Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2631
Revised: February 5, 2024
Accepted: April 8, 2024
Published online: June 15, 2024
Processing time: 157 Days and 14.7 Hours
Previous observational studies have shown that the prevalence of gastroesophageal reflux disease (GERD) and Barrett’s esophagus (BE) is associated with socioeconomic status. However, due to the methodological limitations of tra
To explore the causal relationship between the prevalence of these conditions and socioeconomic status using Mendelian randomization (MR).
We initially screened single nucleotide polymorphisms (SNPs) to serve as proxies for eight socioeconomic status phenotypes for univariate MR analysis. The inverse variance weighted (IVW) method was used as the primary analytical method to estimate the causal relationship between the eight socioeconomic status phe
The study identified three socioeconomic statuses that had a significant impact on GERD. These included household income [odds ratio (OR): 0.46; 95% confidence interval (95%CI): 0.31-0.70], education attainment (OR: 0.23; 95%CI: 0.18-0.29), and the Townsend Deprivation Index at recruitment (OR: 1.57; 95%CI: 1.04-2.37). These factors were found to independently and predominantly influence the genetic causal effect of GERD. Furthermore, the mediating effect of educational attainment on GERD was found to be mediated by MDD (proportion mediated: 10.83%). Similarly, the effect of educational attainment on BE was mediated by MDD (proportion mediated: 10.58%) and the number of cigarettes smoked per day (proportion mediated: 3.50%). Additionally, the mediating effect of household income on GERD was observed to be mediated by sleep duration (proportion mediated: 9.75%)
This MR study shed light on the link between socioeconomic status and GERD or BE, providing insights for the prevention of esophageal cancer and precancerous lesions.
Core Tip: This study linked the Townsend Deprivation Index at recruitment to a higher risk of gastroesophageal reflux disease (GERD), while household income and educational attainment protected against GERD. Additionally, our study provided a deeper understanding of the causal effects of educational attainment and household income on GERD and Barrett’s esophagus. Sleep, major depressive disorder, and smoking mediated the relationship between socioeconomic status and GERD or Barret’s esophagus. This Mendelian randomization study has significant public health implications for primary and secondary prevention of esophageal cancer.
- Citation: Liu YX, Bin CL, Zhang L, Yang WT, An BP. Socioeconomic traits and the risk of Barrett’s esophagus and gastroesophageal reflux disease: A Mendelian randomization study. World J Gastrointest Oncol 2024; 16(6): 2631-2645
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2631.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2631
Gastroesophageal reflux disease (GERD) is a condition where stomach contents flow back into the esophagus, causing discomfort and complications[1,2]. Several risk factors, such as smoking, alcohol consumption, and obesity, can contribute to GERD development. The prevalence of GERD is distinctly regional[3]. Generally, developed countries have higher rates[4]. However, a recent epidemiologic survey conducted in China revealed a 3.1% prevalence of symptomatic GERD, showing an increasing trend over the years[5]. The main mechanism underlying GERD pathogenesis is a weakened barrier function of the lower esophageal sphincter. In severe cases, this weak barrier function can lead to columnar epithelial hyperplasia in the distal esophagus, diagnosed as Barrett’s esophagus (BE)[6-8]. BE is considered a precancerous lesion for esophageal adenocarcinoma. The likelihood of developing esophageal adenocarcinoma with BE lesions ranges from 0.1% to 3.1% in the general population[9]. Therefore, studying modifiable risk factors for GERD and BE is crucial for early disease prevention and reducing the incidence of esophageal adenocarcinoma.
Some observational studies have suggested a potential association between lower socioeconomic characteristics and increased risk of GERD[10-12]. However, the impact of socioeconomic characteristics on BE remains controversial. For instance, one case-control study indicated that higher socioeconomic levels increased the incidence of BE[13]. Conversely, a separate case-control study within a Northern California membership organization demonstrated that higher socioeconomic levels acted as a protective factor against BE[14]. This contradiction may be caused by observational studies being subject to confounding factors and reverse causality[15]. It is worth noting that research on GERD may be constrained by certain methodological limitations as well.
A previous Mendelian randomization (MR) study identified risk factors for GERD and BE, which were mediated through education[16]. Additionally, studies confirmed that alcohol consumption, smoking, obesity, poor sleep, and lifestyle habits were associated with GERD and BE[3,11,14,17,18]. However, it is still unknown to what extent these risk factors mediate the association between socioeconomic traits (including education) and GERD and BE. Although changing socioeconomic levels may not be a realistic approach for disease prevention, targeting these modifiable risk factors could have significant implications for disease prevention.
MR is a method that can estimate causal relationships between exposure and outcome using genetic variants as instrumental variables (IVs)[19]. This approach follows Mendel’s second law of inheritance, where alleles are randomly assigned and fixed at conception[20]. Similar to a traditional randomized controlled trial, subjects are assigned randomly to treatment and control groups following MR rules[21]. By using genetic variants associated with specific risk factors, MR minimizes the influence of confounding variables and reverse causality[20].
To analyze causal relationships, two public genome-wide association study (GWAS) databases can be used for a two-sample MR analysis[21]. Additionally, extensions of MR methods, such as multivariate MR (MVMR) and two-step MR, can provide further insights. MVMR allows for the exploration of multiple exposures that predominantly explain the causal effect[22,23]. On the other hand, two-step MR can identify mediators between exposure and outcome while simultaneously addressing unmeasured confounding[24]. These MR methods provide a robust approach for investigating causal relationships in complex diseases like GERD and BE[20].
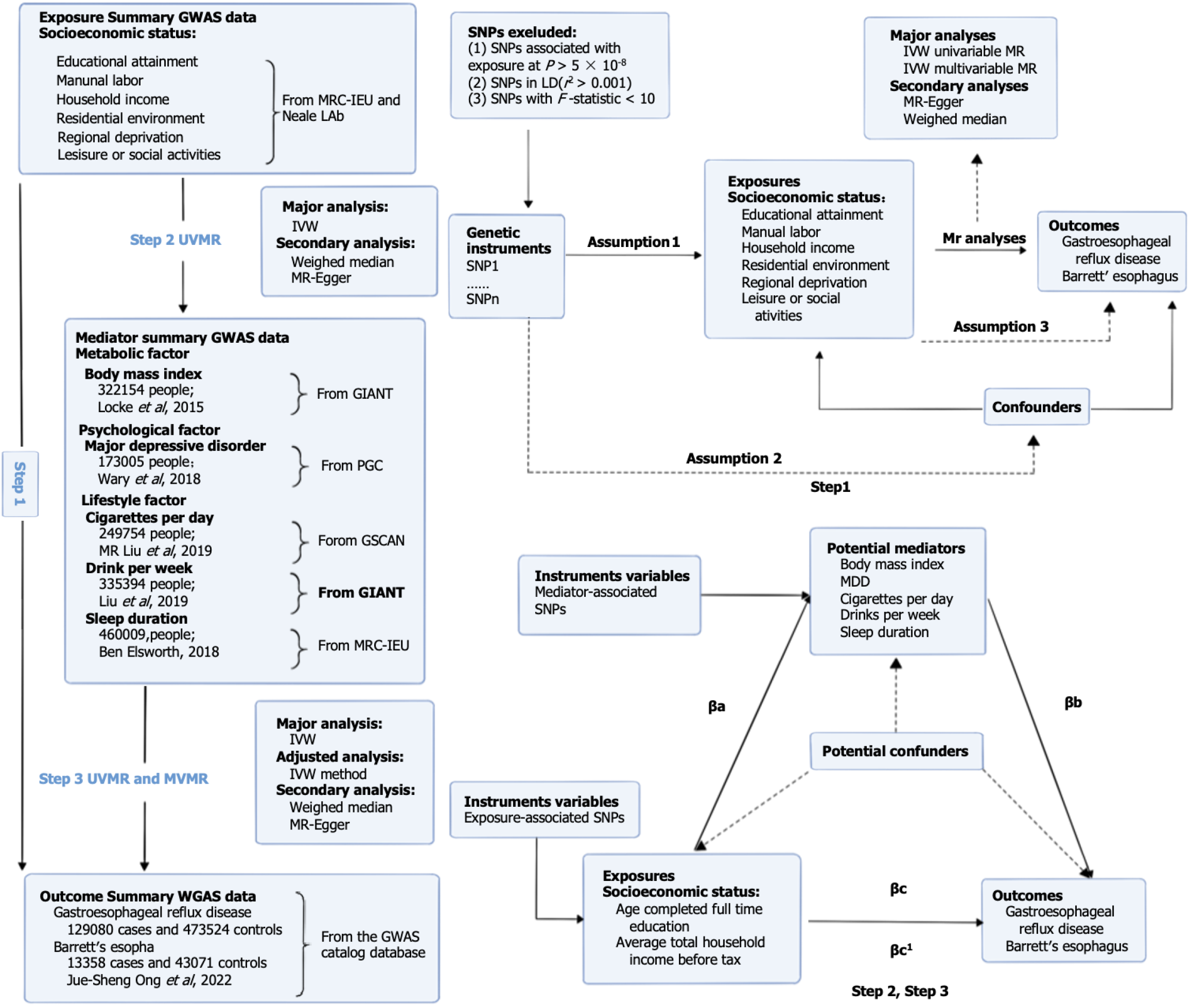
Therefore, to determine the causal association between each phenotype in the six socioeconomic domains (educational attainment, manual labor, household income, residential environment, regional deprivation, and leisure or social activities) and the risk of GERD and BE, we first conducted a series of univariate MR (UVMR) analyses. Afterward, we performed MVMR analyses to identify which of the six socioeconomic characteristics primarily accounted for the risk of GERD and BE. Furthermore, we employed a two-step MR approach to explore whether the effects of GERD and BE were mediated by modifiable risk factors.
We first conducted a UVMR analysis to genetically examine whether each of the eight categories of socioeconomic traits had a causal effect on GERD and BE. We then performed MVMR analyses to determine which one or several socioeconomic traits were causally related to GERD and BE using overlapping single nucleotide polymorphisms (SNPs) as genetic tools. Finally, we performed a two-step MR mediation analysis to assess the proportion of the above associations mediated by modifiable risk factors. An overview of the rationale, design, and procedures for our MR study is exhibited in Figure 1.
GWAS data on socioeconomic status-related phenotypes: We obtained SNPs that are strongly associated with socioeconomic status from two data sources: The Medical Research Council Integrative Epidemiology Unit (MRC-IEU) United Kingdom Biobank and Neale Lab OpenGWAS data infrastructure. Summary statistics were retrieved from the MRC-IEU GWAS database (https://gwas.mrcieu.ac.uk). Socioeconomic status encompassed eight characteristics: Educational attainment (age at completion of full-time education); manual labor (work involving heavy physical or manual labor); household income (average total household income before tax); regional deprivation [Townsend Deprivation Index (TDI) at recruitment]; residential environment [type of accommodation lived in (apartment/ duplex/condominium)]; and leisure or social activities [leisure/social activities (pub/social club or sports club/gym)]. For more details on the specific GWAS data, please refer to Table 1[25,26].
| Trait | Phenotype | Sample size | SNPs | Ancestry | Consortium | Link |
| Educational attainment | Age completed full time education | 307897 | 9851867 | European | MRC-IEU | https://gwas.mrcieu.ac.uk/datasets/ukb-b-6134/ |
| Manual labor | Job involves heavy manual or physical work | 263615 | 9851867 | European | MRC-IEU | https://gwas.mrcieu.ac.uk/datasets/ukb-b-2002/ |
| Household income | Average total household income before tax | 397751 | 9851867 | European | MRC-IEU | https://gwas.mrcieu.ac.uk/datasets/ukb-b-7408/ |
| Residential environment | Type of accommodation lived in: Apartment, duplex, or condominium | 360088 | 13586555 | European | Neale Lab | https://gwas.mrcieu.ac.uk/datasets/ukb-d-670_2/ |
| Type of accommodation lived in: A house or bungalow | 360088 | 13586555 | European | Neale Lab | https://gwas.mrcieu.ac.uk/datasets/ukb-d-670_1/ | |
| Regional deprivation | Townsend Deprivation Index at recruitment | 462464 | 9851867 | European | MRC-IEU | https://gwas.mrcieu.ac.uk/datasets/ukb-b-10011/ |
| Leisure or social activities | Leisure/social activities: Pub or social club | 461369 | 9851867 | European | MRC-IEU | https://gwas.mrcieu.ac.uk/datasets/ukb-b-4171/ |
| Leisure/social activities: Sports club or gym | 461369 | 9851867 | European | MRC-IEU | https://gwas.mrcieu.ac.uk/datasets/ukb-b-4000/ | |
| Esophageal diseases | Gastroesophageal reflux disease | 602604 | 2320781 | European | Ong JS | https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90000514/ |
| Barrett’s esophagus | 56429 | 2320520 | European | Ong JS | https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90000515/ | |
| Metabolic factors | Body mass index | 322154 | 2554668 | European | GIANT | https://gwas.mrcieu.ac.uk/datasets/ieu-a-835/ |
| Psychological factors | Major depressive disorder | 173005 | 13554550 | European | PGC | https://gwas.mrcieu.ac.uk/datasets/ieu-a-1188/ |
| Lifestyle factors | Cigarettes smoked per day | 249752 | 12003613 | European | GSCAN | https://gwas.mrcieu.ac.uk/datasets/ieu-b-142/ |
| Drinks per week | 335394 | 11887865 | European | GSCAN | https://gwas.mrcieu.ac.uk/datasets/ieu-b-73/ | |
| Sleep duration | 460099 | 9851867 | European | MRC-IEU | https://gwas.mrcieu.ac.uk/datasets/ukb-b-4424/ |
GWAS data on GERD and BE outcomes: Genetic data for GERD and BE were obtained from a public GWAS study of Ong et al[27] with a sample of 129080 European ancestry cases and 473524 European ancestry controls.
GWAS data for the mediator: The mediators represented three categories of modifiable risk factors containing many variables. Therefore, we selected several variables that have been widely reported to be associated with esophageal diseases found in observational studies, which were metabolic factors [i.e., body mass index (BMI)], psychological factors [i.e., major depressive disorder (MDD)], and lifestyle factors (i.e., amount of smoking and alcohol consumption, sleeping duration). Data on BMI was derived from the GIANT database, including 322154 European descent males and females, and the number of SNPs was 2554668[28]. We obtained data on MDD from a GWAS conducted by the Psychiatric Genomics Consortium using samples from Europeans. The dataset consisted of 13554550 SNPs for 173005 individuals[29]. To obtain genetic association results for cigarettes smoked per day, we relied on a GWAS meta-analysis conducted by the GWAS and Sequencing Consortium of Alcohol and Nicotine Use. The study included a sample size of 249752 individuals and encompassed 12003613 SNPs. Similarly, the data for alcoholic drinks per week was derived from the same consortium’s GWAS meta-analysis, which involved 335394 individuals and 11887865 SNPs[30]. Finally, we collected information on sleep duration from the MRC-IEU database. The database includes data from 460099 individuals of European descent and contains 9851867 SNPs.
Valid IVs must satisfy three important assumptions: (1) There should be a significant association between IVs and exposure factors; (2) IVs should not be associated with all confounders of the outcome; and (3) IVs should only influence the outcome through their association with exposure factors[31]. To fulfill the first hypothesis, IVs should be closely associated with exposure factors. SNPs associated with eight categories of socioeconomic traits were extracted using a significant threshold of P < 5 × 10-8 from the GWAS dataset[32]. Afterward, we obtained independent exposure SNPs by aggregating them based on linkage disequilibrium (LD) with an r2 threshold of < 0.001 and allele distances of > 10000 kb[33]. To identify corresponding SNPs for the outcome variables (GERD and BE), we extracted them from the GWAS dataset while excluding SNPs with a minor allele frequency (MAF) of < 0.01[34]. Additionally, we harmonized the data on socioeconomic traits and outcomes by removing all palindromic SNPs[35]. Furthermore, to assess the presence of weak IV bias, we calculated the F value. The formula used for F was [(N – k – 1)/k] × [R2/(1 – R2)], where N represents the number of samples, k is the total number of SNPs selected for MR analysis, and R2 is the proportion of phenotypic variation explained by all SNPs in the MR analysis[36]. The R2 value was determined using the formula R2 = Σ [2 × (1 – MAF) × MAF × (β/SD)2], where SD and β represent the standard deviations and effect size coefficients, respectively, and MAF represents the MAF for each SNP[37,38]. A threshold of F > 10 was considered indicative of the absence of weak IV bias[39]. This finding further supports the validity of the first assumption. To fulfill the second MR assumption, SNPs associated with major confounders need to be manually removed using Phenoscanner (http://www.phenoscanner.medschl.cam.ac.uk/). Potential confounders for GERD and BE include BMI, alcohol, cigarette, and depressive status[40,41].
To address the potential impact of reverse association, we employed the Steiger filtering approach to determine the correct directions of inference. To conduct the filtering directionality test, we used the TwoSampleMR R package and implemented the Steiger filtering approach.
Three methods were used for UVMR analysis: Inverse variance weighted (IVW) analysis, MR-Egger, and weighted median. The primary focus of the IVW method was to assess the relationship between socioeconomic status and GERD and BE. This method involves calculating the inverse of the variance of each IV as a weight[42]. Additionally, it helps evaluate horizontal pleiotropy by ensuring the validity of all IVs[43]. To account for multiple tests conducted using all five MR methods, we applied the Bonferroni correction[44]. We calculated a Bonferroni-corrected P threshold by dividing 0.05 by the number of tests[45]. A Bonferroni-corrected threshold of 0.005 (0.05/11) was considered statistically significant. To estimate the causal effects of the eight phenotypes of socioeconomic traits on GERD and BE risk, odds ratios (ORs) and corresponding 95% confidence intervals (95%CIs) were calculated.
To further test the stability and reliability of the UVMR, sensitivity analysis was essential. Initially, we conducted a Q statistic calculation to assess the level of heterogeneity of individual SNPs[46]. In addition, we performed a leave-one-out analysis to test the sensitivity of the results, in which we sequentially removed one SNP at a time to assess whether the estimates were biased or driven by outliers[47]. In cases where one or more SNPs were identified as causing a significant change in the overall MR estimate, they were excluded, and the MR analysis was rerun[47]. To assess the horizontal pleiotropy of effect estimates, we utilized the MR-Egger intercept method. Specifically, we employed the “mr_pleiotropy_test” function from the R Two Sample MR package MVMR[48]. Moreover, the MR-PRESSO test enabled us to identify and remove abnormal SNPs, providing us with outlier-adjusted estimates of causal associations[49].
To examine the direct effects of the eight socioeconomic phenotypes on GERD and BE, we performed MVMR analyses. This approach expands upon the UVMR method and enables the identification of causal effects from multiple exposures on the outcome[50]. In our main analysis, we applied the IVW approach with multiplicative random effects. For each exposure, we utilized the combinations of IVs as SNPs to conduct the MVMR analysis[51]. The results of the MVMR analysis were represented as ORs and their corresponding 95%CIs.
First, the effect of each exposure on each mediator was estimated using UVMR. Second, another UVMR was employed to estimate the mediator’s effect on the outcome. As a third step, the individual mediator effects for each mediator were calculated by multiplying the effect of each exposure on each mediator with the effect of each mediator on the outcome while adjusting for the exposure[22]. To obtain unbiased estimates of direct and indirect effects, two-step MR mediation analyses assumed that there was no interaction between exposure and mediator, and there was a linear association between exposure, mediator, and outcome[52]. Finally, we divided the mediator effect by the total effect to estimate the mediated proportion. We used the delta method to compute the estimates and confidence intervals for the mediating effects, and the standard errors were compared to the bounds by normal approximation to obtain the corresponding P values. The delta method was implemented through the “Rmediation” package.
In the analysis of the causal relationship between socioeconomic traits and GERD, we employed several screening steps. First, we removed SNPs with LD or MAF less than 0.01 as well as palindromic SNPs and confounding SNPs. The details of these exclusions can be found in Supplementary Tables 1-12. After this initial screening, we retained a total of 16 SNPs as IVs for “Age completed full-time education”, 12 SNPs for “Involved in heavy physical or manual labor”, 10 SNPs for “Average total household income before tax”, and no SNPs for the categories “Type of accommodation lived in: Apartment, duplex, or condominium” and “Type of accommodation lived in: House or bungalow”. For the “Townsend Deprivation Index at the time of recruitment”, we retained 8 SNPs, and for “Leisure/social activity: Pub or social club” and “Leisure/social activity: Sports club or gym”, we retained 4 SNPs each. These retained SNPs were then used for UVMR analysis. Similarly, when analyzing the causal relationship between socioeconomics and BE, we followed a comparable screening process. After removing SNPs with LD or MAF less than 0.01, palindromic SNPs, and confounding SNPs, we retained a total of 12 SNPs as IVs for “Age completed full-time education” and 12 SNPs for “Involved in heavy physical or manual labor”. For “Average gross household income before taxes,” we retained 18 SNPs, while no SNPs were retained for the categories “Type of accommodation lived in: Apartment, duplex, or condominium” and “Type of accommodation lived in: House or bungalow”. Additionally, we kept 8 SNPs for “Townsend Deprivation Index at the time of recruitment”, 6 SNPs for “Leisure/social activities: Pubs or social clubs”, and 2 SNPs for “Leisure/social activities: Sports club or gym”.
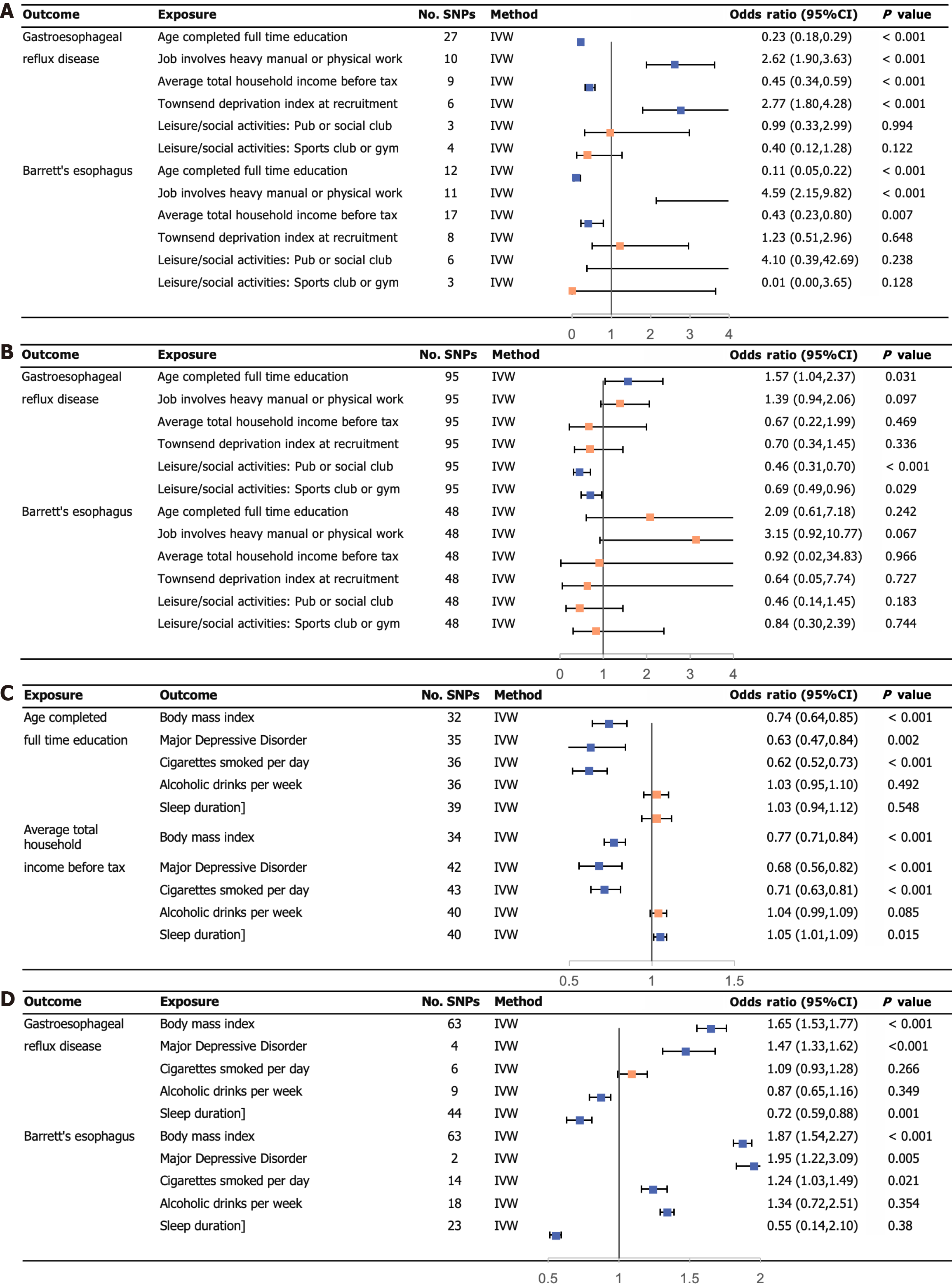
UVMR analysis using the IVW regression method revealed several associations with the risk of GERD. Average total household income before tax (OR: 0.45; 95%CI: 0.34-0.59) and age completed full-time education (OR: 0.23; 95%CI: 0.18-0.29) showed a negative association with GERD risk. Conversely, jobs involving heavy physical or manual labor (OR: 2.62; 95%CI: 1.90-3.63) and the TDI at recruitment (OR: 2.77; 95%CI: 1.80-4.28) were positively associated with the risk of GERD.
Moving on to the investigation of the residential environment and social activity, our analysis using the IVW method did not find any evidence supporting a causal association with GERD. However, when examining the genetic prediction of education attainment (OR: 0.11; 95%CI: 0.05-0.22) and household income (OR: 0.43; 95%CI: 0.29-0.80), we found a significant association with the risk of BE. This means that higher educational attainment and household income were associated with a reduced risk of BE.
Furthermore, jobs involving heavy physical or manual labor showed a positive association with the risk of BE (OR: 4.59; 95%CI: 2.15-9.82). In other words, individuals with heavy physical or manual labor were more than four times as likely to develop BE compared to their peers. However, we did not find any causal relationship between other phenotypes and the risk of BE. Detailed results of the MR analyses can be seen in Figure 2A, while scatter plots illustrating specific findings are provided in Supplementary Figures 1-12. The specific results of the three MR analysis methods are presented in Tables 2 and 3.
| Exposure | Outcome | F statistic | SNP | IVW OR (95%CI) | P value | Weighted median OR (95%CI) | P value | MR-Egger OR (95%CI) | P value |
| Age completed full time education | GERD | 21.564 | 27 | 0.23 (0.18, 0.29) | 3.120E-37 | 0.27 (0.21, 0.35) | 3.890E-24 | 0.26 (0.08, 0.83) | 0.032 |
| Job involves heavy manual or physical work | GERD | 15.312 | 10 | 2.62 (1.90, 3.63) | 5.470E-09 | 2.35 (1.66, 3.34) | 1.760E-06 | 0.37 (0.07, 1.83) | 0.257 |
| Average total household income before tax | GERD | 16.722 | 9 | 0.45 (0.34, 0.59) | 1.090E-08 | 0.55 (0.40, 0.76) | < 0.001 | 0.83 (0.07, 10.68) | 0.891 |
| Type of accommodation lived in: Apartment, duplex, or condominium | GERD | 21.654 | 0 | NA | NA | NA | NA | NA | NA |
| Type of accommodation lived in: House or bungalow | GERD | 17.211 | 0 | NA | NA | NA | NA | NA | NA |
| TDI at recruitment | GERD | 22.719 | 6 | 2.77 (1.80, 4.28) | 3.770E-06 | 2.32 (1.42, 3.79) | 0.001 | 0.12 (0.01, 1.95) | 0.211 |
| Exposure | Outcome | F statistic | SNP | IVW OR (95%CI) | P value | Weighted median OR (95%CI) | P value | MR-Egger OR (95%CI) | P value |
| Age completed full time education | BE | 32.511 | 12 | 0.11 (0.05, 0.22) | 7.830E-10 | 0.14 (0.05, 0.36) | 7.010E-05 | 0.240 (0.02, 3.66) | 0.328 |
| Job involves heavy manual or physical work | BE | 21.688 | 11 | 4.59 (2.15, 9.82) | 8.370E-05 | 2.90 (1.18, 7.12) | 0.020 | 0.480 (0.01, 47.14) | 0.760 |
| Average total household income before tax | BE | 12.432 | 17 | 0.43 (0.23, 0.80) | 0.007 | 0.51 (0.25, 1.03) | 0.061 | 0.670 (0.05, 9.53) | 0.771 |
| Type of accommodation lived in: Apartment, duplex, or condominium | BE | 17.781 | 0 | NA | NA | NA | NA | NA | NA |
| Type of accommodation lived in: House or bungalow | BE | 19.785 | 0 | NA | NA | NA | NA | NA | NA |
| TDI at recruitment | BE | 17.710 | 8 | 1.23 (0.51, 2.96) | 0.648 | 0.92 (0.31, 2.71) | 0.875 | 0.002 (0.00, 4.11) | 0.162 |
For MVMR analysis, we utilized a combination of 95 SNPs associated with GERD and 48 SNPs associated with BE. In our study, three key phenotypes emerged, educational attainment, household income, and regional deprivation, each playing significant and independent roles in the effects of various socioeconomic characteristics on GERD. The OR for educational attainment was 0.46 (95%CI: 0.31-0.70), for household income it was 0.69 (95%CI: 0.49-0.96), and for regional deprivation it was 1.57 (95%CI: 1.04-2.37). However, after adjusting for each other, none of the six socioeconomic phenotypes were found to be significantly associated with BE. You can refer to Figure 2B for specific results obtained through the MVMR analysis.
Effects of socioeconomic traits on GERD and BE: Figure 1 demonstrates the causal effect of each socioeconomic phenotype on GERD and BE. To maintain consistency across several statistical methods, we chose “Age completed full-time education” and “Average total household income before tax” as the exposure factors of the mediation analysis.
Effects of educational attainment and household income on modifiable risk factors: In Figure 2C, the UVMR genetic predictions displayed the impact of two socioeconomic characteristics on five modifiable risk factors. The results from all three MR methods consistently indicated a negative association between “Age completed full-time education” and both “MDD” and “Cigarettes smoked per day”. Furthermore, the predictions revealed a positive association between “Average total household income before tax” and “Sleep duration”. The specific results of the three MR analysis methods are presented in Supplementary Tables 13-22.
Effects of modifiable risk factors on GERD and BE: Figure 2D illustrates the impact of modifiable risk factors predicted by UVMR on the development of GERD and BE. It was observed that genetic prediction of BMI and MDD significantly escalated the risk of GERD. Conversely, adequate sleep duration has a protective effect against GERD. Additionally, increased BMI, presence of MDD, and the number of cigarettes smoked per day contributed to an increased risk of developing BE. The specific results of the three MR analysis methods are presented in Supplementary Tables 23-32.
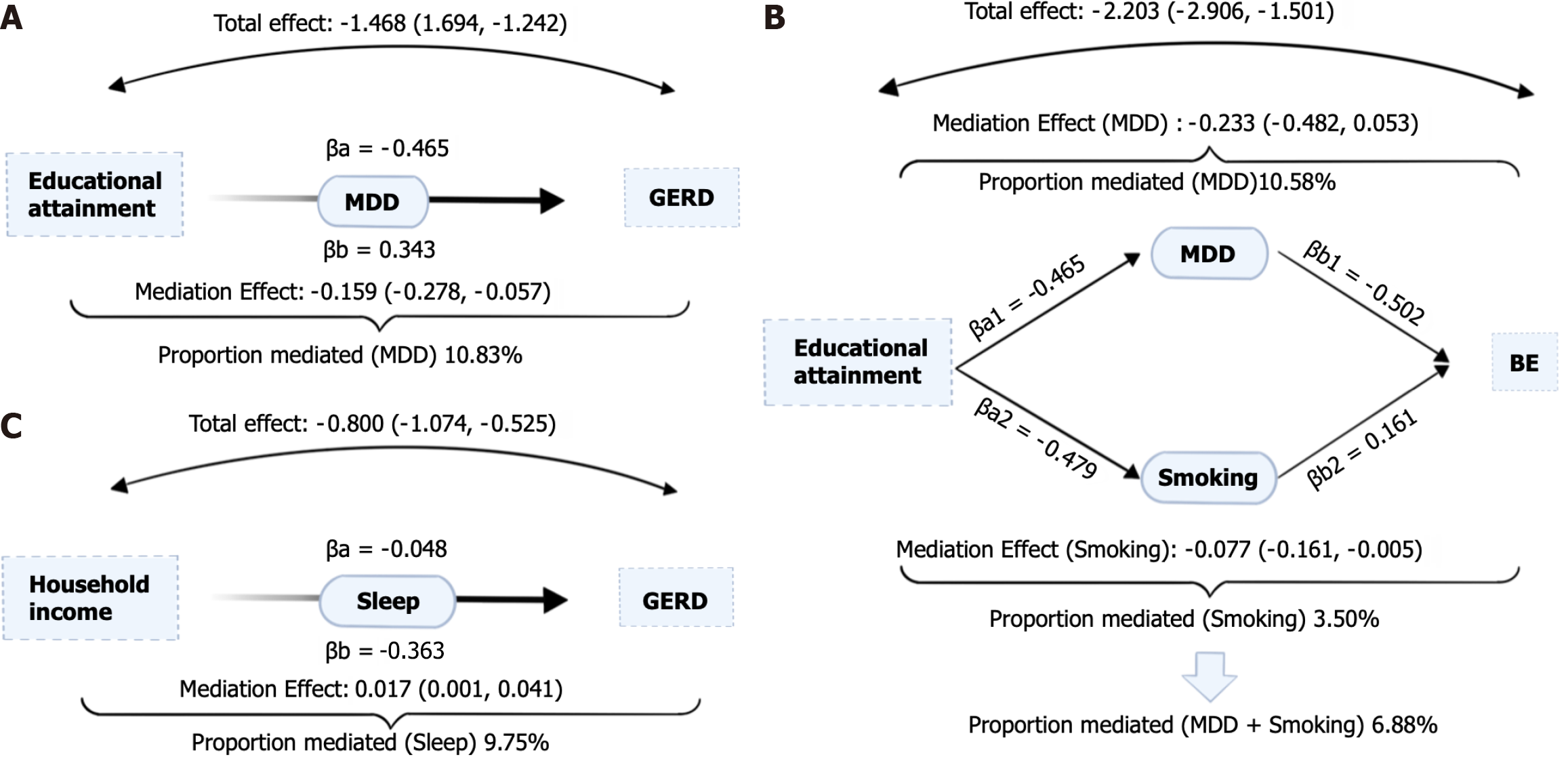
In our study, we focused on modifiable risk factors that have been causally linked through MR analysis to both educational attainment and household income (step one). Additionally, we examined the influence of these risk factors as mediators on GERD and BE (step two). Specifically, we investigated the impact of MDD, cigarettes smoked per day, and sleep duration (Figure 3). The effects of modifiable risk factors on GERD and BE after adjusting for exposure were demonstrated in Table 4. Our findings revealed that the relationship between educational attainment and GERD was partially mediated by MDD, with approximately 10.83% of the effect being explained through this mediator. Similarly, the association between educational attainment and BE was mediated by both MDD (10.58% of the effect) and cigarettes smoked per day (3.50% of the effect). On the other hand, household income was found to have a mediating effect of 9.75% on GERD through sleep duration. However, no mediation was observed between household income and BE through the identified modifiable risk factors. The specific results of the mediation analysis are presented in Supplementary Table 33.
| Exposure | Outcome | OR (95%CI) | P value | Mediation effect |
| Age completed full time education | GERD | 0.27 (0.23, 0.33) | 6.410E-46 | 10.83% |
| Age completed full time education | BE | 0.13 (0.08, 0.23) | 2.140E-13 | 10.58% |
| Age completed full time education | BE | 0.11 (0.07, 0.20) | 2.900E-14 | 3.50% |
| Age completed full time education | BE | 0.14 (0.08, 0.24) | 1.290E-13 | 6.88% |
| Average total household income before tax | GERD | 0.36 (0.30, 0.44) | 3.240E-23 | 9.75% |
Consistency was observed between the results of the three MR statistics, and the direction and effect sizes of the MR-Egger method matched those of the main analyses. However, for our primary analysis, we relied on the effect values from the IVW method. This decision was made because some of the results from the MR-Egger method may not have been sta
This study comprehensively explored the causal relationship of socioeconomic traits on GERD and BE using UVMR and MVMR. The mediating role of five modifiable risk factors in the causal relationship was further analyzed by two-step MR. MVMR found that genetically predicted educational attainment and household income were negatively associated with the onset of GERD. This means that better education and higher household income can reduce the risk of GERD. Also, the TDI at recruitment was positively associated with the risk of GERD. The two-step Mendelian study found that depression mediated 10.83% of the total effect of education on GERD. Depression and smoking mediated 10.58% and 3.50%, respectively, of the total effect of education on BE. The effect of household income on GERD was mediated by sleep duration, with the proportion mediated at 9.75%.
The study contributed to the secondary prevention of esophageal cancer and the primary prevention of GERD and BE. The incidence of esophageal cancer is on the rise globally, and the prevalence of GERD and BE has shifted from developed to developing countries[53]. It is helpful to consider socioeconomic status when formulating strategies to reduce the risk of GERD and BE, both of which are precancerous lesions. Factors such as education, income, and regional poverty should be considered. Furthermore, this is of great significance in the prevention of further development of precancerous lesions into esophageal cancer. It is easier to control modifiable risk factors than to change one’s socioeconomic status. Our findings provide strong support for a randomized controlled trial to test the effects of treating depression, quitting smoking, and improving sleep disorders on the risk of GERD and BE among those with lower levels of education and those with lower levels of household income.
Several previous observational studies have found that educational attainment and household income were protective factors for GERD[54,55], and our MR genetic prediction further strengthened this association. TDI, an indicator of socioeconomic deprivation for an area, is often used as a proxy for individual deprivation[56]. It is associated with higher levels of socioeconomic deprivation, resulting in a higher TDI[57]. However, no study has explored the relationship between TDI and GERD. In our study, we aimed to fill this research gap and found a positive causal relationship between the TDI at recruitment and a higher risk of GERD. While our MVMR analyses did not reveal a causal relationship between education, household income, and BE, the consistent estimates from the UVMR analyses lend credibility to the causal evidence. Filiberti et al[58] showed a decrease in the risk of BE with increasing socioeconomic status, such as education attainment and household income. However, these studies are observational, and there are limited MR studies exploring the causal association between education and BE at the genetic level. To bridge this gap, we conducted UVMR analyses to demonstrate the role of education and household income in BE. Our findings further support the notion that higher education levels and household incomes are associated with a decreased risk of BE.
Observational studies have demonstrated that many modifiable risk factors, including obesity, smoking, alcohol consumption, depression, and sleep, are associated with outcomes[59-61]. However, it is important to consider that observational studies may be influenced by confounding factors and reverse causation[19]. Green et al[62] conducted an MR study that demonstrated an association between fat distribution, waist-to-hip ratio (representing obesity), and the risk of GERD. Similarly, Thrift et al[63] found that obesity is positively associated with the risk of BE. Another MR study by Miao et al[64] showed a positive association between genetic susceptibility to MDD and GERD and its subtypes. Regarding smoking and alcohol consumption, a study by Yuan and Larsson[65] using MR found that genetic prediction of smoking was statistically associated with a higher incidence of GERD. However, no causal association was found between alcohol consumption and GERD incidence. The findings may be influenced by inadequate statistical power or a possible association of heavy alcohol consumption or alcohol abuse with GERD.
A prospective study by Nejatinamini et al[66] found that modifiable risk factors, such as obesity, smoking, and excessive alcohol consumption, accounted for 45.6% of the link between low socioeconomic position and cancer incidence and mortality. Although few studies examined how socioeconomic position is linked to both BE and GERD through modifiable risk factors, an MR study demonstrated the mediating role of factors such as BMI, smoking, and alcohol consumption in linking education with GERD and BE[16]. These findings are consistent with our study. However, it is crucial to note that this study did not investigate the potential effects of other socioeconomic factors influenced by these modifiable risk factors, as education represents only one aspect of socioeconomic status.
First, we hypothesized that individuals with higher education and income possess greater knowledge of health prevention as well as awareness of medical checkups. This heightened awareness is vital for reducing the risk of developing GERD and BE[67]. In addition, these individuals have access to better healthcare services, which further contributes to the prevention and control of these conditions. The TDI is a deprivation index derived from four census variables: Unemployment; non-home ownership; households without cars; and overcrowded households. It assigns positive values to areas with high material deprivation[56]. This index essentially reflects the level of deprivation, which is similar to the mechanisms underlying GERD and BE. In essence, areas with higher levels of material deprivation tend to have a higher incidence of both GERD and BE.
Smoking can be associated with GERD through two main mechanisms. First, smoking weakens the lower esophageal sphincter, which results in the flow of stomach contents into the esophagus[18]. Second, tobacco reduces salivary bicarbonate secretion, which plays a crucial role in neutralizing gastric acid[68]. Additionally, tobacco use can induce systemic inflammatory pathways, influencing the development and progression of GERD and its progression to BE[69]. In the case of MDD and BE, psychological and psychiatric factors can impact body sensory thresholds, leading to altered perception and reporting of esophageal irritation in individuals with reflux symptoms[68]. Furthermore, a bidirectional relationship has been observed between sleep and GERD. Poor sleep quality can cause nocturnal acid breakthrough of the lower esophageal sphincter, resulting in the reflux of gastric acid into the esophagus[70].
We identified several strengths in our study. First, we conducted a comprehensive analysis that investigated the causal association between eight socioeconomic phenotypes and GERD and BE. Unlike previous MR studies that focused on individual characteristics in socioeconomic status, our analysis compensated for these limitations. Second, we have utilized pooled data from GWAS with larger sample sizes and more SNPs, which improved the quality of analysis and avoided biases such as unobserved confounding, misclassification, and reverse causation[71]. Furthermore, we employed a two-step MR approach to explore the potential mediating effect of modifiable risk factors in the relationship between socioeconomic status and disease. This approach provided additional protection against non-differential measurement errors in the mediator, enhancing the reliability of our findings[72].
However, it is important to acknowledge the limitations of our study. First, we observed significant heterogeneity in the MR analysis. Despite this, our main results remained consistent after using MR-Egger, IVW, and outlier-corrected MR-PRESSO analyses to address potential outliers. Second, it is worth noting that our study only included European populations, which may limit the generalizability of our findings to other ethnic populations. Lastly, we obtained different causal effect estimates between the MR-Egger method and other MR methods. The MR-Egger method, due to its calculation of horizontal pleiotropy, may have weaker statistical efficacy compared to other MR methods[73]. Our primary approach was to rely on the findings from the IVW method.
This study examined the relationship between socioeconomic status and GERD or BE. Our findings revealed that educational attainment and household income have a protective effect against GERD, while TDI was associated with a higher risk of GERD. Furthermore, our study provided a deeper understanding of the causal effects of educational attainment and household income on GERD and BE. We found that these effects were partially influenced by daily smoking, MDD, and sleep duration. These factors mediated the relationship between socioeconomic status and GERD or BE. The implications of this MR study are significant in terms of public health. By understanding the association between socioeconomic status and GERD or BE, we gain new insights for primary prevention strategies against esophageal cancer and secondary prevention of precancerous lesions.
We thank the contributors of the original GWAS datasets.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade A
Creativity or Innovation: Grade A
Scientific Significance: Grade A
P-Reviewer: Verma V, United States S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal Reflux Disease: A Review. JAMA. 2020;324:2536-2547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 2. | Mittal R, Vaezi MF. Esophageal Motility Disorders and Gastroesophageal Reflux Disease. N Engl J Med. 2020;383:1961-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 365] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 4. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1264] [Article Influence: 114.9] [Reference Citation Analysis (2)] |
| 5. | He J, Ma X, Zhao Y, Wang R, Yan X, Yan H, Yin P, Kang X, Fang J, Hao Y, Li Q, Dent J, Sung JJ, Zou D, Wallander MA, Johansson S, Liu W, Li Z. A population-based survey of the epidemiology of symptom-defined gastroesophageal reflux disease: the Systematic Investigation of Gastrointestinal Diseases in China. BMC Gastroenterol. 2010;10:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Yadlapati R, DeLay K. Proton Pump Inhibitor-Refractory Gastroesophageal Reflux Disease. Med Clin North Am. 2019;103:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Patti MG. An Evidence-Based Approach to the Treatment of Gastroesophageal Reflux Disease. JAMA Surg. 2016;151:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med. 2014;371:836-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 10. | Jansson C, Nordenstedt H, Johansson S, Wallander MA, Johnsen R, Hveem K, Lagergren J. Relation between gastroesophageal reflux symptoms and socioeconomic factors: a population-based study (the HUNT Study). Clin Gastroenterol Hepatol. 2007;5:1029-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 438] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 12. | Wang SE, Kendall BJ, Hodge AM, Dixon-Suen SC, Dashti SG, Makalic E, Williamson EM, Thomas RJS, Giles GG, English DR. Demographic and lifestyle risk factors for gastroesophageal reflux disease and Barrett's esophagus in Australia. Dis Esophagus. 2022;35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Ford AC, Forman D, Reynolds PD, Cooper BT, Moayyedi P. Ethnicity, gender, and socioeconomic status as risk factors for esophagitis and Barrett's esophagus. Am J Epidemiol. 2005;162:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Kubo A, Levin TR, Block G, Rumore GJ, Quesenberry CP Jr, Buffler P, Corley DA. Alcohol types and sociodemographic characteristics as risk factors for Barrett's esophagus. Gastroenterology. 2009;136:806-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318:1925-1926. [RCA] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 2168] [Article Influence: 271.0] [Reference Citation Analysis (0)] |
| 16. | Zhang X, Yang X, Zhang T, Yin X, Man J, Lu M. Association of educational attainment with esophageal cancer, Barrett's esophagus, and gastroesophageal reflux disease, and the mediating role of modifiable risk factors: A Mendelian randomization study. Front Public Health. 2023;11:1022367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 17. | Ishii D, Miyagi H, Hirasawa M. Risk factors for recurrent gastroesophageal reflux disease after Thal fundoplication. Pediatr Surg Int. 2021;37:1731-1735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Ness-Jensen E, Lagergren J. Tobacco smoking, alcohol consumption and gastro-oesophageal reflux disease. Best Pract Res Clin Gastroenterol. 2017;31:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Sekula P, Del Greco M F, Pattaro C, Köttgen A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J Am Soc Nephrol. 2016;27:3253-3265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 1398] [Article Influence: 155.3] [Reference Citation Analysis (0)] |
| 20. | Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3525] [Cited by in RCA: 2999] [Article Influence: 176.4] [Reference Citation Analysis (0)] |
| 21. | O'Donnell CJ, Sabatine MS. Opportunities and Challenges in Mendelian Randomization Studies to Guide Trial Design. JAMA Cardiol. 2018;3:967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Sanderson E. Multivariable Mendelian Randomization and Mediation. Cold Spring Harb Perspect Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 472] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 23. | Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, Evans DM, Smith GD. Recent Developments in Mendelian Randomization Studies. Curr Epidemiol Rep. 2017;4:330-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 734] [Cited by in RCA: 730] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 24. | Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89-R98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2280] [Cited by in RCA: 2911] [Article Influence: 264.6] [Reference Citation Analysis (0)] |
| 25. | Zhang W, Ge J, Qu Z, Wu W, Lei H, Pan H, Chen H. Evaluation for causal effects of socioeconomic traits on risk of female genital prolapse (FGP): a multivariable Mendelian randomization analysis. BMC Med Genomics. 2023;16:125. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Harrison S, Davies AR, Dickson M, Tyrrell J, Green MJ, Katikireddi SV, Campbell D, Munafò M, Dixon P, Jones HE, Rice F, Davies NM, Howe LD. The causal effects of health conditions and risk factors on social and socioeconomic outcomes: Mendelian randomization in UK Biobank. Int J Epidemiol. 2020;49:1661-1681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Ong JS, An J, Han X, Law MH, Nandakumar P; 23andMe Research team; Esophageal cancer consortium, Schumacher J, Gockel I, Bohmer A, Jankowski J, Palles C, Olsen CM, Neale RE, Fitzgerald R, Thrift AP, Vaughan TL, Buas MF, Hinds DA, Gharahkhani P, Kendall BJ, MacGregor S. Multitrait genetic association analysis identifies 50 new risk loci for gastro-oesophageal reflux, seven new loci for Barrett's oesophagus and provides insights into clinical heterogeneity in reflux diagnosis. Gut. 2022;71:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 28. | Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Mägi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Zhao JH, Zhao W, Chen J, Fehrmann R, Hedman ÅK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Leach IM, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stančáková A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Ärnlöv J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Blüher M, Böhringer S, Bonnycastle LL, Böttcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Chen YI, Clarke R, Daw EW, de Craen AJM, Delgado G, Dimitriou M, Doney ASF, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Gräßler J, Grönberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson Å, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Lo KS, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PKE, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Müller G, Müller-Nurasyid M, Musk AW, Nagaraja R, Nöthen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Smith AV, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q; LifeLines Cohort Study, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gådin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee JY, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JRB, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, van 't Hooft FM, Vinkhuyzen AAE, Westra HJ, Zheng W, Zondervan KT; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium, Heath AC, Arveiler D, Bakker SJL, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrières J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hyppönen E, Illig T, Jacobs KB, Jarvelin MR, Jöckel KH, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, Kastelein JJP, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Marchand LL, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PAF, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PEH, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tönjes A, Trégouët DA, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Völker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PIW, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, März W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njølstad I, Oostra BA, Palmer CNA, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann HE, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O'Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJF, Speliotes EK. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3250] [Article Influence: 325.0] [Reference Citation Analysis (0)] |
| 29. | Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, Adams MJ, Agerbo E, Air TM, Andlauer TMF, Bacanu SA, Bækvad-Hansen M, Beekman AFT, Bigdeli TB, Binder EB, Blackwood DRH, Bryois J, Buttenschøn HN, Bybjerg-Grauholm J, Cai N, Castelao E, Christensen JH, Clarke TK, Coleman JIR, Colodro-Conde L, Couvy-Duchesne B, Craddock N, Crawford GE, Crowley CA, Dashti HS, Davies G, Deary IJ, Degenhardt F, Derks EM, Direk N, Dolan CV, Dunn EC, Eley TC, Eriksson N, Escott-Price V, Kiadeh FHF, Finucane HK, Forstner AJ, Frank J, Gaspar HA, Gill M, Giusti-Rodríguez P, Goes FS, Gordon SD, Grove J, Hall LS, Hannon E, Hansen CS, Hansen TF, Herms S, Hickie IB, Hoffmann P, Homuth G, Horn C, Hottenga JJ, Hougaard DM, Hu M, Hyde CL, Ising M, Jansen R, Jin F, Jorgenson E, Knowles JA, Kohane IS, Kraft J, Kretzschmar WW, Krogh J, Kutalik Z, Lane JM, Li Y, Lind PA, Liu X, Lu L, MacIntyre DJ, MacKinnon DF, Maier RM, Maier W, Marchini J, Mbarek H, McGrath P, McGuffin P, Medland SE, Mehta D, Middeldorp CM, Mihailov E, Milaneschi Y, Milani L, Mill J, Mondimore FM, Montgomery GW, Mostafavi S, Mullins N, Nauck M, Ng B, Nivard MG, Nyholt DR, O'Reilly PF, Oskarsson H, Owen MJ, Painter JN, Pedersen CB, Pedersen MG, Peterson RE, Pettersson E, Peyrot WJ, Pistis G, Posthuma D, Purcell SM, Quiroz JA, Qvist P, Rice JP, Riley BP, Rivera M, Saeed Mirza S, Saxena R, Schoevers R, Schulte EC, Shen L, Shi J, Shyn SI, Sigurdsson E, Sinnamon GBC, Smit JH, Smith DJ, Stefansson H, Steinberg S, Stockmeier CA, Streit F, Strohmaier J, Tansey KE, Teismann H, Teumer A, Thompson W, Thomson PA, Thorgeirsson TE, Tian C, Traylor M, Treutlein J, Trubetskoy V, Uitterlinden AG, Umbricht D, Van der Auwera S, van Hemert AM, Viktorin A, Visscher PM, Wang Y, Webb BT, Weinsheimer SM, Wellmann J, Willemsen G, Witt SH, Wu Y, Xi HS, Yang J, Zhang F; eQTLGen; 23andMe, Arolt V, Baune BT, Berger K, Boomsma DI, Cichon S, Dannlowski U, de Geus ECJ, DePaulo JR, Domenici E, Domschke K, Esko T, Grabe HJ, Hamilton SP, Hayward C, Heath AC, Hinds DA, Kendler KS, Kloiber S, Lewis G, Li QS, Lucae S, Madden PFA, Magnusson PK, Martin NG, McIntosh AM, Metspalu A, Mors O, Mortensen PB, Müller-Myhsok B, Nordentoft M, Nöthen MM, O'Donovan MC, Paciga SA, Pedersen NL, Penninx BWJH, Perlis RH, Porteous DJ, Potash JB, Preisig M, Rietschel M, Schaefer C, Schulze TG, Smoller JW, Stefansson K, Tiemeier H, Uher R, Völzke H, Weissman MM, Werge T, Winslow AR, Lewis CM, Levinson DF, Breen G, Børglum AD, Sullivan PF; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668-681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2291] [Cited by in RCA: 1988] [Article Influence: 284.0] [Reference Citation Analysis (0)] |
| 30. | Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, Datta G, Davila-Velderrain J, McGuire D, Tian C, Zhan X; 23andMe Research Team; HUNT All-In Psychiatry, Choquet H, Docherty AR, Faul JD, Foerster JR, Fritsche LG, Gabrielsen ME, Gordon SD, Haessler J, Hottenga JJ, Huang H, Jang SK, Jansen PR, Ling Y, Mägi R, Matoba N, McMahon G, Mulas A, Orrù V, Palviainen T, Pandit A, Reginsson GW, Skogholt AH, Smith JA, Taylor AE, Turman C, Willemsen G, Young H, Young KA, Zajac GJM, Zhao W, Zhou W, Bjornsdottir G, Boardman JD, Boehnke M, Boomsma DI, Chen C, Cucca F, Davies GE, Eaton CB, Ehringer MA, Esko T, Fiorillo E, Gillespie NA, Gudbjartsson DF, Haller T, Harris KM, Heath AC, Hewitt JK, Hickie IB, Hokanson JE, Hopfer CJ, Hunter DJ, Iacono WG, Johnson EO, Kamatani Y, Kardia SLR, Keller MC, Kellis M, Kooperberg C, Kraft P, Krauter KS, Laakso M, Lind PA, Loukola A, Lutz SM, Madden PAF, Martin NG, McGue M, McQueen MB, Medland SE, Metspalu A, Mohlke KL, Nielsen JB, Okada Y, Peters U, Polderman TJC, Posthuma D, Reiner AP, Rice JP, Rimm E, Rose RJ, Runarsdottir V, Stallings MC, Stančáková A, Stefansson H, Thai KK, Tindle HA, Tyrfingsson T, Wall TL, Weir DR, Weisner C, Whitfield JB, Winsvold BS, Yin J, Zuccolo L, Bierut LJ, Hveem K, Lee JJ, Munafò MR, Saccone NL, Willer CJ, Cornelis MC, David SP, Hinds DA, Jorgenson E, Kaprio J, Stitzel JA, Stefansson K, Thorgeirsson TE, Abecasis G, Liu DJ, Vrieze S. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51:237-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1465] [Cited by in RCA: 1300] [Article Influence: 216.7] [Reference Citation Analysis (0)] |
| 31. | Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1354] [Cited by in RCA: 2595] [Article Influence: 370.7] [Reference Citation Analysis (0)] |
| 32. | Li M, Lin J, Liang S, Chen Z, Bai Y, Long X, Huang S, Mo Z. The role of age at menarche and age at menopause in Alzheimer's disease: evidence from a bidirectional mendelian randomization study. Aging (Albany NY). 2021;13:19722-19749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Shen J, Zhou H, Liu J, Zhang Y, Zhou T, Yang Y, Fang W, Huang Y, Zhang L. A modifiable risk factors atlas of lung cancer: A Mendelian randomization study. Cancer Med. 2021;10:4587-4603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Cui Z, Hou G, Meng X, Feng H, He B, Tian Y. Bidirectional Causal Associations Between Inflammatory Bowel Disease and Ankylosing Spondylitis: A Two-Sample Mendelian Randomization Analysis. Front Genet. 2020;11:587876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016;45:1717-1726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 556] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 36. | Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181:251-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 532] [Cited by in RCA: 1082] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 37. | Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48:713-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 732] [Article Influence: 122.0] [Reference Citation Analysis (0)] |
| 38. | Gormley M, Dudding T, Kachuri L, Burrows K, Chong AHW, Martin RM, Thomas SJ, Tyrrell J, Ness AR, Brennan P, Munafò MR, Pring M, Boccia S, Olshan AF, Diergaarde B, Hung RJ, Liu G, Tajara EH, Severino P, Toporcov TN, Lacko M, Waterboer T, Brenner N, Smith GD, Vincent EE, Richmond RC. Investigating the effect of sexual behaviour on oropharyngeal cancer risk: a methodological assessment of Mendelian randomization. BMC Med. 2022;20:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Zhang J, Taylor EW, Bennett K, Saad R, Rayman MP. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111:1297-1299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 40. | Hallan A, Bomme M, Hveem K, Møller-Hansen J, Ness-Jensen E. Risk factors on the development of new-onset gastroesophageal reflux symptoms. A population-based prospective cohort study: the HUNT study. Am J Gastroenterol. 2015;110:393-400; quiz 401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 41. | De Ceglie A, Fisher DA, Filiberti R, Blanchi S, Conio M. Barrett's esophagus, esophageal and esophagogastric junction adenocarcinomas: the role of diet. Clin Res Hepatol Gastroenterol. 2011;35:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Yuan S, Tang B, Zheng J, Larsson SC. Circulating Lipoprotein Lipids, Apolipoproteins and Ischemic Stroke. Ann Neurol. 2020;88:1229-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Yin KJ, Huang JX, Wang P, Yang XK, Tao SS, Li HM, Ni J, Pan HF. No Genetic Causal Association Between Periodontitis and Arthritis: A Bidirectional Two-Sample Mendelian Randomization Analysis. Front Immunol. 2022;13:808832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 44. | Magnus MC, Guyatt AL, Lawn RB, Wyss AB, Trajanoska K, Küpers LK, Rivadeneira F, Tobin MD, London SJ, Lawlor DA, Millard LAC, Fraser A. Identifying potential causal effects of age at menarche: a Mendelian randomization phenome-wide association study. BMC Med. 2020;18:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | Millard LAC, Munafò MR, Tilling K, Wootton RE, Davey Smith G. MR-pheWAS with stratification and interaction: Searching for the causal effects of smoking heaviness identified an effect on facial aging. PLoS Genet. 2019;15:e1008353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology. 2017;28:30-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 1284] [Article Influence: 183.4] [Reference Citation Analysis (0)] |
| 47. | Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 2880] [Article Influence: 360.0] [Reference Citation Analysis (0)] |
| 48. | Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2275] [Cited by in RCA: 6244] [Article Influence: 624.4] [Reference Citation Analysis (0)] |
| 49. | Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 5365] [Article Influence: 766.4] [Reference Citation Analysis (0)] |
| 50. | Rosoff DB, Davey Smith G, Mehta N, Clarke TK, Lohoff FW. Evaluating the relationship between alcohol consumption, tobacco use, and cardiovascular disease: A multivariable Mendelian randomization study. PLoS Med. 2020;17:e1003410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 51. | Chen L, Fan Z, Sun X, Qiu W, Chen Y, Zhou J, Lv G. Mendelian Randomization Rules Out Causation Between Inflammatory Bowel Disease and Non-Alcoholic Fatty Liver Disease. Front Pharmacol. 2022;13:891410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 52. | Burgess S, Daniel RM, Butterworth AS, Thompson SG; EPIC-InterAct Consortium. Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol. 2015;44:484-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 351] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 53. | Manabe N, Matsueda K, Haruma K. Epidemiological Review of Gastroesophageal Junction Adenocarcinoma in Asian Countries. Digestion. 2022;103:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 54. | Pendergrast TR, Jain S, Trueger NS, Gottlieb M, Woitowich NC, Arora VM. Prevalence of Personal Attacks and Sexual Harassment of Physicians on Social Media. JAMA Intern Med. 2021;181:550-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 55. | Manterola C, Grande L, Bustos L, Otzen T. Prevalence of gastroesophageal reflux disease: a population-based cross-sectional study in southern Chile. Gastroenterol Rep (Oxf). 2020;8:286-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 56. | Lyall LM, Wyse CA, Graham N, Ferguson A, Lyall DM, Cullen B, Celis Morales CA, Biello SM, Mackay D, Ward J, Strawbridge RJ, Gill JMR, Bailey MES, Pell JP, Smith DJ. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91 105 participants from the UK Biobank. Lancet Psychiatry. 2018;5:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 57. | Luben R, Hayat S, Khawaja A, Wareham N, Pharoah PP, Khaw KT. Residential area deprivation and risk of subsequent hospital admission in a British population: the EPIC-Norfolk cohort. BMJ Open. 2019;9:e031251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Filiberti RA, Fontana V, De Ceglie A, Blanchi S, Lacchin T, De Matthaeis M, Ignomirelli O, Cappiello R, Rosa A, D'Onofrio V, Iaquinto G, Conio M. Dietary Habits and Risk of Esophagitis and Barrett's Esophagus: A Multicenter Italian Case-Control Study. Dig Dis Sci. 2021;66:3448-3460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Sun X, Elston RC, Barnholtz-Sloan JS, Falk GW, Grady WM, Faulx A, Mittal SK, Canto M, Shaheen NJ, Wang JS, Iyer PG, Abrams JA, Tian YD, Willis JE, Guda K, Markowitz SD, Chandar A, Warfe JM, Brock W, Chak A. Predicting Barrett's Esophagus in Families: An Esophagus Translational Research Network (BETRNet) Model Fitting Clinical Data to a Familial Paradigm. Cancer Epidemiol Biomarkers Prev. 2016;25:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Jansson C, Johansson AL, Nyrén O, Lagergren J. Socioeconomic factors and risk of esophageal adenocarcinoma: a nationwide Swedish case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14:1754-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 61. | Conio M, Filiberti R, Blanchi S, Ferraris R, Marchi S, Ravelli P, Lapertosa G, Iaquinto G, Sablich R, Gusmaroli R, Aste H, Giacosa A; Gruppo Operativo per lo Studio delle Precancerosi Esofagee (GOSPE). Risk factors for Barrett's esophagus: a case-control study. Int J Cancer. 2002;97:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 62. | Green HD, Beaumont RN, Wood AR, Hamilton B, Jones SE, Goodhand JR, Kennedy NA, Ahmad T, Yaghootkar H, Weedon MN, Frayling TM, Tyrrell J. Genetic evidence that higher central adiposity causes gastro-oesophageal reflux disease: a Mendelian randomization study. Int J Epidemiol. 2020;49:1270-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 63. | Thrift AP, Shaheen NJ, Gammon MD, Bernstein L, Reid BJ, Onstad L, Risch HA, Liu G, Bird NC, Wu AH, Corley DA, Romero Y, Chanock SJ, Chow WH, Casson AG, Levine DM, Zhang R, Ek WE, MacGregor S, Ye W, Hardie LJ, Vaughan TL, Whiteman DC. Obesity and risk of esophageal adenocarcinoma and Barrett's esophagus: a Mendelian randomization study. J Natl Cancer Inst. 2014;106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 64. | Miao Y, Yuan S, Li Y, Chen J, Li X, Larsson SC, Zhang Q. Bidirectional Association between Major Depressive Disorder and Gastroesophageal Reflux Disease: Mendelian Randomization Study. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 65. | Yuan S, Larsson SC. Adiposity, diabetes, lifestyle factors and risk of gastroesophageal reflux disease: a Mendelian randomization study. Eur J Epidemiol. 2022;37:747-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 66. | Nejatinamini S, Godley J, Minaker LM, Sajobi TT, McCormack GR, Cooke MJ, Nykiforuk CIJ, Koning L, Olstad DL. Quantifying the contribution of modifiable risk factors to socio-economic inequities in cancer morbidity and mortality: a nationally representative population-based cohort study. Int J Epidemiol. 2021;50:1498-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Chapelle N, Ben Ghezala I, Barkun A, Bardou M. The pharmacotherapeutic management of gastroesophageal reflux disease (GERD). Expert Opin Pharmacother. 2021;22:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 68. | Chou PH, Lin CC, Lin CH, Tsai CJ, Cheng C, Chuo YP, Chan CH, Lan TH. Prevalence of gastroesophageal reflux disease in major depressive disorder: a population-based study. Psychosomatics. 2014;55:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Etemadi A, Gandomkar A, Freedman ND, Moghadami M, Fattahi MR, Poustchi H, Islami F, Boffetta P, Dawsey SM, Abnet CC, Malekzadeh R. The association between waterpipe smoking and gastroesophageal reflux disease. Int J Epidemiol. 2017;46:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Lim KG, Morgenthaler TI, Katzka DA. Sleep and Nocturnal Gastroesophageal Reflux: An Update. Chest. 2018;154:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 71. | Nordestgaard BG. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ Res. 2016;118:547-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 756] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 72. | Huang Y, Xu P, Fu X, Ren Z, Cheng J, Lin Z, Tan J, Huang B, Huang Z, Xu H, Zhang D, Gao Y. The effect of triglycerides in the associations between physical activity, sedentary behavior and depression: An interaction and mediation analysis. J Affect Disord. 2021;295:1377-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Xu L, Borges MC, Hemani G, Lawlor DA. The role of glycaemic and lipid risk factors in mediating the effect of BMI on coronary heart disease: a two-step, two-sample Mendelian randomisation study. Diabetologia. 2017;60:2210-2220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |