Published online Jul 15, 2024. doi: 10.4251/wjgo.v16.i7.2915
Revised: May 16, 2024
Accepted: May 28, 2024
Published online: July 15, 2024
Processing time: 116 Days and 4.5 Hours
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal-derived tumors of the GI tract. They can occur throughout the GI tract, and the survival time of some patients can be improved by first-line targeted therapy with imatinib. However, there are some limitations with imatinib treatment. Immunotherapy for GIST has attracted much attention in recent years, and as one of the most abundant cells in the GIST microenvironment, M2 macrophages play an important role in disease progression. They have unique anti-inflammatory and pro-tumorigenic effects and are one target for immunotherapy. This review summarizes the connection between different factors and the programmed death receptor-1/programmed death ligand-1 pathway and M2 macrophages to reactivate or enhance anti-tumor immunity and improve imatinib efficacy, and to provide new ideas for GIST immunotherapy.
Core Tip: The place of imatinib in gastrointestinal stromal tumor (GIST) treatment is indisputable, but it has some limitations and is not accepted by all patients. In this review, we summarize the interaction between M2 macrophages and the programmed death receptor-1/programmed death ligand-1 pathway, which can improve the efficacy of imatinib by reactivating or enhancing the anti-tumor effect of the host immune system and provide new ideas for GIST immunotherapy.
- Citation: Wang XK, Yang X, Yao TH, Tao PX, Jia GJ, Sun DX, Yi L, Gu YH. Advances in immunotherapy of M2 macrophages and gastrointestinal stromal tumor. World J Gastrointest Oncol 2024; 16(7): 2915-2924
- URL: https://www.wjgnet.com/1948-5204/full/v16/i7/2915.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i7.2915
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of the GI tract[1]. The biological behavior of GIST ranges from benign to malignant, and the global incidence is 10-15 cases per million per year, and they are seen predominantly in middle-aged and older people, with a median age of onset in the 60 seconds, and an even sex distribution[2]. The most common site of occurrence is the stomach (60%-70%), followed by the small intestine (20%-25%), colon and rectum (5%) and esophagus (< 5%)[2,3]. Surgery remains the mainstay of curative treatment for GIST, but adjuvant therapies with targeted agents such as imatinib have become particularly important when metastasis occurs or when surgery is not possible due to large tumor size. Tumor immunotherapy is a rapidly developing and promising cancer treatment that harnesses the host's immune system to inactivate relevant tumor cells. The presence of large infiltration of immune cells into the GIST tumor tissue makes it possible to activate the anti-tumor effects of the host immune system by stimulating these immune cells. This may become a new strategy to enhance GIST monotherapy with imatinib. As the main immunosuppressive cells in the tumor microenvironment (TME), M2 macrophages mainly suppress immune responses by secreting chemokines and cytokines. They have important implications for tumor progression. Therefore, in this review, we explore and discuss the roles of M2 macrophages in GIST tumor progression and examine the current application of combined immunotherapy and imatinib targeting therapy to inhibit M2 macrophages. This discussion aims to provide therapeutic ideas and targets for improving the immunosuppressive GIST microenvironment, thereby enhancing the efficacy of immunotherapy and improving patient prognosis.
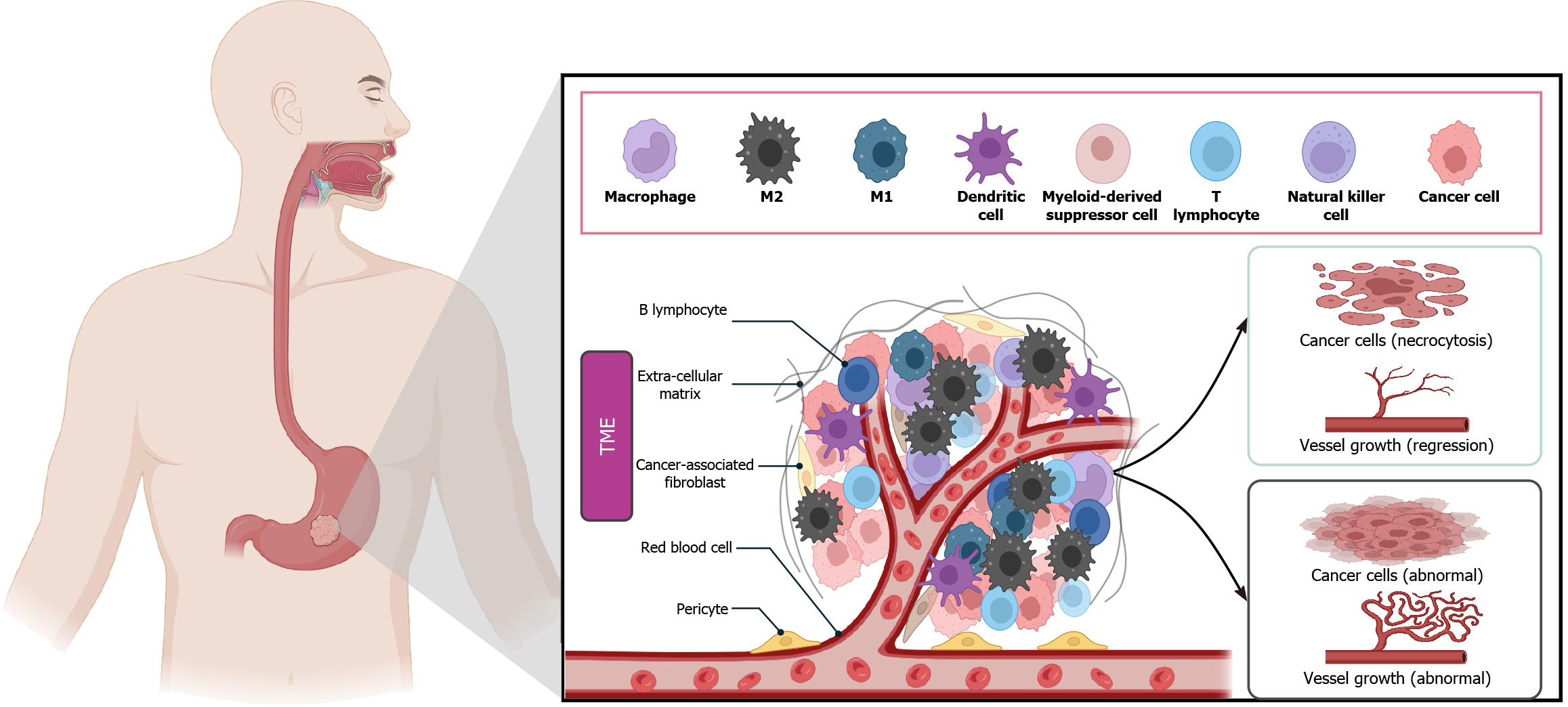
The TME is of significant consequence in the context of human tumor progression. Macrophages are one of the most abundant normal cells in the TME and are a “double-edged sword”, as they mediate cytotoxicity and phagocytosis, causing vascular damage and tumor cell necrosis. They also promote tumor cell survival and proliferation, angiogenesis, and inhibit innate and adaptive immune responses through a variety of mechanisms to promote tumor progression and metastasis[4] (Figure 1). The polarization of macrophages in tumors is a complex process due to the complexity of the TME. Macrophages will only transition into tumor-associated macrophages (TAMs) under specific environmental conditions[5]. Inhibitory cytokines released by tumor cells in the TME or receptors from tumor-infiltrating macrophages in contact with immune checkpoint proteins on tumor cells cause macrophages to differentiate into M2-type macrophages[6]. TAMs can help tumor cells evade immune surveillance and clearance by establishing an immunosuppressive microenvironment[7]. For example, in non-small cell lung cancer (NSCLC), the brain is a common site of metastasis. The TME in brain metastasis is suppressed to decrease CD4 T cells and M1 TAMs and increase M2 TAM infiltration[8]. These results indicate that the density of TAMs is related to the poor prognosis of malignant tumors[6], in which M2 TAMs predominate.
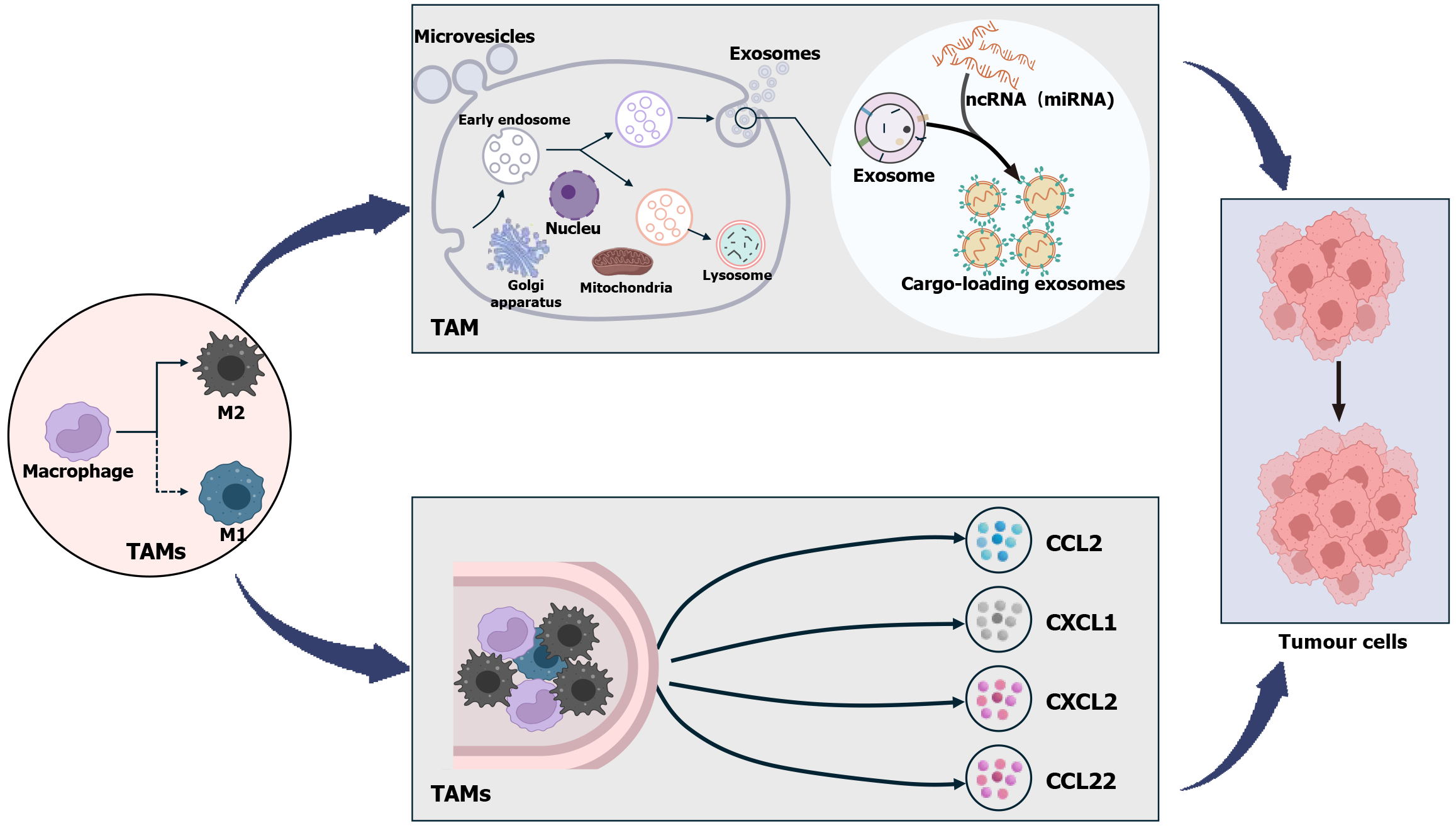
The relationship between TAMs and malignant tumors has become increasingly obvious. TAMs directly impact relevant cancer cells by transferring some noncoding RNA and other substances via exosomes. For example, TAM-derived miR-21-5p and miR-155-5p enter colorectal cancer cells, promoting colorectal cancer cell motility and cancer invasion and migration[9]. By evading CD8 cells, LINC01232 can promote immune escape of glioma cells[10], miR-23a-3p enhances hepatocellular carcinoma (HCC) metastasis by promoting epithelial-mesenchymal transition (EMT), angiogenesis, and vascular permeability[11]. Finally, the lncRNA ADPGK-AS1 alters the phenotypic status of TAMs to promote lung cancer progression[12]. TAMs produce a variety of cytokines that influence tumor cells[13]. TAM-derived IL-6 increases the expression of CC ligand 2 (CCL2) and EIF4A3 in tumor cells, which results in increased proliferation and invasion of breast cancer cells[14]; TAMs also promote HCC cell resistance to sorafenib via chemokine CXC ligand (CXCL) 1 and CXCL2[15]. In addition, TAMs can also indirectly affect relevant tumor cells by regulating TME using other immune cells. Among them, high levels of chemokine CCL22 secreted by TAMs can inhibit T cell proliferation and activity, thus promoting tumor cell growth[16] (Figure 2). Therefore, TAM is a crucial therapeutic target for a range of cancers, with implications for tumor diagnosis and prognosis[17]. GIST tissues are mainly infiltrated by a large number of macrophages and T cells[18], where macrophages are predominantly M2 TAMs and T cells are predominantly CD3 and CD8 T cells and a small number of forkhead box (Fox) p3 T regulatory (Treg) cells[19]. It is clear that the presence of M2 TAMs is crucial in influencing the course of GIST.
It is well established that inflammation plays a significant role in the development of tumors and is regarded as one of the defining characteristics of cancer. TAMs are among the most prevalent inflammatory cells in the TME of GIST, and it is important to objectively evaluate their role in the disease. TAMs are activated and polarized into two main subpopulations under specific circumstances: M1 TAMs and M2 TAMs, both of which play different roles in the TME[20]. M1 TAMs can be induced by microbial products or proinflammatory cytokines, and in terms of cellular functions, M1 TAMs have proinflammatory and anti-tumorigenic roles. M1 TAMs exert their anti-tumorigenic activity by phagocytosis of tumor cells, by exposure of tumor cell antigens and their presentation to T cells, as well as by the production of proinflammatory cytokines [interleukin (IL)-1β, IL-18, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α]. In contrast, the inflammatory factors IL-4 and IL-13 bind to the IL-4Rα receptor and induce M2 TAMs, but unlike the M1 phenotype, M2 TAMs tends to have pro-tumorigenic roles in anti-inflammation, recruitment of Treg cells and induction of angiogenesis[7,17]. Despite the homology between the M1 and M2 phenotypes, they are distinct in their functional roles, yet closely interrelated and interdependent.
In the early tumor stage, M1 TAMs initiate an inflammatory response together with other immune cells, while in the advanced tumor stage, M1 TAMs are transformed into M2 TAMs[21]. This also implies that the inflammatory response can respond to different tumor stages, whereas a large number of M2 TAMs suppresses the inflammatory response and also largely predict tumor progression. Metastatic GIST have approximately twice the number of macrophages as primary GIST, and high levels of M2 TAMs induce the emergence of a large number of Treg cells[19], which affect tumor progression by suppressing the local cytotoxic immune response. A low CD8 T/Foxp3 Treg cell ratio has previously been found to be a significant independent poor prognostic factor in cervical cancer[22]. A low ratio of cytotoxic T cells/Treg cells suggests the presence of an immunosuppressive microenvironment within the tumor, whereas the ratio of the two in GIST is lower than that of cervical cancer, suggesting that there is a more potent microenvironment of immunosuppression[19]. Inflammation and the hypoxic environment of tumor tissues lead to aggregation of a large number of macrophages, and hypoxia is the main driver of tumor angiogenesis. A large number of TAMs recruited by chemokines, such as CCL2, CCL5, and CXCL12, aggregate in peritumoral blood vessels, which are mainly responsible for tumor angiogenesis[23,24], and in this case, the TAMs are more skewed towards M2 phenotype[25]. The inflammatory state is higher at the primary site of metastatic GIST compared to nonmetastatic GIST[18]. This may be because high levels of proinflammatory factors in inflammation contribute to the generation of M2 TAMs, resulting in a high expression level of M2 TAMs, which in turn favor metastasis and largely predicts that the associated tumors are progressing or have progressed and deteriorated. However, the presence of inflammation does not only predict deterioration, but its presence also has a beneficial side for the patient, such as the chronic inflammatory response that occurs in the tertiary lymphoid structures (TLSs), including tumors. TLSs are common in localized GIST and seem to be positively correlated with better OS and lower risk of recurrence[26].
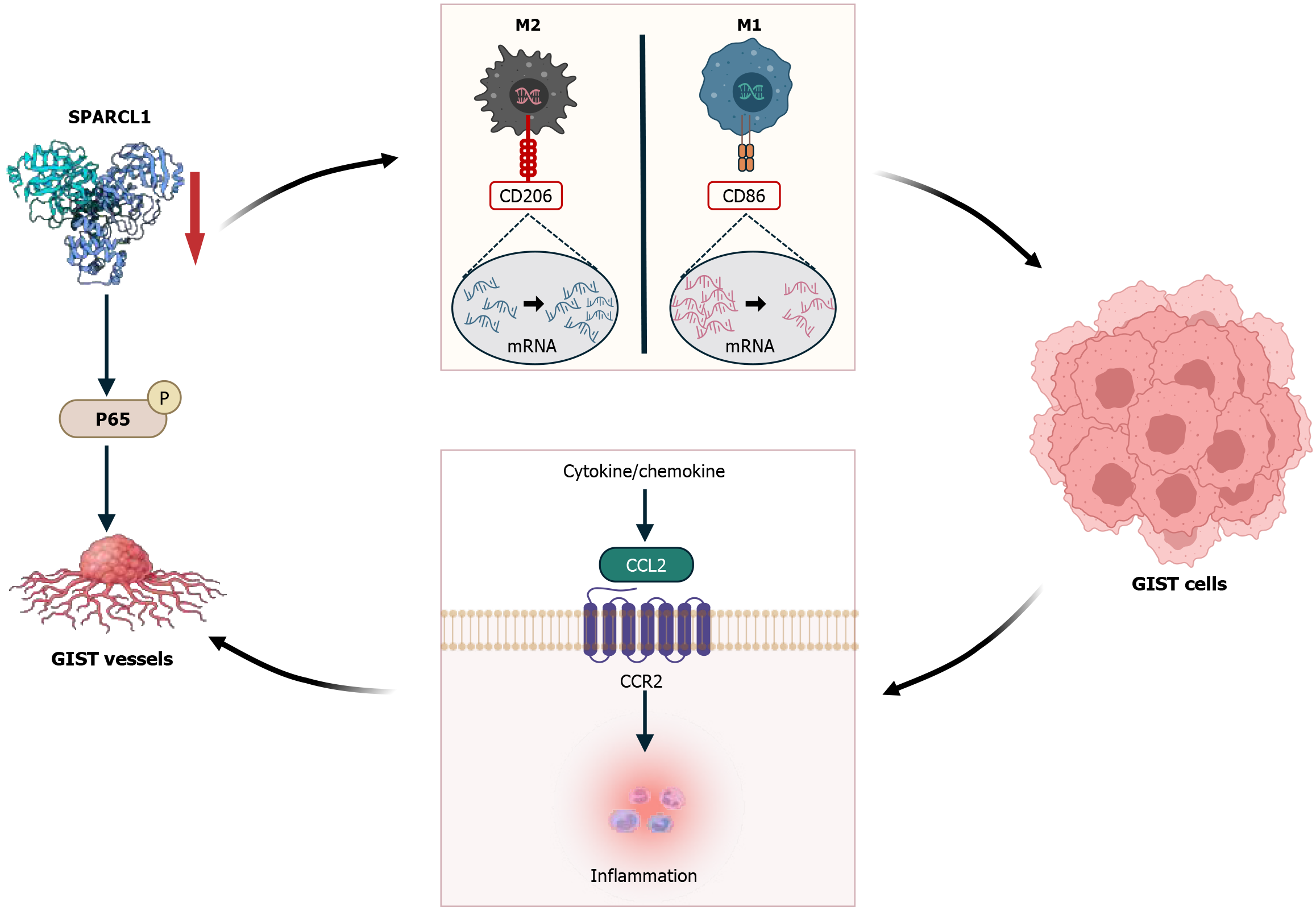
As the main immunosuppressive cells in the TME, M2 TAMs primarily suppress immune responses by secreting chemokines and cytokines. For example, inflammatory chemokines such as CCL2, CXCL2, and CCL3 are highly expressed in GIST[18]. Activation of the CCL2-CCR2 axis promotes TAM recruitment into the TME[27]. It was recently demonstrated that the invasiveness of breast cancer co-expressing epidermal growth factor receptor (EGFR) and human EGFR 2 (HER2) was associated with the CCL2-induced recruitment of TAMs[28]. Similarly, CCL2 activation and upregulation in GIST lead to TAM recruitment, which in turn affects the TME and promotes tumor progression and metastasis[29]. CXCL2, a chemokine secreted by M2 TAMs, promotes invasion, migration, and EMT of tumor cells in GIST. Animal experiments have demonstrated that CXCL2 promotes hepatic metastasis of GIST in vivo[30]. The matrix protein secreted protein acidic and rich in cysteine 1 (SPARCL1) is typically downregulated in most cancers, and its expression in GIST is no exception. A high expression level in the reactive vasculature of both benign and inflammatory lesions has been shown to be immunoregulatory and pro-immune[31]. In other malignant lesions, SPARCL1 expression is opposite; for example, SPARCL1 is associated with angiogenesis in GIST by accelerating p65 phosphorylation and nuclear translocation, but SPARCL1 expression is negatively correlated with microvessel density in GIST[32]. SPARCL1 is closely associated with neovascularization in GIST and involved in GIST cell progression. Inhibition of SPARCL1 increases mRNA expression of the M2 TAM polarization marker CD206 and decreases mRNA expression of M1 TAM polarization marker CD86 in GIST cells. SPARCL1 knockdown markedly increases the migratory and invasive ability of GIST-882 cells[32]. This suggests that SPARCL1 alters the TME in which GIST cells survive by affecting TAM polarization and recruitment and promoting CCL2 release from GIST cells to promote tumor progression. This shows that high SPARCL1 expression in tumor tissues is significant for GIST (Figure 3).
Immune checkpoint inhibitors (ICIs) are a promising form of immunotherapy that are advancing cancer treatment by blocking the signals that allow cancer cells to evade detection by the immune system. Programmed death receptor-1/programmed death ligand-1 (PD-1/PD-L1) blockers are one of the most widely investigated therapies to date. They have gained good results in many cancers, such as non-small-cell lung cancer[33], advanced renal cell carcinoma[34], advanced melanoma[35], and others. Among them, PD-1 is an inhibitory co-receptor mainly expressed on T cells, and PD-1 can bind to PD-L1 on tumor cells to effectively inhibit T-cell activity, thus reducing T-cell recognition of tumor cells and allowing tumor cells to evade immune surveillance[36]. For example, TAMs are increased in patients with multiple myeloma, where they inhibit cytotoxic-T-lymphocyte function through the PD-1/PD-L1 signaling pathway and are involved in the occurrence of immune escape of myeloma cells[37]. It is important to emphasize that PD-1 expressed alone inhibits the activity of antitumor CD8 T cells, promotes the polarization of M2 TAMs, and downregulates the activity of antigen-specific T cells. M2 PD-1 TAMs increase with time and tumor stage, and the phagocytosis of tumor cells is reduced, which contributes to tumor growth[38]. PD-L1 T cells infiltrating the tumor act on PD-1 macrophages within the tumor and induce polarization of M2 TAMs. The interaction between PD-L1 and PD-1 leads to an increase in protumor immunosuppressive factors, such as IL-10, IL-17, and transforming growth factor (TGF)-β, as well as inhibition of CD8 T cell activation, expansion, and cytotoxicity[36-39]. PD-1/PD-L1 expression alone or in combination induces M2 TAM polarization to weaken tumor-specific immunity and promote tumor progression.
High PD-1/PD-L1 expression is associated with M2 TAM polarization, and treatment with anti-PD-1/PD-L1 antibodies reverses the M1/M2 phenotypic switch and elicits the antitumor activity of TAMs with a locally proinflammatory M1-like phenotype[40]. There is a strong link between macrophage polarization and CD8 T cells, and M2 TAMs promote CD8 T-cell depletion, whereas M1 TAMs restore CD8 T-cell migration and infiltration. Thus, TAM polarization plays a key role in PD-1/PD-L1 blockade resistance by inducing T-cell rejection[40]. M2 TAMs not only secrete certain biologically active molecules by affecting the layout of CD8 T cells in tumor tissues, but also interfere with the activation and function of CD8 T cells through the indoleamine 2,3-dioxygenase (IDO) derived from them, leading to T cell exhaustion and PD-1/PD-L1 blockade resistance, allowing immune escape of tumor cells to further promote tumor progression[40]. IDO is a rate-limiting enzyme that catabolizes tryptophan to kynurenine and also induces immunosuppression or participates in the immune escape of tumor cells through high expression levels in the TME[41], thereby promoting tumor cell survival and growth. It has been found that IDO is expressed in PDGFRA-mutant GIST and is accompanied by a significant increase in the number of CD4 cells[42]. In a KitV558Δ/+ mouse model with confirmed tumors, IDO inhibitors enhance the anti-tumor effects of imatinib and anti-PD1 antibodies by activating CD8 T cells and inducing Treg cell apoptosis[43].
In GIST, PD-1 is expressed at low levels on T cells, whereas PD-L1 is predominantly found in GIST cells[43]. A previous study showed that among all sarcoma tissue specimens, GIST had the highest PD-L1 expression[44]. Patients with active GIST with plasma PD-L1 concentrations above a critical value tend to have a worse prognosis, and plasma PD-L1 has the potential to serve as a prognostic biomarker for GIST patients[45]. PD-L1 expression is linked to unfavorable prognostic features, including tumor size, proliferation index, high-risk GIST, and drug resistance. However, there is no association with RFS, metastasis, and OS[18], demonstrating the importance of PD-L1 expression in GIST. As far as GIST patients are concerned, the poor efficacy exerted by ICIs in clinical trials and the lack of significant synergism between ICIs and tyrosine kinase inhibitors (TKIs) may be why their clinical application has not yet been realized. It has been found that the limited efficacy of PD-1/PD-L inhibitors in GIST may be related to the immunosuppressive TME resulting from activation of TAMs and the IDO pathway[46]. Nevertheless, it cannot be concluded that ICIs are ineffective in the treatment of GIST; for example, it seems that patients with PDGFRA D842V mutation and high expression of PD-L1 in GIST are more likely to benefit from ICIs and should be prioritized[18]. Some studies have shown that the number of CD8 T cells is positively correlated with the expression of PD-L1. In addition, CD8 T-cell numbers are higher in WT non-gastric GIST, suggesting that these patients may benefit more from PD-1/PD-L1 inhibitors[47]. In addition, since patients with advanced disease need to be treated with long-term imatinib and multiline TKIs, their anti-tumor immunity is suppressed and weakened, and ICIs should be administered as early in the disease as possible[18]. The combination of anti-PD-1/PD-L1 antibody and imatinib has been found to enhance the anti-tumor effect of imatinib in a mouse GIST model[43]. Therefore, it is important to explore more reliable markers of ICIs, in the hope that they can be used in combination with TKIs in sensitive patients to realize a new era of precision immunotherapy.
Imatinib (STI571) was first used clinically in 2002 and achieved significant efficacy in the treatment of a patient with metastatic GI mesenchymal stromal tumor[48]. Since then, GIST treatment with imatinib has entered a new era. Imatinib works by blocking the KIT pathway to inhibit GIST tumor cell proliferation and survival. It exerts anti-tumor activity by directly killing GIST tumor cells and indirectly affecting immune cells, especially in patients with large, difficult-to-resect tumors, locally advanced tumors, and post-operative adjuvant therapy[49]. The efficacy of imatinib as a TKI depends on the exons involved in KIT and PDGFRA mutations. About 14% of GIST patients initially develop resistance to imatinib, and about 50% develop resistance after 2 years of treatment[50]. Therefore, it is crucial to analyze the type of mutation exhibited by GIST before choosing to treat with imatinib. Patients with KIT exon 11 mutations are sensitive to the standard dose of imatinib (400 mg/d); for exon 9 mutations, the results tend to be suboptimal at the standard dose of imatinib; and PDGFRA exon 18, D842V, shows high resistance to imatinib[51]. A retrospective cohort study in the Netherlands found that patients with GIST showed an increase in 1-year net survival, 5-year net survival, and median overall survival[52], which shows that the widespread use of imatinib has achieved significant efficacy.
Imatinib has been shown to benefit most sensitized patients in the treatment of GIST. It is important to note that imatinib treatment can be a “double-edged sword” for patients. On the one hand, short-term administration enhances the infiltration and activity of CD8 T cells, dendritic cells (DCs), and natural killer cells, as well as the secretion of IFN-γ. It also decreases the infiltration of Treg cells and expression of PD-L1, thus providing an immunological benefit. On the other hand, prolonged administration of imatinib polarizes intratumoral M2 TAMs and decreases expression of CD8 T cells, DCs, and MHC-I, thereby weakening the antitumor response[18]. TAMs in untreated mouse models and imatinib-resistant GIST are functionally more similar to M1 TAMs, whereas TAMs in sensitive GIST are functionally more similar to M2 TAMs. This may be because imatinib polarizes TAMs toward M2 phenotype by inhibiting oncogene activity and is related to TAM phagocytosis of imatinib-induced apoptotic cells, which promotes M2 TAMs[19]. GIST sensitive to imatinib treatment promotes more TAMs to polarize towards the M2 rather than the M1 phenotype in the long term, which is often detrimental to patient prognosis. Most patients on imatinib treatment show IDO overexpression, and the large level of metabolites produced through IDO involvement also makes it more likely for macrophages to be polarized towards the M2 phenotype, which is a major factor in immune escape[26]. PD-L1 can induce TAM polarization to the M2 phenotype, but imatinib can reduce immune escape by decreasing PD-L1 expression on tumor cells[26], which may be due to the reduced PD-L1-induced expression of M2 TAMs, thus limiting tumor progression.
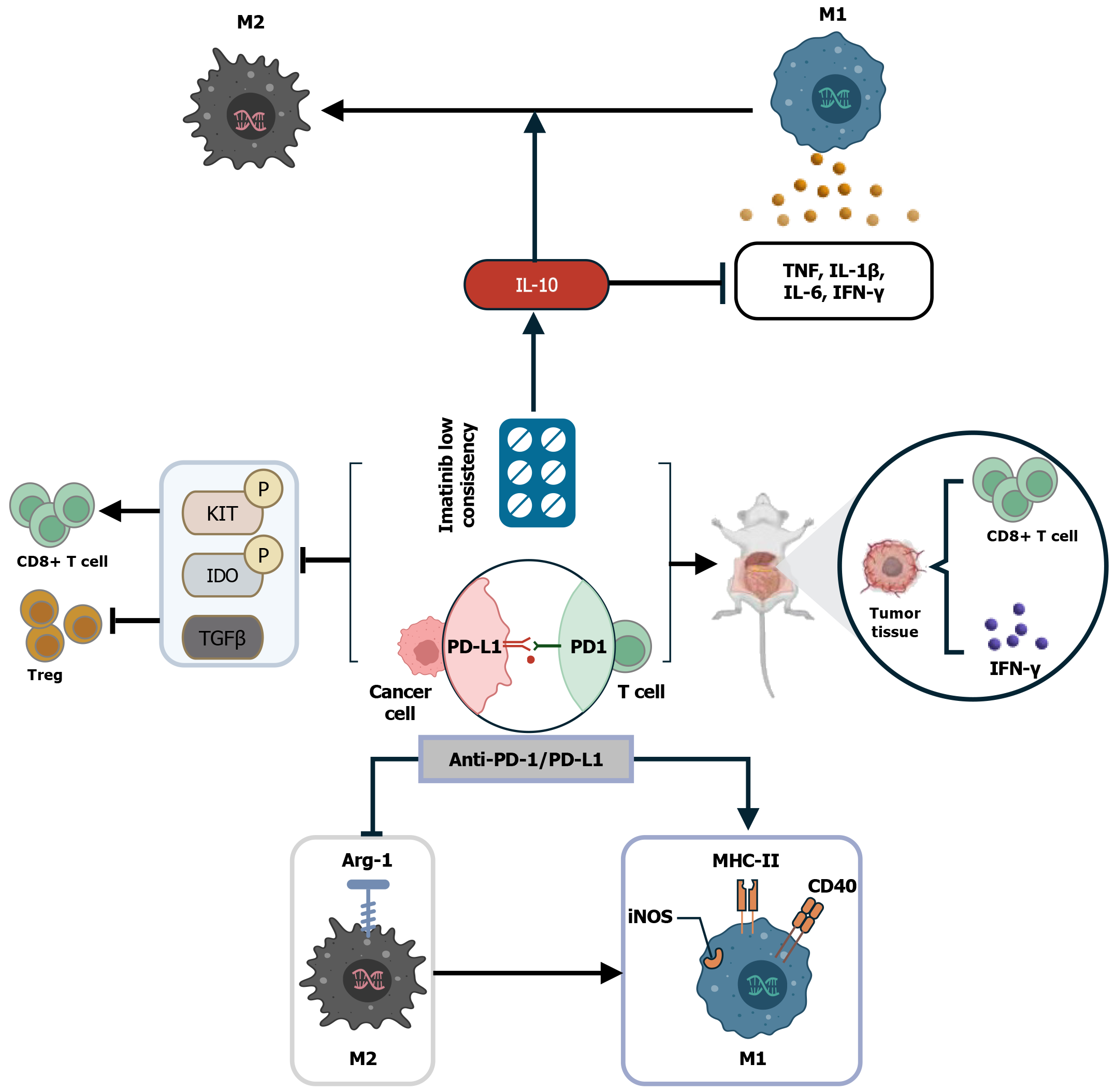
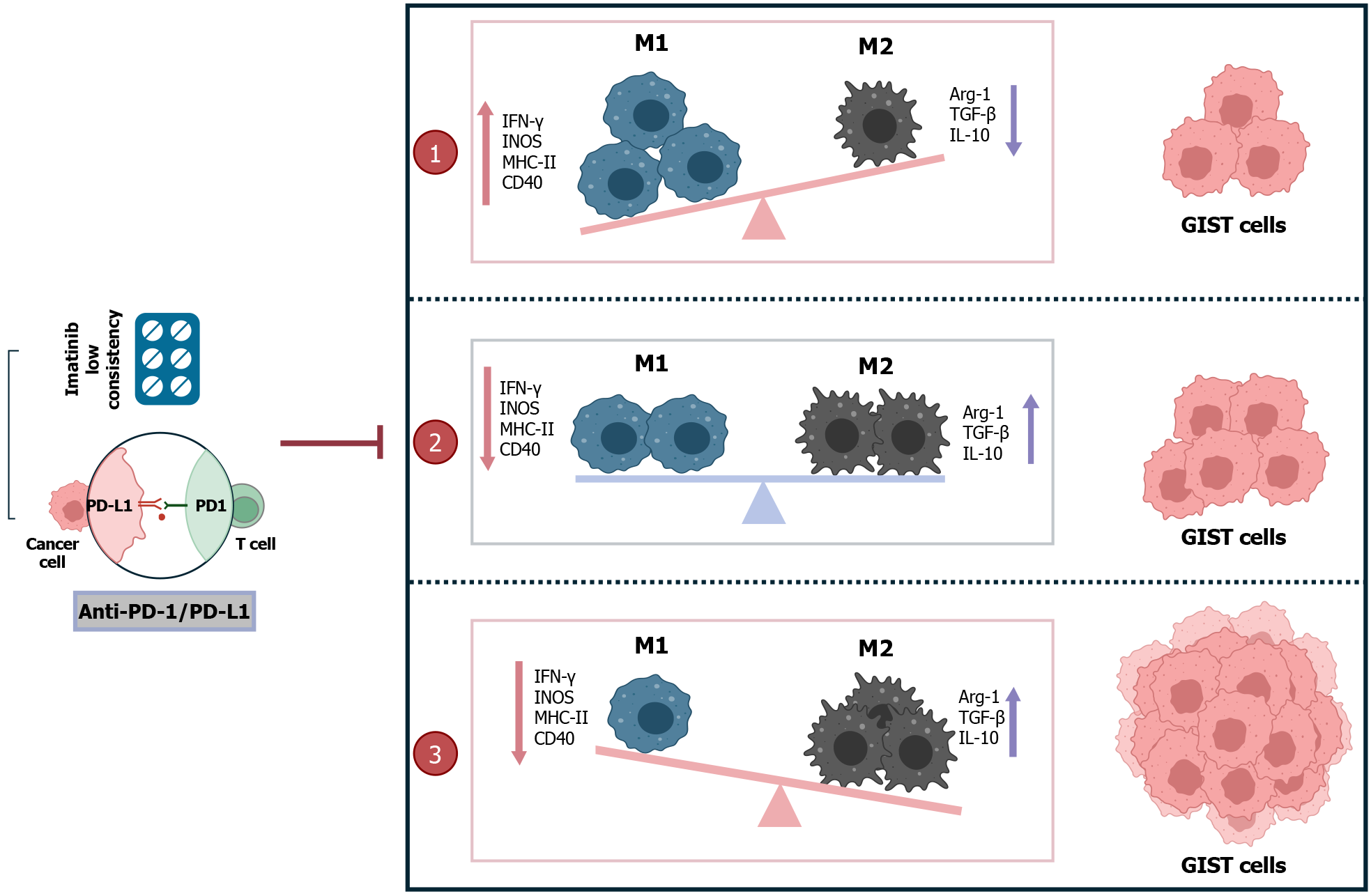
M2 TAMs in GIST recruit large numbers of Treg cells, independent of CD8 T cells and imatinib treatment[19,43]. It has been shown that low imatinib concentrations induce the secretion of the anti-inflammatory cytokine IL-10 by M1 TAMs. This skews the functional expression of M2 TAMs and allows for a significant increase in the number of M2 TAMs and Treg cells to promote immune evasion[19]. The anti-inflammatory factor IL-10 promotes the transition from M1 to M2 TAMs through positive feedback, but also inhibits the synthesis and expression of proinflammatory cytokines, including IL-1β, IL-6, TNF, and IFN-γ[54]. However, in an in vivo mouse model, combination of anti-PD-1/PD-L1 with imatinib for 1 wk resulted in increased CD8 T cell proliferation and production of inflammatory cytokines (IFN-γ) in tumor tissues compared to treatment with imatinib alone[43]. Anti-PD-L1 treatment reduces the levels of TAM markers such as Arg-1 and increases TAM markers such as iNOS, MHC II, and CD40, and enhances polarization of macrophages towards a proinflammatory phenotype and inhibition of polarization into anti-inflammatory and immunosuppressive macrophages that support tumor growth[13]. In GIST, increased IFN-γ inhibits the polarization of M1 TAMs toward M2, which in turn reduces CD8 T-cell depletion as a means to inhibit tumor progression. Anti-PD-1 and anti-PD-L1 altered tumor weight only in combination with imatinib, and reduced phosphorylated KIT, phosphorylated IDO and TGF-β, whereas IDO inhibition enhanced the anti-tumor effects of anti-PD-1[43]. In a mouse model of lung cancer, the combination of anti-PD-1/PD-L1 with an anti-angiogenic drug (apatinib) improved TME, which enhanced the anti-tumor effects of PD-1/PD-L1 inhibitors by inducing polarization of M2 to M1 TAMs[55], which was validated in a later study of brain metastasis in lung cancer[8]. In contrast to imatinib alone, the combination of PD-1/PD-L1 inhibitor and imatinib can not only promote the production of CD8 T cells and IFN-γ, but also promote the depletion of Treg cells to inhibit tumor cells and inhibiting the polarization of M2-TAMs (Figure 4). Thus, the anti-tumor effects of imatinib and anti-PD1 antibody were enhanced, and the GIST progression and metastasis were further inhibited.
GIST is a potentially malignant tumor in the GI tract. Radical resection and TKIs remain the mainstay of treatment for localized and recurrent/metastatic GIST, respectively. Although it is well documented that adjuvant therapy with TKIs prolongs the survival of GIST patients, the singularity of the target makes them still limited, which is a major factor affecting disease progression. The infiltration of M2 TAMs has a severe immunosuppressive effect on the GIST microenvironment and is associated with a variety of substances that participate in the immune escape of tumor cells, thus exacerbating tumor growth and metastasis. Infiltration of M2 TAMs also plays an important role in tumor development and progression and is a potential target for immunotherapy. Low concentrations as well as prolonged imatinib treatment promote M2 TAM polarization, and treatment with ICIs alone fails to achieve clinically significant efficacy. However, the combination of the two in a mouse model inhibited tumor progression and suppressed M1 to M2 polarization, which in turn reduced M2 TAM infiltration in GIST, thereby inhibiting tumor progression. In conclusion, M2 TAMs have been identified as potential targets in the immune microenvironment of GIST. Combination therapy of the two can induce M2 TAMs to M1 conversion or inhibit M1 to M2 TAMs conversion, thereby reducing M2 TAMs infiltration in tumor tissues, enhancing imatinib efficacy while improving the host immune system (Figure 5). However, many experimental studies are needed to test whether this combination therapy can be truly applied in future clinical work, with a view to providing a theoretical basis for more effective therapeutic measures for GIST patients.
In recent years, immunotherapy in GIST has attracted much attention, and immunotherapy targeting M2 macrophages is expected to overcome the bottleneck of GIST targeted therapy to more effectively inhibit tumor progression.
| 1. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (33)] |
| 2. | Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 520] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 3. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1177] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 4. | Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21:799-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1029] [Article Influence: 343.0] [Reference Citation Analysis (0)] |
| 5. | Pęczek P, Gajda M, Rutkowski K, Fudalej M, Deptała A, Badowska-Kozakiewicz AM. Cancer-associated inflammation: pathophysiology and clinical significance. J Cancer Res Clin Oncol. 2023;149:2657-2672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 6. | Mishra AK, Banday S, Bharadwaj R, Ali A, Rashid R, Kulshreshtha A, Malonia SK. Macrophages as a Potential Immunotherapeutic Target in Solid Cancers. Vaccines (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Zhang Q, Sioud M. Tumor-Associated Macrophage Subsets: Shaping Polarization and Targeting. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 146] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 8. | Wang H, Liu F, Chen X, Zhao C, Li X, Zhou C, Hu J, Chu Q, Jiang T. Outcome differences between PD-1/PD-L1 inhibitors-based monotherapy and combination treatments in NSCLC with brain metastases. Exp Hematol Oncol. 2023;12:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | Lan J, Sun L, Xu F, Liu L, Hu F, Song D, Hou Z, Wu W, Luo X, Wang J, Yuan X, Hu J, Wang G. M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer Res. 2019;79:146-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 479] [Article Influence: 68.4] [Reference Citation Analysis (2)] |
| 10. | Li J, Wang K, Yang C, Zhu K, Jiang C, Wang M, Zhou Z, Tang N, Wang Q, Wang S, Shu P, Yuan H, Xiong Z, Li J, Liang T, Rao J, Wang X, Jiang X. Tumor-Associated Macrophage-Derived Exosomal LINC01232 Induces the Immune Escape in Glioma by Decreasing Surface MHC-I Expression. Adv Sci (Weinh). 2023;10:e2207067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 11. | Zhang C, Wei S, Dai S, Li X, Wang H, Zhang H, Sun G, Shan B, Zhao L. The NR_109/FUBP1/c-Myc axis regulates TAM polarization and remodels the tumor microenvironment to promote cancer development. J Immunother Cancer. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 12. | Karger A, Mansouri S, Leisegang MS, Weigert A, Günther S, Kuenne C, Wittig I, Zukunft S, Klatt S, Aliraj B, Klotz LV, Winter H, Mahavadi P, Fleming I, Ruppert C, Witte B, Alkoudmani I, Gattenlöhner S, Grimminger F, Seeger W, Pullamsetti SS, Savai R. ADPGK-AS1 Long noncoding RNA switches macrophage metabolic and phenotypic state to promote lung cancer growth. EMBO J. 2023;42:e111620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 13. | Zhang H, Liu L, Liu J, Dang P, Hu S, Yuan W, Sun Z, Liu Y, Wang C. Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer. 2023;22:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 181] [Reference Citation Analysis (0)] |
| 14. | Zhou B, Mo Z, Lai G, Chen X, Li R, Wu R, Zhu J, Zheng F. Targeting tumor exosomal circular RNA cSERPINE2 suppresses breast cancer progression by modulating MALT1-NF-κB-IL-6 axis of tumor-associated macrophages. J Exp Clin Cancer Res. 2023;42:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 15. | Wang HC, Haung LY, Wang CJ, Chao YJ, Hou YC, Yen CJ, Shan YS. Tumor-associated macrophages promote resistance of hepatocellular carcinoma cells against sorafenib by activating CXCR2 signaling. J Biomed Sci. 2022;29:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 16. | Yang YL, Yang F, Huang ZQ, Li YY, Shi HY, Sun Q, Ma Y, Wang Y, Zhang Y, Yang S, Zhao GR, Xu FH. T cells, NK cells, and tumor-associated macrophages in cancer immunotherapy and the current state of the art of drug delivery systems. Front Immunol. 2023;14:1199173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 17. | Chen S, Saeed AFUH, Liu Q, Jiang Q, Xu H, Xiao GG, Rao L, Duo Y. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8:207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 762] [Reference Citation Analysis (0)] |
| 18. | Li B, Chen H, Yang S, Chen F, Xu L, Li Y, Li M, Zhu C, Shao F, Zhang X, Deng C, Zeng L, He Y, Zhang C. Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers. Mol Cancer. 2023;22:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 19. | van Dongen M, Savage ND, Jordanova ES, Briaire-de Bruijn IH, Walburg KV, Ottenhoff TH, Hogendoorn PC, van der Burg SH, Gelderblom H, van Hall T. Anti-inflammatory M2 type macrophages characterize metastasized and tyrosine kinase inhibitor-treated gastrointestinal stromal tumors. Int J Cancer. 2010;127:899-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Aehnlich P, Powell RM, Peeters MJW, Rahbech A, Thor Straten P. TAM Receptor Inhibition-Implications for Cancer and the Immune System. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 4082] [Article Influence: 177.5] [Reference Citation Analysis (0)] |
| 22. | Jordanova ES, Gorter A, Ayachi O, Prins F, Durrant LG, Kenter GG, van der Burg SH, Fleuren GJ. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clin Cancer Res. 2008;14:2028-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Liu M, Liu L, Song Y, Li W, Xu L. Targeting macrophages: a novel treatment strategy in solid tumors. J Transl Med. 2022;20:586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 24. | Li M, Yang Y, Xiong L, Jiang P, Wang J, Li C. Metabolism, metabolites, and macrophages in cancer. J Hematol Oncol. 2023;16:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 179] [Reference Citation Analysis (0)] |
| 25. | Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126:3672-3679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 409] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 26. | Roulleaux Dugage M, Jones RL, Trent J, Champiat S, Dumont S. Beyond the Driver Mutation: Immunotherapies in Gastrointestinal Stromal Tumors. Front Immunol. 2021;12:715727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Li F, Kitajima S, Kohno S, Yoshida A, Tange S, Sasaki S, Okada N, Nishimoto Y, Muranaka H, Nagatani N, Suzuki M, Masuda S, Thai TC, Nishiuchi T, Tanaka T, Barbie DA, Mukaida N, Takahashi C. Retinoblastoma Inactivation Induces a Protumoral Microenvironment via Enhanced CCL2 Secretion. Cancer Res. 2019;79:3903-3915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 28. | You D, Kim H, Jeong Y, Yoon SY, Lo E, Kim S, Lee JE. Tumorigenicity of EGFR- and/or HER2-Positive Breast Cancers Is Mediated by Recruitment of Tumor-Associated Macrophages. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Mu J, Sun P, Ma Z, Sun P. BRD4 promotes tumor progression and NF-κB/CCL2-dependent tumor-associated macrophage recruitment in GIST. Cell Death Dis. 2019;10:935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Cai H, Chen Y, Chen X, Sun W, Li Y. Tumor-associated macrophages mediate gastrointestinal stromal tumor cell metastasis through CXCL2/CXCR2. Cell Immunol. 2023;384:104642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 31. | Esposito I, Kayed H, Keleg S, Giese T, Sage EH, Schirmacher P, Friess H, Kleeff J. Tumor-suppressor function of SPARC-like protein 1/Hevin in pancreatic cancer. Neoplasia. 2007;9:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Shen C, Han L, Liu B, Zhang G, Cai Z, Yin X, Yin Y, Chen Z, Zhang B. The KDM6A-SPARCL1 axis blocks metastasis and regulates the tumour microenvironment of gastrointestinal stromal tumours by inhibiting the nuclear translocation of p65. Br J Cancer. 2022;126:1457-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6945] [Cited by in RCA: 7518] [Article Influence: 751.8] [Reference Citation Analysis (0)] |
| 34. | Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4375] [Cited by in RCA: 4594] [Article Influence: 459.4] [Reference Citation Analysis (0)] |
| 35. | Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A; KEYNOTE-006 investigators. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4026] [Cited by in RCA: 4473] [Article Influence: 447.3] [Reference Citation Analysis (1)] |
| 36. | Long Y, Yu X, Chen R, Tong Y, Gong L. Noncanonical PD-1/PD-L1 Axis in Relation to the Efficacy of Anti-PD Therapy. Front Immunol. 2022;13:910704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Zhang J, Liu Z, Cao P, Wang H, Liu H, Hua L, Xue H, Fu R. Tumor-associated macrophages regulate the function of cytotoxic T lymphocyte through PD-1/PD-L1 pathway in multiple myeloma. Cancer Med. 2022;11:4838-4848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 38. | Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, Ring AM, Connolly AJ, Weissman IL. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1465] [Cited by in RCA: 1640] [Article Influence: 205.0] [Reference Citation Analysis (0)] |
| 39. | Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, Wu K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 566] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 40. | Li W, Wu F, Zhao S, Shi P, Wang S, Cui D. Correlation between PD-1/PD-L1 expression and polarization in tumor-associated macrophages: A key player in tumor immunotherapy. Cytokine Growth Factor Rev. 2022;67:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 41. | Fujiwara Y, Kato S, Nesline MK, Conroy JM, DePietro P, Pabla S, Kurzrock R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat Rev. 2022;110:102461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 166] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 42. | Sun X, Sun J, Yuan W, Gao X, Fu M, Xue A, Li H, Shu P, Fang Y, Hou Y, Shen K, Sun Y, Qin J, Qin X. Immune Cell Infiltration and the Expression of PD-1 and PD-L1 in Primary PDGFRA-Mutant Gastrointestinal Stromal Tumors. J Gastrointest Surg. 2021;25:2091-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Seifert AM, Zeng S, Zhang JQ, Kim TS, Cohen NA, Beckman MJ, Medina BD, Maltbaek JH, Loo JK, Crawley MH, Rossi F, Besmer P, Antonescu CR, DeMatteo RP. PD-1/PD-L1 Blockade Enhances T-cell Activity and Antitumor Efficacy of Imatinib in Gastrointestinal Stromal Tumors. Clin Cancer Res. 2017;23:454-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 44. | D'Angelo SP, Shoushtari AN, Agaram NP, Kuk D, Qin LX, Carvajal RD, Dickson MA, Gounder M, Keohan ML, Schwartz GK, Tap WD. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol. 2015;46:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 240] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 45. | Brinch CM, Hogdall E, Junker N, Moeller HJ, Sandfeld-Paulsen B, de Heer P, Penninga L, Rossen PB, Krarup-Hansen A, Aggerholm-Pedersen N. The Prognostic Value of Plasma Programmed Death Protein-1 (PD-1) and Programmed Death-Ligand 1 (PD-L1) in Patients with Gastrointestinal Stromal Tumor. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Toulmonde M, Penel N, Adam J, Chevreau C, Blay JY, Le Cesne A, Bompas E, Piperno-Neumann S, Cousin S, Grellety T, Ryckewaert T, Bessede A, Ghiringhelli F, Pulido M, Italiano A. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 320] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 47. | Sun X, Shu P, Fang Y, Yuan W, Zhang Q, Sun J, Fu M, Xue A, Gao X, Shen K, Hou Y, Sun Y, Qin J, Qin X. Clinical and Prognostic Significance of Tumor-Infiltrating CD8+ T Cells and PD-L1 Expression in Primary Gastrointestinal Stromal Tumors. Front Oncol. 2021;11:789915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, Demetri GD. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1324] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 49. | Everling EM, Marchet D, DE-Antoni NM, Isbert BBM, Alves GV, Grezzana-Filho TJM. GASTROINTESTINAL STROMAL TUMOR: OUTCOMES OF THE PAST DECADE IN A REFERENCE INSTITUTION IN SOUTHERN BRAZIL. Arq Bras Cir Dig. 2022;35:e1658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 50. | Hu X, Wang Z, Su P, Zhang Q, Kou Y. Advances in the research of the mechanism of secondary resistance to imatinib in gastrointestinal stromal tumors. Front Oncol. 2022;12:933248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 51. | Huang WK, Wu CE, Wang SY, Chang CF, Chou WC, Chen JS, Yeh CN. Systemic Therapy for Gastrointestinal Stromal Tumor: Current Standards and Emerging Challenges. Curr Treat Options Oncol. 2022;23:1303-1319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 52. | Luyendijk M, Visser O, Blommestein HM, de Hingh IHJT, Hoebers FJP, Jager A, Sonke GS, de Vries EGE, Uyl-de Groot CA, Siesling S. Changes in survival in de novo metastatic cancer in an era of new medicines. J Natl Cancer Inst. 2023;115:628-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 53. | Cavnar MJ, Zeng S, Kim TS, Sorenson EC, Ocuin LM, Balachandran VP, Seifert AM, Greer JB, Popow R, Crawley MH, Cohen NA, Green BL, Rossi F, Besmer P, Antonescu CR, DeMatteo RP. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. J Exp Med. 2013;210:2873-2886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 54. | Zheng X, Yu X, Wang C, Liu Y, Jia M, Lei F, Tian J, Li C. Targeted co-delivery biomimetic nanoparticles reverse macrophage polarization for enhanced rheumatoid arthritis therapy. Drug Deliv. 2022;29:1025-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 55. | Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao C, Jia Y, Shi J, Zhang L, Liu X, Qiao M, Chen X, Su C, Yu H, Zhou C, Zhang J, Camidge DR, Hirsch FR. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol Res. 2019;7:630-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |