Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2742
Revised: March 20, 2024
Accepted: April 16, 2024
Published online: June 15, 2024
Processing time: 156 Days and 13 Hours
Hepatocellular carcinoma (HCC) is the most common malignant liver disease in the world. Platelets (PLTs) are known to play a key role in the maintenance of liver homeostasis and the pathophysiological processes of a variety of liver diseases. Aspirin is the most classic antiplatelet agent. However, the molecular mechanism of platelet action and whether aspirin can affect HCC progression by inhibiting platelet activity need further study.
To explore the impact of the antiplatelet effect of aspirin on the development of HCC.
Platelet-rich plasma, platelet plasma, pure platelet, and platelet lysate were prepared, and a coculture model of PLTs and HCC cells was established. CCK-8 analysis, apoptosis analysis, Transwell analysis, and real-time polymerase chain reaction (RT-PCR) were used to analyze the effects of PLTs on the growth, metastasis, and inflammatory microenvironment of HCC. RT-PCR and Western blot were used to detect the effects of platelet activation on tumor-related signaling pathways. Aspirin was used to block the activation and aggregation of PLTs both in vitro and in vivo, and the effect of PLTs on the progression of HCC was detected.
PLTs significantly promoted the growth, invasion, epithelial-mesenchymal transition, and formation of an inflammatory microenvironment in HCC cells. Activated PLTs promoted HCC progression by activating the mitogen-activated protein kinase/protein kinase B/signal transducer and activator of transcription three (MAPK/ AKT/STAT3) signaling axis. Additionally, aspirin inhibited HCC progression in vitro and in vivo by inhibiting platelet activation.
PLTs play an important role in the pathogenesis of HCC, and aspirin can affect HCC progression by inhibiting platelet activity. These results suggest that antiplatelet therapy has promising application prospects in the treatment and combined treatment of HCC.
Core Tip: Previous studies have revealed the role of platelet plasma and its derivatives in tumorigenesis and development. Aspirin has also been shown to reduce the risk of hepatocellular carcinoma (HCC). However, the molecular mechanism of platelet action and whether aspirin can affect the progression of HCC by inhibiting platelet activity need to be further studied. Therefore, our study focused on exploring the functional mechanisms of platelets in the progression of HCC and using aspirin as an example to evaluate the application prospects of antiplatelet therapy. These findings will provide a potential strategy for the treatment of HCC and combination therapy.
- Citation: Zhao LJ, Wang ZY, Liu WT, Yu LL, Qi HN, Ren J, Zhang CG. Aspirin suppresses hepatocellular carcinoma progression by inhibiting platelet activity. World J Gastrointest Oncol 2024; 16(6): 2742-2756
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2742.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2742
Platelets (PLTs), the main component of the coagulation system, are produced by megakaryocytes in the hematopoietic tissues of the bone marrow and play a physiological role in coagulation, hemostasis and wound healing[1]. However, subsequent research has shown that PLTs also play a critical role in other pathological processes, including inflammation, tissue repair, innate immunity, and tumor growth and metastasis[2]. In recent years, increasing clinical evidence has suggested that an increased platelet count may be a predictive factor for certain cancers, such as bladder cancer, esophageal cancer, breast cancer, lung cancer, colorectal cancer, gallbladder cancer, pancreatic cancer, cervical cancer, and stomach cancer, and it is also a method for monitoring tumor progression[3]. Furthermore, many preclinical studies have revealed the close association between PLTs and tumor cells in processes such as tumor cell proliferation, angiogenesis, tumor-related inflammation, and chemotherapy resistance[1,4], highlighting their potential as diagnostic and prognostic biomarkers and therapeutic targets[5].
Hepatocellular carcinoma (HCC) is the most common malignant liver disease in the world, accounting for more than 90% of primary liver cancers, and it is also a malignant disease that seriously threatens the health and life of the Chinese people[6]. Studies have shown that the liver is an important organ for the regulation of platelet production and clearance[7]. PLTs play a key role in the maintenance of liver homeostasis and in the pathological and physiological processes of various liver diseases[8]. Under physiological conditions, PLTs promote hemostasis and maintain vascular integrity. They also regulate the immune system to protect the liver from pathogen invasion[9]. However, under pathological conditions, the function of PLTs seems to be dual, exerting either protective or harmful effects in different pathological stages[9]. For example, in acute viral hepatitis, PLTs can enhance adaptive immunity[10], while in other types of liver injury, PLTs can promote damage, fibrosis, and HCC[11]. Overall, patients with chronic liver disease (CLD) often have abnormalities in platelet count or quality[8]. These findings suggest that PLTs may play a significant role in the occurrence and development of liver cancer and that in-depth research on the role and molecular mechanisms of PLTs in the occurrence and development of HCC is highly important.
Aspirin is one of the three classic drugs in the history of medicine and is widely used in clinical practice for its anti-inflammatory and analgesic effects, cardiovascular and cerebrovascular events, postoperative thrombosis prevention and so on[12]. It is still the most widely used antipyretic, analgesic, and anti-inflammatory drug in the world and is also used as a standard preparation for comparing and evaluating other drugs[13]. Aspirin appears to be a panacea that is effective in treating a variety of diseases, including cancer[13]. Recently, aspirin was found to effectively inhibit the release of cyclooxygenase-1 from PLTs, leading to platelet dysfunction and thus inhibiting the metastasis of tumor cells[14]. Clinical studies have shown that low-dose aspirin has been linked to a reduced risk of various cancers[15]. Aspirin can improve the survival rate by reducing liver inflammation and thus improving liver function[16]. Aspirin can reduce the mortality rate of CLD and can reduce the risk of liver cancer onset[17]. However, whether aspirin inhibits HCC progression by inhibiting PLTs remains to be studied.
In this study, we found that PLTs could significantly promote growth, invasion, epithelial-mesenchymal transition (EMT), and the formation of an inflammatory microenvironment in HCC cells through platelet and HCC cell coculture experiments. Furthermore, we found that activated PLTs promoted HCC progression by activating the mitogen-activated protein kinase/protein kinase B/signal transducer and activator of transcription three (MAPK/AKT/STAT3) signaling axis in HCC and that aspirin inhibited HCC progression in vitro and in vivo by inhibiting platelet activation. In conclusion, our study showed that PLTs play an important role in the pathogenesis of HCC. Moreover, aspirin has promising application prospects in the treatment of HCC and combination therapy, which will provide potential ideas for the clinical treatment of liver cancer diseases and a theoretical basis for the risks of clinical blood transfusion therapy.
Whole blood (20 mL) was collected from healthy adults in our research group and centrifuged to obtain platelet-rich plasma (PRP). After centrifugation, the supernatant was collected, which was platelet-poor plasma (PPP), and the remaining plasma in the lower layer was PRP with a high platelet content (the platelet concentration in PRP used in this project was 800 × 109/L). After separation, a purified PLT was obtained (the PLT concentration used in this study was 800 × 109/L). Finally, PLTs are subjected to several cycles of repeated freeze-thaw cycles at a platelet concentration of 800 × 109/L to disrupt the membrane structure and release the growth factors stored in PLTs to form platelet lysates (PLs).
The human HCC cell lines HepG2 (#CBP60199, COBIOER) and SMMC-7721 (#CBP60210, COBIOER) and the mouse hepatoma cell line HepA1-6 (#CBP60574, COBIOER) were purchased from Nanjing Cobioer Biosciences Co., Ltd. All HCC cell lines were cultured in DMEM (#12100, Solarbio) supplemented with 10% fetal bovine serum (FBS, #P08X20, G-P Link) supplemented with 100 U/mL penicillin/streptomycin (#C100C5, New Cell & Molecular Biotech Co., Ltd.). All cell lines were maintained in a 5% CO2 incubator at 37 °C.
For cell coculture experiments, HepG2 and SMMC-7721 cells were seeded in 6-well plates at 5 × 106 cells/well the day before the experiment, the cells were mounted, remained intact for 8 h and then starved for 4 h. Then, the supernatant was replaced with different configured cell coculture media (10% PPP medium, 10% PRP medium, 10% PLT medium, and 10% PL medium) for 24 h. Note: Heparin (#07980, Stemcell Technologies) was added to the PL at a concentration of 2 IU/mL to prevent coagulation.
HepG2 cells and SMMC-7721 cells were seeded in 96-well plates at 2 × 103 cells/well, and the medium was changed to platelet-containing coculture medium according to experimental needs after the cells were adherent. Cell viability was measured for the first time (0 h) after cell attachment, followed by 24 h, 48 h, 72 h, and 96 h. The cell viability assay was performed according to the manufacturer’s instructions. CCK8 (10 μL, #B34304, Bimake) reagent was added to each well, and the plates were incubated in a 37 °C incubator for 2 h, after which the absorbance was determined at 450 nm using a Multiskan MK3 microplate reader (Thermo).
Transwell assays were used to test the invasion of HCC cells according to the manufacturer’s instructions. Briefly, 1 × 105 cells were seeded in serum-free culture media and placed inside a Transwell chamber (8 μm pore size, #19521030, Costay) with complete medium containing 20% FBS in the lower chamber. After 28 h of incubation at 37 °C, a cotton swab was used to remove the residual cells on the upper surface of the inner chamber. Cells that migrated to the bottom of the membrane were fixed with 4% paraformaldehyde (#SL1830, Coolaber) and stained with 0.1% crystal violet (#G1064, Solarbio). Images were taken under a light microscope (Leica DMI8), and the number of cells in 5 randomly selected fields of view was counted for quantification.
Aspirin (#HY-14654, MCE) was purchased from MCE and dissolved in dimethylsulfoxide (DMSO, #D8371, Solarbio). HepG2 and SMMC-7721 cells were seeded in 96-well culture plates at a density of 6 × 104 cells/well (100 μL per well) and incubated overnight at 37 °C, 5% CO2, and saturated humidity. After adherence, aspirin (#195036, MCE) was added to reach final concentrations of 102.4 μM, 51.2 μM, 25.6 μM, 12.8 μM, 6.4 μM, 3.2 μM, 1.6 μM, 0.8 μM, 0.4 μM, 0.2 μM, 0.1 μM, and 0 μM. Each group was set up with 4 compound wells, and the final volume of each well was 100 μL. The control group was supplemented with an equal amount of DMEM. After 24 h of incubation, the medium was replaced, and 10 μL of CCK8 (#B34304, Bimake) reagent was added according to the manufacturer’s instructions. The absorbance was measured at 450 nm using a Multiskan MK3 microplate reader (Thermo).
Cells from the different experimental treatment groups were collected and washed three times with precooled 4 °C PBS. Fluorescein isothiocyanate-labeled Annexin V (Annexin V-FITC) single staining, propidium iodide (PI) single staining, and Annexin V-FITC and PI double staining were performed according to the instructions of the apoptosis kit (#AK10303, Elabscience). All samples were analyzed using a BD FACS CaliburTM Flow Cytometer (#342975, BD Biosciences).
Total RNA was isolated from cell lines using TRIzol reagent (#R401-01, Vazyme) according to the manufacturer’s protocol. HiSCRIPTR II Q RT SuperMix (#R223-01, Vazyme) was used for reverse transcription. HiSCRIPTR Universal SYBR qPCR Master Mix (#Q511-02, Vazyme) and the Pikorea196 Real-Time PCR Detection System (Thermo Fisher Scientific) were used for real-time polymerase chain reaction (RT-PCR). GAPDH was used as an endogenous normalization control, and relative expression levels were calculated using the 2−ΔΔCt method. All of the experiments were performed in three biological replicates. Information on the primers used is given in Supplementary Table 1.
RIPA buffer (#R0020, Solarbio) was used to extract total protein. Then, 5 × protein buffer (#WB2001, New Cell & Molecular Biotech Co., Ltd.) was mixed with the lysates. Proteins were denatured at 95 °C for 10 min and then separated on an 8%-12% sodium dodecyl sulfate polyacrylamide gel. After the proteins were transferred to a PVDF membrane (#IPVH00010, Millipore), the PVDF membrane was blocked in blocking solution (#P0252, Beyotime) for 2 h at room temperature. After that, the membranes were incubated with the corresponding primary antibodies at 4 °C according to the manufacturer’s instructions. After treatment with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody, the membrane was incubated with an enhanced chemiluminescence (ECL) kit (#KE0101, Kemix). The ECL signals were detected using an ECL imaging system (GE Amersham Imager 680, United States). The following antibodies were used: p-MAPK (#4390S, CST), p-AKT (#4060T, CST), P-STAT3 (#9139s, CST), GAPDH (AB0037, abways), anti-mouse IgG (#P03801M, Gplink), and anti-rabbit IgG (#P03802M, Gplink).
All animal experiments were carried out in accordance with a protocol approved by the Ethics Committee of Xinxiang Medical College (number: XYLL-2020537, date: 2020.08.01). C57BL/6 black mice (5 wk old) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd., and maintained under specific pathogen-free (SPF) conditions.
As previously described[18], HCC mouse models were constructed using diethylnitrosamine chemically induced in 5-wk-old C57BL/6 mice. After 25 wk, the experimental group was treated with 30 mg/kg/d aspirin and intragastrically treated for two weeks every other day. At the end of the experiment, whole blood samples were collected from the mice via an EDTA anticoagulant tube for routine blood testing. EDTA anticoagulated plasma was collected for blood biochemical analysis. Mouse liver tissue was collected, fixed with 10% neutral formaldehyde, or stored at 80 °C for immunohistochemistry experiments and subsequent analysis.
For the subcutaneous tumorigenesis experiment in mice, we used 5-wk-old C57BL/6 male mice (Beijing Vital River Laboratory Animal Technology Co., Ltd.). Mouse HepA1-6 cells were subcutaneously injected with 1.2 × 106/0.1 mL of saline. After injection, the animals were monitored for 1-2 wk, and when the tumor volume reached an average of 50 mm3, the experimental group was given 30 mg/kg/d aspirin and gavaged for two weeks every other day. At the end of the experiment, the mice were sacrificed, the tumors were collected, and tumor volume and weight were measured. The tumor volume (V) was calculated as follows: V = L × W2 × 0.5.
The specimens were fixed in 4% paraformaldehyde (#SL1830, Coolaber) overnight and then paraffin-embedded. Specimens with a thickness of 8 μm were cut, placed on glass slides, and dried overnight. Sections were dewaxed and rehydrated before staining. Antigen repair was performed with citric antigen repair solution (#G1202, Sevier), and endogenous peroxidase activity was blocked with 3% hydrogen peroxide solution. The samples were incubated with 3% BSA for 1 h to block specific antibody binding. The slides were incubated with a primary antibody against Ki67 (1:100) at 4 °C overnight. Then, the secondary antibody (HRP-labeled) was added to the corresponding primary antibody and incubated for 50 min at room temperature. Then, freshly prepared DAB was used for color development, and the positive signal was a brownish-yellow color. Next, the sections were counterstained with hematoxylin for approximately 3 min, washed with tap water, returned to blue with hematoxylin and then washed again with running water. Finally, the samples were dehydrated and sealed. Images were acquired using a Nikon ECLIPSE 90i microscope. Images were saved, and were acquired with NIS-Elements AR 3.2 software. The different channels (RGBs) of the immunofluorescence image were fused with ImageJ software. Blind quantification was performed using ImageJ software, where the binary image of each channel was converted to a binary image and staining on the threshold image was automatically detected macroscopically.
All experiments were performed independently and repeated at least three times. All the data are presented as the mean ± SD. Two groups were compared using a two-tailed Student’s t test. Multiple groups were compared using one-way or two-way ANOVA. Correlations were calculated using Pearson’s correlation coefficient. All the statistical analyses were performed in GraphPad Prism 9. P < 0.05 was considered to indicate statistical significance. aP < 0.05, bP < 0.01, cP < 0.001.
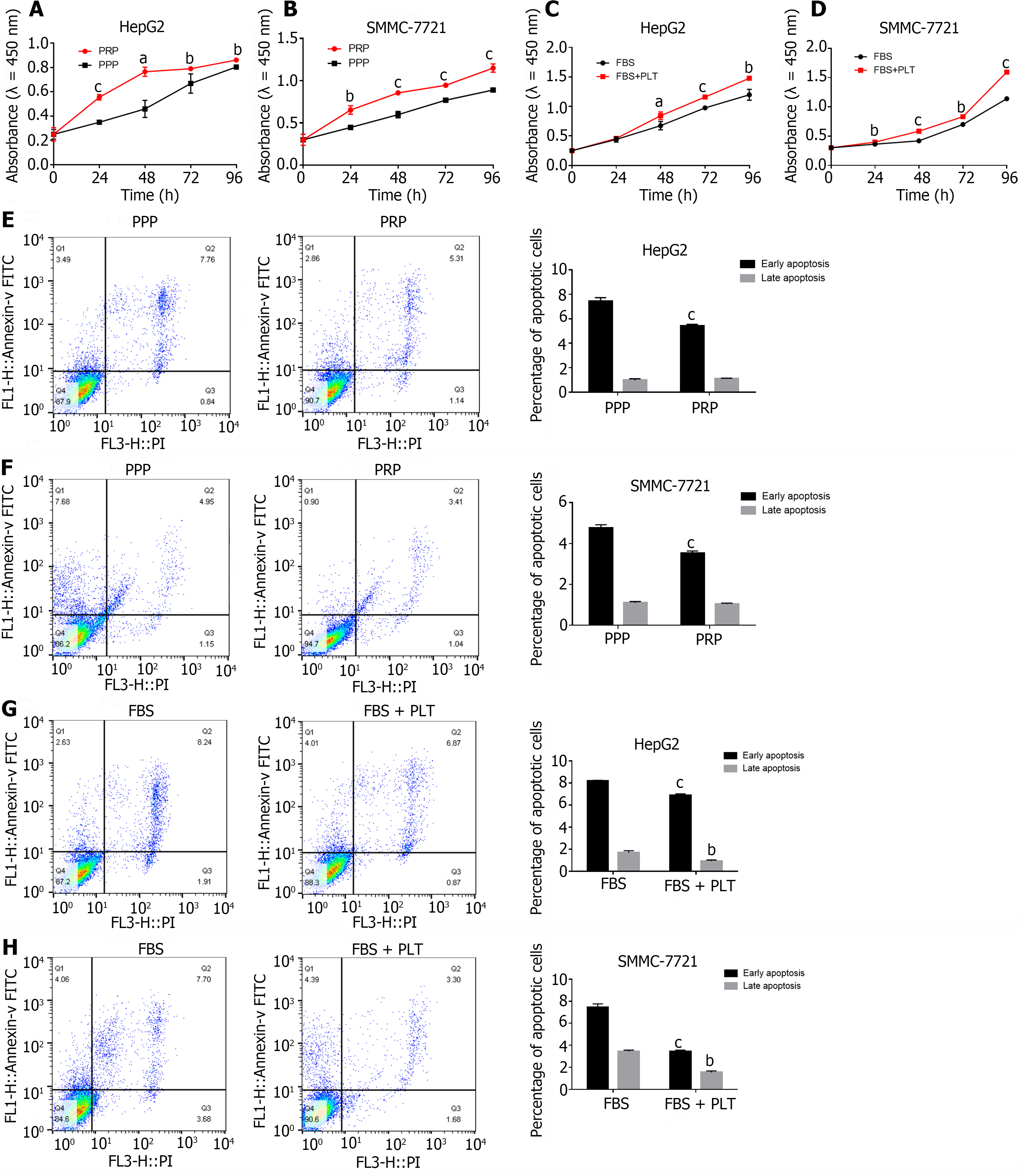
To determine the role of PLTs in the occurrence and development of HCC, we cocultured PPP and PRP with HCC cells in vitro. After 24 h, the effects of PPP and PRP on HCC cell proliferation were detected by CCK-8 analysis. Compared with those in the PPP group, the proliferative capacities of the HepG2 (Figure 1A) and SMMC-7721 (Figure 1B) HCC cells in the PRP group were significantly greater. Given the complex composition of PRP, we separated PRP to obtain purified PLTs. We cocultured PLTs with HCC cells while maintaining the basic growth of HCC cells at a low concentration of FBS (1%)[19] and tested the effect of PLTs on HCC cell proliferation by CCK-8. The results showed that the proliferation of HepG2 (Figure 1C) and SMMC-7721 (Figure 1D) cells was significantly greater after the addition of PLT than in the control group.
Since cell growth is a balance between growth stimulation and apoptosis, we further examined the effect of PLTs on apoptosis by flow cytometry. Compared with the PPP, PRP inhibited the early apoptosis of HepG2 (Figure 1E; Supplementary Figure 1A, 7.45% ± 0.28% vs 5.43% ± 0.11%) and SMMC-7721 (Figure 1F; Supplementary Figure 1B, 4.77% ± 0.15% vs 3.53% ± 0.11%) cells, and the difference in the proportion of cells that underwent early apoptosis between the groups was statistically significant (P < 0.05). However, the effects on late apoptosis (1.13% ± 0.02% vs 0.79% ± 0.12%, 1.04% ± 0.03% vs 1.10% ± 0.05%) were not significant. The purified and concentrated PLTs were cocultured with HCC cells for 24 h, and the effect of PLTs on the apoptosis of HCC cells was detected. PLTs significantly inhibited the apoptosis of HepG2 (Figure 1G; Supplementary Figure 1C) and SMMC-7721 (Figure 1H; Supplementary Figure 1D) HCC cells, and there was a significant difference in the percentage of early apoptotic cells (8.20% ± 0.05% vs 6.89% ± 0.13%, 7.46% ± 0.30% ± 3.45% ± 0.11%) and late apoptotic cells (1.71% ± 0.16% vs 0.94% ± 0.10%, 3.45% ± 0.11% vs 1.54% ± 0.13%) (P < 0.05). The above studies showed that PLTs significantly promote the growth of HCC cells.
PLTs play an important role in tumorigenesis and metastasis[20]. Activated PLTs can interact with tumor cells to secrete a variety of cytokines, promoting EMT and enhancing tumor cell migration and invasion[21]. Therefore, we further investigated the effects of PLTs on HCC cell invasion and EMT ability.
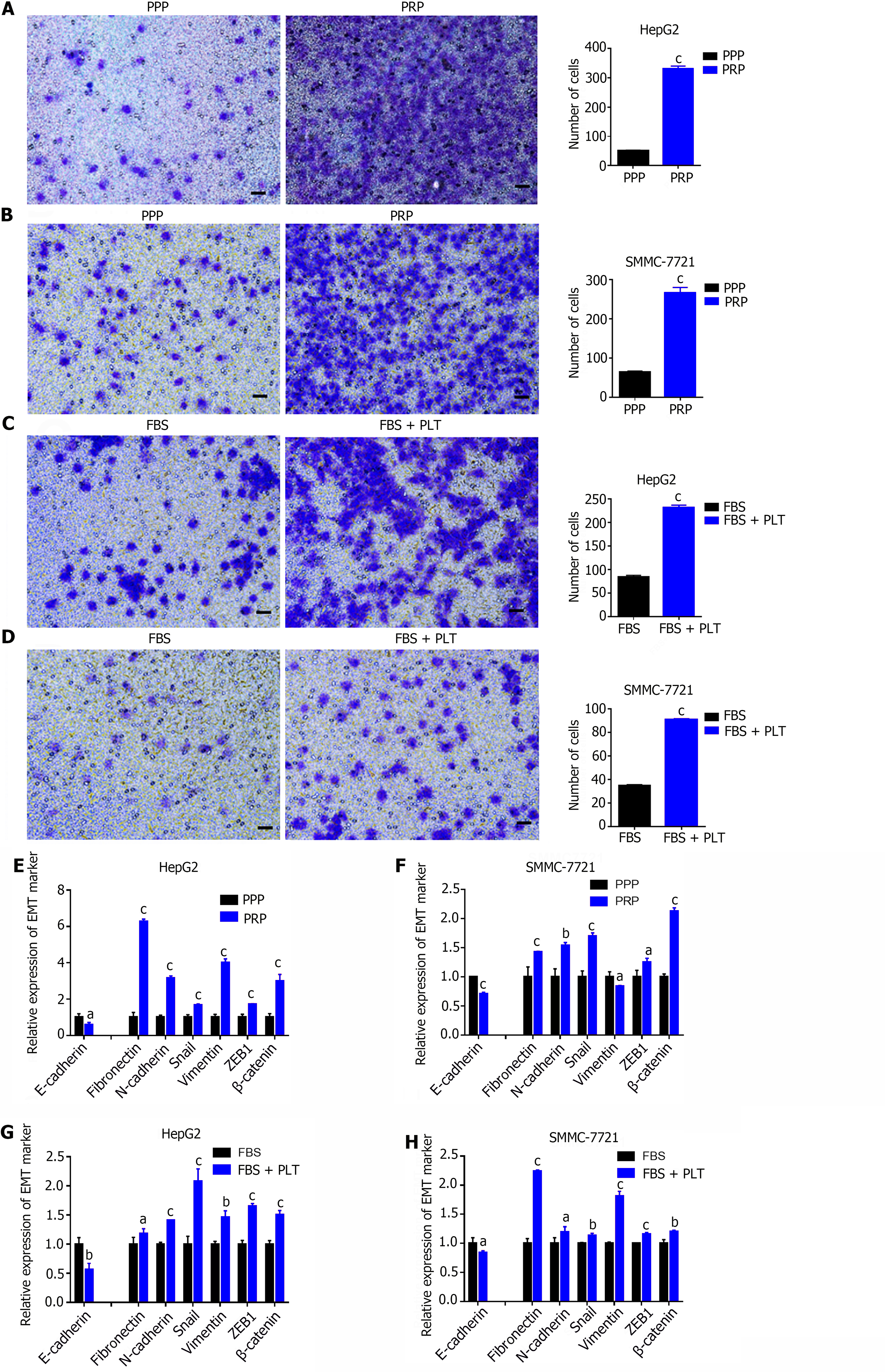
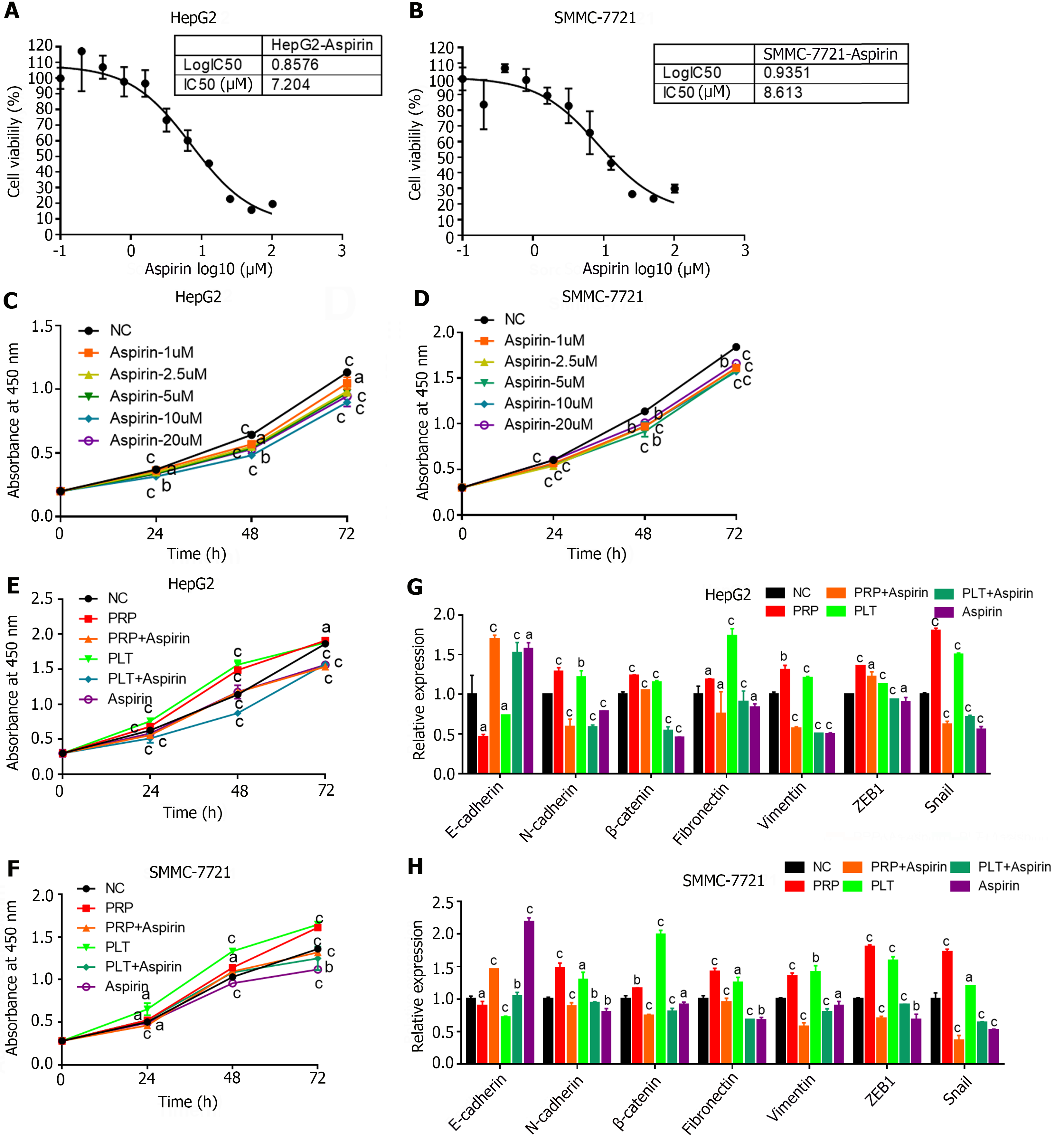
Transwell assays revealed that PRP significantly promoted the invasion ability of HepG2 (Figure 2A) and SMMC-7721 (Figure 2B) cells compared with PPP. Similarly, the invasion capacity of HepG2 (Figure 2C) and SMMC-7721 (Figure 2D) cells was significantly enhanced after PLT addition compared with that of the control group. The RT-PCR results showed that PRP significantly reduced the expression of an epithelial cell marker (E-cadherin) and promoted the expression of mesenchymal cell markers (Fibronectin, N-cadherin, Snail, Vimentin, ZEB1 and β-catenin) in HepG2 (Figure 2E) and SMMC-7721 (Figure 2F) cells. Similarly, the EMT capacity of HepG2 (Figure 2G) and SMMC7721 (Figure 2H) cells was significantly enhanced after the addition of PLT compared to that of the control group. These results indicate that PLTs promote HCC cell invasion and EMT.
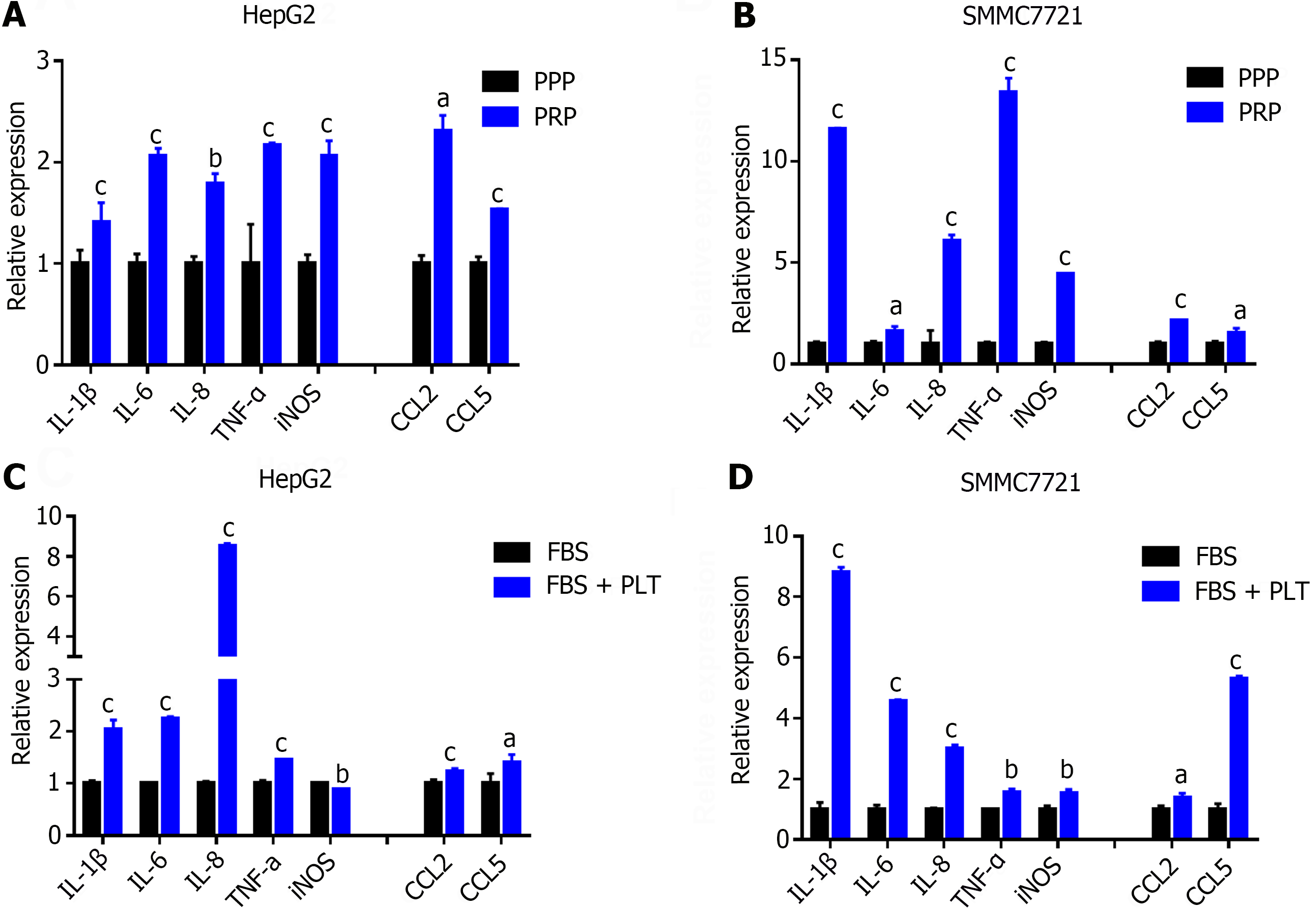
Moreover, previous studies have shown that HCC is a typical inflammation-related tumor, and the tumor immune microenvironment (TIME) plays a key role in HCC development and the response to antitumor therapy[22]. Therefore, we further examined the effect of PLTs on the HCC TIME. We used PPP, PRP and purified and concentrated PLTs to coculture HCC cells, collected the cell pellet after 24 h, extracted total RNA and performed reverse transcription. Finally, the effect of PLTs on the HCC TIME was detected by RT-PCR. Compared with the PPP, PRP stimulated the secretion of inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-a, and iNOS) and chemokines (CCL2 and CCL5) in HepG2 cells (Figure 3A) and SMMC7721 cells (Figure 3B). Similarly, compared with those in the control group, the PLT significantly promoted the secretion of inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α, and iNOS) and chemokines (CCL2 and CCL5) in HCC cells (Figure 3C and D). These results suggest that PLTs promote the formation of an inflammatory microenvironment in HCC.
In summary, our study demonstrated that PLTs can not only significantly promote the proliferation, invasion and EMT of HCC cells but also promote the secretion of more inflammatory cytokines and chemokines by HCC cells, which further aggravates the inflammatory microenvironment of tumors and promotes the growth and survival of tumor cells.
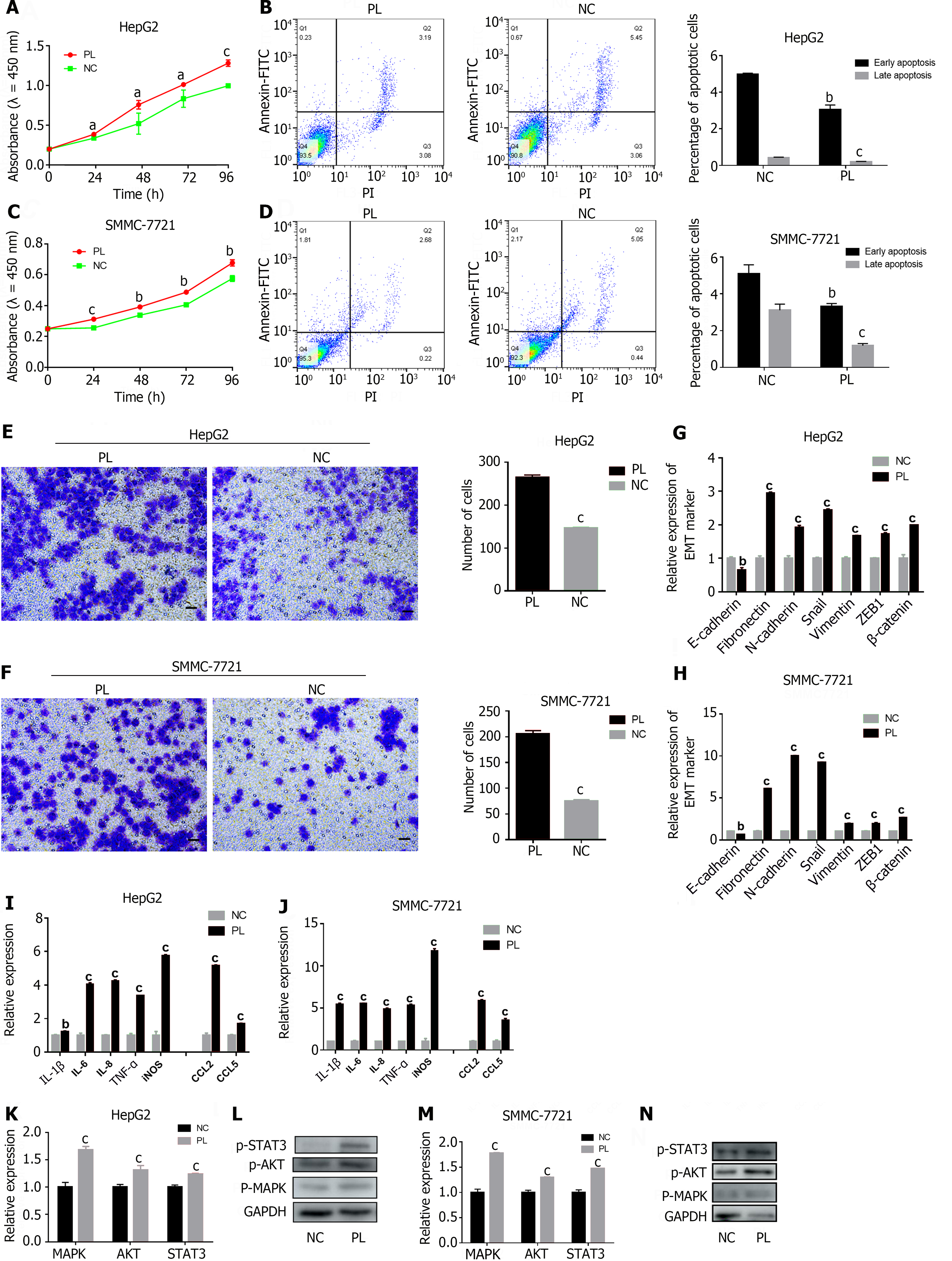
To further explore the mechanism by which PLTs promote the progression of HCC and the formation of an inflammatory microenvironment. We first induced platelet activation to release factors by repeated freezing and thawing cycles. Subsequently, PL was cocultured with HCC cells, and the effect of activated PLTs on the biological activity of HCC cells was monitored in vitro. Compared with that in the control group, the proliferation ability of HCC cells in the PL group was significantly enhanced (Figure 4A and C), while the apoptosis was significantly inhibited (Figure 4B and D; Supplementary Figure 1E and F). The percentages of cells in early apoptosis (4.97% ± 0.06% vs 3.04% ± 0.25%, 5.09% ± 0.49% vs 3.30% ± 0.17%) and late apoptosis (0.42% ± 0.03% vs 0.19% ± 0.02%, 3.11% ± 0.32% vs 1.18% ± 0.11%) were significantly different (P < 0.05). Transwell assays and RT-PCR detection of EMT markers also yielded similar results, that is, compared with the control group, the PL group significantly promoted the invasion of HepG2 cells and SMMC-7721 cells (Figure 4E and F) and EMT ability (Figure 4G and H). In addition, the ability of PL-treated HepG2 cells and SMMC-7721 cells to secrete proinflammatory cytokines was significantly enhanced (Figure 4I and J).
Previous studies have shown that the effects of cytokines mediated by pathways such as the MAPK and JAK/STAT pathways are evidence that inflammation promotes tumorigenesis[23]. Therefore, we hypothesized that activated PLTs could exert proinflammatory and protumor effects on HCC by activating the MAPK/AKT/STAT3 signaling axis. The results showed that PL indeed activated the MAPK/AKT/STAT3 signaling axis (Figure 4K and M) and promoted the protein expression of p-MAPK, p-AKT, and p-STAT3 in HCC (Figure 4L and N). In conclusion, our study revealed that activated PLTs can have proinflammatory and protumor effects on HCC by releasing their components to activate the MAPK/AKT/STAT3 signaling axis.
In addition, studies have shown that aspirin is a classic antiplatelet agent that is effective in treating a variety of diseases, including cancer[13]. To further determine whether the inhibitory effect of aspirin on PLTs can affect the progression of HCC, we performed relevant tests at the cellular and animal levels. We first measured the IC50 of aspirin in the HCC cell lines HepG2 and SMMC-7721 (Figure 5A and B), set up different concentration gradients for cell proliferation experiments (Figure 5C and D), and finally selected the appropriate dose for “rescue” experiments. After coculture of PLTs and HCC cells, aspirin was used for 24 h, and the proliferation and EMT of HCC cells in different experimental groups were detected. The results showed that PRP and PLTs could significantly promote the proliferation and EMT ability of HCC cells. And the proliferation and EMT transformation of PRP and PLTs were significantly inhibited after aspirin treatment (Figure 5E-H). These results indicated that aspirin could inhibit HCC tumor growth and EMT by inhibiting platelet activity.
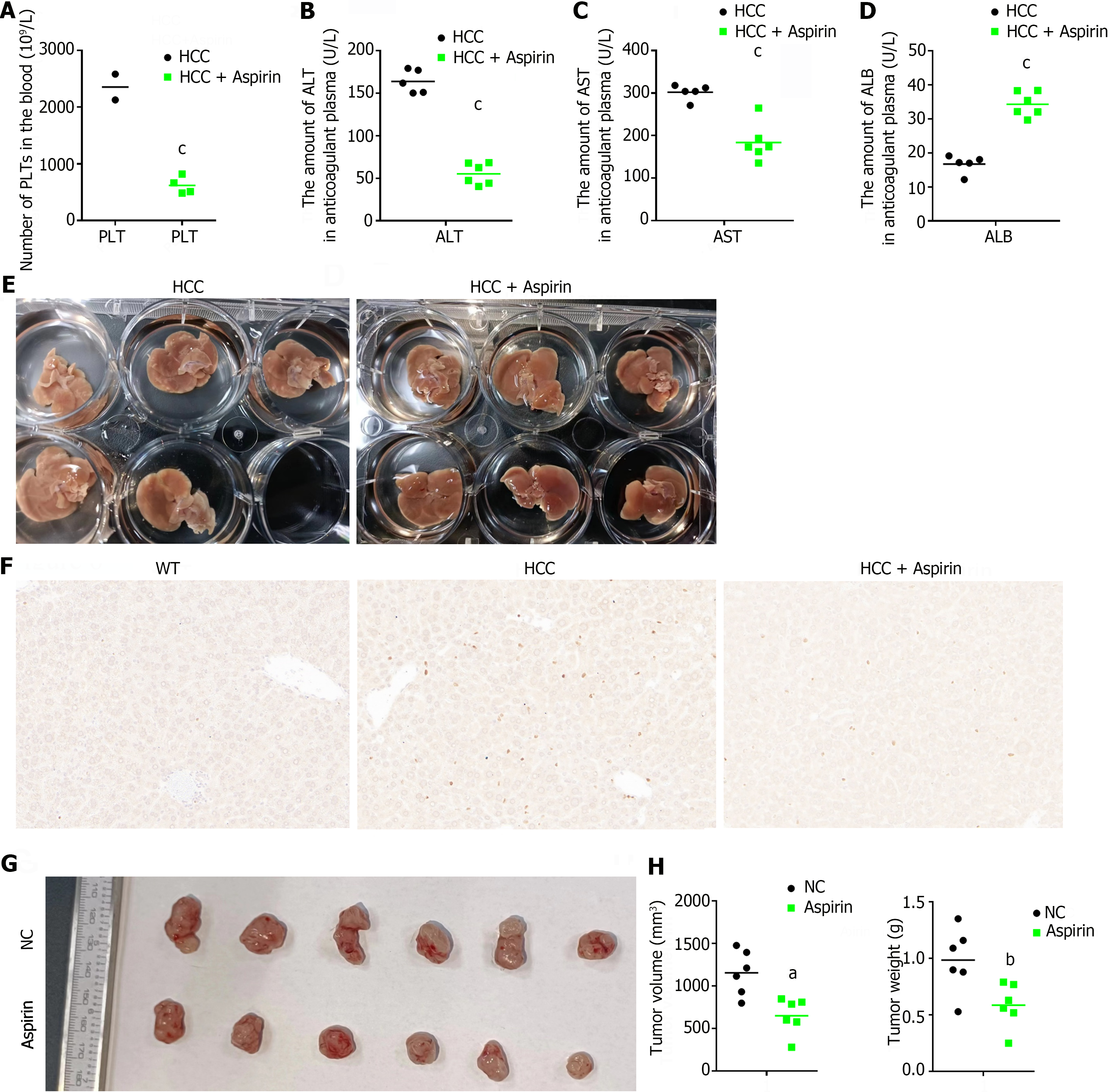
Second, we tested the platelet-blocking effect of aspirin and whether blocking platelet activation could inhibit the growth of HCC tumors in a constructed animal model of HCC. We first collected whole blood from DEN-induced HCC mice and aspirin-treated HCC mice for routine blood testing to determine the inhibitory effect of aspirin on PLTs. As shown in Figure 6A, the platelet content in the whole blood of the successfully induced HCC model mice was abnormally increased and was significantly greater than the normal value (450-1590 × 109/L). In contrast, the platelet content was significantly reduced in the aspirin-treated HCC model mice (Figure 6A). Next, we measured the effect of aspirin on HCC progression in vivo. Elevated alanine transaminase (ALT) and aspartate transaminase (AST) are the typical manifestations of all liver diseases[24], and a low content of albumin (ALB) often indicates severe liver disease or poor reserve function, which is at the end stage of liver disease[25]. EDTA-K2 anticoagulant plasma was collected, and the levels of ALT, AST and ALB in the different experimental groups were biochemically measured. After 25 wk of induction by the carcinogenic compound DEN, the ALT and AST levels of all mice in the HCC group were significantly greater than the normal values (ALT: 10.06-96.47 U/L, AST: 36.31-235.48 U/L), but the ALB level was significantly lower than the normal value (ALB: 21.22-39.15 U/L), indicating different degrees of liver injury. However, after aspirin treatment, these abnormal indicators were significantly reduced (Figure 6B-D).
We also collected liver tissue from different experimental groups of mice, and macroscopic evaluation revealed a significant reduction in the number of tumors on the cell surface of mice treated with aspirin (Figure 6E). Tumor cells are known to have a greater rate of proliferation[26]. To further evaluate whether aspirin treatment affects tumor cell proliferation in the liver, we performed immunohistochemical staining of liver tissue using the proliferation marker Ki67. The results showed a significant decrease in the percentage of Ki67-positive nuclei in tumor tissue after aspirin treatment (Figure 6F). Finally, to better visualize the effect of aspirin on HCC tumor growth, we treated HCC tumor-bearing mice with aspirin, and the results showed that aspirin significantly inhibited HCC tumor growth (Figure 6G and H). In summary, our study suggested that aspirin can inhibit HCC progression by inhibiting platelet activity.
HCC is the most common malignant tumor in the world[27]. HCC has a low incidence in Western countries and a high incidence in parts of Asia and Africa, particularly China, where it has the third highest mortality rate among digestive malignancies, after gastric and esophageal cancers[28]. Early-stage HCC lesions are usually small and can often be cured through surgical resection, local ablative therapies, or liver transplantation[29]. However, approximately 50% of patients are diagnosed after the manifestation of advanced HCC symptoms, which limits treatment options[29]. Moreover, the five-year recurrence rate after HCC treatment is more than 70%, and the five-year survival rate is unsatisfactory even in patients with early-stage HCC undergoing surgical treatment[30]. Therefore, exploring and studying the pathological mechanism underlying the occurrence and development of HCC, identifying specific or sensitive biological markers as early as possible, and laying a solid foundation for early clinical diagnosis and prognosis evaluation can improve the treatment and prognosis of HCC patients.
PLTs, which are the smallest cells in the peripheral blood and are approximately 2-3 mm in diameter and have no nucleated or discal structure, are derived from megakaryocytes. PLTs are predominantly found in the bone marrow, two-thirds of which are found in the peripheral blood circulation, and the remaining one-third are reversibly sequestered in the spleen[31]. PLTs play multiple roles in physiological and pathological processes, including hemostasis, maintenance of vascular integrity, angiogenesis, inflammatory response, nonspecific immunity, wound healing, and tumorigenesis[32]. However, the role of PLTs in HCC appears to be controversial. Clinical studies have shown a direct correlation between high platelet counts and greater tumor invasiveness as well as poorer survival rates in HCC patients. Conversely, thrombocytopenia can promote patient survival[33]. Furthermore, platelet count has been incorporated as a prognostic biomarker and a criterion for treatment selection in patients at risk of developing HCC and in patients with viral cirrhosis[34]. Notably, other studies suggest that PLTs can also play a protective role in liver regeneration, CLD, and fibrosis, as well as in the prevention of HCC. For instance, platelet-derived growth factor mediates platelet production following liver resection, which can reduce liver fibrosis and promote liver regeneration[35]. PLTs can decrease the progression of liver fibrosis through MMP-9 induction and TGF-β downregulation[36]. PLTs can inhibit HCC growth by enhancing the CD8+ T-cell-dependent antitumor immune response through P2Y12/Leukotriene-dependent CD40L release[37]. Taken together, these data support that PLTs are relevant regulators of CLD and liver cancer, however, their effects are likely dependent on the context.
Currently, platelet transfusion is a commonly used method for the treatment of tumors[38]. Clinical studies have shown that appropriate platelet supplementation can improve and enhance the anticoagulant function of patients for the purpose of coagulation and hemostasis[38]. However, repeated blood transfusions have been found to lead to immune tolerance in patients[39], which subsequently promotes tumor metastasis or recurrence[40]. In our study, to explore the role of PLTs and their components in the development of HCC, we cocultured PRP, PLTs and PL with HCC cells to observe the effect of PLTs and their components on the biological function of HCC cells. The results showed that PLTs and their components play a significant role in influencing the phenotype of HCC and closely contribute to disease progression and malignant processes. These findings further suggest that clinical platelet transfusion therapy has the risk of promoting the progression of HCC.
Furthermore, studies have shown that PLTs are active mediators in the HCC microenvironment[41]. In HCC, severe liver fibrosis and cirrhosis are typically present, leading to platelet aggregation and activation[41]. Activated PLTs not only interact with various types of cells in the HCC microenvironment, including hepatic stellate cells, inflammatory cells, cancer cells, and macrophages[42] but also release multiple growth factors and mitogens, such as PDGF-β, TGF-β, VEGF, interleukin-1, and serotonin[43]. These platelet-derived factors participate in fibroblast signaling, regulate hepatic inflammatory responses, and promote HCC invasion and metastasis[44]. Moreover, this intercellular communication stimulates tumor cells to release various cytokines to induce additional platelet generation or activate platelet secretion of proangiogenic, prometastatic, and tumor cell growth-stimulating factors, further enabling tumor cells to evade immune system attacks[45]. In this study, we found that PLTs could induce HCC cells to secrete more interleukins (IL-1β, IL-6, and IL-8), members of the TNF-α superfamily, proinflammatory cytokines (iNOS), chemokines (CCL2 and CCL5) and other cytokines. These results indicate that PLTs can support the progression of HCC in vivo by stimulating tumor cell proliferation and regulating the surrounding liver microenvironment and further suggest that excessive platelet transfusion has the risk of generating an inflammatory cytokine storm.
In recent years, an increasing number of preclinical and clinical studies have demonstrated that systemic antiplatelet therapy is a promising option for improving the outcomes and efficacy of chemotherapy and immunotherapy treatment. However, the increased risk of bleeding and complications associated with platelet depletion (low platelet count) hinder the targeted use of PLTs as cancer therapeutic agents[46]. Therefore, identifying targets that can effectively block platelet-tumor cell interactions without affecting the normal physiological function of PLTs is highly important[47]. As aspirin is the most classical antiplatelet drug, there have been many studies on the prevention and treatment of tumors with aspirin in recent years[48]. Studies have shown that aspirin can reduce the risk of liver fibrosis in patients with chronic hepatitis B and C, and it has preventive and therapeutic effects on liver cancer[49]. Our research also demonstrated that aspirin inhibits the growth and metastasis of HCC tumors by inhibiting platelet function. This suggests that antiplatelet therapy, including the use of aspirin, holds great potential for preventing or delaying the progression of HCC in patients with CLD, as well as for the treatment and combination therapy of HCC, and warrants further exploration. However, recent clinical data also indicate that daily low-dose aspirin intake is associated with an increased incidence of high-grade cancer at the time of diagnosis[50], particularly an increased risk of cancer-related mortality in older individuals[51]. Therefore, it is important to consider using antiplatelet therapy in combination with chemotherapy, radiation therapy, or immune modulators. In summary, understanding the interaction between PLTs and HCC is vital for elucidating the mechanisms of HCC development and developing new treatment strategies.
PLTs play an important role in the pathogenesis of HCC, and antiplatelet therapies such as aspirin still have wide application prospects for preventing or delaying the progression of HCC in patients with CLD, HCC treatment and combination therapy, which are worthy of further exploration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Papazafiropoulou A, Greece S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Maouia A, Rebetz J, Kapur R, Semple JW. The Immune Nature of Platelets Revisited. Transfus Med Rev. 2020;34:209-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 2. | Daskalaki MG, Tsatsanis C, Kampranis SC. Histone methylation and acetylation in macrophages as a mechanism for regulation of inflammatory responses. J Cell Physiol. 2018;233:6495-6507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Hsieh RW, Ravindran A, Hook CC, Begna KH, Ashrani AA, Pruthi RK, Marshall AL, Hogan W, Litzow M, Hoyer J, Oliveira JL, Vishnu P, Call TG, Al-Kali A, Patnaik M, Gangat N, Pardanani A, Tefferi A, Go RS. Etiologies of Extreme Thrombocytosis: A Contemporary Series. Mayo Clin Proc. 2019;94:1542-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Watson SP, Harrison P, Halford GM. Platelets: the next decade. Platelets. 2020;31:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 5. | Li S, Lu Z, Wu S, Chu T, Li B, Qi F, Zhao Y, Nie G. The dynamic role of platelets in cancer progression and their therapeutic implications. Nat Rev Cancer. 2024;24:72-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 6. | Alenezi AO, Krishna S, Mendiratta-Lala M, Kielar AZ. Imaging and Management of Liver Cancer. Semin Ultrasound CT MR. 2020;41:122-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | López JA. Introduction to a review series on platelets and cancer. Blood. 2021;137:3151-3152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Papapanagiotou A, Daskalakis G, Siasos G, Gargalionis A, Papavassiliou AG. The Role of Platelets in Cardiovascular Disease: Molecular Mechanisms. Curr Pharm Des. 2016;22:4493-4505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Cuesta ÁM, Palao N, Bragado P, Gutierrez-Uzquiza A, Herrera B, Sánchez A, Porras A. New and Old Key Players in Liver Cancer. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 10. | Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, Chisari FV, Ruggeri ZM, Guidotti LG. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167-1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, Leone V, Peiseler M, Surewaard BGJ, Rath D, Ali A, Wolf MJ, Drescher H, Healy ME, Dauch D, Kroy D, Krenkel O, Kohlhepp M, Engleitner T, Olkus A, Sijmonsma T, Volz J, Deppermann C, Stegner D, Helbling P, Nombela-Arrieta C, Rafiei A, Hinterleitner M, Rall M, Baku F, Borst O, Wilson CL, Leslie J, O'Connor T, Weston CJ, Chauhan A, Adams DH, Sheriff L, Teijeiro A, Prinz M, Bogeska R, Anstee N, Bongers MN, Notohamiprodjo M, Geisler T, Withers DJ, Ware J, Mann DA, Augustin HG, Vegiopoulos A, Milsom MD, Rose AJ, Lalor PF, Llovet JM, Pinyol R, Tacke F, Rad R, Matter M, Djouder N, Kubes P, Knolle PA, Unger K, Zender L, Nieswandt B, Gawaz M, Weber A, Heikenwalder M. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25:641-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 12. | Hybiak J, Broniarek I, Kiryczyński G, Los LD, Rosik J, Machaj F, Sławiński H, Jankowska K, Urasińska E. Aspirin and its pleiotropic application. Eur J Pharmacol. 2020;866:172762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Santos-Gallego CG, Badimon J. Overview of Aspirin and Platelet Biology. Am J Cardiol. 2021;144 Suppl 1:S2-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Ornelas A, Zacharias-Millward N, Menter DG, Davis JS, Lichtenberger L, Hawke D, Hawk E, Vilar E, Bhattacharya P, Millward S. Beyond COX-1: the effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 2017;36:289-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 15. | Tsoi KKF, Ho JMW, Chan FCH, Sung JJY. Long-term use of low-dose aspirin for cancer prevention: A 10-year population cohort study in Hong Kong. Int J Cancer. 2019;145:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Lange NF, Radu P, Dufour JF. Prevention of NAFLD-associated HCC: Role of lifestyle and chemoprevention. J Hepatol. 2021;75:1217-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 17. | Ricciotti E, Wangensteen KJ, FitzGerald GA. Aspirin in Hepatocellular Carcinoma. Cancer Res. 2021;81:3751-3761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Heindryckx F, Mertens K, Charette N, Vandeghinste B, Casteleyn C, Van Steenkiste C, Slaets D, Libbrecht L, Staelens S, Starkel P, Geerts A, Colle I, Van Vlierberghe H. Kinetics of angiogenic changes in a new mouse model for hepatocellular carcinoma. Mol Cancer. 2010;9:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Carr BI, Cavallini A, D'Alessandro R, Refolo MG, Lippolis C, Mazzocca A, Messa C. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer. 2014;14:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Zhang Y, Ding Y, Zhuang R. Platelet-mediated tumor metastasis mechanism and the role of cell adhesion molecules. Crit Rev Oncol Hematol. 2021;167:103502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Wang X, Zhao S, Wang Z, Gao T. Platelets involved tumor cell EMT during circulation: communications and interventions. Cell Commun Signal. 2022;20:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Thushara RM, Hemshekhar M, Basappa, Kemparaju K, Rangappa KS, Girish KS. Biologicals, platelet apoptosis and human diseases: An outlook. Crit Rev Oncol Hematol. 2015;93:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Garcia-Lezana T, Lopez-Canovas JL, Villanueva A. Signaling pathways in hepatocellular carcinoma. Adv Cancer Res. 2021;149:63-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 24. | Zhou L, Wang SB, Chen SG, Qu Q, Rui JA. Prognostic Value of ALT, AST, and AAR in Hepatocellular Carcinoma with B-Type Hepatitis-Associated Cirrhosis after Radical Hepatectomy. Clin Lab. 2018;64:1739-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Staufer K, Huber H, Zessner-Spitzenberg J, Stauber R, Finkenstedt A, Bantel H, Weiss TS, Huber M, Starlinger P, Gruenberger T, Reiberger T, Sebens S, McIntyre G, Tabibiazar R, Giaccia A, Zoller H, Trauner M, Mikulits W. Gas6 in chronic liver disease-a novel blood-based biomarker for liver fibrosis. Cell Death Discov. 2023;9:282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Wang J, He H, Jiang Q, Wang Y, Jia S. CBX6 Promotes HCC Metastasis Via Transcription Factors Snail/Zeb1-Mediated EMT Mechanism. Onco Targets Ther. 2020;13:12489-12500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Zheng L, Lv W, Zhou Y, Lin X, Yao J. Progress on the Mechanism for Aspirin's Anti-tumor Effects. Curr Drug Targets. 2021;22:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Gilles H, Garbutt T, Landrum J. Hepatocellular Carcinoma. Crit Care Nurs Clin North Am. 2022;34:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 104] [Reference Citation Analysis (0)] |
| 29. | Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 466] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 30. | Guan Y, Chen J, Nepovimova E, Long M, Wu W, Kuca K. Aflatoxin Detoxification Using Microorganisms and Enzymes. Toxins (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 31. | Ramai D, Ofosu A, Lai JK, Gao ZH, Adler DG. Fibrolamellar Hepatocellular Carcinoma: A Population-Based Observational Study. Dig Dis Sci. 2021;66:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Lee EJ, Lee AI. Thrombocytopenia. Prim Care. 2016;43:543-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Scheiner B, Kirstein M, Popp S, Hucke F, Bota S, Rohr-Udilova N, Reiberger T, Müller C, Trauner M, Peck-Radosavljevic M, Vogel A, Sieghart W, Pinter M. Association of Platelet Count and Mean Platelet Volume with Overall Survival in Patients with Cirrhosis and Unresectable Hepatocellular Carcinoma. Liver Cancer. 2019;8:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Best MG, Wurdinger T. Tumor-educated platelets for the earlier detection of hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2020;44:794-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Murata S, Hashimoto I, Nakano Y, Myronovych A, Watanabe M, Ohkohchi N. Single administration of thrombopoietin prevents progression of liver fibrosis and promotes liver regeneration after partial hepatectomy in cirrhotic rats. Ann Surg. 2008;248:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Watanabe M, Murata S, Hashimoto I, Nakano Y, Ikeda O, Aoyagi Y, Matsuo R, Fukunaga K, Yasue H, Ohkohchi N. Platelets contribute to the reduction of liver fibrosis in mice. J Gastroenterol Hepatol. 2009;24:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Ma C, Fu Q, Diggs LP, McVey JC, McCallen J, Wabitsch S, Ruf B, Brown Z, Heinrich B, Zhang Q, Rosato U, Wang S, Cui L, Berzofsky JA, Kleiner DE, Bosco DB, Wu LJ, Lai CW, Rotman Y, Xie C, Korangy F, Greten TF. Platelets control liver tumor growth through P2Y12-dependent CD40L release in NAFLD. Cancer Cell. 2022;40:986-998.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 38. | Franchini M, Veneri D, Lippi G. Thrombocytopenia and infections. Expert Rev Hematol. 2017;10:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Yu Z, Shibazaki M, Otsuka H, Takada H, Nakamura M, Endo Y. Dynamics of Platelet Behaviors as Defenders and Guardians: Accumulations in Liver, Lung, and Spleen in Mice. Biol Pharm Bull. 2019;42:1253-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Mattson MK, Groves C, Smith MM, Christensen JM, Chen D, Stubbs JR, Karon BS, Nuttall GA. Platelet transfusion: The effects of a fluid warmer on platelet function. Transfusion. 2021;61:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Li X, Zhao K, Lu Y, Wang J, Yao W. Genetic Analysis of Platelet-Related Genes in Hepatocellular Carcinoma Reveals a Novel Prognostic Signature and Determines PRKCD as the Potential Molecular Bridge. Biol Proced Online. 2022;24:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Pavlović N, Kopsida M, Gerwins P, Heindryckx F. Activated platelets contribute to the progression of hepatocellular carcinoma by altering the tumor environment. Life Sci. 2021;277:119612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Guo Y, Cui W, Pei Y, Xu D. Platelets promote invasion and induce epithelial to mesenchymal transition in ovarian cancer cells by TGF-β signaling pathway. Gynecol Oncol. 2019;153:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 44. | Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1398] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 45. | Atasheva S, Shayakhmetov DM. Cytokine Responses to Adenovirus and Adenovirus Vectors. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 46. | Tesfamariam B. Impact of Reticulated Platelets on Platelet Reactivity in Neonates. J Cardiovasc Pharmacol Ther. 2021;26:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Sabrkhany S, Kuijpers MJE, Griffioen AW, Oude Egbrink MGA. Platelets: the holy grail in cancer blood biomarker research? Angiogenesis. 2019;22:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Tao DL, Tassi Yunga S, Williams CD, McCarty OJT. Aspirin and antiplatelet treatments in cancer. Blood. 2021;137:3201-3211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 49. | Poujol-Robert A, Boëlle PY, Conti F, Durand F, Duvoux C, Wendum D, Paradis V, Mackiewicz V, Chazouillères O, Corpechot C, Poupon R. Aspirin may reduce liver fibrosis progression: Evidence from a multicenter retrospective study of recurrent hepatitis C after liver transplantation. Clin Res Hepatol Gastroenterol. 2014;38:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | McNeil JJ, Gibbs P, Orchard SG, Lockery JE, Bernstein WB, Cao Y, Ford L, Haydon A, Kirpach B, Macrae F, McLean C, Millar J, Murray AM, Nelson MR, Polekhina G, Reid CM, Richmond E, Rodríguez LM, Shah RC, Tie J, Umar A, Londen GJV, Ronaldson K, Wolfe R, Woods RL, Zalcberg J, Chan AT; ASPREE Investigator Group. Effect of Aspirin on Cancer Incidence and Mortality in Older Adults. J Natl Cancer Inst. 2021;113:258-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 51. | McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, Kirpach B, Shah RC, Ives DG, Storey E, Ryan J, Tonkin AM, Newman AB, Williamson JD, Margolis KL, Ernst ME, Abhayaratna WP, Stocks N, Fitzgerald SM, Orchard SG, Trevaks RE, Beilin LJ, Donnan GA, Gibbs P, Johnston CI, Radziszewska B, Grimm R, Murray AM; ASPREE Investigator Group. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N Engl J Med. 2018;379:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 586] [Article Influence: 83.7] [Reference Citation Analysis (0)] |