Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2793
Revised: March 1, 2024
Accepted: April 16, 2024
Published online: June 15, 2024
Processing time: 187 Days and 1.3 Hours
Hepatocellular carcinoma (HCC) ranks sixth globally in cancer incidence and third in mortality rates. Unfortunately, over 70% of HCC patients forego the opportunity for curative surgery or liver transplantation due to inadequate physical examinations, poor physical condition, and limited organ availability upon diagnosis. Clinical guidelines endorse transarterial chemoembolization (TACE) as the frontline treatment for intermediate to advanced-stage HCC. Cryoablation (CRA) is an emerging local ablative therapy increasingly used in HCC management. Recent studies suggest that combining CRA with TACE offers complementary and synergistic effects, potentially improving long-term survival rates. However, the superiority of combined TACE + CRA therapy over TACE alone for HCC lesions equal to or exceeding 5 cm requires further investigation.
To compare the efficacy and safety of TACE combined with CRA vs TACE alone in the treatment of HCC with a diameter of ≥ 5 cm.
PubMed, EMBASE, Cochrane Library, CNKI, Wanfang, and VIP databases were searched to retrieve all relevant studies on TACE and CRA up to July 2022. Meta-analysis was performed using RevMan 5.3 software.
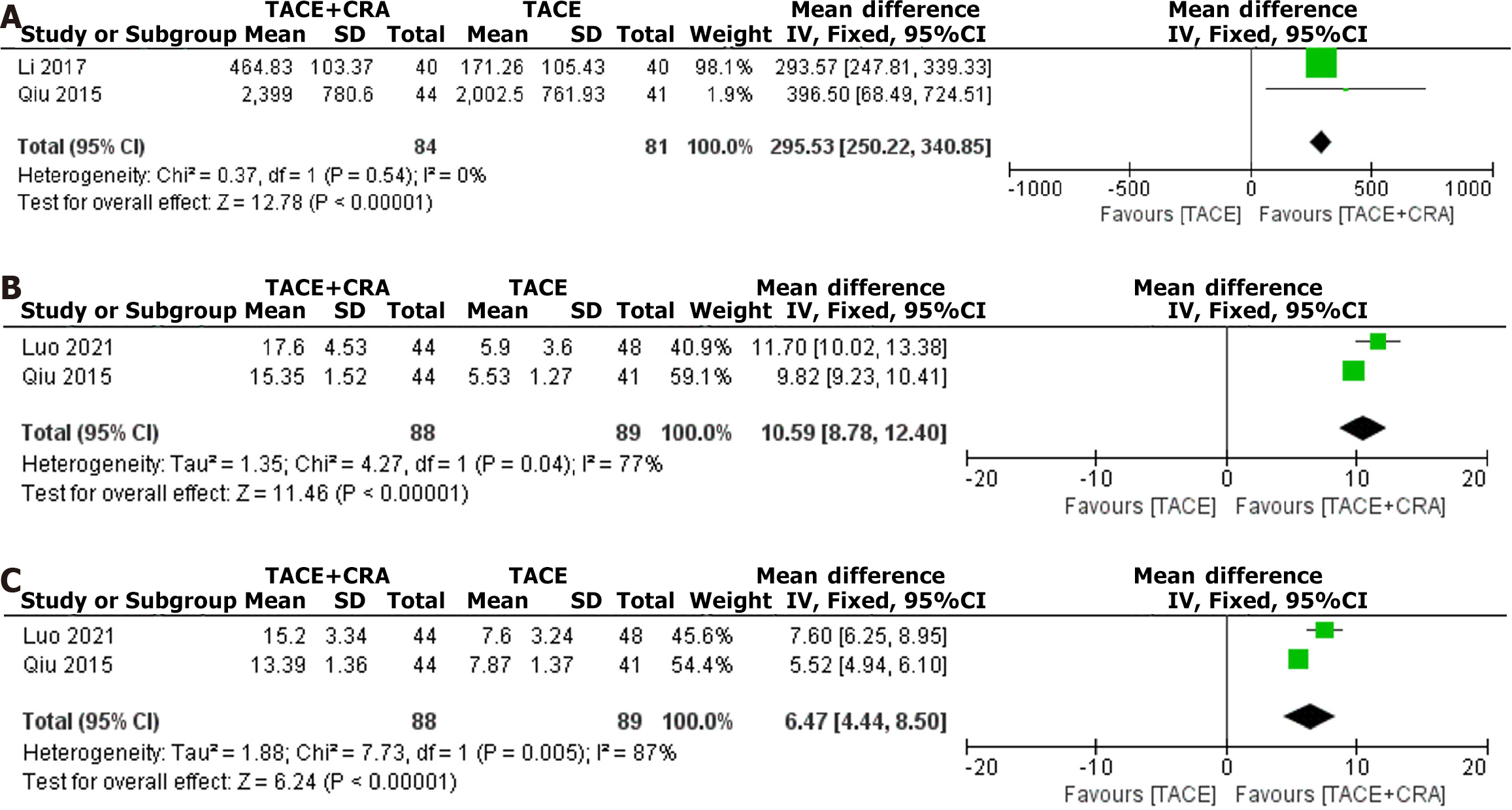
After screening according to the inclusion and exclusion criteria, 6 articles were included, including 2 randomized controlled trials and 4 nonrandomized controlled trials, with a total of 575 patients included in the meta-analysis. The results showed that the objective response rate [odds ratio (OR) = 2.56, 95% confidence interval (CI):1.66-3.96, P < 0.0001), disease control rate (OR = 3.03, 95%CI: 1.88-4.89, P < 0.00001), 1-year survival rate (OR = 3.79, 95%CI: 2.50-5.76, P < 0.00001), 2-year survival rate (OR = 2.34, 95%CI: 1.43-3.85, P = 0.0008), and 3-year survival rate (OR = 3.34, 95%CI: 1.61-6.94, P = 0.001) were all superior to those of the control group; the postoperative decrease in alpha-fetoprotein value (OR = 295.53, 95%CI: 250.22-340.85, P < 0.0001), the postoperative increase in CD4 value (OR = 10.59, 95%CI: 8.78-12.40, P < 0.00001), and the postoperative decrease in CD8 value (OR = 6.47, 95%CI: 4.44-8.50, P < 0.00001) were also significantly higher than those in the TACE-alone treatment group.
Compared with TACE-alone treatment, TACE + CRA combined treatment not only improves the immune function of HCC patients with a diameter of ≥ 5 cm, but also enhances the therapeutic efficacy and long-term survival rate, without increasing the risk of complications. Therefore, TACE + CRA combined treatment may be a more recommended treatment for patients with HCC with a diameter of ≥ 5 cm.
Core Tip: To compare the efficacy and safety of transarterial chemoembolization (TACE) combined with cryoablation (CRA) vs TACE alone in the treatment of hepatocellular carcinoma (HCC) with a diameter of ≥ 5 cm. Compared with TACE-alone treatment, TACE + CRA combined treatment not only improves the immune function of HCC patients with a diameter of ≥ 5 cm, but also enhances the therapeutic efficacy and long-term survival rate, without increasing the risk of complications. Therefore, TACE + CRA combined treatment may be a more recommended treatment for patients with HCC with a diameter of ≥ 5 cm.
- Citation: Cheng JF, Sun QL, Tang L, Xu XJ, Huang XZ. Meta-analysis of transarterial chemoembolization combined with cryoablation vs transarterial chemoembolization alone for ≥ 5 cm hepatocellular carcinoma. World J Gastrointest Oncol 2024; 16(6): 2793-2803
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2793.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2793
Hepatocellular carcinoma (HCC) ranks sixth in global cancer incidence and third in mortality due to its highly malignant nature[1,2]. Over 70% of HCC patients lose the opportunity for curative surgery or liver transplantation due to a lack of regular physical examinations, poor physical condition, and limited donor organ availability when liver cancer is detected. Transarterial chemoembolization (TACE) is the first-line treatment, as recommended by guidelines for intermediate and advanced HCC[3]. Cryoablation (CRA) is a newly developed local ablation therapy that has been gradually applied for the local treatment of HCC. Recent studies have shown that the combination of CRA and TACE for the treatment of primary liver cancer has complementary and synergistic effects that can prolong the long-term survival of patients[4]; however, it remains unclear whether TACE + CRA combination therapy is superior to TACE alone in the treatment of HCC patients with lesions greater than or equal to 5 cm. In this study, a meta-analysis was performed to compare the efficacy and safety of TACE + CRA and TACE alone in the clinical treatment of patients with HCC ≥ 5 cm in diameter and aimed to provide a reference for the TACE + CRA treatment approach for such patients.
A systematic literature review was conducted to assess the clinical efficacy and safety of hepatic arterial TACE combined with CRA and TACE alone for HCC patients with large tumors. The review included blinded and nonblinded studies, as well as concealed and nonconcealed allocation studies. The databases searched included PubMed, EMBASE, the Cochrane Library, CNKI, Wanfang, and VIP, and the search was limited to studies written in English or Chinese. The search period was unrestricted and started at database inception and ended on May 1, 2022. The methodology followed the guidelines specified in the Cochrane Handbook of Systematic Reviews and involved the use of a combination of subject-specific and free-text terms, such as “liver cancer”, “hepatic carcinoma”, “hepatoma”, “liver carcinoma”, “liver neoplasms”, “HCC”, “cancer of liver”, “hepatocellular”, “transcatheter arterial chemoembolization”, “cryosurgery”, and “cryotherapy”. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses recommendations. If the results reported had possible overlap, only the most recent study was included in the meta-analysis.
The inclusion criteria were as follows: (1) Patients underwent TACE combined with CRA or TACE alone; (2) Clinical guidelines were used to diagnose HCC, regardless of demographic factors such as sex, race, and nationality, with a tumor diameter greater than or equal to 5 cm; (3) Patients experienced no severe organ failure of the heart, liver, or kidney or significant hematological abnormalities before treatment; (4) No indication for surgical resection; (5) Study contained a description of the general clinical characteristics of each study group with comparability; and (6) Patients had at least one primary outcome measure. The exclusion criteria were as follows: (1) Studies that compare non-TACE + CRA and TACE; (2) Reviews, nonclinical studies, case reports, and conference abstracts; and (3) Studies lacking necessary data or relevant outcome measures.
The experimental group received TACE combined with CRA, and the control group received TACE alone. The literature screening, review, and data extraction were performed independently by two researchers. Any discrepancies were resolved through discussion. If the necessary data were unclear or unavailable, the authors of the relevant articles were contacted by phone or email to obtain the required information. The extracted data included: (1) General information such as the title, authors, publication date, and source of the study; (2) Baseline characteristics and details of the intervention measures studied; and (3) Outcome measures.
To assess the quality of the included studies, the researchers used the Cochrane bias risk tool. The Cochrane Handbook for Systematic Reviews of Interventions, including descriptions of randomization, allocation concealment, blinding methods, incomplete outcome data, selective reporting, and other forms of bias, was used to evaluate the limitations of the included studies.
Data from the included studies were independently extracted by two researchers. Any discrepancies were resolved through discussion until a consensus was reached. The extracted data included the following information: Author(s), publication year, study design, country or region, study population, sample size, patient age, tumor size, intervention measures, and outcome data. The 1-year, 2-year, and 3-year survival rates, objective response rate (ORR), disease control rate (DCR), postoperative alpha-fetoprotein (AFP) decrease, decrease in CD8+ T cells, and increase in CD4+ T cells were directly extracted from the original data. If discrepancies arose, a third investigator was involved to reach a conclusion.
In our study, we used Excel 2010 software to extract the data and RevMan 5.3 software for the statistical analysis. Dichotomous variables in the experimental and control groups were evaluated using odds ratio (OR) and 95% confidence interval (CI), while continuous variables were evaluated using mean difference (MD) and 95%CI. To assess the heterogeneity of the included studies, a χ2 test and I2 statistic were used to test statistical heterogeneity. P value ≥ 0.1 and I2 > 50% indicated significant statistical heterogeneity, and a random-effects model was used. If no statistical heterogeneity was found, a fixed-effects model was used. Sensitivity analysis was performed by excluding individual trials, and publication bias was assessed using Egger’s test.
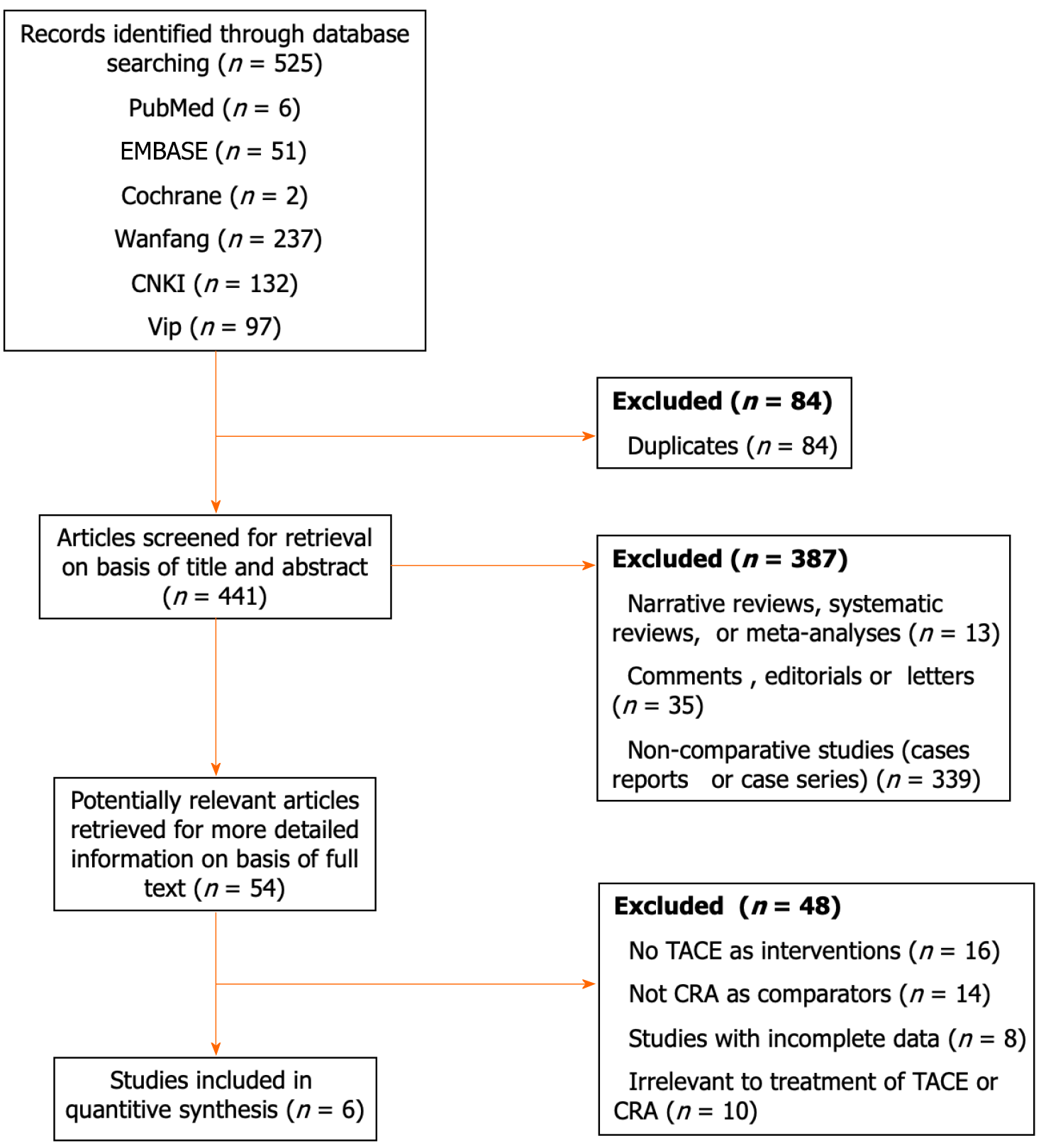
In all, 525 articles were obtained from the literature search, including 466 in Chinese and 59 in English. After duplicate articles were removed, 441 articles were screened. The titles and abstracts were reviewed to eliminate articles that did not meet the established inclusion criteria and included duplicates, reviews, basic research, uncontrolled studies, incomplete outcome measures, reviews or letters, consensus or recommendations, abstracts, ongoing clinical trials, and research proposals. In all, 4 Chinese nonrandomized controlled trials[5-8] and 2 Chinese randomized controlled trials (RCTs)[9,10] were included in the final analysis, and these studies included 575 HCC patients. Of these patients, 285 were in the experimental group and received TACE followed by CRA treatment, while 290 were in the control group and received TACE alone (Figure 1).
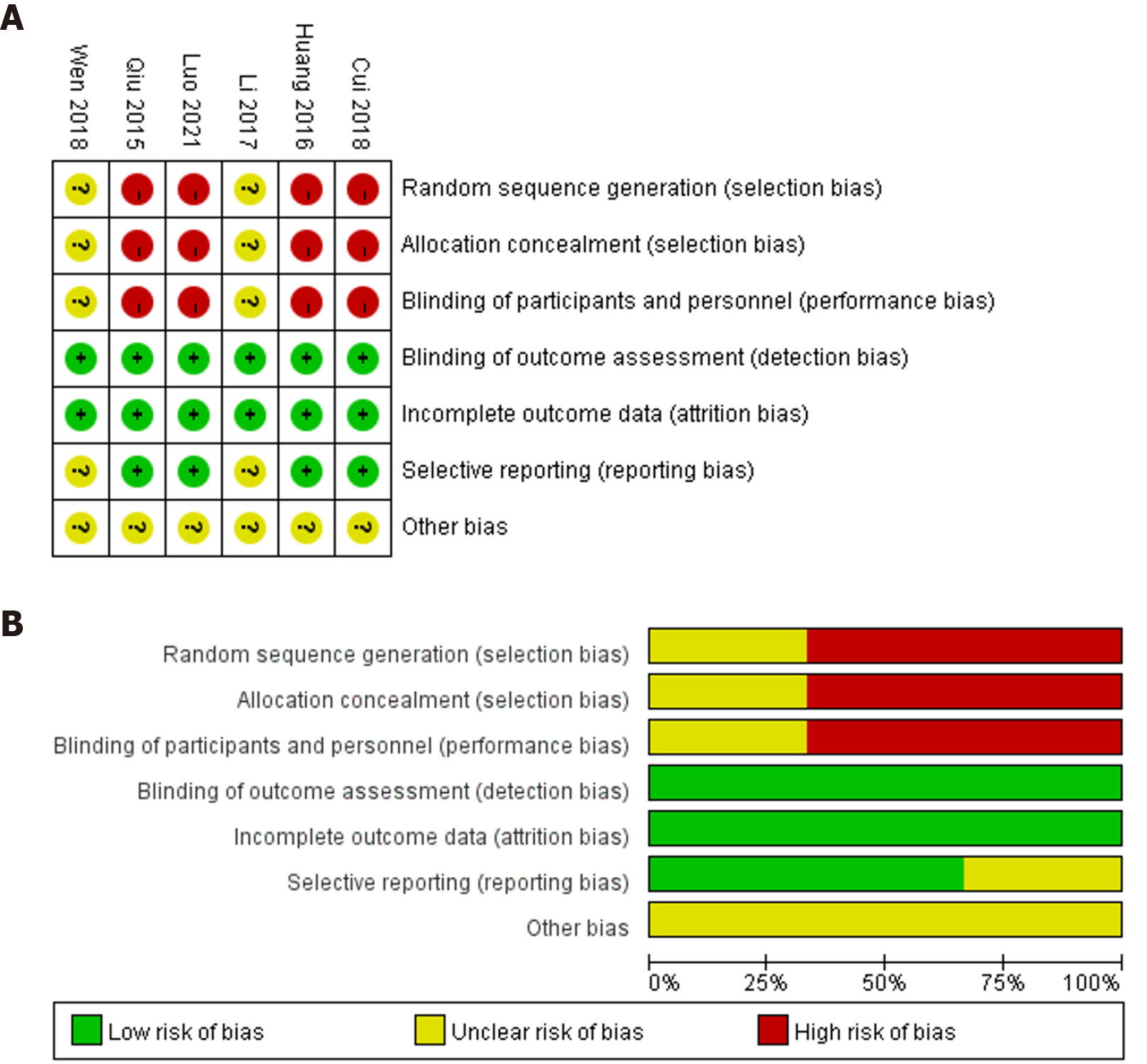
All 6 included studies were peer-reviewed articles published in open access journals. The basic information of the included studies is shown in Table 1. Moreover, the risk of bias depicted in Figure 2 indicated a significantly elevated potential for selection bias and performance bias, which can be attributed in part, to the absence of RCTs in the current meta-analysis.
| Ref. | Country | Design | Inclusion criteria | Intervention | Sample size (n) | Sex (male/female) | Mean age (yr) | Tumor size (cm) | Child-Pugh (A/B) | BCLC (B/C) |
| Cui et al[5], 2018 | China | Retrospective cohort study | HCC diagnosed by clinical history, imaging and laboratory examinations | TACE + CRA | 56 | 49/7 | 50.5 ± 11.3 | ≥ 5 | 52/4 | - |
| TACE | 54 | 49/5 | 52.7 ± 10.4 | ≥ 5 | 50/4 | - | ||||
| Wen and Lin[10], 2018 | China | Retrospective cohort study | HCC diagnosed by clinical history, imaging and laboratory examinations | TACE + CRA | 43 | 35/8 | 54. 8 ± 10. 2 | ≥ 5 | 37/6 | - |
| TACE | 43 | 37/6 | 53. 2 ± 12. 3 | ≥ 5 | 35/8 | - | ||||
| Qiu et al[7], 2015 | China | Retrospective cohort study | HCC diagnosed by clinical history, imaging and laboratory examinations | TACE + CRA | 44 | 28/16 | 51.0 ± 26.1 | 15.87 ± 4.53 | 29/15 | - |
| TACE | 41 | 30/11 | 53.0 ± 23.6 | 14.92 ± 3.97 | 27/14 | - | ||||
| Luo et al[8], 2021 | China | Retrospective cohort study | HCC diagnosed by clinical history, imaging and laboratory examinations | TACE + CRA | 44 | 34/10 | 50.3 ± 7.4 | 15.5 ± 4.2 | 16/28 | 18/26 |
| TACE | 48 | 36/12 | 51.8 ± 8.6 | 14.9 ± 4.3 | 18/30 | 19/29 | ||||
| Li et al[9], 2017 | China | Retrospective cohort study | HCC diagnosed by clinical history, imaging and laboratory examinations | TACE + CRA | 40 | - | 48.7 ± 6.3 | 8.4 ± 3.5 | - | - |
| TACE | 40 | - | - | - | ||||||
| Huang et al[6], 2016 | China | Retrospective cohort study | HCC diagnosed by clinical history, imaging and laboratory examinations | TACE + CRA | 58 | 51/7 | 52 | > 5 | 54/4 | 27/31 |
| TACE | 64 | 59/5 | > 5 | 59/5 | 23/41 |
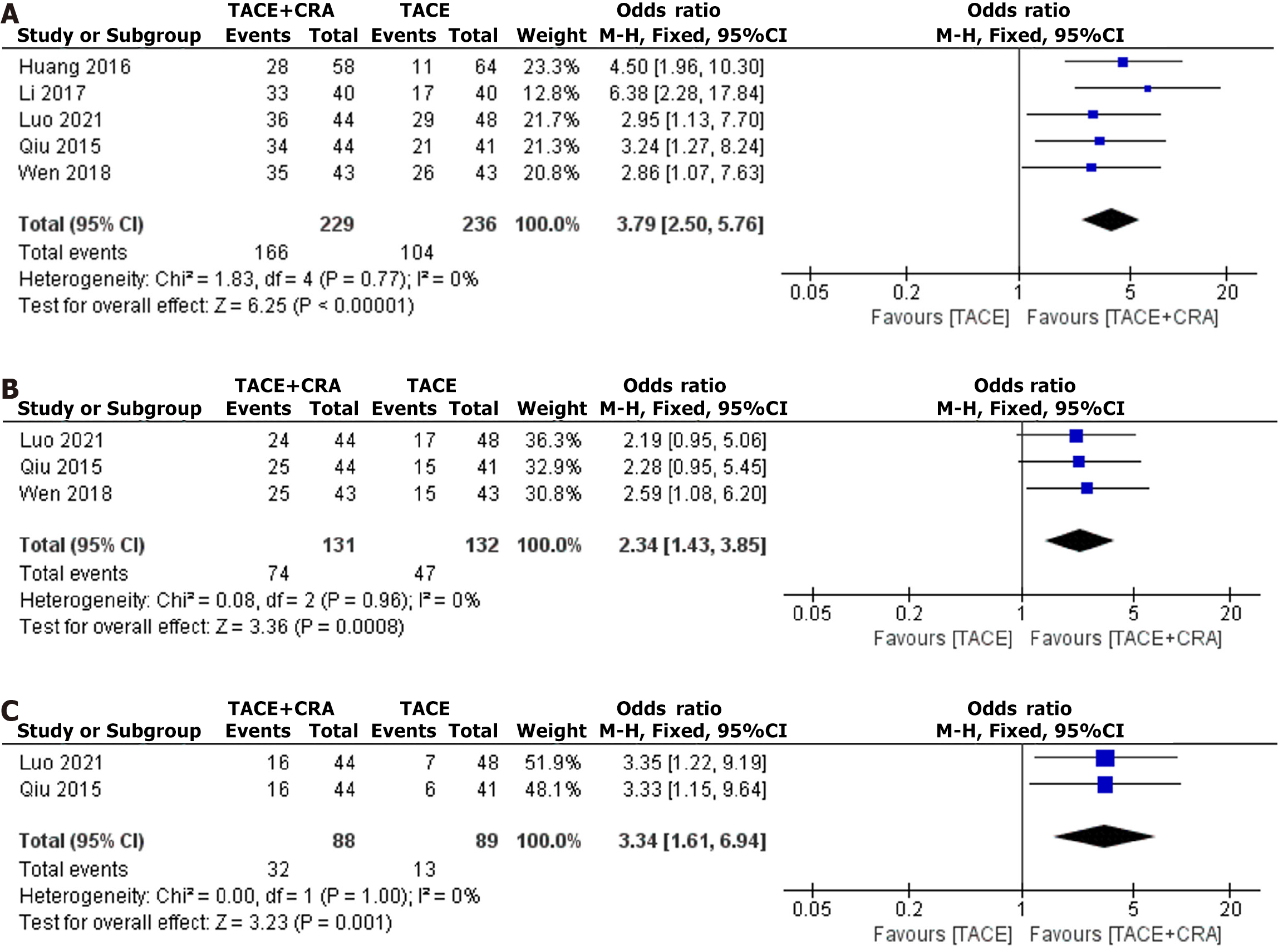
1-year survival: Five studies reported the 1-year survival rate, and as heterogeneity testing revealed no significant difference (I2 = 0%, P = 0.77), a fixed-effects model was used for analysis[6-10]. The difference in the 1-year survival rate between the two groups was statistically significant (OR = 3.79, 95%CI: 2.50-5.76, P < 0.00001, as shown in Figure 3A) and indicates that the 1-year OS rate in the combined treatment group was significantly longer than that in the TACE group. Egger’s test did not reveal any significant evidence of publication bias (bias = -0.0408085; 95%CI: -15.01327 to 14.93165; P = 0.994).
2-year survival: Three studies reported the 2-year survival rate, and as heterogeneity testing revealed no significant difference (I2 = 0%, P = 0.96), a fixed-effects model was used for analysis[7,8,10]. The difference in the 2-year survival rate between the two groups was statistically significant (OR = 2.34, 95%CI: 1.43-3.85, P = 0.0008, as shown in Figure 3B) and indicates that the 2-year OS rate in the combined treatment group was significantly longer than that in the TACE group. Egger’s test did not reveal any significant evidence of publication bias (bias = 6.151379; 95%CI: -72.4699 to 84.77266; P = 0.502).
3-year survival: Two studies reported the 3-year survival rate, and since heterogeneity testing revealed no significant difference (I2 = 0%, P = 1.00), a fixed-effects model was used for analysis[7,8]. The difference in the 3-year survival rate between the two groups was statistically significant (OR = 3.34, 95%CI: 1.61-6.94, P = 0.001, as shown in Figure 3C) and indicates that the 3-year OS rate in the combined treatment group was significantly longer than that in the TACE group.
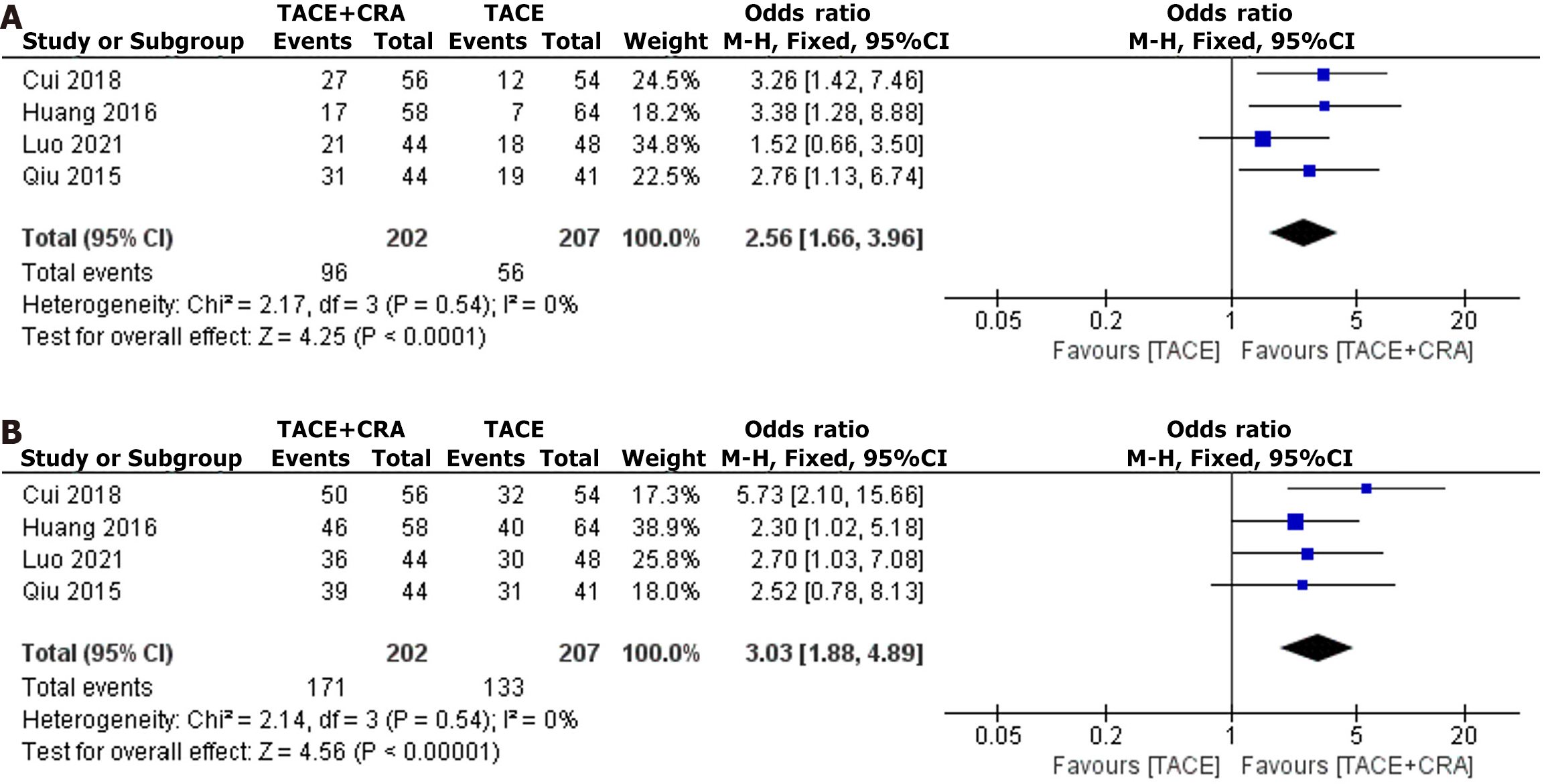
Four studies reported the ORR, and since the heterogeneity test revealed no significant difference (I2 = 0%, P = 0.54), a fixed-effects model was used for analysis[5-8]. The difference in ORR between the two groups was statistically significant (OR = 2.56, 95%CI: 1.66-3.96, P < 0.0001, as shown in Figure 4A), which indicates that the ORR in the combined treatment group was significantly greater than that in the TACE group. Egger’s test did not reveal any significant evidence of publication bias (bias = 5.776609; 95%CI: -25.82534 to 37.37856; P = 0.514).
Four studies reported the DCR, and since the heterogeneity test revealed no significant difference (I2 = 0%, P = 0.54), a fixed-effects model was used for analysis[5-8]. The difference in the DCR between the two groups was statistically significant (OR = 3.03, 95%CI: 1.88-4.89, P < 0.00001, as shown in Figure 4B), which indicates that the DCR in the combined treatment group was significantly greater than that in the TACE group. Egger’s test did not reveal any significant evidence of publication bias (bias = 1.765798; 95%CI: -14.41025 to 17.94184; P = 0.685).
Two studies reported a decrease in postoperative AFP levels, and since the heterogeneity test revealed no significant difference (I2 = 0%, P = 0.54), a fixed-effects model was used for analysis[7,9]. The difference in postoperative AFP decrease between the two groups was statistically significant (OR = 295.53, 95%CI: 250.22-340.85, P < 0.00001, as shown in Figure 5A), which indicates that the postoperative AFP decrease in the combined treatment group was significantly greater than that in the TACE group.
Two studies reported an increase in postoperative CD4+ T cells, and since the heterogeneity test revealed a significant difference (I2 = 77%, P = 0.04), a random-effects model was used for analysis[7,8]. The difference in the postoperative CD4+ increase between the two groups was statistically significant (OR = 10.59, 95%CI: 8.78-12.40, P < 0.00001, as shown in Figure 5B), which indicates that the postoperative CD4+ increase in the combined treatment group was significantly greater than that in the TACE group.
Two studies reported a decrease in postoperative CD8+ T cells, and since heterogeneity testing revealed a significant difference (I2 = 87%, P = 0.005) a random-effects model was used for analysis[7,8]. The difference in the postoperative decrease in CD8+ T-cell density between the two groups was statistically significant (OR = 6.47, 95%CI: 4.44-8.50, P < 0.00001, as shown in Figure 5C), which shows that the postoperative decrease in CD8+ T-cell density in the combined treatment group was significantly greater than that in the TACE group.
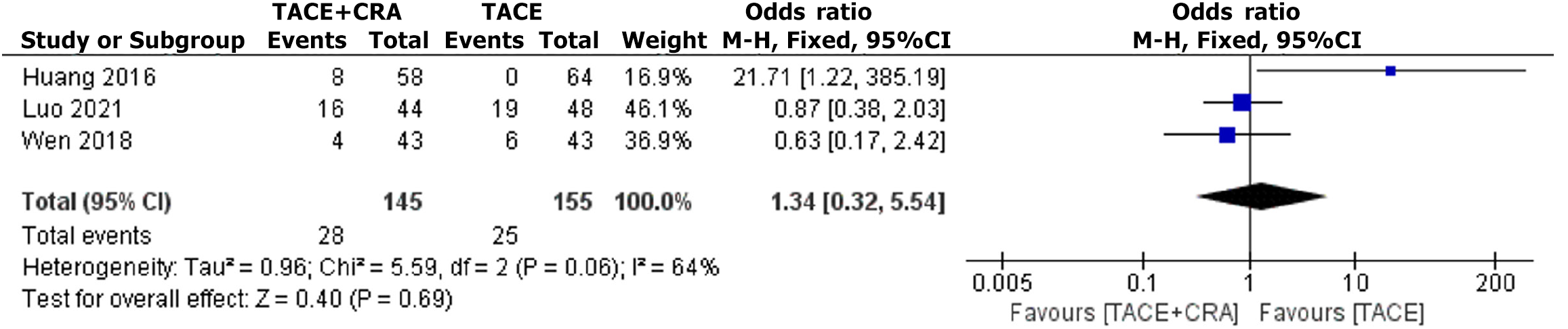
Three studies reported the incidence of complications, and heterogeneity testing revealed a significant difference (I2 = 64%, P = 0.04), and thus a random-effects model was used for analysis[6,8,10]. No statistically significant difference in the incidence of complications was observed between the two groups (OR = 1.34, 95%CI: 0.32-5.54, P = 0.69, as shown in Figure 6). Egger’s test did not reveal any significant evidence of publication bias (bias = 2.509422; 95%CI: -21.12182 to 26.14066; P = 0.0.406).
We conducted a sensitivity analysis to assess the stability of the included studies, the results of which showed that the combined OR did not change significantly with the exclusion of any individual study. Therefore, based on the impact of individual studies on the overall combined effect size, the meta-analysis results are robust.
The treatment of HCC patients with lesions ≥ 5 cm has always been challenging in clinical practice. Currently, TACE is the first-line treatment for HCC patients with lesions ≥ 5 cm[11]. This approach involves arterial infusion of chemotherapy drugs and embolization of the tumor-feeding artery to achieve therapeutic effects including ischemic necrosis. Despite the confirmed safety and efficacy of TACE in clinical practice, its application still has inevitable limitations. On the one hand, the complex blood supply of liver tumor lesions ≥ 5 cm often originates from the hepatic artery and portal vein, and thus, complete occlusion of the tumor-feeding artery is difficult. This results in the establishment of collateral circulation and leads to recurrence and metastasis of the lesion. On the other hand, compared with small tumors, large tumors require multiple TACE sessions to achieve the desired therapeutic effect. However, repeated TACE sessions may affect liver function and even lead to severe complications of liver failure, which exacerbates the disease[12,13]. Furthermore, some studies have suggested that the hypoxic environment in tumor tissue after TACE may induce the upregulation of vascular endothelial growth factor expression, which can promote collateral circulation establishment or increase the extrahepatic blood supply, thereby promoting tumor recurrence and metastasis[14]. Moreover, other studies have shown that lesions ≥ 5 cm are the main factor in the failure of initial TACE to achieve a complete response, as the complete tumor necrosis rate ranges from 40% to 50%[15]. Therefore, the establishment of safer and more effective treatment options has become a challenge in the treatment of HCC patients with lesions ≥ 5 cm.
CRA destroys tumor cells primarily by freezing through ultrarapid cooling and thawing in a single treatment session. Ice crystals form when the temperature decreases and then expand and destroy cells during rewarming[16]. Therefore, compared with single freezing or heating therapy, CRA has greater destructiveness and more precise efficacy. Due to the alternating cold and heat treatment process and the ability to completely destroy lesion tissue, CRA has become an important tool for the treatment of liver cancer. CRA is associated with minimal trauma, significant therapeutic effects, and fewer postoperative complications, especially in the treatment of small liver cancer, which yields satisfactory results. Current research has shown that for early liver cancer patients with tumors < 3 cm, CRA ablation therapy can be an alternative to surgery[17]. However, the use of CRA for the treatment of liver cancer patients with lesions ≥ 5 cm has limitations. Due to the limited maximum ablation range of the freezing needle and the heat loss caused by the rich blood supply of the liver, the effectiveness of CRA is somewhat limited. In addition, for large liver cancers with irregular shapes or larger volumes, even with multiple needle placements or multiple sessions, fully encompassing the tumor and avoiding damage to surrounding tissues can be challenging. Therefore, the use of CRA alone may not fully meet the treatment requirements for patients with liver cancer with lesions ≥ 5 cm.
The results of this study showed that for liver cancer patients with lesions ≥ 5 cm, the ORR and DCR in the combination therapy group were significantly greater than the corresponding values in the TACE-alone group. TACE + CRA combination therapy showed better efficacy, which may be related to the complementary advantages of TACE and CRA in tumor treatment. First, after TACE treatment, blood flow in the lesion decreases, which weakens the heat sink effect and accelerates ice ball formation during CRA; this in turn reduces the risk of bleeding during the ablation process and significantly increases the tumor necrosis rate[18]. Second, angiography can detect small lesions that are difficult to visualize, and after iodized oil deposition, it is more conducive to target area positioning and the evaluation of ablation boundaries. Third, CRA can effectively kill residual tumor cells after TACE, while TACE can enhance the control of satellite foci around the ablated tumor to reduce the recurrence rate. Finally, the permeability of tumor cell membranes increases after CRA, which allows for easier entry of chemotherapy drugs into cancer cells[19], where they play a more significant role.
AFP is an important indicator for monitoring the recurrence and progression of primary liver cancer[20]. Compared with TACE alone, combination therapy resulted in a more significant decrease in postoperative AFP levels, which indirectly suggests that TACE + CRA combination therapy is more effective than TACE alone for liver cancer patients with lesions ≥ 5 cm. This study also revealed that the postoperative survival rates at 1, 2, and 3 years in the combination therapy group were significantly longer than those in the TACE-alone group. This finding is consistent with the higher ORR and DCR observed in the combination therapy group than in the TACE-alone group. It is evident that a higher ORR and DCR indicate that more patients in the combination therapy group were in a state of remission or stability than those in the TACE-alone group, which leads to longer survival times; this finding is also consistent with the findings of previous studies[21].
CD4+ cells play an important role in both humoral and cellular immunity, while CD8+ cells have a negative regulatory effect on immune function. Studies have shown that liver cancer patients often exhibit varying degrees of immune dysfunction, which is manifested by a decrease in the number of CD4+ cells and an increase in the number of CD8+ cells in the peripheral blood T lymphocyte subset[22]. This phenomenon may be related to significant damage to normal liver tissue due to repeated TACE treatments and the subsequent inhibition of immune function against tumors. This study revealed that combination therapy significantly increased the number of CD4+ cells and reduced the number of CD8+ cells compared with TACE alone, which suggests that CRA combined with TACE has the potential to improve immune suppression and enhance the immune function of patients. Studies have suggested that the enhancement of immune function through CRA may be attributable to the necrosis and breakdown of tumor tissue after CRA treatment and the continuous release of antigen-active microtumor tissue into the blood[23,24]. This prompts the body to produce a tumor-specific immune response that inhibits tumor growth and eradicates residual cancer cells. Compared with the TACE treatment group, the combination therapy group showed a significant decrease in AFP levels, a significant increase in CD4+ T cells, and a significant decrease in CD8+ T cells after surgery, which indicates that CRA combined with TACE is more beneficial for protecting or enhancing the immune function of the body than TACE alone. This finding has important clinical significance in the treatment of liver cancer patients with lesions ≥ 5 cm, and the longer postoperative survival of patients in the combination therapy group may also be related to improvements in immune suppression. Studies have shown that good immune function can significantly prolong the survival of cancer patients[25].
This study revealed no statistically significant difference in the incidence of complications between the combination therapy group and the TACE alone group (OR = 1.34, 95%CI: 0.32-5.54; P = 0.69). According to the included literature, no significant increase in complications is observed with combination therapy, and some studies have even shown that combination therapy may be beneficial for reducing the occurrence of complications[6]. One possible reason is that most studies included in our analysis had strict selection criteria, especially for assessing liver function, which may be a key factor in reducing the risk of related complications. On the contrary, treating physicians with sufficient experience can assess the general condition of the patient, liver function, tumor size, tumor location, and tumor number before treatment to determine the best puncture path and appropriate ablation range. This can help avoid damage to adjacent organs and achieve a safe ablation margin under the premise of ensuring safety. In summary, according to the literature, both treatments are relatively safe and have demonstrated good feasibility for clinical application.
The limitations of this study include the following: (1) Due to the inclusion of literature from different research centers, differences in the selection criteria for cases were apparent, and selection bias was difficult to avoid; (2) RCTs that lacked detailed blinding and random allocation methods were included, which increased the risk of related biases; (3) Although we systematically searched six major databases (PubMed, EMBASE, the Cochrane Library, Wanfang, CNKI, and VIP) to obtain all possible related literature, all studies included in this analysis originated in China. We speculate that this may be because approximately 50% of new liver cancer cases worldwide occur in China, while other countries and regions might have a shortage of sufficient cases to conduct such studies; consequently, the majority of relevant research was performed in China[26,27]. Hence, the generalizability of the study findings to a broader population necessitates further validation through larger-scale controlled studies; (4) The varying time intervals between TACE and sequential CRA treatment might have contributed to the heterogeneity of the results; and (5) Due to the different standards for complications in the included literature, some studies may not have defined adverse reactions such as postoperative mild postembolization syndrome or mild symptoms as complications, or they may not have reported such data in detail. Therefore, well-designed and clearly defined large-scale RCTs are still needed to evaluate treatment safety.
In summary, compared with TACE alone, TACE + CRA combination therapy can not only improve the immune function of patients with liver cancer with a diameter ≥ 5 cm, but this therapy also results in better treatment efficacy and long-term survival rates and does not increase the risk of complications. TACE + CRA combination therapy may therefore be a more recommended treatment method for this patient group.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Elpek GO, Türkiye S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4109] [Article Influence: 587.0] [Reference Citation Analysis (6)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55840] [Article Influence: 7977.1] [Reference Citation Analysis (132)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6064] [Article Influence: 866.3] [Reference Citation Analysis (3)] |
| 4. | Galanakis N, Kehagias E, Matthaiou N, Samonakis D, Tsetis D. Transcatheter arterial chemoembolization combined with radiofrequency or microwave ablation for hepatocellular carcinoma: a review. Hepat Oncol. 2018;5:HEP07. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Cui W, Fan W, Huang K, Wang Y, Lu M, Yao W, Li J. Large hepatocellular carcinomas: treatment with transarterial chemoembolization alone or in combination with percutaneous cryoablation. Int J Hyperthermia. 2018;35:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Huang K, Fan W, Zhang Y, Wang Y, Cui W, Li J. [Transarterial chemoembolization combined with cryoablation for unresectable large hepatocellular carcinoma: a controlled study]. Zhonghua Yixue Zazhi. 2016;96:2978-2982. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Qiu G, Xu Li, Luo P, Chen Y. [Clinical observation of argon-helium knife cryotherapy combined with transcatheter arterial chemoembolization(tace) on huge liver cancer]. Linchuang Zhongliuxue Zazhi. 2015;20:540-544. |
| 8. | Luo J, Sheng W, Ma J, Li J. [The effect on immune function of patients with huge liver cancer by argon-helium knife cryoablation combined with TACE]. Zhonghua Puwaike SHoushuxue Zazhi. 2021;15:298-301. [DOI] [Full Text] |
| 9. | Li J. [Clinical efficacy of hepatic artery embolization chemotherapy combined with argon-helium knife for middle and advanced hepatocellular carcinoma]. Dangdai Yixue. 2017;23:104-106. [DOI] [Full Text] |
| 10. | Wen C, Lin Zhi. [Efficacy and safety of transcatheter arterial chemoembolization combined with cryoablation in the treatment of unresectable large hepatocellular carcinoma]. Zhongguo Zonghe Linchuang. 2018;34:339-343. [DOI] [Full Text] |
| 11. | Massani M, Stecca T, Ruffolo C, Bassi N. Should we routinely use DEBTACE for unresectable HCC? cTACE versus DEBTACE: a single-center survival analysis. Updates Surg. 2017;69:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Li Z, Mi DH, Yang KH, Cao N, Tian JH, Ma B, Liu YL. [Effectiveness and Safety of TACE Combined with AHCS for Primary Hepatic Carcinomas: A Systematic Review]. Zhongguo Xunzheng Yixue Zazhi. 2013;13:31-38. |
| 13. | Yan F. [Clinical efficacy of double-intervention treatment of advanced liver cancer]. Linchuang Shiyan Yixue Zazhi. 2011;10:1282-1283. |
| 14. | Deng K, Yang R, Huang Z, Meng H, Deng M. [Clinical value of serum VEGF testing after TACE in primary liver cancer]. Dangdai Yiyong Yingxiangxue. 2019;28:1893-1895. |
| 15. | Kim BK, Kim SU, Kim KA, Chung YE, Kim MJ, Park MS, Park JY, Kim DY, Ahn SH, Kim MD, Park SI, Won JY, Lee DY, Han KH. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. 2015;62:1304-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 16. | Chen Y, Li R, Guo W. [Progress of antitumor immunological mechanisms of Argon-helium cryosurgical system]. Mianyixue Zazhi. 2018;34:259-264. |
| 17. | Yan QH, Xu DG, Shen YF, Yuan DL, Bao JH, Li HB, Lv YG. Observation of the effect of targeted therapy of 64-slice spiral CT combined with cryoablation for liver cancer. World J Gastroenterol. 2017;23:4080-4089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Mala T, Frich L, Aurdal L, Clausen OP, Edwin B, Søreide O, Gladhaug IP. Hepatic vascular inflow occlusion enhances tissue destruction during cryoablation of porcine liver. J Surg Res. 2003;115:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Morris DL, Horton MD, Dilley AV, Warlters A, Clingan PR. Treatment of hepatic metastases by cryotherapy and regional cytotoxic perfusion. Gut. 1993;34:1156-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Wang X, Gu Y, Jin C, Chen Y, Wu Z. [Characteristics and clinical signifcance of tumor markers in sera of patients with liver carcinoma]. Zhongguo Zhongliu Fangzhi Zazhi. 2006;. |
| 21. | Ni M. [Clinical effect of transcatheter arterial chemoembolization combined with argon-helium cryoablation in patients with advanced unresectable primary hepatocellular carcinoma]. Linchuang Gandan Zazhi. 2015;. [DOI] [Full Text] |
| 22. | Liu J. [Effects of Postoperative Pain on Cellular Immune Function and Early Prognosis in patients with Primary liver cancer]. Guangzhou: Southern Medical University, 2017. |
| 23. | Zhang CX, Cheng Y, Ma LX, Jin FY, Liu YX. The immune-enhancing effect of Ar-He targeted cryoablation for the treatment of advanced non - small cell lung cancer. Tumor. 2007;27: 741-743. |
| 24. | Ravindranath MH, Wood TF, Soh D, Gonzales A, Muthugounder S, Perez C, Morton DL, Bilchik AJ. Cryosurgical ablation of liver tumors in colon cancer patients increases the serum total ganglioside level and then selectively augments antiganglioside IgM. Cryobiology. 2002;45:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054-1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (2)] |
| 26. | Zheng R, Sun KX, Zhang S, Zeng H, Zou X, Chen R, Gu X, Wei W, He J. [Report of cancer epidemiology in China, 2015]. Zhonghua zhongliu zazhi. 2019;41:19-28. [RCA] [DOI] [Full Text] [Cited by in RCA: 277] [Reference Citation Analysis (0)] |
| 27. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20516] [Article Influence: 2051.6] [Reference Citation Analysis (20)] |