Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2487
Revised: April 4, 2024
Accepted: April 11, 2024
Published online: June 15, 2024
Processing time: 135 Days and 14.5 Hours
The influence of Helicobacter-pylori (H. pylori) infection and the characteristics of gastric cancer (GC) on tumor-infiltrating lymphocyte (TIL) levels has not been extensively studied. Analysis of infiltrating-immune-cell subtypes as well as survival is necessary to obtain comprehensive information.
To determine the rates of deficient mismatch-repair (dMMR), HER2-status and H. pylori infection and their association with TIL levels in GC.
Samples from 503 resected GC tumors were included and TIL levels were evaluated following the international-TILs-working-group recommendations with assessment of the intratumoral (IT), stromal (ST) and invasive-border (IB) compartments. The density of CD3, CD8 and CD163 immune cells, and dMMR and HER2-status were determined by immunohistochemistry (IHC). H. pylori infection was evaluated by routine histology and quantitative PCR (qPCR) in a subset of samples.
dMMR was found in 34.4%, HER2+ in 5% and H. pylori-positive in 55.7% of samples. High IT-TIL was associated with grade-3 (P = 0.038), while ST-TIL with grade-1 (P < 0.001), intestinal-histology (P < 0.001) and no-recurrence (P = 0.003). dMMR was associated with high TIL levels in the ST (P = 0.019) and IB (P = 0.01) compartments, and ST-CD3 (P = 0.049) and ST-CD8 (P = 0.05) densities. HER2- was associated with high IT-CD8 (P = 0.009). H. pylori-negative was associated with high IT-TIL levels (P = 0.009) when assessed by routine-histology, and with high TIL levels in the 3 compartments (P = 0.002-0.047) and CD8 density in the IT and ST compartments (P = 0.001) when assessed by qPCR. A longer overall survival was associated with low IT-CD163 (P = 0.003) and CD8/CD3 (P = 0.001 in IT and P = 0.002 in ST) and high IT-CD3 (P = 0.021), ST-CD3 (P = 0.003) and CD3/CD163 (P = 0.002).
TIL levels were related to dMMR and H. pylori-negativity. Low CD8/CD3 and high CD163/CD3 were associated with lower recurrence and longer survival.
Core Tip: Absence of Helicobacter pylori was associated with high tumor-infiltrating lymphocyte (TIL) levels and CD8 density. Deficient mismatch-repair was associated with high TIL levels, and CD3 and CD8 density. Longer overall survival was associated with a low CD8/CD3 ratio, and high CD3 and CD3/CD163 ratio.
- Citation: Castaneda CA, Castillo M, Bernabe LA, Sanchez J, Fassan M, Tello K, Wistuba II, Chavez Passiuri I, Ruiz E, Sanchez J, Barreda F, Valdivia D, Bazan Y, Abad-Licham M, Mengoa C, Fuentes H, Montenegro P, Poquioma E, Alatrista R, Flores CJ, Taxa L. Association between Helicobacter pylori infection, mismatch repair, HER2 and tumor-infiltrating lymphocytes in gastric cancer. World J Gastrointest Oncol 2024; 16(6): 2487-2503
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2487.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2487
Gastric cancer (GC) is one of the most common cancers throughout the world and the second most frequent in Peruvian males, and carries a poor prognosis[1-3]. The pathological features of GC can define tumor behavior and prognosis[4]. Helicobacter pylori (H. pylori) infection, which is highly prevalent in Peru, is an accepted trigger of GC and has recently been suggested to predict a lower effect of immunotherapy with checkpoint immune inhibitors[5].
Microsatellite instability (MSI) defines one of the four molecular GC subtypes[6]. It is usually detected by deficient mismatch-repair (dMMR) and has been associated with high levels of neoantigens, and along with HER2 positive status are biomarkers of response to checkpoint immune inhibitors and trastuzumab treatment, respectively[7-11]. A recent study found that the addition of checkpoint inhibitors to antiHER2 therapy in HER2 positive cases increases clinical response[12].
High levels of tumor-infiltrating lymphocytes (TILs) have been associated with longer survival and greater response to checkpoint inhibitors in different malignancies[13,14]. Information on the type, density and location of TIL subpopulations has been associated with tumor features, such as Epstein-Barr-Virus infection[4] and survival in gastrointestinal malignancies[15,16], and predicts response to checkpoint inhibitors in advanced GC[11,17-19]. Despite the relevance of H. pylori infection, dMMR and HER2 status in GC, few studies have evaluated their association with TIL levels in resected non-metastatic tumors. The studies available differ in the methodologies used to evaluate TIL and very few have included South American populations.
In the present study, TIL levels were determined following the International Immuno-Oncology Biomarkers Working Group: Part 2 recommendations[20] as well as the density of CD3+ T lymphocytes, CD8+ cytotoxic T lymphocytes and CD163+ M2 macrophages. In addition, the relationship of TIL levels with the clinicopathological features, including H. pylori infection, dMMR, HER2 status and survival in resected GC, was evaluated.
We included information from 503 GC patients who underwent surgery at the Instituto Nacional de Enfermedades Neoplasicas in Lima, Peru from January 2008 to December 2018 and in whom pathology material was available. Clinical-pathological features were obtained from medical histories and pathology reports of the patients, and hematoxylin and eosin (HE)-stained slides were prospectively reviewed when no specific information was found[21,22].
This single-center retrospective cohort study was approved by the Research and Ethics Committee (Protocol Number 050-2015-CIE/INEN), and the patients provided signed informed consent.
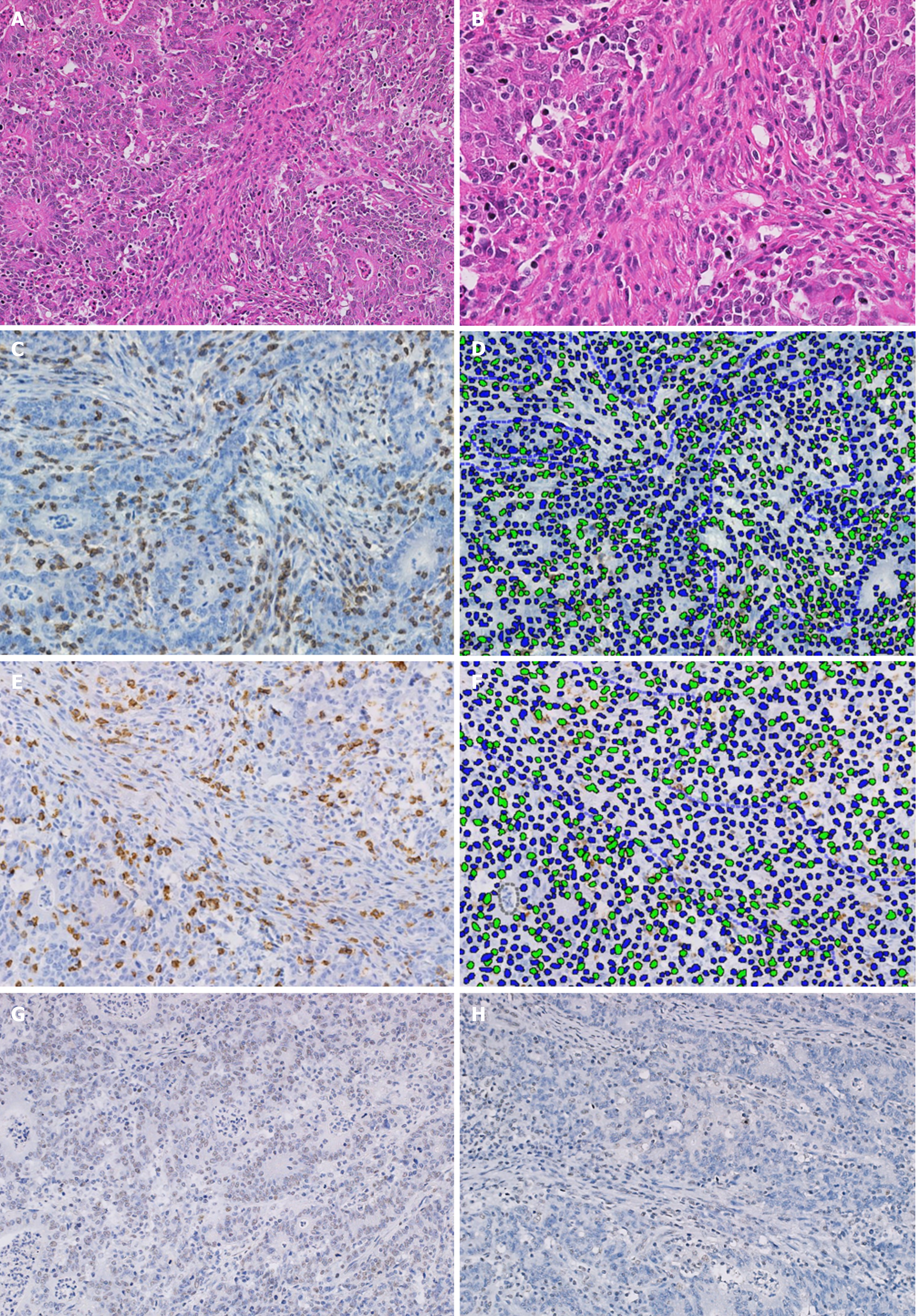
Several original sections from each primary tumor were re-examined and the most representative tissue block, with a 5 µm thickness and stained in HE was selected. Original and new sections were examined by experienced histopathologists (Sanchez J & Taxa L) for review of the standard pathological features, including the presence of H. pylori, and contrasted with original reports. The level of TILs was estimated avoiding ulcerated or necrotic areas and classified by spatial location [intratumoral (IT), stromal (ST) and invasive-border (IB) compartments (Figures 1 and 2)]. TIL levels above the median (calculated for every compartment) were classified as high[20].
Core tissue biopsies (6 mm in diameter) were taken from tumoral areas with a high density of TIL in every individual paraffin-embedded tumor and 10-12 cores were re-arranged in a new recipient paraffin block (tissue array block) using the Quick-Ray Manual Tissue Microarrayer (Unitma Co., Ltd., Seoul, Korea). Sections (4 mm) were taken from each tissue array block, deparaffinized, and dehydrated[23].
Staining of GC tissue sections was performed using the EnVision FLEX Kit (K8000, Dako Glostrup, Denmark) in paraffin-embedded sections. Briefly, each paraffin section was deparaffinized, followed by antigen retrieval with Epitope Retrieval Solution, Tris/EDTA buffer pH 9 (DM830, Dako, Glostrup, Denmark) in a preheated water bath (95°C, 20 min). Endogenous peroxidase was blocked, and antihuman primary antibodies were applied for 25 to 45 min at 25ºC. Immune cells were evaluated using anti-mouse CD3 antibody (Is503, Dako), anti-human CD8 (IS623, Dako) and CD163 antibodies (clone EP324, Master Diagnostica, Granada, Spain).
The MMR proteins evaluated were mouse anti-human MutL protein homolog 1 (MLH1, ES01 Dako), mouse anti-human MutS protein homolog 2 (MSH2, FE11 Dako), rabbit anti-human MutS protein homolog 6 (MSH6, EP49 Dako) and rabbit anti-human postmeiotic segregation increased 2 (PMS2, EP51 Dako). HER2 was evaluated with the polyclonal rabbit anti-human c-erbB-2 oncoprotein (AO485 Dako).
Thereafter, the sections were incubated with secondary Abs as per the EnVision FLEX Kit, and counter-stained with hematoxylin. Positive staining controls were performed with paraffin sections of normal human tonsil.
Assessment of HER-2 status was performed following standard scoring criteria specific for GC[7,8,24], while dMMR was determined when the expression of at least one of the 4 MMR proteins evaluated was lost (Figure 3)[11,17-19].
H. pylori gene expression was determined by the constitutive hspA and UreA genes in DNA obtained from frozen gastric samples by quantitative PCR (qPCR) in the LightCycler 96 Instrument Thermal Cycler (Roche, Mannheim, Germany).
Values were considered positive when ≥ 10 copies/μL were detected. The virulence of the cagA, vacAs, and vacAm genes as well as vacAs1 and vacAm1 alleles was tested by an experienced cancer biologist (NS) in H. pylori-positive patients, as described in a previous study by our group[3].
Immunostained slides were scanned with a digital virtual microscope BX63 Olympus (Tokyo, Japan), and the region with the highest immune cell density was selected. Five high power fields (HPF; or three when there was not enough stained tissue) within the IT and ST compartments were captured at 20 × magnification and analyzed using Visiopharm Tissuemorph Digital Pathology image analysis software (Visiopharm, Hoersholm, Denmark) under the supervision of a pathologist (Sanchez J). The density of the immune cells was calculated by the mean of positive cells in the captured HPF[25] (Figure 3). The optimal cutoff values for defining a higher density of immune cells (CD3, CD8 and CD163) were calculated using the maximally selected rank statistics according to Lausen [in relation to overall survival (OS)][26].
The non-paired Student’s t-test was used to examine differences between groups. Correlations between values were evaluated using the non-parametric Spearman rank correlation. The intraclass correlation test was used to compare TIL levels and the density of immune cells in different compartments. OS was calculated from the date of surgery until death or until the date patients were last known to be alive (obtained from National Registry of Identification and Marital Status). Disease-free survival (DFS) was calculated from the date of surgery until relapse (patient records) or last known alive status. The last review of state of life was carried out in May 2022. In the univariate analysis, the survival curves were compared according to clinical characteristics using the log-rank or Breslow test, and in multivariate analysis using the Cox regression model with a stepwise selection method. A P value < 0.05 was considered significant. Analyses were performed using the SPSS statistical software (IBM SPSS Statistical 19) and R program. The statistical methods of this study were reviewed by Flores CJ from Oncosalud-AUNA.
The clinicopathological features of the patients included are described in Table 1. Most cases underwent subtotal gastrectomy (62.4%). Adjuvant chemotherapy was administered in 45.1% and radiation in 13.9%.
| Feature | Total (n = 503) | % | Median | 5 yr DFS | P value | Median | 5 yr OS | P value |
| Age, yr (19-95 yr) | ||||||||
| < 60 | 231 | 45.9 | 4.6 | 49.1 | 0.043 | 7.0 | 52.5 | 0.009 |
| ≥ 60 | 272 | 54.1 | 3.0 | 39.6 | 3.7 | 41.6 | ||
| Sex | ||||||||
| Female | 252 | 50.1 | 3.3 | 43.7 | 0.582 | 3.8 | 45.3 | 0.319 |
| Male | 251 | 49.9 | 4.0 | 44.6 | 4.5 | 48.2 | ||
| Bormann | ||||||||
| I-II | 90 | 18.1 | NA | 65.4 | < 0.001 | NA | 66.0 | 0.002 |
| III | 311 | 62.7 | 2.9 | 40.6 | 4.0 | 44.4 | ||
| IV-V | 95 | 19.2 | 2.6 | 36.8 | 2.8 | 37.6 | ||
| Lauren (n = 496) | ||||||||
| Intestinal | 222 | 44.8 | 4.3 | 39.9 | 0.716 | 4.8 | 49.3 | 0.598 |
| Diffuse | 181 | 36.5 | 3.0 | 46.2 | 4.1 | 43.9 | ||
| Mixed | 93 | 18.8 | 2.3 | 42.9 | 3.2 | 43.2 | ||
| Grade | ||||||||
| 1 | 46 | 9.1 | NA | 62.2 | 0.025 | NA | 67.8 | 0.044 |
| 2 | 160 | 31.8 | 4.4 | 46.9 | 4.5 | 49.0 | ||
| 3 | 297 | 59.1 | 2.5 | 39.7 | 3.6 | 42.3 | ||
| ILV | ||||||||
| No | 149 | 29.6 | 6.9 | 62.4 | < 0.001 | 8.1 | 66.4 | < 0.001 |
| Yes | 354 | 70.4 | 2.4 | 36.5 | 3.0 | 38.5 | ||
| Antrum | ||||||||
| Yes | 323 | 64.2 | 2.7 | 47.9 | 0.064 | 3.5 | 51.0 | 0.104 |
| No | 180 | 35.8 | 4.5 | 38.8 | 5.1 | 40.8 | ||
| Clinical stage | ||||||||
| I | 64 | 12.7 | NA | 80.7 | < 0.001 | NA | 83.0 | < 0.001 |
| II | 138 | 27.4 | 7.7 | 59.6 | 7.7 | 59.5 | ||
| III | 301 | 59.8 | 1.9 | 29.3 | 2.3 | 33.2 | ||
| Node involvement | ||||||||
| No | 139 | 27.6 | 10.5 | 67.0 | < 0.001 | NA | 68.2 | < 0.001 |
| Yes | 364 | 72.4 | 2.3 | 35.4 | 3.0 | 38.4 | ||
| Recurrence | ||||||||
| No | 322 | 64.0 | - | - | - | NA | 68.5 | < 0.001 |
| Yes | 181 | 36.0 | - | - | 1.7 | 12.3 | ||
| H. pylori HE (n = 415) | ||||||||
| Absent | 184 | 44.3 | 3.8 | 42.9 | 0.252 | 4.5 | 46.0 | 0.637 |
| Present | 231 | 55.7 | 7.3 | 48.3 | 5.3 | 51.2 | ||
| H. pylori qPCR (n = 234) | ||||||||
| Negative | 85 | 36.3 | 2.5 | 36.8 | 0.229 | 3.1 | 39.1 | 0.219 |
| Positive | 149 | 63.7 | 3.8 | 38.3 | 4.0 | 39.7 | ||
| dMMR (n = 479) | ||||||||
| No | 269 | 65.6 | 4.3 | 45.8 | 0.396 | 4.7 | 47.9 | 0.158 |
| Yes | 141 | 34.4 | 3.1 | 42.6 | - | 3.6 | 44.7 | - |
| HER-2 status (n = 468) | ||||||||
| Negative | 434 | 95.0 | 3.5 | 43.4 | 0.868 | 4.3 | 46.2 | 0.892 |
| Positive | 23 | 5 | 3.1 | 39.8 | 3.3 | 39.3 | ||
| IT TIL (n = 462) | ||||||||
| < 10 | 186 | 40.3 | 2.7 | 41.8 | 0.763 | 3.7 | 43.6 | 0.395 |
| ≥ 10 | 276 | 59.7 | 3.7 | 44.0 | 4.5 | 47.1 | ||
| ST TIL (n = 461) | ||||||||
| < 30 | 258 | 55.9 | 2.6 | 41.5 | 0.458 | 3.8 | 45.0 | 0.853 |
| ≥ 30 | 203 | 44.0 | 3.8 | 44.8 | 4.4 | 46.3 | ||
| IB TIL (n = 332) | ||||||||
| < 70 | 178 | 53.6 | 3.3 | 46.0 | 0.991 | 4.4 | 47.8 | 0.660 |
| ≥ 70 | 154 | 46.4 | 4.4 | 46.4 | 4.9 | 49.4 | ||
| IT CD3/HPF (n = 453) | ||||||||
| < 58 | 126 | 27.8 | 2.4 | 34.3 | 0.028 | 2.9 | 36.5 | 0.021 |
| ≥ 58 | 327 | 72.2 | 4.4 | 47.0 | 5.4 | 50.5 | ||
| IT CD8/ HPF (n = 443) | ||||||||
| < 70 | 231 | 52.1 | 4.4 | 47.4 | 0.060 | 4.8 | 49.9 | 0.142 |
| ≥ 70 | 212 | 47.9 | 2.6 | 40.4 | 4.0 | 43.8 | ||
| IT CD163/HPF (n = 205) | ||||||||
| < 240 | 183 | 89.3 | 2.8 | 42.7 | 0.002 | 3.8 | 45.3 | 0.003 |
| ≥ 240 | 22 | 10.7 | 1.4 | 0.0 | 1.5 | 0.0 | ||
| IT CD8/CD3 ratio (n = 432) | ||||||||
| < 0.63 | 210 | 48.6 | 4.5 | 49.1 | <0.001 | 6.0 | 52.9 | 0.001 |
| ≥ 0.63 | 222 | 51.4 | 2.3 | 36.0 | 2.8 | 38.5 | ||
| IT CD3/CD163 (n = 179) | ||||||||
| < 8 | 137 | 76.5 | 22 | 33.9 | 0.004 | 2.6 | 36.6 | 0.002 |
| ≥ 8 | 42 | 23.5 | NA | 59.5 | NA | 64.3 | ||
| ST CD3/HPF (n = 363) | ||||||||
| < 95 | 180 | 49.6 | 2.6 | 36.6 | 0.014 | 2.9 | 38.7 | 0.003 |
| ≥ 95 | 183 | 50.4 | 5.2 | 50.7 | 7.0 | 54.6 | ||
| ST CD8/HPF (n = 341) | ||||||||
| < 68 | 216 | 63.3 | 3.8 | 43.5 | 0.578 | 4.0 | 45.0 | 0.382 |
| ≥ 68 | 125 | 36.7 | 3.6 | 43.0 | 4.5 | 47.5 | ||
| ST CD8/CD3 ratio (n = 328) | ||||||||
| < 1.2 | 277 | 84.5 | 4.3 | 45.9 | 0.005 | 4.6 | 49.1 | 0.002 |
| ≥ 1.2 | 51 | 15.5 | 1.9 | 26.1 | 2.0 | 26.6 |
H. pylori (+) was found in 231 of 415 (55.7%) cases when evaluated in HE staining and in 149 of 234 (63.7%) cases when evaluated with qPCR. The cagA gene was detected in 87.2% (130/149) of H. pylori (+) patients identified by qPCR, while the vacAs gene was detected in 79.1% (117/148) and vacAm in 75.2% (112/149) of H. pylori (+) patients. VacAs1 and vacAm1 alleles were detected in 47.9% (56/117) and 70.5% (79/112) of H. pylori (+) patients, respectively, and concurrent presence was found in 40.2% (39/97).
The presence of dMMR was found in 141 (34.4%; negative in 269 cases and not conclusive staining in 69), HER2-positive status in 23 (5%; negative in 434 and equivocal in 11 cases) and both features were found in 8 (2.2%) cases (Table 1). dMMR was associated with grade 1 (P = 0.028). HER2 overexpression was associated with an intestinal subtype (P < 0.001), grade 3 (P < 0.001) and low stage (P = 0.009).
Regarding survival analysis, the median follow-up was 6.4 years (95%CI 5.9-6.8 years), and recurrence and death were found in 181 cases and 270 cases, respectively. The clinicopathological features related to survival are described in Table 1.
The median TIL level in the IT compartment was 10% (1%-80%) (n = 462), being 30% in the ST (1%-95%; n = 461) and 70% (1%-95%; n = 332) in the IB compartments, with a significant correlation among the three compartments [IT vs ST, intraclass correlation coefficient (ICC) = 0.613; IT vs IB, ICC = 0.340; IB vs ST, ICC = 0.726]. A high IT TIL level was associated with grade 3 (P = 0.038), lymphovascular invasion+ (P = 0.028) and stage 2 (P = 0.016). High ST TIL levels were associated with age ≥ 60 years (P < 0.001), non-antrum location (P = 0.049), intestinal histology (P < 0.001), grade 1 (P < 0.001), stage 2 (P = 0.001) and no recurrence (P = 0.003). Lastly, a high IB TIL level was associated with intestinal histology (P = 0.002), lymph node negative (P = 0.029) and earlier stages (P = 0.001; Table 2).
| Features | IT TILs | P value | ST TILs | P value | IB TILs | P value |
| Median | > 10% | > 30% | > 70% | |||
| Age | 0.762 | < 0.001 | 0.279 | |||
| < 60 | 59.1 | 33.3 | 43 | |||
| ≥ 60 | 60.4 | 54.8 | 48.9 | |||
| Sex | 0.382 | 0.074 | 0.678 | |||
| Female | 57.7 | 39.8 | 45.1 | |||
| Male | 61.7 | 48.1 | 47.4 | |||
| Gastric region location | 0.450 | 0.049 | 0.160 | |||
| No antrum | 62 | 50 | 51.1 | |||
| Antrum | 58.4 | 40.5 | 43.3 | |||
| Bormann | 0.221 | 0.379 | 0.094 | |||
| 1 | 54.5 | 50 | 44.4 | |||
| 2 | 47.3 | 36.4 | 38.8 | |||
| 3 | 63.3 | 47.6 | 52.2 | |||
| 4 | 61 | 38.3 | 33.3 | |||
| 5 | 50 | 50 | 28.6 | |||
| Lauren histology | 0.891 | < 0.001 | 0.002 | |||
| Intestinal | 58.7 | 58.74 | 53.1 | |||
| Diffuse | 58.9 | 20.4 | 28.6 | |||
| Mixed | 61.6 | 50 | 42.6 | |||
| Grade | 0.038 | < 0.001 | 0.716 | |||
| 1 | 50 | 58.3 | 52.8 | |||
| 2 | 53.5 | 55.5 | 45.6 | |||
| 3 | 64.6 | 35.6 | 45.8 | |||
| Lymphovascular invasion | 0.028 | 0.520 | 0.968 | |||
| No | 51.9 | 41.7 | 46.2 | |||
| Yes | 62.9 | 44.9 | 46.5 | |||
| Lymph node involvement | 0.403 | 0.714 | 0.029 | |||
| Yes | 60.9 | 43.5 | 42.5 | |||
| No | 56.6 | 45.5 | 55.6 | |||
| Pathology stage | 0.016 | 0.001 | 0.001 | |||
| I | 42 | 28 | 41.5 | |||
| II | 65.4 | 55.8 | 60.4 | |||
| III | 60.3 | 41.5 | 38.9 | |||
| Recurrence | 0.230 | 0.003 | 0.369 | |||
| No | 61.9 | 49.5 | 48 | |||
| Yes | 56.3 | 35.2 | 42.7 | |||
| H. pylori HE | 0.009 | 0.445 | 0.411 | |||
| Absent | 65.9 | 47.6 | 47.5 | |||
| Present | 52.4 | 43.6 | 42.5 | |||
| H. pylori qPCR | 0.002 | 0.047 | 0.010 | |||
| Absent | 67.1 | 48.6 | 50.9 | |||
| Present | 43.7 | 34.1 | 29.9 | |||
| CagA/H. pylori+ | 0.761 | 0.275 | 0.002 | |||
| CagA- | 40 | 46.7 | 64.3 | |||
| CagA+ | 44.1 | 32.4 | 24.1 | |||
| VacAs/H. pylori+ | 0.332 | 0.719 | 0.045 | |||
| VacAs- | 51.9 | 37 | 47.6 | |||
| VacAs+ | 41.4 | 33.3 | 25 | |||
| VacAs1/H. pylori+ | 0.530 | 0.887 | 0.894 | |||
| VacAs1- | 38.5 | 32.7 | 24.4 | |||
| VacAs1+ | 44.7 | 34 | 25.7 | |||
| VacAm/H. pylori+ | 0.824 | 0.535 | 0.265 | |||
| VacAm- | 41.9 | 38.7 | 38.5 | |||
| VacAm+ | 44.2 | 32.6 | 26.8 | |||
| VacAm1/H. pylori+ | 0.154 | 0.093 | 0.508 | |||
| VacAm1- | 55.2 | 44.8 | 21.7 | |||
| VacAm1+ | 39.4 | 27.3 | 29.2 | |||
| VacAs1+m1+/H. pylori+ | 0.937 | 0.953 | 0.527 | |||
| No VacAs1+m1+ | 44.2 | 32.7 | 24.4 | |||
| VacAs1+m1+ | 43.3 | 66.7 | 31.8 | |||
| dMMR | 0.875 | 0.019 | 0.010 | |||
| No | 58.7 | 38.9 | 38.6 | |||
| Yes | 59.6 | 51.5 | 54.2 | |||
| HER2 positive | 0.498 | 0.420 | 0.55 | |||
| No | 60.1 | 44.1 | 47.2 | |||
| Yes | 52.4 | 47.6 | 35.3 |
There was poor correlation between IT TIL levels and the densities of CD3+ (ICC = 0.106), CD8+ (ICC = 0.151) and CD163+ (ICC = 0.065) cells.
The mean densities of IT CD3+ (n = 453) and CD8+ (n = 443) cells/HPF were 108.6 (0.2-925.2) and 66.6 (1-777.4), respectively, while the mean density of IT CD163+ was 43 (0-519)/HPF (n = 205), and those of ST CD3+ (n = 363) and CD8+ (n = 341) cells/HPF were 95.2 (0.2-858.6) and 45.8 (0.5-581.8), respectively (Table 1). There was almost perfect agreement between the densities of CD3+ and CD8+ cells in the IT (ICC = 0.692) as well as in the ST compartments (ICC = 0.606). There was moderate agreement between the density of IT CD163+ and both CD3+ (ICC = 0.327) and CD8+ cells (ICC = 0.259) in the IT compartment.
A high density of IT CD3+ cells were associated with diffuse histology (P = 0.03), grade 3 (P < 0.001), absence of recurrence (P = 0.02) and longer DFS and OS (P = 0.028 and 0.021, respectively). A high density of IT CD8+ cells were associated with grade 3 (P < 0.001; Table 3) and a high density of IT CD163+ cells were associated with a non-antrum location (P = 0.031), grade 3 (P = 0.02) and shorter DFS and OS (P = 0.002 and P = 0.003, respectively). Cases with a high IT CD3/CD163 ratio presented a longer DFS (P = 0.004) and OS (P = 0.002; Table 1 and Figure 3).
| Features | IT CD3 | P value | ST CD3 | P value | IT CD8 | P value | ST CD8 | P value | IT CD163 | P value |
| Median | > 58 | > 95 | > 70 | > 68 | > 240 | |||||
| Age | 0.714 | 0.275 | 0.026 | 0.224 | 0.162 | |||||
| < 60 | 73 | 47 | 42.1 | 32.9 | 7.4 | |||||
| ≥ 60 | 71.5 | 52.8 | 52.7 | 39.3 | 13.5 | |||||
| Sex | 0.039 | 0.557 | 0.814 | 0.699 | 0.354 | |||||
| Female | 76.5 | 52 | 48.4 | 37.7 | 8.7 | |||||
| Male | 67.8 | 49 | 47.3 | 35.7 | 12.7 | |||||
| Gastric region location | 0.503 | 0.585 | 0.795 | 0.901 | 0.031 | |||||
| No antrum | 74.1 | 52.4 | 48.7 | 36.2 | 16.7 | |||||
| Antrum | 71.1 | 49.4 | 47.4 | 36.9 | 7.1 | |||||
| Bormann | 0.648 | 0.441 | 0.338 | 0.006 | 0.462 | |||||
| I-II | 70.4 | 47.4 | 41.8 | 22.6 | 5.3 | |||||
| III | 73.6 | 55.6 | 50.7 | 43 | 11.7 | |||||
| IV-V | 68.9 | 52.9 | 46.1 | 30 | 13 | |||||
| Lauren histology | 0.03 | 0.003 | 0.308 | 0.003 | 0.038 | |||||
| Intestinal | 66.2 | 57.4 | 43.7 | 40.3 | 8.2 | |||||
| Diffuse | 78 | 36.6 | 50 | 22.2 | 17.8 | |||||
| Mixed | 75.9 | 50.8 | 52.7 | 46.3 | 2.9 | |||||
| Grade | <0.001 | 0.311 | <0.001 | 0.083 | 0.02 | |||||
| 1 | 59 | 55.3 | 26.8 | 20.5 | 3.8 | |||||
| 2 | 62.9 | 54.2 | 40.7 | 38.2 | 4.3 | |||||
| 3 | 79.5 | 46.4 | 55.3 | 39.2 | 16.4 | |||||
| Lymphovascular invasion | 0.602 | 0.241 | 0.092 | 0.117 | 0.865 | |||||
| No | 73.9 | 55.2 | 41.8 | 30.4 | 11.3 | |||||
| Yes | 71.5 | 48.4 | 50.5 | 39.3 | 10.5 | |||||
| Lymph node involvement | 0.723 | 0.983 | 0.487 | 0.141 | 0.252 | |||||
| Yes | 72.6 | 50.4 | 48.9 | 39.1 | 12.1 | |||||
| No | 71 | 50.5 | 45.2 | 30.6 | 6.3 | |||||
| Pathology stage | 0.942 | 0.101 | 0.674 | 0.058 | 0.197 | |||||
| I | 70.9 | 50 | 42.9 | 20 | 9.5 | |||||
| II | 73.2 | 59 | 47.2 | 41 | 4.1 | |||||
| III | 72 | 46.3 | 49.2 | 37.8 | 13.3 | |||||
| Recurrence | 0.02 | 0.079 | 0.448 | 0.475 | 0.389 | |||||
| No | 76 | 53.9 | 46.6 | 35.2 | 9.3 | |||||
| Yes | 65.9 | 44.4 | 50 | 39.1 | 13.2 | |||||
| H. pylori HE | 0.547 | 0.817 | 0.356 | 0.97 | 0.637 | |||||
| Absent | 71.1 | 51.4 | 49.1 | 36 | 13.4 | |||||
| Present | 73.9 | 52.8 | 44.2 | 36.2 | 11.1 | |||||
| H. pylori qPCR | 0.143 | 0.335 | 0.001 | 0.001 | 0.105 | |||||
| Absent | 83.1 | 55.9 | 62.7 | 49.1 | 26.1 | |||||
| Present | 74 | 48.1 | 37 | 22.8 | 14.1 | |||||
| CagA/H. pylori+ | 0.254 | 0.603 | 0.023 | 0.54 | 0.729 | |||||
| CagA- | 82.9 | 42.9 | 57.5 | 21.2 | 14.3 | |||||
| CagA+ | 74.2 | 48 | 37.2 | 26.6 | 17.2 | |||||
| VacAs/H. pylori+ | 0.992 | 0.843 | 0.071 | 0.855 | 0.823 | |||||
| VacAs- | 76.8 | 45.8 | 51.7 | 24.4 | 15.2 | |||||
| VacAs+ | 76.9 | 47.6 | 37.3 | 25.9 | 16.9 | |||||
| VacAs1/H. pylori+ | 0.397 | 0.432 | 0.942 | 0.234 | 0.543 | |||||
| VacAs1- | 79.2 | 53.5 | 36.4 | 18.4 | 14.7 | |||||
| VacAs1+ | 72.2 | 44.7 | 37 | 30 | 20.8 | |||||
| VacAm/H. pylori+ | 0.1 | 0.248 | 0.255 | 0.884 | 0.865 | |||||
| VacAm- | 69.4 | 40.4 | 47.6 | 24.5 | 17.1 | |||||
| VacAm+ | 80.6 | 50.6 | 38.7 | 25.6 | 15.8 | |||||
| VacAm1/H. pylori+ | 0.145 | 0.098 | 0.242 | 0.822 | 0.877 | |||||
| VacAm1- | 89.7 | 65.2 | 48.1 | 23.8 | 16.7 | |||||
| VacAm1+ | 77 | 44.8 | 35.4 | 26.3 | 15.2 | |||||
| VacAs1+m1+/H. pylori+ | 0.113 | 0.289 | 0.913 | 0.253 | 0.345 | |||||
| No VacAs1+m1+ | 86 | 57.5 | 37.7 | 21.6 | 12.9 | |||||
| VacAs1+m1+ | 72.2 | 44 | 38.9 | 34.6 | 23.5 | |||||
| dMMR | 0.393 | 0.049 | 0.095 | 0.05 | 0.169 | |||||
| No | 75.4 | 47.6 | 45.5 | 32.3 | 11.5 | |||||
| Yes | 71.3 | 59.5 | 54.7 | 43.9 | 5.1 | |||||
| HER2 status | 0.06 | 0.204 | 0.009 | 0.404 | 0.835 | |||||
| Negative | 74.4 | 52.4 | 49.9 | 37.7 | 11.1 | |||||
| Positive | 56.5 | 38.1 | 21.7 | 28.6 | 9.1 |
A high density of ST CD3+ cells was associated with an intestinal subtype (P = 0.003), and longer DFS and OS (P = 0.014 and P = 0.003, respectively), while a high density of ST CD8+ cells was associated with Bormann III GC (P = 0.006) and an intestinal subtype (P = 0.003; Table 3).
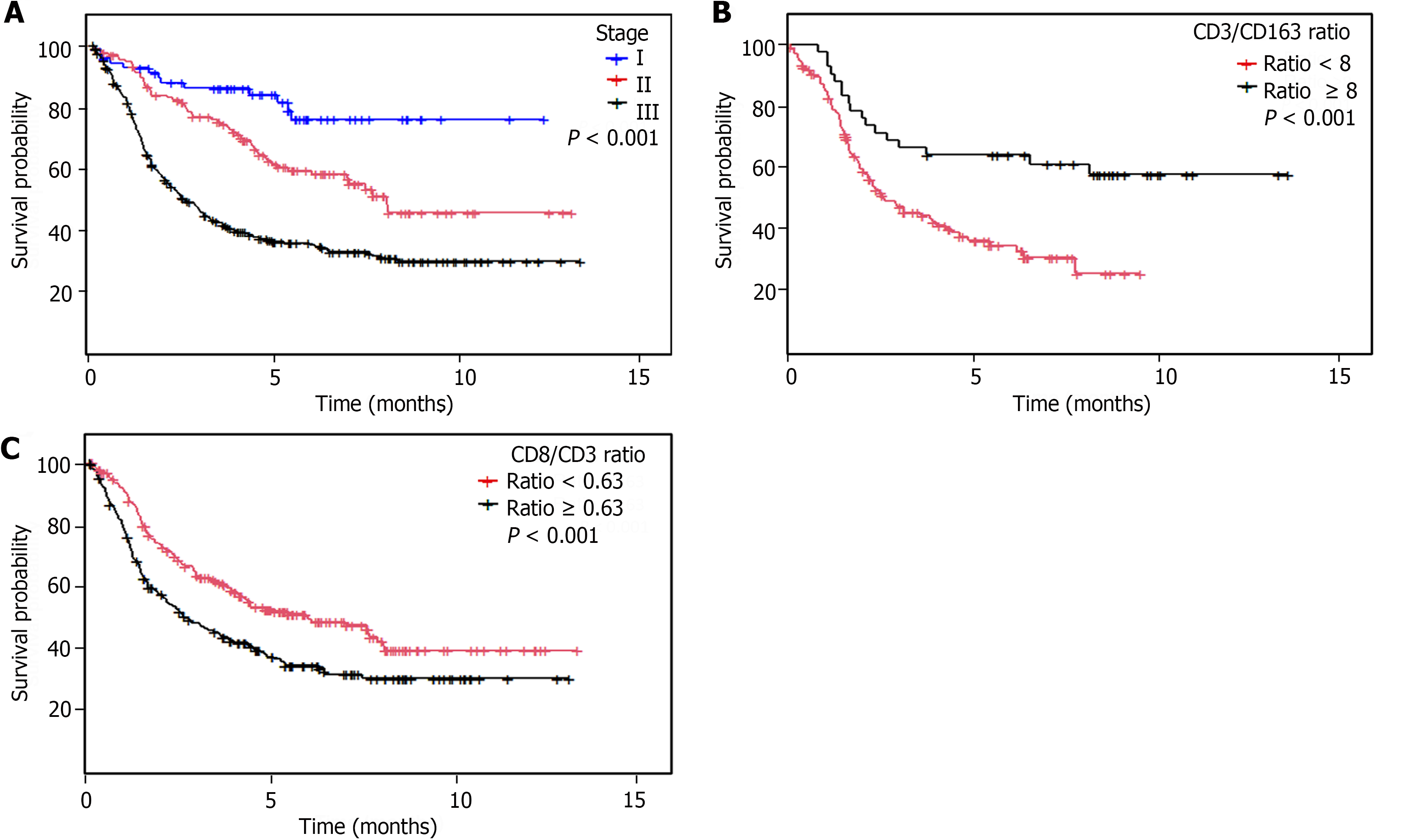
Patients with a low CD8/CD3 ratio in the IT and ST compartments had a longer DFS (P < 0.001 and P = 0.005, respectively) and OS (P = 0.001 and P = 0.002, respectively; Table 1 and Figure 3).
Multivariate analysis showed that age, tumor grade, stage as well as a high density of IT CD3+ cells and a low IT CD8/CD3 ratio were associated with a longer DFS and OS (Table 4).
| Features | DFS | OS | ||
| HR | P value | HR | P value | |
| Age | ||||
| < 60 | Reference | - | Reference | - |
| > 60 | 1.8 (1.2, 2.5) | 0.002 | 1.9 (1.3, 2.8) | < 0.001 |
| Histological grade | ||||
| Differentiated | Reference | Reference | ||
| Undifferentiated | 1.7 (1.01, 2.7) | 0.043 | 2.0 (1.2, 3.3) | 0.007 |
| WHO classification | ||||
| 1-2 | Reference | Reference | ||
| 3-4 | 2.5 (1.6, 3.8) | < 0.001 | 2.4 (1.5, 3.7) | < 0.001 |
| Clinical stage | ||||
| I-II | Reference | Reference | ||
| III | 2.1 (1.4, 3-2) | < 0.001 | 1.9 (1.2, 2.9) | 0.004 |
| IT CD3/HPF | ||||
| High (> 58) | Reference | Reference | ||
| Low (< 58) | 1.5 (1.02, 2.2) | 0.037 | 1.6 (1.1, 2.4) | 0.018 |
| IT CD8/CD3 ratio | ||||
| < 0.63 | Reference | Reference | ||
| > 0.63 | 1.7 (1.2, 2.5) | 0.002 | 1.6 (1.1, 2.3) | 0.008 |
| Non-significant variables | ||||
| Sex | - | 0.939 | - | 0.51 |
| Cardia-fundus | - | 0.398 | - | 0.212 |
| Bormann | - | 0.328 | - | 0.315 |
| Lauren | - | 0.311 | - | 0.291 |
| Lymph nodes | - | 0.836 | - | 0.663 |
| lymphovascular invasion | - | 0.972 | - | 0.277 |
| H. pylori | - | 0.123 | - | 0.140 |
| Deficient mismatch-repair | - | 0.973 | - | 0.365 |
| HER2 | - | 0.222 | - | 0.114 |
| IT TIL | - | 0.940 | - | 0.547 |
| Stromal TIL | - | 0.818 | - | 0.806 |
| IT CD8/HPF | - | 0.077 | - | 0.340 |
A high IT TIL level was associated with absence of H. pylori (P = 0.009) when evaluated by HE in the whole series. When evaluated by qPCR (n = 234), H. pylori (-) was associated with high IT (P = 0.002), ST (P = 0.047) and IB (P = 0.01) TIL levels. High IB TIL levels were associated with infection by H. pylori+ cagA- (P = 0.002) and vacA- (P = 0.045; Table 2).
High densities of IT CD8+ (P = 0.001) and ST CD8+ (P = 0.001) cells were associated with H. pylori (-) and a high density of IT CD8+ cells were associated with H. pylori+ cagA- (P = 0.023; Table 3).
High ST (P = 0.019) and IB (P = 0.01) TIL levels were associated with dMMR. High densities of ST CD3+ (P = 0.049) and CD8+ (P = 0.05) cells were associated with dMMR (Table 3) and a high density of IT CD8+ cells were associated with HER2-negative (P = 0.009; Tables 2 and 3).
Our series shows that the association between TIL levels and clinical-pathological features varies according to the tumor compartments in which TILs are determined. This is the first study to describe that high TIL levels in the IT compartment are associated with the absence of H. pylori infection, while high TIL levels in both the IB and ST compartments are associated with dMMR, and ST TIL levels are also associated with low disease recurrence. High densities of CD3+ and CD8+ T lymphocytes were associated with dMMR in the ST compartment, and CD8+ T lymphocytes with HER2 negative in the IT compartment.
We found that patients with tumors with a high density of CD3+ T lymphocytes in the IT and ST compartments, a low CD8/CD3 ratio in the IT and ST compartments, a low density of CD163+ macrophages, and a high CD3/CD163 ratio had greater survival than the remaining patients.
H. pylori infection detected by 2 methodologies was consistently associated with low TIL levels in the IT compartment. In addition, a low TIL level in the IB compartment was associated with H. pylori detected by qPCR, as well as with strains without strong virulence factors (CagA- and VacA-). Furthermore, the high density of CD8+ T lymphocytes in the IT and ST compartments was also associated with the absence of H. pylori (determined by qPCR). Different studies find that the intestinal microbiota modulates the activity of the immune system against cancer and even the activity of checkpoint inhibitors[27,28]. However, to our knowledge, this is the first time that a strong association between H. pylori and immune activity against cancer in GC has been described. Our results need further validation and suggest that H. pylori status evaluated by qPCR should be analyzed in clinical trials evaluating checkpoint inhibitors in GC.
Different series have evaluated the association between MSI and TIL, and Angell et al[29] found that 18.9% of GC cases were MSI- high and were associated with a high density of CD3+ and CD8+ T lymphocytes, as well as a better OS in a series including 380 cases of GC[30-33]. We found that the association between high levels of TIL and dMMR depends on the compartment evaluated, since the association was found in the ST and IB compartments but not in the IT compartment. Similarly, high densities of CD3+ and CD8+ T lymphocytes were associated with dMMR in the ST compartment but not in the IT compartment.
We found that a low density of CD8+ T lymphocytes in the IT compartment was associated with HER2 positivity. Similarly, Lv et al[34] reported a HER2 positive rate of 14% and an inverse relationship with the density of CD8+ T lymphocytes in a series of 120 patients with GC. However, other studies have described different findings[29].
The TIL levels were highest in the IB and ST compartments and were associated with less aggressive clinicopathological features (grade 1 and intestinal subtype for IB and ST, and negative lymph nodes for IB). The association between increased survival and a strong lymphocyte infiltrate has been described by different studies including up to 400 GC cases; however, these studies used different and non-standard methodologies (reporting rates of strong lymphocyte infiltration of 14% to 47%)[35-37].
On the other hand, aggressive features such as grade 3 were associated with high TIL levels in the IT compartment but low TIL levels in the ST compartment. Similarly, grade 3 was associated with high density of CD3 in the IT compartment but not in the ST compartment. This different activity of TILs depending on their spatial location in relation to malignant cells could be explained by a higher percentage of anergic immune cells in the IT compartment[38].
We found that a high density of CD3+ T lymphocytes in the IT and ST compartments, as well as tumors with a low CD8/CD3 ratio in the IT and ST compartments, were associated with increased survival in univariate and multivariate analyses. In addition, as densities were calculated in small tumor cores (tissue microarray-stained samples), we expect that the prognostic value of the density of CD3+ T lymphocytes and the CD8/CD3 ratio would be maintained when evaluated in samples obtained from gastroscopies or conservative surgery. Therefore, we recommend that these densities be evaluated in prospective GC trials.
The poor prognosis associated with the CD8/CD3 ratio has also been described by other groups and could be related to an anergic status of tumor-infiltrating CD8+ T lymphocytes[16,39]. Recent studies suggest that 30% to 38% of cases with a high CD8 T- cell density also have high levels of positive PD-L1[40], and Wang et al[41] found that the presence of both stains was associated with a shorter survival in a series of 147 GC cases.
In addition, our finding of a poor prognosis associated with high levels of CD163+ M2 macrophages has also been described by other groups and confirms the protumoral activity of these immune cells. The high ratio of CD3/CD163 was also associated with a favorable prognosis even in the smaller population size in which it was tested. Nonetheless, further studies on the ratio of immune cells in GC are necessary to confirm these findings[42,43].
Finally, this is the largest South American series evaluating molecular markers in GC, reporting dMMR and HER2 overexpression rates of 29% and 4.7% in early GC, respectively. The prevalence of both biomarkers is similar to what has previously been described in Caucasian series (5%-33% for dMMR and less than 18% for HER2)[10,44-46].
The levels of TILs are significantly related to dMMR and a H. pylori-negative status. However, the association of TIL levels with tumor features depends on the tumor compartment evaluated. Low CD8/CD3 and high CD163/CD3 values were strongly associated with a lower rate of recurrence and longer survival.
The authors thank the Universidad Cientifica del Sur, Grupo de Estudios Clinicos Oncologicos del Peru (GECO Peru) and the Instituto Nacional de Enfermedades Neoplasicas for the administrative support provided to carry out this research and language revision by a native English reviewer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Peru
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Zhang JW, China S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer. 1998;1:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 195] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Ruiz E, Sanchez J, Celis J, Payet E, Berrospi F, Chavez I, Young F. [Surgical outcome of 801 patients with localized gastric cancer treated with d2 lymphadenectomy]. Rev Gastroenterol Peru. 2009;29:124-131. [PubMed] |
| 3. | Castaneda CA, Castillo M, Chavez I, Barreda F, Suarez N, Nieves J, Bernabe LA, Valdivia D, Ruiz E, Dias-Neto E, Landa-Baella MP, Bazan Y, Rengifo CA, Montenegro P. Prevalence of Helicobacter pylori Infection, Its Virulent Genotypes, and Epstein-Barr Virus in Peruvian Patients With Chronic Gastritis and Gastric Cancer. J Glob Oncol. 2019;5:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Castañeda C, Castillo M, Bernabe L, Suarez N, Fassan M, Sanchez J, Tello K, Alatrista R, Chavez I, Ruiz E, Bazan Y, Barreda F, Valdivia D, Meng W, Chakravarti A, Taxa L, Montenegro P. The relationship between tumour infiltrating lymphocytes, Epstein-Barr virus and Helicobacter pylori infection in gastric cancer. Ecancermedicalscience. 2022;16:1362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 5. | Tsai KF, Liou JM, Chen MJ, Chen CC, Kuo SH, Lai IR, Yeh KH, Lin MT, Wang HP, Cheng AL, Lin JT, Shun CT, Wu MS; Taiwan Gastrointestinal Disease and Helicobacter Consortium. Distinct Clinicopathological Features and Prognosis of Helicobacter pylori Negative Gastric Cancer. PLoS One. 2017;12:e0170942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4845] [Article Influence: 440.5] [Reference Citation Analysis (2)] |
| 7. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5309] [Article Influence: 353.9] [Reference Citation Analysis (3)] |
| 8. | Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, Saito K, Kawaguchi Y, Kamio T, Kojima A, Sugihara M, Yamaguchi K; DESTINY-Gastric01 Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med. 2020;382:2419-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 820] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 9. | Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, Chou JF, Segal MF, Simmons MZ, Momtaz P, Shcherba M, Ku GY, Zervoudakis A, Won ES, Kelsen DP, Ilson DH, Nagy RJ, Lanman RB, Ptashkin RN, Donoghue MTA, Capanu M, Taylor BS, Solit DB, Schultz N, Hechtman JF. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 263] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 10. | Cangiano J, Centeno BA, Garrett CR, Cáceres W, de Jesús A, Lee JH, Pavía O, Jove R, Báez L, Sullivan DM, Muro-Cacho CA, Muñoz-Antonia T. Signal transduction proteins in tumors from Puerto Rican and Caucasian gastric adenocarcinoma patients: expression differences with potential for specific targeted therapies. Dig Dis Sci. 2008;53:2090-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Joshi SS, Maron SB, Catenacci DV. Pembrolizumab for treatment of advanced gastric and gastroesophageal junction adenocarcinoma. Future Oncol. 2018;14:417-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Janjigian Y, Kawazoe A, Weber P, Luo S, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, Wyrwicz L, Shitara K, Qin S, Van Cutsem E, Tabernero J, Li L, Shih C, Bhagia P, Chung H. LBA-4 Initial data from the phase 3 KEYNOTE-811 study of trastuzumab and chemotherapy with or without pembrolizumab for HER2-positive metastatic gastric or gastroesophageal junction (G/GEJ) cancer. Ann Oncol. 2021;32 Suppl 3:S227. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S; International TILs Working Group 2014. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1473] [Cited by in RCA: 2217] [Article Influence: 201.5] [Reference Citation Analysis (0)] |
| 14. | Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, Peng H, Cui L, Li C. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1595-1605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 245] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 15. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 4903] [Article Influence: 258.1] [Reference Citation Analysis (0)] |
| 16. | Fang T, Wang Z, Yin X, Wang H, Zhang L, Lin X, Zhang X, Wang Y, Xue Y. Evaluation of Immune Infiltration Based on Image Plus Helps Predict the Prognosis of Stage III Gastric Cancer Patients with Significantly Different Outcomes in Northeastern China. Dis Markers. 2022;2022:2893336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, McRee AJ, Lin CC, Pathiraja K, Lunceford J, Emancipator K, Juco J, Koshiji M, Bang YJ. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 909] [Article Influence: 101.0] [Reference Citation Analysis (1)] |
| 18. | Wu TH, Hsiue EHC, Yuan CT, Tseng LH, Lin CC, Yeh KH. Durable response to programmed death-1 (PD-1) blockade in a metastatic gastric cancer patient with mismatch repair deficiency and microsatellite instability. J Cancer Res Pract. 2017;4:72-75. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2120] [Cited by in RCA: 2004] [Article Influence: 400.8] [Reference Citation Analysis (0)] |
| 20. | Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, Sanders M, Solomon B, Solinas C, Van den Eynden GGGM, Allory Y, Preusser M, Hainfellner J, Pruneri G, Vingiani A, Demaria S, Symmans F, Nuciforo P, Comerma L, Thompson EA, Lakhani S, Kim SR, Schnitt S, Colpaert C, Sotiriou C, Scherer SJ, Ignatiadis M, Badve S, Pierce RH, Viale G, Sirtaine N, Penault-Llorca F, Sugie T, Fineberg S, Paik S, Srinivasan A, Richardson A, Wang Y, Chmielik E, Brock J, Johnson DB, Balko J, Wienert S, Bossuyt V, Michiels S, Ternes N, Burchardi N, Luen SJ, Savas P, Klauschen F, Watson PH, Nelson BH, Criscitiello C, O'Toole S, Larsimont D, de Wind R, Curigliano G, André F, Lacroix-Triki M, van de Vijver M, Rojo F, Floris G, Bedri S, Sparano J, Rimm D, Nielsen T, Kos Z, Hewitt S, Singh B, Farshid G, Loibl S, Allison KH, Tung N, Adams S, Willard-Gallo K, Horlings HM, Gandhi L, Moreira A, Hirsch F, Dieci MV, Urbanowicz M, Brcic I, Korski K, Gaire F, Koeppen H, Lo A, Giltnane J, Rebelatto MC, Steele KE, Zha J, Emancipator K, Juco JW, Denkert C, Reis-Filho J, Loi S, Fox SB. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv Anat Pathol. 2017;24:311-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 556] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 21. | Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4321] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 22. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Geneva: World Health Organization, 2010: 417. |
| 23. | Muraca P. Oncology tissue microarrays. United States patent US20030049701A1. 2003 Mar 13. |
| 24. | Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 432] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 25. | Castaneda CA, Castillo M, Aliaga K, Bernabe LA, Casavilca S, Sanchez J, Torres-Cabala CA, Gomez HL, Mas L, Dunstan J, Cotrina JM, Abugattas J, Chavez I, Ruiz E, Montenegro P, Rojas V, Orrego E, Galvez-Nino M, Felix B, Landa-Baella MP, Vidaurre T, Villa MR, Zevallos R, Taxa L, Guerra H. Level of tumor-infiltrating lymphocytes and density of infiltrating immune cells in different malignancies. Biomark Med. 2019;13:1481-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Lausen B, Schumacher M. Maximally selected rank statistics. Biometrics. 1992;1:73-85. [RCA] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 366] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 27. | Shi Y, Zheng H, Wang M, Ding S. Influence of Helicobacter pylori infection on PD‐1/PD-L1 blockade therapy needs more attention. Helicobacter. 2022;27:e12878. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | McCulloch JA, Davar D, Rodrigues RR, Badger JH, Fang JR, Cole AM, Balaji AK, Vetizou M, Prescott SM, Fernandes MR, Costa RGF, Yuan W, Salcedo R, Bahadiroglu E, Roy S, DeBlasio RN, Morrison RM, Chauvin JM, Ding Q, Zidi B, Lowin A, Chakka S, Gao W, Pagliano O, Ernst SJ, Rose A, Newman NK, Morgun A, Zarour HM, Trinchieri G, Dzutsev AK. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med. 2022;28:545-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 281] [Article Influence: 93.7] [Reference Citation Analysis (0)] |
| 29. | Angell HK, Lee J, Kim KM, Kim K, Kim ST, Park SH, Kang WK, Sharpe A, Ogden J, Davenport A, Hodgson DR, Barrett JC, Kilgour E. PD-L1 and immune infiltrates are differentially expressed in distinct subgroups of gastric cancer. Oncoimmunology. 2019;8:e1544442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A, Ochiai A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 31. | Pectasides E, Chatzidakis I, Kotoula V, Koliou GA, Papadopoulou K, Giannoulatou E, Giannouzakos VG, Bobos M, Papavasileiou C, Chrisafi S, Florou A, Pectasides D, Fountzilas G. Prognostic Biomarkers in Early-stage Gastric Adenocarcinoma Treated With Adjuvant Chemoradiotherapy. Cancer Genomics Proteomics. 2020;17:277-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Shin SJ, Kim SY, Choi YY, Son T, Cheong JH, Hyung WJ, Noh SH, Park CG, Kim HI. Mismatch Repair Status of Gastric Cancer and Its Association with the Local and Systemic Immune Response. Oncologist. 2019;24:e835-e844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Morihiro T, Kuroda S, Kanaya N, Kakiuchi Y, Kubota T, Aoyama K, Tanaka T, Kikuchi S, Nagasaka T, Nishizaki M, Kagawa S, Tazawa H, Fujiwara T. PD-L1 expression combined with microsatellite instability/CD8+ tumor infiltrating lymphocytes as a useful prognostic biomarker in gastric cancer. Sci Rep. 2019;9:4633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Lv H, Zhang J, Sun K, Nie C, Chen B, Wang J, Xu W, Wang S, Liu Y, Chen X. Expression of Human Epidermal Growth Factor Receptor-2 Status and Programmed Cell Death Protein-1 Ligand Is Associated With Prognosis in Gastric Cancer. Front Oncol. 2020;10:580045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Setälä LP, Kosma VM, Marin S, Lipponen PK, Eskelinen MJ, Syrjänen KJ, Alhava EM. Prognostic factors in gastric cancer: the value of vascular invasion, mitotic rate and lymphoplasmacytic infiltration. Br J Cancer. 1996;74:766-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Yu CC, Levison DA, Dunn JA, Ward LC, Demonakou M, Allum WH, Hallisey MT. Pathological prognostic factors in the second British Stomach Cancer Group trial of adjuvant therapy in resectable gastric cancer. Br J Cancer. 1995;71:1106-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Xiangming C, Iwashige H, Aridome K, Hokita S, Aikou T. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Lett. 2000;159:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Fukuda K, Tsujitani S, Maeta Y, Yamaguchi K, Ikeguchi M, Kaibara N. The expression of RCAS1 and tumor infiltrating lymphocytes in patients with T3 gastric carcinoma. Gastric Cancer. 2002;5:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Jin K, Cao Y, Gu Y, Fang H, Fei Y, Wang J, Liu X, Lv K, He X, Lin C, Liu H, Li H, He H, Li R, Zhang H, Xu J. Poor clinical outcomes and immunoevasive contexture in CXCL13+CD8+ T cells enriched gastric cancer patients. Oncoimmunology. 2021;10:1915560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Valentini AM, Di Pinto F, Coletta S, Guerra V, Armentano R, Caruso ML. Tumor microenvironment immune types in gastric cancer are associated with mismatch repair however, not HER2 status. Oncol Lett. 2019;18:1775-1785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Wang W, Wang K, Chen Z, Chen L, Guo W, Liao P, Rotroff D, Knepper TC, Liu Z, Zhang W, Mcleod HL, He Y. Immunoclassification characterized by CD8 and PD-L1 expression is associated with the clinical outcome of gastric cancer patients. Oncotarget. 2018;9:12164-12173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Zhou W, Zhang Y, He F, Lv S, Zhang X, Fei C. Abundance of CD163-Positive Tumor-Associated Macrophages in the Early Gastric Cancer Predicts the Recurrence after Curative Resection. Dig Dis. 2020;38:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Hu J, Ma Y, Ma J, Yang Y, Ning Y, Zhu J, Wang P, Chen G, Liu Y. M2 Macrophage-Based Prognostic Nomogram for Gastric Cancer After Surgical Resection. Front Oncol. 2021;11:690037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Pereira MA, Ramos MFKP, Dias AR, Faraj SF, Ribeiro RRE, de Castria TB, Zilberstein B, Alves VAF, Ribeiro U Jr, de Mello ES. Expression Profile of Markers for Targeted Therapy in Gastric Cancer Patients: HER-2, Microsatellite Instability and PD-L1. Mol Diagn Ther. 2019;23:761-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Cruz-Reyes C, Gamboa-Dominguez A. HER2 amplification in gastric cancer is a rare event restricted to the intestinal phenotype. Int J Surg Pathol. 2013;21:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Alvarado-Cabrero I, Gil-Hernández S, Ruelas-Perea A, Villaverde-Rodríguez D, Montes-Ochoa JR, Medrano-Guzmán R. Immunohistochemical assessment of HER2 expression in gastric cancer. A clinicopathologic study of 93 cases. Cir Cir. 2017;85:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |