Published online Jul 15, 2024. doi: 10.4251/wjgo.v16.i7.3331
Revised: May 1, 2024
Accepted: May 22, 2024
Published online: July 15, 2024
Processing time: 132 Days and 0.7 Hours
Metastatic breast cancer originating in the gastrointestinal tract is a rare occur
Here, we report a case of breast metastasis from gastric cancer in a 43-year-old woman. This patient was admitted to our hospital with complaints of discomfort in the upper and middle abdomen persisting for two months, as well as black stools for over ten days. She underwent radical distal gastrectomy for gastric cancer, followed by postoperative chemotherapy. Three years later, the patient developed bilateral breast nodules. Imaging studies indicated a high probability of malignancy. She subsequently underwent a right modified radical mastectomy and excision of a left breast mass. Postoperative pathology revealed the right breast tumor was consistent with primary gastric cancer.
We present a case of breast metastasis from gastric cancer to contribute to the limited foundation of research into this rare disease.
Core Tip: Gastric cancer is a common malignancy worldwide, with the peritoneum, liver, and lungs being frequent sites of distant metastasis. However, metastasis to the breast is extremely rare. Breast metastases from gastric cancer are easily overlooked given the low incidence, and the pathogenesis and treatment principles have been infrequently studied. We present a case of breast metastasis from gastric cancer and analyze similar published cases indexed on PubMed to explore the potential epidemiology, etiology, pathogenesis, and treatment. This provides an initial research foundation for this rare manifestation of gastric cancer spread.
- Citation: Liu JH, Dhamija G, Jiang Y, He D, Zhou XC. Gastric cancer metastatic to the breast: A case report. World J Gastrointest Oncol 2024; 16(7): 3331-3340
- URL: https://www.wjgnet.com/1948-5204/full/v16/i7/3331.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i7.3331
Primary breast cancer is one of the most common malignancies affecting women. Although notable advancements have been made in early screening, diagnosis and treatment in recent years, leading to a gradual decline in mortality, breast cancer remains the leading cause of cancer-related deaths among women globally[1]. Metastatic disease is uncommon, accounting for approximately 0.5%-2.0% of breast malignancies[2]. The most frequent primary sources are malignant melanoma, lymphoma, lung cancer, ovarian cancer and soft tissue sarcoma[3,4]. Metastases originating from gastro
However, accurately distinguishing metastatic disease from primary breast malignancies is important for prognosis and guiding appropriate management, especially as survival improves for metastatic breast cancer with expanding treat
A 43-year-old Chinese woman presented to the General Surgery Department with a 1-month history of bilateral breast lumps.
The patient incidentally palpated bilateral breast masses while bathing. She reported no associated breast pain, skin dimpling, erythema, ulceration, fever, nipple discharge, or bleeding.
Three years ago, the patient developed mild epigastric and periumbilical discomfort associated with acid reflux and belching. She did not seek medical attention until two months later when she noted the onset of small amounts of black, tarry stools, along with worsening abdominal pain. Her past medical, family, genetic, and psychosocial history were otherwise unremarkable.
Gastroscopy revealed chronic gastritis and an ulcerated lesion in the gastric body. Biopsy of the ulcer demonstrated poorly differentiated adenocarcinoma. The patient was admitted and underwent a distal radical gastrectomy with D2 lymphadenectomy, proximal gastric remnant, and Roux-en-Y gastrojejunocolonic anastomosis for gastric cancer. Intraoperatively, the tumor was found to invade the nerves. Metastatic carcinoma was identified in 6/13 lesser curvature lymph nodes, 4/4 greater curvature lymph nodes, and 3/3 perigastric lymph nodes. The greater omentum and margins were negative for tumor.
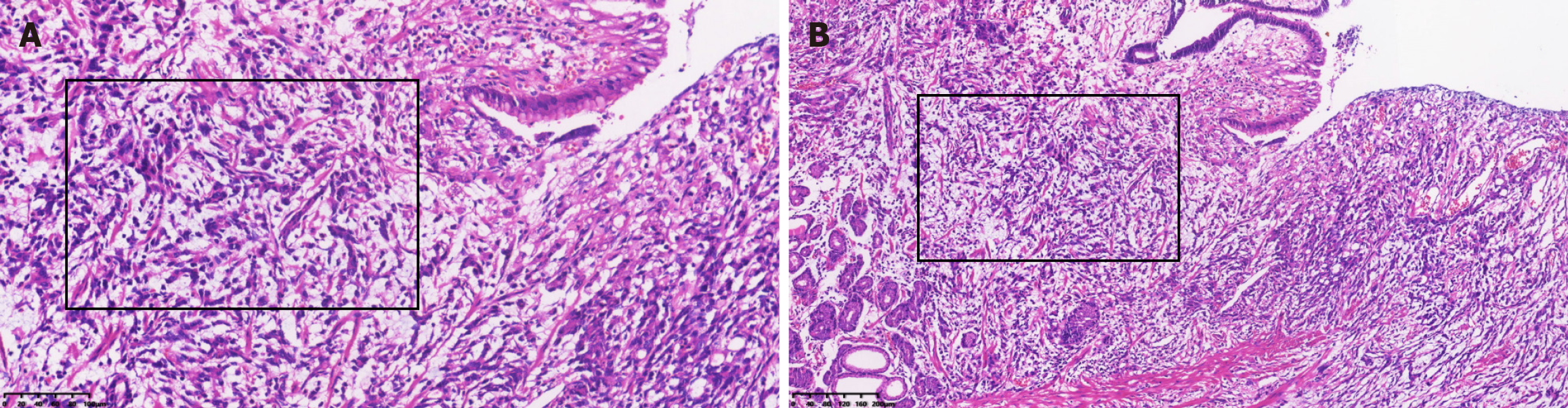
Postoperative pathology showed ulcerative, invasive, poorly differentiated adenocarcinoma involving the entire gastric wall (Figure 1). Immunohistochemistry showed estrogen receptor (ER) (-), progesterone receptor (PR) (-), tumor protein 53 (p53) (+), human epidermal factor receptor 2 (HER2) (-), cytokeratin 20 (CK20) (-), CK7 (+), carcinoembryonic antigen (CEA) (+), and < 10% Ki-67(+) cells. The final diagnosis was stage T3N2M0 poorly differentiated invasive gastric adenocarcinoma. The patient completed six cycles of postoperative FOLFOX chemotherapy with oxaliplatin 0.20 d1, calcium folinate 0.25 d1-5, and 5-fluorouracil 0.5 d1-5.
The patient reported no family history of malignant tumors.
Vital signs: Temperature 37.4 °C, blood pressure 110/78 mmHg, heart rate 80 bpm, respiratory rate 20 rpm. Exam revealed a 2.5 cm mass in the right upper outer breast quadrant. The mass was firm, mobile, with smooth borders and no tenderness.
No abnormality was found in routine blood and urine analyses.
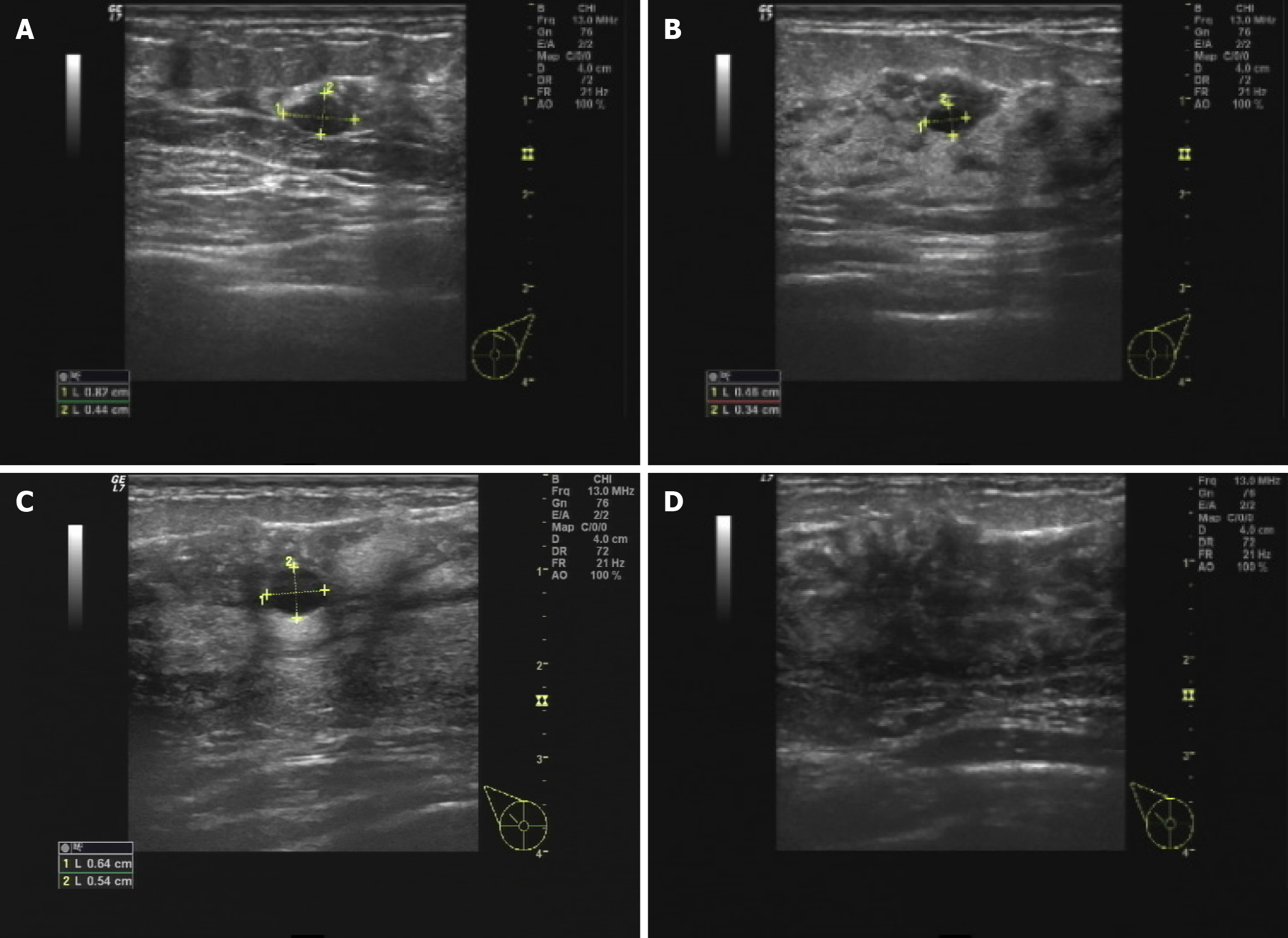
Breast ultrasound showed a 6 mm × 5 mm hypoechoic cystic mass in the right breast at 10 o’clock position. The mass had well-defined borders and posterior acoustic enhancement. There were scattered punctate echogenic foci within the mass measuring 1-2 mm, along with multiple enlarged right axillary lymph nodes (Figure 2).
Mammography revealed scattered sheet-like, flocculent and granular densities in both breasts, with punctate and rod-like calcifications more numerous in the left breast and clustered in the right upper outer quadrant. No distinct mass was visible. BI-RADS assessment was IV for the right breast and III for the left (Figure 3).
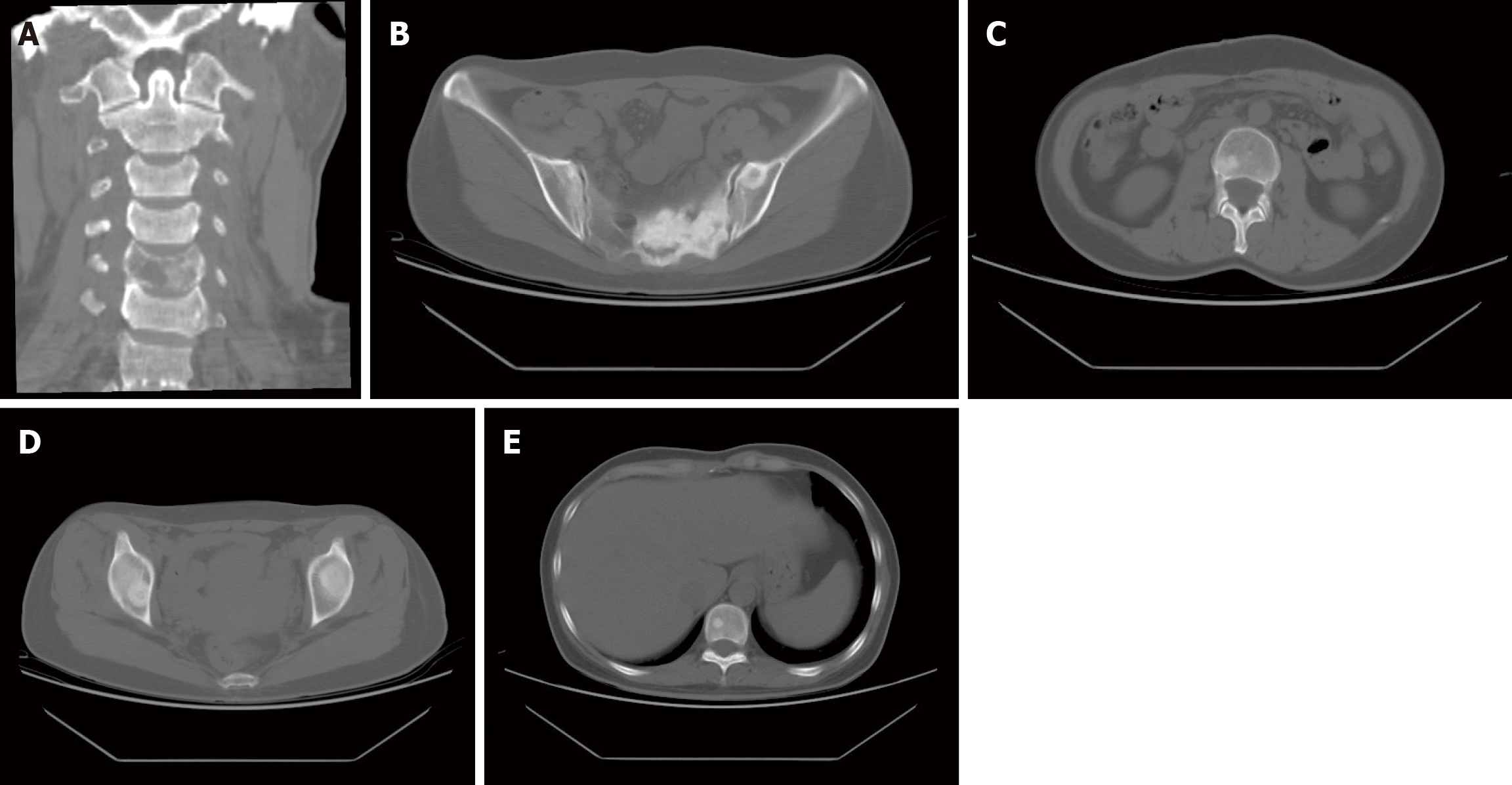
Computed tomography (CT) imaging detected multiple osseous metastases throughout the body (Figure 4). Computed tomography of the chest and abdomen was unremarkable.
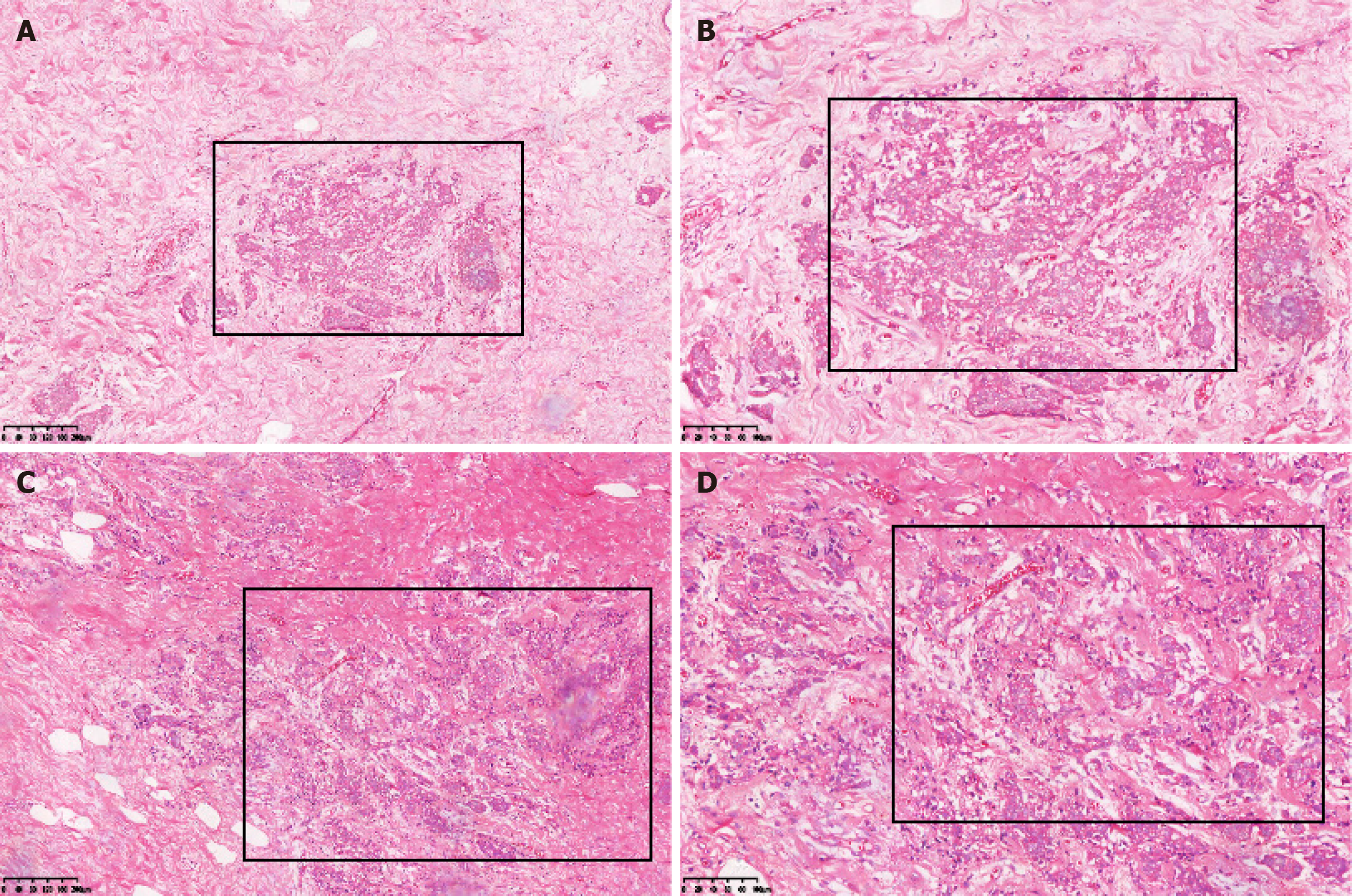
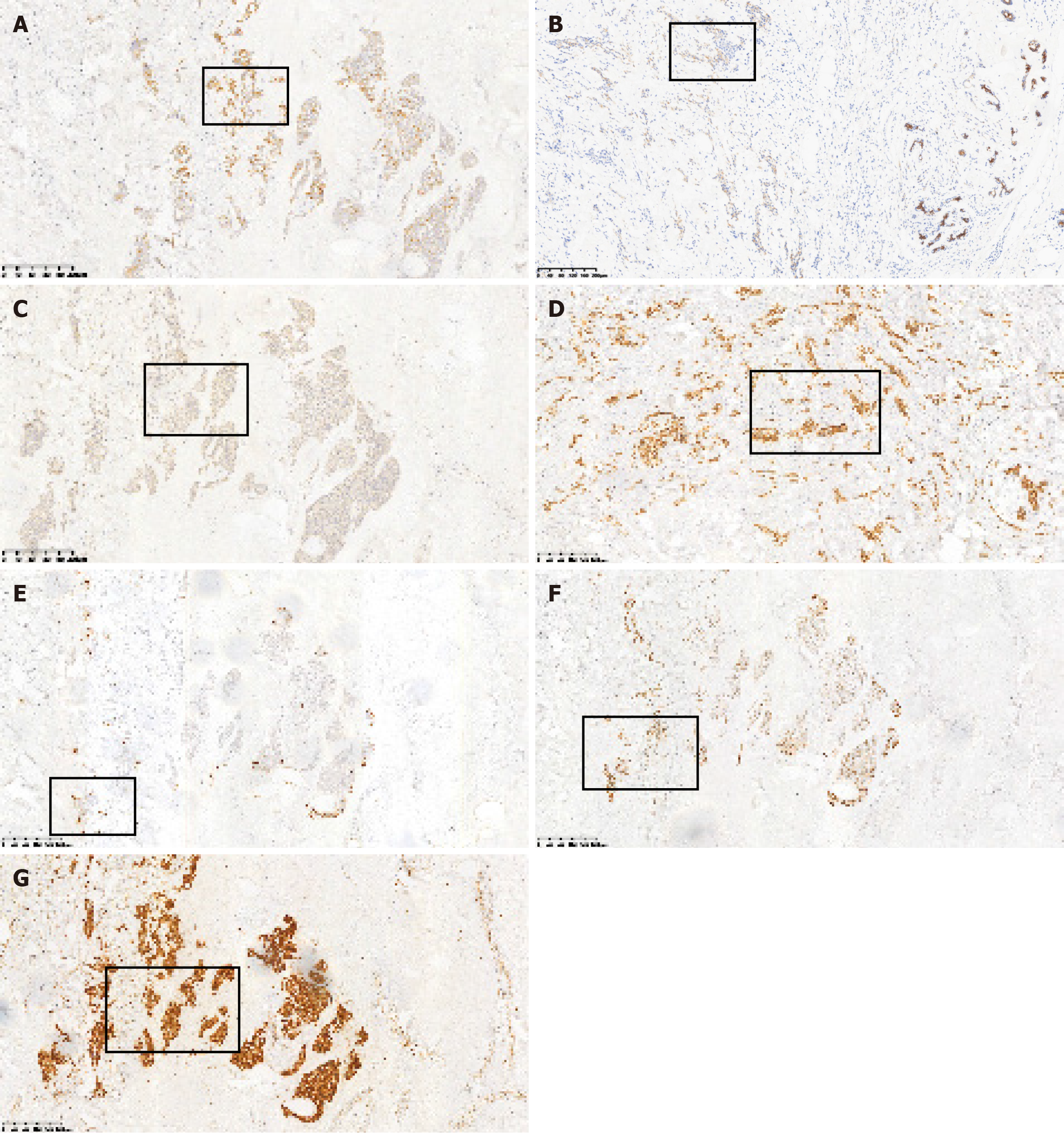
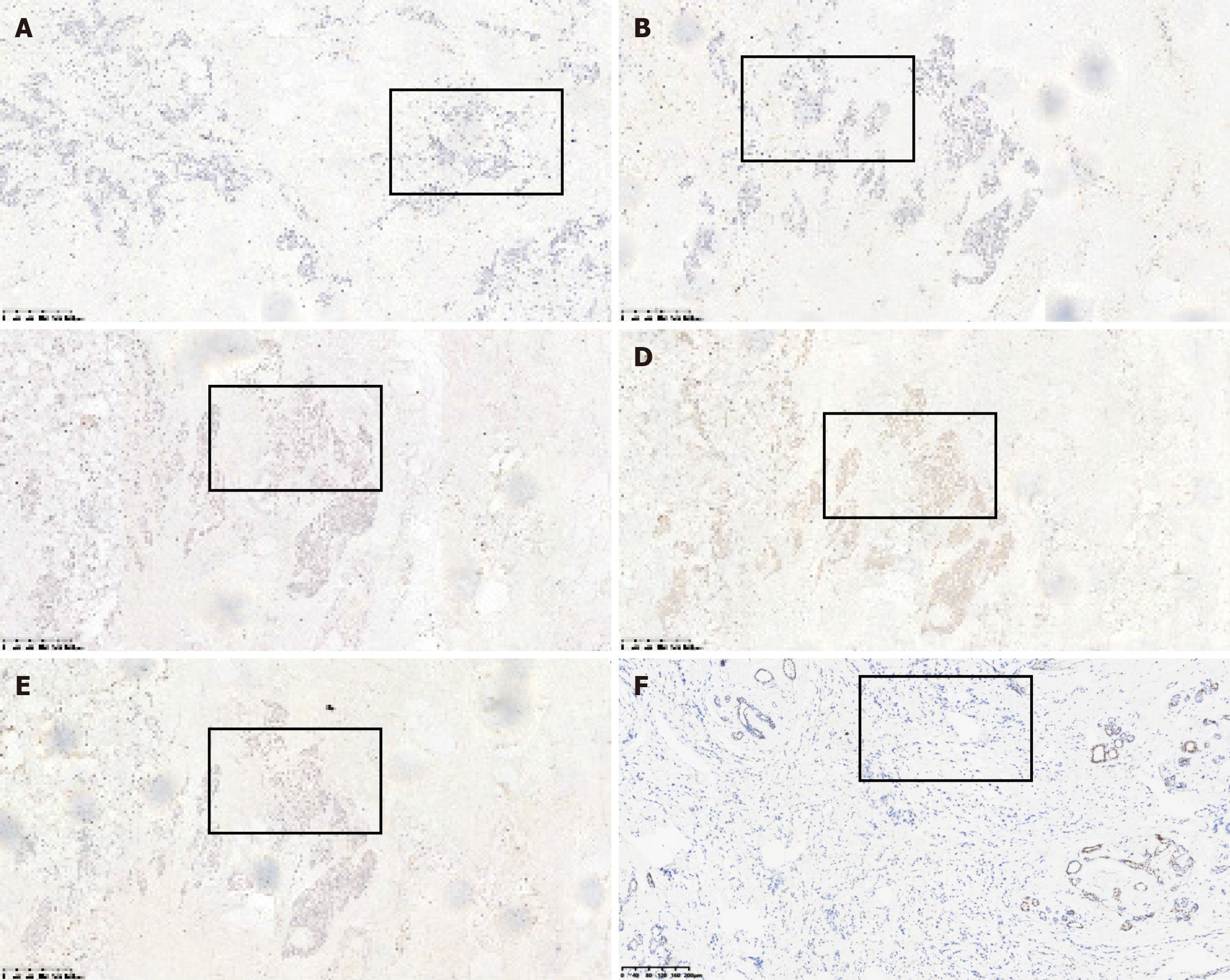
The patient underwent a modified radical surgery for the right breast cancer and excision of the left breast mass under general anesthesia. The final diagnosis revealed invasive carcinoma in the right breast. The postoperative pathology report indicated the following: (1) Left breast hyperplasia with fibroadenoma and cancerous thrombus within the ducts. Immunohistochemical markers showed: E-cadherin (E-cad) (+), 34βE12 (+), P120 membrane (+), CK5/6 (-); and (2) right breast hyperplasia with fibroadenoma, diffuse infiltration of epithelioid cells displaying heterogeneity in the mesenchyme, some with a striated structure. This was considered as postoperative metastasis of gastric cancer, taking into account the patient’s medical history and immunohistochemistry results. Immunohistochemical marker results were: ER (-), PR (-), HER2 (-), E-cad (+), P120 membrane (+), CK5/6 (+), 34βE12 (+), epidermal growth factor receptor (-), P53 (+), Ki-67 10% (+), CK7 (+), CK20 (-), Villin (+), and GATA binding protein 3 (GATA3) (-) (Figures 5-7).
Based on the medical history and workup, the final diagnosis was metastatic breast cancer from gastric primary.
The patient underwent a modified radical surgery for the right breast cancer and excision of the left breast mass under general anesthesia.
The patient experienced symptomatic improvement initially but bone metastases developed on December 13, 2013, and the patient received radiation therapy to the C5 vertebrae, DT: 3000 cGy/12 F. In January 2014, redness and swelling in the area of the incision on the right chest wall appeared, and four sessions of docetaxel 100 mg d1 + cisplatin 120 mg d1 + 5-fluorouracil 0.75 d1-5 chemotherapy combined with radiation therapy to the right chest wall, DT: 2800 cGy /14 F. Oxaliplatin 0.2 d1 + capecitabine 1.0 g bid d1-14 chemotherapy was started on September 26, 2014, for 2 sessions, followed by single-agent capecitabine 1.5 g bid d1-14 chemotherapy for 3 sessions. Symptoms did not improve significantly. February 29, 2015, continued right chest wall radiation therapy, DT: 5000 cGy/25 F, and targeted therapy by apatinib. Still no significant improvement. She ultimately succumbed on October 2, 2015, due to bone marrow suppression, sepsis, and pneumonia in the setting of prior chemotherapy.
Gastric cancer is a global health concern, with an estimated one million individuals affected annually. It ranks as the fifth most prevalent malignancy worldwide and stands as the third leading cause of cancer-related mortality in both women and men[1]. In cases of distant metastasis from gastric cancer, common sites involve the peritoneum, liver, lymph nodes, and lungs[6,7]. In contrast, breast metastases from gastric cancer are exceedingly rare, often eluding detection due to their low incidence. Consequently, the pathogenesis and treatment principles specific to this type of metastasis have been subject to limited study.
Based on available reports, patients with breast metastases from gastric cancer tend to be younger compared to those with primary breast malignancies[7]. One study found a mean age of 47 years with premenopausal women more fre
The pathogenesis of breast metastases from gastric cancer is unclear. Proposed routes include lymphatic and hematogenous spread[9]. The rich breast vasculature has been hypothesized to facilitate metastases in premenopausal women[7,10-12]. On the other hand, some reports note the left breast is more frequently involved in over 50% of cases, potentially related to drainage through the left supraclavicular nodes[2,4,7,8,13,14].
As an endocrine organ, hormonal factors may also contribute. Estrogen has been proposed to promote extramammary tumorigenesis[11]. The ER-β is expressed in some gastric adenocarcinomas, particularly indolent cell carcinoma, and may mediate estrogen-induced proliferation of tumor cells in extramammary regions[15,16]. Additionally, young gastric cancers associated with hormonal factors appear more aggressive, and gastric cancer growth may be influenced by the hormonal environment[17]. In one report, gastric cancers metastasized to the breast and ovary, respectively. This tendency of gastric indolent cell carcinomas to grow on endocrine target organs led to the hypothesis that the premenopausal hormonal environment and gastric cancer tissue type may be associated with metastasis to the breast and ovary[18].
Underlying genetic mutations have additionally been implicated. It has been suggested that individuals carrying germline mutations in BRCA2 are at increased risk of breast and gastric cancer[19-21]. A case report of breast metastasis from gastric cancer genetically tested the patient’s blood and revealed the presence of a heterozygous variant in exon 10 of BRCA2, though the significance of this finding is unknown[14].
Differentiating between metastatic and primary cancer in patients is crucial for prognosis and determining the appropriate treatment approach. In cases where a clinically indistinguishable breast mass is encountered, the diagnosis often hinges on either mammapuncture biopsy or intraoperative frozen section analysis. Additionally, breast aspiration biopsy, involving core needle or fine needle aspiration, plays a significant role in distinguishing primary from metastatic breast tumors[22]. Notably, aspiration biopsy was utilized in 59.3% of cases, effectively preventing unnecessary mastectomies[4].
In the context of metastasis diagnosis, immunohistochemistry stands out as a reliable and widely employed tool[23]. Specific markers such as ER, PR, and gross cystic disease fluid protein 15 staining are indicative of metastatic breast cancer, while CK20 and CK7 staining exhibit high specificity for primary gastric[4,10,15,18,24,25]. In the case of metastatic gastrointestinal tumors, positive markers include CEA, CK7, and CK20, while ER and PR markers remain negative. Thus, a combination of CK20, CEA, ER, and PR staining can be utilized to diagnose gastrointestinal metastases[26].
In our case, diffuse infiltrating striated epithelioid cells were seen filling the mammary mesenchyme in hematoxylin and eosin (H&E) staining, similar to the H&E staining picture of primary gastric cancer. Meanwhile, immunohistochemical staining showed negative ER, PR, HER2, and GATA3 indicators, which basically excluded breast primary, and positive CK7, Villin indicators, which were highly suggestive of gastrointestinal origin. Finally, combined with the patient’s medical history, metastatic breast cancer was considered.
A recent study revealed that hepatocyte nuclear factor 4 alpha was expressed in 99% of gastric cancers and 0% of breast cancers, while CK20 and CK7 were only positive in approximately 50% of gastric cancers. Consequently, the researchers concluded that HNF4A surpasses CK20 and can complement immunohistochemistry in effectively distinguishing pri
In terms of treatment, breast metastases originating from gastric cancer typically coincide with highly malignant tumors, indicating advanced disease. Consequently, Prognosis is generally poor once breast metastases develop, with survival under one year[9]. The current systemic treatment options encompass neoadjuvant chemotherapy and curative surgery[12]. However, certain reports have raised doubts about the efficacy of surgical intervention and chemotherapy in improving survival rates for breast metastases[4].
Fortunately, recent advances such as trastuzumab and apatinib have brought about improved prognosis, especially for cases of unresectable, advanced, or recurrent/metastatic gastric cancer[25,28]. Surgery primarily serves a palliative role in this context[9,29].
Overall, studies on breast metastases from gastric cancer are still scarce, not only because of their rarity, but also due to the high malignancy and short survival in these patients. It is important to identify them early and manage such patients appropriately to avoid unnecessary and potentially harmful treatment. It is also hoped that the case we have shared will provide some modest help in advancing knowledge of this disease.
Gastric cancer ranks as the fifth most prevalent malignancy globally, and it stands as the third leading cause of cancer-related fatalities among both women and men. Common sites for distant metastases typically involve the peritoneum, liver, lymph nodes, and lungs. Conversely, metastasis to the breast from gastric cancer is an exceptionally rare occurrence, often escaping detection due to its low incidence. Moreover, research pertaining to its pathogenesis and treatment principles has been limited. Therefore, we present a case of breast metastasis originating from gastric cancer, with the aspiration of contributing valuable insights for the study of this rare condition.
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11922] [Article Influence: 2980.5] [Reference Citation Analysis (4)] |
| 2. | Lee SK, Kim WW, Kim SH, Hur SM, Kim S, Choi JH, Cho EY, Han SY, Hahn BK, Choe JH, Kim JH, Kim JS, Lee JE, Nam SJ, Yang JH. Characteristics of metastasis in the breast from extramammary malignancies. J Surg Oncol. 2010;101:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Domanski HA, Mas-Morillas A. Breast metastases from pancreatic and ovarian carcinoma. Diagn Cytopathol. 1999;21:154-155. [PubMed] [DOI] [Full Text] |
| 4. | Ma Y, Liu W, Li J, Xu Y, Wang H. Gastric cancer with breast metastasis: Clinical features and prognostic factors. Oncol Lett. 2018;16:5565-5574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Alva S, Shetty-Alva N. An update of tumor metastasis to the breast data. Arch Surg. 1999;134:450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Koch A, Richter-Marot A, Wissler MP, Baratte A, Mathelin C. [Mammary metastasis of extramammary cancers: current knowledge and diagnostic difficulties]. Gynecol Obstet Fertil. 2013;41:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Iesato A, Oba T, Ono M, Hanamura T, Watanabe T, Ito T, Kanai T, Maeno K, Ishizaka K, Kitabatake H, Takeuchi D, Suzuki A, Nakayama J, Ito K. Breast metastases of gastric signet-ring cell carcinoma: a report of two cases and review of the literature. Onco Targets Ther. 2015;8:91-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Parrell Soler C, Palacios Marqués A, Saco López L, Bermejo De Las Heras R, Pertusa Martínez S. Breast metastatic localization of signet-ring cell gastric carcinoma. ISRN Obstet Gynecol. 2011;2011:426150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Wei LY, Kong M, Zhang Z, Zhang XC. Breast metastasis of gastric signet-ring cell carcinoma. J Zhejiang Univ Sci B. 2017;18:1026-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Boutis AL, Andreadis C, Patakiouta F, Mouratidou D. Gastric signet-ring adenocarcinoma presenting with breast metastasis. World J Gastroenterol. 2006;12:2958-2961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Vergier B, Trojani M, de Mascarel I, Coindre JM, Le Treut A. Metastases to the breast: differential diagnosis from primary breast carcinoma. J Surg Oncol. 1991;48:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Shiraishi M, Itoh T, Furuyama K, Yamasaki S, Shimada Y, Hosotani R, Nakashima Y, Imamura M. Case of metastatic breast cancer from esophageal cancer. Dis Esophagus. 2001;14:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Tian Q, Zeng J, Tao X, Zhang Z, Zhou X, Wang Y. Clinical pathology of metastatic gastric carcinoma to the breast: A report of two cases and a review of literature. Oncol Lett. 2016;11:3081-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Dulskas A, Al Bandar M, Choi YY, Shin SJ, Beom SH, Son T, Kim HI, Cheong JH, Hyung WJ, Noh SH. A case of gastric cancer metastasis to the breast in a female with BRCA2 germline mutation and literature review. Acta Chir Belg. 2019;119:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, Nakachi K, Gustafsson JA, Hayashi S. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol. 2002;128:319-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Buerba-Vieregge HH, Fernández-Ferreira R, Soberanis-Piña PD, De la Peña-López IR, Navarro-García LM, Macari-Jorge A. Breast Metastasis of Gastric Signet Ring Cell Carcinoma: A Case Report and Literature Review. Case Rep Oncol. 2021;14:165-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Maeta M, Yamashiro H, Oka A, Tsujitani S, Ikeguchi M, Kaibara N. Gastric cancer in the young, with special reference to 14 pregnancy-associated cases: analysis based on 2,325 consecutive cases of gastric cancer. J Surg Oncol. 1995;58:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Di Cosimo S, Ferretti G, Fazio N, Mandalà M, Curigliano G, Bosari S, Intra M, Latronico A, Goldhirsch A. Breast and ovarian metastatic localization of signet-ring cell gastric carcinoma. Ann Oncol. 2003;14:803-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Jakubowska A, Scott R, Menkiszak J, Gronwald J, Byrski T, Huzarski T, Górski B, Cybulski C, Debniak T, Kowalska E, Starzyńska T, Ławniczak M, Narod S, Lubinski J. A high frequency of BRCA2 gene mutations in Polish families with ovarian and stomach cancer. Eur J Hum Genet. 2003;11:955-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Friedenson B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed. 2005;7:60. [PubMed] |
| 21. | Moran A, O’Hara C, Khan S, Shack L, Woodward E, Maher ER, Lalloo F, Evans DG. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012;11:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 22. | Deshpande AH, Munshi MM, Lele VR, Bobhate SK. Aspiration cytology of extramammary tumors metastatic to the breast. Diagn Cytopathol. 1999;21:319-323. [PubMed] [DOI] [Full Text] |
| 23. | Tang T, Zhang L, Li C, Zhou T. Gastric and adrenal metastasis from breast cancer: Case report and review of literature. Medicine (Baltimore). 2020;99:e18812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | O’Connell FP, Wang HH, Odze RD. Utility of immunohistochemistry in distinguishing primary adenocarcinomas from metastatic breast carcinomas in the gastrointestinal tract. Arch Pathol Lab Med. 2005;129:338-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | He CL, Chen P, Xia BL, Xiao Q, Cai FL. Breast metastasis of gastric signet-ring cell carcinoma: a case report and literature review. World J Surg Oncol. 2015;13:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Kiani Markani L, Kiani Markani F, Kadivar M, Hossinaei N, Safari E. Breast metastasis from gastric carcinoma: A case report. Caspian J Intern Med. 2022;13:132-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | van der Post RS, Bult P, Vogelaar IP, Ligtenberg MJ, Hoogerbrugge N, van Krieken JH. HNF4A immunohistochemistry facilitates distinction between primary and metastatic breast and gastric carcinoma. Virchows Arch. 2014;464:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Miller KD, Goding Sauer A, Ortiz AP, Fedewa SA, Pinheiro PS, Tortolero-Luna G, Martinez-Tyson D, Jemal A, Siegel RL. Cancer Statistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68:425-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 318] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 29. | Sato T, Muto I, Fushiki M, Hasegawa M, Sakai T, Sekiya M. Metastatic breast cancer from gastric and ovarian cancer, mimicking inflammatory breast cancer: report of two cases. Breast Cancer. 2008;15:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |