Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2610
Revised: April 6, 2024
Accepted: April 12, 2024
Published online: June 15, 2024
Processing time: 161 Days and 23.4 Hours
Gastric signet ring cell carcinoma (GSRC) represents a specific subtype of gastric cancer renowned for its contentious epidemiological features, treatment principles, and prognostic factors.
To investigate the epidemiology of GSRC and establish an improved model for predicting the prognosis of patients with locally advanced GSRC (LAGSRC) after surgery.
The annual rates of GSRC incidence and mortality, covering the years 1975 to 2019, were extracted from the Surveillance, Epidemiology, and End Results (SEER) database to explore the temporal trends in both disease incidence and mortality rates using Joinpoint software. The clinical data of 3793 postoperative LAGSRC patients were collected from the SEER database for the analysis of survival rates. The Cox regression model was used to explore the independent prognostic factors for overall survival (OS). The risk factors extracted were used to establish a prognostic nomogram.
The overall incidence of GSRC increased dramatically between 1975 and 1998, followed by a significant downward trend in incidence after 1998. In recent years, there has been a similarly optimistic trend in GSRC mortality rates. The trend in GSRC showed discrepancies based on age and sex. Receiver operating characteristic curves, calibration curves, and decision curve analysis for 1-year, 3-year, and 5-year OS demonstrated the high discriminative ability and clinical utility of this nomogram. The area under the curve indicated that the performance of the new model outperformed that of the pathological staging system.
The model we established can aid clinicians in the early prognostication of LAGSRC patients, resulting in improved clinical outcomes by modifying management strategies and patient health care.
Core Tip: In the United States, there has been a downward trend in the incidence and mortality rates of gastric signet ring cell carcinoma (GSRC) over time, and these optimistic trends may be credited to the implementation of cancer screenings and the advancements in novel treatment approaches in the past few decades. Additionally, the model we established can help clinicians in early identification of the prognosis of locally advanced GSRC patients, resulting in enhanced clinical outcomes by modifying management strategies and patient healthcare.
- Citation: Yu ZH, Zhang LM, Dai ZQ, Zhang MN, Zheng SM. Epidemiology and prognostic nomogram for locally advanced gastric signet ring cell carcinoma: A population-based study. World J Gastrointest Oncol 2024; 16(6): 2610-2630
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2610.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2610
Gastric cancer (GC) is a formidable disease that poses a significant threat to human health. According to multipopulation observations conducted by the GLOBOCAN 2020, it ranks fifth in terms of incidence and fourth in terms of mortality among all cancers worldwide[1]. Gastric adenocarcinoma (GA) is the predominant histological subtype of GC, constituting approximately 95% of all diagnosed cases[2]. Gastric signet ring cell carcinoma (GSRC) belongs to the diffuse-type GA category and is distinguished by the presence of signet-ring cells. These cells possess an abundance of mucin, which displaces the nucleus to the cell periphery. GSRC arises from undifferentiated stem cells situated in the glandular neck region of the mucosal epithelium. Disruption of cell-cell adhesion complexes occurs due to the loss of E-cadherin, a calcium-dependent cell adhesion protein encoded by the tumor suppressor gene CDH1. Consequently, the infiltration of GSRC into both the mucosal layer and submucosal layer occurs gradually. However, once the submucosal layer is breached, swift dissemination and metastasis of cancer cells ensue[3].
Despite significant advancements in understanding the epidemiology, pathology, molecular mechanisms, and treatment alternatives and approaches associated with GSRC, the diagnostic and therapeutic challenges remain substantial. Recent studies have shown that GSRC represents a significant proportion, ranging from approximately 35% to 45%, of GA cases in Asia, the United States, and Europe[4]. Moreover, the majority of individuals with GSRC often present with a locally progressive stage upon initial diagnosis and typically manifest an advanced disease state involving invasion of lymph nodes and adjacent organs, and a significant proportion of patients experience recurrence or complications subsequent to surgical resection[5,6].
Although prior studies have substantiated the increasing incidence of GSRC between 1973 and 2000[7,8], attributed to several factors including alterations in dietary patterns and Helicobacter pylori infection, a comprehensive investigation into the precise epidemiology of GSRC subsequent to 2000 has been noticeably lacking. The present study endeavors to address this knowledge gap by providing an in-depth examination of the subject matter. In addition to identifying and evaluating the epidemiology of GSRC, this study aimed to develop a dynamic prognostic nomogram for patients with a postoperative pathological stage of locally advanced GSRC (LAGSRC, i.e., T1-2N+M0 or T3-4bNxM0, as defined by the AJCC/UICC 8th edition TNM staging of GC, combined with anatomical definitions[9,10]). Although current management guidelines for GA propose surgery supplemented with adjuvant chemotherapy, predicting patient outcomes under this regimen remains a conundrum. A dynamic nomogram, a predictive tool that provides individualized risk assessment, could significantly aid in developing personalized treatment strategies and predicting patient survival, thus improving overall patient management.
The incidence data and age-adjusted incidence-based mortality rates of GSRC patients from 1975 to 2019 were obtained through an extensive analysis and interpretation of the Surveillance, Epidemiology, and End Results (SEER) 8 region database. The demographic data, including sex and age (i.e., < 60 years/60-69 years/> 70 years) at the time of diagnosis, were retrieved from the SEER database. The same method was used to collect data on GA for further comparative analysis with GSRC. The SEER program, initiated in 1973, has been committed to collecting cancer-related information from across the United States, thereby offering comprehensive and detailed insights into cancer, including incidence, survival rates, mortality rates, treatment approaches, and demographic statistics. This extensive and widely utilized database holds significant importance in the medical field, particularly in the realm of epidemiological research. It serves as a valuable reference and guide, facilitating the development of preventive, diagnostic, and therapeutic strategies for cancer.
All rates were age-adjusted based on the 2000 United States standard population. The annual percentage change (APC) and corresponding 95% confidence intervals (95%CIs) were calculated using Joinpoint software (version 4.9). In 2000, Kim et al[11], pioneered the Joinpoint regression model. This model fundamentally maps out segmented regression constructed on the temporal characteristics seen in disease distribution. This is achieved by partitioning the study period into different intervals through various junction points, followed by trend fitting and optimization for each interval. Therefore, this approach allows for a comprehensive evaluation of the unique evolutions in disease patterns across different segments within a total time frame. A t test was conducted to ascertain whether the APC differed significantly from zero. The estimates were deemed statistically significant at α = 0.05.
This investigation meticulously complied with the provisions presented in the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis statement, aiming to guarantee transparency and comprehensiveness in the reporting of its research outcomes. The data pertaining to the studied patients with GSRC were acquired from the SEER database encompassing the period from January 1, 2000 to December 31, 2015. According to the International Classification of Diseases for Oncology (ICD-O), Third Edition, GSRC patients were identified. The ICD-O topography code for GSRC is C16, which corresponds to the location in the stomach. The histological subtype was signet ring cell carcinoma (ICD-O histology codes: 8490/2, 8490/3). The exclusion criteria were as follows: (1) Unclear survival time or incomplete follow-up information, as well as mortality within 30 days of diagnosis; (2) absence of surgical resection for the primary tumor; (3) simultaneous occurrence of tumors in multiple sites; (4) inability to determine pathological staging, and not meeting the criteria of T1-2N+M0 or T3-4bNxM0; and (5) onset of disease at an age younger than 18 years. In this study, we included the following covariates for analysis: age, year of diagnosis, sex, race, histological grade, involved lymph node count, tumor infiltration depth, tumor size, tumor site, positive lymph node count, pathological staging, chemotherapy, radiation therapy, survival status, cause of death, and survival time.
The differences in the distributions of clinicopathological characteristics between the chemotherapy group and the nonchemotherapy group were compared using chi-square tests and independent sample t tests/Mann-Whitney U-tests. Survival analyses were conducted considering both overall survival (OS) and cancer-specific survival (CSS), with Kaplan-Meier survival curves being generated, and the associated P values were determined using the log-rank test. Cox regression analysis was utilized to conduct multifactorial survival analysis, aiming to assess the influence of individual covariates on survival time. The resulting impact was typically quantified in terms of hazard ratios (HRs). To facilitate a more intuitive portrayal of these HRs, along with their associated confidence levels, we generated a forest plot incorporating the 95% confidence intervals.
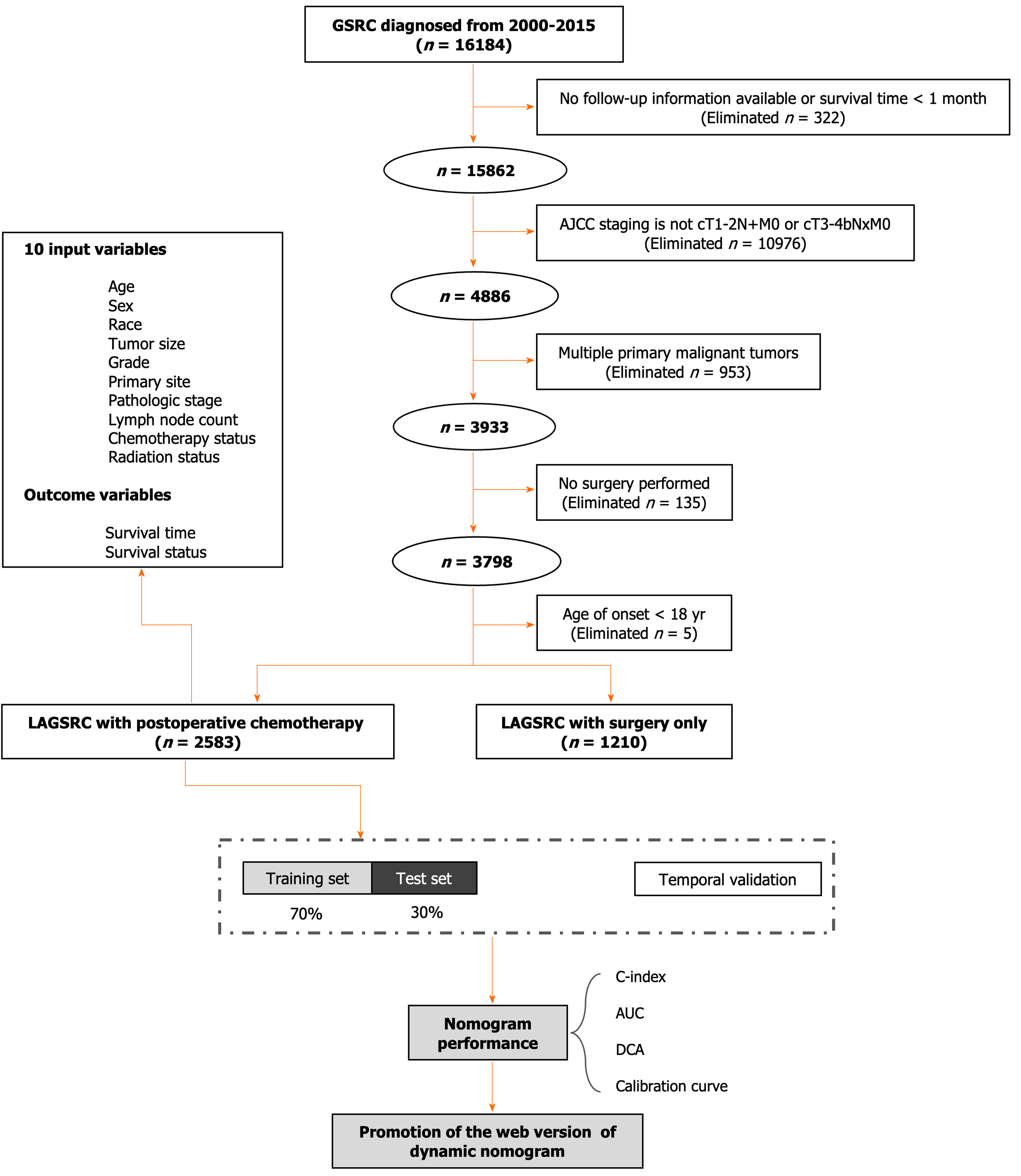
LAGSRC patients who underwent radical surgery for primary tumors were randomly allocated to a training set or an internal test set at a ratio of 7:3. The backward stepwise selection method within the Cox regression model was applied to the training cohort, facilitating the identification of pertinent variables. Utilizing these established prognostic factors, we devised a nomogram to ascertain the probabilities of 1-year, 3-year, and 5-year OS for LAGSRC patients. The model underwent internal validation using the bootstrap method with 1000 repetitions. Subsequently, the calibration curve was utilized to calibrate the established nomogram. The evaluation of the model included the concordance index (C-index), area under the receiver operating characteristic (ROC) curve (AUC), and decision curve analysis (DCA). The observed results were compared to the predicted probabilities using the calibration curve. Additionally, this study included patients who were diagnosed with LAGSRC from 2018 to 2019 to serve as a temporal validation group for assessing the generalizability of this prognostic model. Figure 1 presents a visual framework.
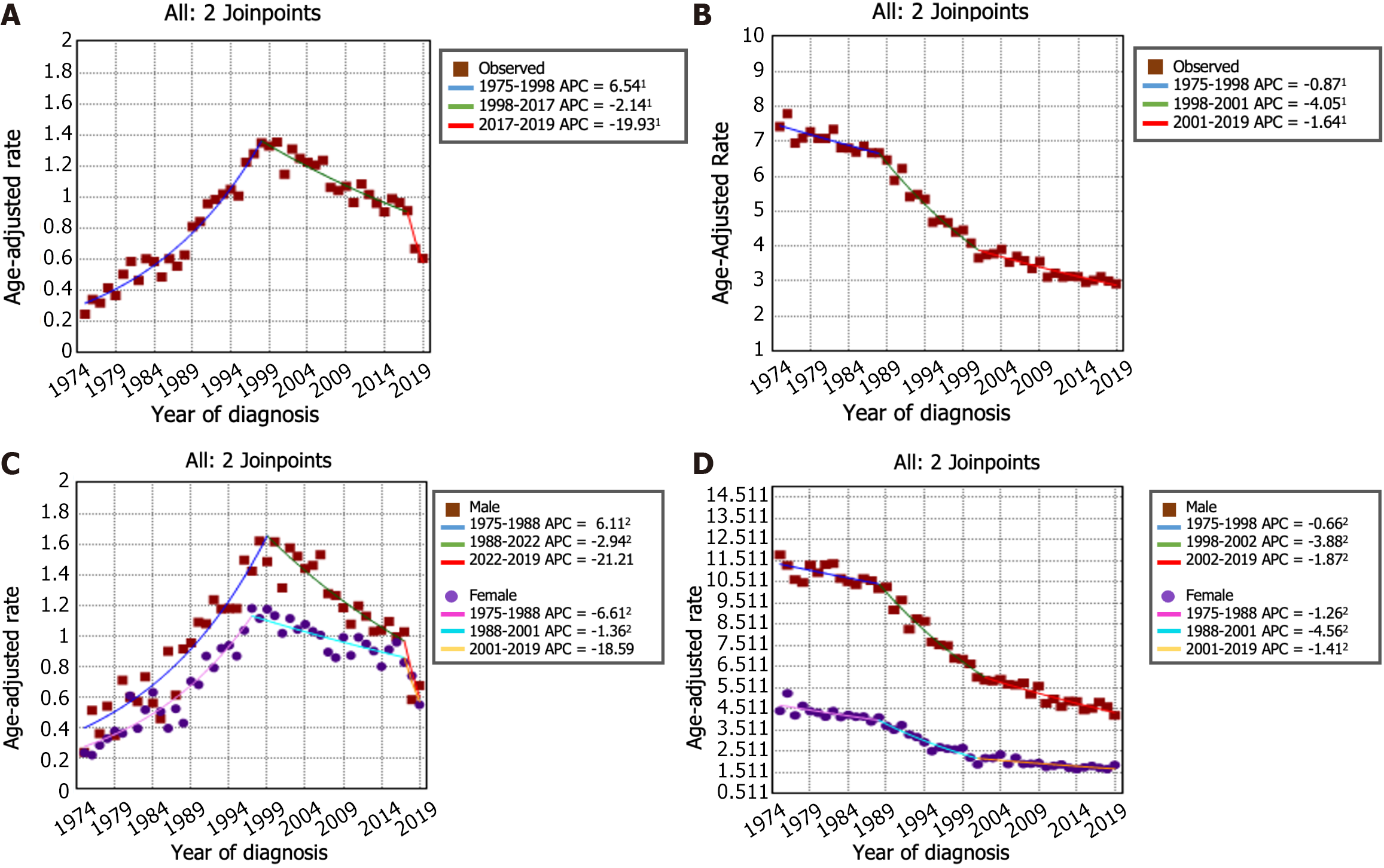
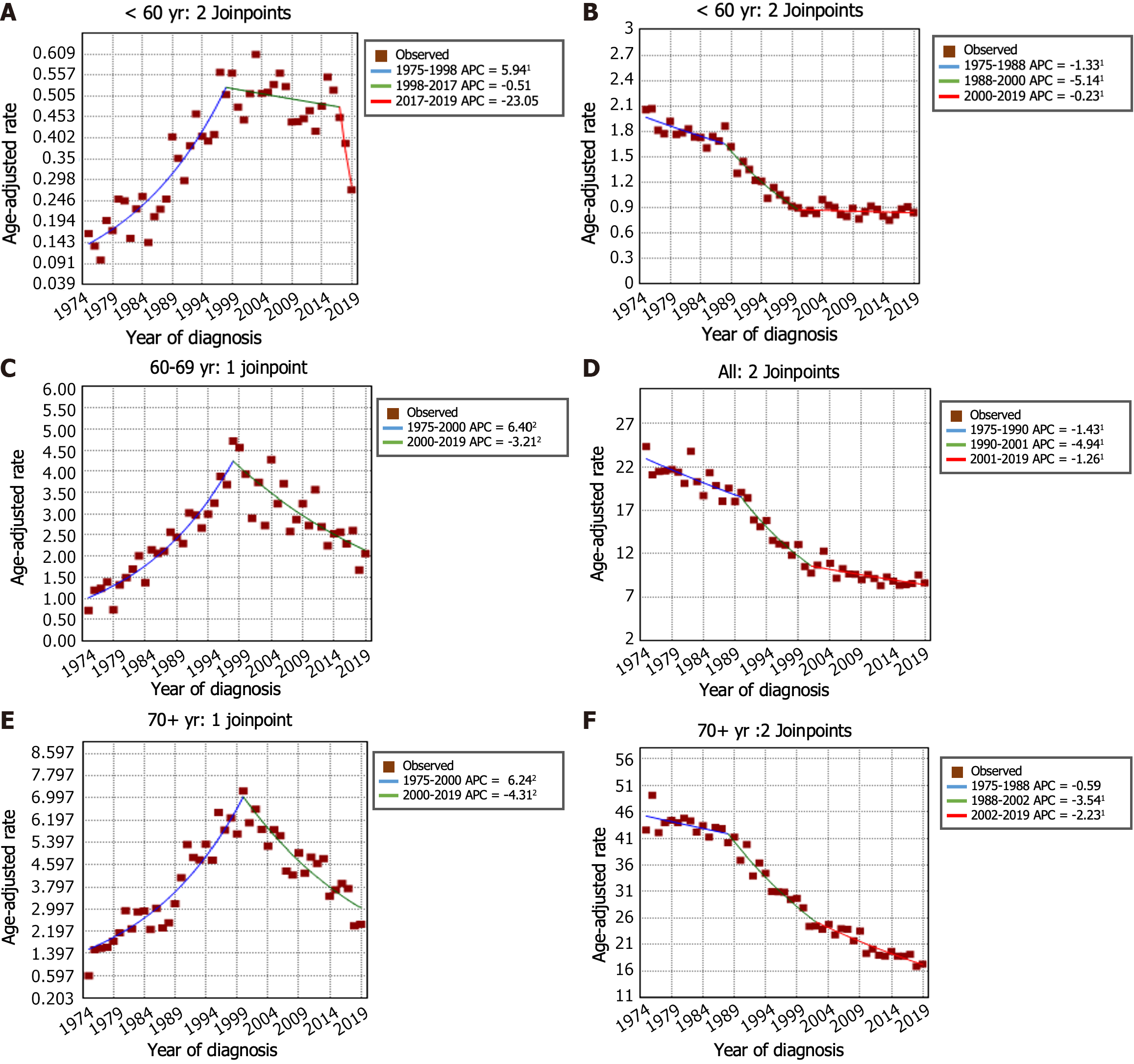
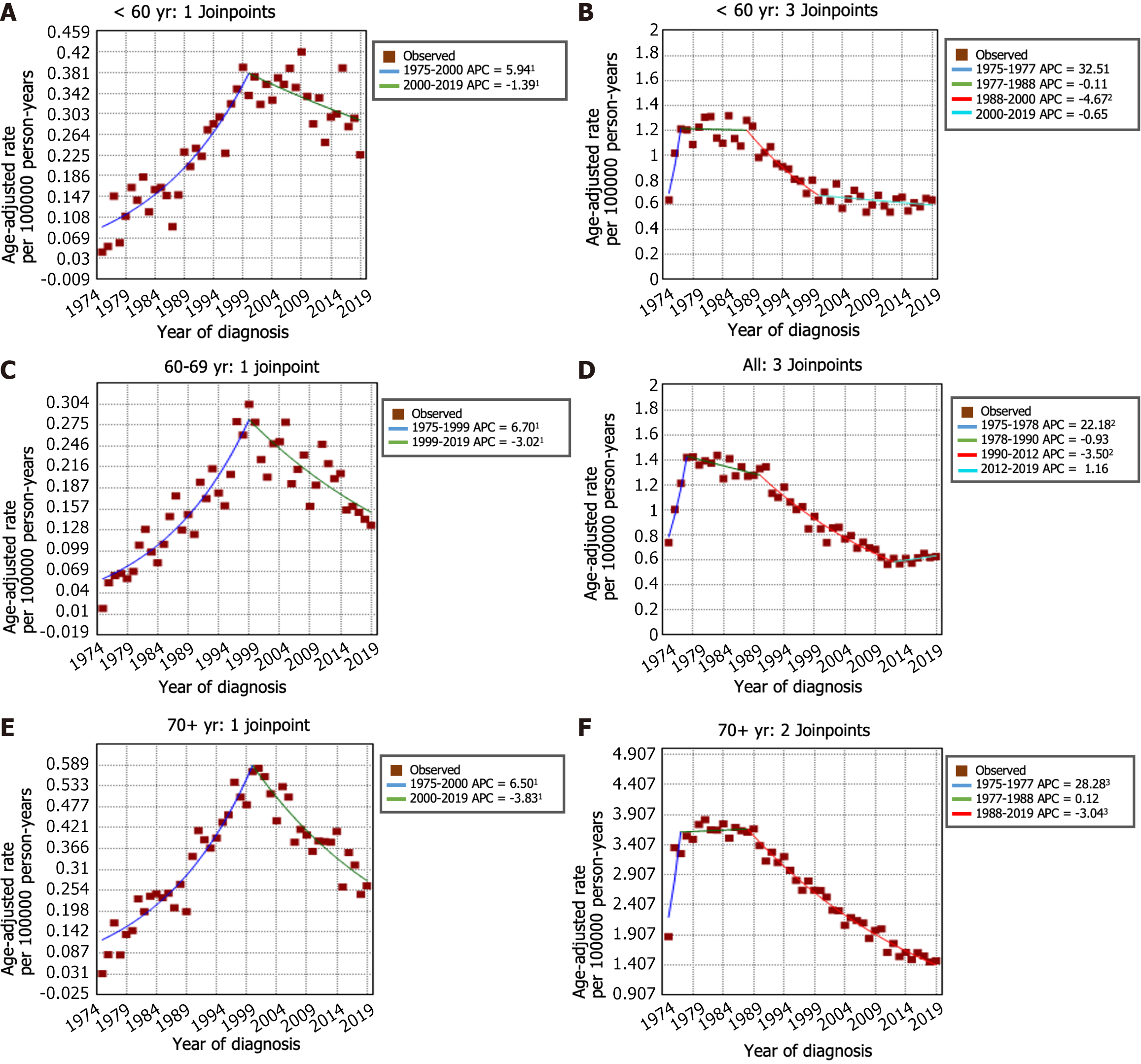
The incidence trends of GSRC and GA can be seen in Figure 2A and B. From 1975 to 1998, the incidence of GSRC increased (APC: 6.5, 95%CI: 5.8 to 7.2). Then, the incidence decreased from 1998 to 2017 (APC: -2.1, 95%CI: -2.7 to -1.6), and it continued to decrease, at a faster rate, during 2017-2019 (APC: -19.9, 95%CI: -35.4 to -0.7). The incidence rate of GA decreased from 7.42 per 100000 person-years in 1975 to 2.93 per 100000 person-years in 2019. In the period spanning from 1975 to 1988, the incidence rate of GA decreased (APC: -0.9, 95%CI: -1.4 to -0.4). This decreasing trend persisted and even accelerated from 1988 to 2001 (APC: -4.0, 95%CI: -4.6 to -3.5). Nevertheless, a deceleration in the rate of decrease was noted from 2001 to 2019 (APC: -1.6, 95%CI: -2.0 to -1.3). Figure 2C and D illustrates the trends in incidence rates stratified by sex indicating a markedly greater proportion of females in the GSRC group than in the GA group. The overall trend of incidence rates between males and females was generally similar. Furthermore, the age-stratified analysis of the incidence rate trends for GSRC and GA can be observed in Figure 3.
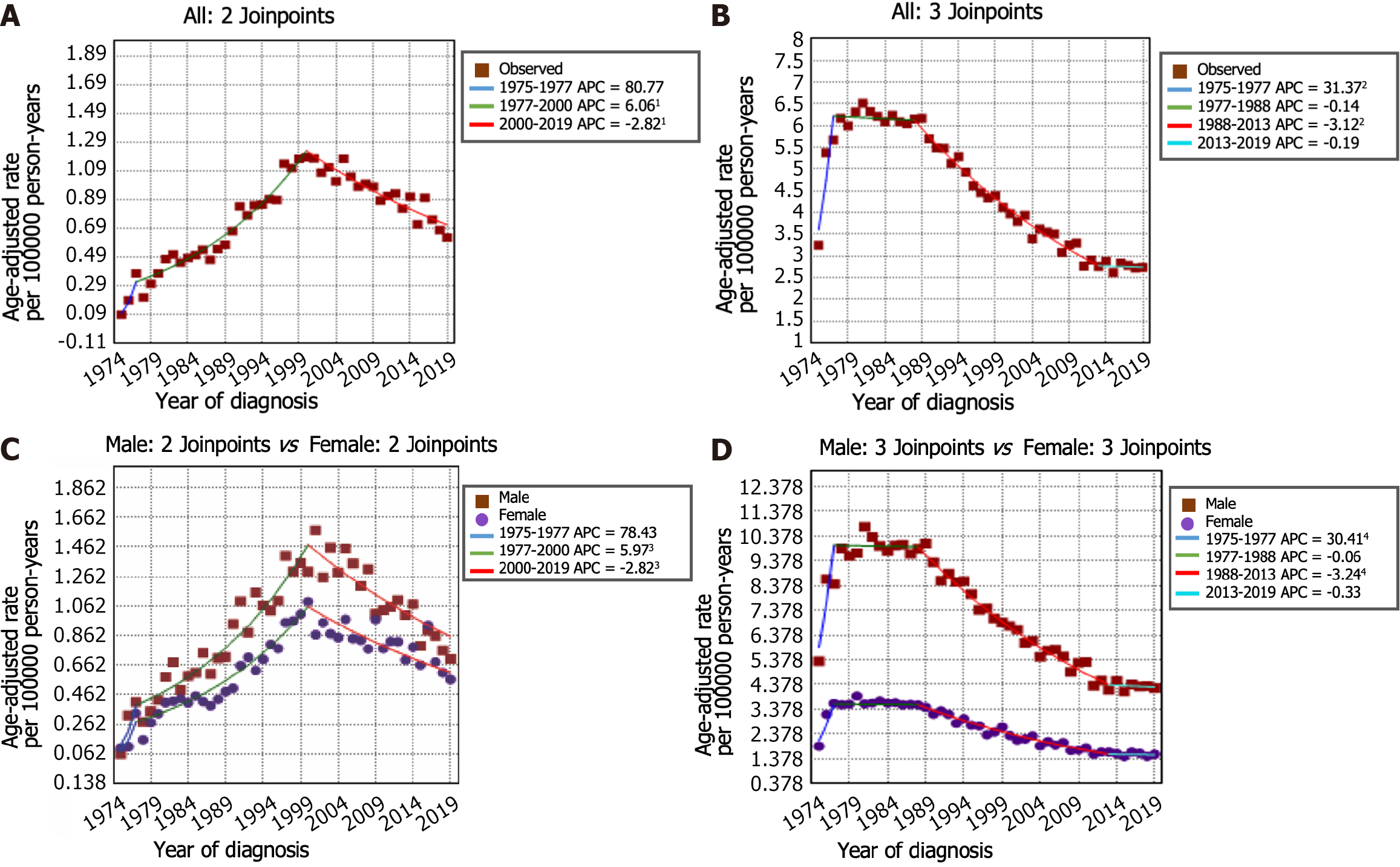
The incidence-based mortality rate trends for GSRC and GA are shown in Figure 4A and B. During the 1975-1977 timeframe, the mortality rate for GSRC exhibited a notably greater increase (APC: 80.8, 95%CI: -22.6 to 322.2) than that during the 1977-2000 period (APC: 6.1, 95%CI: 5.3 to 6.9). Nonetheless, a significant reduction in the mortality rate for GSRC was observed during the period spanning from 2000 to 2019 (APC: -2.8, 95%CI: -3.4 to -2.2). There were 4 trends with 3 Joinpoints in the mortality rate of GA. Between 1975 and 1977, there was an increase in the mortality rate for GA (APC: 31.4, 95%CI: 14.9 to 50.2). Subsequently, from 1977 to 2019, a decrease in the mortality rate for GA was observed, with a decrease from 5.67 per 100000 person-years in 1977 to 2.72 per 100000 person-years in 2019. Similarly, the results of the mortality rate analysis stratified by sex and age are shown in Figure 4C and D and Figure 5. Comparatively, the male-to-female mortality ratio in the GSRC was more closely aligned.
Of the 3793 LAGSRC patients included in the analysis, 2583 (68.1%) received gastrectomy combined with adjuvant chemotherapy and 1210 (31.9%) did not receive adjuvant chemotherapy after surgery. As indicated in Table 1, significant differences were observed with respect to age, sex, year of diagnosis, tumor site, T-stage, number of lymph nodes examined, and postoperative radiation therapy status between patients receiving chemotherapy and those not receiving chemotherapy. The data were arbitrarily distributed into a training set and an internal test set (7:3 ratio). Table 2 describes the clinical and pathological features of the training set and internal test set, and shows that the deviations were purely random.
| Characteristic | LAGSRC (n = 3793) | P value2 | |
| Untreated/unknown n = 12101 | Chemotherapy, n = 25831 | ||
| Age (yr) | 66 (14) | 57 (13) | < 0.001 |
| Sex | 0.039 | ||
| Female | 621 (51) | 1233 (48) | |
| Male | 589 (49) | 1350 (52) | |
| Year of diagnosis | < 0.001 | ||
| 2000-2003 | 411 (34) | 582 (23) | |
| 2004-2007 | 388 (32) | 742 (29) | |
| 2008-2011 | 246 (20) | 656 (25) | |
| 2012-2015 | 165 (14) | 603 (23) | |
| Race | 0.34 | ||
| White | 832 (69) | 1718 (67) | |
| Black | 139 (11) | 304 (12) | |
| Other | 239 (20) | 561 (22) | |
| Tumor size (cm) | 0.92 | ||
| < 2 | 59 (4.9) | 134 (5.2) | |
| 2-5 | 440 (36) | 937 (36) | |
| > 5 | 495 (41) | 1035 (40) | |
| Unknown | 216 (18) | 477 (18) | |
| Grade | 0.19 | ||
| G1/G2 | 42 (3.5) | 63 (2.4) | |
| G3/G4 | 1101 (91) | 2378 (92) | |
| Unknown | 67 (5.5) | 142 (5.5) | |
| Primary site | < 0.001 | ||
| Body | 118 (9.8) | 257 (9.9) | |
| Cardia | 116 (9.6) | 371 (14) | |
| Fundus | 26 (2.1) | 66 (2.6) | |
| Gastric antrum | 364 (30) | 690 (27) | |
| Greater curvature | 46 (3.8) | 160 (6.2) | |
| Lesser curvature | 171 (14) | 330 (13) | |
| Pylorus | 68 (5.6) | 129 (5.0) | |
| Overlapping lesion | 139 (11) | 317 (12) | |
| Unknown | 162 (13) | 263 (10) | |
| PT 8th | < 0.001 | ||
| T1 | 55 (4.5) | 103 (4.0) | |
| T2 | 65 (5.4) | 171 (6.6) | |
| T3 | 392 (32) | 954 (37) | |
| T4a | 387 (32) | 838 (32) | |
| T4b | 311 (26) | 517 (20) | |
| PN 8th | 0.067 | ||
| N0 | 220 (18) | 398 (15) | |
| N1 | 234 (19) | 466 (18) | |
| N2 | 243 (20) | 596 (23) | |
| N3a | 324 (27) | 731 (28) | |
| N3b | 189 (16) | 392 (15) | |
| Pathologic stage | 0.5 | ||
| IB | 33 (2.7) | 64 (2.5) | |
| IIA | 152 (13) | 307 (12) | |
| IIB | 159 (13) | 340 (13) | |
| IIIA | 257 (21) | 622 (24) | |
| IIIB | 334 (28) | 703 (27) | |
| IIIC | 275 (23) | 547 (21) | |
| Lymph node count | 17 (13) | 19 (13) | < 0.001 |
| Radiation status | < 0.001 | ||
| No/unknown | 1120 (93) | 983 (38) | |
| Yes | 90 (7.4) | 1600 (62) | |
| Characteristics | LAGSRC (n = 3793) | P value2 | |
| Train set (n = 2655)1 | Internal test set (n = 1138)1 | ||
| Age (yr) | 61.0 (13) | 59.5 (13) | 0.33 |
| Sex | 0.96 | ||
| Female | 1299 (48.9) | 555 (48.8) | |
| Male | 1356 (51.1) | 583 (51.2) | |
| Year of diagnosis | 0.87 | ||
| 2000-2003 | 687 (25.9) | 306 (26.9) | |
| 2004-2007 | 788 (29.7) | 342 (30.1) | |
| 2008-2011 | 638 (24) | 264 (23.2) | |
| 2012-2015 | 542 (20.4) | 226 (19.9) | |
| Race | 0.57 | ||
| White | 1771 (66.7) | 779 (68.5) | |
| Black | 316 (11.9) | 127 (11.2) | |
| Other | 568 (21.4) | 232 (20.4) | |
| Tumor size (cm) | 0.81 | ||
| < 2 | 140 (5.3) | 53 (4.7) | |
| 2-5 | 962 (36.2) | 415 (36.5) | |
| > 5 | 1063 (40) | 467 (41) | |
| Unknown | 490 (18.5) | 203 (17.8) | |
| Grade | 0.26 | ||
| G1/G2 | 81 (3.1) | 24 (2.1) | |
| G3/G4 | 2426 (91.4) | 1053 (92.5) | |
| Unknown | 148 (5.6) | 61 (5.4) | |
| Primary site | 0.88 | ||
| Body | 258 (9.7) | 117 (10.3) | |
| Cardia | 335 (12.6) | 152 (13.4) | |
| Fundus | 63 (2.4) | 29 (2.5) | |
| Gastric antrum | 755 (28.4) | 299 (26.3) | |
| Greater curvature | 147 (5.5) | 59 (5.2) | |
| Lesser curvature | 353 (13.3) | 148 (13) | |
| Pylorus | 131 (4.9) | 66 (5.9) | |
| Overlapping lesion | 314 (11.8) | 142 (12.5) | |
| Unknown | 299 (11.3) | 126 (11.1) | |
| PT 8th | 0.67 | ||
| T1 | 118 (4.4) | 40 (3.5) | |
| T2 | 142 (5.3) | 94 (8.3) | |
| T3 | 976 (36.8) | 370 (32.5) | |
| T4a | 851 (32.1) | 374 (32.9) | |
| T4b | 568 (21.4) | 260 (22.8) | |
| PN 8th | 0.84 | ||
| N0 | 423 (15.9) | 195 (17.1) | |
| N1 | 497 (18.7) | 203 (17.8) | |
| N2 | 585 (22) | 254 (22.3) | |
| N3a | 737 (27.8) | 318 (27.9) | |
| N3b | 413 (15.6) | 168 (14.8) | |
| Pathologic stage | 0.11 | ||
| IB | 78 (2.9) | 19 (1.7) | |
| IIA | 322 (12.1) | 137 (12) | |
| IIB | 337 (12.7) | 162 (14.2) | |
| IIIA | 599 (22.6) | 280 (24.6) | |
| IIIB | 742 (27.9) | 295 (25.9) | |
| IIIC | 577 (21.7) | 245 (21.5) | |
| Lymph node count | 16 (13) | 16 (14) | 0.52 |
| Chemotherapy status | 0.18 | ||
| No/unknown | 865 (32.6) | 345 (30.3) | |
| Yes | 1790 (67.4) | 793 (69.7) | |
| Radiation status | 0.45 | ||
| No/unknown | 1461 (55) | 289 (37) | |
| Yes | 1194 (45) | 486 (63) | |
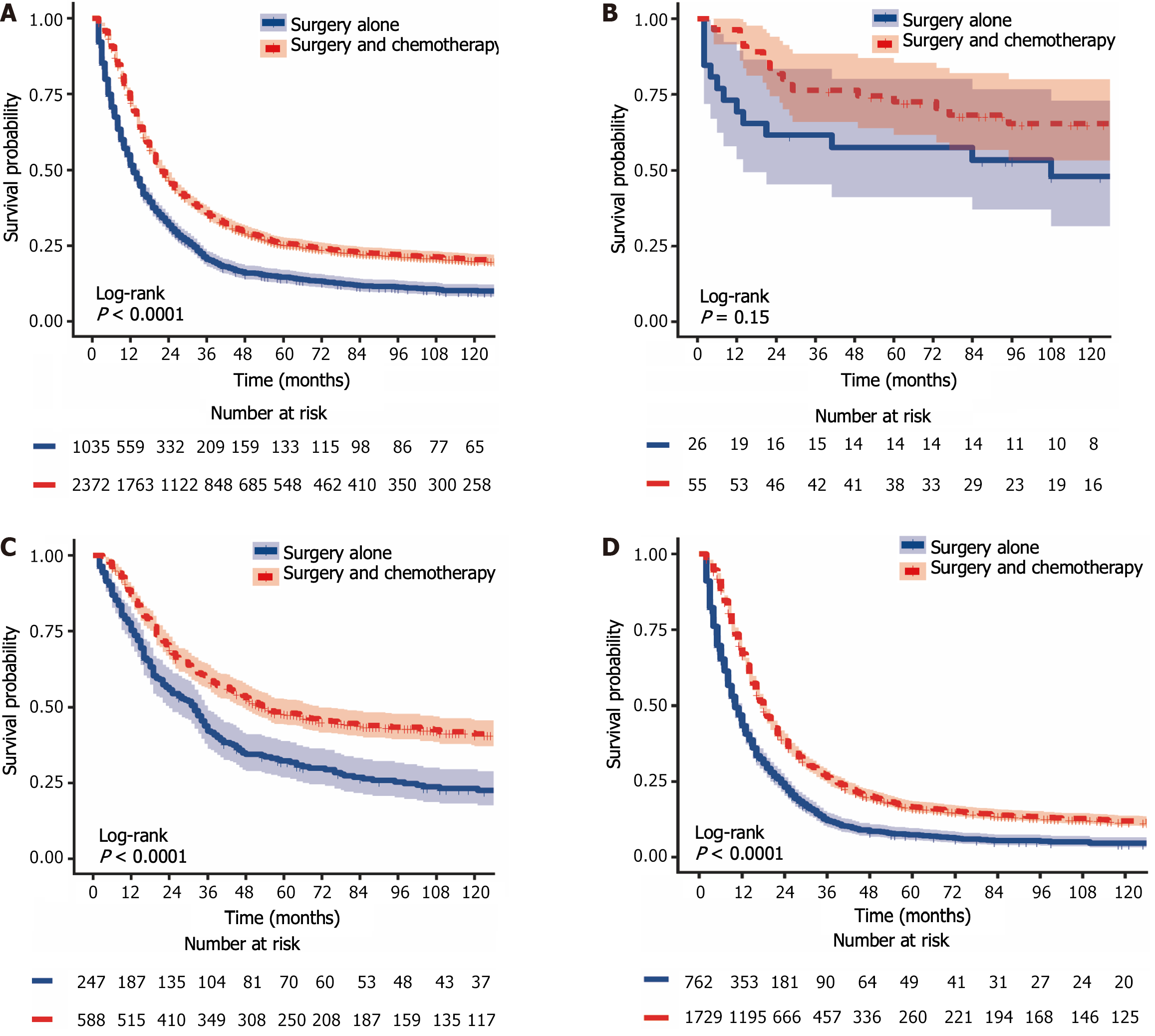
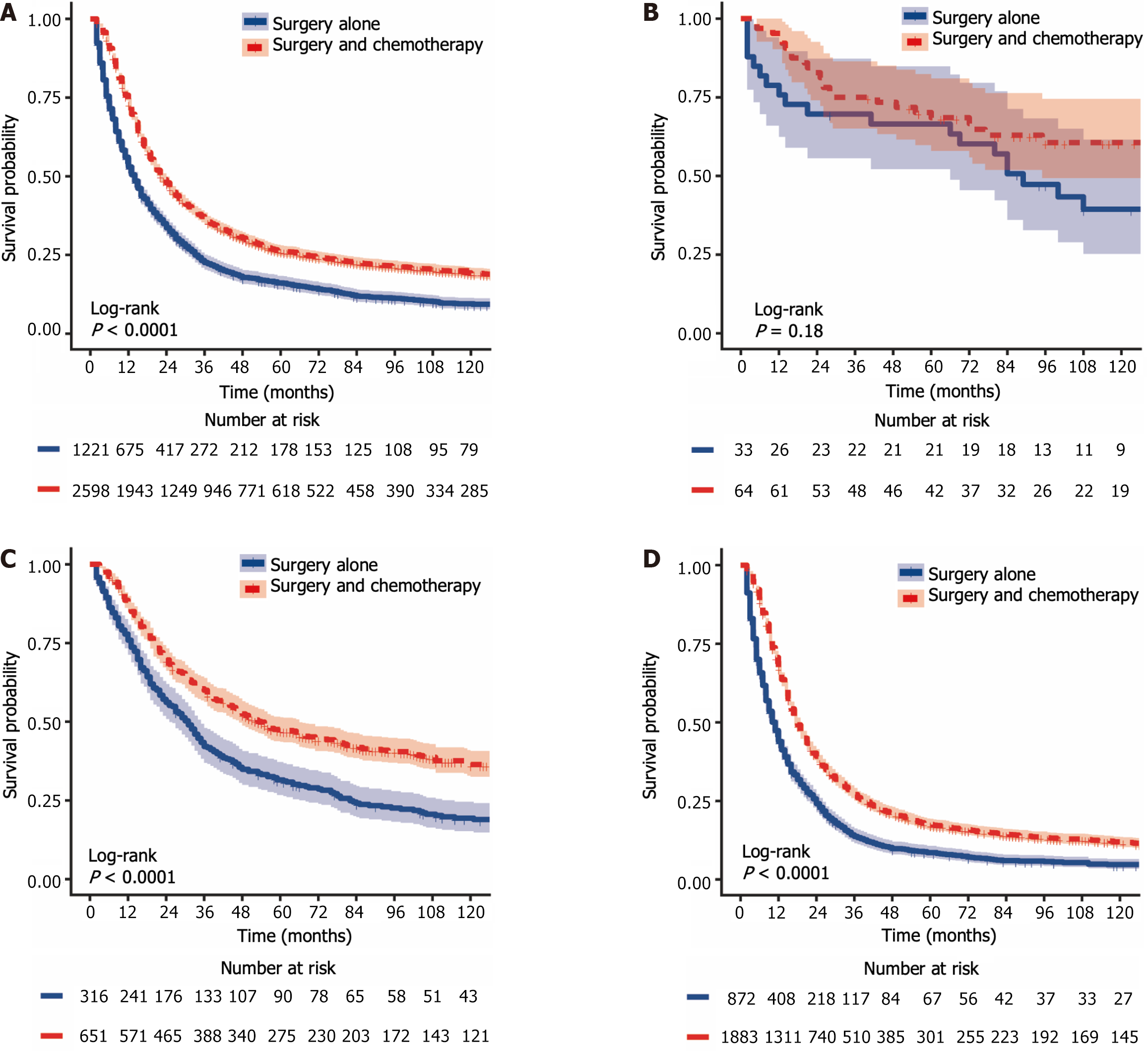
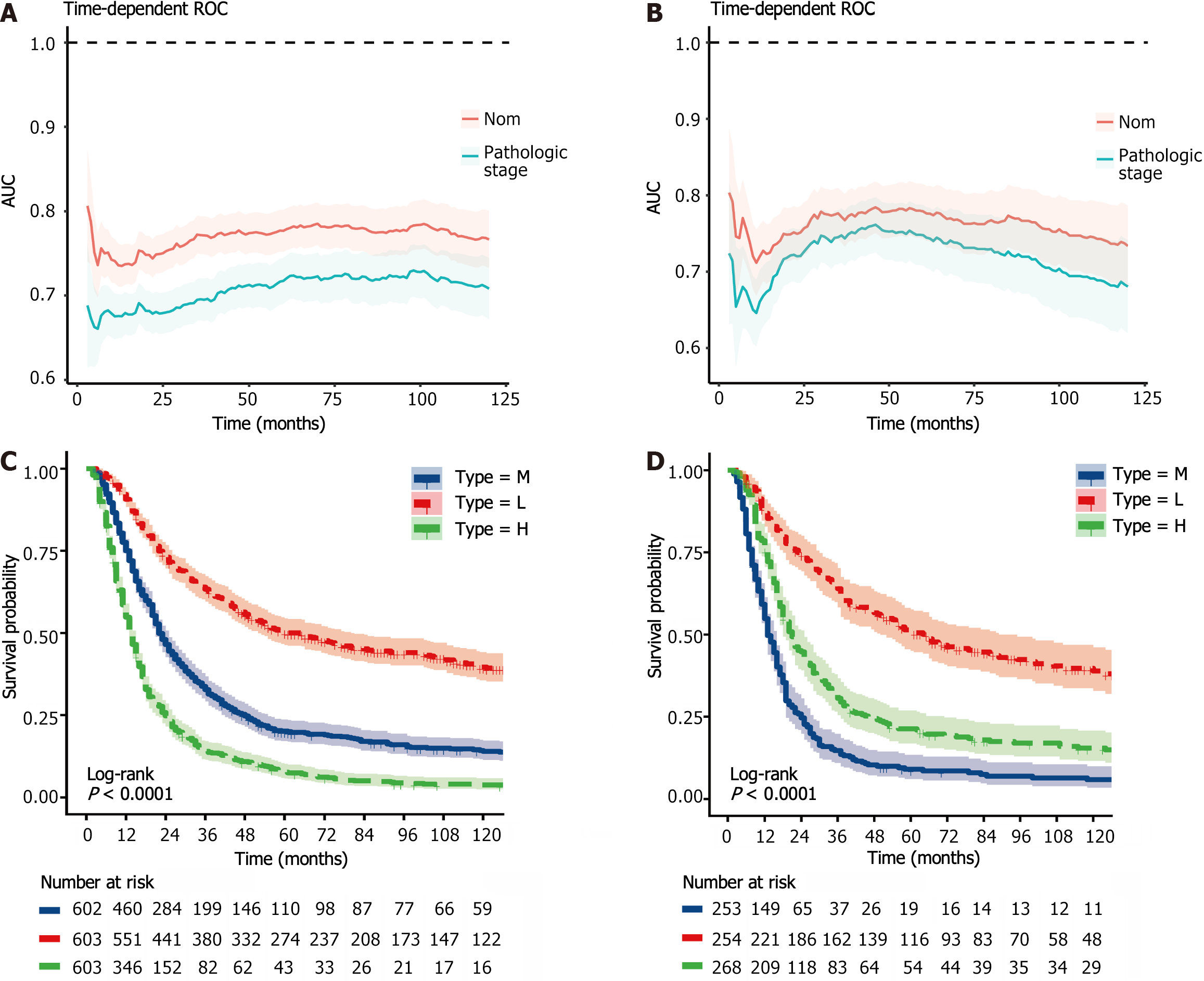
The 1-year, 3-year, and 5-year CSS rates for LAGSRC patients who received postoperative chemotherapy were 72.7% (95%CI: 70.9% to 74.5%), 35.9% (95%CI: 34.0% to 37.9%), and 25.8% (95%CI: 24.1% to 27.7%), respectively. The 1-year, 3-year, and 5-year CSS rates for the surgery-only group were 51.6% (95%CI: 48.6% to 54.7%), 20.48% (95%CI: 18.1% to 23.1%), and 14.6% (95%CI: 12.6% to 17.0%), respectively. For CSS, the median survival times in the two groups were 22 months and 13 months respectively, with a log-rank test P < 0.001 (Figure 6A). The 1-year, 3-year, and 5-year OS rates for LAGSRC patients who underwent postoperative chemotherapy were 73.0%, 36.5%, and 26.3%, respectively, compared with 53.0%, 22.6%, and 16.1%, respectively, for the surgery-only group (log-rank test P < 0.001; Figure 7A).
Further analysis was conducted to evaluate the impact of postoperative adjuvant chemotherapy on the survival of LAGSRC patients stratified by various pathological stages. Among patients with stage I disease, the groups receiving postoperative adjuvant chemotherapy exhibited 1-year, 3-year, and 5-year CSS rates of 96.4%, 76.4%, and 72.5% respectively. Conversely, the CSS rates for the surgery-only group at 1 year, 3 years, and 5 years were 69.2%, 61.5%, and 57.4%, respectively. Based on the log-rank test for CSS, there was no statistically significant difference among the groups, with a p-value of 0.152 (Figure 6B). Similar results were obtained for OS (Figure 7B). Among LAGSRC patients with stage II/III disease, significant differences were observed based on the log-rank test for both CSS (P < 0.001) and OS (P < 0.001). Figure 6C and D and Figure 7C and D depict the trends in survival rates for stage II/III patients, showing nonoverlapping survival curves between the two groups. At any follow-up time point, the surgical combined with chemotherapy group exhibited significantly greater survival rates than did the surgery-only group.
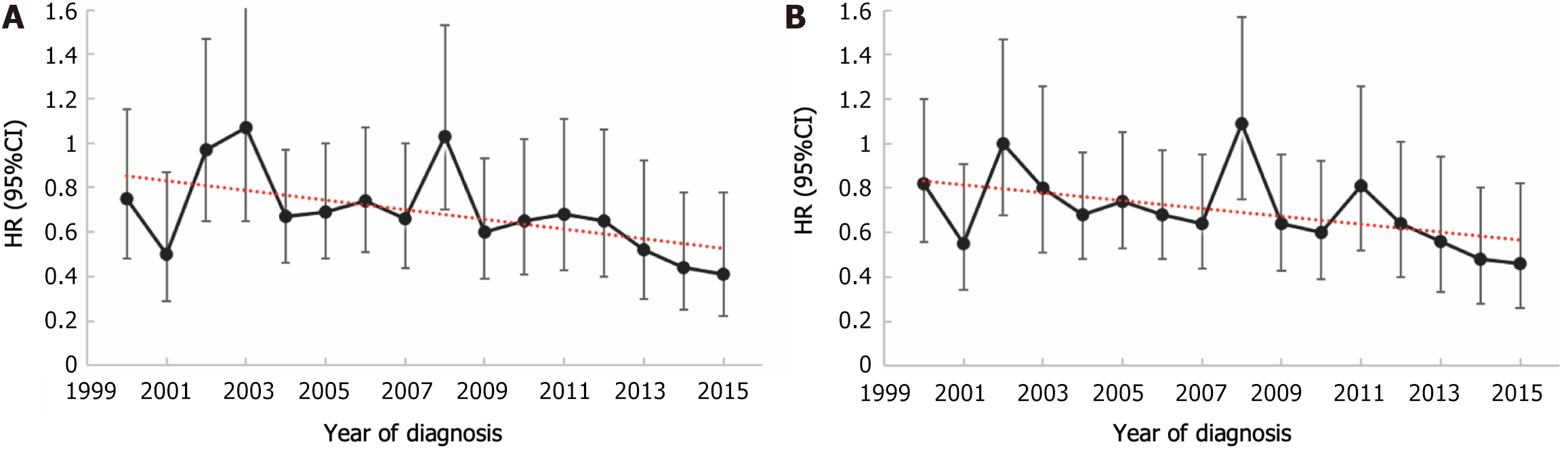
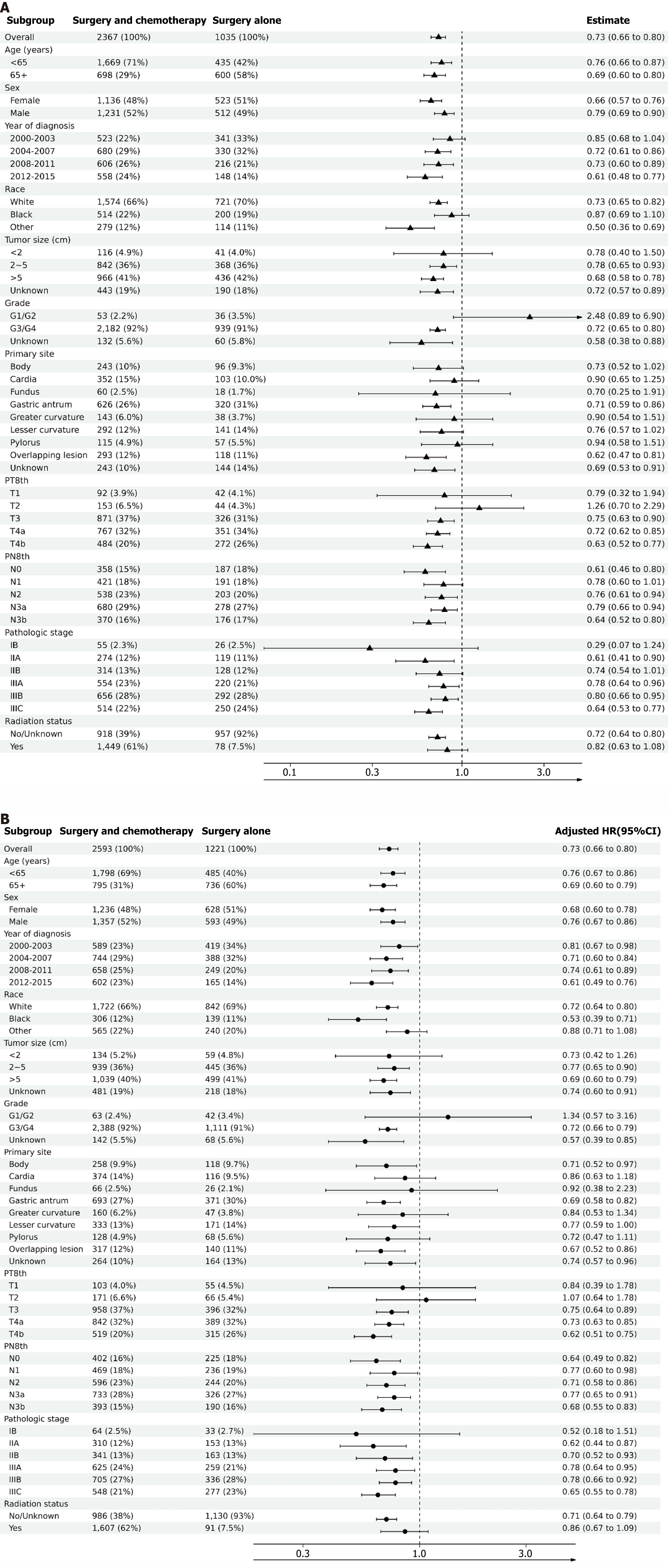
This study comprehensively assessed the survival benefits conferred by postoperative chemotherapy in patients diagnosed with LAGSRC across various diagnostic years. This study included a cohort of patients diagnosed with LAGSRC between 2000 and 2015, during which multiple adjustments were made to the chemotherapy regimens. To account for the potential influence of diagnostic year variations, an analysis was conducted employing Cox proportional hazards models with adjustments for multiple covariates. Figure 8 illustrates the trends in HRs of adjuvant chemotherapy for LAGSRC after adjusting for confounding factors such as age, sex, histological differentiation, tumor location, tumor size, pathological stage, and adjuvant radiotherapy. In this context, the HR of adjuvant chemotherapy for LASRC patients in terms of CSS decreased with increasing diagnostic years, and this decrease was statistically significant (P = 0.033). For OS, the HR of adjuvant chemotherapy for LASRC patients also decreased with increasing years, but the difference did not reach statistical significance (P = 0.056). In addition, based on the multivariable Cox regression, the forest plot illustrates the effect estimates and confidence intervals of the survival benefits from postoperative adjuvant chemotherapy in different subgroups, along with potential statistical significance (Figure 9).
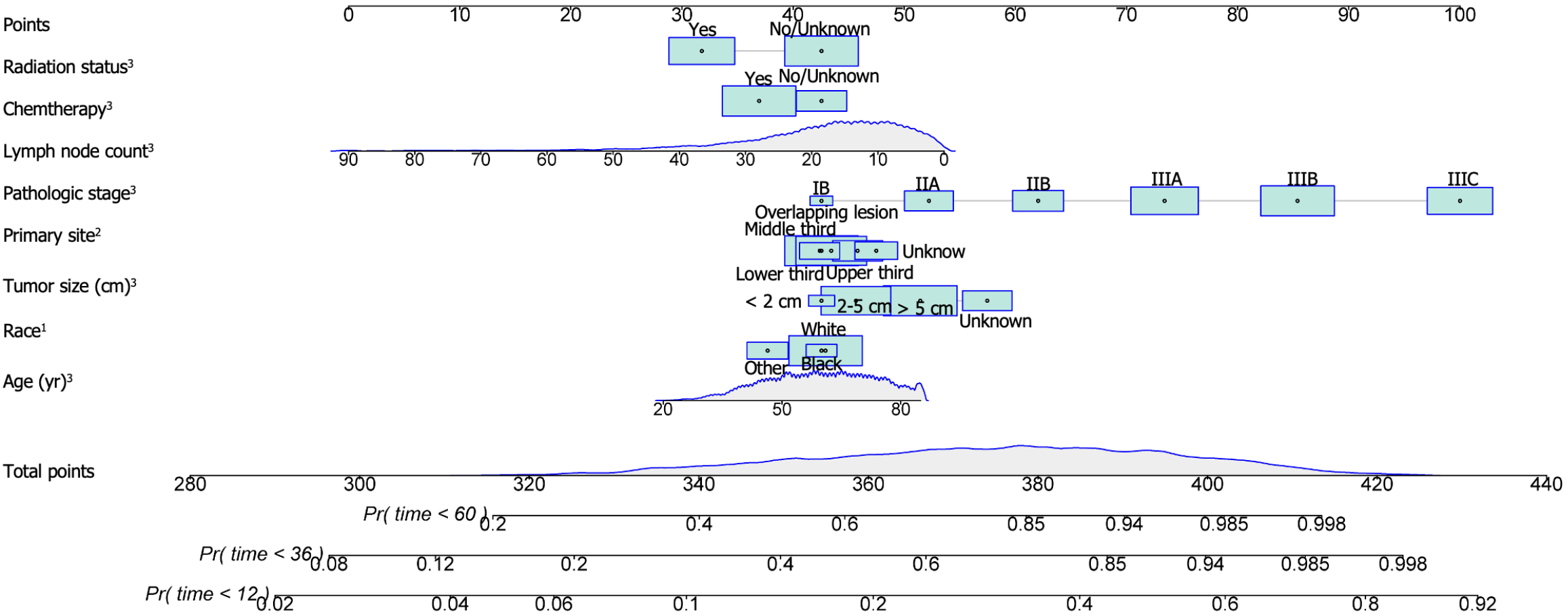
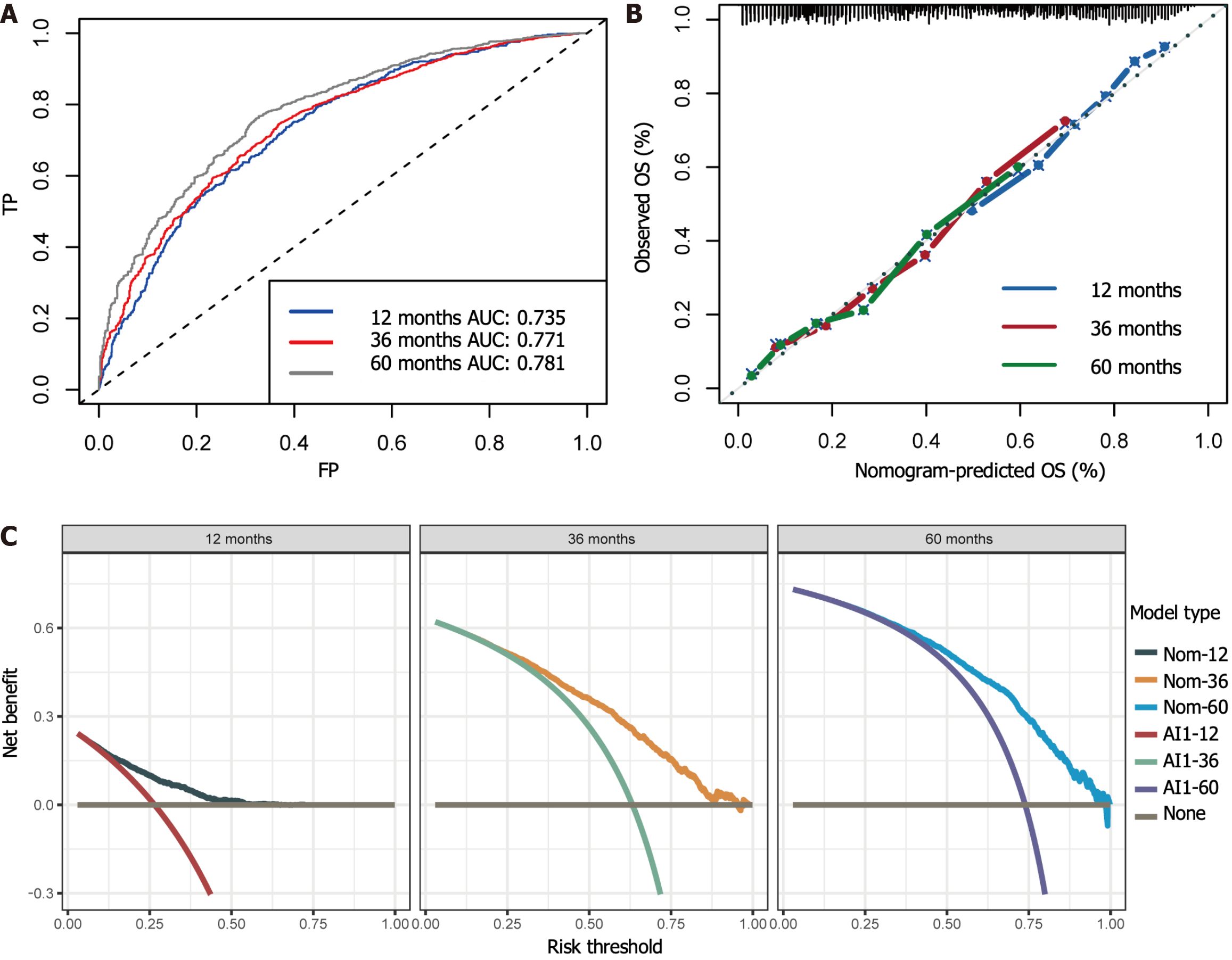
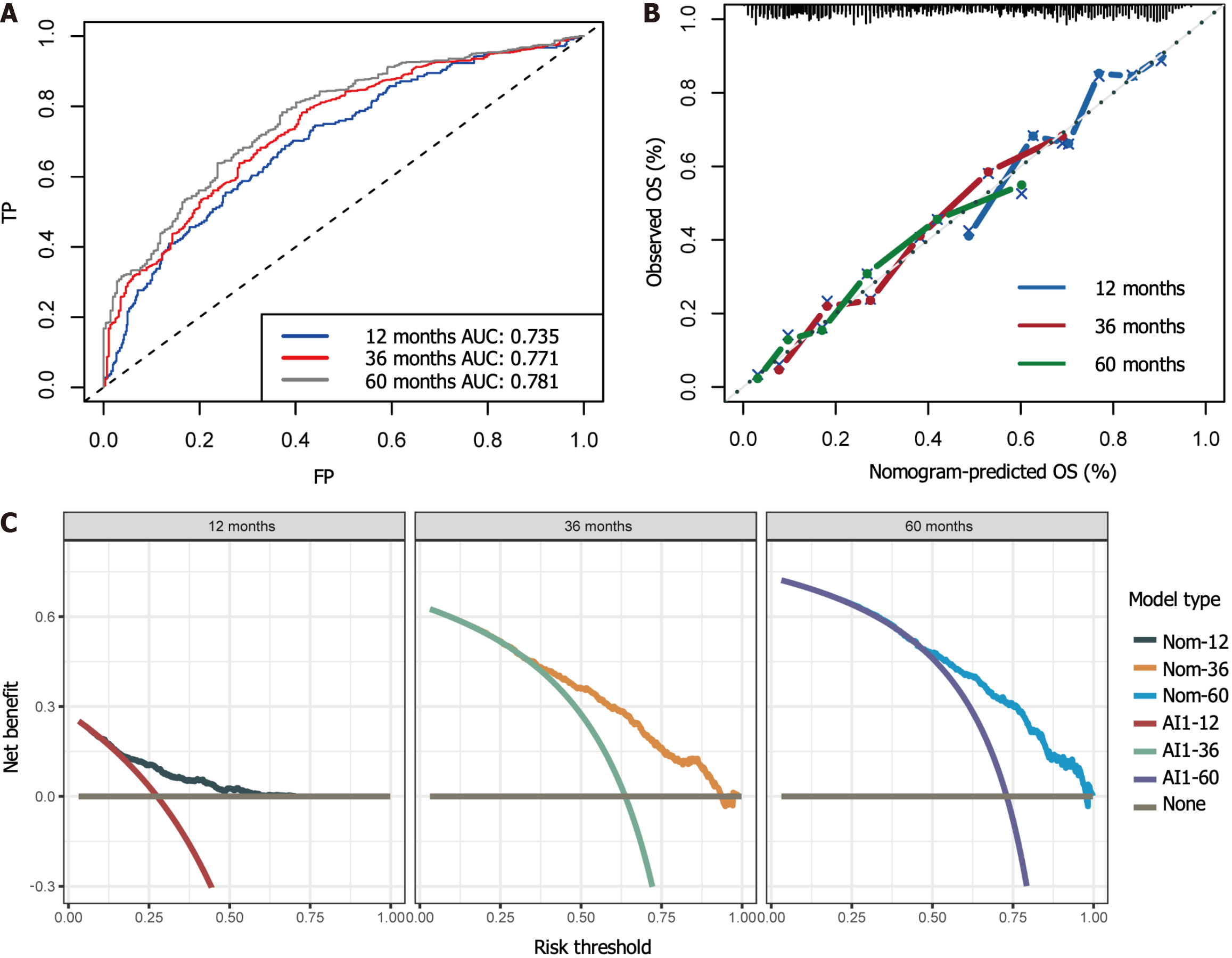
Using the stepwise regression method in Cox regression modeling, several independent prognostic factors were identified, including age, race, tumor size, tumor site, pathological stage, number of lymph nodes examined, postoperative chemotherapy and radiation therapy status (Table 3). This comprehensive analysis highlighted the key factors that significantly impacted prognosis, and these key factors were utilized to construct a prognostic nomogram with the aim of predicting OS probabilities in LAGSRC patients at 1, 3, and 5 years (Figure 10). We subsequently plotted ROC curves for both the training and test sets. For the 1-, 3-, and 5-year intervals within the training set, the AUC values were 0.735, 0.771, and 0.781, respectively (Figure 11A). On the other hand, within the test set, the designated AUC values revealed a sequential pattern of 0.704, 0.759, and 0.767, respectively, for the same time intervals (Figure 12A). The C-index of this OS nomogram was 0.745 in the training set and 0.712 in the test set. According to the calibration curves of this prediction model, the estimated survival rates closely aligned with the actual observed values, indicating a high level of concordance (Figure 11B and 12B). The DCA curves confirmed the clinical value of this nomogram (Figure 11C and 12C).
| Dependent: Survival (time, status) | HR (univariable) | HR (multivariable) | |
| Age | Mean (SD) | 1.02 (1.01-1.02, P < 0.001) | 1.01 (1.01-1.02, P < 0.001) |
| Sex | Female | - | - |
| Male | 1.00 (0.93-1.07, P = 0.999) | - | |
| Race | White | - | - |
| Black | 0.91 (0.82-1.02, P = 0.109) | 0.89 (0.75-1.05, P = 0.165) | |
| Other | 0.77 (0.71-0.85, P < 0.001) | 0.84 (0.73-0.96, P = 0.009) | |
| Tumor size | < 2 | - | - |
| 2-5 | 1.27 (1.07-1.52, P = 0.008) | 1.08 (0.83-1.40, P = 0.558) | |
| > 5 | 1.98 (1.66-2.36, P < 0.001) | 1.41 (1.09-1.82, P = 0.009) | |
| Unknown | 2.24 (1.86-2.70, P < 0.001) | 1.69 (1.29-2.22, P < 0.001) | |
| Grade | G1/G2 | - | - |
| G3/G4 | 1.11 (0.89-1.37, P = 0.348) | - | |
| Unknown | 1.16 (0.90-1.51, P = 0.245) | - | |
| Primary site | Body | - | - |
| Cardia | 1.16 (1.00-1.35, P = 0.051) | 1.23 (0.98-1.53, P = 0.071) | |
| Fundus | 1.07 (0.83-1.38, P = 0.608) | 1.14 (0.78-1.65, P = 0.503) | |
| Gastric antrum | 1.11 (0.97-1.26, P = 0.135) | 1.06 (0.87-1.30, P = 0.551) | |
| Greater curvature | 1.02 (0.84-1.23, P = 0.878) | 1.25 (0.96-1.64, P = 0.103) | |
| Lesser curvature | 0.90 (0.78-1.05, P = 0.190) | 1.00 (0.79-1.27, P = 0.972) | |
| Pylorus | 1.07 (0.88-1.30, P = 0.492) | 1.09 (0.82-1.46, P = 0.545) | |
| Overlapping lesion | 1.53 (1.32-1.78, P < 0.001) | 1.38 (1.10-1.73, P = 0.005) | |
| Unknown | 1.45 (1.24-1.69, P < 0.001) | 1.34 (1.06-1.69, P = 0.014) | |
| Pathologic stage | IB | - | - |
| IIA | 1.37 (1.01-1.85, P = 0.045) | 1.59 (0.98-2.59, P = 0.062) | |
| IIB | 1.94 (1.44-2.61, P < 0.001) | 2.37 (1.47-3.84, P < 0.001) | |
| IIIA | 5.54 (4.14-7.40, P < 0.001) | 3.41 (2.13-5.44, P < 0.001) | |
| IIIB | 2.73 (2.05-3.65, P < 0.001) | 4.86 (3.05-7.76, P < 0.001) | |
| IIIC | 3.83 (2.87-5.10, P < 0.001) | 7.42 (4.62-11.92, P < 0.001) | |
| Lymph node count | Mean (SD) | 0.99 (0.99-1.00, P < 0.001) | 0.98 (0.98-0.98, P < 0.001) |
| Chemotherapy status | No/unknown | - | |
| Yes | 0.64 (0.59-0.69, P < 0.001) | ||
| Radiation status | No/unknown | - | - |
| Yes | 0.63 (0.59-0.68, P < 0.001) | 0.69 (0.62-0.77, P < 0.001) |
To further explore the rationality of this nomogram, time-dependent ROC curve analysis was performed, and the results showed that the nomogram model had greater predictive accuracy and discriminatory ability than did pathological staging across various observation time intervals (Figure 13A and B). In addition, we calculated the total score for each patient based on the nomogram and divided them into a high-risk group (total score > 217.4), an intermediate-risk group (total score 185.9-217.4), and a low-risk group (total score < 185.9) according to the tertile distribution. Subsequently, we conducted Kaplan-Meier survival analysis to explore potential variations in survival outcomes among these three patient cohorts (Figure 13C and D).
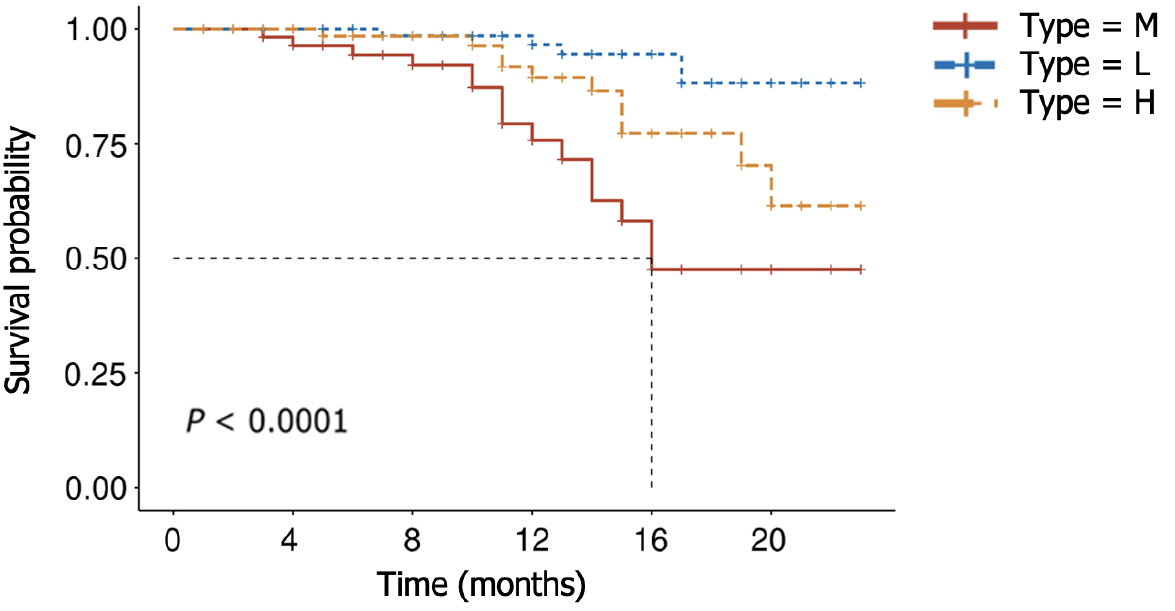
To temporally validate this nomogram model, we collected data from a total of 232 LAGSRC patients who underwent surgical procedures between 2018 and 2019. The findings indicated that the model yielded elevated AUC values of specifically 0.83 and 0.79 for 1-year and 2-year predictive accuracy, respectively. According to the nomogram, each patient's total score was calculated. Patients were then classified into three risk strata, including high (total score > 217.4), middle (total score 185.9-217.4), and low (total score < 185.9), based on their total scores. Subsequently, a survival analysis was performed. The results demonstrated significant differences in survival among these three categories (Figure 14).
We have successfully developed a user-friendly web application, accessible via the URL https://idealeimingzhang.shinyapps.io/DynNomapp/. By inputting pertinent patient characteristics, an immediate estimation of survival probability can be obtained. This online prediction tool shows exceptional convenience for implementation in clinical practice.
According to previous studies, the incidence rate of GSRC ranged from 15.1% to 34.9% among cases of GA, and the incidence of GSRC in the United States gradually increased from 0.3 cases per 100000 people in 1973 to 1.8 cases per 100000 people in 2000[8]. It is worth noting that previous studies have predominantly focused on GSRC incidence up to 2000[7,8], and limited information is available for this period thereafter. The novelty of our study lies in extending the analysis up to 2019, thus providing insights into the more recent trends in GSRC incidence. The findings of this study revealed a sustained downward trend in the incidence of GA in the United States since 1975, with a particularly noteworthy decrease observed between 1988 and 2001 (APC: -4.0, 95%CI: -4.6 to -3.5). Remarkably, an unexpected decline in the occurrence of GSRC was also observed in the United States after 1998 (1998 to 2017 APC -2.1, P < 0.05; 2017 to 2019 APC -19.9, P < 0.05). In this study, we analyzed the incidence of GSRC by sex and age and found several distinct trends. First, the incidence rate of GSRC was greater in males than in females, but the male-female ratio of incidence was close to 1:1. Second, we observed that the decrease in the incidence rate was more pronounced in males than in females. Third, in patients under the age of 60 years, there was no significant decline in the incidence rate over the studied period. However, in patients aged over 60 years, there was a significant decrease in morbidity. This indicates that current preventive measures, early detection, and treatment strategies may be more effective in older individuals. This could be attributed to several factors, including more frequent and comprehensive tumor screening protocols and increased awareness of risk factors among the older population.
We also conducted an analysis of incidence-based mortality rate trends for GSRC patients from 1975 to 2019. The research findings indicate a striking similarity between the trends in mortality rates and incidence rates, with a notable decline observed in the overall incidence-based mortality rate of GSRC in the United States after 2000. When analyzing the incidence-based mortality rates of GSRC stratified by sex and age, several noteworthy trends emerged. First, the mortality rate among males was found to be greater than that among females. Second, both sexes exhibited similar patterns of mortality rate fluctuations over time. Third, the rate of decline in mortality was relatively slower for GSRC patients under the age of 60 years than for those aged over 60 years.
The decreasing trend in the incidence and mortality rates of GSRC can largely be attributed to advancements in precision prevention and GC screening based on novel biomarkers[12]. Precision prevention strategies based on novel biomarkers contribute to the identification of individuals at high risk for GSRC and provide them with more targeted preventive measures. Personalized prevention guidance can be developed by understanding individual genetic factors, environmental exposures, and lifestyle factors, including improving dietary habits and reducing exposure to carcinogens. Furthermore, advancements in genetic diagnostics can directly impact the mortality rate of GC patients. The molecular characteristics of human epidermal growth factor receptor 2 (HER2) expression in GC can guide anti-HER2 targeted therapy[13]. These methods can effectively reduce the overall mortality rate of GC patients. Screening methods, including imaging, electronic endoscopy, and magnetically controlled capsule endoscopy, have also demonstrated their significant role in the diagnosis and management of GC[14,15].
In Western countries, a significant majority of GCs are diagnosed in advanced stages[16]; however, a vast majority of these cases involve patients with locally advanced disease who are still amenable to surgical resection[10]. The paradigm for treating LAGC has evolved from a single surgical strategy to a comprehensive, surgery-centered multidisciplinary approach. However, numerous gaps remain. Currently, definitive, internationally endorsed standard treatment protocols are still lacking in several aspects, including preoperative staging, indications and selection of neoadjuvant and adjuvant therapies, and handling of unresectable LAGC. In this study, from the perspective of different pathological stages, adjuvant chemotherapy following surgery significantly prolonged the CSS and OS of patients with stage II/III GSRC, however, it did not provide protective benefits for patients with stage I GSRC. These findings aligned with the results of several large randomized controlled clinical trials, such as ACTS-GC, CLASSIC and JACCOR GC-07[17-19]. Considering that the SEER database research cohort spans from 2000 to 2015 and incorporates multiple improvements in GC treatment strategies, this study conducted a comprehensive evaluation of the survival advantages derived from postoperative chemotherapy in patients diagnosed with LAGSRC. Linear regression analysis revealed that the HR of CSS after adjuvant chemotherapy significantly decreased with increasing diagnostic year, indicating an enhanced protective effect. In part, this observation signifies that in recent years, the survival rate of patients with GSRC may have improved owing to the standardized utilization of mainstream chemotherapy regimens, such as the combination of fluorouracil and platinum-based drugs.
In this study, we employed backward stepwise selection to systematically eliminate factors with minimal impact, ultimately retaining only the predictive variables that exert a substantial influence on the model. Among these variables, the extent of lymph node dissection held crucial predictive value for survival prognosis. Both the CSCO (Chinese Society of Clinical Oncology) 2020 and the 5th edition JGCA (Japanese Gastric Cancer Association) guidelines for the diagnosis and treatment of GC have the same principles for lymph node dissection in advanced GC; that is, patients with T2 or N+ disease are recommended for standard D2 lymph node dissection[20]. However, the scope of lymph node dissection for LAGC remains controversial in Europe and the United States. Considering the need for extensive training and expertise in D2 lymphadenectomy, along with the execution of standard surgery in well-versed medical centers, the National Comprehensive Cancer Network guidelines in the United States recommend that patients with LAGC undergo D1 or modified D2 lymph node dissection[21]. Despite this study's findings suggesting a survival advantage for patients with LAGSRC undergoing postoperative chemoradiotherapy, as opposed to receiving only adjuvant chemotherapy after surgery, the clinical utility of postoperative adjuvant chemoradiotherapy in GC management continues to elicit substantial debate. The American INT0116 study revealed that the use of fluorouracil combined with radiotherapy after D0/D1 surgery significantly improved patient survival compared to surgery alone[22]. However, the results of the Korean ARTIST and ARTIST 2 studies showed that there were no survival benefits with adjuvant chemoradiation compared to adjuvant chemotherapy after D2 surgery alone[23,24]. This disparity may be related to the different lymph node dissection methods used for radical GC surgery in Eastern and Western countries.
In the realm of clinical practice, it is customary for physicians and researchers to utilize postoperative pathological staging as the benchmark approach to appraise tumor prognoses and identify suitable treatment strategies[25]. Despite the acknowledgment of postoperative pathological staging as a significant predictive variable, it may not be the sole determinant and needs to be considered in conjunction with other significant clinical, pathological, and biological characteristics to comprehensively assess and predict patient survival outcomes. The nomogram survival prediction model represents an invaluable predictive instrument developed by leveraging a wealth of clinical variables and individual patient characteristics. Through the application of mathematical functions, this model adeptly converts the values of each feature into their respective survival prediction probabilities. Thus, it provides physicians and patients with more comprehensive decision support, enabling informed clinical judgments and treatment strategies[26,27]. GSRC poses significant challenges in terms of treatment and prognosis evaluation. Although prognostic models for GC exist, they often neglect the unique characteristics of GSRC, especially in predicting the prognosis of locally advanced patients who require a surgery-centered multidisciplinary approach. Our study sought to bridge this knowledge gap by developing an innovative computational tool for predicting patient survival outcomes. Furthermore, we established an online prediction tool to facilitate the widespread clinical implementation of this model.
There are some issues and limitations in our research that deserve further discussion. First, we focused exclusively on the changing trends in the incidence and mortality rates of GSRC within the United States, and additional research and exploration are necessary to understand the global trends in incidence and mortality rates. Further international comparative studies will enhance our understanding of the epidemiological characteristics of this cancer and its evolving global burden. Second, our study employed a retrospective study design, which inherently presents certain limitations such as information bias. Therefore, future prospective studies will provide more profound and reliable validation of our findings, providing clinical practice with more robust evidence. Third, the SEER database we utilized does not encompass certain specific biochemical parameters, such as CEA and CA19-9. Finally, despite conducting temporal validation of our nomogram, spatial and domain validation are still warranted to evaluate the generalizability of the model.
This study utilized an innovative Joinpoint regression method to investigate and unveil novel trends in the incidence and mortality rates of GSRC in recent years. Our findings shed light on the evolving patterns of GSRC incidence and mortality rates over different time periods, offering valuable insights into the epidemiological characteristics of this disease. Additionally, our study successfully established a prognostic survival model for individuals with LAGSRC. This model enabled us to accurately predict the survival duration of LAGSRC patients and furnish pivotal decision-making resources for health care professionals and individuals affected by this condition.
We extend our profound gratitude to the SEER program for granting approval for registration and providing access to the SEER database.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Papazafiropoulou A, Greece S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64447] [Article Influence: 16111.8] [Reference Citation Analysis (176)] |
| 2. | Zhao W, Jia Y, Sun G, Yang H, Liu L, Qu X, Ding J, Yu H, Xu B, Zhao S, Xing L, Chai J. Single-cell analysis of gastric signet ring cell carcinoma reveals cytological and immune microenvironment features. Nat Commun. 2023;14:2985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 3. | Mariette C, Carneiro F, Grabsch HI, van der Post RS, Allum W, de Manzoni G; European Chapter of International Gastric Cancer Association. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 4. | Bamboat ZM, Tang LH, Vinuela E, Kuk D, Gonen M, Shah MA, Brennan MF, Coit DG, Strong VE. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014;21:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Voron T, Messager M, Duhamel A, Lefevre J, Mabrut JY, Goere D, Meunier B, Brigand C, Hamy A, Glehen O, Mariette C, Paye F. Is signet-ring cell carcinoma a specific entity among gastric cancers? Gastric Cancer. 2016;19:1027-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1465] [Article Influence: 162.8] [Reference Citation Analysis (0)] |
| 7. | Antonioli DA, Goldman H. Changes in the location and type of gastric adenocarcinoma. Cancer. 1982;50:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 301] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4396] [Article Influence: 549.5] [Reference Citation Analysis (4)] |
| 10. | Rausei S, Boni L, Rovera F, Dionigi G. Locally advanced gastric cancer: a new definition to standardise. J Clin Pathol. 2013;66:164-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 108] [Reference Citation Analysis (0)] |
| 12. | Shinozuka T, Kanda M, Kodera Y. Site-specific protein biomarkers in gastric cancer: a comprehensive review of novel biomarkers and clinical applications. Expert Rev Mol Diagn. 2023;23:701-712. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, Yuan XL, Liu TS, Li GX, Wu Q, Xu HM, Ji JF, Li YF, Wang X, Yu S, Liu H, Guan WL, Xu RH. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). 2019;39:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 311] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 14. | Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K, Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 405] [Article Influence: 67.5] [Reference Citation Analysis (1)] |
| 15. | Fan X, Qin X, Zhang Y, Li Z, Zhou T, Zhang J, You W, Li W, Pan K. Screening for gastric cancer in China: Advances, challenges and visions. Chin J Cancer Res. 2021;33:168-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 16. | Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the "different disease" hypothesis. Cancer. 2000;88:921-932. [PubMed] |
| 17. | Ji J, Liang H, Zhan Y, Liu Y, He Y, Ye Y, Sun Y, Huang C, Yan M, Shi Y, Wu A. [Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): Chinese subgroup analysis]. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:133-138. [PubMed] |
| 18. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ; CLASSIC trial investigators. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 775] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 19. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K; ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1941] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 20. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1332] [Article Influence: 333.0] [Reference Citation Analysis (2)] |
| 21. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 954] [Article Influence: 318.0] [Reference Citation Analysis (0)] |
| 22. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2435] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 23. | Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, Kang JH, Oh SY, Hwang IG, Ji JH, Shin DB, Yu JI, Kim KM, An JY, Choi MG, Lee JH, Kim S, Hong JY, Park JO, Park YS, Lim HY, Bae JM, Kang WK; ARTIST 2 investigators. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial(☆). Ann Oncol. 2021;32:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 24. | Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, Sohn I, Jung SH, Choi MG, Lee JH, Bae JM, Kim S, Kim ST, Park JO, Park YS, Lim HY, Kang WK. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol. 2015;33:3130-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 300] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 25. | Asplund J, Gottlieb-Vedi E, Leijonmarck W, Mattsson F, Lagergren J. Prognosis after surgery for gastric adenocarcinoma in the Swedish Gastric Cancer Surgery Study (SWEGASS). Acta Oncol. 2021;60:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Brateanu A, Yu C, Kattan MW, Olender J, Nielsen C. A nomogram to predict the probability of passing the American Board of Internal Medicine examination. Med Educ Online. 2012;17:18810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Cao J, Yuan P, Wang L, Wang Y, Ma H, Yuan X, Lv W, Hu J. Clinical Nomogram for Predicting Survival of Esophageal Cancer Patients after Esophagectomy. Sci Rep. 2016;6:26684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |