Published online Jul 15, 2024. doi: 10.4251/wjgo.v16.i7.3308
Revised: May 17, 2024
Accepted: May 20, 2024
Published online: July 15, 2024
Processing time: 79 Days and 5.4 Hours
Combination therapy has emerged as the focus of research for unresectable hepatocellular carcinoma (HCC). In recent years, several studies have explored the clinical efficacy and safety of the combination therapies of transarterial chemoembolization (TACE) with tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs).
To conduct an updated meta-analysis verifying the clinical benefits and adverse effects of the triple combination therapy for unresectable HCC.
All eligible cohort, non-randomized controlled, and randomized controlled trial studies from the PubMed, Web of Science, Embase, Cochrane Library, and MedLine databases up to March 20, 2024 were screened for the present meta-analysis. The study endpoints included complete response (CR), objective re
A total of 29 studies with 1754 patients were included. Among the patients who received the TACE therapy with TKIs and ICIs, the tumor response results revealed a pooled CR, ORR, and DCR of 14% [95%CI (0.11–0.18)], 61% [95%CI (0.55–0.66)], and 85% [95%CI (0.83–0.87)], respectively. In terms of the survival outcomes, the pooled median PFS and OS were 10.25 months [95%CI (9.31–11.18)] and 20.47 months [95%CI (18.98–21.97)], respectively. The pooled prevalence of all-grade AEs during the triple treatment was 90% [95%CI (0.84–0.94)] and that of grade ≥ 3 AEs was 32% [95%CI (0.24–0.42)].
The combination therapy of TACE, TKIs, and ICIs exhibits great clinical benefits for unresectable HCC in terms of tumor responses and survival outcomes without increasing the risk of severe AEs.
Core Tip: Consensus regarding the treatment of unresectable hepatocellular carcinoma (HCC) currently remains lacking. The triple combination therapy of transarterial chemoembolization with tyrosine kinase and immune checkpoint inhibitors has attracted significant attention as an aggressive treatment strategy and has been used for treatment in recent years. We conducted a systematic review and updated meta-analysis to verify the clinical benefits and adverse effects of triple therapy in 29 studies with 1754 patients with unresectable HCC. The complete response, objective response rate, disease control rate, overall survival, progression-free survival, and adverse events induced by the triple therapy were evaluated.
- Citation: Han F, Wang XH, Xu CZ. Clinical benefits of transarterial chemoembolization combined with tyrosine kinase and immune checkpoint inhibitors for unresectable hepatocellular carcinoma. World J Gastrointest Oncol 2024; 16(7): 3308-3320
- URL: https://www.wjgnet.com/1948-5204/full/v16/i7/3308.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i7.3308
Primary liver cancer is the most common and fatal solid malignancy worldwide[1]. In 2020, 905700 people were diagnosed with liver cancer, with 830200 fatalities globally[2]. The 5-year survival rate for liver cancer was 18% between 2006 and 2012[3]. The mortality rate of liver cancer increased by 43% from 2000 to 2016 in the United States[4]. By 2040, new cases and deaths from liver cancer may increase by > 55%[2]. Hepatocellular carcinoma (HCC) is the most common histological type of liver cancer, accounting for approximately 90% of cases[1]. Many curative treatments, including surgical resection, radiofrequency ablation, and liver transplantation, are available for patients with early HCC [Barcelona Clinic Liver Cancer (BCLC) 0 and A][5]. However, owing to the late presentation of symptoms, > 70% of patients are diagnosed with the intermediate (BCLC B) and advanced (BCLC C) HCC stages and mostly receive locoregional and systemic therapies[5-7].
Transarterial chemoembolization (TACE) is the most common and standard locoregional therapy in the management of patients with intermediate HCC[8]. TACE improved the survival outcomes of patients with unresectable HCC in random controlled trials conducted in Europe and Asia[5]. However, repeated TACE may impair liver function and even result in the development of TACE resistance. Consequently, TACE therapy alone is not sufficient for patients with advanced stages, especially portal vein invasion or extrahepatic spread[9,10]. Additionally TACE is generally not successful in controlling tumor progression because of the high incidence of incomplete embolization and embolization-related changes in the tumor microenvironment[11]. For systemic therapy, sorafenib, a multikinase inhibitor, was the first drug approved for the first-line systemic regimen. Treatment with sorafenib resulted in a longer median overall survival (OS) than the placebo group (10.7 vs 7.9 months) in patients with advanced HCC[12]. Lenvatinib is also an approved multikinase inhibitor for advanced HCC, and it demonstrated a comparable median OS of 13.6 vs 12.3 months for sorafenib in REFLECT trial[13]. Many other tyrosine kinase inhibitors (TKIs) and/or antiangiogenic VEGFR2 antagonists, such as regorafenib, cabozantinib, and ramucirumab, have shown significant improvements in the median OS of patients with HCC[14]. Immune checkpoint inhibitors (ICIs), including atezolizumab/bevacizumab, are also systemic first-line therapies for advanced HCC. Compared with sorafenib and lenvatinib, treatment with these ICIs resulted in the longest median OS of 15.03 months, median progression-free survival (PFS)of 7.97 months, and highest objective response rate (ORR) of 31.6%[15]. Recently, the combination of locoregional and systemic treatments has yielded impressive clinical outcomes[14]. A multicenter retrospective matched-cohort study of patients with HCC from 59 hospitals in China found that TACE combination therapy with PD-(L)1 inhibitors and molecular targeted agents significantly improved the median PFS, median OS, and ORR compared with TACE monotherapy[16]. A single-arm phase II trial based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST) revealed that TACE plus lenvatinib and PD-1 inhibitors exhibited a high ORR of 60.0%, disease control rate (DCR) of 86.7%, long median OS of 18.4 months, and median PFS of 8.0 months, with 93.3% adverse events (AEs) of any grade, 40.0% grade 3 TRAEs, and no grade 4/5 TRAEs in patients with advanced HCC[17]. To date, numerous clinical trials and studies with or without control or intervention measures have explored the benefits of combination therapies of TACE with TKIs and ICIs using different treatment regimens and have achieved encouraging results.
Combination therapies with remarkable therapeutic potential have become the focus of research on unresectable HCC[6]. Survival outcomes, tumor responses, and adverse reactions caused by triple therapy have attracted much attention from academic researchers and scholars. Therefore, in this study, we conducted a meta-analysis to evaluate the clinical benefits and side effects of triple therapy of TACE combined with TKIs and ICIs in unresectable HCC using systematic and up-to-date data. Our study may serve as a reference for the selection of treatment regimens by clinicians.
We identified the eligible studies from the PubMed, Web of Science, Embase, Cochrane Library, and MedLine databases up to March 20, 2024. The following terms were used: transarterial chemoembolization OR transcatheter arterial chemoembolization OR TACE AND tyrosine kinase inhibitors OR TKIs OR sorafenib OR lenvatinib AND immune checkpoint inhibitors OR ICIs OR programmed cell death protein 1 OR programmed cell death ligand 1 OR PD-1/L1 inhibitors OR atezolizumab OR bevacizumab AND liver cancer OR liver neoplasms OR hepatocellular carcinoma OR hepatocarcinoma OR HCC.
The inclusion criteria included: (1) Patients diagnosed with HCC; (2) Intervention using triple therapy of TACE with TKIs and ICIs; (3) No restriction on whether a control group or intervention was established; (4) Studies reporting at least one of the endpoints, such as complete response (CR), ORR, DCR, median PFS, median OS, all-grade AEs, and grade ≥ 3 AEs; and (5) Study design including cohort, non-randomized controlled, and randomized controlled trials. The exclusion criteria were as follows: (1) The use of monotherapy or combination therapies other than TACE with TKIs and ICIs; (2) Studies with insufficient data; (3) Studies that were not available; (4) Duplicate studies; and (5) Studies not reported in English.
The data were extracted by two professionals, and the following information was collected: (1) Characteristics of studies: first author's name, publication date, country, subjects, study design, treatment regimens, and sample size; (2) Characteristics of patients: median age, sex, whether hepatitis B virus-positive, α-fetoprotein levels, Child-Pugh grade, BCLC stage, extrahepatic metastasis, and portal vein tumor thrombus; (3) Endpoints reported in the articles: tumor responses (CR, ORR, and DCR), survival outcomes (median PFS and median OS), adverse effects (all-grade AEs and grade ≥ 3 AEs).
The tumor response was evaluated according to the mRECIST. ORR was defined as CR or partial response (PR), whereas DCR was defined as the sum of CR, PR, and stable disease. AEs and grade ≥ 3 AEs were evaluated based on the Common Terminology Criteria for Adverse Events. A meta-analysis of the pooled rates of CR, ORR, DCR, and AEs and the effect size of median PFS and OS with 95%CI was performed using Stata 16/18. The heterogeneity was evaluated using I2 statistics, and P > 0.10 or I2 ≥ 50% was considered as apparent heterogeneity. A random-effect or fixed-effect model was used. P < 0.05 was considered statistically significant.
A flowchart of the study selection is illustrated in Figure 1. After excluding ineligible studies, 29 articles[17-45] with 1754 patients were included. Among the included studies, 27 were retrospective studies and 2 were prospective studies. In this meta-analysis, regardless of the presence or absence of control or intervention measures, patients who underwent triple combination therapy were included. The characteristics of the included studies and patients are presented in Table 1.
| Ref. | Country | Subjects | Study design | Treatment regimen | Sample size | Age (yr) | Male (%) | Positive of HBV (%) | AFP < 400 ng/mL (%) | Child-Pugh A (%) | BCLC stage A/B/C | EHM (%) | PVTT (%) |
| Yuan et al[18], 2024 | China | Patients with unresectable HCC | Retrospective | TACE + TKIs + ICIs | 139 | 59 (50, 67) | 121 (87.05) | 115 (82.73) | 103 (74.10) | 119 (85.61) | 0/99/40 | 23 (16.55) | 31 (22.3) |
| Sun et al[19], 2024 | China | Patients with advanced HCC | Retrospective | TACE + lenvatinib + sintilimab | 40 | 55 ± 9 | 34 (85.0) | 30 (75.0) | 26 (65.0) | 34 (85.0) | 0/0/40 | 14 (35.0) | 29 (72.5) |
| Sheng et al[20], 2024 | China | Patients with unresectable HCC | Retrospective | TACE + lenvatinib + PD-1 inhibitor | 113 | 64.48 ± 10.83 | 95 (84.1) | 72 (63.7) | 90 (79.6) | 88 (77.9) | 0/54/59 | 15 (13.3) | 29 (25.7) |
| Cai et al[17], 2024 | China | Patients with advanced HCC | Retrospective | TACE + lenvatinib + sintilimab | 30 | 49.4 ± 9.9 | 26 (86.7) | 25 (83.3) | NR | 29 (96.7) | NR | 13 (43.3) | NR |
| Gao et al[21], 2023 | China | Patients with advanced HCC | Retrospective | TACE + TKIs + ICIs | 41 | 52 (46, 57) | 35 (85.4) | 29 (70.7) | 9 (22.0) | 32 (78.0) | 0/11/30 | 10 (24.4) | 29 (70.7) |
| Li et al[22], 2023 | China | Patients with advanced HCC | Prospective | TACE + TKIs + camrelizumab | 87 | 56 (34, 75) | 81 (93.1) | 75 (86.2) | 51 (58.6) | 51 (58.6) | NR/NR/69 | 43 (49.4) | 65 (74.7) |
| Hu et al[44], 2023 | China | Patients with unresectable HCC | Retrospective | TACE + TKIs + ICIs | 98 | 52 (42, 62) | 87 (88.8) | 85 (86.7) | NR | 75 (76.5) | 0/12/86 | 49 (50.0) | 14 (14.3) |
| Zhang et al[23], 2024 | China | Patients with unresectable HCC | Retrospective | TACE + TKIs + ICIs | 54 | ≤ 60: 38 (72.5); > 60: 16 (27.5) | 46 (85.0) | 53 (98.1) | 26 (48.1) | 54 (100) | 0/23/31 | 19 (35.2) | NR |
| Wu et al[24], 2024 | China | Patients with unresectable HCC | Prospective | TACE + lenvatinib + camrelizumab | 55 | 54 (46, 62) | 45 (81.8) | 27 (49.1) | 23 (41.8) | 55 (100) | 0//12/43 | 10 (18.2) | 37 (67.3) |
| Gao et al[25], 2023 | China | Patients with TACE-refractory HCC | Retrospective | TACE + lenvatinib + PD-1 inhibitor | 57 | 57.5 ± 9.4 | 45 (78.9) | 43 (75.4) | NR | 34 (59.6) | 0/17/40 | 24 (42.1) | NR |
| Lu et al[26], 2023 | China | Patients with unresectable HCC | Retrospective | TACE + donafenib + toripalimab | 81 | 51.9±12.4 | 65 (80.2) | 54(66.7) | NR | 46 (56.8) | 0/22/59 | NR | NR |
| Pan et al[27], 2023 | China | Patients with unresectable HCC | Retrospective | TACE + TKIs + ICIs | 49 | < 65: 38 (77.6); ≥ 65: 11 (22.4) | 46 (93.9) | 41 (83.7) | 21 (42.9) | 40 (81.6) | 5/14/30 | 6 (12.2) | 27 (55.1) |
| Wu et al[28], 2023 | China | Patients with BCLC-defined stage C HCC | Retrospective | TACE + lenvatinib + camrelizumab | 57 | 53.18 ± 9.25 | 49 (86.0) | 44 (77.2) | 25 (43.9) | 52 (91.2) | 0/0/57 | 23 (40.4) | NR |
| Wang et al[29], 2023 | China | Patients with unresectable HCC | Retrospective | TACE + lenvatinib + PD-1 inhibitor | 45 | 54 (18, 79) | 42 (93.33) | 42 (93.33) | 13 (28.89) | NR | NR | 18 (40.0) | 20 (44.44) |
| Xin et al[30], 2023 | China | Patients with unresectable HCC | Retrospective | TACE + lenvatinib + PD-1 inhibitor | 60 | 57.5 (26, 76) | 54 (90.0) | 56 (93.4) | 32 (53.3) | NR | 0/21/39 | 18 (30.0) | 28 (46.7) |
| Wang et al[31], 2023 | China | Patients with unresectable HCC | Retrospective | TACE + lenvatinib + PD-1 inhibitor | 46 | 55.54 ± 11.92 | 41 (89.1) | 42 (91.3) | 18 (39.1) | 38 (82.6) | 0/8/38 | 24 (52.2) | NR |
| Sun et al[32], 2023 | China | Patients with advanced HCC | Retrospective | TACE + TKIs + camrelizumab | 70 | 53.8 ± 10.4 | 58 (82.9) | 54 (77.1) | 30 (42.9) | 57 (81.4) | NR | NR | NR |
| Ju et al[33], 2021 | China | Patients with unresectable HCC | Retrospective | TACE + apatinib + camrelizumab | 56 | 52 (26, 75) | 46 (82.1) | 48 (85.7) | NR | 43 (76.8) | 0/13/43 | NR | NR |
| Cai et al[34], 2022 | China | Patients with advanced HCC | Retrospective | TACE + lenvatinib + PD-1 inhibitor | 41 | 51.9 ± 10.3 | 37 (90.2) | 35 (85.4) | 20 (48.8) | 37 (90.2) | NR | 17 (41.5) | NR |
| Li et al[35], 2022 | China | Patients with unresectable HCC | Retrospective | TACE + lenvatinib + PD-1 inhibitor | 114 | 53 (24, 79) | 102 (89.5) | 102 (89.5) | NR | 111 (97.4) | 3/42/69 | 23 (20.2) | NR |
| Teng et al[36], 2022 | China | Patients with unresectable HCC | Retrospective | TACE + lenvatinib + PD-1 inhibitor | 53 | 56.9 (37, 75) | 45 (84.9) | 45 (84.9) | 35 (66.0) | 34 (64.2) | 0/23/30 | 42 (79.2) | NR |
| Qu et al[37], 2022 | China | Patients with unresectable HCC | Retrospective | TACE + lenvatinib + PD-1 inhibitor | 56 | 51 (24, 82) | 51 (91.1) | 43 (76.8) | 34 (60.7) | 53 (94.6) | 0/17/39 | 29 (51.8) | NR |
| Yang et al[38], 2022 | China | Patients with unresectable HCC | Retrospective | TACE + TKIs + ICIs | 53 | 59 ± 10.6 | 45 (85.0) | 47 (89.0) | 34 (64.0) | 34 (64.0) | 2/29/22 | NR | NR |
| Yang et al[39], 2021 | China | Patients with unresectable HCC | Retrospective | TACE + TKIs + ICIs | 31 | 57.5 ± 9.4 | 25 (80.6) | 26 (83.9) | 23 (74.2) | 27 (87.1) | 2/18/11 | NR | NR |
| Liu et al[45], 2021 | China | Patients with advanced HCC | Retrospective | TACE + lenvatinib + camrelizumab | 22 | 57.7 ± 9.9 | 17 (77.3) | 15 (68.2) | 7 (31.2) | 16 (72.7) | 0/12/10 | 8 (36.4) | 11 (50.0) |
| Wu et al[40], 2021 | China | Patients with unresectable HCC | Retrospective | TACE + lenvatinib + Anti-PD-1 antibodies | 62 | 57 (23, 75) | 56 (90.3) | 57 (91.9) | 30 (48.4) | 62 (100.0) | 6/21/35 | 6 (9.7) | 15 (24.2) |
| Cao et al[41], 2021 | China | Patients with unresectable HCC | Retrospective | TACE + lenvatinib + sintilimab | 52 | ≤ 65: 40 (76.9); > 65: 12 (23.1) | 45 (86.4) | 47 (90.4) | 34 (65.4) | 46 (88.5) | 0/13/39 | 21 (40.4) | NR |
| Zheng et al[42], 2020 | China | Patients with advanced TACE-refractory HCC | Retrospective | TACE + sorafenib + ICIs | 22 | < 55: 10 (45.45); ≥ 55: 12 (54.55) | 19 (86.36) | 17 (77.27) | 7(31.82) | 13 (59.03) | 0/11/11 | 7(31.82) | 7(31.82 |
| Chen et al[43], 2022 | China | Patients with unresectable HCC | Retrospective | TACE + lenvatinib + pembrolizumab | 70 | 58 (36, 69) | 37 (52.9) | 38 (54.3) | 25 (35.7) | 70 (100.0) | 0/47/23 | NR | NR |
Tumor responses are presented as forest plots in Figure 2. Tumor responses, including CR, ORR, and DCR, were reported in 21, 24, and 23 studies, respectively. The results revealed the pool CR rate (Figure 3A), ORR (Figure 3B), and DCR (Figure 3C) as 14% [95%CI (0.11–0.18)], 61% [95%CI (0.55–0.66)], and 85% [95%CI (0.83–0.87)], respectively. A random-effect model was used for CR (I2 = 50.98%; P < 0.01) and ORR (I2 = 70.47%; P < 0.01), and a fixed-effect model was used for DCR (I2 = 28.27%; P = 0.10).
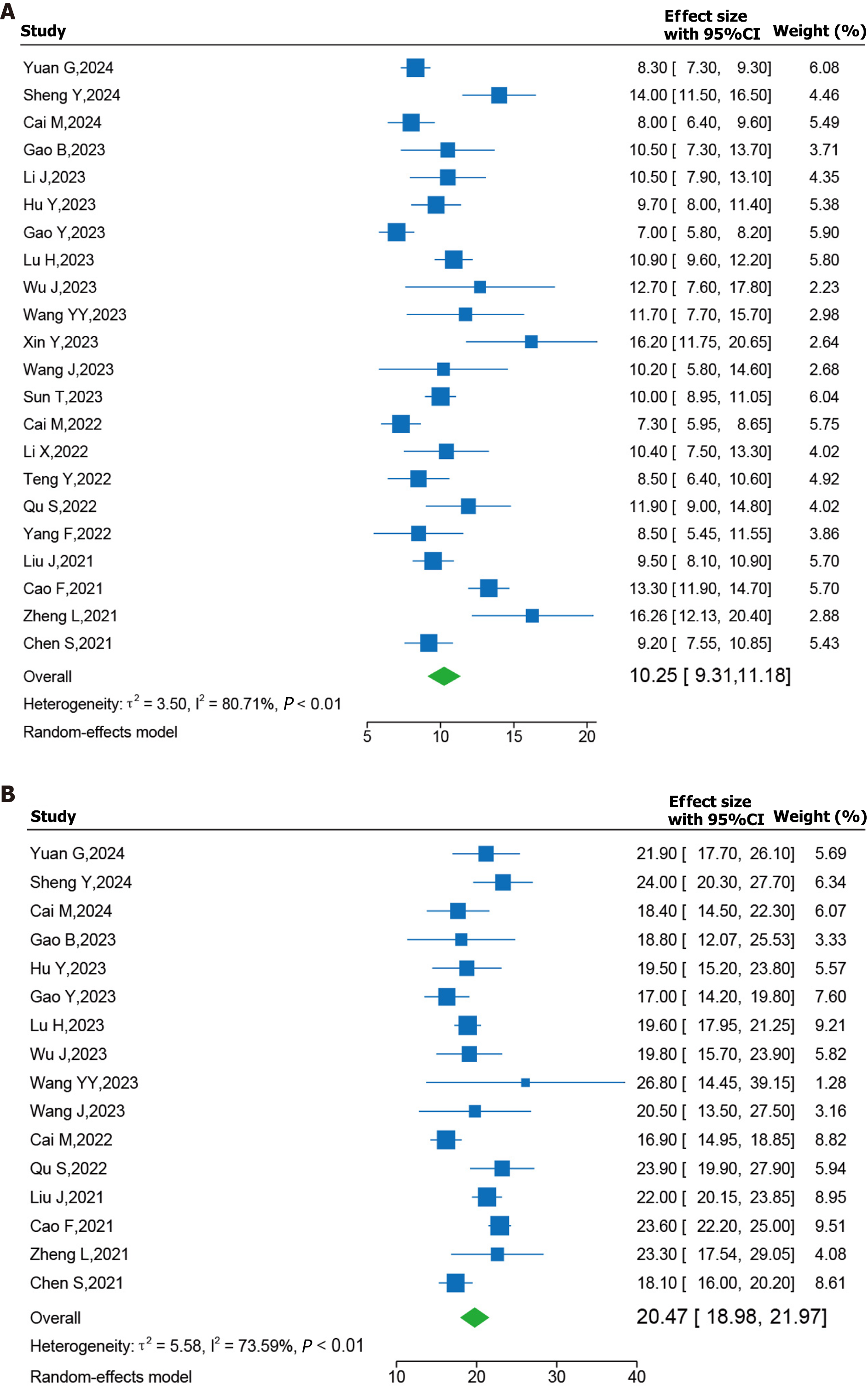
Forest plots of survival outcomes are presented in Figure 3. In terms of survival outcomes, 22 and 16 studies reported PFS and OS data, respectively. The pooled results demonstrated that patients who underwent triple combination therapy exhibited a promising median PFS [10.25 months; 95%CI (9.31–11.18); Figure 2A]. Moreover, the triple therapy was associated with a long median OS [20.47 months; 95%CI (18.98–20.97); Figure 2B]. A random-effect model was employed because of the high I2 values for the analysis of median PFS (I2 = 80.71%; P < 0.01) and OS (I2 = 73.59%; P < 0.01).
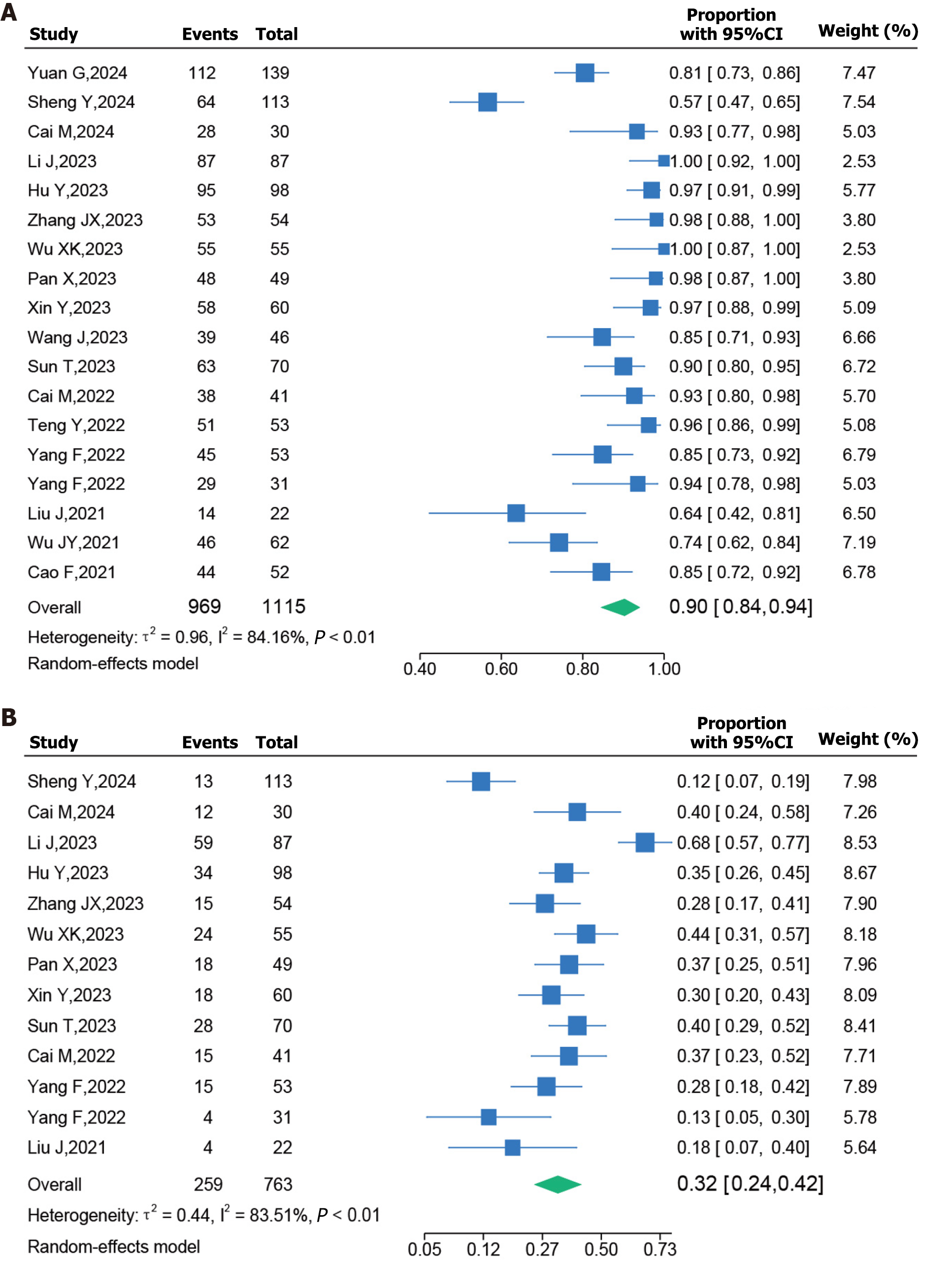
Forest plots for AEs are presented in Figure 4. AEs mainly included hypertension, elevated alanine aminotransferase levels, fatigue, diarrhea, vomiting, decreased appetite, thrombocytopenia, and hypothyroidism. In this analysis, 18 studies reported all-grade AEs, and 13 studies reported grade ≥ 3 AEs. The pooled incidence of all-grade AEs was 90% [95%CI (0.84–0.94)] and that of grade ≥ 3 AEs was 32% [95%CI (0.24–0.42)]. A random-effect model was employed, and the I2 values for all-grade AEs and grade ≥ 3 AEs were 84.16% (P < 0.01) and 83.51% (P < 0.01), respectively.
Using locoregional therapy along with systemic therapy may be a promising choice for unresectable HCC. In this study, 29 studies with 1754 patients who underwent TACE therapy in combination with TKIs and ICIs were included, and the findings revealed an encouraging CR, ORR, DCR, OS, PFS, and acceptable AEs in patients with unresectable HCC.
The efficacy and safety of triple TACE therapy with TKIs and ICIs in patients with advanced HCC were first reported in 2021[45]. In that study, patients receiving TACE therapy with lenvatinib and camrelizumab exhibited an ORR of 72.7%, and the DCR reached 95.5% at the third month. Further, the median OS and PFS were 24 and 11.4 months, respectively, with no serious AEs or deaths[45]. Thereafter, numerous studies have explored the benefits of combination therapies using different treatment options. The superiority of the triple combination modality over TACE monotherapy, TACE + TKIs, and TKIs + ICIs has been demonstrated in many controlled studies. In 2020, the TACTICS trial compared the effectiveness of TACE and sorafenib with that of TACE monotherapy, and reported a significant improvement in PFS (25.2 vs 13.5 months; P = 0.006)[46]. Notably, the ORR of TACE plus lenvatinib treatment reached 88.7% for unresectable HCC in the TACTICS-L trial[47]. A propensity score matching retrospective study reported that the triple combination of TACE with TKIs and ICIs demonstrated better ORR (52.5% vs 32.8%; P < 0.001) and DCR (82.7% vs 59.6%; P < 0.001), and achieved longer OS (median OS, 21.9 vs 16.3 months; P = 0.022) and PFS (median PFS, 8.3 vs 5.1 months; P < 0.0001) than TACE alone, with AEs similar to those reported in previous TACE-related studies[18]. Single-agent ICIs have shown an ORR of 15%–20% in patients with advanced HCC, mostly with no significant benefit on OS, resulting in approximately 30% of HCCs exhibiting intrinsic resistance to ICIs[48]. Using a PD-1 inhibitor in addition to TACE and lenvatinib has shown significant improvements in efficiency and safety[20]. Compared with TACE with lenvatinib, TACE with lenvatinib and PD-1 inhibitor resulted in longer PFS (14.0 vs 9.0 months; P < 0.001) and OS (24.0 vs 15.0 months; P < 0.001), and a better overall ORR (54.0% vs 32.8%; P = 0.001), with no significant difference in the incidence of AEs (56.64% vs 46.09%; P = 0.102) and grade ≥ 3 AEs (11.50% vs 9.38%; P = 0.588)[20]. In 2022, the IMbrave150 trial demonstrated that atezolizumab with bevacizumab results in a higher OS than sorafenib (19.2 vs 13.4 months; P < 0.001) in patients with untreated HCC[49]. The OS of the TACE with TKI plus ICI group was significantly longer than that of the TKI plus ICI group (19.5 vs 10.8 months; P = 0.005), and major AEs were comparable in both groups (34.7% vs 30.6%; P = 0.621)[44].
The findings of the present study revealed that the triple combination therapy exhibited an encouraging pooled CR rate, ORR, and DCR of 14%, 61%, and 85%, respectively. Further, the pooled median PFS and OS were 10.25 and 20.47 months, respectively. Moreover, the pooled prevalence of all-grade AEs was 90%, and that of grade ≥ 3 AEs was 32%. Our data support the potential application of triple combination therapy for unresectable HCC, which may have great clinical benefits in tumor responses and survival outcomes without increasing the risk of severe AEs. The findings are consistent with a recent largest, multicenter, retrospective cohort study in China, which indicated that combining TACE with ICIs plus anti-VEGF antibody/TKIs can significantly improve the OS, PFS, and ORR, with an acceptable safety profile[50]. Additionally, two multicenter international study registered in the United States were conducted to evaluate TACE in combination with ICIs and lenvatinib therapy in patients with locoregional/incurable/non-metastatic HCC (NCT05301842, NCT04246177). The results of the two ongoing trials have been not posted yet, but we are looking forward to the improved clinical effects of the triple therapy for HCC. Table 2 presents the ongoing clinical trials exploring the clinical benefits of the triple combination modality.
| Combination regimen | Comparator | Cancer stage | NCT number | Phase | Primary endpoint |
| TACE + lenvatinib + sintilimab/camrelizumab | None | Advanced unresectable HCC | NCT04997850 | Ⅰ/Ⅱ | Conversion resection rate |
| TACE + lenvatinib + ICIs | None | Intermediate/advanced HCC | NCT04974281 | Ⅰ | Conversion resection rate |
| TACE + donafenib + ICIs | None | Advanced HCC | NCT05262959 | Ⅱ | PFS |
| TACE + sorafenib + ICIs | None | Intermediate/advanced HCC | NCT04518852 | Ⅱ | ORR, OS |
| TACE + sorafenib + tilelizumab | None | Advanced HCC | NCT04992143 | Ⅱ | 1-yr survival rate |
| TACE + lenvatinib + tislelizumab | None | Advanced unresectable HCC | NCT05131698 | Ⅰ | ORR |
| TACE + sorafenib + tilelizumab | None | Advanced HCC | NCT04599777 | Ⅱ | OS |
| TACE + lenvatinib + durvalumab + tremelimumab | TACE | Locoregional HCC not amenable to curative therapy | NCT05301842 | Ⅲ | PFS |
| TACE + lenvatinib + pembrolizumab | TACE | Incurable/Non-metastatic HCC | NCT04246177 | Ⅲ | PFS, OS |
This study has some limitations. First, the retrospective design of the most included studies may have caused recall bias in this study. Second, conducting the subgroup analysis was difficult because of the small sample size of all included studies and insufficient data on TACE, TKIs, and ICIs in some of the studies. Third, as all eligible studies were from China, our conclusions may not be widely relied upon and generalized because of the notable heterogeneity in etiology. Fourth, different treatment regimens of triple combination therapy could also be a major contributor to heterogeneity. Finally, the sequence of triple therapy administration was not uniform across the included studies, and a broad consensus on this is required in the future.
We conducted a meta-analysis to evaluate the clinical efficacy and side effects of a combination of TACE with TKIs, and ICIs for unresectable HCC using current data. Our data support the potential application of triple therapy, which may have great clinical benefits for unresectable HCC in terms of tumor responses and survival outcomes without increasing the risk of severe AEs. Future randomized controlled trials with large sample sizes and multiple centers must be conducted to assess the efficacy and safety of the triple combination therapy and identify the optimal treatment regimen for potential beneficiaries with unresectable HCC.
| 1. | Zhang J, Zhang Z, Wu Z, Wang Y, Xia L. The switch triggering the invasion process: Lipid metabolism in the metastasis of hepatocellular carcinoma. Chin Med J (Engl). 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 2. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1052] [Article Influence: 350.7] [Reference Citation Analysis (0)] |
| 3. | Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ, Weir HK. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 1109] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 4. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3161] [Article Influence: 526.8] [Reference Citation Analysis (37)] |
| 5. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 2889] [Article Influence: 481.5] [Reference Citation Analysis (17)] |
| 6. | Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, Wang C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20:203-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 392] [Reference Citation Analysis (0)] |
| 7. | Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 939] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 8. | Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 590] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 9. | Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, Wei W, Zhang YJ, Guo Y, Guo RP, Chen MS, Shi M. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J Clin Oncol. 2022;40:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 240] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 10. | Li S, Mei J, Wang Q, Shi F, Liu H, Zhao M, Lu L, Ling Y, Guo Z, Guo Y, Chen X, Shi M, Lau WY, Wei W, Guo R. Transarterial infusion chemotherapy with FOLFOX for advanced hepatocellular carcinoma: a multi-center propensity score matched analysis of real-world practice. Hepatobiliary Surg Nutr. 2021;10:631-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Raoul JL, Gilabert M, Piana G. How to define transarterial chemoembolization failure or refractoriness: a European perspective. Liver Cancer. 2014;3:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10258] [Article Influence: 603.4] [Reference Citation Analysis (2)] |
| 13. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3816] [Article Influence: 545.1] [Reference Citation Analysis (1)] |
| 14. | Himmelsbach V, Koch C, Trojan J, Finkelmeier F. Systemic Drugs for Hepatocellular Carcinoma: What Do Recent Clinical Trials Reveal About Sequencing and the Emerging Complexities of Clinical Decisions? J Hepatocell Carcinoma. 2024;11:363-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Mahn R, Glüer OA, Sadeghlar F, Möhring C, Zhou T, Anhalt T, Monin MB, Kania A, Glowka TR, Feldmann G, Brossart P, Kalff JC, Schmidt-Wolf IGH, Strassburg CP, Gonzalez-Carmona MA. First-Line Treatment for Advanced Hepatocellular Carcinoma: A Three-Armed Real-World Comparison. J Hepatocell Carcinoma. 2024;11:81-94. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, Zhong BY, Sun JH, Jin ZC, Chen JJ, Ge NJ, Ding WB, Li WH, Huang JH, Mu W, Gu SZ, Li JP, Zhao H, Wen SW, Lei YM, Song YS, Yuan CW, Wang WD, Huang M, Zhao W, Wu JB, Wang S, Zhu X, Han JJ, Ren WX, Lu ZM, Xing WG, Fan Y, Lin HL, Zhang ZS, Xu GH, Hu WH, Tu Q, Su HY, Zheng CS, Chen Y, Zhao XY, Fang ZT, Wang Q, Zhao JW, Xu AB, Xu J, Wu QH, Niu HZ, Wang J, Dai F, Feng DP, Li QD, Shi RS, Li JR, Yang G, Shi HB, Ji JS, Liu YE, Cai Z, Yang P, Zhao Y, Zhu XL, Lu LG, Teng GJ; CHANCE001 Investigators. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 150] [Article Influence: 75.0] [Reference Citation Analysis (1)] |
| 17. | Cai M, Huang W, Liang W, Guo Y, Liang L, Lin L, Xie L, Zhou J, Chen Y, Cao B, Wu J, Zhu K. Lenvatinib, sintilimab plus transarterial chemoembolization for advanced stage hepatocellular carcinoma: A phase II study. Liver Int. 2024;44:920-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Yuan G, Li W, Zang M, Li R, Li Q, Hu X, Zhang Q, Huang W, Ruan J, Pang H, Chen J. Transarterial chemoembolization with/without immune checkpoint inhibitors plus tyrosine kinase inhibitors for unresectable hepatocellular carcinoma: a single center, propensity score matching real-world study. Discov Oncol. 2024;15:68. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Sun SS, Guo XD, Li WD, Chen JL. Lenvatinib combined with sintilimab plus transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma. World J Clin Cases. 2024;12:285-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (4)] |
| 20. | Sheng Y, Wang Q, Liu H, Chen W, Xing W. Prognostic nomogram model for selecting between transarterial chemoembolization plus lenvatinib, with and without PD-1 inhibitor in unresectable hepatocellular carcinoma. Br J Radiol. 2024;97:668-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 21. | Gao B, Yang F, Zheng D, Hu S, Liu J, Liu H, Liu Y, Liu L, Wang R, Zhao Y, Cui C, Fang C, Yang J, Su S, Han Y, Yang X, Li B. Transarterial Chemoembolization Combined with Tyrosine Kinase Inhibitors Plus Immune Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma: A Propensity Score Matching Analysis. J Hepatocell Carcinoma. 2023;10:2265-2276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Li J, Kong M, Yu G, Wang S, Shi Z, Han H, Lin Y, Shi J, Song J. Safety and efficacy of transarterial chemoembolization combined with tyrosine kinase inhibitors and camrelizumab in the treatment of patients with advanced unresectable hepatocellular carcinoma. Front Immunol. 2023;14:1188308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Zhang JX, Hua HJ, Cheng Y, Liu S, Shi HB, Zu QQ. Role of Transarterial Chemoembolization in the Era of Tyrosine Kinase Inhibitor and Immune Checkpoint Inhibitor Combination Therapy for Unresectable Hepatocellular Carcinoma: A Retrospective Propensity Score Matched Analysis. Acad Radiol. 2024;31:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 24. | Wu XK, Yang LF, Chen YF, Chen ZW, Lu H, Shen XY, Chi MH, Wang L, Zhang H, Chen JF, Huang JY, Zeng YY, Yan ML, Zhang ZB. Transcatheter arterial chemoembolisation combined with lenvatinib plus camrelizumab as conversion therapy for unresectable hepatocellular carcinoma: a single-arm, multicentre, prospective study. EClinicalMedicine. 2024;67:102367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 25. | Gao Y, Lu H, Xiong Z. Efficacy and safety of tyrosine kinase inhibitors plus PD-1 inhibitor in patients with transarterial chemoembolization- refractory hepatocellular carcinoma: a two-center retrospective study. Front Oncol. 2023;13:1231359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Lu H, Liang B, Xia X, Zheng C. Efficacy and safety analysis of TACE + Donafenib + Toripalimab versus TACE + Sorafenib in the treatment of unresectable hepatocellular carcinoma: a retrospective study. BMC Cancer. 2023;23:1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 27. | Pan X, Wu SJ, Tang Y, Zhou YF, Luo JW, Fang ZT. Safety and Efficacy of Transarterial Chemoembolization Combined with Tyrosine Kinase Inhibitor and Immune Checkpoint Inhibitors for Unresectable Hepatocellular Carcinoma: A Single Center Experience. J Hepatocell Carcinoma. 2023;10:883-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Wu J, Zeng J, Wang H, Huo Z, Hou X, He D. Efficacy and safety of transarterial chemoembolization combined with lenvatinib and camrelizumab in patients with BCLC-defined stage C hepatocellular carcinoma. Front Oncol. 2023;13:1244341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | Wang YY, Yang X, Wang YC, Long JY, Sun HS, Li YR, Xun ZY, Zhang N, Xue JN, Ning C, Zhang JW, Zhu CP, Zhang LH, Yang XB, Zhao HT. Clinical outcomes of lenvatinib plus transarterial chemoembolization with or without programmed death receptor-1 inhibitors in unresectable hepatocellular carcinoma. World J Gastroenterol. 2023;29:1614-1626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 30. | Xin Y, Zhang X, Liu N, Peng G, Huang X, Cao X, Zhou X, Li X. Efficacy and safety of lenvatinib plus PD-1 inhibitor with or without transarterial chemoembolization in unresectable hepatocellular carcinoma. Hepatol Int. 2023;17:753-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 31. | Wang J, Zhao M, Han G, Han X, Shi J, Mi L, Li N, Yin X, Duan X, Hou J, Yin F. Transarterial Chemoembolization Combined With PD-1 Inhibitors Plus Lenvatinib Showed Improved Efficacy for Treatment of Unresectable Hepatocellular Carcinoma Compared With PD-1 Inhibitors Plus Lenvatinib. Technol Cancer Res Treat. 2023;22:15330338231166765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Sun T, Ren Y, Sun B, Chen L, Zhu L, Zhang L, Zheng C. The Feasibility of TACE Combined with TKIs Plus PD-1 Antibody for Advanced HCC. J Hepatocell Carcinoma. 2023;10:447-457. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Ju S, Zhou C, Yang C, Wang C, Liu J, Wang Y, Huang S, Li T, Chen Y, Bai Y, Yao W, Xiong B. Apatinib Plus Camrelizumab With/Without Chemoembolization for Hepatocellular Carcinoma: A Real-World Experience of a Single Center. Front Oncol. 2021;11:835889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 34. | Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, Zhou J, Lin L, Cao B, Chen Y, Zhu K. Transarterial Chemoembolization Combined With Lenvatinib Plus PD-1 Inhibitor for Advanced Hepatocellular Carcinoma: A Retrospective Cohort Study. Front Immunol. 2022;13:848387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 35. | Li X, Fu Z, Chen X, Cao K, Zhong J, Liu L, Ding N, Zhang X, Zhai J, Qu Z. Efficacy and Safety of Lenvatinib Combined With PD-1 Inhibitors Plus TACE for Unresectable Hepatocellular Carcinoma Patients in China Real-World. Front Oncol. 2022;12:950266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 36. | Teng Y, Ding X, Li W, Sun W, Chen J. A Retrospective Study on Therapeutic Efficacy of Transarterial Chemoembolization Combined With Immune Checkpoint Inhibitors Plus Lenvatinib in Patients With Unresectable Hepatocellular Carcinoma. Technol Cancer Res Treat. 2022;21:15330338221075174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Qu S, Zhang X, Wu Y, Meng Y, Pan H, Fang Q, Hu L, Zhang J, Wang R, Wei L, Wu D. Efficacy and Safety of TACE Combined With Lenvatinib Plus PD-1 Inhibitors Compared With TACE Alone for Unresectable Hepatocellular Carcinoma Patients: A Prospective Cohort Study. Front Oncol. 2022;12:874473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 38. | Yang F, Xu GL, Huang JT, Yin Y, Xiang W, Zhong BY, Li WC, Shen J, Zhang S, Yang J, Sun HP, Wang WS, Zhu XL. Transarterial Chemoembolization Combined With Immune Checkpoint Inhibitors and Tyrosine Kinase Inhibitors for Unresectable Hepatocellular Carcinoma: Efficacy and Systemic Immune Response. Front Immunol. 2022;13:847601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 39. | Yang F, Yang J, Xiang W, Zhong BY, Li WC, Shen J, Zhang S, Yin Y, Sun HP, Wang WS, Zhu XL. Safety and Efficacy of Transarterial Chemoembolization Combined With Immune Checkpoint Inhibitors and Tyrosine Kinase Inhibitors for Hepatocellular Carcinoma. Front Oncol. 2021;11:657512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, Zhou JY, Li YN, Qiu FN, Li B, Yan ML. Lenvatinib Combined with Anti-PD-1 Antibodies Plus Transcatheter Arterial Chemoembolization for Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Study. J Hepatocell Carcinoma. 2021;8:1233-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 41. | Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, Feng D, Chen Y, Zheng J. The Efficacy of TACE Combined With Lenvatinib Plus Sintilimab in Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Study. Front Oncol. 2021;11:783480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 42. | Zheng L, Fang S, Wu F, Chen W, Chen M, Weng Q, Wu X, Song J, Zhao Z, Ji J. Efficacy and Safety of TACE Combined With Sorafenib Plus Immune Checkpoint Inhibitors for the Treatment of Intermediate and Advanced TACE-Refractory Hepatocellular Carcinoma: A Retrospective Study. Front Mol Biosci. 2020;7:609322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 43. | Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, Zhuang W, Chen X, Chen H, Xu B, Lai J, Guo W. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. 2022;148:2115-2125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 44. | Hu Y, Pan T, Cai X, He QS, Zheng YB, Huang MS, Jiang ZB, Chen JW, Wu C. Addition of transarterial chemoembolization improves outcome of tyrosine kinase and immune checkpoint inhibitors regime in patients with unresectable hepatocellular carcinoma. J Gastrointest Oncol. 2023;14:1837-1848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 45. | Liu J, Li Z, Zhang W, Lu H, Sun Z, Wang G, Han X. Comprehensive Treatment of Trans-Arterial Chemoembolization Plus Lenvatinib Followed by Camrelizumab for Advanced Hepatocellular Carcinoma Patients. Front Pharmacol. 2021;12:709060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 46. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 500] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 47. | Kudo M, Ueshima K, Saeki I, Ishikawa T, Inaba Y, Morimoto N, Aikata H, Tanabe N, Wada Y, Kondo Y, Tsuda M, Nakao K, Ito T, Hosaka T, Kawamura Y, Kuzuya T, Nojiri S, Ogawa C, Koga H, Hino K, Ikeda M, Moriguchi M, Hisai T, Yoshimura K, Furuse J, Arai Y. A Phase 2, Prospective, Multicenter, Single-Arm Trial of Transarterial Chemoembolization Therapy in Combination Strategy with Lenvatinib in Patients with Unresectable Intermediate-Stage Hepatocellular Carcinoma: TACTICS-L Trial. Liver Cancer. 2024;13:99-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 48. | Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 2023;79:506-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 166] [Reference Citation Analysis (0)] |
| 49. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 951] [Article Influence: 317.0] [Reference Citation Analysis (0)] |
| 50. | Jin ZC, Chen JJ, Zhu XL, Duan XH, Xin YJ, Zhong BY, Chen JZ, Tie J, Zhu KS, Zhang L, Huang M, Piao MJ, Li X, Shi HB, Liu RB, Xu AB, Ji F, Wu JB, Shao GL, Li HL, Huang MS, Peng ZY, Ji JS, Yuan CW, Liu XF, Hu ZC, Yang WZ, Yin GW, Huang JH, Ge NJ, Qi X, Zhao Y, Zhou JW, Xu GH, Tu Q, Lin HL, Zhang YJ, Jiang H, Shao HB, Su YJ, Chen TS, Shi BQ, Zhou X, Zhao HT, Zhu HD, Ren ZG, Teng GJ; CHANCE2201 Investigators. Immune checkpoint inhibitors and anti-vascular endothelial growth factor antibody/tyrosine kinase inhibitors with or without transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma (CHANCE2201): a target trial emulation study. EClinicalMedicine. 2024;72:102622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 55.0] [Reference Citation Analysis (0)] |