Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2804
Revised: April 14, 2024
Accepted: April 28, 2024
Published online: June 15, 2024
Processing time: 146 Days and 5 Hours
Non-invasive differential diagnosis between hepatocellular carcinoma (HCC) and other liver cancer (i.e. cholangiocarcinoma or metastasis) is highly challenging and definitive diagnosis still relies on histological exam. The patterns of enhancement and wash-out of liver nodules can be used to stratify the risk of malignancy only in cirrhotic patients and HCC frequently shows atypical features. Dynamic contrast-enhanced ultrasound (DCEUS) with standardized software could help to overcome these obstacles, providing functional and quantitative parameters and potentially improving accuracy in the evaluation of tumor perfusion.
To explore clinical evidence regarding the application of DCEUS in the differential diagnosis of liver nodules.
A comprehensive literature search of clinical studies was performed to identify the parameters of DCEUS that could relate to histological diagnosis. In accordance with the study protocol, a qualitative and quantitative analysis of the evidence was planned.
Rise time was significantly higher in HCC patients with a standardized mean difference (SMD) of 0.83 (95%CI: 0.48-1.18). Similarly, other statistically significant parameters were mean transit time local with a SMD of 0.73 (95%CI: 0.20-1.27), peak enhancement with a SMD of 0.37 (95%CI: 0.03-0.70), area wash-in area under the curve with a SMD of 0.47 (95%CI: 0.13-0.81), wash-out area under the curve with a SMD of 0.55 (95%CI: 0.21-0.89) and wash-in and wash-out area under the curve with SMD of 0.51 (95%CI: 0.17-0.85). SMD resulted not significant in fall time and wash-in rate, but the latter presented a trend towards greater values in HCC compared to intrahepatic cholangiocarcinoma.
DCEUS could improve non-invasive diagnosis of HCC, leading to less liver biopsy and early treatment. This quantitative analysis needs to be applied on larger cohorts to confirm these preliminary results.
Core Tip: Dynamic contrast enhanced ultrasound (DCEUS) is a novel technique that could help to overcome the diagnostic limits of standard contrast enhanced ultrasound. The parameters derived from the quantitative analysis of the time-intensity curve could give pivotal information upon the histotype of liver nodules. The aim of this paper is to explore clinical evidence regarding DCEUS application for differential diagnosis of liver nodules. To the best of our knowledge, this is the first systematic review and meta-analysis on this topic.
- Citation: Esposto G, Santini P, Termite F, Galasso L, Mignini I, Ainora ME, Gasbarrini A, Zocco MA. Dynamic contrast enhanced ultrasound in differential diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. World J Gastrointest Oncol 2024; 16(6): 2804-2815
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2804.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2804
Primary liver cancer ranks as the sixth most diagnosed cancer globally and stands respectively as the third and second leading cause of cancer-related death and of premature cancer-related death worldwide. Its incidence is expected to rise by 55% in the next two decades, affirming it as a universal health burden[1]. Among primary liver cancers, hepatocellular carcinoma (HCC) constitutes roughly 80%-85% of cases[2], while intrahepatic cholangiocarcinoma (ICC) accounts for 15%-20% of all primary liver malignancies[3,4].
Differential diagnosis between these histotypes is often challenging and seldom requires histological exam. In this context, imaging techniques that assess contrast enhancement patterns, particularly contrast-enhanced ultrasound (CEUS), have been pivotal in defining liver lesions detected incidentally[5,6]. Indeed, HCC often displays distinct vascular alterations, such as gradual decrease in portal blood flow and the development of new blood vessels, resulting in increased arterial supply[7]. The arterial phase hyperenhancement (APHE) followed by gradual wash-out during the portal-venous phase, serve as defining features of HCC, exhibiting a specificity that almost reaches 100%[8,9]. The American College of Radiology Liver Imaging Reporting and Data System (LI-RADS) relies on these distinct vascular patterns to categorize the risk of malignancy in liver nodules of cirrhotic patients[10,11]. Nevertheless, HCC occasionally displays atypical features leading to a considerable number of patients requiring liver biopsy for diagnosis[12,13]. According to the CEUS LI-RADS non diagnostic categories are LR-M, LR-3, and LR-4[10].
Several studies have explored the diagnostic accuracy of CEUS for distinguishing HCC from ICC with a recent meta-analysis affirming a sensitivity of 92% (95%CI: 0.84–0.96), a specificity of 87% (95%CI: 0.79–0.92) and an AUC of 0.95 (95%CI: 0.93–0.97)[14]. In this meta-analysis three CEUS features suggestive of HCC were APHE, mild washout and late washout (> 60 s) (in accordance with what reported in European Association for the Study of the Liver Clinical Practice Guidelines), while the three CEUS features indicating ICC were arterial rim enhancement, marked washout and early washout (< 60 s). Subgroup analyses revealed that the diagnostic performance may be influenced by factors such as liver background: The diagnostic odds ratio for the non-cirrhotic group exhibited greater improvement (89.67, 95%CI: 12.77–629.94) compared to the cirrhotic group, suggesting that CEUS demonstrates a superior detection ability in the non-cirrhotic group compared to the cirrhotic group[14].
Despite the CEUS features described above, ICC may resemble patterns observed in HCC[15] and therefore, the diagnosis continues to rely exclusively on tumor biopsy[16].
Dynamic CEUS (DCEUS) with standardized software could help to overcome these obstacles, providing functional and quantitative parameters and potentially improving accuracy in the evaluation of tumor perfusion[4,17].
Although literature have started to explore DCEUS application in diagnosis of liver lesions[17,18], the small sample sizes of enrolled patients limit the findings in daily clinical practice.
This systematic review and meta-analysis aims to comprehensively consolidate existing literature data. Its primary focus is to isolate potential parameters derived from dynamic CEUS studies that could significantly enhance clinical practice for differential diagnosis of HCC, potentially reducing the requirement for more invasive diagnostic procedures.
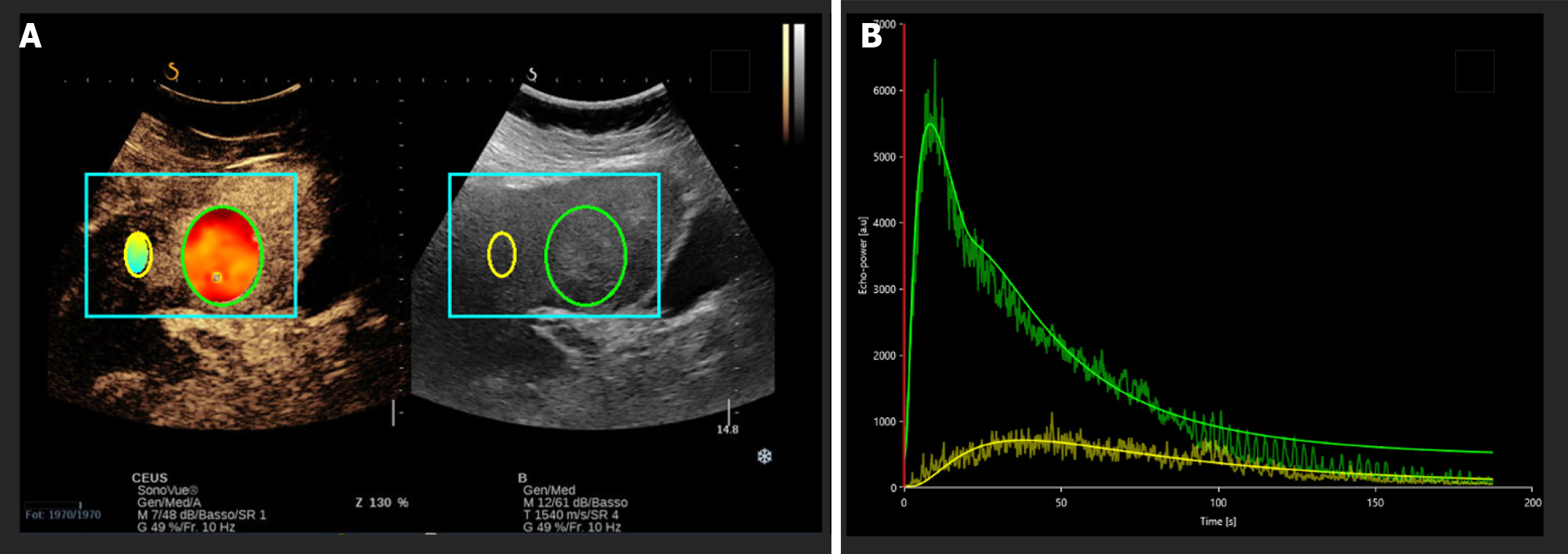
After CEUS evaluation, the captured clip is exported in digital imaging and communications in medicine format and then analyzed using specific analysis software, such as VueBox® (Bracco, Milan, Italy). The analysis entails delineation of a region of interest (ROI) that includes the identified liver lesion and a ROI that includes normal liver parenchyma. The software processes the time-intensity curves, deriving continuous parameters (Figure 1).
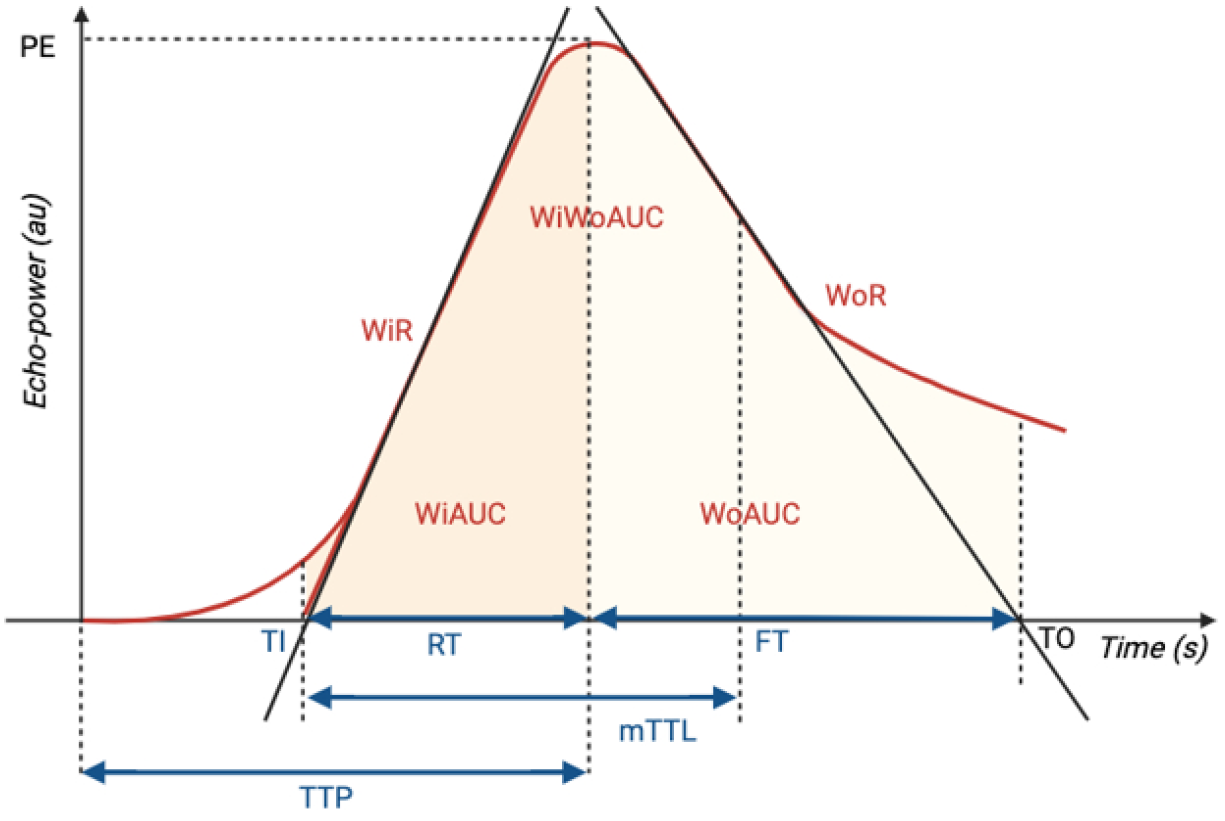
The perfusion parameters extracted from time-intensity curves provide a comprehensive assessment of blood volume, flow dynamics, and contrast agent behavior within the tissue (Figure 2).
Here is a breakdown of each parameter.
Peak enhancement (PE) measures the maximum intensity of the contrast agent within the tissue in arbitrary units (AU).
Wash-in area under the curve (WiAUC) represents the cumulative enhancement of the contrast agent during the initial phase of its entry into the tissue, measured in AU.
Wash-in rate (WiR) indicates the rate at which the contrast agent enters the tissue, calculated in AU per unit time. It reflects the speed of the contrast agent's accumulation during the initial phase.
Rise time (RT) measures the time taken for the contrast agent to reach its PE from its initial entry into the tissue, usually quantified in seconds (s).
Time to peak (TTP) signifies the duration taken for the contrast agent to reach its maximum enhancement within the tissue, measured in seconds from the start of the imaging.
Mean transit time local (mTTL) indicates the average time taken for contrast agents to pass through the tissue being analyzed. It is measured in seconds and is a representation of the average transit time of the contrast agent within the tissue.
Wash-out area under the curve (WoAUC) signifies the cumulative decrease in contrast agent concentration during the washout phase, measured in AU. This phase represents the decline or removal of the contrast agent from the tissue.
Wash-in and wash-out area under the curve (WiWoAUC) encompasses the total contrast agent exposure by combining the area under both the wash-in and wash-out phases of the time-intensity curve, measured in AU. It encapsulates the overall contrast agent behavior throughout the imaging duration.
Fall time (FT) is the time required to return from the PE to the baseline level.
Wash-out rate (WoR) indicates the rate at which the contrast agent leaves the tissue, calculated in AU per unit time. It reflects the speed of the contrast agent's regression during the portal and late phases.
Ratio of each parameter is defined as the percent change between the focal liver lesion and the surrounding parenchyma [i.e., (ROI lesion–ROI parenchyma)/ROI parenchyma].
A systematic literature review was conducted to answer the following research question: Can dynamic contrast enhanced ultrasound be used to improve the diagnostic accuracy of standard qualitative CEUS in diagnosis of liver cancer?
The study was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA)[19,20] and synthetized with meta-analysis. The study protocol for this systematic review and meta-analysis was written and submitted to the International Register of Systematic Reviews (PROSPERO, ID: CRD4202349104) prior to the completion of the literature search.
The search was conducted in the following electronic bibliographic databases: Medline (via PubMed), EMBASE (via Ovid), and Web of Science. To assure an adequate sensitivity, the search strategy only included terms related to the diagnostic technique being evaluated and the target population of patients affected by liver cancer. Therefore, three domains were combined, regarding DCEUS and HCC. The search string for each database can be consulted in the Supplementary Table 1. The search was complemented by manually reviewing references of retrieved articles and the prior systematic reviews on this topic.
Studies were considered eligible if they met the following inclusion criteria: (1) Prospective and retrospective cohort studies; (2) Written in English; and (3) Describing patients with suspect liver cancer who had undergone a contrast enhanced ultrasound for diagnosis. Meeting abstracts and oral or poster communications at scientific congresses were excluded. The results of the literature search were merged, and duplicates were removed using EndNoteTM. Individual records were manually screened with title and abstract analysis by two independent reviewers (Esposto G and Termite F). Any disagreement was resolved by discussion. Records considered appropriate were eligible for full-text analysis. Study selection, full-text analysis, and data extraction have been performed by two reviewers (Esposto G and Termite F). In the case of multiple records reporting on a single study, we focused on the most recent published paper in which the outcomes of the review were reported in the most exhaustive and complete way.
The following data were collected: Author, location, year of publication, study design, total number of patients, histology of liver cancer, DCEUS continuous parameters derived from time-intensity curves, type of contrast agent used, software used for analysis of time-intensity curves and confounding factors as reported in each study. Missing data were requested from study authors.
In accordance with the study protocol, a qualitative analysis of the evidence was planned. The results were sum
| Ref. | Risk of bias | Applicability concerns | |||||
| Patients selection | Index test | Reference standard | Flow and timing | Patients selection | Index test | Reference standard | |
| Ainora et al[5], 2023 | Low | Unclear | Low | Low | Low | Low | Low |
| Dong et al[15], 2023 | Unclear | Low | Low | Low | Low | Low | Low |
| Wildner et al[23], 2019 | High | Unclear | Unclear | High | Low | Low | Low |
| Wildner et al[17], 2014 | High | High | Low | High | Low | Low | Low |
| Ref. | Study design | Outcome | No. patients | Ultrasound contrast agent | Software for analysis of time-intensity curves |
| Ainora et al[5], 2023, Italy | Prospective | HCC vs ICC | 82 | SonoVue® | Vuebox® |
| Dong et al[15], 2023, China | Retrospective | HCC vs ICC | 54 | SonoVue® | Vuebox® |
| Wildner et al[23], 2019, Germany | Prospective | HCC vs ICC vs metastases vs FNH | 148 | SonoVue® | Vuebox® |
| Wildner et al[17], 2014, Germany | Prospective | HCC vs ICC | 43 | SonoVue® | Vuebox® |
Data synthesis was performed by dividing the selected studies into groups defined by tumor’s histology. The results were summarized with a comprehensive summary table of the time-intensity curve analysis (Table 3).
| Ref. | Age (yr) | Sex (male/female) | Origin of the study population | No. HCC | No. non HCC |
| Ainora et al[5], 2023 | 68 ± 11 | 55/27 | Cirrhotic and non-cirrhotic patients | 38 | 44 |
| Dong et al[15], 2023 | 59.4 ± 8.6 (ICC) | 27/27 | Non-cirrhotic patients | 24 | 30 |
| 60.9 ± 12.1 (HCC) | |||||
| Wildner et al[23], 2019 | 63.8 ± 12.6 | 84/64 | Cirrhotic and non-cirrhotic patients | 41 | 107 |
| Wildner et al[17], 2014 | 67 | 30/13 | Cirrhotic and non-cirrhotic patients | 23 | 20 |
The quantitative synthesis of the data was carried out through analysis of the Hedges' g standardized mean difference (SMD) of each DCEUS parameter. This method is more conservative than simple mean difference analysis and accounts for the differences in the ultrasound machines used in the studies. Whenever the original paper did not report the results in terms of mean and standard deviation, the relative median and interquartile range were converted according to the indications of the Cochrane Library[21]. Heterogeneity was assessed by calculation of τ2 and I2 statistics. The first account for the between-studies variance, while the second one represents the proportion of total variation across studies due to heterogeneity rather than chance. SMDs were meta-analyzed using a random effect model when the heterogeneity was high (I2 > 50%) or very high (I2 > 75%). When heterogeneity was moderate (I2 < 50%) or low (I2 < 25%), SMDs were meta-analyzed using a fixed effect inverse variance model.
Risk of bias of eligible studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool[22]. Risk-of-bias assessment was carried out by two authors (Esposto G and Termite F), and any disagreement between the two independent reviewers was resolved by discussion, with involvement of a third review author where necessary.
The number of studies was too small to allow a graphical assessment of publication bias by funnel plot or statistical assessment by Egger’s test. However, all the identified studies showed a statistic difference between HCC and ICC patients in term of DCEUS parameters. Consequently, it cannot be excluded that this evidence could reflect the presence of a certain degree of unmeasurable publication bias.
The main outcome of the current systematic review is the presence of a different distribution in DCEUS parameters between histological examination of liver cancer.
DCEUS parameters comprehend wash-out time (s), WoR ratio, FT (s), FT ratio, PE lesion, PE Ratio, WiAUC lesion, WiAUC ratio, RT lesion, mTTL lesion, mTTL ratio, TTP lesion, TTP ratio, WiR lesion, WiR ratio, WoAUC lesion, WoAUC ratio, WiWoAUC lesion, WiWoAUC ratio.
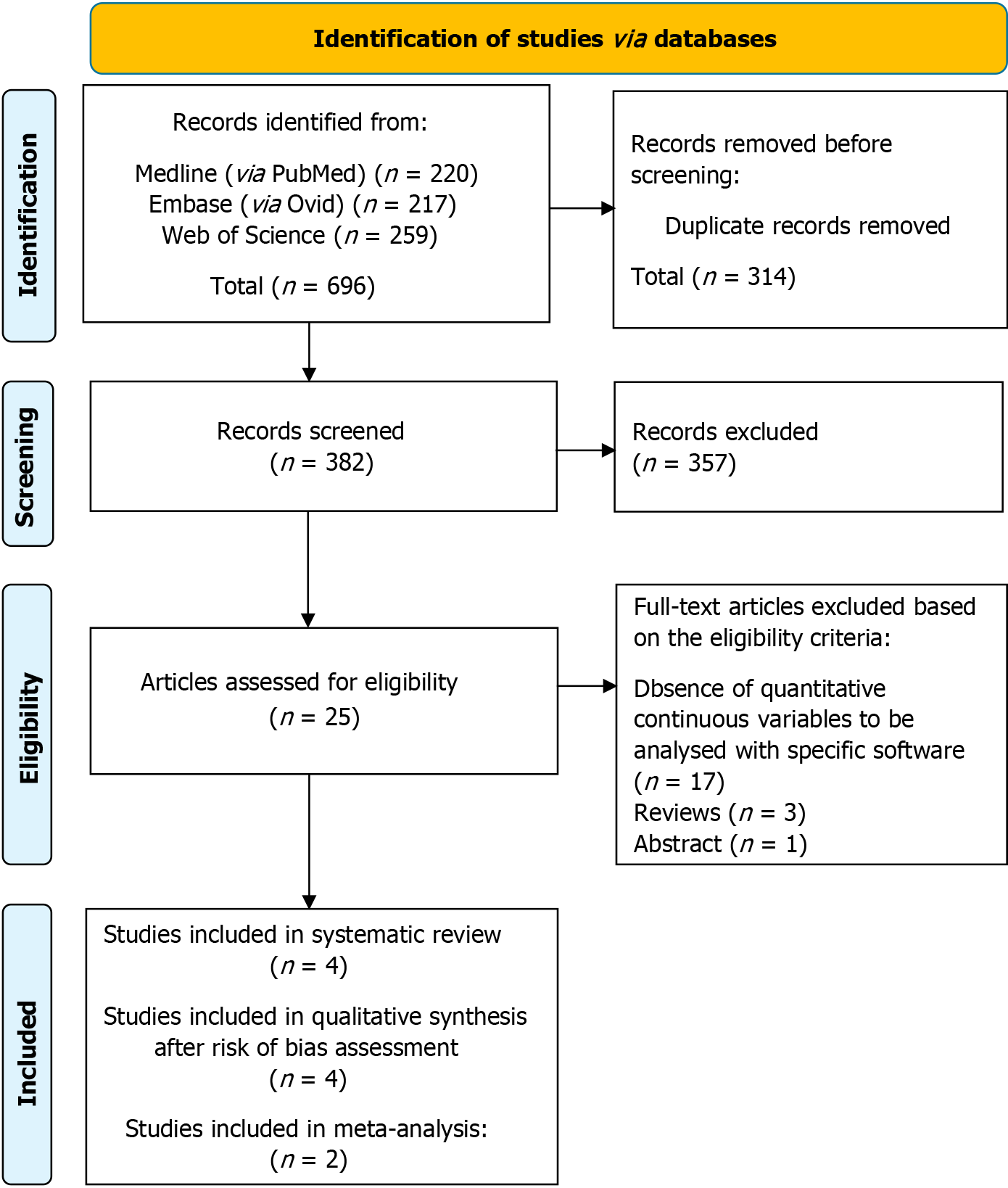
Three biomedical databases were screened using the prespecified search methods on November 21, 2023, and a total of 696 studies were found (Medline via PubMed: 220, EMBASE via Ovid: 217, and Web of Science: 259). After removal of duplicates, 382 records underwent primary eligibility screening based on titles and abstracts. As a result, 25 papers met eligibility criteria for full-text analysis. 21 studies were excluded: 1 was only present as an abstract and 17 were excluded due to the absence of quantitative continuous variables to be analyzed with specific software. Three narrative reviews previously published were excluded from further analysis. Finally, 4 studies matched the predetermined eligibility requirements for this systematic review.
After a structured risk-of-bias assessment, all 4 original papers were included in the qualitative synthesis. Figure 3 shows the PRISMA selection flow diagram that describes the study-selection process in detail.
To evaluate the internal and external validity of each included study, a structured analysis of the risk of bias was carried out using the QUADAS-2 tool[22] for quality assessment of diagnostic accuracy studies (Table 1). It is noteworthy that risk-of-bias assessment evaluates each included study in the context of the research questions of the current systematic review and meta-analysis and does not analyze the general scientific worth or quality of the individual study.
Two studies were at high risk of bias in the patients selection domain; the exclusion criteria are not available in the paper of Wildner et al[17], while Wildner et al[23] did not exclude patients treated for HCC. Treatment, both pharmacological or physical (i.e., transcatheter arterial chemoembolization or transarterial radioembolization), could lead to changes in the distribution of the contrast ultrasound agent and therefore could be a bias for the analysis of the time-intensity curves. Moreover, despite both studies were conducted prospectively, one did not include consecutive patients[17], while the other one did not specify whether patients were consecutive[23]. In the same domain, one study was categorized as having an unknown risk of bias, because it was not clear if patients were consecutive or not[15].
The index test domain was judged to be at low risk of bias for one study[15], although the question about the use of a prespecified threshold was ignored due to the absence of a validated cutoff for HCC diagnosis. Two studies were cate
Three studies were judged to be at low risk of bias in the reference standard domain[5,15,17], while one study[23] had an unknown risk, because it was not clear if the reference standard was interpreted blind to the DCEUS evaluation.
The flow and timing domain was at high risk for bias in two studies, due to the absence of an appropriate interval between the index test and the reference standard[17,23]. Indeed, if patients are treated in the interval between DCEUS and liver biopsy, the distribution of the contrast agent could be altered. Moreover, in both studies patients did not receive the same reference standard, as some patients were diagnosed with radiological findings [x-ray micro-computed tomography (CT), magnetic resonance imaging (MRI), or CEUS] and not with histological exam.
The evaluation of concerns about applicability was found to be at low risk for each domain in all four studies[5,15,17,23].
A visual summary of distribution of risk of bias and applicability concerns across QUADAS-2 domains can be found in Supplementary Figure 1.
The four studies considered eligible for qualitative synthesis were homogenous in terms of study design, outcomes, type of contrast agent and software for time-intensity curve analysis (Table 2), while there was a certain grade of heterogenicity in the population included and in the ultrasound machines used for CEUS examination.
The baseline characteristics of patients included in each study are summarized in Table 3. The number of study participants ranges from 43 to 148, for a total of 327 patients.
The four studies considered eligible for qualitative synthesis analyzed the time-intensity curves with specific software. The results of the measured continuous variables are summarized in Table 4.
| Ainora et al[5], 2023 | Dong et al[15], 2023 | |||
| HCC (n = 38) | ICC (n = 44) | HCC (n = 24) | ICC (n = 30) | |
| Rise time lesion (s) | 10.9 ± 3.8 | 8.1 ± 4.1 | 11.9 ± 5.3 | 7.5 ± 3.0 |
| Fall time lesion (s) | 37.5 ± 19.5 | 35.4 ± 23.9 | 27.7 ± 10.8 | 14.9 ± 5.9 |
| mTTL lesion (s) | 67.0 ± 52.0 | 42.9 ± 46.1 | 68.9 ± 32.4 | 42.2 ± 18.0 |
| TTP lesion (s) | 12.5 ± 4.3 | 9.1 ± 5.8 | 17.6 ± 9.6 | 12.8 ± 6.4 |
| PE percent change | 0.66 ± 1.35 | -0.04 ± 1.38 | 1.28 ± 0.47 | 1.16 ± 0.90 |
| WiR percent change | 2.41 ± 3.96 | 1.32 ± 1.91 | 2.33 ± 1.19 | 2.38 ± 2.33 |
| WiAUC percent change | -0.22 ± 0.72 | -0.52 ± 0.36 | 0.79 ± 0.45 | 0.61 ± 0.51 |
| WoAUC percent change | -0.11 ± 0.74 | -0.41 ± 0.53 | 0.85 ± 0.53 | 0.53 ± 0.42 |
| WiWoAUC percent change | -0.13 ± 0.70 | -0.39 ± 0.39 | 0.83 ± 0.50 | 0.55 ± 0.44 |
Ainora et al[5] included 82 consecutive patients candidate to biopsy of focal liver lesions. Patients undergone both ultrasound B mode and CEUS evaluation with 2.4 mL of SonoVue® (Bracco, Milan, Italy). The reference standard was histopathological examination.
Ultrasound B mode characteristics showed no statistically significant difference between HCC and ICC. CEUS displayed arterial homogenous hyperenhancement in 68.5% of HCC and 22.7% of ICC lesions, while one HCC nodule (2.6%) and 15 ICC nodules (34.1%) had rim hyperenhancement. The 89% of nodules showed portal or late phase wash-out with a mean value of 59.4 s ± 26.9 s in HCC and 45.4 s ± 17.2 s in ICC lesions (P = 0.01). Marked wash-out was found in 28.9% (11/38) of HCC lesions, in contrast to 52.3% (23/44) of ICC lesions (P = 0.03).
CEUS clips were then analyzed with Vuebox® software and significant differences were seen in four blood volume parameters: PE (P < 0.03), WiAUC, WoAUC and WiWoAUC (P < 0.01).
When the authors considered the ratio between nodules and the surrounding parenchyma, PE and WiAUC were statistically higher in HCC respect to ICC; higher WiR values were also observed (P < 0.01). On the other side, mTTL, which is associated with portal and late-phase wash-out, was statistically shorter for ICC compared to HCC (P = 0.03). At univariate analysis PE ratio (OR, 0.62; 95%CI: 0.43–0.91, P = 0.01) and WiR ratio (OR, 0.87; 95%CI: 0.78–0.98, P = 0.02) were associated with histological diagnosis. At multivariate analysis PE ratio (P = 0.02) was independent predictor of HCC or ICC. Other independent factors were liver cirrhosis (P < 0.01) and shear-wave elastography (P = 0.01). The authors proposed a score based on these three factors for differential diagnosis of liver cancer, with an area under the receiver operating characteristic (ROC) curve of 0.836.
Dong et al[15] retrospectively included 54 patients with histological diagnosis of ICC or HCC in non-cirrhotic liver. Patients prior to surgery or liver biopsy, were subjected to both ultrasound B mode and CEUS examination with 1.5 mL of SonoVue® (Bracco, Milan, Italy). The reference standard was histopathological examination. CEUS clips were analyzed with Vuebox® software.
On ultrasound B mode evaluation there were no significant differences in size between ICC and HCC. On CEUS examination, all HCC nodules showed hyperenhancement (P < 0.05), while ICC displayed various enhancement patterns. As concerns wash-out, 83.3% of ICC had arterial phase wash-out, while only 15.7% of them had portal venous phase wash-out. On the opposite HCC showed a 41.7% portal venous phase wash-out and 16.7% late phase wash-out (P < 0.05).
Time-intensity curve analysis revealed four parameters (RT, mTTL, TTP, and FT) that were statistically lower in ICC nodules compared to HCC (P < 0.05). Moreover, both WoAUC and WiWoAUC ratios were statistically lower in the ICC patients (P < 0.05). No other parameter showed a significant difference between the two groups. On ROC curve analysis, FT had an AUROC of 0.903 (95%CI: 0.823−0.982) with a derived cutoff value of 16.9 s (95.8% sensitivity, 73.3% specificity, 83.3% accuracy) for distinction between ICC and HCC nodules in non-cirrhotic liver.
The combined AUROC of the RT, mTTL, TTP, FT, WoAUC ratio and WiWoAUC ratio was 0.946 (95%CI: 0.888−1.000) with 86.7% sensitivity, 95.8% specificity, and 90.7% accuracy. Overall, the authors concluded that these results improved the diagnostic accuracy of CEUS alone.
Wildner et al[23] in 2019 included 148 patients with liver nodules undergoing liver biopsy or radiological diagnosis. 138 patients were diagnosed by histological exam, while the remaining 10 were diagnosed by typical findings in CT, MRI, or CEUS. Patients undergone both ultrasound B-mode evaluation and CEUS examination with 1.5 mL of SonoVue® (Bracco, Milan, Italy). CEUS clips were analyzed with Vuebox® software. PE had significantly higher values for HCC compared to metastasis. RT, mTTL, and FT showed no statistical difference among the described groups. WiWoAUC was significantly higher in HCC compared to metastasis.
Wildner et al[17] in 2014 included 43 consecutive patients with focal liver lesions subjected to liver biopsy or radiological diagnosis. The authors performed both ultrasound B-mode and CEUS evaluation with 1.2 mL of SonoVue® (Bracco, Milan, Italy). CEUS clips were analyzed with Vuebox® software. As concerns DCEUS, the wash-in parameters were not significantly different from HCC and ICC, while mTTL and FT were statistically lower in ICC [mTTL, P = 0.0209; HCC (118.4 s ± 88.4 s) vs ICC (64.8 s ± 49.7 s); FT, P = 0.0433; HCC (42.5 s ± 27.7 s) vs ICC (27.7 s ± 16.2 s)]. Wash-out after PE was significantly higher in ICC compared to HCC at definite timepoints: 40 s after PE (P = 0.0001), 80 s after PE (P = 0.0007), 100 s after PE (P = 0.0029), and 120 s after PE (P = 0.0181).
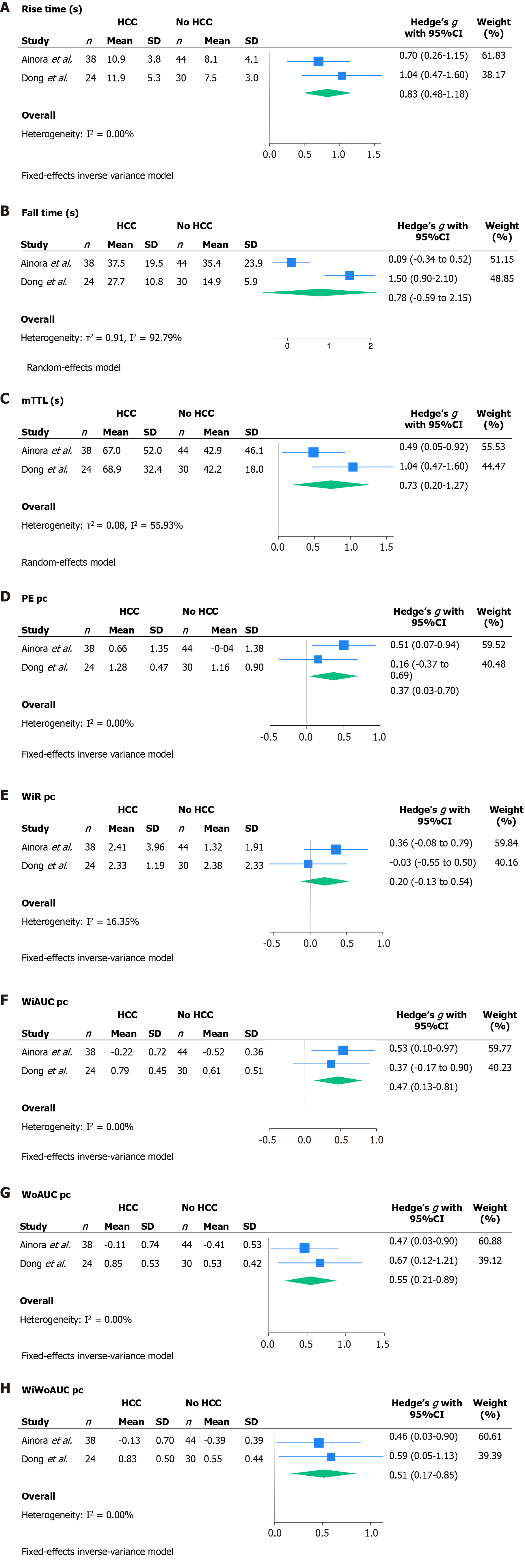
The quantitative synthesis focused on the mean distribution of each continuous variable of the time-intensity curve across the HCC and ICC groups. The SMDs, calculated as Hedges' g SMD, are reported in Figure 4. SMDs were meta-analyzed using a random effect model or a fixed effect inverse variance model when respectively the heterogeneity was high (I2 > 50%) or very high (I2 > 75%) and when heterogeneity was moderate (I2 < 50%) or low (I2 < 25%). RT was significantly higher in HCC patients with a SMD of 0.83 (95%CI: 0.48-1.18). Similarly, mTTL showed a statistically significant SMD of 0.73 (95%CI: 0.20-1.27); PE showed a statistically significant SMD of 0.37 (95%CI: 0.03-0.70); WiAUC showed a statistically significant SMD of 0.47 (95%CI: 0.13-0.81); WoAUC showed a statistically significant SMD of 0.55 (95%CI: 0.21-0.89); WiWoAUC showed a statistically significant SMD of 0.51 (95%CI: 0.17-0.85). On the other hand, SMD resulted not statistically significant in FT and WiR, but the latter presented a trend towards greater values in HCC compared to ICC.
A sensitivity analysis was conducted in the subgroup of patients who developed HCC or ICC in a non-cirrhotic liver. The SMDs, calculated as Hedges' g SMD, are reported in Supplementary Figure 2. In this subgroup, mTTL showed a statistically significant SMD of 0.91 (95%CI: 0.44-1.39), WoAUC showed a statistically significant SMD of 0.61 (95%CI: 0.15-1.07), WiWoAUC showed a statistically significant SMD of 0.59 (95%CI: 0.13-1.05), PE and WiAUC showed a non-significant SMD, although a trend was shown toward greater values in HCC compared to ICC. SMD resulted not statistically significant in RT and FT.
In this meta-analysis we only included two studies, for a total of 136 patients. The exclusion of Wildner et al[23] in 2019 and Wildner et al[17] in 2014 from the quantitative synthesis is due to the heterogeneity and lack of data to be compared with the remaining two studies and to a high risk of bias.
The quantitative analysis of Ainora et al[5] and Dong et al[15] showed a statistical significance for RT, mTTL, PE, WiAUC, WoAUC, and WiWoAUC. In our analysis, these values are higher in HCC nodules compared to ICC nodules and these results are consistent with the contrastographic behavior described by Chen et al[14]. Their meta-analysis on the capability of CEUS in differential diagnosis between HCC and ICC showed indeed that HCC is associated with APHE, mild washout and late washout (> 60 s), while ICC is associated with arterial rim enhancement, marked washout and early washout (< 60 s). DCEUS could come into aid in this context, as it provides quantification software for standardized analysis of enhancement microvasculature kinetics[24].
The sensitivity analysis conducted in the subgroup of non-cirrhotic patients revealed a statistical significance for mTTL, WoAUC, and WiWoAU. Compared to the above-mentioned analysis, PE and WiAUC lost significance, although a trend was shown toward greater values in HCC compared to ICC. These differences could be both explained by the smaller number of patients included and by the different distribution of contrast agent in cirrhotic liver compared to non-cirrhotic ones.
Indeed, CEUS features indicative of HCC in non-cirrhotic liver manifest typically as APHE and relatively rapid wash-out in the portal venous phase[15]. These characteristics diverge from those observed in HCC in cirrhotic liver and align more closely with ICC. The observed microvascular behavior can be explained by the fact that HCC in cirrhotic liver primarily obtains nourishment from branches of the hepatic artery, with minimal reliance on portal branches.
Moreover, a rim-like hyperenhancement pattern is more commonly detected in ICC occurring in individuals with a non-cirrhotic liver background. In contrast, ICC developing in the context of liver cirrhosis may display complete hyperenhancement, resembling patterns observed in HCC[15].
This can be understood by the evidence that significantly increased fibrous stroma and necrosis (features responsible for the rim-like hyperenhancement) are more common in ICC cases without liver cirrhosis compared to those with cirrhosis. Conversely, ICC developing in cirrhotic conditions may exhibit sustained augmentation of vasculature due to the enlargement and dilation of arterial branches[25].
This meta-analysis has several limitations, mainly due to the small number of studies and patients included. Whereas literature lacks studies specifically designed to explore each of the above discussed variables, our results look promising and are to be intended solely as a starting point for future studies. Our aim and hope are that this paper could be used as a model to design protocols on larger cohorts and hopefully find a diagnostic tool to improve HCC non-invasive differential diagnosis.
DCEUS could in future represent a valid tool for non-invasive diagnosis of HCC, possibly leading to less liver biopsy and early treatment. In our analysis numerous DCEUS parameters (PE, mTTL, RS, WiAUC, WoAUC, and WiWoAUC) were indeed statistically higher in HCC nodules compared to ICC ones. This meta-analysis is limited by the paucity of data found in current literature. Despite the lack of strong evidence, our results show that DCEUS could help to overcome the limits of CEUS alone for differential diagnosis of HCC with atypical features. The quantitative analysis of CEUS parameters needs to be implemented and studied on larger cohorts, in order to confirm and eventually consolidate these preliminary results.
| 1. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1052] [Article Influence: 350.7] [Reference Citation Analysis (0)] |
| 2. | Motta BM, Masarone M, Torre P, Persico M. From Non-Alcoholic Steatohepatitis (NASH) to Hepatocellular Carcinoma (HCC): Epidemiology, Incidence, Predictions, Risk Factors, and Prevention. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Yang JQ, Wang XG, Wu B. Incidence trend and prognosis of intrahepatic cholangiocarcinoma: a study based on the SEER database. Transl Cancer Res. 2023;12:3007-3015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Pascale A, Rosmorduc O, Duclos-Vallée JC. New epidemiologic trends in cholangiocarcinoma. Clin Res Hepatol Gastroenterol. 2023;47:102223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 5. | Ainora ME, Cerrito L, Liguori A, Mignini I, De Luca A, Galasso L, Garcovich M, Riccardi L, Ponziani F, Santopaolo F, Pompili M, Gasbarrini A, Zocco MA. Multiparametric Dynamic Ultrasound Approach for Differential Diagnosis of Primary Liver Tumors. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Vidili G, Arru M, Solinas G, Calvisi DF, Meloni P, Sauchella A, Turilli D, Fabio C, Cossu A, Madeddu G, Babudieri S, Zocco MA, Iannetti G, Di Lembo E, Delitala AP, Manetti R. Contrast-enhanced ultrasound Liver Imaging Reporting and Data System: Lights and shadows in hepatocellular carcinoma and cholangiocellular carcinoma diagnosis. World J Gastroenterol. 2022;28:3488-3502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Pinto E, Pelizzaro F, Farinati F, Russo FP. Angiogenesis and Hepatocellular Carcinoma: From Molecular Mechanisms to Systemic Therapies. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Reference Citation Analysis (0)] |
| 8. | Schellhaas B, Bernatik T, Bohle W, Borowitzka F, Chang J, Dietrich CF, Dirks K, Donoval R, Drube K, Friedrich-Rust M, Gall C, Gittinger F, Gutermann M, Haenle MM, von Herbay A, Ho CH, Hochdoerffer R, Hoffmann T, Hüttig M, Janson C, Jung EM, Jung N, Karlas T, Klinger C, Kornmehl A, Kratzer W, Krug S, Kunze G, Leitlein J, Link A, Lottspeich C, Marano A, Mauch M, Moleda L, Neesse A, Petzold G, Potthoff A, Praktiknjo M, Rösner KD, Schanz S, Schultheiß M, Sivanathan V, Stock J, Thomsen T, Vogelpohl J, Vogt C, Wagner S, Wiegard C, Wiesinger I, Will U, Ziesch M, Zimmermann P, Strobel D. Contrast-Enhanced Ultrasound Algorithms (CEUS-LIRADS/ESCULAP) for the Noninvasive Diagnosis of Hepatocellular Carcinoma - A Prospective Multicenter DEGUM Study. Ultraschall Med. 2021;42:e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Meitner-Schellhaas B, Jesper D, Goertz RS, Zundler S, Strobel D. Washout appearance of hepatocellular carcinomas using standardized contrast-enhanced ultrasound (CEUS) including an extended late phase observation - Real-world data from the prospective multicentre DEGUM study. Clin Hemorheol Microcirc. 2023;84:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Kim TK, Noh SY, Wilson SR, Kono Y, Piscaglia F, Jang HJ, Lyshchik A, Dietrich CF, Willmann JK, Vezeridis A, Sirlin CB. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 - a review of important differences compared to the CT/MRI system. Clin Mol Hepatol. 2017;23:280-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 11. | Shin J, Lee S, Bae H, Chung YE, Choi JY, Huh YM, Park MS. Contrast-enhanced ultrasound liver imaging reporting and data system for diagnosing hepatocellular carcinoma: A meta-analysis. Liver Int. 2020;40:2345-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Li F, Li Q, Liu Y, Han J, Zheng W, Huang Y, Zheng X, Cao L, Zhou JH. Distinguishing intrahepatic cholangiocarcinoma from hepatocellular carcinoma in patients with and without risks: the evaluation of the LR-M criteria of contrast-enhanced ultrasound liver imaging reporting and data system version 2017. Eur Radiol. 2020;30:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Dong Y, Wang WP, Lee WJ, Meloni MF, Clevert DA, Chammas MC, Tannapfel A, Forgione A, Piscaglia F, Dietrich CF. Contrast-Enhanced Ultrasound Features of Histopathologically Proven Hepatocellular Carcinoma in the Non-cirrhotic Liver: A Multicenter Study. Ultrasound Med Biol. 2022;48:1797-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Chen Y, Zhu Y, Chen K, Wang H, Zhang W, Bao J, Wang W. Differentiation between hepatocellular carcinoma and intrahepatic cholangiocarcinoma using contrast-enhanced ultrasound: A systematic review and meta-analysis. Clin Hemorheol Microcirc. 2021;79:293-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Dong Y, Chen S, Möller K, Qiu YJ, Lu XY, Zhang Q, Dietrich CF, Wang WP. Applications of Dynamic Contrast-Enhanced Ultrasound in Differential Diagnosis of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma in Non-cirrhotic Liver. Ultrasound Med Biol. 2023;49:1780-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol. 2023;79:181-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 17. | Wildner D, Pfeifer L, Goertz RS, Bernatik T, Sturm J, Neurath MF, Strobel D. Dynamic contrast-enhanced ultrasound (DCE-US) for the characterization of hepatocellular carcinoma and cholangiocellular carcinoma. Ultraschall Med. 2014;35:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Guo HL, Zheng X, Cheng MQ, Zeng D, Huang H, Xie XY, Lu MD, Kuang M, Wang W, Xian MF, Chen LD. Contrast-Enhanced Ultrasound for Differentiation Between Poorly Differentiated Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Ultrasound Med. 2022;41:1213-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2603] [Cited by in RCA: 4415] [Article Influence: 1103.8] [Reference Citation Analysis (33)] |
| 20. | McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM; and the PRISMA-DTA Group, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, Hunt HA, Hyde CJ, Korevaar DA, Leeflang MMG, Macaskill P, Reitsma JB, Rodin R, Rutjes AWS, Salameh JP, Stevens A, Takwoingi Y, Tonelli M, Weeks L, Whiting P, Willis BH. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. 2018;319:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 2077] [Article Influence: 296.7] [Reference Citation Analysis (0)] |
| 21. | Higgins JPT, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). United States: Bloomsbury, 2023. |
| 22. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9557] [Article Influence: 682.6] [Reference Citation Analysis (0)] |
| 23. | Wildner D, Schellhaas B, Strack D, Goertz RS, Pfeifer L, Fiessler C, Neurath MF, Strobel D. Differentiation of malignant liver tumors by software-based perfusion quantification with dynamic contrast-enhanced ultrasound (DCEUS). Clin Hemorheol Microcirc. 2019;71:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Dietrich CF, Averkiou MA, Correas JM, Lassau N, Leen E, Piscaglia F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012;33:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 244] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 25. | Yuan MX, Li R, Zhang XH, Tang CL, Guo YL, Guo DY, Luo MK. Factors Affecting the Enhancement Patterns of Intrahepatic Cholangiocarcinoma (ICC) on Contrast-Enhanced Ultrasound (CEUS) and their Pathological Correlations in Patients with a Single Lesion. Ultraschall Med. 2016;37:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |