an acute phase reactant[23].
There is overall a scarcity of studies investigating the association between serum ferritin and other patient- and disease-specific characteristics in EO-CRC. Limited evidence suggests that serum ferritin may be an informative measure in younger populations, where a more pronounced association between low serum ferritin and with CRC risk may exist[24]. For instance, in a prospective cohort study of United States veterans age 18-49 years, those with IDA (serum ferritin < 15 ng/mL) exhibited over a 10-fold increased risk for developing CRC compared to individuals without IDA within five years[25]. This association was more robust in those age 40-49 years compared to those under 30 years. Sensitivity analysis yielded similar findings when iron deficiency was defined as serum ferritin < 45 ng/mL. Similarly, another study of women age 35-65 years revealed significantly lower serum ferritin levels in those with CRC compared to healthy controls (HCs), with average serum ferritin 70.7 ng/mL compared to 88.4 ng/mL in controls[26].
Given the rising incidence of EO-CRC, often complicated by more advanced diagnoses, it is imperative to improve screening methods and identify a reliable marker for detecting CRC in younger populations. This study aims to compare serum ferritin levels between patients diagnosed with EO-CRC to healthy individuals, and identify patient- and disease-specific characteristics, including age, and sex, and stage that may further stratify individual risk in this population.
We performed a retrospective analysis of patients diagnosed with invasive CRC age 18-50 years at Oregon Health & Science University (OHSU) and affiliated inpatient and outpatient community sites in the Portland, Oregon metropolitan area from 1/2013-12/2023. This study was approved by the OHSU Institutional Review Board prior to initiation (STUDY00026428). Patients with an incident CRC diagnosis were identified using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes for “malignant neoplasm of the colon” (C18) and “malignant neoplasm of the rectum” (C20). Index date was defined as the date of histologic CRC diagnosis. Serum ferritin obtained at diagnosis was defined as within 2 weeks of histologic diagnosis. Patients were included if they had serum ferritin measured within 2 years prior to 1 year following CRC histologic diagnosis. Exclusion criteria included a documented medical history of inflammatory bowel disease, malignancy unrelated to the indexed diagnosis of colon cancer, any diagnosed iron overload disorder such as hemochromatosis, patients currently undergoing intravenous iron supplementation or chelation therapy (although these individuals were included if serum ferritin levels were obtained prior to treatment initiation), pregnancy at the time of serum ferritin collection or CRC diagnosis, histological diagnoses of benign or non-dysplastic conditions based on surgical pathology specimens, cancer in situ, or stage 0 disease. Charts were independently reviewed for inclusion and exclusion criteria.
To supplement the analysis, we identified a cohort of age-matched HCs meeting similar inclusion and exclusion criteria. HCs were included if they had an available serum ferritin between the same time interval (1/2013-12/2023). Patients were excluded if they had a known diagnosis of inflammatory bowel disease (to minimize confounders of bleeding and inflammation) or CRC. CRC patients were identified by the ICD-10-CM codes above and inflammatory bowel disease patients were identified by ICD 10 codes for ulcerative colitis (K50) and Crohn’s disease (K51).
Patient demographics, including age and sex, and pertinent laboratory variables including serum ferritin, hemoglobin, and mean corpuscular volume were ascertained. For the EO-CRC cohort, the closest available serum ferritin level obtained before diagnosis was selected if multiple levels were available. Furthermore, if a patient required intravenous iron, the serum ferritin value before the infusion was used. Cancer-specific variables including date of diagnosis, American Joint Committee on Cancer (AJCC) staging, and months from diagnosis to serum ferritin check were also obtained.
For the HC cohort we conducted a comprehensive search of our patient database to identify individuals with serum ferritin levels falling within four predefined ranges: ≤ 20.0 ng/mL, 20.1-45.0 ng/mL, 45.1-100.0 ng/mL, and > 100.0 ng/mL. Given the large sample size and the possibility of patients having multiple serum ferritin levels over time, it is acknowledged that some individuals may have been counted in more than one category. Our aim was to include a comprehensive HC group that represented a diverse sample of the population. Therefore, all patients meeting the inclusion criteria within the specified ranges were included in our analysis. This approach ensured the inclusivity and representativeness of our HC cohort.
Our primary outcome of interest was to compare serum ferritin between EO-CRC and HC cohorts. To conduct this, we defined four distinct serum ferritin groups: ≤ 20.0 ng/mL, 20.1-45.0 ng/mL, 45.1-100.0 ng/mL, and > 100.0 ng/mL. These groups were further stratified by age and sex to minimize bias. Fisher’s Exact Test was used in an omnibus analysis to determine the overall significance of the model by testing proportion comparisons of the aforementioned serum ferritin categories between EO-CRC and HCs. A post-hoc two-sample Z-test of proportions was then utilized to examine which of the pairwise comparisons were significant.
Non-parametric tests were used due to non-normal distribution. We reported central tendency using median and inter-quartile range (IQR). Within EO-CRC patients, Kruskal-Wallis was performed to compare serum ferritin levels based on AJCC stage. This analysis was further stratified by sex. Post-hoc Mann Whitney U-tests were executed to discern individual differences between the four AJCC stages: Stage I, II, III, and IV. Additionally, using Mann Whiteny U-tests, anemia status (defined as hemoglobin < 12.0 g/dL in females and < 13.5 g/dL in males) and biologic sex by serum ferritin levels were compared. Spearman’s Rho was used to assess the correlation between serum ferritin and the time of serum ferritin ascertainment from diagnosis. A partial correlation, using Spearman’s Rho, was performed to assess the potential association between serum ferritin and age after adjusting for AJCC stage. Findings were considered significant at P < 0.05, and outliers were systematically excluded from all analyses using a strict criterion of > quartile 3 (75th percentile) plus 5 times IQR.
In a separate sensitivity analysis, the tests described above were repeated, including only patients with serum ferritin obtained at or before diagnosis to limit potential biases, including the use systemic chemotherapy or surgical resection. Statistical analysis was performed using IBM SPSS version 29.
We identified 85 patients with EO-CRC, including 37 males and 48 females. Demographic and clinical characteristics are presented in Table 1. The mean age at diagnosis was 40.5 years ± 5.1 years, with the majority within the range of 29-49 years. There was only one patient under the age of 29 (specifically, 22 years old). Median serum ferritin level was 26 ng/mL (IQR: 85.5 ng/mL). Within the EO-CRC cohort, 39 of 85 individuals (45.9%, 24 females) had serum ferritin level < 20 ng/mL, 54 of 85 (63.5%, 35 females) had a serum ferritin level < 45 ng/mL, 65 of 85 (76.5%, 41 females) had a serum ferritin level < 100 ng/mL, and 20 of 85 (23.5%, 7 females) had a serum ferritin level > 100 ng/mL. Three outliers with serum ferritin levels exceeding 500 ng/mL were excluded from subsequent analyses.
| Females (n = 48, 56.5%) | Males (n = 37, 43.5%) | Total (n = 85) | |
| Demographic characteristics | |||
| Age (years, mean ± SD) | 39.8 ± 4.9 | 41.5 ± 5.2 | 40.5 ± 5.1 |
| Serum ferritin level | |||
| ≤ 20 ng/mL | 24 (50.0) | 15 (40.5) | 39 (45.9) |
| 21-45 ng/mL | 11 (22.9) | 4 (10.8) | 15 (17.6) |
| 46-100 ng/mL | 6 (12.5) | 5 (13.5) | 11 (12.9) |
| ≥ 101 ng/mL | 7 (14.6) | 13 (35.1) | 20 (23.5) |
| AJCC stage1 | |||
| I | 4 (8.3) | 1 (2.7) | 5 (6.0) |
| II | 3 (6.3) | 6 (16.2) | 9 (10.7) |
| III | 19 (39.6) | 9 (24.3) | 28 (33.3) |
| IV | 21 (43.8) | 21 (56.8) | 42 (50.0) |
| Characteristics at diagnosis | |||
| Anemia | 39 (81.3) | 31 (83.8) | 70 (82.4) |
| MCV < 80 fL | 20 (41.7) | 21 (56.8) | 41 (48.2) |
| MCV >100 fL | 2 (4.2) | 1 (2.7) | 3 (3.5) |
| Ferritin obtained before | 9 (18.8) | 5 (13.5) | 14 (16.5) |
| Ferritin obtained at diagnosis | 18 (37.5) | 16 (43.2) | 34 (40.0) |
| Ferritin obtained after | 21 (43.8) | 16 (43.2) | 37 (43.5) |
Of the 85 patients in the cohort, 70 were anemic (82.4%) with mean hemoglobin of 10.0 g/dL ± 2.4 g/dL (standard deviation, SD) in females and 10.4 g/dL ± 3.0 g/dL in males. AJCC staging was available for all but one patient (patient opted to not complete workup for staging). Of the 84 patients with available staging, 5 (6.0%, 4 females) were diagnosed with stage I CRC, 9 (10.7%, 3 females) with stage II CRC, 28 (33.3%, 19 females) with stage III CRC, and 42 (50.0%, 21 females) with stage IV CRC. Regarding the timing of serum ferritin measurements, 14/85 patients (16.5%, 9 females) were obtained before diagnosis, 34/85 (40%, 18 females) were obtained at diagnosis, and 37/85 (43.5%, 21 females) had serum ferritin measurements obtained after diagnosis.
In our cohort of age-matched HCs with available serum ferritin, we identified 34035 females and 14149 males within age range of 29-39 years, and 32750 females and 7534 males within range of 40-49 years.
To minimize potential bias and considering that our CRC sample included only one patient below age 29 years, we excluded this patient from the comparative analyses of HCs. Table 2 demonstrates comparisons between the EO-CRC and HC cohorts stratified by age, sex, and serum ferritin range. Notably, there were significant differences in serum ferritin range between both cohorts among females and males 29-39 years, as well as in males 40-49 years (P = 0.029, P = 0.0098, P < 0.00001, respectively). There was no significant difference in females 40-49 years (P = 0.456). Both females and males 29-39 years with CRC had a higher proportion of serum ferritin < 20 ng/mL (female 65%, male 40%) compared to HCs (female 32.1%, male 7.2%) (P = 0.002 and P < 0.00001, respectively). Males aged 40-49 years with CRC had a higher proportion of serum ferritin < 20 ng/mL than HCs (38.5% vs 6.9%; P < 0.00001). In females aged 40-49 years, although the proportion of serum ferritin < 20 ng/mL was higher in CRC vs HCs (39.3% vs 28.6%), the difference was non-significant
| EO-CRC | Healthy controls | P value | |
| Female: Age 29-39 | Fisher’s exact: P = 0.029 | ||
| Ferritin ≤ 20.0 ng/mL | 13/20 (65.0%) | 10931/34035 (32.1%) | 0.002 |
| 20.1-45.0 ng/mL | 3/20 (15.0%) | 10626/34035 (31.2%) | 0.116 |
| 45.1-100.0 ng/mL | 2/20 (10.0%) | 8039/34035 (23.6%) | 0.153 |
| ≥ 100.1 ng/mL | 2/20 (10.0%) | 4439/34035 (13.0%) | 0.689 |
| Male: Age 29-39 | Fisher’s exact: P = 0.0098 | ||
| Ferritin ≤ 20.0 ng/mL | 4/10 (40.0%) | 423/5892 (7.2%) | < 0.00001 |
| 20.1-45.0 ng/mL | 1/10 (10.0%) | 825/5892 (14.0%) | 0.719 |
| 45.1-100.0 ng/mL | 1/10 (10.0%) | 1738/5892 (29.5%) | 0.177 |
| ≥ 100.1 ng/mL | 4/10 (40.0%) | 2906/5892 (49.3%) | 0.555 |
| Female: Age 40-49 | Fisher’s exact: P = 0.456 | ||
| Ferritin ≤ 20.0 ng/mL | 11/28 (39.3%) | 9364/32750 (28.6%) | 0.211 |
| 20.1-45.0 ng/mL | 8/28 (28.6%) | 9947/32750 (30.4%) | 0.834 |
| 45.1-100.0 ng/mL | 4/28 (14.3%) | 8230/32750 (25.1%) | 0.187 |
| ≥ 100.1 ng/mL | 5/28 (17.9%) | 5209/32750 (15.9%) | 0.779 |
| Male: Age 40-49 | Fisher’s exact: P < 0.00001 | ||
| Ferritin ≤ 20.0 ng/mL | 10/26 (38.5%) | 519/7534 (6.9%) | < 0.00001 |
| 20.1-45.0 ng/mL | 3/26 (11.5%) | 920/7534 (12.2%) | 0.920 |
| 45.1-100.0 ng/mL | 4/26 (15.4%) | 2021/7534 (26.8%) | 0.187 |
| ≥ 100.1 ng/mL | 9/26 (34.6%) | 4074/7534 (54.1%) | 0.047 |
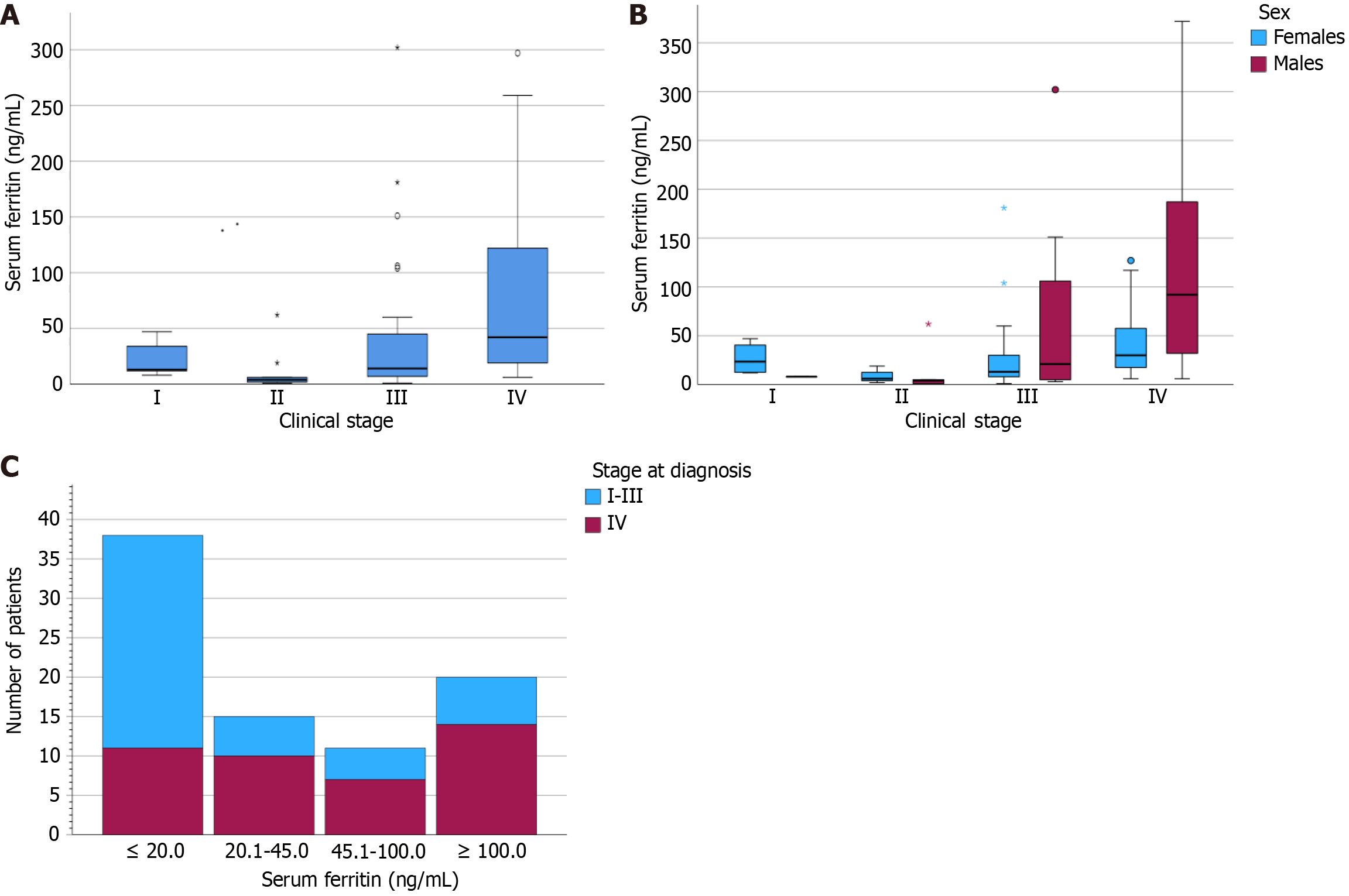
Median serum ferritin values within the EO-CRC cohort, stratified by AJCC stage, anemia status, and sex are summarized in Table 3, with corresponding statistical tests outlined in Table 4. Patients with concurrent anemia (median: 19 ng/mL, IQR: 49 ng/mL) demonstrated significantly lower serum ferritin compared to those without anemia (median: 60 ng/mL, IQR: 130 ng/mL; P = 0.018). Stage IV disease was associated with significantly higher serum ferritin compared to less advanced stages (P < 0.001) (Figure 1A). Specifically, stage II disease was associated with significantly lower serum ferritin compared to stage III (P = 0.032) and stage IV (P < 0.001), and stage III was associated with significantly lower serum ferritin than stage IV (P = 0.003). When stratified by sex, serum ferritin by stage differed significantly among males (P = 0.009) and approached significance in females (P = 0.051) (Figure 1B). When comparing patients with locally advanced stage I-III to those with advanced disease stage IV, we found a higher proportion of patients with early-stage disease with serum ferritin levels < 20 ng/mL, whereas a higher proportion of patients with stage IV disease had serum ferritin levels > 100 ng/mL (Figure 1C).
| Median (IQR), ng/mL | Females (n = 45) | Males (n = 37) | Total (n = 82) |
| AJCC stage | |||
| I | 23.5 (31.50) | 8.01 | 13 (30.5) |
| II | 6.02 | 4.0 (18.25) | 9.0 (11.0) |
| III | 13.0 (23.25) | 21.0 (124.00) | 14.0 (51.0) |
| IV | 30.0 (45.00) | 92.0 (170.00) | 42.0 (106.0) |
| Anemia status | |||
| Yes | 17.5 (25.50) | 32.0 (127.00) | 19.0 (49.00) |
| No | 34.0 (47.00) | 131.5 (97.00) | 60.0 (130.00) |
| Sex | 19.0 (31.50) | 41.0 (142.00) | - |
| Item | r | P value |
| Serum ferritin by age1 | ||
| Total | 0.281 | 0.011 |
| Females | 0.391 | 0.009 |
| Males | 0.086 | 0.618 |
| Serum ferritin by months from diagnosis | ||
| Total | 0.272 | 0.014 |
| Females | 0.052 | 0.733 |
| Males | 0.436 | 0.007 |
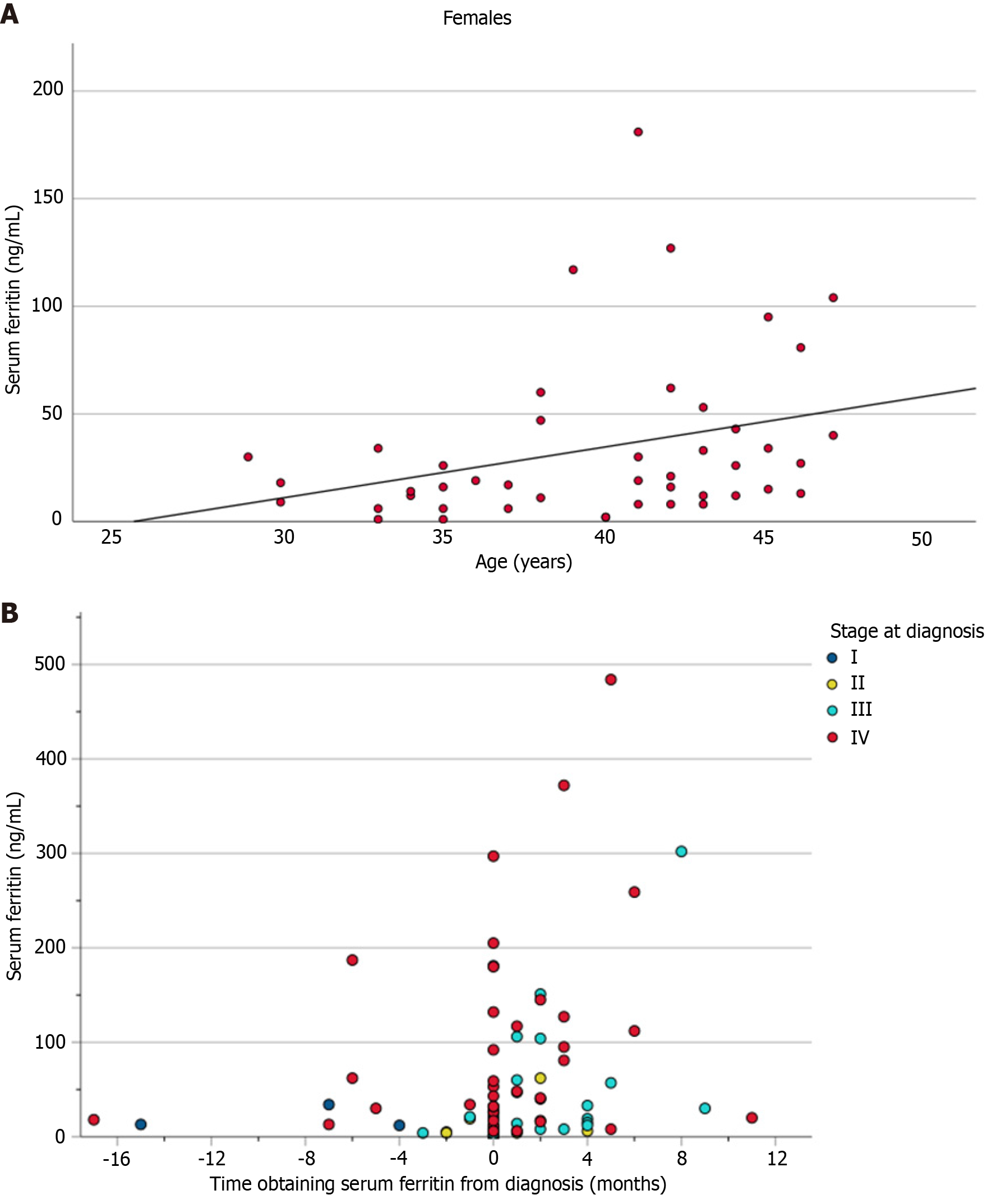
When comparing serum ferritin by sex, we found a trend towards a lower median serum ferritin for females (median: 19 ng/mL, IQR: 31 ng/mL) compared to males that did not reach statistical significance (median: 41 ng/mL, IQR: 142 ng/mL; P = 0.127). After adjusting for stage of diagnosis, we found that serum ferritin was positively correlated with age among females (r = 0.391; P = 0.009), which was not demonstrated in males (r = 0.086; P = 0.618) (Figure 2A and Table 4). In the unadjusted analysis, we detected a significant positive correlation between serum ferritin level and the time obtained in relation to diagnosis (Figure 2B), with lower serum ferritin levels obtained prior to or at diagnosis and higher levels obtained post-diagnosis.
A separate sensitivity analysis was conducted to include only patients within the EO-CRC cohort with serum ferritin obtained at or before diagnosis. A total of 46 patients were included in the sensitivity analysis within the EO-CRC cohort. Similar findings were observed between EO-CRC and HC cohorts, with a higher proportion of patients within EO-CRC cohort with serum ferritin < 20 ng/mL (Supplementary Table 1).
Median serum ferritin values, stratified by stage, anemia status, and sex, among those in the EO-CRC cohort are reported in Supplementary Table 2, while the corresponding statistical tests comparing these groups are outlined in Supplementary Table 3. Median serum ferritin was 18.5 ng/mL and IQR was 44.5 ng/mL. In this cohort, 52.1% of patients had serum ferritin levels < 20.0 ng/mL, 75.0% had serum ferritin levels < 45.0 ng/mL, 83.3% had serum ferritin levels < 100.0 ng/mL and 17.3% had serum ferritin levels > 100.0 ng/mL. Overall, most comparisons were consistent with our main analyses, with stronger effect sizes between serum ferritin based on stage, except for stage II vs III, which was not significant.
Correlation analyses are summarized in Supplementary Table 4. Similar to our primary analysis, we observed a significant positive correlation between serum ferritin and age among females that was not significant among males. In contrast, serum ferritin did not correlate by time from diagnosis when only levels obtained before/at diagnosis were included.
In this large, retrospective study of young patients diagnosed with EO-CRC, we found a higher proportion of individuals with serum ferritin < 20 ng/mL compared to a control population of healthy individuals. Furthermore, lower serum ferritin in patients with EO-CRC was associated with an earlier stage of disease, earlier time to serum ferritin measurement preceding diagnosis compared to after diagnosis, and a younger age among females. Taken together, these findings suggest that serum ferritin may be a particularly useful marker to aid in the diagnosis of EO-CRC in patients with more localized disease, emphasizing the importance of early and appropriate GI screening in patients found to have iron deficiency.
Consistent with prior analyses[2,3], our study population consisted primarily of patients with advanced stage III and IV disease in over 83% of individuals. Previous work has explored the reasoning for more advanced disease in EO-CRC, suggesting it could be related to lack of screening, failure to recognize and evaluate symptoms and/or histological differences (more mucinous and signet-ring histology with poor or no differentiation)[27,28]. There was a significant association between stage IV disease and higher serum ferritin compared to locally advanced stage I-III, possibly reflecting underlying systemic inflammation or the impact of chemotherapy. This difference was predominantly observed among males with EO-CRC, potentially due to concurrent causes of blood loss among menstruating individuals leading to lower iron stores. A previous study of older patients with CRC also found a significant association between higher serum ferritin and more advanced stage disease[21]. Furthermore, elevated serum ferritin has also been independently associated with higher mortality in patients with CRC[29]. While AGA guidelines specifically recommend GI screening for certain individuals with iron deficiency, they acknowledge the limitations of false elevations of serum ferritin as an acute phase reactant[16]. These findings highlight the diagnostic challenges of relying on serum ferritin to guide workup for CRC, particularly in patients with advanced disease, yet demonstrate potential utility in early screening to guide further workup.
In addition to the association between serum ferritin and stage of diagnosis, the timing of serum ferritin in relation to diagnosis has also yielded important findings. The results of our analysis showed a positive correlation between serum ferritin and later time to measurement that was particularly pronounced in males. When adjusted to only include patients with serum ferritin levels obtained before or at the time of their diagnosis, there was no significant correlation observed, suggesting that higher serum ferritin levels were mainly observed in those obtained after the time of diagnosis, perhaps owing to treatment-specific factors leading to systemic inflammation. This can be seen in the sensitivity analysis which demonstrated a lower median serum ferritin (18.5 ng/mL) as compared to our primary analysis (26.0 ng/mL). Future studies are needed to determine whether the routine incorporation of serum ferritin in health maintenance screening may provide a useful tool in detecting early diagnosis of CRC in young patients.
Our analysis also found a higher proportion of patients with severe iron deficiency (serum ferritin < 20 ng/mL) in patients with EO-CRC compared to HCs, particularly in males and in younger individuals. When comparing characte
While current AGA guidelines recommend bidirectional endoscopy in males and postmenopausal females with serum ferritin < 45 ng/mL[16], only a conditional recommendation exists for premenopausal females with iron deficiency. Furthermore, although the AGA defines iron deficiency as serum ferritin < 45 ng/mL, there’s no universal consensus of the diagnostic threshold. The World Health Organization defines iron deficiency as serum ferritin < 15 ng/mL[31] while other societies have defined iron deficiency as < 30 ng/mL[32,33]. In our cohort, 63.5% of patients with CRC (75.0% in the sensitivity analysis) had a serum ferritin < 45 ng/mL. Reliable data to define GI malignancy risk at different ages or anemia levels weren’t found in the literature review that informed the AGA guidelines[16]. Given the higher prevalence of iron deficiency in women, the feasibility of bidirectional endoscopy in this population is challenging. However, insights from this study and future trials may establish updated thresholds for workup in this age group (e.g., serum ferritin < 20 ng/mL). Thus, we believe our study’s results can provide initial data for future risk stratification in this population. Further studies are necessary to delineate the appropriate endoscopic workup in premenopausal women, including screening measures such as fecal immunochemical testing and/or serologic testing of malabsorptive syndromes.
While our study predominantly consisted of patients with iron deficiency anemia in 82.4% of individuals, there was a subset of patients who were iron deficient without concurrent anemia. To this point, in a study of postmenopausal women and men, iron deficiency anemia was associated with a 31-times higher risk of GI cancer compared to non-anemic, non-iron deficient individuals[17]. Additionally, iron deficiency without anemia has shown a 5-times increased risk of GI malignancy compared to non-iron deficient individuals, albeit at a lower risk compared to iron deficiency anemia[17]. It is worth acknowledging that the high proportion of anemia observed in our study might be attributed to patients who were found first to be anemic on routine labs, then found to have iron deficiency on further diagnostic workup. The importance of early detection of iron deficiency cannot be underscored, given the potential morbidity associated with under-recognition, as well as potential clinical implications such as missed diagnoses of CRC.
We acknowledge several limitations due to the inherent nature of this retrospective analysis. Although efforts were made to mitigate selection bias in the HC cohort, we were unable to fully match HCs with the EO-CRC cohort or minimize all potential confounding variables, including underlying bleeding or inflammatory disorders that may impact serum ferritin. However, our sample population of HCs did include a large sample size of over 60000 females and 13000 males to allow for a sample distribution of the general population. Additionally, our study did not include a control group comparing older individuals with CRC with younger individuals, though the primary goal of our analysis was to identify patient- and disease-related characteristics pertinent to the younger population. Among patients in the EO-CRC cohort, there were very few patients with limited stage I and II disease, and thus conclusions in this population are overall limited. We could not fully rule out other sources of blood loss in the study population, including heavy menstrual bleeding. Assessing the incidence of heavy menstrual bleeding in the cohort presents challenges due to variability in provider documentation and screening practices, and thus highlights a limitation of the retrospective nature of our analysis. While this may be a confounder to serum ferritin assessment, ensuring a representative population of premenopausal individuals was a priority in our study design. We also acknowledge that serum ferritin levels obtained after the time of diagnosis may be confounded by other factors owing to treatment, though reassuringly similar findings were observed within our sensitivity analyses. It is important to acknowledge that our decision to select the closest serum ferritin level to diagnosis, rather than the earliest available serum ferritin result, may have influenced the results of our sensitivity analysis. Given that serum ferritin is not routinely ordered as part of standard clinical assessment, conducting a retrospective study with a more stringent time interval for serum ferritin (e.g., 6 months prior, as illustrated in Figure 2B) would likely have restricted our sample size. Considering the emerging evidence of intracellular iron deposition in various tumors, it’s important to acknowledge that serum ferritin levels may not always correlate with serum iron. Future prospective studies are needed to address these limitations, further validate the findings of this study, and potentially explore alternative iron measures such as transferrin saturation or serum iron when assessing the risk of CRC.
To our knowledge, this is the first study that has evaluated the diagnostic role of serum ferritin in young patients with EO-CRC. Our results show a significantly higher proportion of patients with EO-CRC with severe iron deficiency with serum ferritin < 20 ng/mL compared to a healthy population, and demonstrated significant associations between lower serum ferritin and earlier stage and younger age in females. The study findings suggest that in EO-CRC, bleeding may occur early in the course of the disease, and that chronic inflammation and/or systemic therapy may contribute to elevated serum ferritin in more advanced disease. Ongoing research is crucial to establish the optimal serum ferritin threshold for defining iron deficiency and to determine its usefulness in identifying individuals at risk for CRC, parti
| 1. | National Cancer Institute. An interactive website for SEER cancer statistics. Surveillance Research Program. [cited 13 September 2023]. Available from: https://seer.cancer.gov/statistics-network/explorer/. |
| 2. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1513] [Reference Citation Analysis (3)] |
| 3. | Chen FW, Sundaram V, Chew TA, Ladabaum U. Advanced-Stage Colorectal Cancer in Persons Younger Than 50 Years Not Associated With Longer Duration of Symptoms or Time to Diagnosis. Clin Gastroenterol Hepatol. 2017;15:728-737.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 199] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 4. | Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC, Shih YT, Walter LC, Andrews KS, Brawley OW, Brooks D, Fedewa SA, Manassaram-Baptiste D, Siegel RL, Wender RC, Smith RA. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1303] [Article Influence: 186.1] [Reference Citation Analysis (0)] |
| 5. | Anderson JC, Samadder JN. To Screen or Not to Screen Adults 45-49 Years of Age: That is the Question. Am J Gastroenterol. 2018;113:1750-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Karahalios A, English DR, Simpson JA. Weight change and risk of colorectal cancer: a systematic review and meta-analysis. Am J Epidemiol. 2015;181:832-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Patel P, De P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15-49-year-olds in Canada, 1969-2010. Cancer Epidemiol. 2016;42:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 8. | Li Z, Chen H, Fritz CD, Zheng X, Zong X, Nickel KB, Tipping A, Nguyen LH, Chan AT, Giovannucci EL, Colditz GA, Olsen MA, Campbell PT, Davidson NO, Fields RC, Cao Y. Type 2 Diabetes and Risk of Early-Onset Colorectal Cancer. Gastro Hep Advances. 2022;1:186-193. [DOI] [Full Text] |
| 9. | McNabb S, Harrison TA, Albanes D, Berndt SI, Brenner H, Caan BJ, Campbell PT, Cao Y, Chang-Claude J, Chan A, Chen Z, English DR, Giles GG, Giovannucci EL, Goodman PJ, Hayes RB, Hoffmeister M, Jacobs EJ, Joshi AD, Larsson SC, Le Marchand L, Li L, Lin Y, Männistö S, Milne RL, Nan H, Newton CC, Ogino S, Parfrey PS, Petersen PS, Potter JD, Schoen RE, Slattery ML, Su YR, Tangen CM, Tucker TC, Weinstein SJ, White E, Wolk A, Woods MO, Phipps AI, Peters U. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int J Cancer. 2020;146:861-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 574] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 11. | O’Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13:691-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 767] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 12. | Lieu CH, Golemis EA, Serebriiskii IG, Newberg J, Hemmerich A, Connelly C, Messersmith WA, Eng C, Eckhardt SG, Frampton G, Cooke M, Meyer JE. Comprehensive Genomic Landscapes in Early and Later Onset Colorectal Cancer. Clin Cancer Res. 2019;25:5852-5858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 13. | Yang Y, Du L, Shi D, Kong C, Liu J, Liu G, Li X, Ma Y. Dysbiosis of human gut microbiome in young-onset colorectal cancer. Nat Commun. 2021;12:6757. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 14. | Glover M, Mansoor E, Panhwar M, Parasa S, Cooper GS. Epidemiology of Colorectal Cancer in Average Risk Adults 20-39 Years of Age: A Population-Based National Study. Dig Dis Sci. 2019;64:3602-3609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Chardalias L, Papaconstantinou I, Gklavas A, Politou M, Theodosopoulos T. Iron Deficiency Anemia in Colorectal Cancer Patients: Is Preoperative Intravenous Iron Infusion Indicated? A Narrative Review of the Literature. Cancer Diagn Progn. 2023;3:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 16. | Ko CW, Siddique SM, Patel A, Harris A, Sultan S, Altayar O, Falck-Ytter Y. AGA Clinical Practice Guidelines on the Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology. 2020;159:1085-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 17. | Ioannou GN, Rockey DC, Bryson CL, Weiss NS. Iron deficiency and gastrointestinal malignancy: a population-based cohort study. Am J Med. 2002;113:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Lee JG, Sahagun G, Oehlke MA, Lieberman DA. Serious gastrointestinal pathology found in patients with serum ferritin values < or = 50 ng/ml. Am J Gastroenterol. 1998;93:772-776. [PubMed] [DOI] [Full Text] |
| 19. | Wilcox CM, Alexander LN, Clark WS. Prospective evaluation of the gastrointestinal tract in patients with iron deficiency and no systemic or gastrointestinal symptoms or signs. Am J Med. 1997;103:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Sawhney MS, Lipato T, Nelson DB, Lederle FA, Rector TS, Bond JH. Should patients with anemia and low normal or normal serum ferritin undergo colonoscopy? Am J Gastroenterol. 2007;102:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Cao LL, Liu H, Yue ZH, Pei L, Wang H, Jia M. Ferritin is a potential tumor marker for colorectal cancer and modulates histone methylation in colorectal cancer cells. 2019. [cited 13 September 2023]. Available from: https://api.semanticscholar.org/CorpusID:197636931. |
| 22. | Bird CL, Witte JS, Swendseid ME, Shikany JM, Hunt IF, Frankl HD, Lee ER, Longnecker MP, Haile RW. Plasma ferritin, iron intake, and the risk of colorectal polyps. Am J Epidemiol. 1996;144:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Bermejo F, García-López S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J Gastroenterol. 2009;15:4638-4643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Kim H, Han K, Ko SH, An HJ. Association between serum ferritin levels and colorectal cancer risk in Korea. Korean J Intern Med. 2022;37:1205-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 25. | Demb J, Liu L, Murphy CC, Doubeni CA, Martínez ME, Gupta S. Young-onset colorectal cancer risk among individuals with iron-deficiency anaemia and haematochezia. Gut. 2020;70:1529-1537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Kato I, Dnistrian AM, Schwartz M, Toniolo P, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Akhmedkhanov A, Riboli E. Iron intake, body iron stores and colorectal cancer risk in women: a nested case-control study. Int J Cancer. 1999;80:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Ballester V, Rashtak S; Boardman L. Clinical and molecular features of young-onset colorectal cancer. World J Gastroenterol. 2016;22:1736-1744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 28. | Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, Guiffre S, Axilbund J, Spiegel A, You YN. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 342] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 29. | Tingting H, Di S, Xiaoping C, Xiaohong W, Dong H. High preoperative serum ferritin predicted poor prognosis in non-metastatic colorectal cancer. Saudi Med J. 2017;38:268-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Hreinsson JP, Jonasson JG, Bjornsson ES. Bleeding-related symptoms in colorectal cancer: a 4-year nationwide population-based study. Aliment Pharmacol Ther. 2014;39:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in populations: World Health Organization. 2020. [cited 13 September 2023]. Available from: https://www.who.int/publications/i/item/9789240000124. |
| 32. | Short MW, Domagalski JE. Iron deficiency anemia: evaluation and management. Am Fam Physician. 2013;87:98-104. [PubMed] |
| 33. | The Royal College of Pathologists of Australasia. The Use of Iron Studies, Ferritin and Other Tests of Iron Status 2020. [cited 18 September 2023]. Available from: https://www.rcpa.edu.au/Library/College-Policies/Position-Statements/The-Use-of-Iron-Studies,-Ferritin-and-Other-Tests.. |