Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3445
Revised: May 19, 2024
Accepted: June 18, 2024
Published online: August 15, 2024
Processing time: 120 Days and 14.4 Hours
The incidence of colorectal cancer (CRC) in China is steadily rising, with a high proportion of advanced-stage diagnoses. This highlights the significance of early detection and prevention measures to enhance survival rates. Fecal immunochemical testing (FIT) is a globally recommended CRC screening method; however, limited research has been conducted on its application in Hainan.
To assess the efficacy and adherence of FIT screening among average-risk individuals in Hainan, while also examining the risk factors associated with positive FIT results.
This population-based cross-sectional study implemented FIT screening for CRC in 2000 asymptomatic participants aged 40-75 years from five cities and 21 community health centers in Hainan Province. The study was conducted from August 2022 to April 2023, employing a stratified sampling method to select participants. Individuals with positive FIT results subsequently underwent colonoscopy. Positive predictive values for confirmed CRC and advanced adenoma were calculated, and the relationship between relevant variables and positive FIT results was analyzed using χ2 tests and multivariate logistic regression.
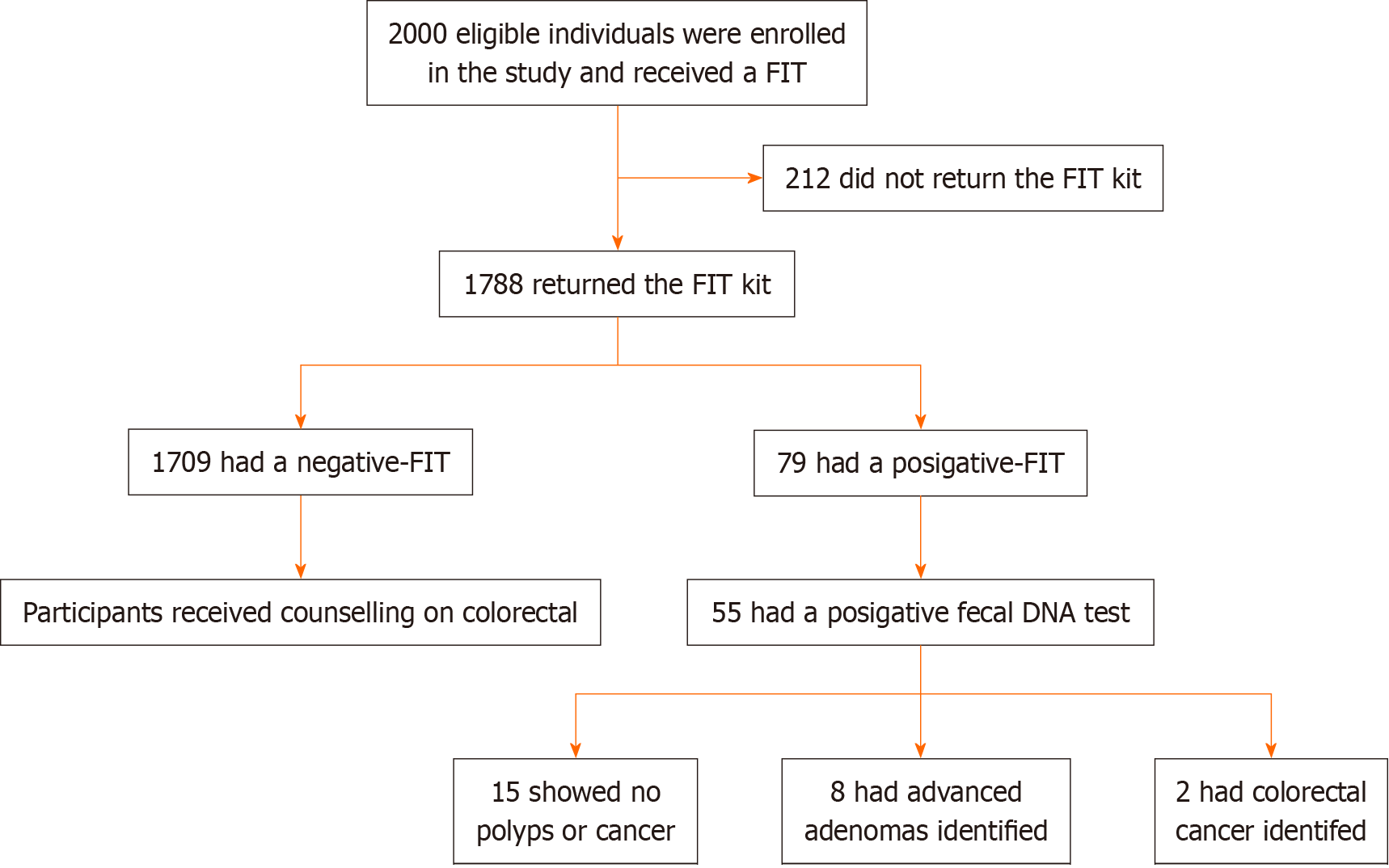
A total of 1788 participants completed the FIT screening, with a median age of 57 years (interquartile range: 40-75). Among them, 503 (28.1%) were males, and 1285 (71.9%) were females, resulting in an 89.4% compliance rate for FIT screening. The overall positivity rate of FIT was 4.4% [79 out of 1788; 95% confidence interval (CI): 3%-5%]. The specific positivity rates for Haikou, Sanya, Orient City, Qionghai City, and Wuzhishan City were 9.6% (45 of 468; 95%CI: 8%-11%), 1.3% (6 of 445; 95%CI: 0.1%-3.1%), 2.7% (8 of 293; 95%CI: 1.2%-4.3%), 3.3% (9 of 276; 95%CI: 1.0%-6.3%), and 4.2% (11 of 406; 95%CI: 1.2%-7.3%), respectively. Significant associations were found between age, dietary habits, and positive FIT results. Out of the 79 participants with positive FIT results, 55 underwent colonoscopy, demonstrating an 82.2% compliance rate. Among them, 10 had a clean gastrointestinal tract, 43 had polyps or adenomas, and 2 were confirmed to have CRC, yielding a positive predictive value of 3.6% (95%CI: 0.9%-4.2%). Among the 43 participants with polyps or adenomas, 8 were diagnosed with advanced adenomas, resulting in an advanced adenoma rate of 14.5% (95%CI: 10.1%-17.7%).
In the Hainan region, FIT screening for CRC among asymptomatic individuals at average risk is feasible and well-received.
Core Tip: The incidence of colorectal cancer (CRC) in China is steadily rising, fecal immunochemical testing (FIT) is a globally recommended CRC screening method. To assess the efficacy and adherence of FIT screening among average-risk individuals in Hainan, while also examining the risk factors associated with positive FIT results. This population-based cross-sectional study implemented FIT screening for CRC in 2000 asymptomatic participants aged 40-75 years from five cities and 21 community health centers in Hainan Province. This study found that in the Hainan region, FIT screening for CRC among asymptomatic individuals at average risk is feasible and well-received.
- Citation: Zeng F, Zhang DY, Chen SJ, Chen RX, Chen C, Huang SM, Li D, Zhang XD, Chen JJ, Mo CY, Gao L, Zeng JT, Xiong JX, Chen Z, Bai FH. Application of fecal immunochemical test in colorectal cancer screening: A community-based, cross-sectional study in average-risk individuals in Hainan. World J Gastrointest Oncol 2024; 16(8): 3445-3456
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3445.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3445
Colorectal cancer (CRC) is a significant threat to the lives and well-being of the Chinese population, resulting in a substantial societal burden. According to the most recent data from 2020[1], there were 387600 new cases of CRC in China in 2015, accounting for 9.87% of all malignant tumors[2]. Additionally, in 2016, rectal cancer ranked third among all malignant tumor incidences and fourth in terms of mortality in Hainan Province[2]. The development of CRC typically follows the “adenoma-carcinoma” sequence, with a progression period of 5-10 years from precancerous lesions to cancer. This extended timeframe offers a crucial window for early diagnosis and clinical intervention[2]. Numerous clinical studies have demonstrated that early CRC screening effectively reduces the occurrence and mortality rates of the disease. Although CRC screening commenced in China during the 1970s in regions with high CRC incidence (such as Jiashan County and Haining City in Zhejiang Province), there has been limited focus on CRC screening, related awareness campaigns, and reports in Hainan Province.
The fecal occult blood test serves as a simple and convenient initial screening method for CRC. The conventional guaiac-based stool occult blood test is known to be influenced by dietary factors or medications and exhibits low sensitivity. In contrast, the fecal immunochemical testing (FIT) utilizes an immunoassay technique to measure fecal hemoglobin levels in the body. FIT offers advantages such as ease of use, increased uptake, quantitative analysis, and enhanced sensitivity for advanced colorectal tumors. Consequently, it has gradually replaced the guaiac-based fecal occult blood test in CRC screening programs[3,4]. Notably, the United Kingdom Bowel Cancer Screening Programme implemented FIT in 2018 and it is now widely employed for CRC screening[5]. Its single low threshold sensitivity reaches 90%[5]. In 2020, the National Cancer Center’s China Guidelines for Colorectal Cancer Screening, Early Diagnosis, and Early Treatment[6] endorsed the accuracy, efficacy, and safety of FIT for CRC screening. The guidelines emphasized FIT’s suitability for CRC screening due to its high sensitivity for CRC diagnosis, albeit with limited sensitivity for precancerous lesions (strong recommendation, quality of evidence: Moderate)[6]. Currently, there is a scarcity of research on CRC screening utilizing FIT in Hainan Province. This study aimed to assess the impact and efficacy of FIT screening in asymptomatic individuals at average risk of CRC across five regions in Hainan.
The study was conducted in the Hainan region of China, encompassing five cities (Haikou, Sanya, Qionghai, Dongfang, and Wuzhishan) and 21 health service stations from August 2022 to April 2023. Asymptomatic adults between the ages of 40 and 75 were recruited for the screening. This age range aligns with the 2018 screening recommendations provided by the American Society for Gastrointestinal Endoscopy and corresponds to the age range of early-onset CRC[7-9]. The researchers distributed stool collection tubes to participants, accompanying them with written and verbal instructions on providing an adequate stool specimen. Participants were requested to return the specimen within 48 hours of defecation for subsequent processing by the study team. Participants with positive FIT results will undergo free colonoscopy examination with general anesthesia unless anesthesia contraindications by trained endoscopists skilled with an acceptable completion and adenoma detection rate at the gastroscopy center in the urban area within 8 weeks. Cecal intubation and quality of bowel preparation (poor, fair, good, or excellent) were recorded as per American Society for Gastrointestinal Endoscopy and American College of Gastroenterology Taskforce on Quality in Colonoscopy recommendations[10]. Each participant’s colon needed to be adequately cleaned and meet the endoscopic cleanliness score before the next endoscopic procedure could be performed. Structural lesions were biopsied, removed, or tattooed for later identification as per surgeon or institutional preference and guidelines. Any pathology identified was documented on a colonoscopy study proforma. Participants undergoing colonoscopy completed a post procedure questionnaire to ascertain the acceptability of the procedure in the context of CRC screening follow-up.
The primary study indicators included the positive rate of FIT screening results, the positive predictive value of FIT for CRC, and the positive predictive value of FIT for advanced adenoma[11], whereby advanced adenoma was defined as adenoma measuring ≥ 10 mm or exhibiting high-grade dysplasia or chorionic histological features ≥ 25% (Figure 1). Ethical approval for this study was obtained from the Institutional Research Committee of the Second Affiliated Hospital of Hainan Medical College (approval number: LW20222142), and all participants provided signed consent forms approved by the Institutional Research Committee.
Inclusion criteria: (1) Individuals aged 40-75 years; (2) Local residents of Hainan Province; (3) Willing participants who have voluntarily consented to the study by signing the informed consent form; (4) No previous history of CRC or hemorrhoids; and (5) Women who are three days post-menstruation.
Exclusion criteria: (1) Individuals with a history of CRC or rectal bleeding within the past six months; (2) Individuals with a first-degree relative diagnosed with CRC; and (3) Individuals with comorbidities that would prevent sedation or general anesthesia in a conscious state.
In this study, we made improvements to the questionnaires used in previous studies in China[12-14]. Trained interviewers administered the questionnaires to each participant, asking a series of questions. The questionnaire covered various domains, including demographic characteristics (such as age, gender, ethnicity, occupation, marital status, and body mass index), socioeconomic status (including education level and personal annual income), hygiene habits (such as utensil sharing and hand hygiene before and after meals, tooth brushing), lifestyle factors (such as smoking, frequency of weekly exercise, work pressure, and sleep quality), dietary habits (including eating out, water consumption, regular meals, tea drinking, and fruit and vegetable consumption), and a history of gastrointestinal disorders (such as CRC, intestinal polyps, chronic diarrhea, chronic appendicitis, or appendectomy, chronic cholecystitis, or gallbladder surgery). The data was entered by two individuals and double-checked to reduce errors. Smoking was defined as the act of smoking or having smoked a minimum of one cigarette per day within the past year. Alcohol consumption was defined as consuming at least 100 g of alcohol per week in the past year. “Often” refers to at least once per day, while “occasionally” is defined as once every 2-3 days or more.
The FIT kit utilized in this study was established by the manufacturer (Anhui Tongkang Medical Technology Co., Ltd., China) with a positive hemoglobin detection threshold of 100 ng/mL.
According to published literature, the average FIT positivity rate in China is approximately 17.6%[1]. To determine the sample size for the study, a two-sided test with an allowable error α of 0.05 was selected, as well as an allowable error δ of ± 1.7%. With Z(1-α/2) = 1.96, P = 5%, the calculation indicated that 1985 cases needed to be included. Accounting for a potential dropout rate of 10%-20%, 2000 cases were included in the study finally, ensuring the accuracy and scientific rigor of the research findings.
The data analysis was conducted utilizing SPSS 25.0 software. Continuous variables were presented as mean ± SD, and a comparison between groups was performed using an independent-sample t-test. Categorical variables were expressed as frequency and percentage [n (%)], and comparisons between groups were assessed using a χ2 test or a trend χ2 test. The univariate analysis included all variables with a P value < 0.10, which were then examined further using multivariate logistic regression analysis (stepwise regression analysis, SLS = 0.10, SLE = 0.05) to identify the risk factors associated with FIT positivity. Results were reported as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was considered present when the P value was less than 0.05.
A total of 1788 individuals successfully underwent FIT screening. The median age of the participants was 57 years (interquartile range: 40-75), comprising 503 males (28.1%) and 1285 females (71.9%). The compliance rate for FIT screening was 89.4%. Among the participants, 1% (n = 18) reported having previously been screened for other types of cancer, with cervical cancer being the most commonly mentioned. Moreover, 20 participants (1%) had prior experience with CRC screening, while 15 participants (0.8%) were advised to undergo further colonoscopy. Interestingly, the participants in this study displayed limited awareness regarding the main symptoms associated with CRC.
The overall positivity rate for FIT was 4.4% (79 out of 1788), with varying rates observed across different cities: 11% in Haikou (45 out of 468), 1.3% in Sanya (6 out of 445), 2.7% in Orient City (8 out of 293), 3.3% in Qionghai City (9 out of 276), and 4.2% in Wuzhishan City (11 out of 406) (P < 0.05). When considering age groups, the ≥ 71 group exhibited a significantly higher FIT positivity rate than the other age groups (P < 0.05). In terms of gender, the positivity rate for males (43.4%) was slightly higher than that for females (41.9%), although the difference was not statistically significant (P > 0.05) (Table 1).
| Number of examples (n = 1788) | FIT-positive (n = 79) | χ2 | P value | |
| Sex | 121.386 | 0.000 | ||
| Male | 603 | 37 | ||
| Female | 1285 | 42 | ||
| Age | 6.966 | 0.008 | ||
| 41-50 | 667 | 19 | ||
| 51-60 | 504 | 25 | ||
| 61-70 | 397 | 17 | ||
| ≥ 71 | 225 | 19 | ||
| Region | 6.372 | 0.027 | ||
| Haikou | 443 | 45 | ||
| Sanya | 425 | 6 | ||
| Dongfan | 268 | 8 | ||
| Qionghai | 245 | 9 | ||
| Wuzhishan | 381 | 11 | ||
| Total | 1788 | 79 |
The univariate analysis revealed significant correlations between the positive rate of FIT and age, frequency of consuming pickled vegetables, and frequency of consuming betel nuts. Specifically, the positive rate of FIT was found to be positively associated with the elderly (≥ 71 years) (OR = 2.160, 95%CI: 1.086-4.298) and regular consumption of betel nut (OR = 1.310, 95%CI: 1.007-1.703). Conversely, weekly exercise (OR = 0.572, 95%CI: 0.484-0.676) and regular consumption of fruits and vegetables (OR = 0.630, 95%CI: 0.528-0.752) showed negative associations with the FIT positivity rate (Tables 2 and 3).
| Factor | Number of examples (n = 1788) | FIT-positive (n = 79) | χ2 | P value |
| Sleep quality | 5.746 | 0.057 | ||
| Better | 543 | 17 | ||
| So so | 681 | 53 | ||
| Difference | 565 | 19 | ||
| Anxiety/stress | 4.833 | 0.089 | ||
| Seldom | 706 | 33 | ||
| Occasionally | 685 | 36 | ||
| Often | 399 | 20 | ||
| Mental illness | 20.021 | 0.000 | ||
| No | 1428 | 50 | ||
| Yes | 360 | 39 | ||
| Level of education | 1.712 | 0.425 | ||
| Elementary school | 292 | 15 | ||
| Junior high school | 825 | 39 | ||
| College degree or above | 168 | 5 | ||
| Exercise time per week | 4.054 | 0.032 | ||
| < 1 hour | 489 | 19 | ||
| 1-4 hours | 513 | 30 | ||
| > 4 hours | 308 | 10 | ||
| Eating regularly or not | 2.655 | 0.103 | ||
| Yes | 1500 | 42 | ||
| No | 238 | 17 | ||
| Smoking | 20.789 | 0.000 | ||
| No | 1102 | 35 | ||
| Yes | 686 | 24 | ||
| Drinking | 17.869 | 0.000 | ||
| No | 922 | 26 | ||
| Yes | 387 | 33 | ||
| Pickled foods | 6.287 | 0.012 | ||
| No | 1434 | 36 | ||
| Yes | 354 | 23 | ||
| Areca catechu | 4.748 | 0.029 | ||
| No | 1571 | 44 | ||
| Yes | 217 | 15 |
| Factor | β | SEB | Wald χ2 | P value | OR (95%CI) |
| Age | 0.237 | 0.108 | 4.152 | 0.008 | 1.121 (0.097-1.453) |
| Pickled foods | 0.583 | 0.251 | 4.382 | 0.012 | 1.421 (1.212-1.671) |
Out of the initial 79 patients who tested positive in the screening, a total of 55 patients underwent colonoscopy, resulting in an overall adherence rate of 69.6%. Analysis revealed a statistically significant difference in colonoscopy compliance rates among different age groups (P < 0.001). However, there was no significant difference in colonoscopy compliance rates between different genders (P > 0.05). Notably, there was a significant difference in colonoscopy compliance rates between different regions, specifically Haikou City and Dongfang City (P < 0.05) (Table 4). FIT screening demonstrated a positive predictive value of 3.6% for CRC and 12.3% for adenomas. The screening also identified no abnormal detection rate concerning sessile serrated polyps, inflammatory polyps, polyps (no pathology), stromal tumors, ulcers, inflammation, and other lesions (Table 5).
| Factor | Total | FIT-positive | Colonoscopy cases | χ2 | P value |
| Sex | |||||
| Male | 603 | 37 | 20 | 1.516 | 0.509 |
| Female | 1285 | 42 | 35 | 0.652 | 0.823 |
| Age | |||||
| 41-50 | 667 | 19 | 13 | 1.907 | 0.167 |
| 51-60 | 504 | 25 | 20 | 4.523 | 0.033 |
| 61-70 | 397 | 17 | 10 | 0.719 | 0.396 |
| ≥ 71 | 225 | 19 | 12 | 9.817 | 0.000 |
| Region | |||||
| Haikou | 443 | 45 | 20 | 5.715 | 0.017 |
| Sanya | 425 | 6 | 6 | 0.556 | 0.782 |
| Dongfan | 268 | 8 | 15 | 0.449 | 0.048 |
| Qionghai | 245 | 9 | 9 | 0.504 | 0.618 |
| Wuzhishan | 381 | 11 | 5 | 11.021 | 0.000 |
| Total | 1788 | 79 | 55 |
| Colonoscopy results | Number of examples |
| Benign results | |
| Hemorrhoids | 6 |
| Enteritis | 4 |
| Normal | 5 |
| Polyps or adenomas | |
| Descending colonic tubular adenoma | 6 |
| Tubular adenoma of the transverse colon | 7 |
| Tubular adenoma of the sigmoid colon | 7 |
| Tubular adenoma of the ascending colon | 6 |
| Sigmoid inflammatory polyps | 4 |
| Sigmoid proliferative polyps | 5 |
| Advanced adenoma | |
| Tubular adenoma of the sigmoid colon | 3 |
| Descending colonic tubular adenoma | 1 |
| Sigmoid villous adenoma | 2 |
| Sigmoid colon villous-tubular adenoma | 1 |
| Tubular adenoma of the transverse colon | 1 |
| CRC | 2 |
| Total | 55 |
The majority of participants in the study expressed positive views about FIT screening. Specifically, 90% of participants (n = 1620) rated their overall experience as favorable (good or very good). Additionally, 89% (n = 1600) found the test kit to be easy to use and 90% (n = 1615) indicated they would recommend the screening test to their family and friends. Moreover, a significant proportion of participants were willing to pay for FIT screening for CRC (75%, n = 1341) and would have participated in the screening if it were available free of charge (90%, n = 1609).
There is growing interest among scholars in implementing organized regional large-scale CRC screening programs. Multiple studies have demonstrated an increase in the incidence and mortality rates of CRC. According to data from GLOBOCAN 2020, there were approximately 1.932 million CRC cases and 935000 deaths globally. More specifically, in China, the number of cases and deaths were 555000 and 286000, respectively, representing 28.73% and 30.59% of the global figures[12]. While many provinces and cities in China have initiated FIT-based CRC screening for individuals at average risk, Hainan Province has not yet implemented a similar program.
Studies have revealed that China experiences over 370000 new cases of CRC and more than 180000 deaths annually[13]. Consequently, reducing the morbidity and mortality rates associated with CRC has become a significant clinical and scientific concern[14]. The malignant transformation of colorectal adenomas accounts for more than 80% of CRC cases, and the removal of these adenomas through colonoscopy can reduce the incidence of CRC by 75%[15]. Effective intervention at this particular stage has the potential to significantly reduce the occurrence of CRC. In 2020, the Expert Group for the National Cancer Center Guidelines for CRC Screening, Early Diagnosis, and Early Treatment in China[6] formulated comprehensive guidelines that outline the selection and evaluation of five screening and early diagnosis tools for CRC. The group concludes that FIT is suitable for CRC screening due to its high sensitivity in diagnosing CRC, despite its limited sensitivity for detecting precancerous lesions (strong recommendation, moderate grade of evidence)[6]. Given China’s large population and limited medical resources, the high cost of colonoscopy for bowel cancer screening is a significant concern. Thus, it is particularly crucial to identify a safe and cost-effective CRC screening strategy with high diagnostic efficiency. By combining the questionnaire on high-risk factors for bowel cancer and using FIT as the primary screening method, followed by subsequent screening of the high-risk group through gold-standard electronic colonoscopy, a screening approach with superior diagnostic performance and low cost can be achieved, with the questionnaire survey serving as a unique screening method in China[6,16].
This study represents the first evaluation of compliance with the FIT test for CRC screening in individuals with an average risk of CRC. It examines demographic factors that contribute to the risk of CRC in this population and explores the effectiveness of FIT screening for CRC in Hainan, China. The questionnaire utilized in this study includes six high-risk factors: Age, gender, smoking history, family history of CRC, body mass index, and self-reported presence or absence of diabetes. These factors can be applied to CRC screening in the Asia-Pacific region[17,18].
In this study, 55 out of 1788 participants tested positive for FIT and subsequently completed colonoscopy, resulting in an FIT screening positive rate of 4.4% for CRC. Age stratification yielded four groups, among which the ≥ 71 age group exhibited a significantly higher FIT-positive rate compared to the other age groups. This finding aligns with a previously conducted study involving 3558 patients aged 20 to 89 years, where the prevalence of adenoma was 1.7% in the 30-39 age group, rising to 3.59% in the 40-49 age group. Notably, the prevalence of adenoma increases in patients over 50 years of age, consistent with conventional assumptions, as the occurrence of adenoma and adenocarcinoma significantly rises after age 50. These results further corroborate the research report by Zhang et al[19], suggesting a relatively high proportion of CRC cases in older age groups. However, it’s important to note that a considerable proportion of CRC cases also exist among younger individuals. Hence, actively carrying out public education campaigns to minimize exposure to CRC high-risk factors and providing tailored screening guidance to high-risk groups is essential in reducing late-stage CRC diagnosis occurrences[20].
In this study, the male-to-female ratio of CRC patients was approximately 1.21:1, indicating a higher incidence in males compared to females. However, no significant difference was observed between the two groups, contrasting with the findings Koo and Leong[21] reported. Such disparities may arise due to varying risk factors for bowel cancer across different regions. Notably, this study identified the consumption of pickled foods and betel nut chewing as high-risk factors associated with positive results in the FIT screening, both of which are also linked to an increased risk of CRC development. Pickled vegetables, bacon, and other pickled foods contain significant amounts of nitrite and secondary amines, which, under suitable acidity or bacteria in the stomach, can give rise to the synthesis of nitrosamine compounds known for their potent carcinogenic effects. The excessive consumption of pickled foods can contribute to the occurrence of CRC. Furthermore, the World Health Organization has classified betel nut as a Class I carcinogen since 2003 due to the formation of nitroso compounds, powerful carcinogens, upon chewing.
Our study determined that engaging in moderate weekly exercise acts as a protective factor against a positive FIT result, aligning with previous findings that the relationship between exercise and lower gastrointestinal disorders is multifaceted[21]. Additionally, the consumption of fruits and vegetables also demonstrates a protective effect against a positive FIT result, which is consistent with expert consensus[6]. Consequently, the identification of high-risk factors for CRC-positive screening in Hainan can be enhanced through questionnaires targeting these factors. Moreover, by tailoring the dissemination of bowel cancer prevention knowledge to the population of Hainan based on local conditions, it is possible to reduce the incidence of bowel cancer to a certain extent.
Our study found that psychiatric factors were risk factors for a positive FIT outcome, which is consistent with previous findings[10,22-25]. In one review, aggressive-hostile personality, negative emotions, high work stress in women, high anxiety levels and depressive symptoms were associated with an increased risk of CRC[21], with the specific mechanism being that depressed patients are chronically exposed to immune activation and inflammation, which can promote tumour growth[10]. In addition, other studies have found increased CRC mortality in patients with psychiatric disorders[23,24], and the greater the degree of psychiatric illness, the greater the risk of dying from CRC[25]. Another study showed a dose-response association between psychotropic medications and participation in CRC screening, with patients being less likely to participate in CRC screening as the class of psychotropic medication used increased[26]. Thus, early CRC screening has the potential to mitigate increased cancer mortality due to psychiatric disorders.
Our study found that smoking was a risk factor for a positive FIT result, which is consistent with epidemiological findings. Cigarette smoking has been shown to increase the risk of colorectal adenomatous polyps, which are considered a precursor to CRC[27]. A large case-control study showed that smoking was strongly associated with microsatellite instability-high and KRAS-WT pathway-associated CRC[28]. In addition, some studies have demonstrated a positive correlation between alcohol consumption and CRC risk, but the exact mechanism is unclear[29,30].
Multi-element logistic regression analysis in this study identified age as a risk factor for positive FIT results, which is consistent with previous studies reported by[31,32], suggesting that people in the higher age groups have a relatively higher risk of developing CRC and that early screening for CRC is extremely important. Similarly, pickled foods have also been suggested to be a risk factor for positive FIT results, probably because the combination of secondary amines produced during the pickling and drying process of pickled foods with nitrites in gastric juices can generate N-nitroso compounds with carcinogenic and mutagenic properties[33-35]. Therefore, consuming more fresh vegetables and fruits instead of preserved foods and adopting healthy eating habits can reduce the risk of CRC.
Compared to similar studies conducted in other regions of China[36,37], the compliance with FIT testing in this study was remarkably high. Several reasons can account for this favorable compliance rate. Firstly, the FIT kits used in this study were provided free of charge. Secondly, comprehensive publicity efforts, such as posters and broadcasts, were undertaken prior to the study, ensuring that the recruited participants had a clear understanding of the study’s objectives and willingly volunteered to take part. Additionally, after the researchers distributed the FIT kits, a significant number of participants promptly returned their samples, indicating a high level of motivation. Combining these factors with the results of the questionnaire survey, the primary screening positive rate in Hainan was found to be 4.4%, lower than that reported in Tianjin[38] and Xinjiang Uygur Autonomous Region[39], but slightly higher than the results of a randomized controlled study on CRC screening conducted in the Netherlands by Grobbee et al[40] (2.0%) and by Pan et al[41] (4.11%). It is important to note that not all individuals with CRC or precancerous lesions exhibit symptoms of blood in the stool. Furthermore, the simple fecal occult blood test has a certain diagnostic miss rate for CRC screening, as blood in the stool can also arise from factors such as inappropriate stool collection methods, menstrual blood in females, hemorrhoidal bleeding, and contamination from hematuria. Consequently, a certain level of false positives is also present. To overcome these limitations, colonoscopy serves as the preferred method for directly visualizing colorectal lesions, exhibiting a sensitivity and specificity exceeding 95%[42]. Internationally recognized CRC screening guidelines establish colonoscopic biopsy as the “gold standard” for CRC diagnosis and screening[43].
In our study, the positive predictive value of FIT screening for CRC was determined to be 3.6%, while the positive predictive value (PPV) for advanced adenoma was 14.5%. Comparatively, the PPV of FIT screening for CRC was slightly higher than that reported in large-scale population screening studies in Guangzhou (2.88% and 14.98%)[44] and Chongqing (0.16% and 15.99%)[43]. Overall, the compliance rate for colonoscopy in our study was slightly higher than the national average of 69.6% for CRC screening[45]. Several factors likely contributed to these results. Firstly, the majority of the screening areas were urban, allowing for effective cancer-related publicity campaigns and ensuring sufficient medical resources and convenient transportation. However, in areas such as Qionghai and Wuzhishan, the compliance rate for colonoscopy was significantly lower. The overall medical informatisation level of Qionghai City is not high, which will result in inefficient use of medical service resources, and it will be more difficult for people to see a doctor[46,47]. Wuzhishan City is quite inadequate in the training and reserve of medical personnel due to the limitations of geography, transport, and financial conditions, and the level of medical care is relatively lagging behind[46,47]. The specific reasons are as follows: (1) These two cities have a sizable rural population with limited knowledge about cancer and screening; (2) The medical resources in these areas are inadequate, and transportation options are not readily accessible; and (3) Elderly individuals, who may possess low awareness of CRC prevention and treatment and have limited education, face substantial challenges in undergoing colonoscopy screening. Moreover, the screening population in these areas may perceive the screening process as overly complicated, experiencing embarrassment and fear of complications[36,48]. During our survey, we encountered individuals who were illiterate and unable to understand the official language of China, which made it challenging to collect their responses to the questionnaire. Additionally, some participants only completed personal information and were unwilling to cooperate with the survey’s content. As a result, a small number of participants had incomplete questionnaire information, limiting the completion of data statistics and rendering their responses merely informative rather than statistically significant. Consequently, our study suggests that a combination of questionnaire surveys, FIT, and colonoscopy can be employed as methods for CRC screening in Hainan Province. Currently, there is limited demographic evidence available to determine the most cost-effective and suitable technical scheme for CRC screening promotion in Hainan Province[49]. Furthermore, the effectiveness of screening is influenced not only by technical accuracy and the age characteristics of the target population but also by factors such as population behavior, economic costs, and habits[40].
The choice of threshold for hemoglobin detection in FIT directly impacts the positive predictive value of the test. However, there is currently no universally accepted optimal threshold, and the selection of thresholds varies across different screening programs[50-52]. The threshold chosen significantly affects the detection rate of advanced adenoma, the likelihood of missed diagnosis of invasive cancer, and the proportion of positive and false-positive screening tests[20,52]. In our study, we opted for the highest existing threshold (100 ng/mL) for commercially available qualitative FIT. Nevertheless, given the substantial number of false-positive results observed in our study, it may be necessary to develop and evaluate a more appropriate threshold for hemoglobin detection in qualitative testing specific to the Hainan region. For instance, Thailand has implemented a national CRC screening program that employs quantitative FIT with a threshold of 150 ng/mL[53]. This threshold yields a higher positive predictive value for advanced tumor testing in the Thai population without overwhelming their limited endoscopy resources.
This study boasts several notable strengths. Firstly, it represents the first FIT analysis conducted to screen CRC in Hainan, China. However, it is important to acknowledge the limitations of this study. One such limitation is the restricted scope of the screening, which encompassed only select areas within Hainan. Furthermore, due to the limited number of samples and sampling areas, large-scale CRC screening in Hainan could not be undertaken.
This study offers valuable insights into the efficacy and acceptability of FIT for CRC screening among individuals at general risk in Hainan. Utilizing a hemoglobin concentration threshold of 100 ng/mL, our findings reveal that 4.4% of the participants obtained a positive result in the FIT test. Notably, we observed high compliance with FIT screening, whereas compliance with a positive result for colonoscopy was relatively low. Thus, there is a need to emphasize the importance of colonoscopy among the general risk population. Additionally, our results indicate a correlation between a positive FIT result, dietary patterns, and lifestyle habits. Consequently, implementing lifestyle modifications, including dietary changes, has the potential to reduce the incidence of CRC.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64636] [Article Influence: 16159.0] [Reference Citation Analysis (176)] |
| 2. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3024] [Article Influence: 504.0] [Reference Citation Analysis (3)] |
| 3. | Allison JE, Fraser CG, Halloran SP, Young GP. Population screening for colorectal cancer means getting FIT: the past, present, and future of colorectal cancer screening using the fecal immunochemical test for hemoglobin (FIT). Gut Liver. 2014;8:117-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Moss S, Mathews C, Day TJ, Smith S, Seaman HE, Snowball J, Halloran SP. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England. Gut. 2017;66:1631-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 5. | National Health Service England, Public Health Commissioning Central Team. Public Health Section 7A. [cited 15 December 2023]. Available from: https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2016/09/public-hlth-comms-intent-2017-18.pdf. |
| 6. | Su Y, Sun X, Zhan HR, Chen R, Sun L, Hao JJ, Tian Y, Chen R. [Global guidelines of colorectal cancer screening for people at average risk:a systematic review]. Zhonghua Zhongliu Fangzhi Zazhi. 2022;29:1236-1242. [DOI] [Full Text] |
| 7. | Gullickson C, Goodman M, Joko-Fru YW, Gnangnon FHR, N'Da G, Woldegeorgis MA, Buziba NG, Karugu C, Manraj SS, Lorenzoni CF, Hansen R, Finesse A, Somdyala NIM, Bukirwa P, Chingonzoh T, Chokunonga E, Liu B, Kantelhardt E, Parkin DM, Jemal A. Colorectal cancer survival in sub-Saharan Africa by age, stage at diagnosis and Human Development Index: A population-based registry study. Int J Cancer. 2021;149:1553-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Paquette IM, Ying J, Shah SA, Abbott DE, Ho SM. African Americans should be screened at an earlier age for colorectal cancer. Gastrointest Endosc. 2015;82:878-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Boer R, Wilschut J, Winawer SJ, Habbema JD. Individualizing colonoscopy screening by sex and race. Gastrointest Endosc. 2009;70:96-108, 108.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Currier MB, Nemeroff CB. Depression as a risk factor for cancer: from pathophysiological advances to treatment implications. Annu Rev Med. 2014;65:203-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Strum WB. Colorectal Adenomas. N Engl J Med. 2016;374:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 12. | National Clinical Research Center for Digestive Diseases (Shanghai); National Early Gastrointestinal-Cancer Prevention & Treatment Center Alliance (GECA); Chinese Society of Digestive Endoscopy; Chinese Society of Health Management; Digestive Endoscopy Professional Committee of Chinese Endoscopist Association; Endoscopic Health Management and Medical Examination Professional Committee of Chinese Endoscopist Association; Endoscopic Diagnosis and Treatment Quality Management and Control Professional Committee of Chinese Endoscopist Association; China Health Promotion Foundation; National Quality Control Center of Digestive Endoscopy; Cancer Endoscopy Professional Committee of China Anti-Cancer Association. [Chinese consensus of early colorectal cancer screening (2019, Shanghai)]. Zhonghua Nei Ke Za Zhi. 2019;58:736-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13213] [Article Influence: 1468.1] [Reference Citation Analysis (3)] |
| 14. | Zheng RS, Zhang SW, Sun KX, Chen R, Wang SM, Li L, Zeng HM, Wei WW, He J. [Cancer statistics in China, 2016]. Zhonghua Zhong Liu Za Zhi. 2023;45:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 15. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3127] [Article Influence: 97.7] [Reference Citation Analysis (1)] |
| 16. | Fang JY, Zheng S, Jiang B, Lai MD, Fang DC, Han Y, Sheng QJ, Li JN, Chen YX, Gao QY. Consensus on the Prevention, Screening, Early Diagnosis and Treatment of Colorectal Tumors in China: Chinese Society of Gastroenterology, October 14-15, 2011, Shanghai, China. Gastrointest Tumors. 2014;1:53-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 17. | Yeoh KG, Ho KY, Chiu HM, Zhu F, Ching JY, Wu DC, Matsuda T, Byeon JS, Lee SK, Goh KL, Sollano J, Rerknimitr R, Leong R, Tsoi K, Lin JT, Sung JJ; Asia-Pacific Working Group on Colorectal Cancer. The Asia-Pacific Colorectal Screening score: a validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut. 2011;60:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (1)] |
| 18. | Wong MC, Lam TY, Tsoi KK, Hirai HW, Chan VC, Ching JY, Chan FK, Sung JJ. A validated tool to predict colorectal neoplasia and inform screening choice for asymptomatic subjects. Gut. 2014;63:1130-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Zhang X, Yang L, Liu S, Li H, Li Q, Li H, Wang N, Ji J. Performance of different colorectal cancer screening strategies: a long-term passive follow-up population-based screening program in Beijing, China. BMC Public Health. 2023;23:1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 20. | Cheng K, Cao X, Li YH, Gan XQ, Liu YP, Ruan L. [Analysis of risk factors for economic toxicity in colorectal cancer patients]. Zhonghua Zhongliu Fangzhi Zazhi. 2023;30:984-989. [DOI] [Full Text] |
| 21. | Koo JH, Leong RW. Sex differences in epidemiological, clinical and pathological characteristics of colorectal cancer. J Gastroenterol Hepatol. 2010;25:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Zainal N, Mohamed SF. Psychosocial Risk Factors for Colorectal Cancer: A Systematic Review. Malaysian J Psychiatry. 2015;24. |
| 23. | Jørgensen MD, Mikkelsen EM, Erichsen R, Thomsen MK. Mental illness and participation in colorectal cancer screening: a scoping review. Scand J Gastroenterol. 2022;57:1216-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Mahar AL, Kurdyak P, Hanna TP, Coburn NG, Groome PA. The effect of a severe psychiatric illness on colorectal cancer treatment and survival: A population-based retrospective cohort study. PLoS One. 2020;15:e0235409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Protani MM, Alotiby MKN, Seth R, Lawrence D, Jordan SJ, Logan H, Kendall BJ, Siskind D, Sara G, Kisely S. Colorectal cancer treatment in people with severe mental illness: a systematic review and meta-analysis. Epidemiol Psychiatr Sci. 2022;31:e82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Kirkøen B, Berstad P, Hoff G, Bernklev T, Randel KR, Holme Ø, de Lange T, Robb KA, Botteri E. Type and Severity of Mental Illness and Participation in Colorectal Cancer Screening. Am J Prev Med. 2023;64:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Kune GA, Kune S, Watson LF, Bahnson CB. Personality as a risk factor in large bowel cancer: data from the Melbourne Colorectal Cancer Study. Psychol Med. 1991;21:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Amitay EL, Carr PR, Jansen L, Roth W, Alwers E, Herpel E, Kloor M, Bläker H, Chang-Claude J, Brenner H, Hoffmeister M. Smoking, alcohol consumption and colorectal cancer risk by molecular pathological subtypes and pathways. Br J Cancer. 2020;122:1604-1610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 456] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 30. | Toriola AT, Kurl S, Laukanen JA, Mazengo C, Kauhanen J. Alcohol consumption and risk of colorectal cancer: the Findrink study. Eur J Epidemiol. 2008;23:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Kong XH, Zhang Z, Deng DH, Yu ZQ, Zhan K, He XS. [Methylated SDC2 testing in stool DNA for early screening of colorectal cancer in Shipai Town, Dongguan City]. Zhonghua Wei Chang Wai Ke Za Zhi. 2023;26:372-379. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Hultcrantz R. Aspects of colorectal cancer screening, methods, age and gender. J Intern Med. 2021;289:493-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 33. | Wu F, Wang B, Zhuang P, Lu Z, Li Y, Wang H, Liu X, Zhao X, Yang W, Jiao J, Zheng W, Zhang Y. Association of preserved vegetable consumption and prevalence of colorectal polyps: results from the Lanxi Pre-colorectal Cancer Cohort (LP3C). Eur J Nutr. 2022;61:1273-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Lee AH, Su D, Pasalich M, Binns CW. Preserved foods associated with increased risk of ovarian cancer. Gynecol Oncol. 2013;129:570-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Jian L, Zhang DH, Lee AH, Binns CW. Do preserved foods increase prostate cancer risk? Br J Cancer. 2004;90:1792-1795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Zhang Y, Rumgay H, Li M, Cao S, Chen W. Nasopharyngeal Cancer Incidence and Mortality in 185 Countries in 2020 and the Projected Burden in 2040: Population-Based Global Epidemiological Profiling. JMIR Public Health Surveill. 2023;9:e49968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 56] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 37. | Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Lieberman D, Levin TR, Rex DK. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017;112:37-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Zhao X, Wang S, Yuan Z, Yan S, Pang W, Liu X, Wang W, Yi B, Han Q, Zhang Q, Zhang X, Zhang C. Colonoscopy compliance and diagnostic yield in a large population-based colorectal cancer screening programme. Int J Colorectal Dis. 2023;38:227. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Gao ZL. [Preliminary results of colorectal cancer screening study in Shawan area, Xinjiang]. Linchuang Xiaohuabing Zazhi. 2011;23:359-361. [DOI] [Full Text] |
| 40. | Grobbee EJ, van der Vlugt M, van Vuuren AJ, Stroobants AK, Mallant-Hent RC, Lansdorp-Vogelaar I, Bossuyt PMM, Kuipers EJ, Dekker E, Spaander MCW. Diagnostic Yield of One-Time Colonoscopy vs One-Time Flexible Sigmoidoscopy vs Multiple Rounds of Mailed Fecal Immunohistochemical Tests in Colorectal Cancer Screening. Clin Gastroenterol Hepatol. 2020;18:667-675.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 41. | Pan X, Wang GZ, Fang DN, Li XH. [Colorectal cancer screening practice in Xuhui District, Shanghai in 2012~2013]. Zhonghua Linchuang Yishi Zazhi (Electronic Edition). 2015;9:3365-3368. [DOI] [Full Text] |
| 42. | Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, Kirk L, Litin S, Simmang C; Gastrointestinal Consortium Panel. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1501] [Cited by in RCA: 1438] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 43. | Li K, Liu HZ, Lin GZ, Li Y, Liang YR, Qin PZ. [Colorectal cancer screening in Guangzhou from 2015 to 2019: Analysis of colonoscopy results in positive populations with different primary screening methods]. Zhongguo Zhongliu. 2021;30:199-205. [DOI] [Full Text] |
| 44. | Du J, Zhang Y, Liu X, Guo Q, Wang F, Wu YZ, Zhou H, He M. [Analysis of colorectal cancer screening results of urban residents in Chongqing from 2012 to 2019]. Zhongguo Zhongliu. 2022;31:355-360. [DOI] [Full Text] |
| 45. | Yuan P, Gu J. [2006 to 2015 China CRC screening population compliance meta-analysis]. Zhongguo Zhongliu. 2017;26:241-248. [DOI] [Full Text] |
| 46. | Zheng SG. [Research on optimization strategy of medical service system in Qionghai City]. Hainan University, 2018. |
| 47. | Chen RX, Zhang DY, Zhang X, Chen S, Huang S, Chen C, Li D, Zeng F, Chen J, Mo C, Gao L, Zeng J, Xiong J, Chen Z, Bai F. A survey on Helicobacter pylori infection rate in Hainan Province and analysis of related risk factors. BMC Gastroenterol. 2023;23:338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Wang H, Liu CC, Bai FZ, Zhu J, Yan XX, Cao MD, Du LB, Wei DH, Wang DB, Liao XZ, Dong D, Gao Y, Dong P, Zhu C, Ma YL, Chai J, Xiao HF, Kong YX, Zhang Q, Zheng WF, Ying RB, Zhou H, Ren JS, Li N, Chen HD, Shi JF, Dai M. [Population's acceptance and attitude toward a novel fecal immunochemical test for colorectal cancer screening: a multi-center survey in China]. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54:760-767. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 49. | Kalich A, Heinemann L, Ghahari S. A Scoping Review of Immigrant Experience of Health Care Access Barriers in Canada. J Immigr Minor Health. 2016;18:697-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 50. | Ribbing Wilén H, Blom J, Höijer J, Hultcrantz R. Fecal immunochemical test in colorectal cancer screening: Colonoscopy findings by different cut-off levels. J Gastroenterol Hepatol. 2019;34:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Knapp GC, Alatise OI, Olasehinde OO, Adeyeye A, Ayandipo OO, Weiser MR, Kingham TP. Is Colorectal Cancer Screening Appropriate in Nigeria? J Glob Oncol. 2019;5:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 52. | Aniwan S, Ratanachu Ek T, Pongprasobchai S, Limsrivilai J, Praisontarangkul OA, Pisespongsa P, Mairiang P, Sangchan A, Sottisuporn J, Wisedopas N, Kullavanijaya P, Rerknimitr R. The Optimal Cut-Off Level of The Fecal Immunochemical Test For Colorectal Cancer Screening in a Country with Limited Colonoscopy Resources: A Multi-Center Study from Thailand. Asian Pac J Cancer Prev. 2017;18:405-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 53. | Gibson DJ, Mooney T, Mooney J, Mulcahy HE, O'Donoghue D. Impact of a higher fecal immunochemistry test cut-off on pathology detected in subsequent rounds of a colorectal screening program. Gastrointest Endosc. 2019;89:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |