Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2476
Revised: March 17, 2024
Accepted: April 18, 2024
Published online: June 15, 2024
Processing time: 137 Days and 18.9 Hours
The objectives of this study were to assess the safety and efficacy of drug-eluting bead transarterial chemoembolization (DEB-TACE) as neoadjuvant therapy before liver transplantation (LT) for advanced-stage hepatocellular carcinoma (HCC) and to analyze the prognostic factors.
To determine whether DEB-TACE before LT is superior to LT for advanced-stage HCC.
A total of 99 individuals diagnosed with advanced HCC were studied retrospectively. The participants were categorized into the following two groups based on whether they had received DEB-TACE before LT: DEB-TACE group (n = 45) and control group (n = 54). The participants were further divided into two subgroups based on the presence or absence of segmental portal vein tumor thrombus (PVTT). The DEB-TACE group consisted of two subgroups: Group A (n = 31) without PVTT and group B (n = 14) with PVTT. The control group also had two subgroups: Group C (n = 37) without PVTT and group D (n = 17) with PVTT. Data on patient demographics, disease characteristics, therapy response, and adverse events (AEs) were collected. The overall survival (OS) and recurrence-free survival (RFS) rates were assessed using Kaplan-Meier curves. Univariate and multivariate Cox regression analyses were conducted to determine the parameters that were independently related to OS and RFS.
The DEB-TACE group exhibited an overall response rate of 86.6%. Following therapy, there was a significant decrease in the median alpha-fetoprotein (AFP) level (275.1 ng/mL vs 41.7 ng/mL, P < 0.001). The main AE was post-embolization syndrome. The 2-year rates of RFS and OS were significantly higher in the DEB-TACE group than in the control group (68.9% vs 38.9%, P = 0.003; 86.7% vs 63.0%, P = 0.008). Within the subgroups, group A had higher 2-year rates of RFS and OS compared to group C (71.0% vs 45.9%, P = 0.038; 83.8% vs 62.2%, P = 0.047). The 2-year RFS rate of group B was markedly superior to that of group D (64.3% vs 23.5%, P = 0.002). Results from multivariate analyses showed that pre-LT DEB-TACE [hazard ratio (HR) = 2.73, 95% confidence interval (CI): 1.44-5.14, P = 0.04], overall target tumor diameter ≤ 7 cm (HR = 1.98, 95%CI: 1.05-3.75, P = 0.035), and AFP level ≤ 400 ng/mL (HR = 2.34; 95%CI: 1.30-4.19, P = 0.009) were significant risk factors for RFS. Additionally, pre-LT DEB-TACE (HR = 3.15, 95%CI: 1.43-6.96, P = 0.004) was identified as a significant risk factor for OS.
DEB-TACE is a safe and efficient therapy for advanced-stage HCC and also enhances patient survival after LT.
Core Tip: This retrospective study investigated the value of drug-eluting bead transarterial chemoembolization (DEB-TACE) as neoadjuvant therapy pre-liver transplantation (LT) for advanced-stage hepatocellular carcinoma. We identified 99 relevant cases among our patient population and followed their overall survival (OS) and recurrence-free survival. The results indicated that DEB-TACE before LT may prolong OS and reduce recurrence rate.
- Citation: Ye ZD, Zhuang L, Song MC, Yang Z, Zhang W, Zhang JF, Cao GH. Drug-eluting bead transarterial chemoembolization as neoadjuvant therapy pre-liver transplantation for advanced-stage hepatocellular carcinoma. World J Gastrointest Oncol 2024; 16(6): 2476-2486
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2476.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2476
Hepatocellular carcinoma (HCC) is the sixth most common form of cancer and the third leading cause of cancer-related deaths worldwide, with an estimated 906000 diagnoses and 830000 deaths annually[1]. Significant risk factors associated with HCC include chronic hepatitis B virus or hepatitis C virus infection, intake of food contaminated with aflatoxin, high alcohol consumption, type 2 diabetes, obesity, and smoking[2].
Liver transplantation (LT) is the gold-standard treatment for unresectable early-stage HCC in the setting of cirrhosis[3]. It is widely recognized that LT provides excellent outcomes for patients with HCC who meet the Milan criteria (solitary tumor ≤ 5 cm in size or no more than three tumors, all < 3 cm in diameter)[4]. However, the Milan criteria are considered too strict, and the Hangzhou criteria (HC) are more suitable for patients with HCC in China. Despite the effectiveness of LT, the development of HCC is gradual and difficult to detect, and there is still a subset of patients beyond the HC, with 30%-40% of patients having portal vein tumor thrombus (PVTT) at the time of initial diagnosis[5-9]. Neoadjuvant therapy (downstaging or bridging) before LT not only prevents tumor progression and reduces the risk of withdrawal from the transplantation list but also improves the prognosis of patients after LT[10,11]. However, due to the scarcity of organs, there is a long waiting period for patients, specifically for those with advanced HCC. Tumor growth also leads to individuals missing the opportunity for transplantation.
Drug-eluting bead transarterial chemoembolization (DEB-TACE) has the advantages of low systemic toxicity, high intratumoral drug concentration, and long drug elution time, showing good pharmacokinetic performance and tolerability in advanced HCC, and has also proven effective as a bridging and downstaging treatment before LT[12-16]. Indeed, it has been employed in our hospital as a conversion therapy before hepatectomy or as a locoregional therapy for patients with unresectable HCC whose Child-Pugh classification is A or B. However, whether it can be used as neoadjuvant therapy for advanced HCC with or without PVTT before LT remains unclear. In this study, we retrospectively analyzed the prognosis of patients who received LT after DEB-TACE for advanced HCC and identified important prognostic factors.
We retrospectively analyzed 99 patients with advanced HCC in Shulan (Hangzhou) Hospital (in Hangzhou, Zhejiang Province, China) between January 2017 and December 2022. The decision to perform DEB-TACE was made by a multidisciplinary team including hepatobiliary and pancreas surgeons, radiologists, and medical oncologists, according to the patient’s tumor stage, liver function, and need for bridging therapy before LT. Patients were divided into the DEB-TACE group and the control group according to whether they received DEB-TACE before LT. Patients in each group were further divided into two subgroups according to the presence or absence of PVTT. The diagnosis of HCC was based on imaging examinations including computer tomography, magnetic resonance imaging before LT, and histology after LT. This study was reviewed and approved by the Ethics Committee of Shulan (Hangzhou) Hospital.
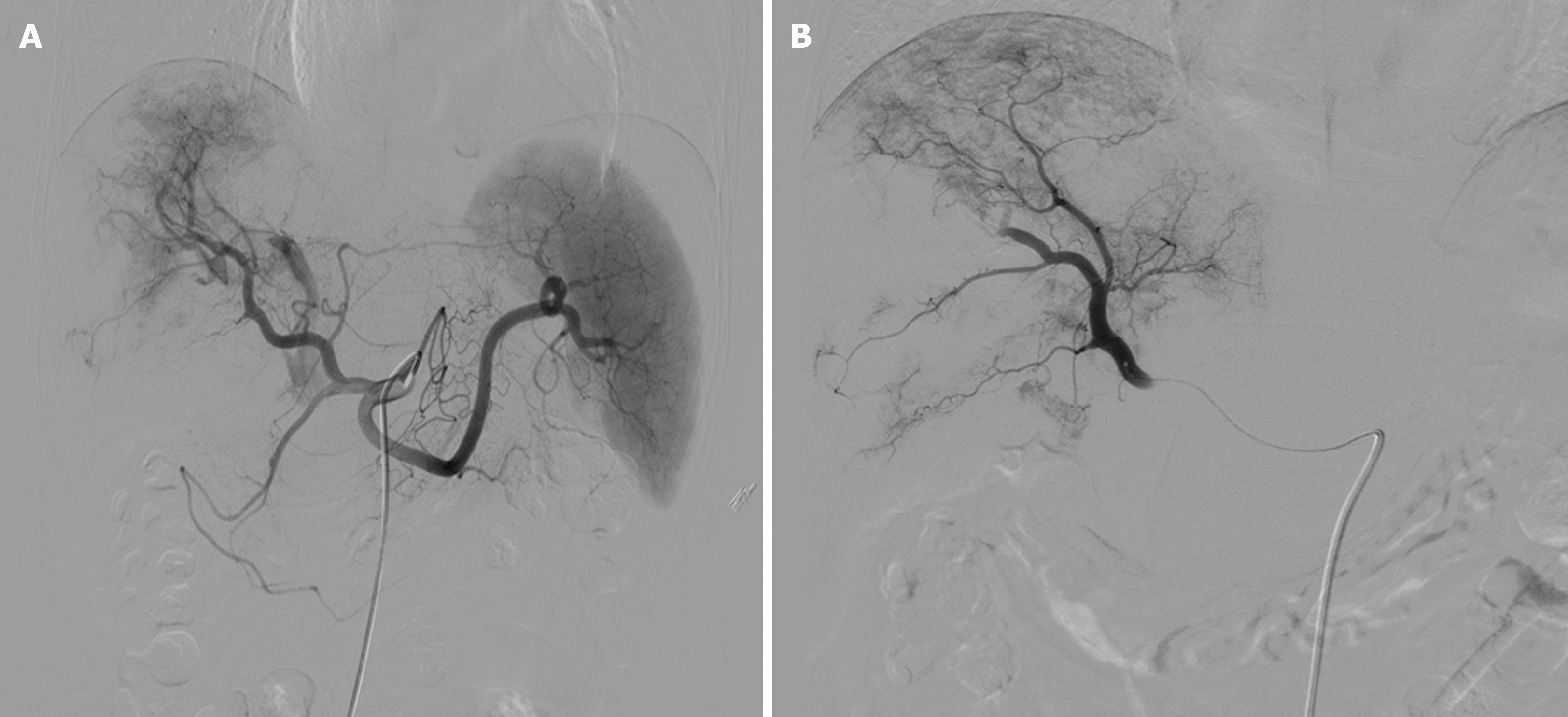
The Seldinger technique was used to access the common femoral artery, with insertion of a 5 F vascular sheath. The tumor blood supply was assessed using celiac and superior mesenteric artery angiography, and a 2.1-F microcatheter was super-selected for the tumor-supplying artery. The DEB-TACE procedure utilized 60 mg epirubicin loaded with 100-300 μm Callispheres® microspheres (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Province, China) to conduct embolization. Polyvinyl alcohol particles or Embosphere® microspheres (BioSphere Medical, Rockland, MA, United States) were used when the embolization endpoint was not achieved. Extrahepatic tumor blood-supplying arteries such as the inferior phrenic, intercostal, or internal mammary arteries were embolized using the same approach, with stasis or near stasis (sluggish) of blood flow as the endpoint of embolization (Figure 1).
Patient baseline clinical and demographic characteristics such as age, sex, Child-Pugh classification, Eastern Cooperative Oncology Group (ECOG) score, time to LT, microvascular invasion (MVI), macrovascular invasion (MAV), overall diameter of target tumors, alpha-fetoprotein (AFP) level, and tumor differentiation were recorded.
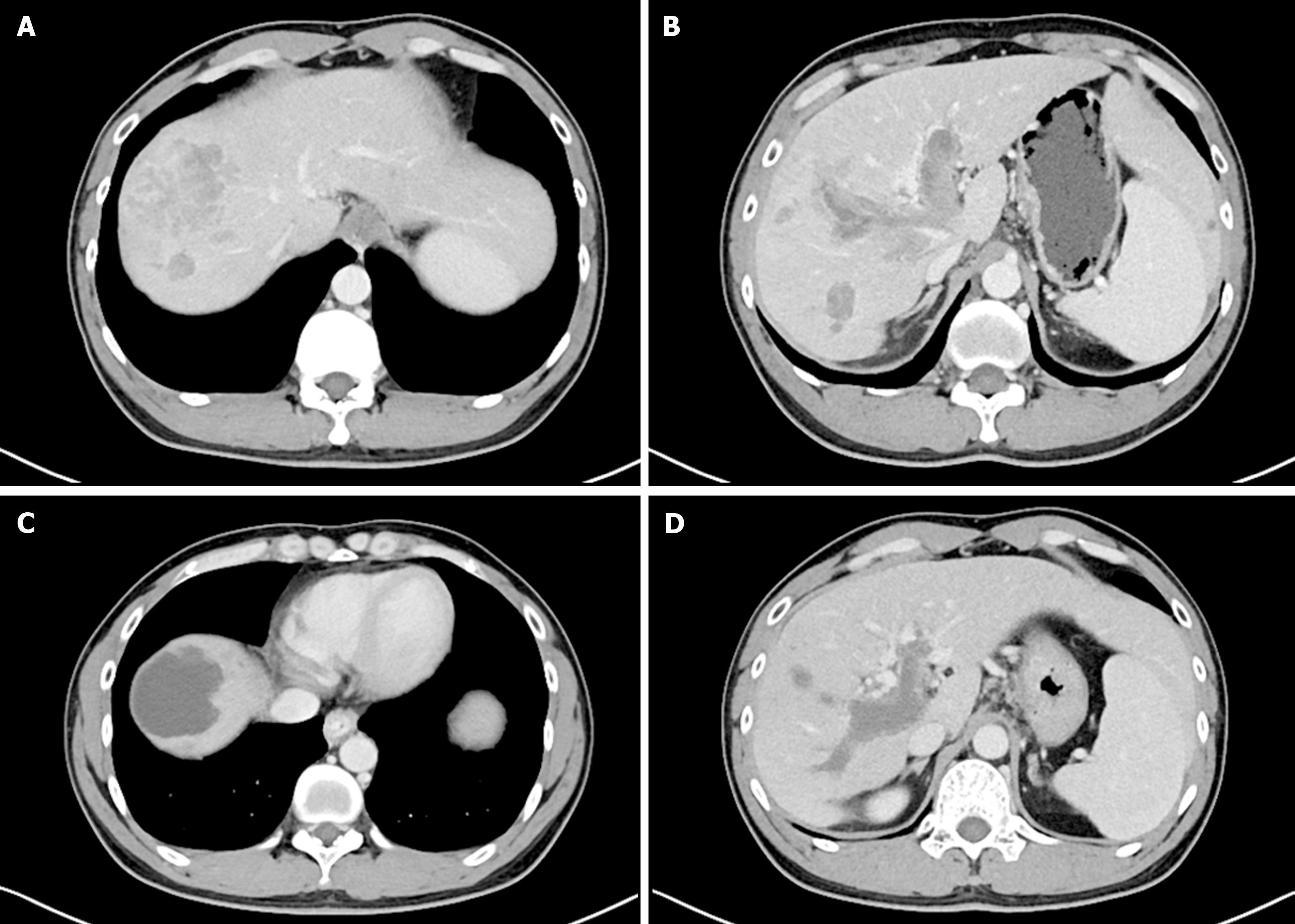
All patients received treatment at our hospital from the time of HCC diagnosis until the LT procedure and subsequent post-transplantation. Patient follow-up was initiated at 3-4 wk after DEB-TACE, at which time two senior radiologists assessed the treatment responses in patients using the modified response evaluation criteria in solid tumors[17], which included complete response (CR), partial response (PR), progressive disease (PD), or stable disease (SD) (Figure 2). The overall response rate (ORR) (%) was defined as (CR + PR/total cases × 100%). A comparison was made between preoperative and postoperative alterations in AFP level in both the DEB-TACE group and its subgroups. The National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 was used for the evaluation of treatment-related adverse events (AEs). Patients were monitored after LT until December 2022. Overall survival (OS) was defined as the time from LT to either death or most recent follow-up. Recurrence-free survival (RFS) was defined as the time between LT and HCC recurrence.
SPSS Statistics 26.0 (IBM Corp., Armonk, NY, United States) was used for all statistical analyses. Continuous variables are reported as the mean and standard deviation or as the median and range, and were compared using the Student’s t-test or Mann-Whitney U test when appropriate. Categorical variables were reported as numbers and percentages and were compared using the χ2 test or Fisher’s exact test. Survival outcomes were assessed with Kaplan-Meier curves and log-rank tests, whereas predictors of OS and RFS were identified using multivariate Cox proportional hazards regression models. P < 0.05 was considered statistically significant.
A total of 99 patients were enrolled in this study and followed up until December 2022 (Table 1). The mean age was 52.6 ± 11.1 years, and 92 (92.9%) were male. All 99 patients had chronic viral hepatitis B. The majority of patients (n = 83, 83.8%) were Child-Pugh class A. Thirty-one (31.3%) patients had segmental PVTT. The median AFP level at admission was 94.7 ng/mL (12.8 ng/mL, 3561.1 ng/mL), and the median AFP level before LT was 41.7 ng/mL (8.0 ng/mL, 601.4 ng/mL). AFP was measured one final time before LT; in 74 (74.7%) patients, the AFP level was ≤ 400 ng/mL. The overall diameter of the target tumor was determined by postoperative pathological measurement; the median overall diameter was 8.0 cm (5.2 cm, 11.3 cm) in 46 (46.5%) patients, and the overall diameter of the target tumors was ≤ 7 cm. Regarding tumor grade, the majority of patients (n = 71, 71.7%) had moderately differentiated tumor cells. Patients were divided into the following two groups: DEB-TACE group (n = 45) and control group (n = 54). DEB-TACE was performed as needed, an average of about 1.3 times. The median time spent on the waiting list was longer in the DEB-TACE group than in the control group (2.0 months vs 1.0 months, P < 0.01). The majority of patients in both groups were Child-Pugh class A, but more patients were class B and C in the control group (A = 43, B = 2, C = 0 vs A = 40, B = 9, C = 4, P < 0.01). There were no statistically significant differences between the two groups in terms of age, sex, ECOG performance status score, MAV, AFP level at admission, AFP level before LT, overall diameter of target tumors, or tumor grade.
| Characteristics | Total, n = 99 | DEB-TACE group, n = 45 | Control group, n = 54 | P value |
| Age in yr | 52.6 ± 11.1 | 52.0 ± 12.1 | 53.0 ± 10.2 | 0.64 |
| Sex | > 0.99 | |||
| Male | 92 (92.9) | 42 (93.3) | 50 (92.6) | |
| Female | 7 (7.1) | 3 (6.7) | 4 (7.4) | |
| ECOG PS score | 0.53 | |||
| 0 | 45 (45.5) | 22 (48.9) | 23 (42.6) | |
| 1 | 54 (54.5) | 23 (51.1 ) | 31 (57.4) | |
| HBV | 99 (100.0) | 45 (100.0) | 54 (100.0) | |
| ChildPugh classification | < 0.01 | |||
| A | 83 (84.7) | 43 (95.6) | 40 (75.5) | |
| B | 11 (11.2) | 2 (4.4) | 9 (17.0) | |
| C | 4 (4.1) | 0 (0.0) | 4 (7.5) | |
| Time to LT in month | 1.0 (1.0, 2.0) | 2.0 (1.0, 3.5) | 1.0 (1.0, 1.0) | < 0.01 |
| Macrovascular invasion | 0.96 | |||
| Negative | 68 (68.7) | 31 (68.9) | 37 (68.5) | |
| Segmental PVTT | 31 (31.3) | 14 (31.1) | 17 (31.5) | |
| AFP level at admission in ng/mL | 94.7 (12.8, 3561.1) | 275.1 (17.2, 5428.8) | 43.1 (9.7, 455.2) | 0.07 |
| AFP level before LT in ng/mL | 41.7 (8.0, 601.4) | 41.7 (4.7, 619.7) | 43.1 (9.7, 455.2) | 0.61 |
| AFP ≤ 400 ng/mL | 74 (74.4) | 32 (71.1) | 42 (77.8) | 0.44 |
| AFP > 400 ng/mL | 25 (25.3) | 13 (28.9) | 12 (22.2) | |
| Overall diameter of target tumors in cm | 8.0 (5.2, 11.3) | 6.9 (4.7, 11.8) | 8.0 (5.4, 11.0) | 0.87 |
| ≤ 7 cm | 46 (46.5) | 24 (53.3) | 22 (40.7) | 0.21 |
| > 7 cm | 53 (53.5) | 21 (46.7) | 32 (59.3) | |
| Tumor grade | 0.97 | |||
| Well differentiated | 3 (3.0) | 1 (2.2) | 2 (3.7) | |
| Moderately differentiated | 71 (71.7) | 33 (73.3) | 38 (70.4) | |
| Undifferentiated | 25 (25.3) | 11 (24.4) | 14 (25.9) |
A notable disparity was found in the extent of MVI between the two groups of patients without segmental PVTT (DEB-TACE group vs control group, P < 0.01). Therefore, we divided the two groups of patients into four subgroups according to the presence or absence of PVTT. In the DEB-TACE group, group A (n = 31) consisted of patients without PVTT and group B (n = 14) included patients with PVTT. Similarly, the control group was also divided into two subgroups; group C (n = 37) comprised patients without PVTT, and group D (n = 17) included patients with PVTT. Tumor characteristics composed the preliminary analyses of the subgroups (Table 2).
| Characteristic | Without segmental PVTT | P value | With segmental PVTT | P value | ||
| Group A, n = 31, 68.9% | Group C, n = 37, 68.5% | Group B, n = 14, 31.1% | Group D, n = 17, 31.5% | |||
| AFP level at admission in ng/mL | 155.9 (11.9, 1386.6) | 22.6 (7.2, 297.9) | 0.121 | 5428.8 (33.5, 20000) | 172.2 (54.5, 7951.4) | 0.233 |
| AFP level before LT in ng/mL | 26.5 (4.6, 200.6) | 22.6 (7.2, 297.9) | 0.703 | 459.9 (4.6, 7670.5) | 172.2 (54.5, 7951.4) | 0.953 |
| Overall diameter of target tumors in cm | 6.3 (4.4, 9.6) | 8.0 (4.9, 11.0) | 0.449 | 9.4 (6.6, 16.6) | 8.0 (6.1, 11.5) | 0.361 |
| Tumor grade, n (%) | 0.925 | > 0.99 | ||||
| Well differentiated | 1 (3.2) | 1 (2.7) | 0 (0.0) | 1 (5.9) | ||
| Moderately differentiated | 22 (71.0) | 27 (73.0) | 11 (78.6) | 11 (64.7) | ||
| Undifferentiated | 8 (25.8) | 9 (24.3) | 3 (21.4) | 5 (29.4) | ||
| MVI, n (%) | < 0.01 | - | ||||
| MVI = M0 | 13 (41.9) | 11 (29.7) | - | - | ||
| MVI = M1 | 10 (32.3) | 6 (16.2) | - | - | ||
| MVI = M2 | 8 (25.8) | 20 (54.1) | - | - | ||
With a 100% technical success rate, 10 (22.2%), 29 (64.5%), 1 (2.2%), and 5 (11.1%) patients achieved CR, PR, SD, and PD respectively, with ORR value of 86.6%. The DEB-TACE group and its subgroups showed a substantial decrease in AFP levels after therapy (Table 3). The most common AEs were post-embolization syndrome including moderate fever (87.5%), abdominal pain (74.7%), and nausea (57.5%). The majority of patients experienced temporary liver function impairment after DEB-TACE. None of the patients experienced severe treatment-related complications.
| Group | AFP before treatment | AFP after treatment | Z | P value |
| DEB-TACE group in ng/mL | 275.1 (17.2-5428.8) | 41.7 (4.7-619.7) | -3.810 | < 0.001 |
| Group A in ng/mL | 155.9 (11.9, 1386.6) | 26.5 (4.6, 200.6) | -2.793 | 0.005 |
| Group B in ng/mL | 5428.8 (33.5, 20000) | 459.9 (4.6, 7670.5) | -2.341 | 0.019 |
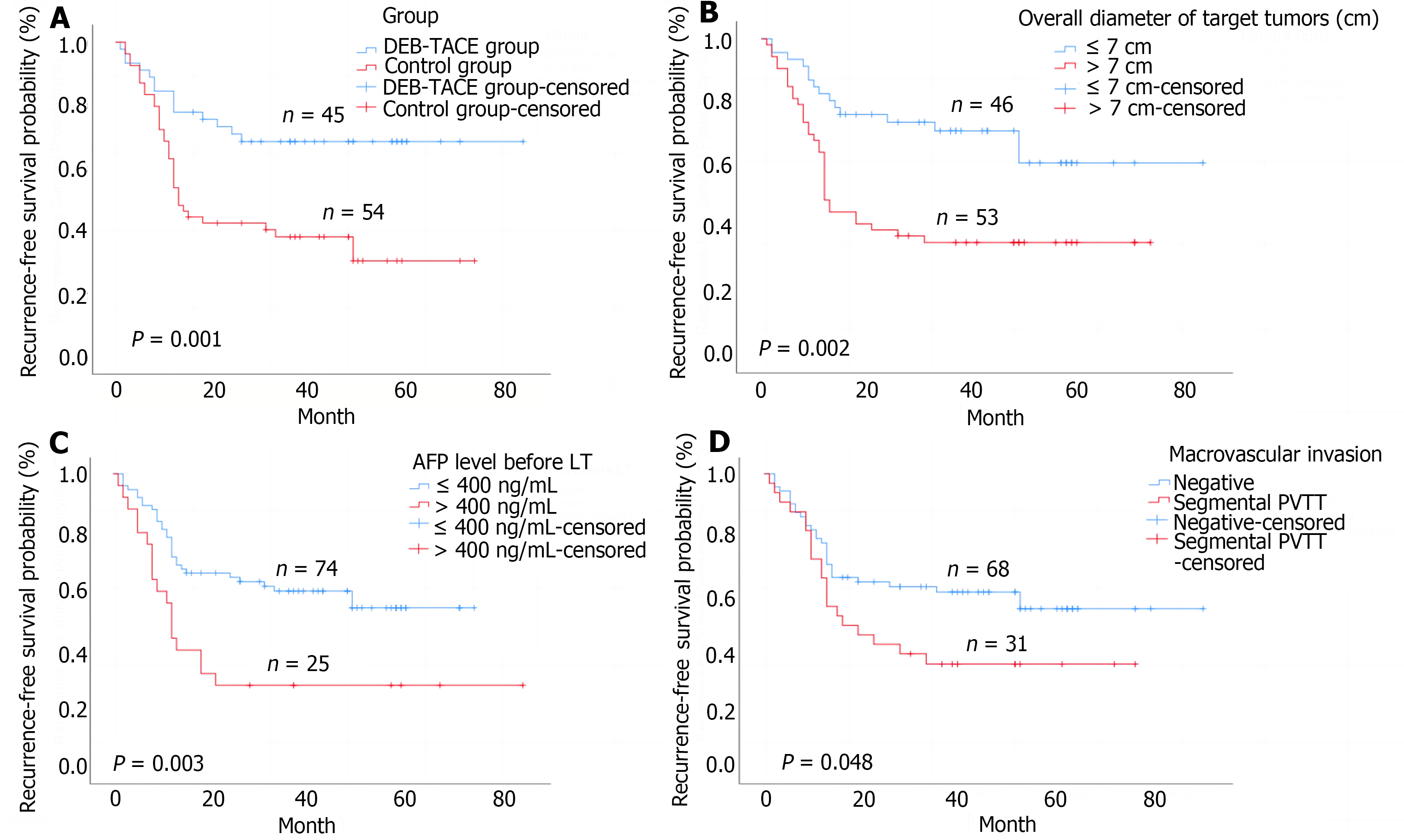
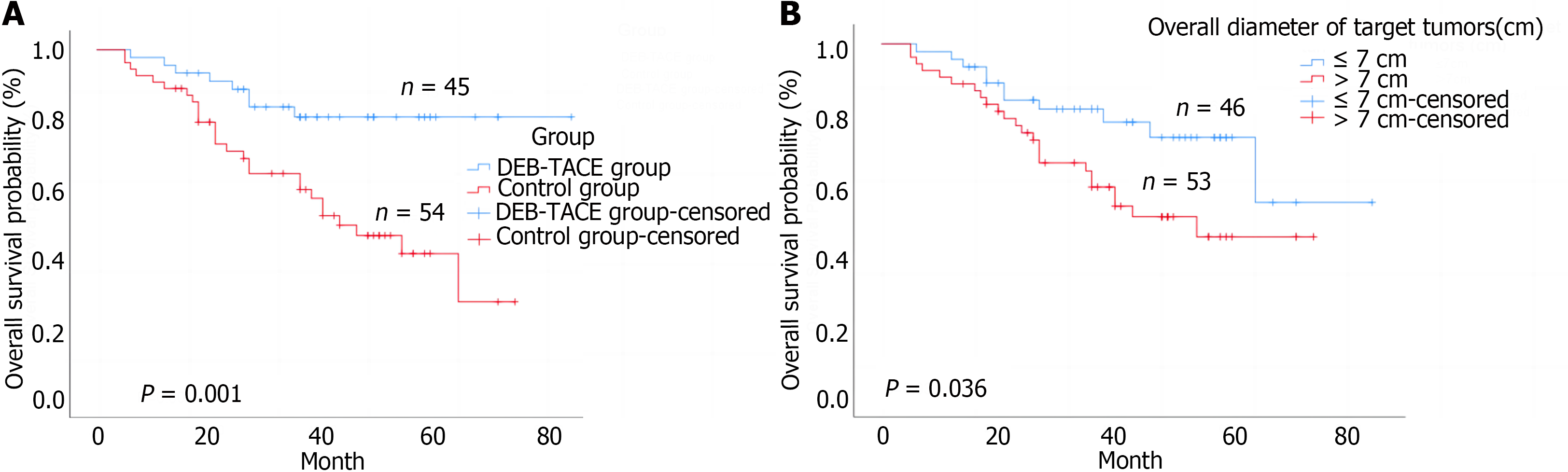
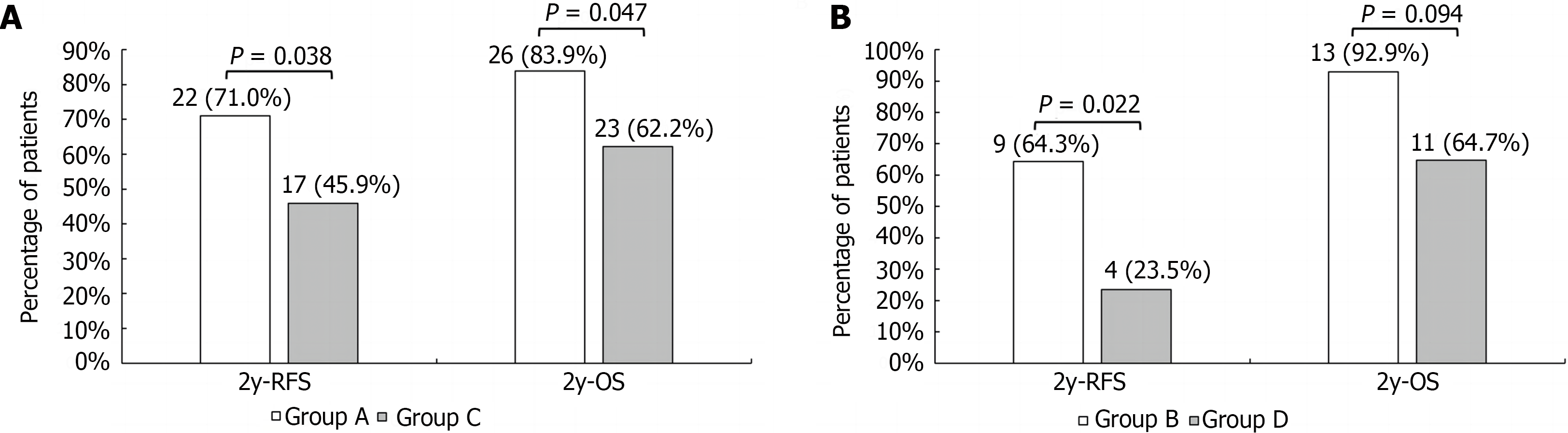
The median post-transplantation follow-up in the DEB-TACE group was 37.0 months, ranging from 27.0 months to 57.0 months, and in the control group was 36.0 months, ranging from 18.0 months to 49.2 months (P = 0.085 compared to the DEB-TACE group). The 2-year RFS and OS were significantly higher in the DEB-TACE group (Figure 3A) than in the control group (Figure 4A) (RFS: 68.9% vs 38.9%, P = 0.003; OS: 86.7% vs 63.0%, P = 0.008). Meanwhile, the overall diameter of target tumors ≤ 7 cm (Figure 3B), AFP level ≤ 400 ng/mL (Figure 3C), and lack of MAV (Figure 3D) were observed to be associated with better RFS (log-rank < 0.05). The overall diameter of target tumors (Figure 4B) ≤ 7 cm was observed to be associated with better OS (log-rank < 0.05). We preliminarily observed the prognosis of patients in the subgroups. Without segmental PVTT, the 2-year RFS rate after LT was 71.0% and 45.9% in groups A and C respectively (P = 0.038; Figure 5A). With segmental PVTT, the 2-year RFS rate after LT was 64.3% and 23.5% in groups B and D respectively (P = 0.022; Figure 5B).
Multivariate analysis indicated that pre-LT DEB-TACE [hazard ratio (HR) = 2.73, 95% confidence interval (CI): 1.44-55.14, P = 0.002], overall diameter of target tumors ≤ 7 cm (HR = 1.98, 95%CI: 1.05-3.75, P = 0.035) and AFP level ≤ 400 ng/mL (HR = 2.27, 95%CI: 1.23-4.21, P = 0.009) were independent factors of tumor recurrence. Further exploration of the independent factors for predicting OS showed that pre-LT DEB-TACE (HR = 3.15, 95%CI: 1.43-6.96, P = 0.004) was an independent factor (Table 4).
| Variable | Univariate analysis | Multivariate analysis | |||||
| Total, n | HR | 95%CI | P value | HR | 95%CI | P value | |
| RFS | |||||||
| Pre-LT DEB-TACE as yes/no | 45/54 | 2.62 | 1.41-4.89 | 0.002 | 2.73 | 1.44-5.14 | 0.002 |
| Child-Pugh classification | |||||||
| A | 83 | Reference | N/A | N/A | N/A | N/A | N/A |
| B | 12 | 2.03 | 0.98-4.23 | 0.056 | N/A | N/A | N/A |
| C | 4 | 1.52 | 0.36-6.35 | 0.559 | N/A | N/A | N/A |
| Macrovascular invasion as yes/no | 31/68 | 1.74 | 0.98-3.09 | 0.056 | N/A | N/A | N/A |
| Overall diameter of target (tumors in cm for ≤ 7 cm/> 7 cm) | 46/53 | 2.55 | 1.38-4.69 | 0.003 | 1.98 | 1.05-3.75 | 0.035 |
| AFP of ≤ 400 ng/mL/> 400 ng/mL) | 74/25 | 2.34 | 1.30-4.19 | 0.004 | 2.27 | 1.23-4.21 | 0.009 |
| Tumor differentiation | |||||||
| Well differentiated | 3 | Reference | N/A | N/A | N/A | N/A | N/A |
| Moderately differentiated | 71 | 1.42 | 0.19-10.45 | 0.726 | N/A | N/A | N/A |
| Undifferentiated | 25 | 2.27 | 0.30-17.13 | 0.426 | N/A | N/A | N/A |
| OS | |||||||
| Pre-LT DEB-TACE as yes/no | 45/54 | 3.33 | 1.51-7.34 | 0.003 | 3.15 | 1.43-6.96 | 0.004 |
| Child-Pugh classification | |||||||
| A | 83 | Reference | N/A | N/A | N/A | N/A | N/A |
| B | 12 | 2.46 | 1.06-5.68 | 0.035 | N/A | N/A | N/A |
| C | 4 | 1.81 | 0.42-7.66 | 0.41 | N/A | N/A | N/A |
| Macrovascular invasion as yes/no | 31/68 | 1.25 | 0.62-2.48 | 0.524 | N/A | N/A | N/A |
| Overall diameter of target (tumors in cm of ≤ 7 cm/> 7 cm) | 46/53 | 2.09 | 1.02-4.28 | 0.042 | N/A | N/A | N/A |
| AFP of ≤ 400 ng/mL/> 400 ng/mL | 74/25 | 1.68 | 0.83-3.39 | 0.145 | N/A | N/A | N/A |
| Tumor differentiation | |||||||
| Well differentiated | 3 | Reference | N/A | N/A | N/A | N/A | N/A |
| Moderately differentiated | 71 | N/A | NA1 | 0.922 | N/A | N/A | N/A |
| Undifferentiated | 25 | N/A | NA1 | 0.920 | N/A | N/A | N/A |
Liver resection is superior to non-surgical treatment for advanced HCC in terms of survival outcome, particularly in patients with segmental PVTT; therefore, it is recommended as the primary therapeutic option for patients with a well-functioning liver[18]. However, even PVTT is limited to the first-order branch. Shi et al[19] found that with surgical resection or palliative resection, the 3-year OS rate was less than 30%. In phase 3 trials of lenvatinib and sorafenib for systemic treatment of unresectable HCC, the median OS was 13.6 months and 12.3 months respectively[3,20]. For patients with PVTT but demonstrating satisfactory liver function, TACE is not a contraindication and is safe and effective in terms of efficacy, especially DEB-TACE as locoregional therapy, showing a non-inferior median OS of 12.3 months compared with systemic therapy[21,22].
In our study, the median total diameter of the target tumor in the DEB-TACE group was 6.9 cm, and 31.3% of patients had segmental PVTT. Nevertheless, DEB-TACE still showed an extremely high ORR, especially after neoadjuvant therapy when the median AFP decreased from 275.1 ng/mL to 41.7 ng/mL. The postoperative AEs primarily presented as post-embolization syndrome, and all patients experienced alleviation following the administration of symptomatic therapy, with no severe AEs observed. Liver function impairment was transient, and all patients experienced a full recovery following liver protective measures. Thus, DEB-TACE showed high efficacy and tolerability in patients with advanced HCC.
In 1996, Mazzaferro et al[4] established the Milan criteria as a standardized method for LT, resulting in 4-year OS and RFS rates of 83% and 75% respectively. In China, HC is not limited to tumor size and number but also introduces tumor differentiation and AFP as reference for tumor biological behavior, resulting in 3-year OS and RFS rates of 81.9% and 78.8% respectively. However, 30% of patients exceed the HC, and PVTT is observed in 44%-62.2% of HCC patients[5,23,24]. In the absence of effective treatment, the duration of PVTT progression from type I to II and type II to III is 8.2 d and 11.5 d respectively, with a median OS of only 2-4 months[1]. Hence, selecting an effective therapy to diminish the tumor burden and manage the advancement of PVTT before LT is of utmost significance.
Previous investigations have shown that HCC with PVTT is a risk factor for poor prognosis and a contraindication to LT[25,26]. However, some researchers believe that segmental PVTT does not inevitably cause immediate distant metastases, and patients with low AFP levels, well-differentiated tumors, and a strong response to neoadjuvant therapy can still benefit from LT[27]. Lee et al[7] found a 3-year RFS rate of 45.5% and 3-year OS rate of 63.6% after LT for HCC with PVTT. Further reports by Choi et al[6] showed that for segmental PVTT, the 3-year RFS and OS rates were 63.9% and 60.3%. In our study, both the 2-year RFS (68.9% vs 38.9%) and OS (86.7% vs 63.0%) rates were higher in the DEB-TACE group than in the control group. However, in the subgroups, especially in patients with PVTT, there were significant differences in RFS rate between group B (64.3%) and group D (23.5%). The 2-year OS rate of group B was still higher than that of group D, although there was no statistically significant difference between the two groups.
We observed an interesting result in the subgroups without PVTT. AFP and MVI can be used as references for tumor biological behavior and are predictors of recurrence and mortality, and patients’ AFP levels are remarkably higher with vascular invasion[28-30]. However, in our study, the median AFP level of group A was significantly higher than that of group C at admission, and patients who received DEB-TACE in group A were more likely to have poor tumor characteristics. The pathological results after LT showed that there was no significant difference in the degree of tumor differentiation between the two groups, but the degree of MVI in group A was lower than that of group C. Regarding whether this was related to the receipt of neoadjuvant therapy, we hypothesize that DEB-TACE can reverse the degree of MVI; however, this theory needs to be confirmed by further studies.
As indicated by multivariate analyses, DEB-TACE pre-LT, AFP level, and overall diameter of target tumors were independent factors for recurrence. More importantly, we found that the DEB-TACE pre-LT was an independent factor for OS. Therefore, we believe that DEB-TACE as neoadjuvant therapy can also improve the prognosis of patients after LT.
This study had some limitations. First, the sample size of the study was small, with patients only from China. Second, for advanced HCC, DEB-TACE as neoadjuvant therapy before LT was not combined with systemic therapy; such combination therapy could lead to better outcomes and thus needs further study.
In our study, DEB-TACE as neoadjuvant therapy before LT was tolerant and effective, revealing a good clinical outcome. Segmental PVTT should not be an absolute contraindication for patients who respond well to neoadjuvant therapy LT.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Filipec Kanizaj T, Croatia S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Khan AR, Wei X, Xu X. Portal Vein Tumor Thrombosis and Hepatocellular Carcinoma - The Changing Tides. J Hepatocell Carcinoma. 2021;8:1089-1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 3. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2593] [Article Influence: 864.3] [Reference Citation Analysis (59)] |
| 4. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5308] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 5. | Xu X, Lu D, Ling Q, Wei X, Wu J, Zhou L, Yan S, Wu L, Geng L, Ke Q, Gao F, Tu Z, Wang W, Zhang M, Shen Y, Xie H, Jiang W, Wang H, Zheng S. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut. 2016;65:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 6. | Choi HJ, Kim DG, Na GH, Hong TH, Bae SH, You YK, Choi JY, Yoon SK. The clinical outcomes of patients with portal vein tumor thrombi after living donor liver transplantation. Liver Transpl. 2017;23:1023-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Lee KW, Suh SW, Choi Y, Jeong J, Yi NJ, Kim H, Yoon KC, Hong SK, Kim HS, Lee KB, Suh KS. Macrovascular invasion is not an absolute contraindication for living donor liver transplantation. Liver Transpl. 2017;23:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10261] [Article Influence: 603.6] [Reference Citation Analysis (2)] |
| 9. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691-700.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 10. | Tabrizian P, Holzner ML, Mehta N, Halazun K, Agopian VG, Yao F, Busuttil RW, Roberts J, Emond JC, Samstein B, Brown RS Jr, Najjar M, Chapman WC, Doyle MM, Florman SS, Schwartz ME, Llovet JM. Ten-Year Outcomes of Liver Transplant and Downstaging for Hepatocellular Carcinoma. JAMA Surg. 2022;157:779-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 11. | Benkö T, König J, Theysohn JM, Schotten C, Saner FH, Treckmann J, Radunz S. Bridging treatment prior to liver transplantation for hepatocellular carcinoma: radioembolization or transarterial chemoembolization? Eur J Med Res. 2022;27:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 12. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6048] [Article Influence: 864.0] [Reference Citation Analysis (3)] |
| 13. | Nicolini D, Svegliati-Baroni G, Candelari R, Mincarelli C, Mandolesi A, Bearzi I, Mocchegiani F, Vecchi A, Montalti R, Benedetti A, Risaliti A, Vivarelli M. Doxorubicin-eluting bead vs conventional transcatheter arterial chemoembolization for hepatocellular carcinoma before liver transplantation. World J Gastroenterol. 2013;19:5622-5632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM, Bruix J. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 719] [Article Influence: 39.9] [Reference Citation Analysis (1)] |
| 15. | Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 16. | Zhou TY, Chen SQ, Wang HL, Weng SM, Zhou GH, Zhang YL, Nie CH, Zhu TY, Wang BQ, Yu ZN, Jing L, Chen F, Sun JH. Safety and efficacy of drug-eluting bead transarterial chemoembolization with CalliSpheres® microsphere for hepatocellular carcinoma with portal vein tumor thrombus: a preliminary study. J Cancer. 2021;12:4522-4529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3301] [Article Influence: 220.1] [Reference Citation Analysis (36)] |
| 18. | Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, Kudo M, Ku Y, Sakamoto M, Nakashima O, Kaneko S, Kokudo N; Liver Cancer Study Group of Japan. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 365] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 19. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011;18:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 20. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3818] [Article Influence: 545.4] [Reference Citation Analysis (1)] |
| 21. | Patidar Y; Basavaraj 1, Mukund A, Sarin SK. Transarterial Chemoembolization in Unresectable Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis: A Tertiary Care Center Experience. Indian J Radiol Imaging. 2021;31:270-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Silva JP, Berger NG, Tsai S, Christians KK, Clarke CN, Mogal H, White S, Rilling W, Gamblin TC. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford). 2017;19:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 23. | Lei J, Yan L. Outcome comparisons among the Hangzhou, Chengdu, and UCSF criteria for hepatocellular carcinoma liver transplantation after successful downstaging therapies. J Gastrointest Surg. 2013;17:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Cheng S, Chen M, Cai J, Sun J, Guo R, Bi X, Lau WY, Wu M. Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus (2018 Edition). Liver Cancer. 2020;9:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 25. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 26. | Xu X, Zheng SS, Liang TB, Wang WL, Jin J, Shen Y, Wu J, Yu J. Orthotopic liver transplantation for patients with hepatocellular carcinoma complicated by portal vein tumor thrombi. Hepatobiliary Pancreat Dis Int. 2004;3:341-344. [PubMed] |
| 27. | Soin AS, Bhangui P, Kataria T, Baijal SS, Piplani T, Gautam D, Choudhary NS, Thiagarajan S, Rastogi A, Saraf N, Saigal S. Experience With LDLT in Patients With Hepatocellular Carcinoma and Portal Vein Tumor Thrombosis Postdownstaging. Transplantation. 2020;104:2334-2345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Liu C, Xiao GQ, Yan LN, Li B, Jiang L, Wen TF, Wang WT, Xu MQ, Yang JY. Value of α-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World J Gastroenterol. 2013;19:1811-1819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Du M, Chen L, Zhao J, Tian F, Zeng H, Tan Y, Sun H, Zhou J, Ji Y. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. 2014;14:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1572] [Article Influence: 92.5] [Reference Citation Analysis (1)] |