INTRODUCTION
Colorectal cancer (CRC) is the third most common malignancy worldwide and the second most deadly malignancy, with approximately 900000 deaths worldwide each year. CRC is also the fifth-leading cause of cancer-related deaths in China[1-3]. After traditional chemoradiotherapy, the clinical application of targeted immunotherapy has greatly improved the survival rate of patients with rectal cancer, but radical surgical resection is still the preferred method to improve the survival rate, improve the quality of life, and even cure CRC[4]. In 1982, Professor Heald proposed the concept of total mesorectal resection (TME)[5]. The implementation of TME significantly reduced the local recurrence of rectal cancer after surgery, so it became the basic principle of rectal cancer surgery. The concept of TME, in addition to the complete removal of the tumor, also emphasizes the complete removal of the lymph nodes in the rectal drainage area[6]. Lymph node metastasis is the most important and common metastasis pathway of CRC, and it is also an important index to judge the stage and prognosis of CRC[7]. Clinically, lymph nodes in the rectal cancer drainage area were divided into three stations: Paracenteric lymph nodes, mesangial lymph nodes, and mesangial root lymph nodes. The proposal of total mesangial resection of rectal cancer emphasizes the “excision” of lymph nodes in the rectal drainage area, which can make the pathological stage of the tumor more accurate and conducive to accurate postoperative treatment. However, the scope of lymph node dissection for rectal cancer is still controversial. A large number of clinical studies have shown that the lymph node metastasis of rectal cancer is mainly through the upper route, through the upper rectal artery, and finally to the peripheral lymph nodes of the abdominal aorta[8-10]. In low rectal cancer, in addition to the upper metastasis pathway, there are also lateral drainage pathways and lower drainage pathways. Lymph node metastases can be continuous or discontinuous, with the latter occurring in about 5% of cases. The continuous route of lymph node metastasis is first to the lymph nodes parallel to the intestinal duct along the marginal artery, then to the mesenteric vessels supplying blood to the intestinal segment where the tumor is located, and finally to the lymph nodes at the beginning of the vascular base. The route of this lymph node metastasis is first parallel to the intestinal duct and then along the blood vessels of the mesentery to the center. In a few cases, lymph node metastasis can also be skipped, especially when the lymph node metastasis in the drainage area is blocked. The lymph node of the cancer focus can also be retrogradely metastasized.
Inferior mesenteric artery (IMA) root lymph node metastasis indicates a poor prognosis in these patients, with a high possibility of local recurrence and distant organ metastasis after surgery. However, further exploration into the value of dissection remains necessary. Some scholars believe that the IMA root lymph node metastasis rate of rectal cancer, especially low rectal cancer, is relatively low, and the difficulty of submesenteric artery root lymph node dissection is increased, which will lead to prolonged operation time, intraoperative collateral damage, increased postoperative complications, decreased postoperative quality of life of patients, prolonged hospital stay, increased hospitalization costs, and other drawbacks. Some studies also believe that lymph node dissection of a submesenteric artery root for rectal cancer can obtain more lymph nodes, reduce the false negative rate, and then provide a better treatment plan to improve the postoperative survival rate of 5 or even 10 years.
Therefore, by searching the literature related to the clinicopathological features affecting lymph node metastasis in the submesenteric artery region, this study deeply studied the rule of IMA root lymph node metastasis and explored the clinicopathological features causing IMA root lymph node metastasis, providing new evidence-based medical evidence for the choice of treatment for rectal cancer patients.
MATERIALS AND METHODS
Literature retrieval
This study was conducted through PubMed, Google Scholar, and other literature search platforms, and the search time was as follows: Published studies and reports on clinical and pathological risk factors for IMA root lymph node metastasis in rectal cancer from the establishment of the database to December 31, 2023. The method of “Subject word + Free word” was used for literature retrieval, and the search terms were “rectal cancer, rectal neoplasms, rectum neoplasms, rectal tumors”, and so on.
Inclusion criteria
The inclusion criteria including: (1) CRC was confirmed by colonoscopy or postoperative pathology; (2) The included study was the first published literature on the risk factors of IMA root lymph node metastasis in rectal cancer at home and abroad; (3) The research purposes and statistical methods of the literatures are the same or similar; and (4) If the search appears to be the same author, or the same institution published duplicate literature, select one paper as the research object.
Exclusion criteria
The exclusion criteria including: (1) Literature types such as comprehensive analysis, review, case reports, and conference reports were excluded; (2) Exclude the literature with incomplete data and cannot extract the required data; and (3) Exclude only the abstract of the article, and cannot obtain the full text or download the full text of the literature.
Data extraction
Author name, publication year, study type, sample size, number of positive cases, and comparative characteristics of study subjects were obtained from the included literature, such as: Preoperative data: gender, age, preoperative carcinoembryonic antigen (CEA) level; intraoperative data: Tumor location and tumor size; postoperative data: Pathological type, degree of tumor differentiation, and depth of tumor invasion.
Quality evaluation of literature
Different scales were used to evaluate the quality of the included literature according to their research types. The literature included in this study was all retrospective studies, scored by the New Castle-Ottawa Scale with a total score of 9. The higher the score, the better the quality; 1-5 was classified as low quality, and 6-9 as high quality.
Data processing and analysis
We used RevMan 5.3 software to analyze the extracted data. The data included in this study were all bicategorical variables, and the odds ratio (OR) and 95% confidence interval (CI) were used as the combined effect index and combined effect interval, respectively, to draw the forest map. P < 0.05 of the Z-test indicated statistically significant differences. The I2 value and P value of the Q test were used to judge the heterogeneity of the included studies. When P > 0.05 or I2 < 50%, a fixed effect model was used. When P < 0.05 or I2 ≥ 50%, there was heterogeneity among the included studies (the greater the I2, the greater the heterogeneity). For studies with heterogeneity and statistical differences, subgroup studies, sensitivity analysis, and meta-regression are needed to find the causes of heterogeneity. References that were significantly off-center were removed, and a quadratic homogeneity test was performed. If heterogeneity was acceptable (I2 < 50%), a fixed-effect model was used for analysis; otherwise, a random effect model was used for analysis.
RESULTS
Literature search results
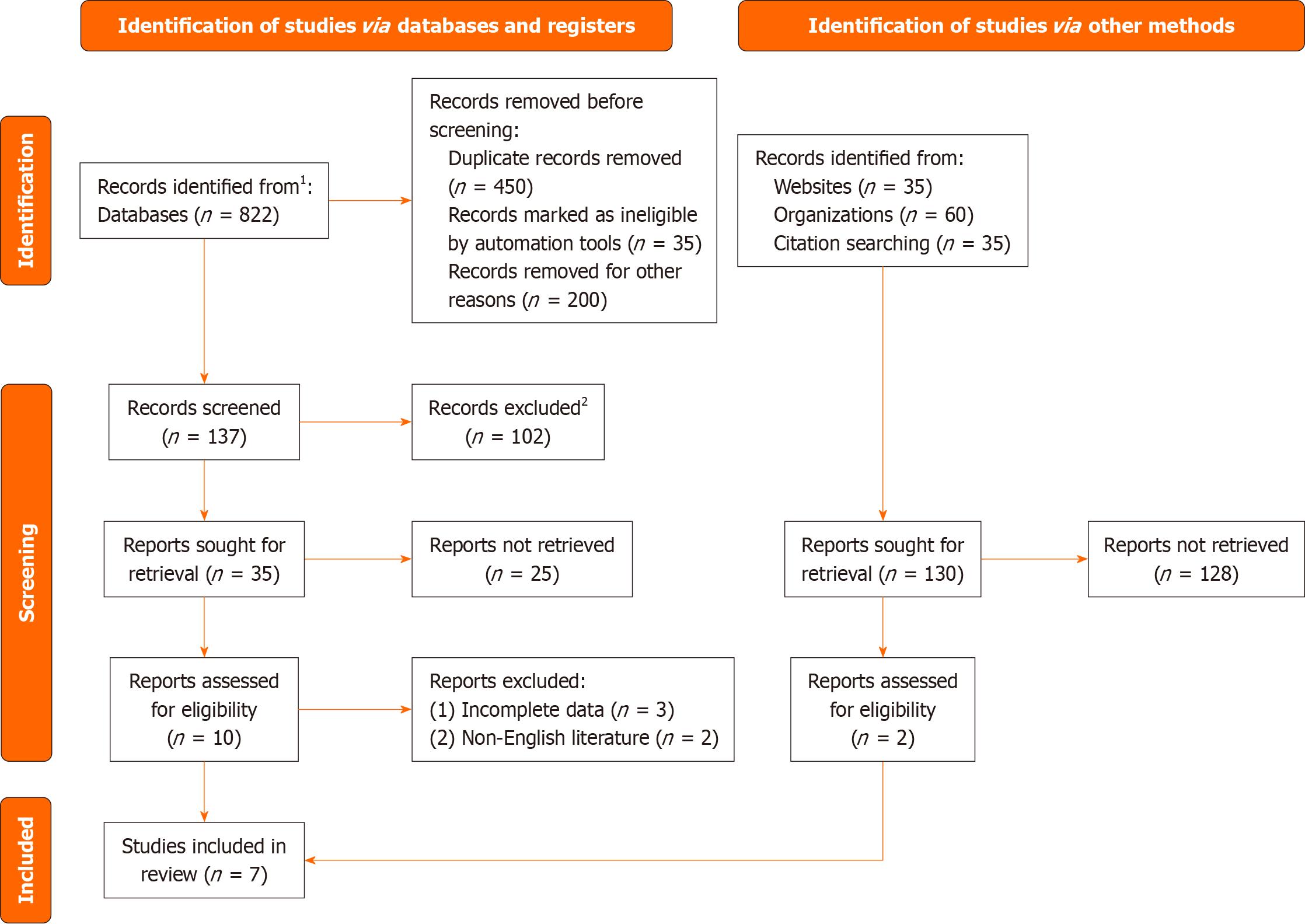
As shown in Figure 1, a total of 322 pieces of literature were retrieved through the database. 31 literatures were obtained after reading the title and abstract of the literatures and excluding the literatures that were inconsistent with the research content. According to the inclusion and exclusion criteria, 7 literatures were finally included in this meta-analysis[11-17].
Figure 1 Document retrieval flow chart.
1Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). 2If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
Meta-analysis of IMA root lymph node metastasis by gender
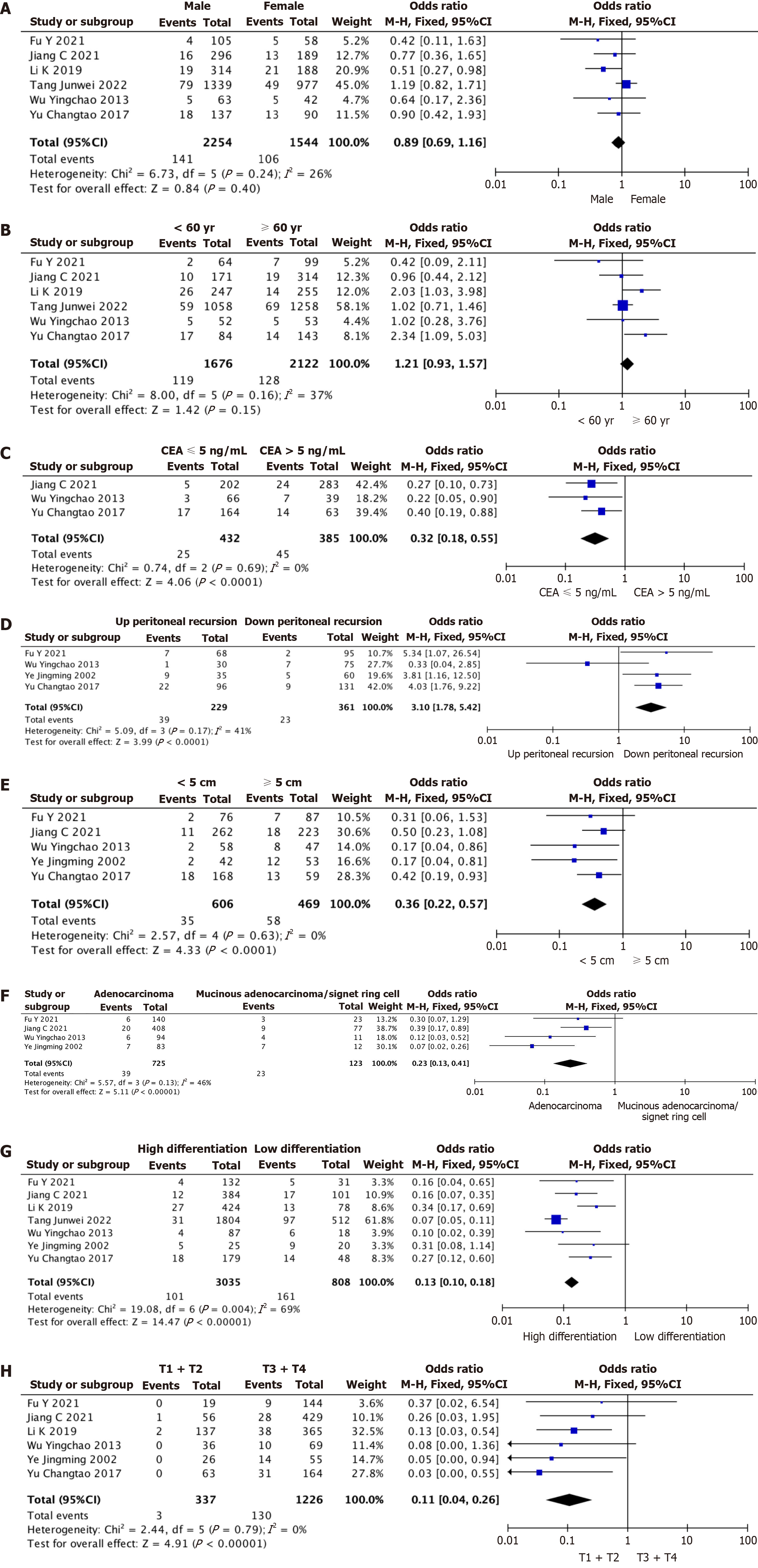
Six of the included studies reported gender as a risk factor for IMA root lymph node metastasis for comprehensive analysis. The results are shown in Figure 2A, where I2 = 26% and P = 0.24 in the Q test. There was slight heterogeneity in the analysis of IMA root lymph node metastasis by gender among the studies. Therefore, we can select the combined effect size of the fixed effects for our meta-analysis. The summary results of the six studies suggested that P = 0.4 of the Z-test was not statistically significant, and gender was not a risk factor for IMA root lymph node metastasis.
Figure 2 Meta-analysis.
A: Meta-analysis of gender as a risk factor; B: Meta-analysis of age as a risk factor; C: Meta-analysis of preoperative carcinoembryonic antigen level as a risk factor; D: Meta-analysis of tumor location as a risk factor; E: Meta-analysis of tumor size as a risk factor; F: Meta-analysis of pathological types as risk factors; G: Meta-analysis of tumor differentiation degree as a risk factor; H: Meta-analysis of T stage as a risk factor. CI: Confidence interval; CEA: Carcinoembryonic antigen.
Meta-analysis of age in relation to IMA root lymph node metastasis
Among the included literature, six studies reported age as a risk factor for IMA root lymph node metastasis. The results are shown in Figure 2B, where I2 = 37% and P = 0.16 in the Q test. There was slight heterogeneity in the analysis of IMA root lymph node metastasis by age among the studies. Therefore, the fixed effects combined effect size can be selected for meta-analysis. The summary results of the six studies suggested that the P = 0.15 of the Z-test was not statistically significant, and age was not a risk factor for IMA root lymph node metastasis.
Meta-analysis of preoperative CEA level on IMA root lymph node metastasis
Among the included literature, 3 studies reported preoperative CEA level as a risk factor for IMA root lymph node metastasis. The results are shown in Figure 2C, where I2 = 0% and P = 0.69 in the Q test. There was no heterogeneity in the analysis of preoperative CEA levels for IMA root lymph node metastasis. Therefore, we can select the combined effect size of fixed effects for meta-analysis. The summary results of the three studies suggested that P < 0.0001 of the Z-test was statistically significant, and preoperative CEA > 5 ng/mL was a risk factor for IMA root lymph node metastasis.
Meta-analysis of tumor location and IMA root lymph node metastasis
Four of the included literatures reported that tumor location was analyzed as a risk factor for IMA root lymph node metastasis, and the results are shown in Figure 2D, where I2 = 41% and P = 0.17 in the Q test. There was slight heterogeneity in the analysis of tumor location for IMA root lymph node metastasis among the studies. Therefore, we can select the combined effect size of the fixed effects for our meta-analysis. The four studies’ results showed that the P < 0.0001 level of significance for the Z-test meant that the tumor’s location above the peritoneal recurrence was a risk factor for IMA root lymph node metastasis.
Meta-analysis of tumor size on IMA root lymph node metastasis
Among the included literature, 5 studies reported that tumor size was analyzed as a risk factor for IMA root lymph node metastasis. The results are shown in Figure 2E, where I2 = 0% and P = 0.63 in the Q test. There was no heterogeneity in the analysis of IMA root lymph node metastasis by tumor location. Therefore, the fixed effects combined effect size can be selected for meta-analysis. The summary results of the five studies suggested that P < 0.0001 of the Z-test was statistically significant, so tumor size ≥ 5 cm was a risk factor for IMA root lymph node metastasis.
Meta-analysis of IMA root lymph node metastasis by pathological type
Among the included literature, 4 studies reported that pathological types were analyzed as risk factors for IMA root lymph node metastasis, and the results are shown in Figure 2F, where I2 = 46% and P = 0.13 in the Q test. There was slight heterogeneity in the analysis of IMA root lymph node metastasis by pathological type. Therefore, we can select the combined effect size of the fixed effects for meta-analysis. The aggregated results of the four studies suggested that P < 0.0001 of the Z-test was statistically significant, so mucinous adenocarcinoma/signet ring cell carcinoma was a risk factor for IMA root lymph node metastasis.
Meta-analysis of IMA root lymph node metastasis by tumor differentiation
The seven pieces of literature included all reported that the degree of tumor differentiation was analyzed as a risk factor for IMA root lymph node metastasis. The results are shown in Figure 2G, where I2 = 69% and P = 0.004 in the Q test. There was heterogeneity in the analysis of the degree of tumor differentiation on IMA root lymph node metastasis. Therefore, random effects combined effect size was selected for meta-analysis. The pooled results of the seven studies suggested that the P < 0.0001 of the Z-test was statistically significant, so low tumor differentiation was a risk factor for IMA root lymph node metastasis.
Meta-analysis of T staging for IMA root lymph node metastasis
Among the included literature, six studies reported that T staging was analyzed as a risk factor for IMA root lymph node metastasis. The results are shown in Figure 2H, where I2 = 0% and P = 0.79 in the Q test. There was no heterogeneity in the analysis of IMA root lymph node metastasis by T staging. Therefore, the fixed effects combined effect size can be selected for meta-analysis. The summary results of the six studies suggested that the P < 0.0001 of the Z-test was statistically significant. Therefore, T-stage T3 and T4 were risk factors for IMA root lymph node metastasis.
Publication offset analysis
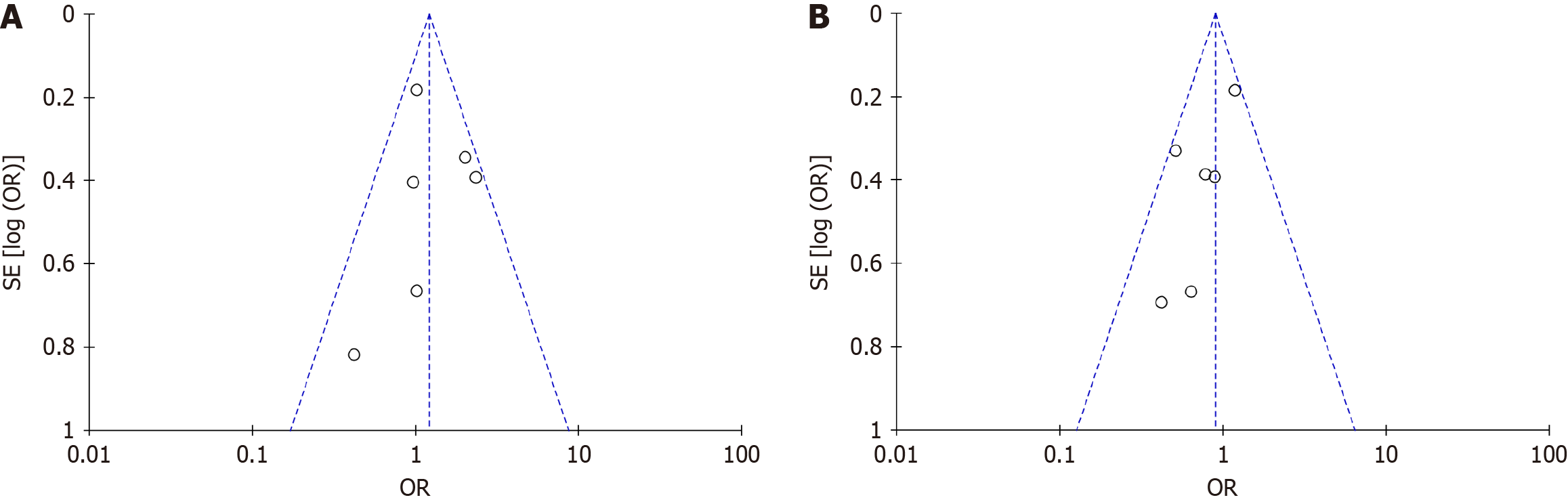
This study conducted a publication bias analysis on the included literature, resulting in a largely symmetrical funnel plot for each analysis outcome. The funnel chart made by the degree of tumor differentiation was used, for example, for analysis. The pattern on the funnel chart was pretty even, which meant that most of the points in the data set used in the study were within the 95%CI. This meant that the data was stable and there wasn’t any major publication bias, so the results could be trusted (Figure 3).
Figure 3 A biased funnel plot analysis.
A: Biased funnel plot of gender as a risk factor; B: Biased funnel plot of age as a risk factor. OR: Odds ratio.
DISCUSSION
Lymph node metastasis is the most common and major metastasis pathway of CRC, and it is also an important indicator to judge the stage and prognosis of CRC[18]. The significance of lymph node metastasis around IMA for prognosis is not very clear, and the value of IMA root lymph node dissection is still controversial[19]. IMA root lymph node metastasis is thought to lead to poor survival outcomes, and many studies have reported that D3 lymph node dissection can reduce paraaortic recurrence and systemic metastasis and improve the prognosis[20-22]. However, studies suggest that the lymph node metastasis rate in the IMA region is low, and patients’ prognosis remains poor even after surgical resection, suggesting that the removal of IMA root lymph nodes holds little significance[23]. Therefore, it is important to review the existing literature and explore its comprehensive impact on patient outcomes. The purpose of this study was to evaluate the risk factors for IMA root lymph node metastasis in rectal cancer and to provide more reference for the selection of surgical methods for these patients.
Many domestic and foreign scholars have carried out in-depth studies on the clinical and pathological risk factors related to IMA root lymph node metastasis in rectal cancer, but the results of various studies are not exactly the same[24-26]. Studies have shown that preoperative neoadjuvant chemoradiotherapy for rectal cancer can reduce the incidence of IMA root lymph node metastasis[28-30]. For patients receiving neoadjuvant chemoradiotherapy before surgery, a high serum CEA level, low tumor differentiation, and rectal cancer with more than peritoneal recursion were risk factors for positive IMA root lymph nodes[31]. Another study found that preoperative CEA level, number of lymph node dissections, and T stage significantly influenced the positive status of lymph nodes at D3 stations in patients with stage III colon cancer[32]. Literature reports[33-35] from various countries indicate that IMA root lymph node metastasis, closely related to the physiological and anatomical structure of the rectum and the pathway of lymphatic reflux, is more likely to occur in high rectal cancer[36]. Specifically, late localization of the tumor was more common, and this study’s analysis results aligned with the literature[37-39]. Through a literature search and review, the risk factors affecting IMA root lymph node metastasis generally include: Gender, age, preoperative CEA level, tumor location, distance from the lower tumor margin to the anus, tumor size, pathological type of tumor, degree of tumor differentiation, nerve and vascular invasion, distant metastasis, tumor budding, T stage, and N stage were summarized[40]. The results of all studies were summarized because there were few reports on risk factors in some literature. A meta-analysis was performed on the 7 literatures (total number of cases: 3893) that were finally included and classified according to preoperative data, intraoperative data, and postoperative data, including patient gender, age, preoperative CEA level, tumor location, tumor size, tumor pathological type, tumor differentiation degree, and T stage, and to explore the effect of IMA root lymph node metastasis in rectal cancer[41].
CONCLUSION
In summary, the positive rate of IMA root lymph node metastasis was related to preoperative CEA level, tumor location, tumor size, tumor pathological type, tumor differentiation degree, and T stage, and the results were similar to those in the literature reviewed. It is still controversial whether the third station lymph node dissection should be performed routinely after radical resection of rectal cancer, because the operation time may be prolonged and postoperative complications increased. The results of this study reflect some of the clinicopathological features that may lead to IMA root lymph node metastasis in rectal cancer, and provide evidence-based medical evidence for the selection of surgical procedures for IMA root lymph node dissection in rectal cancer. However, the sample size of this study is small, the types of studies included in the literature are relatively simple, retrospective analysis, and some of the literature quality is low. We expect to conduct relevant multi-center, multi-type studies with larger sample size, so as to provide more reference evidence for IMA root lymph node dissection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade C
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Anestiadou E, Greece S-Editor: Wang JJ L-Editor: A P-Editor: Zheng XM