Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2463
Peer-review started: January 23, 2024
First decision: January 30, 2024
Revised: February 12, 2024
Accepted: April 1, 2024
Article in press: April 1, 2024
Published online: June 15, 2024
Processing time: 144 Days and 0.8 Hours
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide. Serum biomarkers play an important role in the early diagnosis and prognosis of HCC. Because a certain percentage of HCC patients are negative for alpha-fetoprotein (AFP), the diagnosis of AFP-negative HCC is essential to improve the detection rate of HCC.
To establish an effective model for diagnosing AFP-negative HCC based on serum tumour biomarkers.
A total of 180 HCC patients were enrolled in this study. The expression levels of GP73, des-γ-carboxyprothrombin (DCP), CK18-M65, and CK18-M30 were detected by a fully automated chemiluminescence analyser. The variables were selected by logistic regression analysis. Several models were constructed using stepwise backward logistic regression. The performance of the models was compared using the C statistic, integrated discrimination improvement, net reclassification improvement, and calibration curves. The clinical utility of the nomogram was assessed using decision curve analysis (DCA).
The results showed that the expression levels of GP73, DCP, CK18-M65, and CK18-M30 were significantly greater in AFP-negative HCC patients than in healthy controls (P < 0.001). Multivariate logistic regression analysis revealed that GP73, DCP, and CK18-M65 were independent factors for diagnosing AFP-negative HCC. By comparing the diagnostic performance of multiple models, we included GP73 and CK18-M65 as the model variables, and the model had good discrimination ability (area under the curve = 0.946) and good goodness of fit. The DCA curves indicated the good clinical utility of the nomogram.
Our study identified GP73 and CK18-M65 as serum biomarkers with certain application value in the diagnosis of AFP-negative HCC. The diagnostic nomogram based on CK18-M65 combined with GP73 demonstrated good performance and effectively identified high-risk groups of patients with HCC.
Core Tip: The primary objective of this study was to develop a diagnostic model that can effectively identify patients with alpha-fetoprotein (AFP)-negative hepatocellular carcinoma (HCC) using biomarkers. While previous research has demonstrated the usefulness of combining serological markers for diagnosing HCC, there have been limited investigations on diagnostic models specifically for AFP-negative HCC. Clinicians currently face the challenge of identifying individuals at high risk for early HCC, especially when patients exhibit normal levels of AFP. Early detection plays a crucial role in enabling timely surgical interventions, improving treatment outcomes, and ultimately enhancing the chances of survival for patients with HCC.
- Citation: He L, Zhang C, Liu LL, Huang LP, Lu WJ, Zhang YY, Zou DY, Wang YF, Zhang Q, Yang XL. Development of a diagnostic nomogram for alpha-fetoprotein-negative hepatocellular carcinoma based on serological biomarkers. World J Gastrointest Oncol 2024; 16(6): 2463-2475
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2463.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2463
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide[1]. In the early stages, HCC typically presents without apparent symptoms, leading to a majority of patients being diagnosed at middle or advanced stages. Therefore, early diagnosis is crucial for timely treatment and improved survival rates. Although current ult
Des-γ-carboxyprothrombin (DCP), a marker with high diagnostic value in HCC, was first discovered by Liebman et al[5] in 1984. DCP is associated with vitamin K deficiency. Poté et al[6] showed that DCP could be useful for the diagnosis of early HCC, and it may be a significant diagnostic biomarker for AFP-negative HCC[7,8]. However, there is still some controversy regarding the diagnostic accuracy of DCP due to its elevation in other gastrointestinal tumours. Especially in the early diagnosis of HCC, its sensitivity and specificity are still inadequate. To increase the detection rate of HCC, combining DCP with other biomarkers is recommended.
In recent years, several serum biomarkers, such as GPC3, GP73, CK18, and CK19, have been found to have diagnostic value for HCC. Among these biomarkers, GP73 is an important biomarker for the diagnosis of HCC; it is a protein located on the surface of the Golgi apparatus. GP73 was first discovered in 2000 when Kladney et al[9] studied adult giant cell hepatitis. GP73 has been demonstrated to be closely associated with liver diseases such as hepatitis and HCC. Serum GP73 levels are more sensitive in the early stages of HCC[10], and some studies have shown that GP73 could serve as a diagnostic biomarker for AFP-negative HCC[11,12]. However, GP73 elevation has also been observed in certain other types of malignant cancers[13]. Thus, relying solely on the levels of GP73 might not be sufficient to accurately diagnose HCC. Additional diagnostic methods and markers may be needed to improve the specificity and accuracy of HCC dia
The goal of this study was to construct an effective diagnostic model based on biomarkers for AFP-negative HCC. Related studies of HCC diagnostic models have shown that the combined application of serological markers, such as GALAD and GAAP, has high application value[22,23]. The aMAP score is a newly recommended effective risk stratification tool for predicting the occurrence of HCC, especially in noncirrhotic patients[24]. However, few studies have investigated diagnostic models for AFP-negative HCC. The current challenge for clinicians is finding an effective way to screen individuals at high risk for HCC, which becomes particularly difficult in cases where patients have normal levels of AFP. Through early detection, clinicians can offer timely surgical interventions, enhance treatment outcomes, and ultimately improve the survival rates of HCC patients.
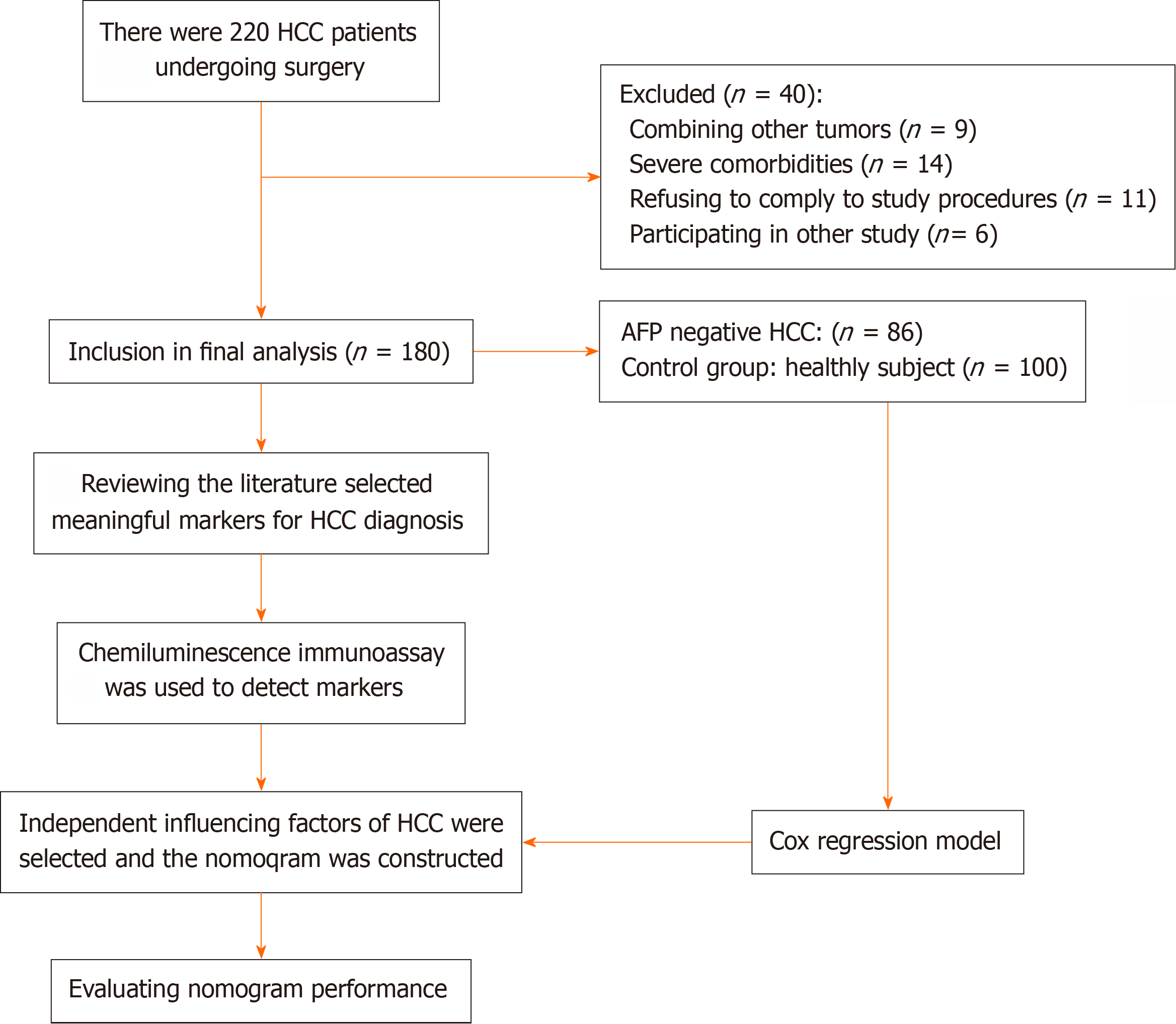
In this study, we recruited a total of 280 individuals from the Third Medical Center of PLA General Hospital between January 2016 and December 2019. We included 180 HCC patients who underwent surgical resection. The age ranged from 30 to 76 years (median age of 52), with 165 males and 15 females. Among the HCC patients, 86 had AFP-negative HCC, which was defined as AFP ≤ 20 ng/mL. In addition, we recruited 100 healthy subjects aged 30 to 68 years (median age of 50 years). The HCC patients were confirmed by radiographic and pathological findings. Other general inclusion criteria included an age of 18 years or older, the ability to comprehend and sign an informed consent form, and the absence of tumour metastasis. Certain exclusion criteria were applied to specific groups. Pregnant or lactating women were exclu
Clinical laboratory test results, including aspartate transaminase, alanine transaminase, alkaline phosphatase, and gamma-glutamyl transferase levels, were collected for the patients.
This study was conducted in accordance with the Declaration of Helsinki and approved by our hospital’s Ethics Committee. Written informed consent was provided by all patients.
In the experiment, all patients were required to fast, and 5 mL peripheral venous blood samples were collected. The blood samples were centrifuged at 3000 r/min for 5 min and immediately frozen at -80 °C until the day of testing. To detect the serum AFP, GP73, DCP, CK18-M65, and CK18-M30 levels, a fully automated chemiluminescence analyser was utilized (C2000). To carry out the analysis, specialized kits designed for the detection of tumour biomarkers were utilized. These kits contain precise reagents and components that interact with the target biomarkers in the serum. The C2000 analyser and kit were obtained from Beijing Hotgen Biotech Co., Ltd.
Univariate analysis and multivariate logistic regression analysis were performed separately to identify independent diagnostic factors. We then constructed multiple models using stepwise backward logistic regression, excluding one variable at a time ranked according to the akaike information criterion (AIC)[25]. To further evaluate the importance of each indicator in the model, random forest importance plots were generated using the “RandomForest” package. Based on the results, a nomogram was constructed using the “regplot package” in R. Additionally, the computational formula for the model was obtained using the “rms package” and the “nomogramEx package” in R. This formula provided a mathematical expression that incorporated the various independent diagnostic factors and their weights, allowing for the calculation of a diagnostic probability for each individual. Overall, this comprehensive approach provided a robust and reliable model for diagnosing the specific condition of interest.
Integrated discrimination improvement: The integrated discrimination improvement (IDI) is a statistical measure used to assess the improvement in predictive accuracy or discrimination of a model when adding new variables or changing the prediction threshold. The IDI measures the difference in the average predicted probabilities between cases and noncases for a given model compared to a reference or baseline model. A positive IDI indicates improved discrimination in the model, meaning that it better distinguishes between individuals with and without the outcome. A negative IDI suggests a decrease in discrimination. The IDI can range from negative infinity to positive infinity, with zero indicating no improvement in discrimination and higher positive values indicating greater improvement.
Net reclassification improvement: The net reclassification improvement (NRI) is a statistical measure used to evaluate the performance improvement of a predictive model when adding new variables or changing the prediction threshold; it quantifies the improvement in correctly predicting the risk of an outcome by comparing the reclassification of individuals into different risk categories using two models: a baseline model and an extended model. The NRI is calculated by estimating the proportions of correctly reclassified individuals across various risk categories. It considers both instances where individuals are correctly reclassified into a higher-risk category and when they are correctly reclassified into a lower-risk category. A positive NRI indicates that the new model improves the classification accuracy compared to the baseline model, while a negative NRI suggests a decrease in accuracy. The NRI ranges from -2 to 2, with 0 indicating no improvement in prediction and values closer to 2 indicating a substantial improvement; it provides a measure of the net improvement achieved by such modifications in correctly classifying individuals into their appropriate risk categories. Risk thresholds for the outcome were defined as less than 20%, 20% to less than 40%, and greater than 40%. Reclassification was also compared using the continuous NRI, which assesses reclassification across a continuous range of risk thresholds.
Calibration curve: The calibration curve is a visual tool used to evaluate the consistency of predictive models. The model compares the predicted values with the actual values to display the accuracy and bias of the model at different prediction ranges. Ideally, the calibration curve should overlap with a perfect diagonal line (y = x), indicating perfect agreement between the model predictions and actual observations. In the calibration curve, the mean absolute error is the average of the prediction errors, indicating the average deviation between the model prediction results and the actual observations. The mean squared error (MSE) is the average of the squared value of the prediction error, which measures the degree of dispersion between the model prediction and the actual observations. The 0.9 quantile of the absolute error represents the 0.9 quantile of the prediction error, where the top 90% of the errors are less than that value. These evaluation metrics reflect the model's prediction performance.
Descriptive statistics are reported as frequencies and proportions for categorical variables and medians (IQRs) or means (SDs) for continuous variables. The continuous variables were compared using the Mann-Whitney U test, and the cate
A total of 180 HCC participants were selected for the study (Figure 1), and an additional 100 healthy individuals were selected as the control group. The baseline characteristics of the patients with HCC and healthy subjects are shown in Table 1. The results showed that the demographic characteristics of the two groups were not significantly different, and the data were comparable.
| HCC (n = 180) | Healthy (n = 100) | F/χ2/Z value | P value | |
| Demographics | ||||
| Age, yr, mean (SD), gender | 52.46 (9.18) | 49.79 (8.26) | 2.318 | 0.129 |
| Male | 165 (91.7) | 85 (85) | 2.987 | 0.084 |
| Female | 15 (8.3) | 15 (15) | ||
| LFTs | ||||
| AST (U/L), median (IQR) | 47 (33, 76.75) | 20 (16, 25) | -11.534 | < 0.001 |
| ALT (U/L), median (IQR) | 38 (26, 59.75) | 21 (18, 25.75) | -9.764 | < 0.001 |
| GGT (U/L), median (IQR) | 86 (45, 147) | 26 (20, 36) | -9.744 | < 0.001 |
| ALP (U/L), median (IQR) | 100.5 (67.25, 149) | 62 (53, 78) | -7.354 | < 0.001 |
| HCC biomarkers | ||||
| AFP (U/L) median (IQR) | 25 (7.49, 572.6) | 4 (3, 5) | -11.269 | < 0.001 |
| GP73 (ng/mL) median (IQR) | 147.5 (111, 217.5) | 54 (45, 73.75) | -11.775 | < 0.001 |
| DCP (ng/mL) median (IQR) | 10 (7, 513) | 6 (2.25, 8) | -8.172 | < 0.001 |
| CK18-M65 (U/L) median (IQR) | 802 (469.5, 1769) | 119 (94, 194.5) | -12.536 | < 0.001 |
| CK18-M30 (U/L) median (IQR) | 407.5 (219, 890.75) | 99 (80, 144) | -10.572 | < 0.001 |
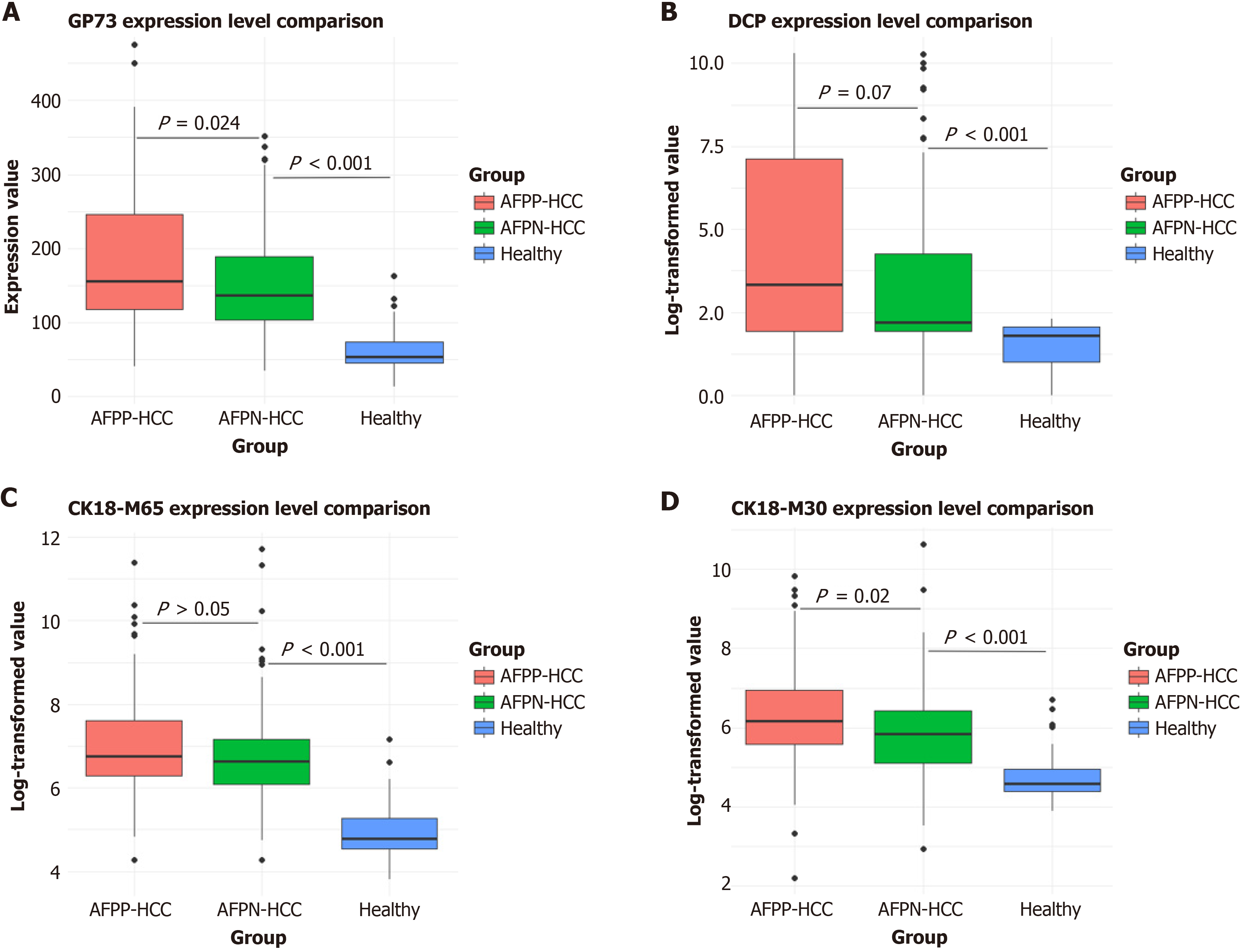
The expression levels of GP73, DCP, CK18-M65, and CK18-M30 were greater in the AFP-positive and AFP-negative HCC groups than in the healthy control group (P < 0.05) (Figure 2). Moreover, the percentage of patients with AFP-negative HCC was greater, and the percentage of patients with AFP-positive HCC was lower in the healthy group. The biomarker with the highest positive rate in AFP-negative HCC patients was CK18-M65 (87.2%), followed by GP73 (75.6%). The biomarker with the lowest positive rate was DCP (72.1%) (Table 2).
| HCC (n = 180) | AFP (-) (n = 86) | Healthy (n = 100) | P value | |
| GP73 | < 0.001 | |||
| Positive rate | 80 (144/180) | 75.6 (65/86) | 7 (7/100) | |
| Negative rate | 20 (36/180) | 24.4 (21/86) | 93 (93/100) | |
| DCP | < 0.001 | |||
| Positive rate | 70 (126/180) | 72.1 (62/86) | 29 (29/100) | |
| Negative rate | 30 (54/180) | 21.4 (24/86) | 71 (71/100) | |
| CK18-M65 | < 0.001 | |||
| Positive rate | 87.8 (158/180) | 87.2 (75/86) | 6 (6/100) | |
| Negative rate | 12.2 (22/180) | 12.8 (11/86) | 94 (94/100) | |
| CK18-M30 | < 0.001 | |||
| Positive rate | 79.4 (143/180) | 73.3 (63/86) | 12 (12/100) | |
| Negative rate | 20.6 (37/180) | 26.7 (23/86) | 88 (88/100) | |
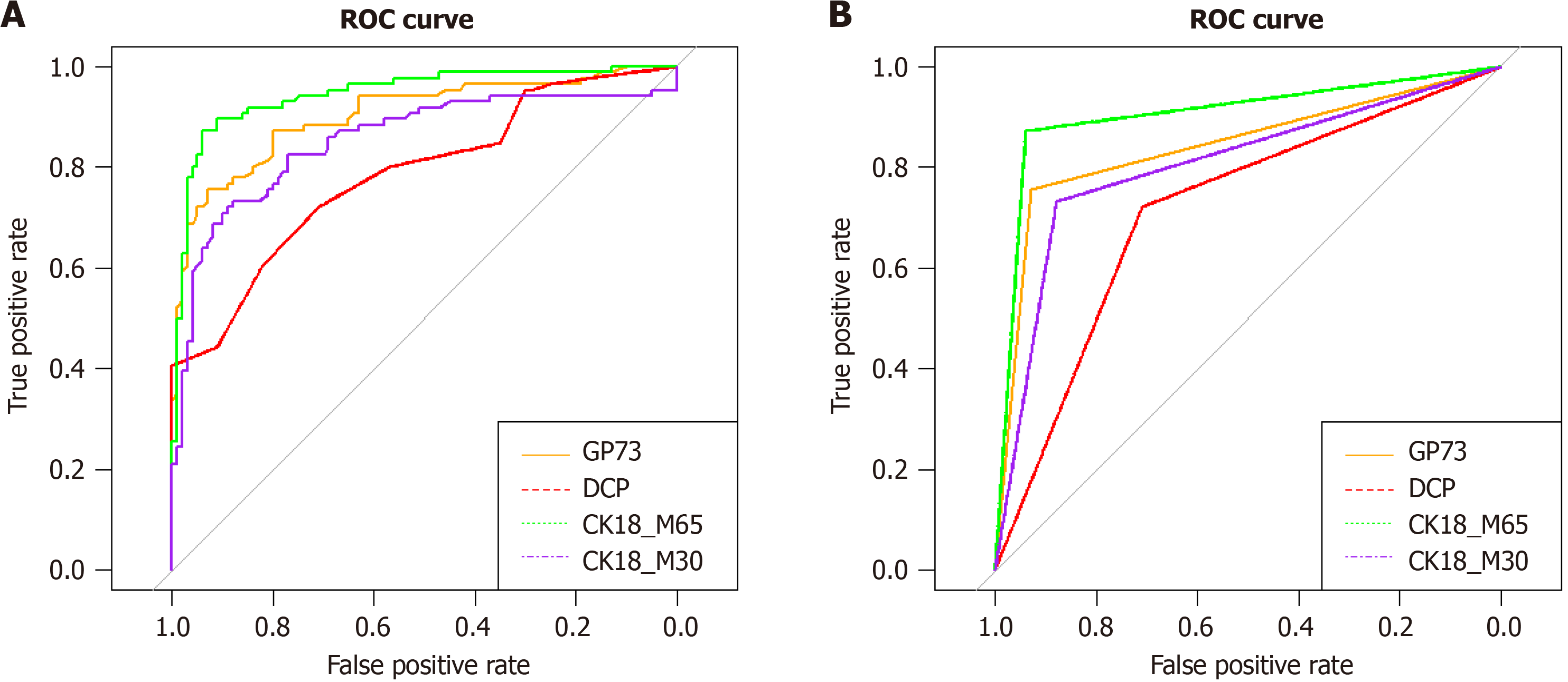
The optimal cut-off value for GP73 for diagnosing AFP-negative HCC was 103 ng/mL. With the optimal cut-off value, the area under the curve (AUC) of GP73 was 0.843, the sensitivity was 75.6%, and the specificity was 93%. The optimal cut-off value for DCP for diagnosing AFP-negative HCC patients was 7 ng/mL. With the optimal cut-off value, the AUC of DCP was 0.715, the sensitivity was 72%, and the specificity was 71%. The optimal cut-off value for CK18-M65 for diagnosing AFP-negative HCC was 329 U/L. With the optimal cut-off value, the AUC of CK18-M65 was 0.9, the sensitivity was 87.2%, and the specificity was 94%. The optimal cut-off value for CK18-M30 for diagnosing AFP-negative HCC was 183 U/L. With the optimal cut-off value, the AUC of CK18-M30 was 0.806, the sensitivity was 73.3%, and the specificity was 88% (Figure 3).
According to the multivariate analysis for the diagnosis of HCC, AFP > 6 ng/mL (OR: 19.99, 95%CI: 5.97-66.86, P < 0.001), GP73 > 103 ng/mL (OR: 16.847, 95% CI: 4.74-59.8, P < 0.001), DCP > 7 ng/mL (OR: 4.154, 95%CI: 1.37-12.59, P = 0.012), and CK18-M65 > 329 U/L (OR: 31.113, 95%CI: 9.25-104.6, P < 0.001) were identified as important factors influencing the diagnosis of HCC (Supplementary Table 1). The AUC of AFP combined with GP73, DCP, and CK18-M65 for the diagnosis of HCC was 0.978 (Supplementary Figure 1A). The results of multivariate analysis for the diagnosis of AFP-negative HCC were as follows: GP73 > 103 ng/mL (OR: 14.13, 95%CI: 4.1-48.8, P < 0.001), CK18-M65 > 329 U/L (OR: 44.14, 95%CI: 13.76-141.56, P < 0.001) and DCP > 7 ng/mL (OR: 3.5, 95%CI: 1.1.15-10.9, P = 0. 028) were identified as independent factors influencing the diagnosis of AFP-negative HCC (Table 3). The AUC of the combination of GP73, DCP and CK18-M65 for the diagnosis of AFP-negative HCC was 0.951 (Supplementary Figure 1B).
| Variable | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| GP73 > 103 ng/mL | 41.122 (16.5, 102.3) | < 0.001 | 14.13 (4.1, 48.8) | < 0.001 |
| DCP > 8 ng/mL | 6.325 (3.34, 12) | < 0.001 | 3.5 (1.15, 10.9) | 0.028 |
| CK18-M65 > 286 U/L | 106.818 (37.76, 302.21) | < 0.001 | 44.141 (13.76, 141.56) | < 0.001 |
| CK18-M30 > 183U/L | 20.08 (9.31, 43.35) | < 0.001 | - | - |
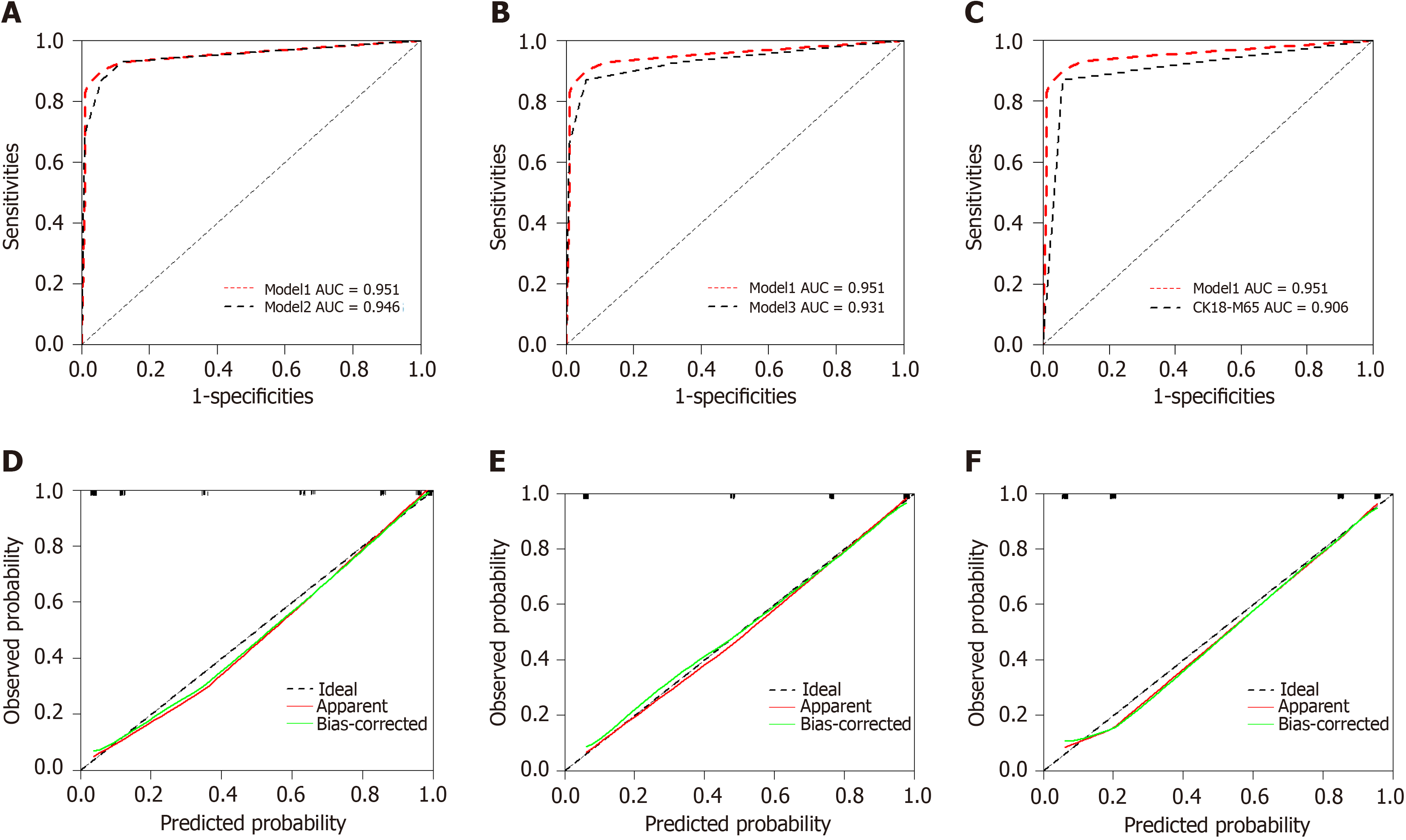
Three variables were associated with a greater risk of progression to AFP-negative HCC: GP73, DCP, and CK18-M65. In contrast to Model 3 (CK18-M65, DCP) and CK18-M65 alone, Model 1 (CK18-M65, GP73, DCP) and Model 2 (CK18-M65, GP73) had higher C statistics and lower AIC values, and the risk classification ability and identification ability significantly improved. However, compared with those of Model 1 and Model 2, there were no marked differences in the C statistics or AIC values, and the classification ability and identification ability did not greatly improve (Table 4). Model 2 had a smaller MSE and 0.9 quantile of absolute error than did Model 1 (MSE: 0.00039; 0.9 quantile of absolute error: 0.025), which showed that Model 2 had a good fit (Figure 4).
| Model performance measures | CK18-M65 | |||
| Model 1 | Model 2 | Model 3 | ||
| CK18-M65, GP73, DCP | CK18-M65, GP73 | CK18-M65, DCP | ||
| AIC | 41 | 44 | 65 | 70.5 |
| Mean absolute error | 0.016 | 0.018 | 0.03 | 0.022 |
| Mean squared error | 0.00048 | 0.00039 | 0.00125 | 0.00049 |
| 0.9 Quantile of absolute error | 0.031 | 0.025 | 0.047 | 0.023 |
| C statistic | 0.951 (0.916-0.986) | 0.946 (0.913-0.979) | 0.931 (0.89-0.97) | 0.906 (0.864-0.948) |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| IDI (95%CI), % | 1 (Reference) | 0.0036 (-0.0239-0.031) | 0.186 (0.1223-0.2496) | 0.0721 (0.0363-0.1079) |
| P value | 0.79 | < 0.001 | < 0.001 | |
| NRI (95%CI), % | ||||
| Categorical | 1 (Reference) | 0.04 (-0.0101-0.0901) | 0.287 (0.1484 - 0.4246) | -0.0019 (-0.0698-0.0661) |
| P value | 0.12 | < 0.001 | 0.96 | |
| Continuous | 1 (Reference) | -0.2326 (-0.5166-0.0515) | 0.866 (0.6135 - 1.1186) | 0.8781 (0.6227-1.1336) |
| P value | 0.11 | < 0.001 | < 0.001 | |
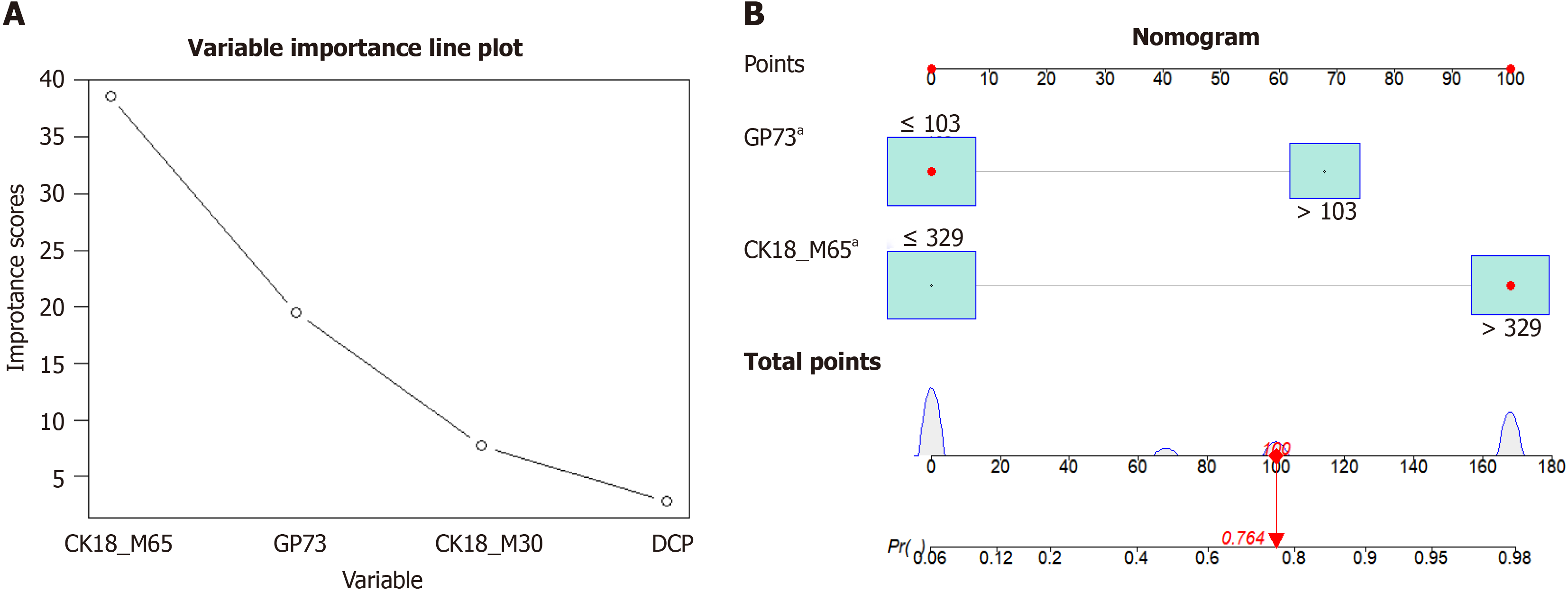
Using the random forest method to compare the importance of various indicators in the model, the importance scores of CK18-M65, GP73, and DCP were 38.61, 19.46, and 2.8, respectively (Figure 5A). According to the above analysis results, the risk factors GP73 and CK18-M65 were used to establish a nomogram for the diagnosis of AFP-negative HCC (Figure 5B). According to the nomogram, the risk score calculation equation for this model was as follows: Risk score = -7.88e-07 × points3 + 0.000165364 × points2 + -0.001956247 × points + 0.097551784). A GP73 concentration ≥ 103 ng/mL was assigned a score of 68 points. Similarly, a CK18-M65 concentration ≥ 329 ng/mL was assigned 100 points, indicating the chance of disease according to the nomogram or formula (Supplementary Table 2).
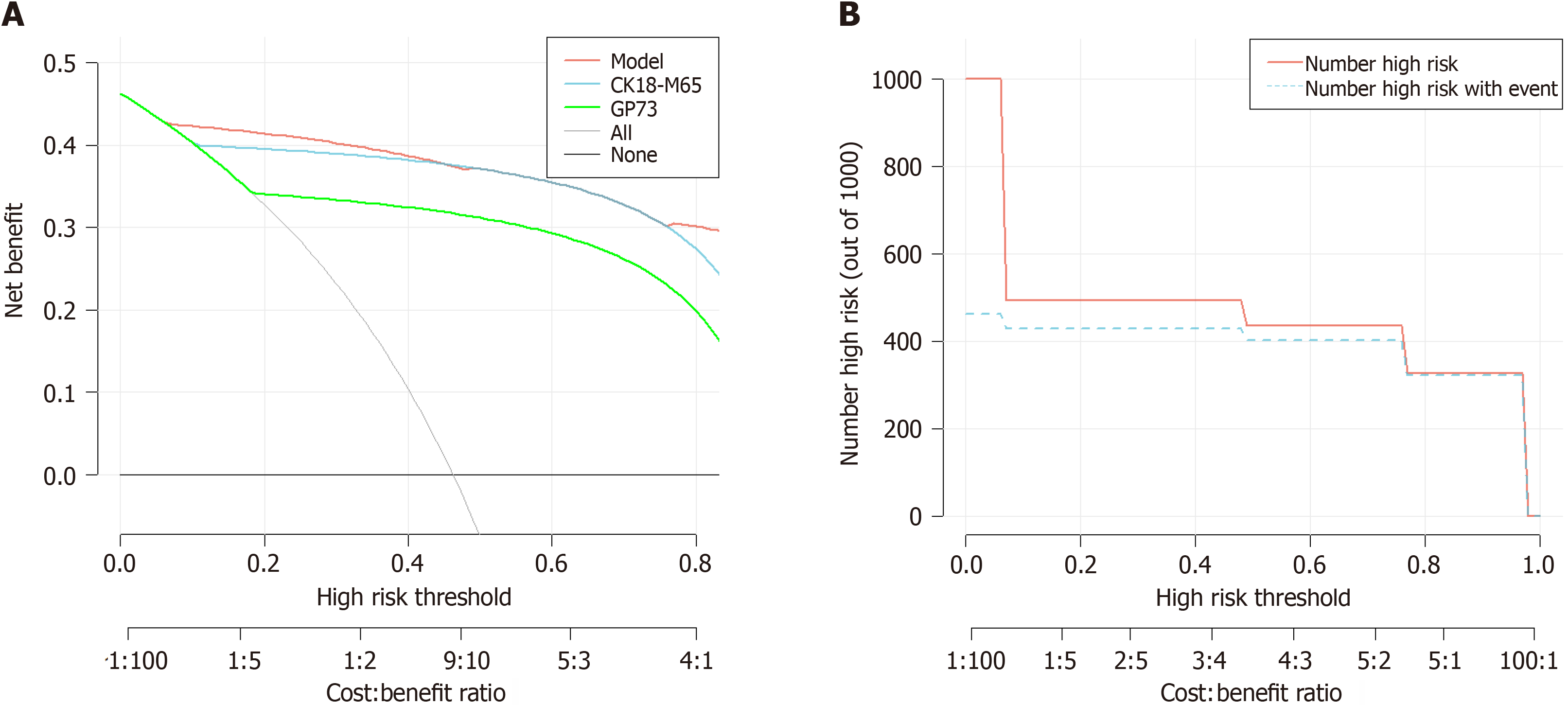
According to the ROC curve results, the AUC of the diagnosis of AFP-negative HCC was 0.946 in healthy subjects (95%CI: 0.913-0.979, sensitivity: 87.2%, specificity: 94%) (Figure 3A). Additionally, the DCA curve results showed that the nomogram has good practicability and is better than applying either CK18-M65 or GP73 alone (Figure 6A). The clinical impact curve indicated that the model has high clinical application value (Figure 6B).
Due to the high mortality rate of HCC, investigators are constantly exploring new treatments. The treatment options for HCC are generally divided into curative treatment and noncurative treatment. Curative treatment includes liver transplantation, resection surgery, or ablation/segmental transarterial radioembolization. Noncurative treatment includes transarterial chemoembolization and systemic treatment. When feasible, transplantation is the most definitive treatment option, but one limitation of transplantation is the shortage of available organs, which restricts the number of patients[26]. According to the Chinese guidelines, a more aggressive multimodal therapy approach has been proposed; this involves combining local therapies with immunotherapy-based systemic therapy[24]. The goal of adding systemic therapies to traditional treatment regimens is to determine whether this combination can further improve overall survival rates. Overall, the treatment landscape for HCC is constantly evolving, with ongoing research investigating the potential benefits of various treatment combinations and approaches. The aim is to provide patients with the best possible outco
While constantly exploring new treatments, we also aim to continuously pursue high standards of diagnosis. Studies have consistently shown that early diagnosis of HCC through surveillance methods leads to significantly better outcomes than relying on symptoms for diagnosis. When HCC is detected early through surveillance, the pooled 3-year survival rate is found to be as high as 50.8%. However, relying on symptoms for diagnosis leads to a significantly lower 3-year survival rate of only 27.9%. This stark contrast highlights the importance of implementing effective surveillance strategies to detect HCC at an early stage[27]. The value of serum markers in the diagnosis of HCC was again mentioned. AFP is a nonspecific marker of HCC[28]. Studies have reported that AFP expression in the human liver impacts programmed cell death, promoting carcinogenesis. AFP inhibits autophagy via activation of the PI3K/AKT/mTOR pathway, and upregulation of the autophagy-related protein mTOR causes aberrant malignant cell behaviour[29]. AFP stimulates cell proliferation mainly through activation of AKT signalling and activation of downstream mTOR signalling; it also activates metastasis by promoting the components required for the activation of the epithelial-mesenchymal transition. Addi
In this study, we confirmed that GP73, DCP, CK18-M65, and CK18-M30 have a certain degree of sensitivity and specificity in the diagnosis of AFP-negative HCC and could aid in the accurate diagnosis of early HCC. The diagnostic value of CK18-M65 was particularly prominent, followed by that of GP73, and that of DCP was the worst. Moreover, we constructed a diagnostic nomogram for AFP-negative HCC based on CK18-M65 and GP73 by comprehensive analysis. This simple nomogram, as evidenced by an AUC of 0.946, sensitivity of 87.2%, and specificity of 94%, has a high diag
Our study showed for the first time that CK18-M65 could serve as an independent influencing factor for AFP-negative HCC diagnosis, demonstrating its potential as a serum biomarker for AFP-negative HCC. CK18-M65 has been considered indicative of hepatocyte necrosis and has been extensively studied in fatty liver disease, in connection with the progr
In our study, the AUC of GP73 for diagnosing AFP-negative HCC was 0.843, indicating its good discriminative ability. The sensitivity and specificity were 75.6% and 93%, respectively. A review reported that serum GP73 is a marker with high sensitivity for HCC and a supplementary marker for identifying AFP-negative HCC[35]. A recent meta-analysis performed a systematic evaluation of 36 studies, and the results demonstrated the high value of GP73 for the diagnosis of HCC[36]. Another meta-analysis included 11 blood markers and revealed that the sum of the AUC and the sensitivity and specificity of AFP and other biomarkers were significantly greater than those of AFP, indicating that the total and specific sensitivity and AUC of GP73 were the highest[37]. Xu et al[11] reported that the AUC of GP73 for distinguishing between AFP-negative HCC patients and controls was 0.781. Our study confirmed this viewpoint. Additionally, several studies have shown that DCP is more effective than GP73[38,39]. We found different results in our study. Relying on DCP for distinguishing AFP-negative HCC patients from healthy individuals did not yield results as satisfactory as utilizing GP73.
Through multivariate analysis, CK18-M65 > 329 U/L (OR = 44.141, P < 0.001) and GP73 > 103 ng/mL (OR = 14.13, P < 0.001) were identified as independent risk factors for diagnosing AFP-negative HCC. A recently published study on models for AFP-negative HCC considered clinical and serum parameters, and the AUC of the model for diagnosing AFP-negative HCC was 0.838[40]. In comparison, our model achieved higher predictive accuracy (AUC: 0.946) using simpler biomarkers. Although we included only healthy individuals in the control group, the nomogram has important clinical significance in assisting with the diagnosis and early screening of AFP-negative HCC in individuals who have not developed hepatitis or liver cirrhosis. With the nomogram, clinicians can calculate the corresponding risk score for diagnosing HCC based on simple indicators. We recommend that CK18-M65 and GP73 be included in the physical examination. In the population with normal AFP expression, the risk of developing HCC was as high as 0.979 when CK18-M65 was > 329 U/L and GP73 was > 103 ng/mL. These individuals are considered high-risk patients and should be given high priority for further examination. When only CK18-M65 > 329 U/L is present, the risk rate is 0.767, and patients should undergo regular follow-up. The high accuracy of the nomogram helps in accurately identifying high-risk individuals from AFP-negative individuals. Furthermore, the nomogram is valuable for the follow-up of HCC patients. These findings can help doctors better understand patients' risk status and enable them to develop targeted treatment plans based on this information, thereby improving treatment outcomes.
Our study has several limitations. First, before the nomogram can be effectively applied in clinical practice, further validation studies using multicentre large samples and prospective cohorts are crucial. Additionally, scalability and sustainability must be considered during the validation process to expand the model's applicability. This is particularly important for enabling broader adoption and ensuring the continued effectiveness of the diagnostic model.
In this study, for the diagnosis of AFP-negative HCC, we constructed a nomogram based on diagnostic serum biomarkers of HCC. Through comprehensive analysis of GP73, DCP, CK18-M65, and CK18-M30, we constructed the final model, namely, CK18-M65 combined with GP73. The nomogram can be used to effectively screen for early-stage HCC in healthy people and for early treatment, ultimately improving the long-term survival rate. In the future, large-scale noninvasive biomarkers will be one of the most promising areas in biomarker research. We need to discover more effective biomarkers capable of detecting AFP-negative HCC patients and build a better diagnostic model. This will enhance the diagnostic accuracy and reliability for early-stage HCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tzeng IS, Taiwan S-Editor: Qu XL L-Editor: A P-Editor: Zhao YQ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Tanaka H. Current role of ultrasound in the diagnosis of hepatocellular carcinoma. J Med Ultrason (2001). 2020;47:239-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Johnson PJ. Role of alpha-fetoprotein in the diagnosis and management of hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14 Suppl:S32-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Hanif H, Ali MJ, Susheela AT, Khan IW, Luna-Cuadros MA, Khan MM, Lau DT. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J Gastroenterol. 2022;28:216-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 136] [Article Influence: 45.3] [Reference Citation Analysis (11)] |
| 5. | Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 420] [Article Influence: 10.2] [Reference Citation Analysis (16)] |
| 6. | Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, Bedossa P, Paradis V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (4)] |
| 7. | Seo SI, Kim HS, Kim WJ, Shin WG, Kim DJ, Kim KH, Jang MK, Lee JH, Kim JS, Kim HY, Lee MS, Park CK. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2015;21:3928-3935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (16)] |
| 8. | Si YQ, Wang XQ, Fan G, Wang CY, Zheng YW, Song X, Pan CC, Chu FL, Liu ZF, Lu BR, Lu ZM. Value of AFP and PIVKA-II in diagnosis of HBV-related hepatocellular carcinoma and prediction of vascular invasion and tumor differentiation. Infect Agent Cancer. 2020;15:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Kladney RD, Bulla GA, Guo L, Mason AL, Tollefson AE, Simon DJ, Koutoubi Z, Fimmel CJ. GP73, a novel Golgi-localized protein upregulated by viral infection. Gene. 2000;249:53-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 208] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Hu JS, Wu DW, Liang S, Miao XY. GP73, a resident Golgi glycoprotein, is sensibility and specificity for hepatocellular carcinoma of diagnosis in a hepatitis B-endemic Asian population. Med Oncol. 2010;27:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Xu WJ, Guo BL, Han YG, Shi L, Ma WS. Diagnostic value of alpha-fetoprotein-L3 and Golgi protein 73 in hepatocellular carcinomas with low AFP levels. Tumour Biol. 2014;35:12069-12074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Zhang Z, Zhang Y, Wang Y, Xu L, Xu W. Alpha-fetoprotein-L3 and Golgi protein 73 may serve as candidate biomarkers for diagnosing alpha-fetoprotein-negative hepatocellular carcinoma. Onco Targets Ther. 2016;9:123-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Liang R, Liu Z, Piao X, Zuo M, Zhang J, Li Y, Lin Y. Research progress on GP73 in malignant tumors. Onco Targets Ther. 2018;11:7417-7421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Linder S, Havelka AM, Ueno T, Shoshan MC. Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Lett. 2004;214:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Morris KL, Tugwood JD, Khoja L, Lancashire M, Sloane R, Burt D, Shenjere P, Zhou C, Hodgson C, Ohtomo T, Katoh A, Ishiguro T, Valle JW, Dive C. Circulating biomarkers in hepatocellular carcinoma. Cancer Chemother Pharmacol. 2014;74:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Chalin A, Turlin B, Ousmen A, Michalak S, Mueller J, Mueller S, Legros L, Bardou-Jacquet E, Viel JF, Samson M, Moirand R. Non-invasive diagnosis of alcohol-related steatohepatitis in patients ongoing alcohol withdrawal based on cytokeratin 18 and transient elastography. Aliment Pharmacol Ther. 2023;58:80-88. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Lavallard VJ, Bonnafous S, Patouraux S, Saint-Paul MC, Rousseau D, Anty R, Le Marchand-Brustel Y, Tran A, Gual P. Serum markers of hepatocyte death and apoptosis are non invasive biomarkers of severe fibrosis in patients with alcoholic liver disease. PLoS One. 2011;6:e17599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Strnad P, Stumptner C, Zatloukal K, Denk H. Intermediate filament cytoskeleton of the liver in health and disease. Histochem Cell Biol. 2008;129:735-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Mueller S, Nahon P, Rausch V, Peccerella T, Silva I, Yagmur E, Straub BK, Lackner C, Seitz HK, Rufat P, Sutton A, Bantel H, Longerich T. Caspase-cleaved keratin-18 fragments increase during alcohol withdrawal and predict liver-related death in patients with alcoholic liver disease. Hepatology. 2017;66:96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Waidmann O, Köberle V, Bettinger D, Trojan J, Zeuzem S, Schultheiß M, Kronenberger B, Piiper A. Diagnostic and prognostic significance of cell death and macrophage activation markers in patients with hepatocellular carcinoma. J Hepatol. 2013;59:769-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Elalfy H, Besheer T, Arafa MM, El-Hussiny MA, El Latif MA, Alsayed SAM. Caspase-Cleaved Cytokeratin 18 Fragment M30 as a Potential Biomarker of Macrovascular Invasion in Hepatocellular Carcinoma. J Gastrointest Cancer. 2018;49:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, Morse J, Hull D, Patman G, Kagebayashi C, Hussain S, Graham J, Reeves H, Satomura S. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 23. | Liu M, Wu R, Liu X, Xu H, Chi X, Wang X, Zhan M, Wang B, Peng F, Gao X, Shi Y, Wen X, Ji Y, Jin Q, Niu J. Validation of the GALAD Model and Establishment of GAAP Model for Diagnosis of Hepatocellular Carcinoma in Chinese Patients. J Hepatocell Carcinoma. 2020;7:219-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Xie DY, Zhu K, Ren ZG, Zhou J, Fan J, Gao Q. A review of 2022 Chinese clinical guidelines on the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2023;12:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 89] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 25. | Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2833] [Cited by in RCA: 3292] [Article Influence: 329.2] [Reference Citation Analysis (0)] |
| 26. | Ganesan P, Kulik LM. Hepatocellular Carcinoma: New Developments. Clin Liver Dis. 2023;27:85-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 229] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 27. | Kanwal F, Singal AG. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology. 2019;157:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 304] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 28. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1637] [Article Influence: 204.6] [Reference Citation Analysis (0)] |
| 29. | Zhu M, Li M. Inhibition of Autophagy and Immune Response: Alpha-fetoprotein Stimulates Initiation of Liver Cancer. Scientific Arch. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Samban SS, Hari A, Nair B, Kumar AR, Meyer BS, Valsan A, Vijayakurup V, Nath LR. An Insight Into the Role of Alpha-Fetoprotein (AFP) in the Development and Progression of Hepatocellular Carcinoma. Mol Biotechnol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Głowska-Ciemny J, Szymański M, Kuszerska A, Malewski Z, von Kaisenberg C, Kocyłowski R. The Role of Alpha-Fetoprotein (AFP) in Contemporary Oncology: The Path from a Diagnostic Biomarker to an Anticancer Drug. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 32. | Huang J, Liu FC, Li L, Zhou WP, Jiang BG, Pan ZY. Nomograms to predict the long-time prognosis in patients with alpha-fetoprotein negative hepatocellular carcinoma following radical resection. Cancer Med. 2020;9:2791-2802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Stoffers P, Guckenbiehl S, Welker MW, Zeuzem S, Lange CM, Trebicka J, Herrmann E, Welsch C. Diagnostic and prognostic significance of cell death markers in patients with cirrhosis and acute decompensation. PLoS One. 2022;17:e0263989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Godin C, Louandre C, Bodeau S, Diouf M, Saidak Z, Conte MA, Chauffert B, Barbare JC, Barget N, Trinchet JC, Ganne N, Galmiche A. Biomarkers of apoptosis and necrosis in patients with hepatocellular carcinoma treated with sorafenib. Anticancer Res. 2015;35:1803-1808. [PubMed] |
| 35. | Samman BS, Hussein A, Samman RS, Alharbi AS. Common Sensitive Diagnostic and Prognostic Markers in Hepatocellular Carcinoma and Their Clinical Significance: A Review. Cureus. 2022;14:e23952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Zhang X, Wu LN, Li XQ, Luo X, Liu SW, Zhang L, Nawaz S, Ma LN, Ding XC. Whether the Golgi protein 73 could be a diagnostic serological marker in hepatocellular carcinoma: a meta analysis. BMC Gastroenterol. 2023;23:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 37. | Pang BY, Leng Y, Wang X, Wang YQ, Jiang LH. A meta-analysis and of clinical values of 11 blood biomarkers, such as AFP, DCP, and GP73 for diagnosis of hepatocellular carcinoma. Ann Med. 2023;55:42-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 38. | Morota K, Nakagawa M, Sekiya R, Hemken PM, Sokoll LJ, Elliott D, Chan DW, Dowell BL. A comparative evaluation of Golgi protein-73, fucosylated hemopexin, α-fetoprotein, and PIVKA-II in the serum of patients with chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Clin Chem Lab Med. 2011;49:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Yamamoto K, Imamura H, Matsuyama Y, Kume Y, Ikeda H, Norman GL, Shums Z, Aoki T, Hasegawa K, Beck Y, Sugawara Y, Kokudo N. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 2010;45:1272-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 40. | Liu L, Wang Q, Zhao X, Huang Y, Feng Y, Zhang Y, Fang Z, Li S. Establishment and validation of nomogram model for the diagnosis of AFP-negative hepatocellular carcinoma. Front Oncol. 2023;13:1131892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |